Abstract
How cord blood (CB) CD34+ cell content and dose, and 8-allele human leukocyte antigen (HLA)-match, vary by patient ancestry is unknown. We analyzed cell content, dose and high-resolution HLA-match of units selected for CB transplantation (CBT) by recipient ancestry. Of 544 units (286 infused, 258 next best back-ups) chosen for 144 racially diverse adult patients (median weight 81 kilograms), the median total nucleated cell (TNC) (x 107) and CD34+ (x 105) content was higher for Europeans than for non-Europeans: 216 versus 197 (p = 0.002) and 160 versus 132 (p = 0.007), respectively. There were marked cell content disparities between ancestry groups with units selected for Africans having the lowest TNC (189 × 107) and CD34+ cell (122 × 105) contents. Units for non-Europeans were also more HLA-mismatched (p = 0.017). When only the 286 transplanted units were analyzed, the adverse effect of reduced cell content was exacerbated by higher weight in some groups. For example, northwestern Europeans (high patient weight, high unit cell content) had the best dosed units whereas Africans (high weight, low unit cell content) had the lowest. In Asians, low cell content was partially compensated by lower weight. Marked differences in 8-allele HLA-match distribution were also observed by ancestry group (e.g. 23% of units for northwestern Europeans were 3–4/8 HLA-matched versus 40% for southern Europeans, 46% for White Hispanics and 51% for Africans). During the study period, 20 additional patients (17 non-European, median weight 98 kilograms) did not receive a CBT due to lack of a suitable graft. CB extends transplant access to most patients but racial disparities exist in cell content, dose and HLA-match.
Introduction
An 8-allele HLA-matched volunteer is widely considered as the standard allogeneic donor choice in the absence of an HLA-identical sibling1–5. However, the majority of non-European, and many mixed ancestry and southern European patients, do not have available 8/8 HLA-matched donors6–8. Moreover, despite the increasing size of volunteer donor registries, we have recently demonstrated that this racial disparity in unrelated donor access is not appreciably improving9. CB is one of the currently available alternative stem cell sources. Due to the reduced stringency of required HLA-match6, 7, 9, CB extends transplant access to many patients without suitable adult donors including racial and ethnic minorities6, 7, 9. However, not all patients have access to optimal CB units10–12. Furthermore, current unit selection guidelines now require consideration of CD34+ cell dose and high resolution 8-allele donor-recipient HLA-match13–16, which could potentially limit the number of suitable units for minorities. How the CB unit characteristics of CD34+ cell content and dose, and high-resolution donor-recipient HLA-match, vary by patient race and ethnicity has not been described. In this study, we investigated this question in a diverse population of adult patients who underwent CB transplantation (CBT) at our center. We also analyzed the characteristics of patients without suitable CB grafts.
Methods
We analyzed the CB Bank reported pre-cryopreservation total nucleated cell (TNC) and total CD34+ cell content, the cryopreserved TNC and CD34+ cell dose (adjusted for patient body weight), and the 4–6/6 HLA-A, -B antigen, -DRB1 allele and 8-allele HLA-match (obtained from confirmatory typing), of infused and back-up units by recipient ancestry in adult patients who received their first unrelated donor CBT at our center between 7/2013–6/2018. The location of the Bank by patient ancestry, as well as the ancestry distribution of patients who did not have a suitable CB graft identified during the study period, were also evaluated.
Patient racial/ ethnic origins were prospectively obtained by detailed patient interview to ascertain the family history. Patients were then grouped as previously described6, 9. In addition, patients self-identified themselves as Black and/or Hispanic. We used similar categories as previous reports17, 18, but additionally divided patients of European ancestry into north-western, eastern, southern, and mixed Europeans. Non-Europeans were divided into Asian, African, white Hispanic, Middle Eastern, and mixed non-European groups. Patients of African ancestry included African Americans, and immigrants from Africa or the Caribbean. Non-European mixes included any patient with at least partial non-European origins even if partly European (but excluded those who self-identified as Black). Racial origins of the CB units were not available in many cases precluding analyses of recipient-donor ancestral matching. Institutional Review Board approval was obtained to perform this analysis.
CB graft selection
Searches of domestic and international CB banks were done via the National Marrow Donor Program (NMDP) or units were obtained directly from the New York Blood Center. Per our center policy for adult CBT nearly all patients received double unit grafts comprised of units that were considered the first and second best. Unit selection was based on: 1) optimal banking practices for all units (including mandatory red blood cell depletion and standard cryovolumes), 2) TNC and CD34+ dose, and 3) 4–6/6 HLA-match and HLA-A, -B, -C, -DRB1 allele match (obtained by confirmatory typing units of interest), as previously described16, 19, 20. Unit selection criteria were a minimum TNC dose of 1.5 × 107/kg/unit and usually a CD34+ dose of at least 1.0 × 105/kg/unit. In addition, units had to be at least 4/6 HLA-A, -B antigen, -DRB1 allele and ≥ 3/8 HLA-allele matched to the recipient. TNC and CD34+ doses were given priority over HLA-match in nearly all patients given the high average weight of our adult patient population. For double unit grafts the total dose and the unit-unit HLA-match were not considered. Presence of donor specific antibodies was taken into consideration but did not preclude CB unit selection21. Domestic units were given priority over international units if possible.
Back-up units were the next best domestic units that were fully typed at 8 HLA-alleles, reserved for the patient, and not shipped but immediately available in the case of an emergency (e.g. problems with shipping, thaw or unit quality)22. Such units are released back into the inventory once the patient has engrafted.
Statistical methods
CB cell dose and HLA characteristics by ancestry group were analyzed using summary statistics. Comparisons of continuous variables between groups were performed by using a permutation-based rank-sum test. To account for multiple units being selected or infused per analyzed patient, permutations were evaluated at the patient level. A similar permutation test was used to evaluate the association between patient ancestry group and unit origin using the odds ratio to characterize the association. Two-sided p-values < 0.05 were considered significant. Analysis was performed using R version 3.5.3 (The R Foundation for Statistical Computing). For original data contact barkerj@mskcc.org.
Results
Patient characteristics
The 144 transplanted patients in this analysis had a median age of 50 years (range 21–67) and a median weight of 81 kilograms (kg, range 48–138). Seventy-eight (54%) patients were male. Nearly all patients (141/144, 98%) were transplanted for hematologic malignancies, predominantly acute leukemia (102/144, 71%). Patients had highly diverse ancestries as listed in Tables 1 and 2, with 75 (52%) patients having either part or full non-European origins.
Table 1:
Characteristics of 544 units (infused or back-up) chosen for 144 patients by recipient ancestry.
| Patient Ancestry N patients (%) Weight Median (range) | N Units | TNC Content Median (range) | CD34+ Content Median (range) | TNC/ kg Dose Median (range) | CD34+/ kg Dose Median (range) | 8-allele HLA-match Median (range) |
|---|---|---|---|---|---|---|
| Europeans(n = 69, 48%) 82 kg (48–126) | 259 | 216 (122–417) | 160 (46–484) | 2.7 (1.5–7.1) | 1.9 (0.7–6.3) | 5 (2–7)* |
| Northwestern(n = 11, 8%) 91 kg (51–126) | 40 | 236 (144–417) | 198 (97–373) | 3 (1.6–5.8) | 2.2 (1.1–4.7) | 5 (3–6) |
| Eastern(n = 17, 12%) 82 kg (49–115) | 65 | 215 (136–397) | 150 (55–369) | 2.7 (1.6–7.1) | 1.9 (0.8–5.6) | 5 (3–7) |
| Southern(n = 26, 18%) 83 kg (48–115) | 100 | 205 (122–376) | 147 (46–484) | 2.5 (1.5–5.4) | 1.8 (0.7–6.3) | 5 (3–6) |
| Mixed(n = 15, 10%) 81 kg (55–116) | 54 | 216 (122–401) | 166 (86–335) | 2.6 (1.5–5) | 2 (0.8–4.7) | 5 (2–6)* |
| Non-Europeans(n = 75, 52%) 79 kg (48–138) | 285 | 197 (108–457) | 132 (29–557) | 2.5 (1.5–8.1) | 1.7 (0.6–8.3) | 5 (2–8)* |
| White Hispanic(n = 14, 10%) 84 kg (56–120) | 55 | 189 (108–441) | 157 (81–398) | 2.5 (1.5–6.4) | 1.8 (0.8–6.2) | 4 (3–6) |
| Asian(n = 21, 14%) 68 kg (48–115) | 79 | 191 (108–457) | 127 (29–320) | 2.7 (1.5–8.1) | 1.8 (0.6–5.4) | 5 (2–7)* |
| African(n = 23, 16%) 81 kg (52–138) | 84 | 189 (88–448) | 122 (47–557) | 2.4 (1.5–5.5) | 1.5 (0.6–6.8) | 5 (3–7) |
| Middle Eastern(n = 4, 3%) 80 kg (59–90) | 15 | 223 (128–369) | 124 (81–177) | 2.7 (1.7–6.2) | 1.7 (1.1–2.5) | 4 (3–5) |
| Mixed non-European(n = 13, 9%) 79 kg (61–109) | 52 | 211 (93–353) | 143 (62–554) | 2.7 (1.3–4.7) | 1.7 (0.7–8.3) | 5 (3–8) |
One Asian patient received a unit that was re-classified as 2/8 HLA-matched upon further expert review after selection. Two additional back-up units were 2/8 HLA-matched (not infused).
Table 2:
Characteristics of 286 transplanted units in 144 patients by recipient ancestry.
| Patient Ancestry N patients (%) Weight Median (range) | N Units | TNC Content Median (range) | CD34+ Content Median (range) | TNC/ kg Dose Median (range) | CD34+/ kg Dose Median (range) | 8-allele HLA-match Median (range) |
|---|---|---|---|---|---|---|
| Europeans(n = 69, 48%) 82 kg (48–126) | 137 | 232 (122–417) | 181 (54–484) | 2.8 (1.5–7.1) | 2.2 (0.7–6.3) | 5/8 (3–7) |
| Northwestern(n = 11, 8%) 91 kg (51–126) | 22 | 267 (177–417) | 222 (104–373) | 3.3 (1.9–5.8) | 2.8 (1.1–4.7) | 5/8 (3–6) |
| Eastern(n = 17, 12%) 82 kg (49–115) | 34 | 230 (136–397) | 179 (55–369) | 2.9 (1.8–7.1) | 2.2 (1.0–5.6) | 5/8 (3–7) |
| Southern(n = 26, 18%) 83 kg (48–115) | 52 | 213 (122–349) | 174 (54–484) | 2.7 (1.5–4.8) | 1.9 (0.7–6.3) | 5/8 (3–6) |
| Mixed(n = 15, 10%) 81 kg (55–116) | 29 | 238 (134–401) | 184 (90–334) | 2.7 (1.6–5.0) | 2.2 (1–4.3.0) | 5/8 (3–6) |
| Non-Europeans(n = 75, 52%) 79 kg (48–138) | 149 | 216 (108–457) | 152 (47–557) | 2.8 (1.5–8.1) | 2.0 (0.6–8.3) | 5/8 (2–8)* |
| White Hispanic(n = 14, 10%) 84 kg (56–120) | 28 | 212 (140–441) | 196 (96–398) | 2.8 (1.6–6.4) | 2.3 (0.8–6.2) | 5/8 (3–6) |
| Asian(n = 21, 14%) 68 kg (48–115) | 42 | 194 (108–457) | 147 (51–320) | 2.8 (1.8–8.1) | 2.0 (0.7–5.4) | 5/8 (2–7)* |
| African(n = 23, 16%) 81 kg (52–138) | 45 | 225 (115–448) | 144 (47–557) | 2.6 (1.5–5.5) | 1.8 (0.6–6.8) | 4/8 (3–7) |
| Middle Eastern(n = 4, 3%) 80 kg (59–90) | 8 | 218 (149–369) | 141 (88–177) | 2.6 (2.0–6.2) | 1.8 (1.2–2.5) | 4.5/8 (3–5) |
| Mixed non-European(n = 13, 9%) 79 kg (61–109) | 26 | 225 (134–353) | 164 (62–554) | 2.8 (1.8–4.0) | 2.0 (0.7–8.3) | 5/8 (3–8) |
One Asian patient received a unit that was re-classified as 2/8 HLA-matched post-selection upon further expert review.
Analysis of all CB units considered for transplantation
To increase the total number of CB units analyzed, the characteristics of all units considered for transplantation (n = 544) for the 144 transplanted patients were analyzed by patient ancestry. Of these, 286 units were infused (142 double unit grafts, 2 single unit grafts) and 258 were fully characterized and reserved as back-up units (0–2 back-ups/ patient).
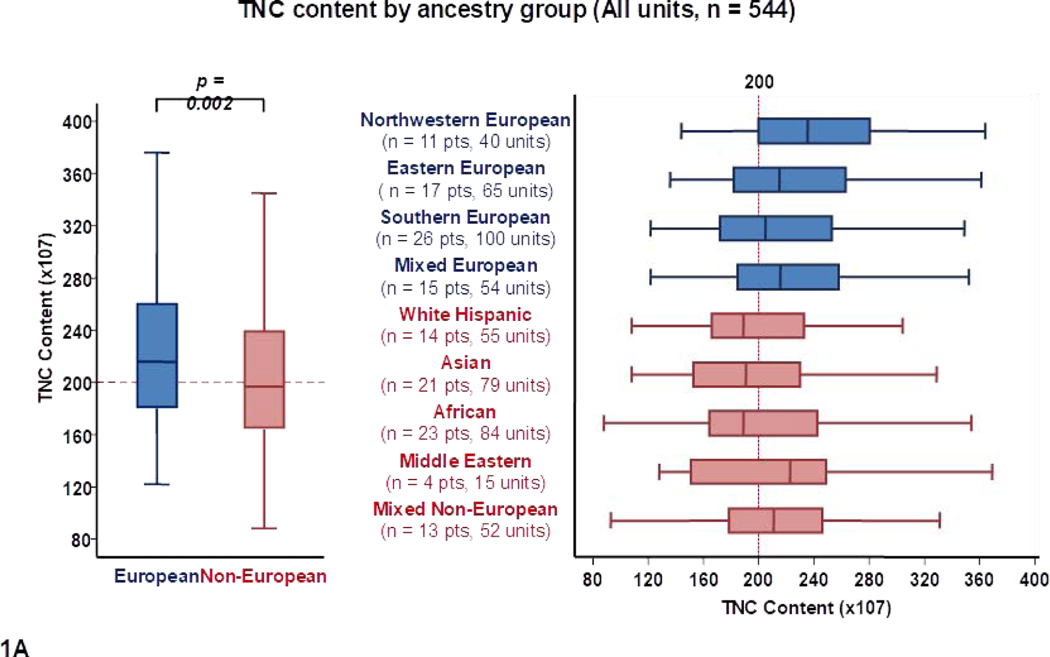
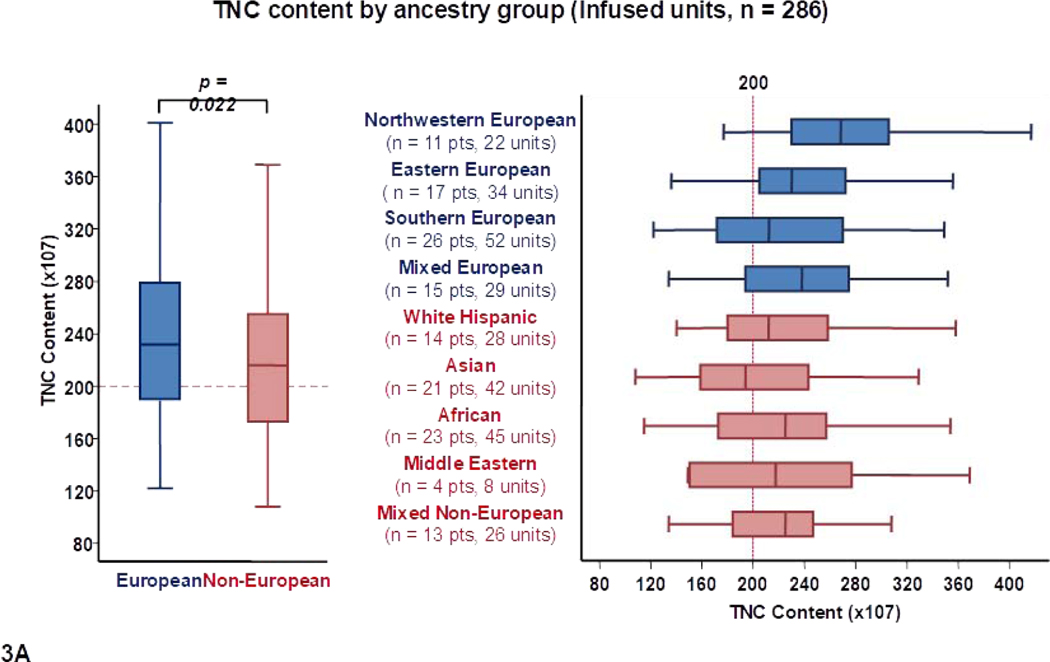
Differences in total TNC content by recipient ancestry are shown in Table 1 and Figure 1A. The median total TNC content of units for Europeans was higher than for non-Europeans (216 × 107 versus 197 × 107, p = 0.002). Units selected for northwestern Europeans had the highest median TNC content (236 × 107). Of all Europeans, units for southern Europeans had the lowest median TNC content (205 × 107). When all patients were considered, median TNC content was the lowest in units selected for Africans (189 × 107) and White Hispanics (189 × 107).
Figure 1.
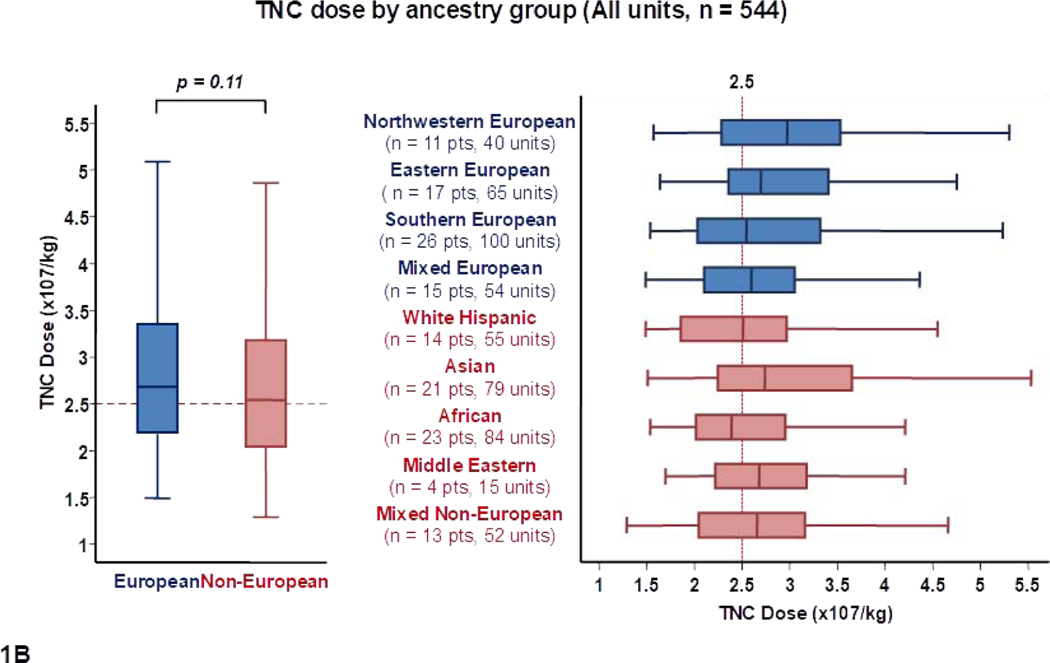
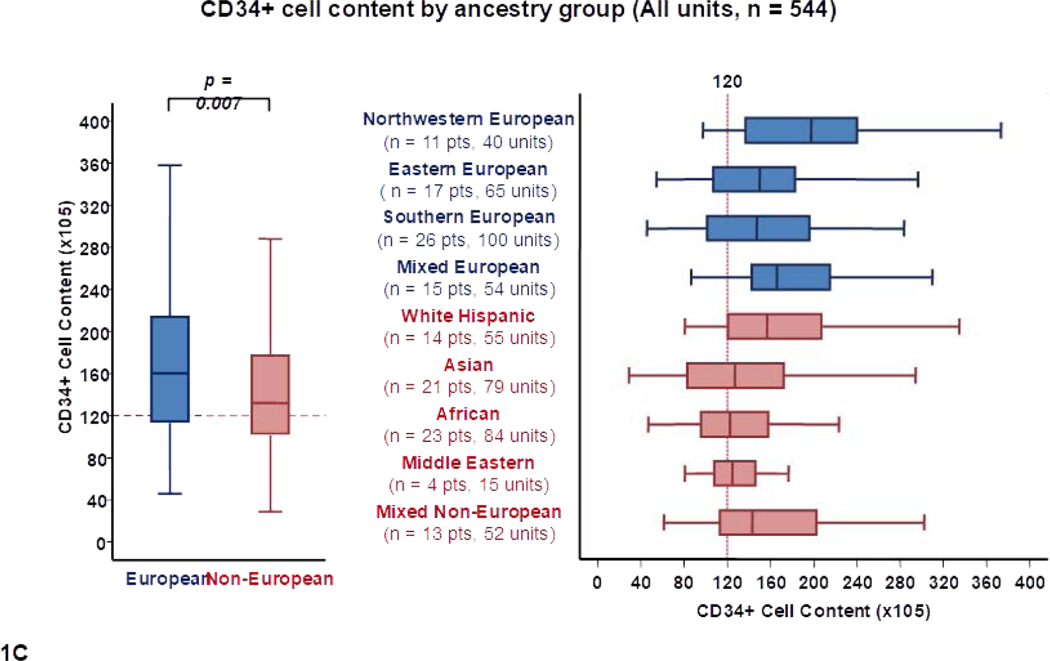
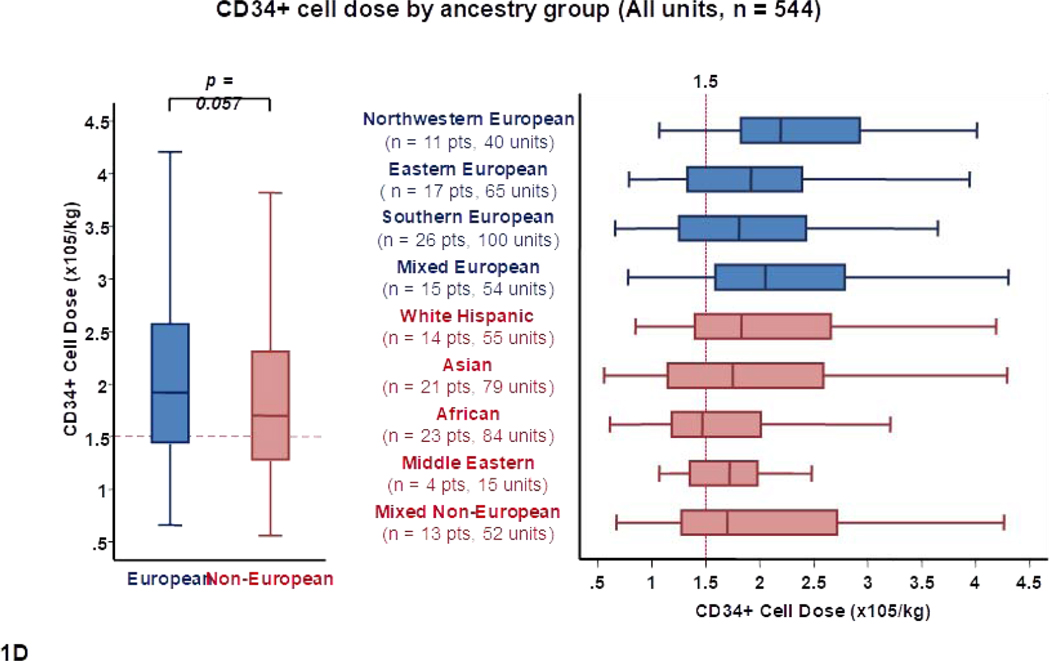
Panel 1A shows TNC content, 1B shows TNC dose, 1C shows CD34+ cell content and 1D shows CD34+ dose of all units analyzed in the study (n = 544) by patient ancestry. The dotted reference lines in 1A and 1C denote the respective TNC content (200 × 107) and CD34+ cell content (120 × 105) thresholds that provide the minimum TNC and CD34+ cell dose for single unit CB grafts for an 80 kg patient. The dotted reference lines in 1B and 1D denote the minimum TNC dose (2.5 × 107) and CD34+ cell dose (1.5 × 105) thresholds for adequate single unit CB grafts.
Footnote: * 14 units in panel 1A (left), 14 in 1A (right), 13 in 1B (left),14 in 1B (right), 24 in 1C (left), 29 in 1C (right), 29 in 1D (left) and 23 in 1D (right) were excluded as outliers (defined as units with cell content or dose 1.5 times outside the respective Interquartile Range).
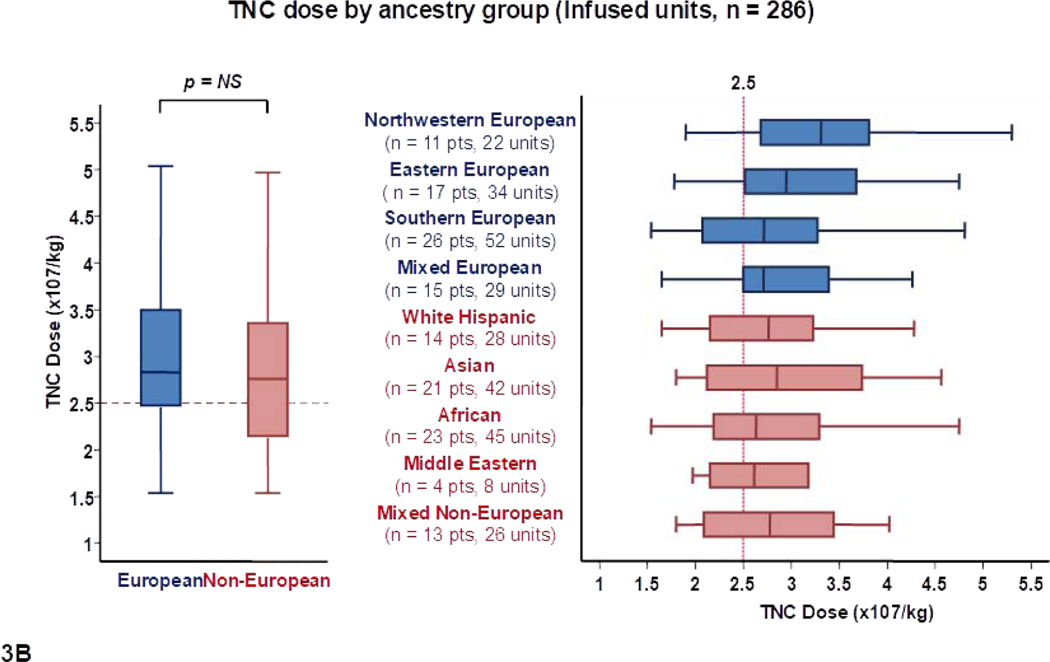
When the TNC dose of the 544 units was analyzed correcting for recipient weight (Table 1, Figure 1B), the median TNC dose in units for European and non-European patients was not significantly different (2.7 × 107/kg versus 2.5 × 107/kg per unit, p = 0.11), and differences between ancestry sub-groups were not as marked.
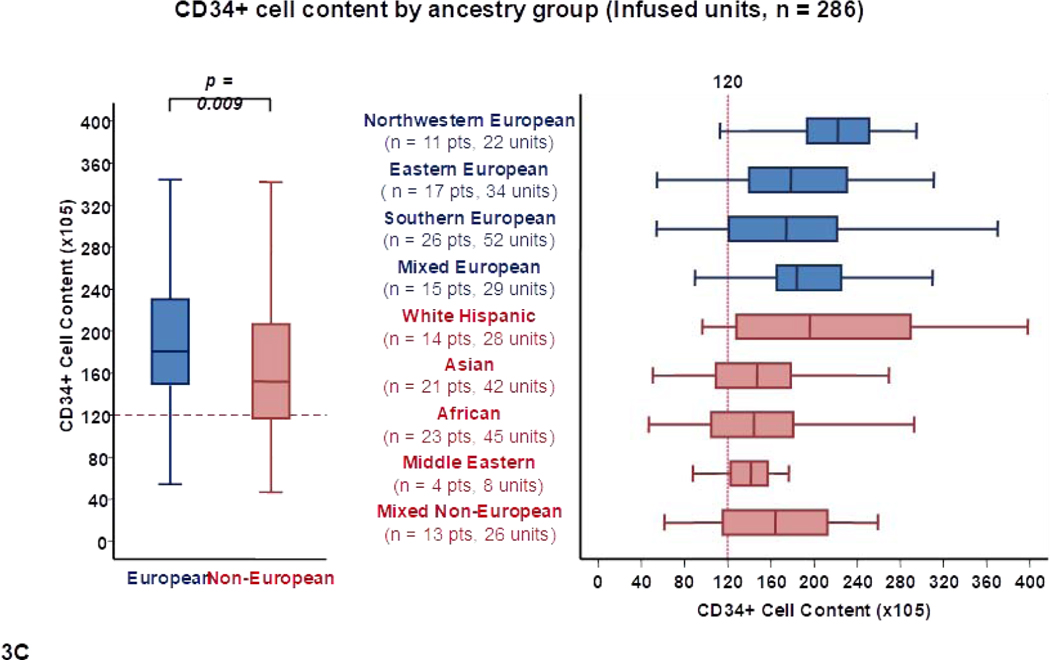
Differences in total CD34+ cell content by recipient ancestry are shown in Table 1 and Figure 1C. As with TNC content, units chosen for Europeans also had a higher median CD34+ cell content than those selected for non-Europeans (160 × 105 versus 132 × 105, p = 0.007). Within European sub-groups, units for northwestern Europeans had the highest median CD34+ cell content (198 × 105) whereas units for southern Europeans had the lowest (147 × 105). The lowest median CD34+ content was observed in units for Africans (122 × 105), Middle Eastern (124 × 105) and Asian patients (127 × 105).
When CD34+ cell dose was considered (Table 1, Figure 1D), there was a trend for units selected for Europeans having a higher median CD34+ dose than units for non-Europeans (1.9 × 105/kg versus 1.7 × 105/kg, p = 0.057). Moreover, there was disparity in the CD34+ cell dose between ancestry subgroups. For example, median CD34+ dose of selected CB units was high at 2.2 × 105/kg for northwestern Europeans, intermediate at 1.8 × 105/kg for southern Europeans, intermediate at 1.8 × 105/kg for Asians and white Hispanics, and lowest at 1.5 × 105/kg for Africans.
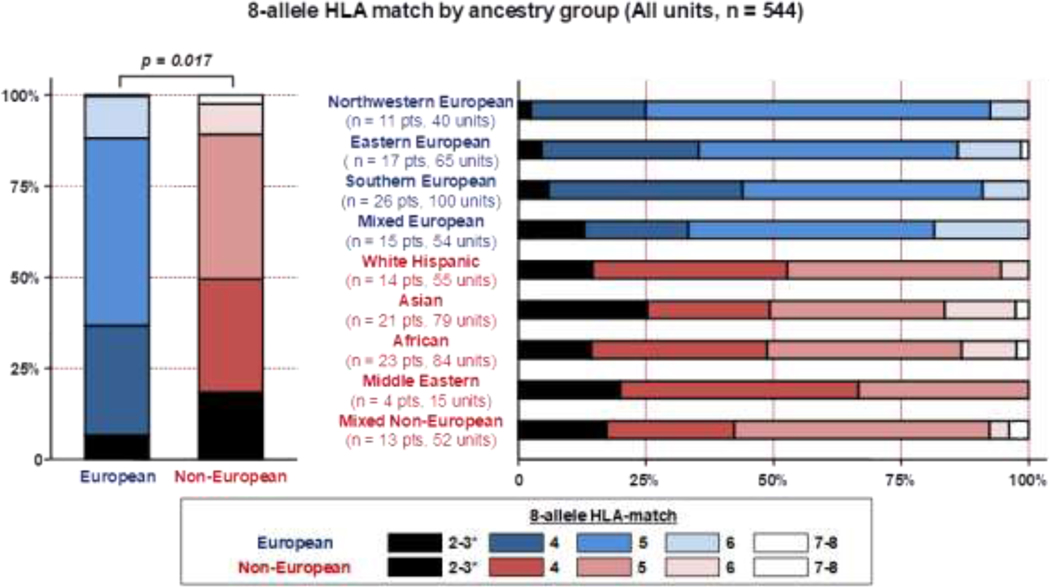
The majority of 544 selected CB units were 4/6 HLA-A, -B antigen, -DRB1 allele matched to the recipients, regardless of patient ancestry (87% for Europeans versus 90% for non-Europeans). Similarly, the median 8-allele HLA-match grade for units considered for Europeans and non-Europeans was 5/8 for both groups (Table 1). However, when HLA-match distribution was analyzed, units identified for non-Europeans had a greater degree of 8-allele HLA-mismatch (p = 0.017) (Figure 2).
Figure 2.
High resolution 8-allele HLA-match of all units evaluated in the study (n = 544) by patient ancestry.
Footnote: * One Asian patient received a unit that was re-classified as 2/8 HLA-matched after selection upon further expert review. Two additional back-up units were 2/8 HLA-matched (not infused).
Analysis of only transplanted CB units
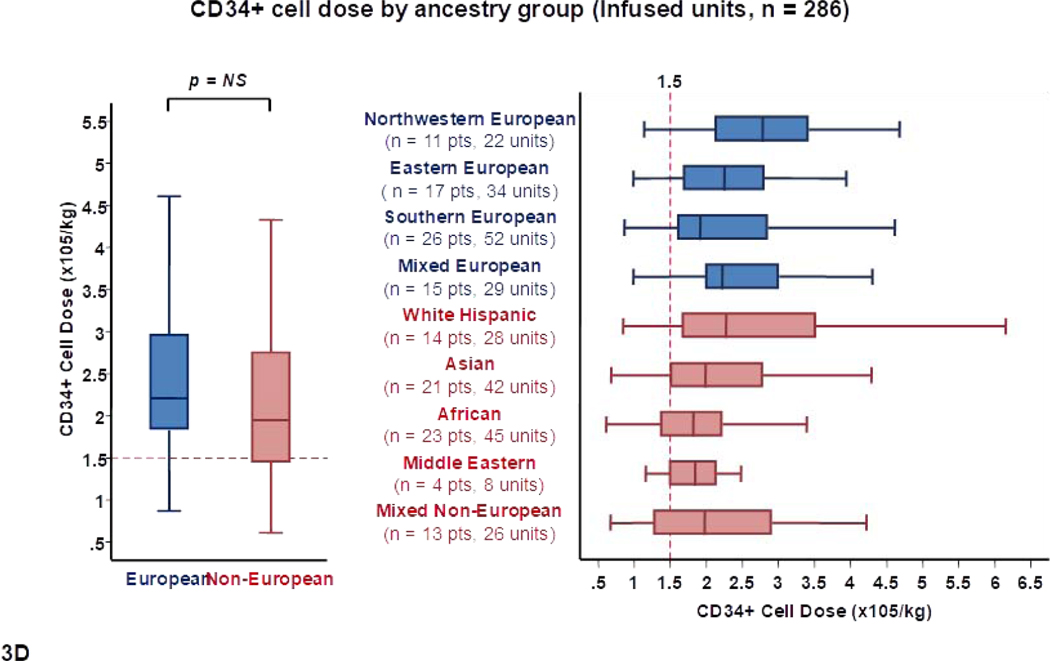
To further examine the characteristics of the available CB units that were considered the best, the 286 transplanted units were analyzed separately (Table 2, Figure 3). These 286 infused units had a median TNC content of 225 × 107 (range 108–457). Overall, 264/286 (92%) infused units had a TNC content ≥ 150 × 107. As with all suitable units, the median TNC and CD34+ cell content of transplanted CB units were higher for Europeans than non-Europeans [232 × 107 versus 216 × 107 (p = 0.022) and 181 × 105 versus 152 × 105 (p = 0.009), respectively] (Table 2, Figures 3A and 3C).
Figure 3.
Panel 3A shows TNC content, 3B shows TNC dose, 3C shows CD34+ cell content and 3D shows CD34+ dose of transplanted units (n = 286) by patient ancestry. The dotted reference lines in 3A and 3C denote the respective TNC content (200 × 107) and CD34+ cell content (120 × 105) that provide the minimum TNC and CD34+ cell dose for single unit CB grafts for an 80 Kg patient. The dotted reference lines in 3B and 3D denoted the minimum TNC (2.5 × 107) and CD34+ cell (1.5 × 105) dose criteria for single unit CB grafts.
Footnote: * 5 units in panel 3A (left), 9 in 3A (right), 9 in 3B (left), 10 in 3B (right), 15 in 3C (left), 17 in 3C (right), 14 in 3D (left) and 13 in 3D (right) were excluded as outliers (defined as units with cell content or dose 1.5 times outside the respective Interquartile Range).
The cell doses of transplanted units by recipient ancestry group are shown in Table 2 and Figures 3B and 3D. There were no overall differences in the median TNC and CD34+ doses of units for Europeans and non-Europeans. However, differences in unit cell content combined with differences in patient body weight resulted in notable TNC and CD34+ dose disparities between ancestry groups. For example, northwestern Europeans (high patient weight, high unit cell content) had the highest median TNC (3.3 × 107/kg) and CD34+ cell dose (2.8 × 105/kg) per unit. Among Europeans, the lowest doses were observed in southern Europeans (median TNC dose 2.7 × 107/kg and median CD34+ dose 1.9 × 105/kg). Among non-European ancestry sub-groups, White Hispanics received units with the highest median TNC (2.8 × 107/kg) and CD34+ cell (2.3 × 105/kg) dose per unit. Low cell content in Asian patients was partially compensated by their lower body weight (median TNC and CD34+ cell doses of 2.8 × 107/kg and 2.0 × 105/kg per unit, respectively). African and Middle Eastern patients (high patient weight, low unit cell content) received the lowest doses (median TNC and CD34+ cell doses of 2.6 × 107/kg and 1.8 × 105/kg per unit, respectively, in each group).
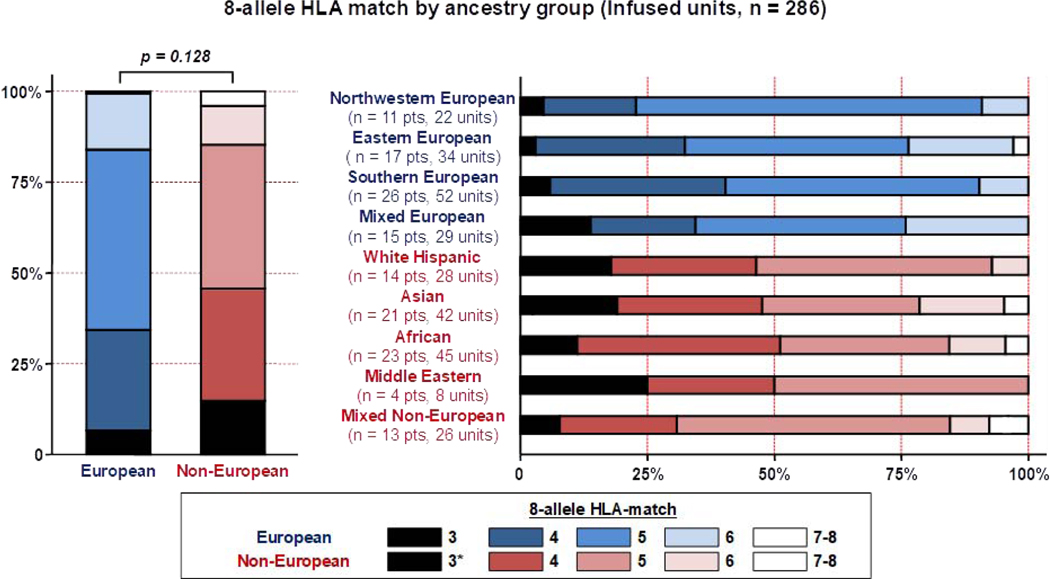
As observed with all CB units considered for transplantation, the high majority of 286 infused units were 4/6 HLA-A, -B antigen, -DRB1 allele matched regardless of European or non-European patient ancestry (86% versus 88%, respectively). Additionally, the median degree of donor-recipient 8-allele HLA-match was not significantly different in European and non-European patients (p = 0.128) (Table 2). However, marked disparities in the donor-recipient 8-allele HLA-match distribution were observed between sub-groups (Figure 4). For example, only 23% of units for northwestern Europeans were 3–4/8 HLA-matched versus 32% for Eastern, 34% for mixed Europeans and 40% for southern Europeans. Of non-Europeans, a higher percentage of units were 3–4/8 HLA-matched: 31% for mixed non-Europeans, 48% for Asian (including a unit reclassified as 2/8, post-selection), 46% of units for white Hispanic, 50% for Middle Eastern and 51% for African patients.
Figure 4.
High resolution 8-allele HLA-match of transplanted units (n = 286) by patient ancestry.
Footnote: * One Asian patient received a unit that was re-classified as 2/8 HLA-matched after selection upon further expert review.
Analysis of the number of transplanted CB units meeting the minimum required cell dose for single unit grafts
Using the published U.S. guideline of an adequate single unit CB graft having a TNC dose ≥ 2.5 × 107/kg and a CD34+ dose ≥ 1.5 × 105/kg16, the number of units that could potentially be considered for single unit grafts by recipient ancestry were analyzed. The vertical reference lines in Figures 3B and 3D indicate these cell dose thresholds for TNC and CD34+ separately. Importantly, however, potentially suitable units had to fulfill both TNC and CD34+ dose criteria. The number of units that fulfilled these criteria by ancestry group is shown in Table 3. Approximately two-thirds of transplanted units for Europeans, and half of those for non-Europeans, had cell doses above the minimum requirement. However, disparities were observed when evaluating patient sub-groups (eg 82% of units for northwestern Europeans met minimum criteria versus only 40% for African patients).
Table 3:
Number of transplanted units that could potentially be considered for single unit grafts according to cell dose (TNC ≥ 2.5 × 107/kg and CD34+ dose ≥ 1.5 × 105/kg) by ancestry group.
| Patient Ancestry N patients Weight, Median (range) | Total N Units Infused | N (%) Units Potential Singles |
|---|---|---|
| Europeans(n = 69) 82 kg (48–126) | 137 | 88 (64%) |
| Northwestern(n = 11) 91 kg (51–126) | 22 | 18 (82%) |
| Eastern(n = 17) 82 kg (49–115) | 34 | 21 (62%) |
| Southern(n = 26) 83 kg (48–115) | 52 | 27 (52%) |
| Mixed(n = 15) 81 kg (55–116) | 29 | 22 (76%) |
| Non-Europeans(n = 75) 79 kg (48–138) | 149 | 75 (50%) |
| White Hispanic(n = 14) 84 kg (56–120) | 28 | 17 (61%) |
| Asian(n = 21) 68 kg (48–115) | 42 | 22 (52%) |
| African(n = 23) 81 kg (52–138) | 45 | 18 (40%) |
| Middle Eastern(n = 4) 80 kg (59–90) | 8 | 5 (63%) |
| Mixed non-European(n = 13) 79 kg (61–109) | 26 | 13 (50%) |
Origin of transplanted CB units
Next, we analyzed the bank of origin for the 286 transplanted units (U.S. versus international) by patient ancestry group (Table 4). Approximately two-thirds (178/286, 62%) of transplanted units originated from U.S. banks. The remaining 108 (38%) units were obtained from international CB banks [predominantly from Europe (46 Spanish, 28 French, 14 German, 9 British, 3 Belgium, 4 Swedish), and 4 from Singapore (n = 3) or Israel (n = 1)]. There was a trend that non-Europeans were more likely to receive U.S. units than European patients [100/149 (67%) versus 78/137 (57%), p = 0.067]. Moreover, among non-European sub-groups, Africans were the most likely to receive U.S. units [33/45 (73%) U.S. versus 12/45 (27%) international units].
Table 4.
Origin of CB units by patient ancestry (n = 286 transplanted units in 144 patients).
| Patient Ancestry N patients (%) | N Units | Unit Origin | |
|---|---|---|---|
| N (%) Domestic | N (%) International | ||
| Europeans (n = 69) | 137 | 78/137 (57%) | 59/137 (43%) |
| Northwestern (n = 11) | 22 | 13/22 (59%) | 9/22 (41%) |
| Eastern (n = 17) | 34 | 16/34 (47%) | 18/34 (53%) |
| Southern (n = 26) | 52 | 30/52 (58%) | 22/52 (42%) |
| Mixed (n = 15) | 29 | 19/29 (66%) | 10/29 (34%) |
| Non-Europeans (n = 75) | 149 | 100/149 (67%) | 49/149 (33%) |
| White Hispanic (n = 14) | 28 | 20/28 (71%) | 8/28 (29%) |
| Asian (n = 21) | 42 | 28/42 (67%) | 14/42 (33%) |
| African (n = 23) | 45 | 33/45 (73%) | 12/45 (27%) |
| Middle Eastern (n = 4) | 8 | 4/8 (50%) | 4/8 (50%) |
| Mixed (n = 13) | 26 | 15/26 (58%) | 11/26 (42%) |
CB graft availability by ancestry group
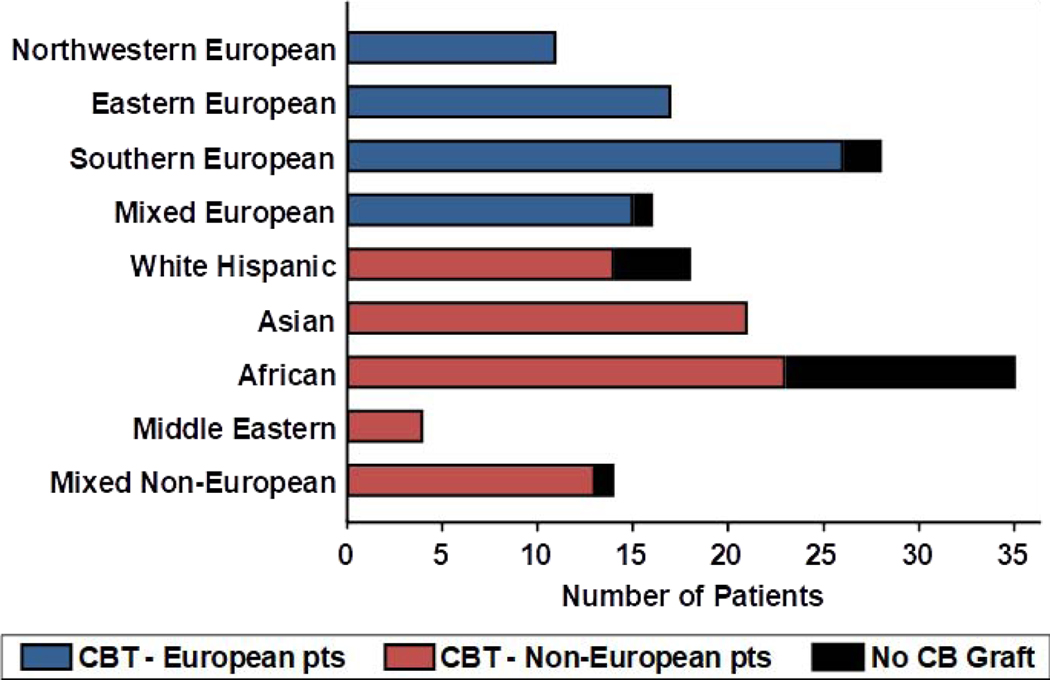
During the study period, 20 additional patients [median weight 98 kg (range 52–143)] were considered for a CB transplant but could not proceed due to lack of a suitable CB graft. Thus, a total of 164 patients were evaluated in this study (144 transplanted with CB and 20 without a CB graft). Of the 20 patients without a CB graft, only 3/20 (15%) were European (2 southern, 1 mixed) and they had a high weight ranging 93–126 kg. The 17 (85%) non-Europeans (12 African, 4 white Hispanic, 1 mixed) without a CB graft also had a high weight [median 98 kg (range 52–143)]. The marked disparity in CB graft availability by ancestry group is shown in Figure 5. For example, 100% of northwestern or eastern European patients had a suitable CB graft whereas only 23/35 (66%) of African patients did. The median weight of the 12 African patients without a suitable CB graft was 94 kg (range 52–143). Of the 20 patients who did not have a graft, 7 received a haplo-identical donor transplant, 2 received mismatched unrelated donor transplants, 3 underwent autologous transplants, and 8 received non-transplant therapy.
Figure 5.
Availability of suitable CB grafts by ancestry group of 164 patients evaluated during study period.
Discussion
Gragert et al7 have previously estimated the availability of CB units suitable for single unit CBT (≥ 4/6 HLA-matched, TNC ≥ 2.5 × 107/kg) in various racial and ethnic groups using population-based genetic models and patient weight distributions from NMDP searches. However, current unit selection guidelines also require consideration of CB banking practices that reflect unit quality, the CD34+ cell dose, and the high-resolution 8-allele HLA-match13–16, 19. Herein, for the first time, we report the racial differences in CB graft availability incorporating CD34+ cell content and dose, and the high-resolution 8-allele HLA-match, in a diverse cohort of 164 adult CBT recipients. This analysis has the advantage that it reflects real world experience in an adult patient population at an experienced CBT center that restricts graft selection to those units considered to be of acceptable quality. To increase the number of units analyzed, we first examined all CB units that were considered for transplantation (i.e. both infused and back-up units22). With this increased sample size of over 500 units, we clearly demonstrate racial/ ethnic disparities in cell content, cell dose, and unit-recipient 8-allele HLA-match.
In general, as compared to European patients, non-Europeans had access to units with a lower TNC and CD34+ cell content, and lower cell doses. In addition, while it was expected that most units would be 4/6 HLA-A, -B antigen, -DRB1 allele matched for this patient population regardless of ancestry7, we observed that units for non-Europeans had a higher degree of 8-allele HLA-mismatch than Europeans. When transplanted units were analyzed separately, overall differences in cell content, cell dose, and HLA-mismatch between Europeans and non-Europeans were less pronounced. This observation suggests that while units meeting minimal dose and HLA-match criteria exist for many non-European patients, the overall number of acceptable units available to choose from is limited.
Importantly, marked disparities were seen between specific ancestry groups. For example, patients originating from northwestern Europe had units with the highest cell content. Therefore, even despite their relatively high body weight, they received the highest TNC and CD34+ cell doses. In general, unit doses were lower for southern European and white Hispanic patients who had similar median weights. As a group, the low cell content for Asian patients was partially compensated by their lower body weight. African patients who had mostly low cell content units, and on average had weights similar to Europeans, received the lowest CD34+ cell doses. Marked disparities were also observed with donor-recipient HLA-match. For example, more than three-quarters of northwestern Europeans received units that were 5–7/8 HLA-matched, whereas more than half of African patients received units that were only 3–4/8 HLA-matched.
The most extreme racial disparity was seen when the origins of the 20 patients without any suitable CB grafts were examined. Specifically, all northwestern and eastern European patients, and nearly all mixed and southern Europeans, evaluated for CBT during the study period had suitable CB grafts. In contrast, only 77% of white Hispanics and 66% of African patients had suitable grafts. It is notable that for some patients, especially minorities, the graft availability is contingent on acceptance of very high degrees of HLA-mismatch in order to ensure adequate cell dose. Therefore, graft availability would have been considerably lower if grafts were restricted to more stringent 8-allele HLA-match or single unit grafts.
Our study would benefit from a larger sample size of racially diverse patients to further substantiate our findings. In addition, ancestry differences in access to single unit grafts require investigation, although that analysis would be complicated as the guidelines defining what is an adequate single CB graft are controversial14–16, 23, 24. Analysis of the likelihood and value of receiving racially matched units by ancestry group would also be of great interest. Finally, the clinical implications of transplanting CB units with relatively low CD34+ dose and/ or high degrees of 8-allele HLA-mismatch, and the potential impact on CBT outcomes according to recipient ancestry, require further study. Such an analysis is underway at our center.
Despite these limitations our study has important implications. We have recently reported that the number of CB units in the U.S. inventory with adequate TNC and CD34+ dose for adults is relatively small12. Notably, however, by utilizing double unit grafts nearly all adult European patients (> 95%) had suitable CB grafts. Moreover, some Europeans, likely those with more common genotypes, had excellent CB grafts. This is highly relevant for patients with high-risk malignancies who may benefit from the robust CB-derived graft-versus-leukemia effects24–27 and those who require urgent transplantation considering the very rapid availability of CB grafts28.
An additional important finding was that most non-Europeans also had acceptable CB grafts, including more than half of the African patients. Therefore, CB significantly extends transplant access to all6, 9. This is especially important for non-Europeans considering their poor access to 8/8 HLA-matched unrelated donors6–9. Additionally, some minorities especially those of African ancestry do not have access to suitable haplo-identical donors10. Nonetheless, in general, non-Europeans received the lowest cell doses and units with a greater HLA-mismatch. Therefore, efforts to augment CB graft dose and to optimize the ability to safely transplant highly mismatched units are the most important for minorities.
This analysis also has implications for CB banking. More than 90% of infused CB units in this study had a TNC content > 150 × 107. Consequently, as previously proposed by Magalon et al29 and Stritesky et al30, our findings would support a major focus on collecting only higher cell content units to improve cost effectiveness of CB banking. This policy is also supported by Querol et al31 who have shown that even a substantial expansion of the CB inventory would only marginally improve the access to better HLA-matched units and would be an inefficient banking strategy. Unfortunately, it has also been shown that units from non-Caucasian donors have lower cell content and banking qualification rates32–36. Accordingly, we would argue that a dedicated focus to selectively bank higher content CB units should further improve minority access given CBT with relatively high degrees of HLA-mismatch is feasible in patients with hematologic malignancies24, 26, 37–39, and CBT across racial boundaries is common11, 40–42. High degrees of HLA-mismatch may not be acceptable in patients with non-malignant diseases43, although these patients are usually smaller and thus more likely to have multiple adequately dosed units to permit optimization of the HLA-match. Finally, while our center utilized international as well as domestic units, non-European patients were more reliant on U.S. CB units. This observation was especially true for patients of African origins and emphasizes the importance of U.S. CB banks for minorities.
Highlights.
Racial disparities exist in unrelated donor CB cell content, dose and HLA-match.
Nearly all European and most non-European adult patients have suitable double unit CB grafts.
Many Europeans have high-dosed, well-matched CB units.
Acceptance of high degrees of HLA-mismatch is needed to permit CBT in many non-Europeans.
Patients without suitable CB units are predominantly non-European with high weight.
Acknowledgements
This research was supported in part by a grant from the National Institute of Health, National Cancer Institute (P30 CA008748).
Footnotes
Conflict-of-Interest disclosure: J.N.B. has received clinical trial funding from Angiocrine Bioscience and unrestricted educational grants from Gamida Cell and Merck. A.S. serves as the Medical Director at the New York Blood Center/National Cord Blood Program. I.P has received an educational grant from Merck and serves on a DSMB for ExCellThera. The authors have no other relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. [DOI] [PubMed] [Google Scholar]
- 2.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119:3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verneris MR, Lee SJ, Ahn KW, et al. HLA Mismatch Is Associated with Worse Outcomes after Unrelated Donor Reduced-Intensity Conditioning Hematopoietic Cell Transplantation: An Analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehn J, Buck K, Maiers M, et al. 8/8 and 10/10 high-resolution match rate for the be the match unrelated donor registry. Biol Blood Marrow Transplant. 2015;21:137–141. [DOI] [PubMed] [Google Scholar]
- 9.Barker JN, Boughan K, Dahi PB, et al. Racial disparities in access to HLA-matched unrelated donor transplants: a prospective 1312-patient analysis. Blood Adv. 2019;3:939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosuri S, Wolff T, Devlin SM, et al. Prospective Evaluation of Unrelated Donor Cord Blood and Haploidentical Donor Access Reveals Graft Availability Varies by Patient Ancestry: Practical Implications for Donor Selection. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker JN, Kempenich J, Kurtzberg J, et al. CD34(+) cell content of 126 341 cord blood units in the US inventory: implications for transplantation and banking. Blood Adv. 2019;3:1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hough R, Danby R, Russell N, et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br J Haematol. 2016;172:360–370. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri A, Paviglianiti A, Gluckman E, Rocha V. Impact of HLA in cord blood transplantation outcomes. HLA. 2016;87:413–421. [DOI] [PubMed] [Google Scholar]
- 16.Barker JN, Kurtzberg J, Ballen K, et al. Optimal Practices in Unrelated Donor Cord Blood Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23:882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60:778–783. [PubMed] [Google Scholar]
- 18.Hurley CK, Fernandez-Vina M, Hildebrand WH, et al. A high degree of HLA disparity arises from limited allelic diversity: analysis of 1775 unrelated bone marrow transplant donor-recipient pairs. Hum Immunol. 2007;68:30–40. [DOI] [PubMed] [Google Scholar]
- 19.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124:2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahi PB, Barone J, Devlin SM, et al. Sustained donor engraftment in recipients of double-unit cord blood transplantation is possible despite donor-specific human leukoctye antigen antibodies. Biol Blood Marrow Transplant. 2014;20:735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponce DM, Lubin M, Gonzales AM, et al. The use of back-up units to enhance the safety of unrelated donor cord blood transplantation. Biol Blood Marrow Transplant. 2012;18:648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127:3450–3457. [DOI] [PubMed] [Google Scholar]
- 24.Wagner JE, Jr., Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015;21:1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016;375:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. [DOI] [PubMed] [Google Scholar]
- 29.Magalon J, Maiers M, Kurtzberg J, et al. Banking or Bankrupting: Strategies for Sustaining the Economic Future of Public Cord Blood Banks. PLoS One. 2015;10:e0143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stritesky G, Wadsworth K, Duffy M, Buck K, Dehn J. Evaluation of the impact of banking umbilical cord blood units with high cell dose for ethnically diverse patients. Transfusion. 2018;58:345–351. [DOI] [PubMed] [Google Scholar]
- 31.Querol S, Mufti GJ, Marsh SG, et al. Cord blood stem cells for hematopoietic stem cell transplantation in the UK: how big should the bank be? Haematologica. 2009;94:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akyurekli C, Chan JY, Elmoazzen H, Tay J, Allan DS. Impact of ethnicity on human umbilical cord blood banking: a systematic review. Transfusion. 2014;54:2122–2127. [DOI] [PubMed] [Google Scholar]
- 33.Page KM, Mendizabal A, Betz-Stablein B, et al. Optimizing donor selection for public cord blood banking: influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion. 2014;54:340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballen KK, Kurtzberg J, Lane TA, et al. Racial diversity with high nucleated cell counts and CD34 counts achieved in a national network of cord blood banks. Biol Blood Marrow Transplant. 2004;10:269–275. [DOI] [PubMed] [Google Scholar]
- 35.Cairo MS, Wagner EL, Fraser J, et al. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–866. [DOI] [PubMed] [Google Scholar]
- 36.George TJ, Sugrue MW, George SN, Wingard JR. Factors associated with parameters of engraftment potential of umbilical cord blood. Transfusion. 2006;46:1803–1812. [DOI] [PubMed] [Google Scholar]
- 37.Brunstein CG, Petersdorf EW, DeFor TE, et al. Impact of Allele-Level HLA Mismatch on Outcomes in Recipients of Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2016;22:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz J, Jaramillo FJ, Planelles D, et al. Impact on outcomes of human leukocyte antigen matching by allele-level typing in adults with acute myeloid leukemia undergoing umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2014;20:106–110. [PubMed] [Google Scholar]
- 39.Yanada M, Konuma T, Kuwatsuka Y, et al. Unit selection for umbilical cord blood transplantation for adults with acute myeloid leukemia in complete remission: a Japanese experience. Bone Marrow Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 40.Ustun C, Bachanova V, Shanley R, et al. Importance of donor ethnicity/race matching in unrelated adult and cord blood allogeneic hematopoietic cell transplant. Leukemia & Lymphoma. 2014;55:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spees LP, Martin PL, Kurtzberg J, et al. Reduction in Mortality after Umbilical Cord Blood Transplantation in Children Over a 20-Year Period (1995–2014). Biol Blood Marrow Transplant. 2019;25:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eapen M, Wang T, Veys PA, et al. Allele-level HLA matching for umbilical cord blood transplantation for non-malignant diseases in children: a retrospective analysis. Lancet Haematol. 2017;4:e325–e333. [DOI] [PMC free article] [PubMed] [Google Scholar]