ABSTRACT
Evidence has demonstrated that miRNAs play an irreplaceable role in tumorigenesis and progression of a broad range of cancers, including gastric cancer. Among these miRNAs, miR-10a and miR-10b have been identified to critically participate in gastric carcinogenesis and malignant progression. In this review, we briefly describe the role of miR-10a and miR-10b in gastric cancer, especially in the regulation of cell proliferation, apoptosis, cell cycle, migration, invasion and metastasis, drug resistance, and cancer stem cells. Furthermore, we highlight several compounds that target the miR-10 family and exhibit antitumor activity in cancer cells. Moreover, we conclude that targeting the miR-10 family might be a promising approach for the treatment of gastric cancer.
KEYWORDS: Gastric cancer, miR-10, treatment, proliferation, invasion
Introduction
Gastric cancer is one of the common tumors in the gastrointestinal tract and one of the leading causes of death from malignancies in the world [1,2] . In recent years, the incidence rate of gastric cancer has been increasing [3]. Because it is difficult to diagnose gastric cancer at the early stage because of no clinical symptoms, some patients are diagnosed at an advanced stage, typically with metastasis [4]. The treatment strategy includes radical surgery, chemotherapy, immune therapy, and radiation therapy; however, the outcome of the treatment is not satisfactory [5–9]. The 5-year survival rate of gastric cancer is still low, indicating a poor prognosis. The disappointing outcome of treatment and poor prognosis are due to the lack of biomarkers for early detection and therapeutics [10]. Therefore, identification of molecular mechanisms regarding gastric tumorigenesis and metastasis and discovery of molecular biomarkers for effective prediction of gastric cancer are pivotal to improve the therapeutic efficacy for gastric cancer patients [11–13].
Evidence has verified that a few oncogenes and tumor suppressive genes are involved in gastric carcinogenesis and progression [4,14,15]. Recently, non-coding RNAs, including microRNAs, have been identified to participate into gastric cancer development due to the regulation of key factors in cellular signaling pathways [16–20]. MiRNAs are a family of non-coding small RNAs with 20–25 nucleotides in length [21,22]. It is clear that miRNAs negatively control protein expression and govern cellular processes, including proliferation, apoptosis, cell cycle, motility and metastasis [23]. Moreover, miRNA-mediated regulation of gene expression acts at the post-transcriptional level. Typically, miRNAs can bind with the 3' untranslated region of target mRNAs via base-pairing, leading to inhibition of gene expression [24,25]. It has been documented that one miRNA can target several downstream genes and one gene can also be regulated by multiple miRNAs. Because the target genes of miRNAs have different biological functions as oncogenes or tumor suppressor genes, miRNAs perform oncogenic or tumor suppressive roles in tumorigenesis [26,27]. Evidence has suggested that miRNAs regulate tumor development in a plenty of human malignancies, including gastric cancer [19,20,28–30]. Among these miRNAs, miR-10a and miR-10b have been identified to have a critical role in gastric carcinogenesis and malignant progression. In this review, we briefly describe the role of miR-10a and miR-10b in gastric cancer, especially in the regulation of cell growth, proliferation, apoptotic cell death, cell cycle progression, migratory ability, invasive capacity, metastasis, drug resistance and cancer stem cells (Table 1). We also highlight multiple compounds that exert anticancer activity in human cancer cells due to targeting the miR-10 family. Notably, we provide the potential questions for the miR-10b family in the current study and future perspective. Lastly, we conclude that targeting the miR-10 family might be a potential approach for gastric cancer therapy.
Table 1.
Role of miR-10b in gastric cancer
| Targets | Signaling pathway | Experimental model | Functions | References |
|---|---|---|---|---|
| HOXD10 | Upregulation of RhoC expression and activation of Akt pathway | MKN45, BGC-823, GES-1 cells | Promotes cell proliferation and invasion | [51,52] |
| PTEN | Activation of pAkt and S6, mTOR pathway | SGC-7901, MGC-803 | Promotes viability and colony formation | [49] |
| KLF4 | N/A | Cancer tissues, cell lines | N/A | [53] |
| KLF11 | Increases TGFβR1 expression | Human skin fibroblast HSF cells | Enhances the migration of fibroblasts | [49] |
| N/A | N/A | 5-FU-resistant SGC-7901 cells | Promotes 5-FU resistance | [59] |
| N/A | N/A | Cisplatin-resistant MGC-803 | Promotes cisplatin resistance | [60] |
| N/A | KLF-4, SOX-2, NANOG | AGS and MKN-45 cells | Promotes cancer stem cells | [64] |
| MAPRE1, HOXD4 | N/A | SNU601, SNU638, MKN1, MKN74 cells | Inhibits cell growth and colony formation | [65] |
| Tiam1 | N/A | HGC-27, MKN-45, MGC-803 and SGC-7901 | Inhibits cell growth, induces apoptosis, represses migration and invasion | [66] |
MiR-10 family
It has been documented that the miR-10 family has two members: miR-10a and miR-10b [31,32]. There is only one base difference between these two members, indicating that miR-10a and miR-10b could have overlapping functions [33,34]. In addition, miR-10a locates upstream of Hoxb4, while miR-10b resides upstream of Hoxd4 in mammals [34]. Moreover, miR-10a/b targets multiple HOX transcripts, such as HOXA1, HOXA3, HOXD10, HOXB1, HOXB3, HOXD4 [33]. Besides these HOX genes, the miR-10 family has been found to regulate many other targets. A mountain of studies have demonstrated that the miR-10 family has an essential function to participate into oncogenesis in multiform types of cancers, such as melanoma, liver cancer, leukemia, colon cancer, glioma, breast cancer, head and neck squamois cell carcinoma [35–40]. Here, we discuss the functions of the miR-10 family in gastric cancer and its underlying molecular mechanisms.
Role of miR-10a in gastric cancer
Oncogenic role of miR-10a
One study used qRT-PCR to obtain the expression of several miRNAs in non-cancer, cancer adjacent, and gastric cancer tissues. This study observed that miR-10a was upregulated in adjacent tissues and gastric cancer tissues compared with non-cancer tissues [41]. Moreover, higher expression of miR-10a in gastric cancer specimens was validated by the TCGA database. Furthermore, miR-10a could be useful for discriminate cancer tissues with big accuracy from non-cancer tissues [41]. One miRNA expression profile was performed to detect the different miRNAs between primary gastric cancer tissues and paired lymph node metastasis (LNM) tissues [42]. This study showed some miRNAs were differentially expressed in these tissues via mercury LNA miRNA array. Specially, 106 miRNAs, including miR-24-1, miR-1284 and miR-510, were increased in five lymph node metastasis specimens compared with five primary gastric tumor tissues by miRNA array [42]. However, 76 miRNAs, including miR-10a, were elevated in metastasis tissues. In line with this point, miR-10a expression was correlated with LNM in 33 patients with gastric cancer. Moreover, this study measured the expression of miR-10a in the three gastric cancer cell lines, MKN-28, SGC7901 and AGSand cells, and human gastric epithelium cell line GES-1 gastric mucosal cell line by qRT-PCR, and found that miR-10a was significantly elevated in gastric cancer cell lines [42]. Furthermore, two gastric cancer cell lines from lymph node metastasis were used and observed that miR-10a expression was elevated compared with the primary tumor origin cell line [42]. Further bioinformatic analysis revealed that miR-10a has several target genes that were associated with a variety of signaling pathways for promoting carcinogenesis and metastasis. This study further suggested that miR-10a was critically involved in gastric cancer metastasis, leading to LNM from primary gastric sites [42]. Lu et al. reported that miR-10a promoted migratory ability in MKN-45 cells by wound healing assay and Matrigel invasion assay [43]. MAPK8IP1, a target of miR-10a, was inhibited by miR-10a overexpression. Upregulation of MAPK8Ip1 abrogated the miR-10a effectors-induced migration and invasion in MKN45 cells [43].
Tumor suppressive role of miR-10a
Jia et al. reported that miR-10a was decreased due to the hypermethylation of the CpG islands in miR-10a [44]. This group measured the expression of miR-10a in 100 gastric cancer patients and four gastric cancer cell lines by real-time PCR. They observed that miR-10a was decreased in patient’s tissues compared with their adjacent non-tumor specimens [44]. Similarly, miR-10a expression was lower in gastric cancer cell lines, including MGC-803, MKN-45, HGC-27 and SGC-7901, compared with GES normal cells. Moreover, the hypermethylation of CpG islands in miR-10a upstream was identified in some gastric cancer tissues and cell lines by quantitative methylation-specific PCR (qMSP) assay [44]. CCK-8 assays and colony formation assays revealed that upregulation of miR-10a inhibited viability of HGC-27 and MGC-803 and suppressed colony formation of HGC-27 cells [44]. Annexin V staining assay was used and found that overexpression of miR-10a increased apoptosis of HGC-27 and MGC-803 cells. Moreover, a wound healing assay and a Matrigel invasion assay illustrated that upregulation of miR-10a repressed migration and invasion of HGC-27 and MGC-803 cells [44]. Bioinformatics and Western blotting analysis revealed that miR-10a exhibited anti-tumor function in gastric cancer cells via downregulation of HOXA1 [44].
Role of miR-10b in gastric cancer
MiR-10b is associated with clinical parameters
One study from Dr. Fan’s group demonstrated that a seven-miRNA signature was correlated with the survival of gastric cancer patients [45]. This group measured the miRNA expression profile by real-time RT-PCR analysis in 100 gastric cancer specimens, and identified that seven miRNAs, including miR-10b, miR-21, let-7a, miR-126, miR-30a-5p, miR-338, were associated with overall survival and relapse-free survival in gastric cancer patients [45]. This finding indicated that miR-10b and other six miRNAs might be prognostic molecular markers in gastric cancer. Another group used mercury LNA Arrays to obtain miRNA expression profiles in 17 gastric carcinomas. They found 49 upregulated miRNAs, including miR-10b, and 39 downregulated miRNAs in gastric cancer [46]. The higher expression of miR-10b was confirmed by RT-PCR in 56 gastric tumor tissues and further validated in 436 paraffin-embedded cancer tissues [46]. Notably, miR-10b expression was linked to tumor sizes, TNM stage, Lauren classification, invasion depth, metastasis, and prognosis in gastric cancer. This study indicated that miR-10b might be useful in prediction of the prognosis of gastric carcinoma [46].
A study examined the expression of miR-10b by qRT-PCR assays in gastric cancer patients located in the Central European population and demonstrated that miR-10b was tendentiously increased in 67 gastric cancer samples in contrast to non-tumor specimens [47]. The increased expression of miR-10b was linked to advanced stages, worse prognosis in patients with gastric cancer [47]. Gao et al. also reported that miR-10b expression was elevated in 109 gastric cancer samples via in situ hybridization and RT-PCR assays compared with paracancerous tissues [48]. Clinically, miR-10b expression was correlated with tumor size, TNM stages, LNM, and distant metastasis in gastric cancer patients. Moreover, miR-10b expression was associated with poor prognosis in gastric cancer patients with stage I, II and III [48]. Yan et al. studied the activity of miR-10b-5p in gastric cancer [49]. They reported that miR-10b-5p was downregulated in normal gastric mucosa tissues compared with gastric cancer tissues. Moreover, miR-10b-5p expression was increased in gastric cancer patients with stages III+IV compared with stages I + II. Importantly, overexpression of miR-10b-5p in serum existed in gastric cancer cases with advanced stages [49]. Strikingly, exosomal miR-10b-5p expression was elevated in gastric cancer patients, especially in cases with advanced stages [49]. Via small RNA sequencing of plasma exosomes, miR-10b-5p was characterized as a biomarker for predicting metastasis of gastric cancer [50]. In gastric cancer patients with LNM, exosomal miR-10b-5p was remarkably increased [50]. Interestingly, exosomal miR-101-3p was highly existed in gastric cancer patients with ovarian metastasis, while miR-143-5p was promoted in gastric cancer cases with liver metastasis [50]. Therefore, different exosomal miRNA biomarkers might be useful for distinguishing patients with gastric cancer, who have various types of metastasis [50].
MiR-10b exerts biological functions via inhibition of downstream targets
There is mounting evidence showing that miR-10b performed its biological functions via the suppression of downstream targets in human cancers. Liu et al. used real-time PCR analysis and revealed that miR-10b expression was elevated in gastric patients with LNM [51]. Consistently, miR-10b was highly expressed in BGC-823 and MKN45 cells, which have the high metastatic ability. Moreover, suppression of miR-10b by its inhibitors retarded invasion of MKN45 cells, while overexpression of miR-10b increased invasiveness of GES-1 cells. The further study validated that miR-10b promoted invasion via repression of HOXD10 in gastric cancer cells, leading to upregulation of RhoC expression and activation of the Akt signaling pathway [51]. Similarly, high expression of miR-10b was reported in multiple gastric cell lines, such as SGC-7901, HCG-27, MKN-28, MKN-45, AGS, BGC-823 cell lines [52]. Inhibition of miR-10b by inhibitors reduced proliferation and migration of BGC-823, MKN-45, and SGC-7901 cells. Moreover, miR-10b enhanced migration and invasion of gastric cancer cells through repression of HOXD10 expression [52].
Ma and colleagues revealed upregulation of miR-10b expression in 65 gastric cancer specimens in contrast to normal adjacent tissue by real-time PCR [53]. Kruppel-like factor 4 (KLF4) was identified as a downstream target of miR-10b in gastric cancer. In consistent, miR-10b expression was negatively correlated with KLF4 expression in gastric tumor specimens [53]. One group reported that miR-10b-5p inhibited the expression of PTEN in SGC-7901 cells, leading to activation of pAkt and S6; the latter is one target of the mTOR signaling pathway [49]. Inhibition of miR-10b-5p repressed viability and colony formation in MGC-803 and SGC-7901 gastric cancer cells. Consistently, exosomal miR-10b-5p promoted cell proliferation in gastric cancer [49]. Interestingly, miR-10b-5p affected its target KLF11 expression and increased TGFβR1 of human skin fibroblast HSF cells, resulting in enhancement of the migration of fibroblasts [49].
Role of miR-10b in drug resistance
Evidence revealed that miR-10b governed drug resistance in various kinds of human cancers [54–57]. For example, miR-10b conferred resistance to the 5-fluorouracil in colorectal cancer cells in part via suppression of pro-apoptotic BIM [54]. In triple-negative breast cancer (TNBC), miR-10b-5p was downregulated in doxorubicin resistance of TNBC cells [55]. In estrogen receptor (ER)-positive breast cancer cells, including MCF-7 and T47D cells, miR-10b facilitated tamoxifen resistance via inhibition of HDAC4 [56]. In esophageal cancer, miR-10b enhanced cisplatin resistance via inhibition of peroxisome proliferator-activated receptor-γ (PPARγ), resulting in activation of Akt/mTOR/p70S6K pathway [57]. In ovarian cancer cells, miR-10b was reported to participate in lncRNA CHRF-mediated resistance of cisplatin [58]. Wang et al. used miRNA microarray to screen the miRNA expression profile in a 5-fluorouracil (5-FU)-resistant SGC-7901 cell line [59]. This profile illustrated 9 upregulated miRNAs, including miR-10b and miR-22, and 18 decreased miRNAs in 5-FU-resistant cells. The expression of miR-10b was further validated by qRT-PCR in 5-FU-resistant cells. Therefore, miR-10b could be useful as a target to develop a chemo-sensitizing agent [59]. In line with this report, miR-10b was found to be increased in cisplatin-resistant MGC-803 gastric cancer cells by qRT-PCR assay, indicating that miR-10b is involved in cisplatin resistance [60].
Role of miR-10b in cancer stem cells
Gastric cancer stem cells have been discoverer and provided new windows for the prevention and treatment of gastric cancer [61]. Several reports have demonstrated that miR-10b participated in the regulation of self-renewal of cancer stem cells [62,63]. One group found the correlation of miR-10b with EMT and melanoma stem cells. Upregulation of miR-10b was observed in melanospheres, suggesting that miR-10b might take part in metastasis and stemness potential in melanoma [63]. Another group reported that miR-10b enhanced self-renewal via inhibition of PTEN and sustained activation of Akt in breast cancer stem cells [62]. Accumulating evidence has supported that miR-10b participates in the development of gastric cancer stem cells. One study reported that gastro-spheres were obtained from AGS and MKN-45 gastric cancer cells and were validated by evaluating the expression of several stemness proteins, including KLF-4, SOX-2, NANOG [64]. Gastro-spheres were also confirmed by increased clonogenicity activity, and enhanced resistance to cisplatin and docetaxel. Moreover, qRT-PCR was utilized and validated that miR-10b is elevated in gastro-spheres derived from AGS and MKN-45 cells. This study implied that miR-10b might take part in controlling cancer stem cells in gastric cancer [64].
Tumor suppressive role of miR-10b
One group found that miR-10b was silenced in gastric tumor cells because of promoter hypermethylation [65]. Moreover, miR-10b methylation was linked to patient age and existed more frequently in intestinal-type gastric cancer than in diffuse-type tumors. One DNA methylation inhibitor 5-aza-CdR (AZA) exposure of SNU601, SNU638, MKN1, MKN74 cells elevated the expression of miR-10a via demethylation [65]. Remarkably, a microtubule-associated protein, RP/EB family member 1 (MAPRE1) was identified as a downstream target of miR-10b in gastric cancer cells. Therefore, miR-10b acted as a tumor suppressor gene via inhibition of MAPRE1 oncoprotein [65]. In line with this report, Li et al. reported that miR-10b acted as a tumor suppressor in gastric cancer [66]. This study found that the level of miR-10b was downregulated in gastric cancer tissues compared with adjacent normal specimens via detecting the measure of miR-10b by qRT-PCR in 100 patients with gastric cancer. Importantly, a lower expression of miR-10b was correlated with lymphatic invasion in gastric cancer patients [66]. In support of this finding, the expression of miR-10b was downregulated in HGC-27, MKN-45, MGC-803, and SGC-7901 gastric cancer cells. Upregulation of miR-10b repressed proliferation of HGC-27 and SGC-7901 via CCK-8 assays, and stimulated cell apoptosis by annexin V staining approach [66]. The data from a wound healing assay and a Tranwell invasion approach showed that miR-10b overexpression blocked migration and invasion in both HGC-27 and SGC-7901 gastric cancer cells [66]. Hypermethylation of miR-10b existed in gastric tumor tissues and tumor cells by MSP assays. AZA treatment increased the level of miR-10b in HGC-27 and SGC-7901 cells. Mechanistically, miR-10b performed antitumor activity via targeting Tiam1 in gastric cancer cells [66]. In addition, a comprehensive study by meta-analysis and TCGA database showed that lower expression of miR-10b was found in gastric tumor tissues than that in adjacent tissues [36]. Meanwhile, miR-10b-5p was linked to worse survival outcomes [36]. Our previous study revealed that miR-10b-5p suppressed the proliferation, migration and invasion via inhibition of TIAM1 in gastric cancer cells [67]. Moreover, we found that tumor suppressor RUNX3 and CBFβ increased the expression of miR-10b-5p and attenuated gastric cancer progression [67].
Targeting miR-10 family for cancer therapy
Dr. Weinberg’s group used miR-10b antagomirs, which is obtained from chemically modified anti-miRNA oligonucleotides, and found that silencing miR-10b suppressed breast cancer metastasis in a mouse mammary tumor model via upregulation of Hoxd10, one of miR-10b targets [68]. Interestingly, miR-10b depletion did not inhibit primary mammary tumor growth but blocked the formation of lung metastases [68]. This study indicated that miR-10b antagomir could be a potential candidate for the suppression of tumor metastasis [68]. Linifanib, a common multi-tyrosine kinase inhibitor, was reported to repress the expression of miR-10b in breast cancer cells and liver cancer cells, leading to suppression of the oncogenic activity of miR-10b [69]. Lidocaine, a local anesthetic in the clinic, has been identified to inhibit oncogenesis in various types of cancers. Zhang et al. reported that lidocaine alleviated resistance of cisplatin and retarded migration and invasion of cisplatin-resistant MGC-803 cells [60]. Moreover, lidocaine promoted cisplatin sensitivity via inhibiting miR-10b expression, leading to suppression of Akt/mTOR and β-catenin pathways [60]. Therefore, targeting the miR-10 family might be a potential approach for treating patients with gastric cancer.
Conclusion and perspective
In conclusion, accumulated evidence demonstrated that the miR-10 family is critically involved in gastric oncogenesis and progression in part via inhibiting the expression of downstream factors in gastric cancer (Figure 1). It is pivotal to note that several questions regarding the role of the miR-10 family in gastric carcinogenesis need to be addressed. Most studies focused on the functions and mechanism of miR-10b in gastric tumorigenesis. Therefore, further investigations are required to determine the role of miR-10a in gastric cancer development. There is a challenge to detect sensitively the expression of miR-10b in gastric cancer patients. Ki et al. developed enzyme-assisted target recycling and LSPR probe for measuring the miR-10b in gastric cancer [70]. Using this system, 5 pM- 10 nM of miR-10b could be detected, indicating that sensitive detection of miR-10b could be realized for future application in monitoring gastric cancer [70]. Moreover, it has been known that one miRNA targets hundreds of genes in oncogenesis. What other downstream genes are regulated by miR-10a and miR-10b in gastric cancer? The miRNA expression often is regulated in a temporal and spatial manner. How miR-10a and miR-10b are temporally and spatiallycontrolled in biological processes? We believe that deeper investigations will provide evidence for targeting the miR-10 family as a potential application for treating gastric cancer patients in future.
Figure 1.
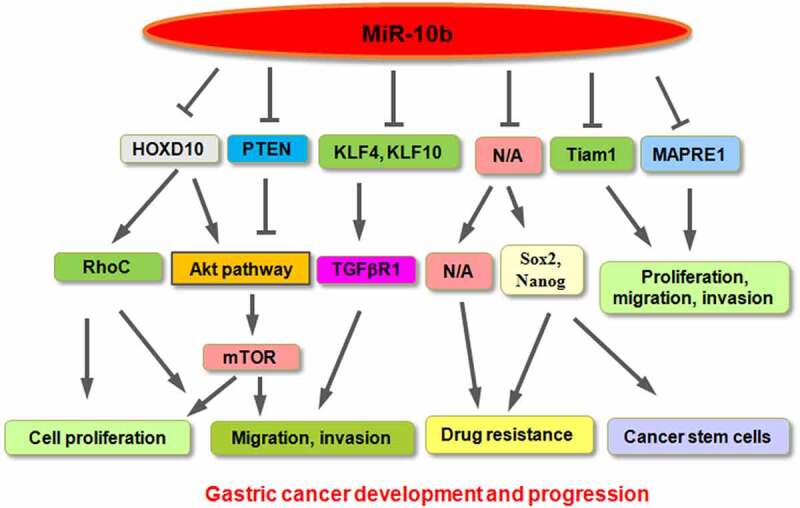
A diagram showing the role of miR-10b in gastric cancer development and progression
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- [2].Joshi SS, Badgwell BD.. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- [4].Seeneevassen L, Bessede E, Megraud F, et al. Gastric cancer: advances in carcinogenesis research and new therapeutic strategies. Int J Mol Sci. 2021;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olnes MJ, Martinson HA. Recent advances in immune therapies for gastric cancer. Cancer Gene Ther. 2021. DOI: 10.1038/s41417-021-00310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ng SP, Leong T. Role of radiation therapy in gastric cancer. Ann Surg Oncol. 2021. DOI: 10.1245/s10434-021-09639-y [DOI] [PubMed] [Google Scholar]
- [7].Yoneda A, Kuroki T, Eguchi S. Immunotherapeutic advances in gastric cancer. Surg Today. 2021. DOI: 10.1007/s00595-021-02236-2 [DOI] [PubMed] [Google Scholar]
- [8].Irino T, Matsuda S, Wada N, et al. Essential updates 2019/2020: perioperative and surgical management of gastric cancer. Ann Gastroenterol Surg. 2021;5:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kawazoe A, Shitara K, Boku N, et al. Current status of immunotherapy for advanced gastric cancer. Jpn J Clin Oncol. 2021;51:20–27. [DOI] [PubMed] [Google Scholar]
- [10].Sasahara M, Kanda M, Kodera Y. Update on molecular biomarkers for diagnosis and prediction of prognosis and treatment responses in gastric cancer. Histol Histopathol. 2021;18326. DOI: 10.14670/HH-18-326 [DOI] [PubMed] [Google Scholar]
- [11].Slagter AE, Vollebergh MA, Jansen EPM, et al. Towards personalization in the curative treatment of gastric cancer. Front Oncol. 2020;10:614907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petrillo A, Smyth EC. Biomarkers for precision treatment in gastric cancer. Visc Med. 2020;36:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ramezankhani R, Solhi R, Es HA, et al. Novel molecular targets in gastric adenocarcinoma. Pharmacol Ther. 2021;220:107714. [DOI] [PubMed] [Google Scholar]
- [14].Tabibzadeh A, Tameshkel FS, Moradi Y, et al. Signal transduction pathway mutations in gastrointestinal (GI) cancers: a systematic review and meta-analysis. Sci Rep. 2020;10:18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, et al. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021;898:173983. [DOI] [PubMed] [Google Scholar]
- [16].Li Z, Lu M, Zhou Y, et al. Role of long non-coding RNAs in the chemoresistance of gastric cancer: a systematic review. Onco Targets Ther. 2021;14:503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shrestha S, Hsu SD, Huang WY, et al. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014;3:878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han BW, Li ZH, Liu SF, et al. A comprehensive review of microRNA-related polymorphisms in gastric cancer. Genet Mol Res. 2016;15. DOI: 10.4238/gmr.15028289 [DOI] [PubMed] [Google Scholar]
- [19].Ouyang J, Xie Z, Lei X, et al. Clinical crosstalk between microRNAs and gastric cancer. Int J Oncol. 2021;58:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kipkeeva F, Muzaffarova T, Korotaeva A, et al. MicroRNA in gastric cancer development: mechanisms and biomarkers. Diagnostics (Basel). 2020;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. [DOI] [PubMed] [Google Scholar]
- [23].Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732. [DOI] [PubMed] [Google Scholar]
- [24].Brennan GP, Henshall DC. MicroRNAs as regulators of brain function and targets for treatment of epilepsy. Nat Rev Neurol. 2020;16:506–519. [DOI] [PubMed] [Google Scholar]
- [25].Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20:5–20. [DOI] [PubMed] [Google Scholar]
- [26].Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21:22–36. [DOI] [PubMed] [Google Scholar]
- [27].Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Giuppi M, La Salvia A, Evangelista J, et al. The role and expression of angiogenesis-related miRNAs in gastric cancer. Biology (Basel). 2021;10. DOI: 10.3390/biology10020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu X, Ma R, Yi B, et al. MicroRNAs are involved in the development and progression of gastric cancer. Acta Pharmacol Sin. 2021;42:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weidle UH, Birzele F, Nopora A. microRNAs promoting growth of gastric cancer xenografts and correlation to clinical prognosis. Cancer Genomics Proteomics. 2021;18:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elgeshy KM, Abdel Wahab AHA. Role, significance and association of microRNA-10a/b in physiology of cancer. Microrna. 2020. DOI: 10.2174/2211536609666201026155519 [DOI] [PubMed] [Google Scholar]
- [32].Sheedy P, Medarova Z. The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res. 2018;8:1674–1688. [PMC free article] [PubMed] [Google Scholar]
- [33].Tehler D, Hoyland-Kroghsbo NM, Lund AH. The miR-10 microRNA precursor family. RNA Biol. 2011;8:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lund AH. miR-10 in development and cancer. Cell Death Differ. 2010;17:209–214. [DOI] [PubMed] [Google Scholar]
- [35].Huang Q, Song Q, Zhong W, et al. MicroRNA-10b and the clinical outcomes of various cancers: a systematic review and meta-analysis. Clin Chim Acta. 2017;474:14–22. [DOI] [PubMed] [Google Scholar]
- [36].Mei L, Lu Z, Shen Z, et al. The prognostic and diagnostic values of MicroRNA-10b in gastric cancer: a comprehensive study based on meta-analysis and TCGA database. Medicine (Baltimore). 2020;99:e20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Balatti V, Oghumu S, Bottoni A, et al. MicroRNA profiling of salivary duct carcinoma versus Her2/Neu overexpressing breast carcinoma identify miR-10a as a putative breast related oncogene. Head Neck Pathol. 2019;13:344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bi L, Sun L, Jin Z, et al. MicroRNA-10a/b are regulators of myeloid differentiation and acute myeloid leukemia. Oncol Lett. 2018;15:5611–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhai L, Li Y, Lan X, et al. MicroRNA-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting BDNF. Exp Ther Med. 2017;14:6147–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bourguignon LY, Wong G, Shiina M. Up-regulation of histone methyltransferase, DOT1L, by matrix hyaluronan promotes MicroRNA-10 expression leading to tumor cell invasion and chemoresistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2016;291:10571–10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pereira AL, Magalhaes L, Moreira FC, et al. Epigenetic field cancerization in gastric cancer: microRNAs as promising biomarkers. J Cancer. 2019;10:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen W, Tang Z, Sun Y, et al. miRNA expression profile in primary gastric cancers and paired lymph node metastases indicates that miR-10a plays a role in metastasis from primary gastric cancer to lymph nodes. Exp Ther Med. 2012;3:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lu Y, Wei G, Liu L, et al. Direct targeting of MAPK8IP1 by miR-10a-5p is a major mechanism for gastric cancer metastasis. Oncol Lett. 2017;13:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jia H, Zhang Z, Zou D, et al. MicroRNA-10a is down-regulated by DNA methylation and functions as a tumor suppressor in gastric cancer cells. PLoS One. 2014;9:e88057. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [45].Li X, Zhang Y, Zhang Y, et al. Survival prediction of gastric cancer by a seven-microRNA signature. Gut. 2010;59:579–585. [DOI] [PubMed] [Google Scholar]
- [46].Wang YY, Ye ZY, Zhao ZS, et al. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278–1285. [DOI] [PubMed] [Google Scholar]
- [47].Obermannova R, Redova-Lojova M, Vychytilova-Faltejskova P, et al. Tumor expression of miR-10b, miR-21, miR-143 and miR-145 is related to clinicopathological features of gastric cancer in a central european population. Anticancer Res. 2018;38:3719–3724. [DOI] [PubMed] [Google Scholar]
- [48].Gao Y, Xu Z, Yuan F, et al. Correlation of expression levels of micro ribonucleic Ccid-10b (miR-10b) and micro ribonucleic acid-181b (miR-181b) with gastric cancer and its diagnostic significance. Med Sci Monit. 2018;24:7988–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yan T, Wang X, Wei G, et al. Exosomal miR-10b-5p mediates cell communication of gastric cancer cells and fibroblasts and facilitates cell proliferation. J Cancer. 2021;12:2140–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang Y, Han T, Feng D, et al. Screening of non-invasive miRNA biomarker candidates for metastasis of gastric cancer by small RNA sequencing of plasma exosomes. Carcinogenesis. 2020;41:582–590. [DOI] [PubMed] [Google Scholar]
- [51].Liu Z, Zhu J, Cao H, et al. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int J Oncol. 2012;40:1553–1560. [DOI] [PubMed] [Google Scholar]
- [52].Wang YY, Li L, Ye ZY, et al. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J Surg Oncol. 2015;13:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ma Z, Chen Y, Min L, et al. Augmented miR-10b expression associated with depressed expression of its target gene KLF4 involved in gastric carcinoma. Int J Clin Exp Pathol. 2015;8:5071–5079. [PMC free article] [PubMed] [Google Scholar]
- [54].Nishida N, Yamashita S, Mimori K, et al. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol. 2012;19:3065–3071. [DOI] [PubMed] [Google Scholar]
- [55].Ouyang M, Li Y, Ye S, et al. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ahmad A, Ginnebaugh KR, Yin S, et al. Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer. 2015;15:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wu K, Hu Y, Yan K, et al. microRNA-10b confers cisplatin resistance by activating AKT/mTOR/P70S6K signaling via targeting PPARgamma in esophageal cancer. J Cell Physiol. 2020;235:1247–1258. [DOI] [PubMed] [Google Scholar]
- [58].Tan WX, Sun G, Shangguan MY, et al. Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci Rep. 2020;10:14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang Y, Gu X, Li Z, et al. microRNA expression profiling in multidrug resistance of the 5Fuinduced SGC7901 human gastric cancer cell line. Mol Med Rep. 2013;7:1506–1510. [DOI] [PubMed] [Google Scholar]
- [60].Zhang X, Gu G, Li X, et al. Lidocaine alleviates cisplatin resistance and inhibits migration of MGC-803/DDP cells through decreasing miR-10b. Cell Cycle. 2020;19:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xiao S, Zhou L. Gastric stem cells: physiological and pathological perspectives. Front Cell Dev Biol. 2020;8:571536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bahena-Ocampo I, Espinosa M, Ceballos-Cancino G, et al. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016;17:648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fomeshi MR, Ebrahimi M, Mowla SJ, et al. Evaluation of the expressions pattern of miR-10b, 21, 200c, 373 and 520c to find the correlation between epithelial-to-mesenchymal transition and melanoma stem cell potential in isolated cancer stem cells. Cell Mol Biol Lett. 2015;20:448–465. [DOI] [PubMed] [Google Scholar]
- [64].Bakhshi M, Asadi J, Ebrahimi M, et al. Increased expression of miR-146a, miR-10b, and miR-21 in cancer stem-like gastro-spheres. J Cell Biochem. 2019;120:16589–16599. [DOI] [PubMed] [Google Scholar]
- [65].Kim K, Lee HC, Park JL, et al. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6:740–751. [DOI] [PubMed] [Google Scholar]
- [66].Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. [DOI] [PubMed] [Google Scholar]
- [67].Hu G, Shi Y, Zhao X, et al. CBFbeta/RUNX3-miR10b-TIAM1 molecular axis inhibits proliferation, migration, and invasion of gastric cancer cells. Int J Clin Exp Pathol. 2019;12:3185–3196. [PMC free article] [PubMed] [Google Scholar]
- [68].Ma L, Reinhardt F, Pan E, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Monroig-Bosque PDC, Shah MY, Fu X, et al. OncomiR-10b hijacks the small molecule inhibitor linifanib in human cancers. Sci Rep. 2018;8:13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ki J, Lee HY, Son HY, et al. Sensitive plasmonic detection of miR-10b in biological samples using enzyme-assisted target recycling and developed LSPR probe. ACS Appl Mater Interfaces. 2019;11:18923–18929. [DOI] [PubMed] [Google Scholar]


