Abstract
Biliary endoscopy is underutilized by interventional radiologists and has the potential to become an effective adjunctive tool to help both diagnose and treat a variety of biliary pathology. This is particularly true in cases where endoscopic retrograde cholangiopancreatography fails or is not feasible due to surgically altered anatomy. Both preoperative clinical and technical procedural factors must be taken into consideration prior to intervention. In this article, clinical evaluation, perioperative management, and procedural techniques for percutaneous biliary endoscopy are reviewed.
Keywords: percutaneous endoscopy, biliary stones, benign biliary strictures, malignant biliary strictures, interventional radiology
The field of interventional radiology is predicated on innovation. Novel techniques are adopted to optimize the care of patients and treat a broad spectrum of pathology. Of these techniques, endoscopy is an underutilized tool that can help expand the procedural repertoire of interventional radiologists. Though the earliest documented case of interventional radiology–operated endoscopy occurred in 1976 when Yamakawa et al utilized it to treat choledocholithiasis, 1 interest ultimately declined. More recently, however, a resurgence of interest has been ignited in the interventional radiology community. 2 3 4 5 6
Interventional radiologists are uniquely equipped to integrate endoscopy into many of their commonly performed image-guided procedures, particularly in biliary, gastrointestinal, and genitourinary interventions. This can be attributed to the high degree of manual dexterity that is often required to execute interventional procedures. 7 Furthermore, endoscopic techniques are comparatively safe and frequently performed as outpatient procedures. These facets allow for a rapid learning curve for the incorporation of endoscopy into the interventional radiologist's repertoire.
Biliary endoscopy is a potent tool that is underutilized by interventional radiologists. Though biliary endoscopy has been more commonly used for the treatment of gallstones with documented excellent results, 7 its role can be expanded to encompass the treatment of other biliary benign and malignant pathologies. This article will review the management of these pathologies with interventional radiology–operated biliary endoscopy.
Management of Biliary Stones
Background
Biliary endoscopy is typically performed in the setting of symptomatic cholelithiasis. It is estimated that 1.4% of population in the United States is afflicted by gallbladder stones per year; among this population, symptomatic duct obstruction occurs in approximately 1 to 4%. 8 9 In the acute setting, these stones can present with cholangitis, pancreatitis, jaundice, and biliary colic. 10 11 In the chronic setting, patients with intraductal stones have an increased risk of ductal strictures, recurrent cholangitis, liver abscess, secondary biliary cirrhosis, and cholangiocarcinoma. 11 12 The preferred method of treatment for these patients is cholecystectomy. However, for those with significant comorbidities, surgery may not be optimal. For those who forego surgery and receive a cholecystostomy, lifelong tube exchanges may be required. This can place a substantial financial burden on the healthcare system. 13 14 15
In the setting of choledocholithiasis, endoscopic retrograde cholangiopancreatography (ERCP) is the preferred first-line treatment. Nonetheless, there are cases where ERCP is technically challenging due to surgically altered biliary anatomy, ductal strictures, or ductal compression from tumor. In these cases, interventional radiology–operated endoscopy can provide a practical solution to this clinical dilemma. 7 Moreover, if the stones are excessively mobile, impacted, bulky, or multiple, direct visualization with percutaneous endoscopy can further optimize treatment outcomes and help distinguish other pathologies that can mimic biliary stones. 16
From the perspective of an interventional radiologist, a significant proportion of the techniques that are required to complete these procedures are within one's skillset. Procurement of the scopes and other ancillary equipment can be prohibitive. Reusable devices require significant capital investment for purchasing, sterilizing, and maintaining the equipment. Due to these exorbitant costs, non-reusable types can be considered. These include the 9.5-Fr LithoVue (Boston Scientific, Marlborough, MA), 10.5-Fr SpyGlass Discover (Boston Scientific), and Neo-flex (NeoScope Inc, Silicon Valley, CA) varieties. However, since most hospitals carry a mobile endoscopy cart, collaboration with other specialties is recommended to create a sustainable plan for percutaneous endoscopy.
Explanation of Technique
Though biliary endoscopy and intervention may be performed under conscious sedation, general anesthesia is recommended to ensure ideal patient comfort, safety, and hemodynamic monitoring. Prior to the beginning of the endoscopy, preprocedural antibiotics should be administered in accordance with the Society of Interventional Radiology guidelines. 7 Intraprocedural monitoring of fluid input/output, electrolytes, and body temperature should ideally be performed. Enteric and rectal tubes can be considered if prolonged intervention is anticipated and can assist with managing fluids infused by the endoscope. The patient is usually placed in the supine position for biliary endoscopy. Initial cholangiography must be done directly before the endoscopy to ensure the location of the stone(s), as their position can easily change. Biliary access through percutaneous drainage must be accomplished 4 to 6 weeks prior to biliary endoscopy. This percutaneous tract should be well-developed to avoid excessive bleeding, which can obstruct visualization. The initial step involves sterilization of the indwelling biliary drain(s) and surrounding skin and then creation of a small hole in an Ioban Incise Drape (3M, Maplewood, MN), which will fit over the biliary drain(s) and adhere to the skin. 16 A neurosurgical drape is then used to fix over the Ioban Incise Drape; a small hole is cut in the window to accommodate for the drain(s). To facilitate a drier setup, the neurosurgical drape consists of a pouch below the window that catches fluid. A safety guide wire must be directed into the duodenum. This can be accomplished by exchanging the biliary drainage catheter over a guide wire for a 25-cm vascular sheath. The initial guide wire can then be removed by isolating the safety guide wire external to a 12-Fr peel-away sheath. 16 This method ensures a friction-free endoscope passage while facilitating the use of saline. Furthermore, utilization of the 12-Fr peel-away sheath avoids extreme pressurization of the biliary tree—which can lead to pain, nausea, and potentially sepsis—while maintaining appropriate distension.
For choledochoscopy, a flexible endoscope is preferred to navigate through the angulations of the biliary tree. These include a 9.5-Fr flexible disposable (Boston Scientific), a 9-Fr flexible reusable endoscope (Olympus America, Center Valley, PA), or a 16.5-Fr flexible reusable endoscope (Olympus America). If pursuing cholecystoscopy, however, the gallbladder should ideally be accessed in long axis through the fundus to permit a more direct trajectory intervention. This facilitates a more effective and comfortable position for eventual stone sweeping and removal maneuvers. 7 The use of a 22.5-Fr rigid endoscope (Olympus America) is preferred by the authors due to the direct course from skin entry to the gallbladder.
The scope device setup requires continuous saline fluid infusion through the working channel for debris clearance. Pressure of the biliary tree must be continuously monitored to avoid the aforementioned complication of over-distension. The endoscope consists of a watertight diaphragm for advancing equipment through both the working channel and the side port; a Check-Flo Adapter (Cook Medical, Bloomington, IN) is used on the working channel. It is recommended to connect the saline to the side port of the working channel—rather than the side port of the Check-Flo Adapter—as the pressure can overwhelm the diaphragm and spray the operator. 16
Up-and-down distal tip deflection is performed to manipulate the scope. Once the stone is localized, direct visual evaluation of the stone is required to determine whether fragmentation with a laser is necessary before removal with a stone basket. In such instances, laser lithotripsy (VersaPulse Holmium Laser; Boston Scientific), electrohydraulic (Gyrus ACMI; Olympus Medical, Southborough, MA), and ultrasonic (Olympus Shock-Pulse SE; Olympus Medical) devices can be used to fragment and improve the removal of the stone. 7 Fragmentation of stones must be minimized to avoid increasing the duration of the procedure. When compared with cholesterol-dominant stones, calcium bilirubinate-dominant stones tend to be stronger. Among six studies that evaluated a total of 323 patients, the technical success rate of percutaneous cholecystolithotomy was 93 to 100%. 17 18 19 20 22
In addition to the removal of the stone(s), the interventional radiologist must assess for the presence of soft debris, which is often associated with stones ( Fig. 1 ). This is particularly salient in cases of previous biliary-enteric anastomosis, in which sutures can extend into the intraluminal space and function as a nidus for stone formation. This can be resolved by removing or unraveling the suture with 3-Fr Piranha Biopsy Forceps (Boston Scientific) through the endoscope under direct visualization to circumvent reorganization of the stone at the site of the anastomosis. 16 Under direct visualization, a Zero Tip Basket is used to remove the stone fragments through the sheath; to enhance clearance, an occlusion balloon can be used to assist in moving the debris into the bowel.
Fig. 1.
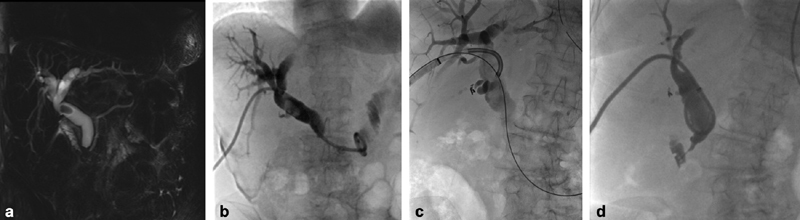
Choledochoscopy in a 68-year-old female with remote history of Roux-en-Y gastric bypass presents with abdominal pain and elevated bilirubin. ( a ) Coronal MIP T2-weighted MRI demonstrates a large stone within the proximal common bile duct with associated internal and external biliary dilatation. ( b ) Cholangiography after placement of an internal/external biliary drain again demonstrates the choledocolith, as well as moderate biliary dilatation. ( c ) Spot radiograph taken during choledocoscopy demonstrates our SpyGlass endoscope directed toward the common bile duct calculus. We placed a safety wire into the bowel during the choledocoscopy to retain access during lithotripsy and stone extraction. ( d ) Cholangiography 2 weeks post choledocoscopy demonstrates a patent common bile duct with resolution of choledocolithasis.
Prior to completion, close scrutiny of the biliary tree with endoscopy must be performed to ensure that there are no additional stones and to rule out additional pathologies. If transhepatic, an external drain catheter can be placed at the end of the procedure after confirmation of biliary patency. For cholecystoscopy, placement of a transcystic internal–external drainage catheter or a cholecystostomy drain may then be performed. 7
Postprocedure, the patient is admitted for overnight observation. A 7- to 10-day course of oral antibiotics (Augmentin, GlaxoSmithKline) is prescribed upon discharge. The recurrence rate of common bile duct stones varies and is documented at 5 to 20%. 21 Some clinicians recommend the prescription of ursodeoxycholic acid 300 mg twice daily to prevent gallstone recurrence, 7 though this is not routinely prescribed in our practice. The patient is ultimately scheduled for tube removal 2 weeks after intervention and completion of a capping trial.
Management of Benign and Malignant Biliary Strictures
Background
Biliary strictures can be attributed to both benign and malignant etiologies. Although the most common benign cause is iatrogenic with 80% attributed to surgical duct injury during orthotopic liver transplantation or cholecystectomy, other examples include inflammatory, autoimmune, or infectious processes. 23 Primary sclerosing cholangitis and IgG4-related sclerosing cholangitis are examples of inflammatory autoimmune causes. 24 Other less common etiologies consist of pyogenic cholangitis, radiation-related strictures, inflammatory bowel pseudotumors, and HIV–cholangiopathy vasculitis-related ischemia. 25
Etiologies of malignant biliary strictures include cholangiocarcinoma, pancreatic adenocarcinoma, gallbladder carcinoma, hepatocellular carcinoma, or lymphoma, though cholangiocarcinoma and pancreatic adenocarcinoma account for more than 72% of cases. 26 Patients with these pathologies typically present with painless jaundice, pruritus, and dilated bile ducts. Magnetic resonance cholangiopancreatography (MRCP) or ERCP can be used to evaluate for malignancy if biliary duct obstruction is suspected. Due to the delayed presentation of these patients' symptoms, curative resection is often not viable.
Irrespective of benign or malignant etiology, if traditional methods of ERCP are not ideal, percutaneous endoscopic treatment of benign and malignant strictures can be an effective tool for diagnostic evaluation of biliary strictures and for subsequent intervention.
Explanation of Technique
Preprocedural planning should involve interdisciplinary decision-making with consultation of surgical, gastrointestinal, oncological, and primary care teams. Surgical interventions and ERCP-guided therapies are usually the first-line standard of care. Nonetheless, in patients with altered anatomy or who are nonoperative candidates, other treatment modalities should be considered.
Assessment of biliary strictures and differentiating between benign and malignant etiologies can be challenging tasks. However, this can be accomplished with cross-sectional imaging—either computed tomography or MRCP—or more invasive diagnostic methods—such as tissue sampling. 26 In cases of altered anatomy—from previous surgical interventions, such as a hepaticojejunostomy, and Roux-en-Y gastric bypass—or restrictive duodenal or papillary stenosis, traditional endoscopic visualization may be difficult. If it is decided to pursue percutaneous endoscopic intervention, standard preprocedural serology evaluation consists of a complete blood count, basic metabolic panel, liver function tests, and coagulation tests. 16 For percutaneous endoscopy, an international normalized ratio value less than 1.5 and a platelet count greater than 50,000/mL are recommended. According to the Society of Interventional Radiology guidelines, it is recommended to administer preprocedural prophylactic antibiotics, such as 1 g ceftriaxone or 1.5 to 3 g ampicillin/sulbactam given intravenously; if the patient is allergic to penicillin, clindamycin and aminoglycoside are recommended. 27 During the procedure, fluid input/output, electrolytes, and body temperature should be monitored.
For transhepatic choledochoscopy, a flexible endoscope is required to navigate through the angulations of the biliary tree. Suitable examples of this include a 9.5-Fr flexible disposable (Boston Scientific), a 9-Fr flexible reusable endoscope (Olympus America), or a 16.5-Fr flexible reusable (Olympus America) endoscope. 26 As previously discussed, the disadvantages of using reusable devices are related to the significant capital investment needed for not only purchasing but also sterilizing and maintaining the equipment. Novel disposable flexible endoscopes are viable options to assist in image-guided interventions. These instruments are more cost-effective, with an average cost of $1,500. 28 Ranging in size from 7.95 to 22.5 Fr, these endoscopes are equipped with a variety of internal channel diameters and can be either rigid or flexible. They often require an endoscopy console and monitor or a video processing unit with the capacity for direct visualization on the fluoroscopy screen. Alternatively, if the objective is cholecystoscopy, the gallbladder should be accessed along its long axis to facilitate a more direct trajectory for intervention. A 22.5-Fr rigid endoscope (Olympus America) is preferred due to the direct course from skin entry to the gallbladder.
Biliary access requires consideration of the endoscope size. Though access can be acquired during the procedure, our authors endorse obtaining access 4 to 6 weeks prior to the procedure to promote tract maturation and to reduce the risk of bile leakage and pain. 22 If using a rigid endoscope, tract dilation with a high-pressure balloon may be necessary. Once access has been successfully obtained, the tract should be secured with an additional safety guidewire in addition to the working wire. A stiff wire—such as the Amplatz wire—is recommended given the size of the scope. 26 A sheath that can adequately accommodate the endoscope should then be advanced over the wire to serve as a conduit. The endoscope itself contains an inner working channel that permits the passage of balloons, catheters, and other devices needed for intervention. 22
Cholangioplasty, internal–external multihole biliary drain placement, and/or ensuing stenting in cases of recalcitrant stenosis are recommended for the management of benign biliary strictures. 26 Often, benign strictures can be managed with cholangioplasty utilizing 8- to 10-mm balloons. For recalcitrant strictures, plastic or covered self-expanding metal stents can be considered. 16 Due to this risk of in-stent stenosis and the subsequent inability to exchange the stent, uncovered metal stents are not ideal for benign pathology, as the objective is to obtain long-term bile duct patency and decompression of the biliary system. Post cholangioplasty and/or stenting, the biliary drain is kept in place and ultimately capped at 24 hours postprocedure.
It is important to know how normal biliary mucosa looks like on endoscopy ( Fig. 2 ). For malignant obstruction, initial endoscopic evaluation provides direct visualization of the tumor and potential for biopsy ( Fig. 3 ). Alternatively, cholangioplasty and stent placement can be utilized for palliation. Stent preference for malignant obstruction remains controversial. Bare metal stents have been reported to fail due to tumoral invasion into the stent, though covered stents have less potential for tumor invasion with longer patency rates. Nonetheless, no difference exists in overall survival. 26
Fig. 2.
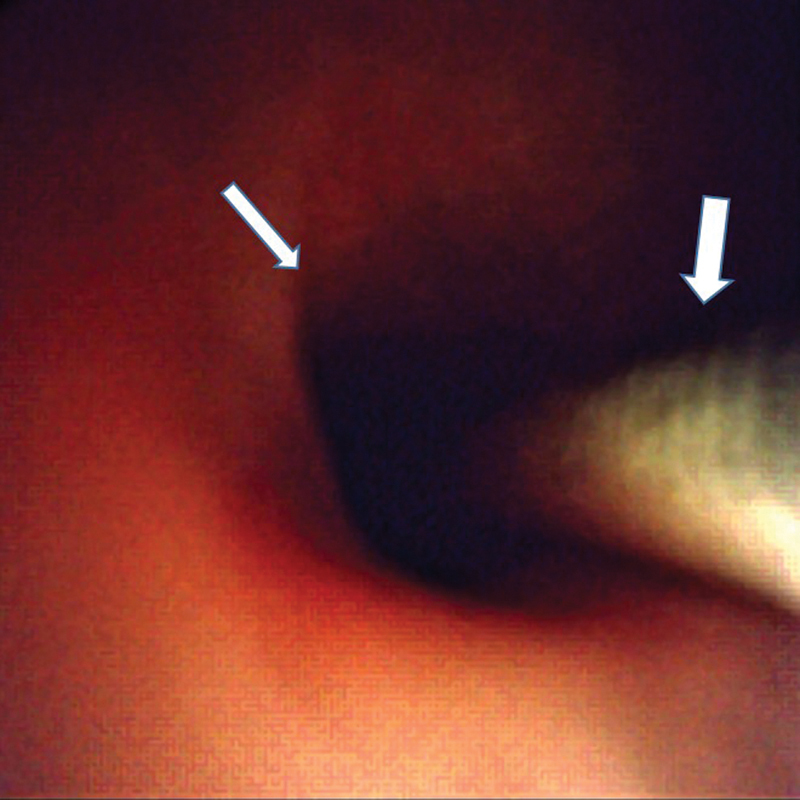
Normal appearing biliary mucosa (small arrow). Wire (big arrow) in the bile duct.
Fig. 3.
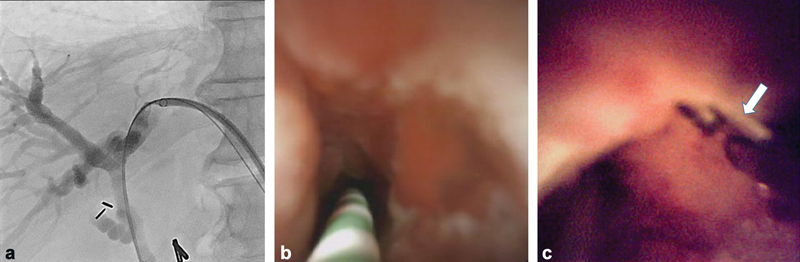
A 53-year-old man with a focal left hepatic duct stricture. ( a ) Percutaneous transhepatic access was obtained demonstrating a significant focal stricture. ( b ) Cholangioscopy demonstrated abnormal mucosa with lobulation along the biliary epithelium. ( c ) Cholangioscopic biopsy was obtained using a forceps (arrow).
Training, Billing, and Credentialing for Endoscopy
Though the value inherent to interventional radiology operated–endoscopy is becoming readily apparent, the infrastructure required to facilitate the employment of these techniques in the United States is unfortunately lacking. 29 The addition of more training opportunities is crucial to further sustain the implementation of these methods. Much can be learned about this from training paradigms abroad. For example, the British Society of Gastrointestinal and Abdominal Radiology provides opportunities for the radiologist to certify in upper gastrointestinal endoscopy. The lack of comparable options in the United States may be attributed to many factors. Nonetheless, the British example emphasizes the importance of organizational support. For the interventional radiologist in the United States, this means that perhaps our own professional societies—such as the Society of Interventional Radiology (SIR) or the American College of Radiology (ACR)—could be involved in producing pragmatic solutions to this problem. Moreover, for those radiologists who have already incorporated endoscopic techniques into their armamentarium, increasing teaching opportunities for colleagues and trainees is another potential method by which this approach can be further disseminated.
For laser therapies, there are existing well-established pathways toward credentialing in the United States under the American Society for Laser Medicine and Surgery (ASLMS). 30 This option should cover the essential aspects of laser operation, tissue interaction, safety, and pre- and postoperative care. A viable alternative to this is the completion of a residency program that integrates such training in laser techniques for residents. Credentialing and sustaining these laser privileges is within the purview of the institution or hospital.
SIR's most recent Interventional Radiology Coding Update delineates the increasing number of services offered by the field. 31 The SIR 2020 Coding Update summarizes multiple codes related to percutaneous biliary endoscopy ( Table 1 ).
Table 1. Current Procedural Terminology (CPT) codes for various pertinent procedures related to biliary endoscopy.
| CPT codes | CPT description |
|---|---|
| 47541 | Placement of access through the biliary tree and into small bowel to assist with an endoscopic biliary procedure (e.g., rendezvous procedure) via a new access; includes cholangiography and all associated imaging |
| 47542 | Balloon dilation of biliary duct(s) or of ampulla (sphincteroplasty), percutaneous, includes all associated imaging |
| 47543 | Endoluminal biopsy(ies) of biliary tree, by any method(s) (e.g., brush, forceps, and/or needle), includes all associated imaging |
| 47544 | Removal of calculi/debris from biliary duct(s) and/or gallbladder, percutaneous, including destruction of calculi by any method (e.g., mechanical, electrohydraulic, lithotripsy), includes all associated imaging |
| 47553 | Biliary endoscopy, with biopsy, single or multiple |
| 47554 | Biliary endoscopy, through skin with stone removal |
| 47555 | Biliary endoscopy, with dilation, no stent (dilation without stent, percutaneous) |
| 47556 | Biliary endoscopy, with dilation and stent (dilation with stent, percutaneous; see also 47801) |
| 47801 | Placement of bile duct stent (e.g., endoprosthesis) |
The fortification of interventional radiologists' repertoire of skills with endoscopy will serve to benefit patients. Though the lack of available training opportunities is a formidable obstacle, the intentional efforts by radiology's professional societies may be an effective approach to overcome this.
Transjejunal Biliary Endoscopy
Background
Modified Hutson loop refers to the surgical affixation of the biliary (afferent/Roux) limb to the anterior abdominal wall (without a stoma). 32 Modified Hutson loops are performed in living donor liver transplants requiring Roux-en-Y hepaticojejunostomies (such as patients with primary sclerosing cholangitis). 33 These have also been created in patients with malignant biliary diseases. 34 35 Percutaneous transjejunal access (PTJA) without surgical affixation has also been described. 36 37 PTJA (with or without the modified Hutson loop) provides an avenue to perform cholangiography and cholangioscopy for diagnostic and therapeutic interventions of the biliary tree in patients with prior Roux-en-Y hepaticojejunostomy.
Explanation of Technique
Prior to the procedure, cross-sectional imaging (such as MRCP) should be obtained and operative reports (including donor biliary anatomy in cases of liver transplantation and presence/absence of a modified Hutson loop) should be reviewed. 33 PTJA should not be performed if the time interval between intervention and surgery (modified Hutson loop creation) is less than 1 month. Moderate sedation or general anesthesia should be considered.
The patient's Roux limb should be accessed with a micropuncture (21-G) needle under ultrasound and fluoroscopic (surgically placed clips in the case of modified Hutson loop) guidance. 33 Dilute contrast should be injected to confirm appropriate intraluminal position of the needle. Following serial dilatation, the sheath should be directed in a retrograde fashion in the Roux limb toward the liver. Fontein et al have reported a small incidence of jejunal limb perforation in their early experience with greater than 14-Fr access which was limited once they used sheaths ≤12 Fr. 37 38
Contrast injection can opacify the biliary-enteric anastomosis. Cholangiography findings should be correlated with preprocedural imaging to ensure all biliary branches are evaluated. If that fails, the following can be considered:
Trendelenburg position to benefit from gravity.
Foley catheter in the jejunum to pressurize the limb and push contrast into the biliary system.
Use an angled catheter to access the ostium under fluoroscopic guidance.
Use an endoscope (LithoVue (Boston Scientific] or SpyGlass Discover (Boston Scientific]) to assist in the visualization of the anastomosis and the bile ducts.
LithoVue, a 9.5-Fr (outer diameter) single-use scope, can be introduced through a sheath ≥10 Fr. LithoVue provides a 3.6-Fr working channel that is used for continuous flushing and advancement of wires, catheters, and devices. It has the ability to deflect 270 degrees in two directions.
SpyGlass Discover, a 10.5-Fr (outer diameter) single-use scope, can be introduced through a sheath ≥11 Fr. This also provides a 3.6-Fr working channel that is used for advancement of wires, catheters, and devices. It has separate channels for insufflation and flushing. The maximum angulation is 30 degrees in four directions.
If cholangiographic/cholangioscopic findings indicate intervention, cholangioplasty and stent placement can be performed via the modified Hutson loop ( Fig. 4 ). Balloons could be advanced through the endoscope if needed. Once the wire/wires is/are in position, Cotton-Leung stents (Cook Medical) can be placed. Percutaneous placement of Cotton-Leung stents is performed using a long peel-away sheath across the stricture through the modified Hutson loop.
Fig. 4.
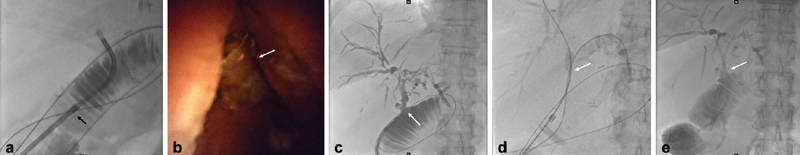
Modified Hutson loop access in a 50-year-old female with a deceased donor liver transplant. ( a ) Endoscope in loop going toward liver guided into the bile duct. The sheath is in the Roux limb (black arrow). ( b ) Debris/stone (white arrow) at the hepaticojejunostomy. ( c ) Cholangiography via a balloon occlusion catheter (white arrow) demonstrating multifocal stricturing consistent with ischemic cholangiopathy. ( d ) Wires (white arrow) advanced into the right and left ducts. ( e ) Plastic stents (white arrow) placed via the Hutson loop into the ducts.
Percutaneous transhepatic access can be considered to opacify the bile ducts and the Roux limb. Archimedean (through and through) access with an 0.014-inch wire can be used to provide tension to advance stents through the modified Hutson loop access.
Before removing the sheath or catheter from the jejunal loop, contrast injection should be performed to confirm that there is no afferent loop obstruction to minimize likelihood of enterocutaneous fistula formation. Patients should typically be observed for 2 hours in the postanesthesia care unit/outpatient recovery room.
Routine clinical/laboratory follow-up is performed to evaluate therapeutic efficacy of the procedure. If Cotton-Leung stents are placed, patients are brought back every 4 to 6 weeks for modified Hutson loop access to retrieve existing stents, evaluate patency, and obtain repeat stenting. The working channel of the endoscope allows insertion of a 10-mm Goose Neck snare and stent snaring under direct endoscopic/fluoroscopic guidance ( Fig. 5 ).
Fig. 5.
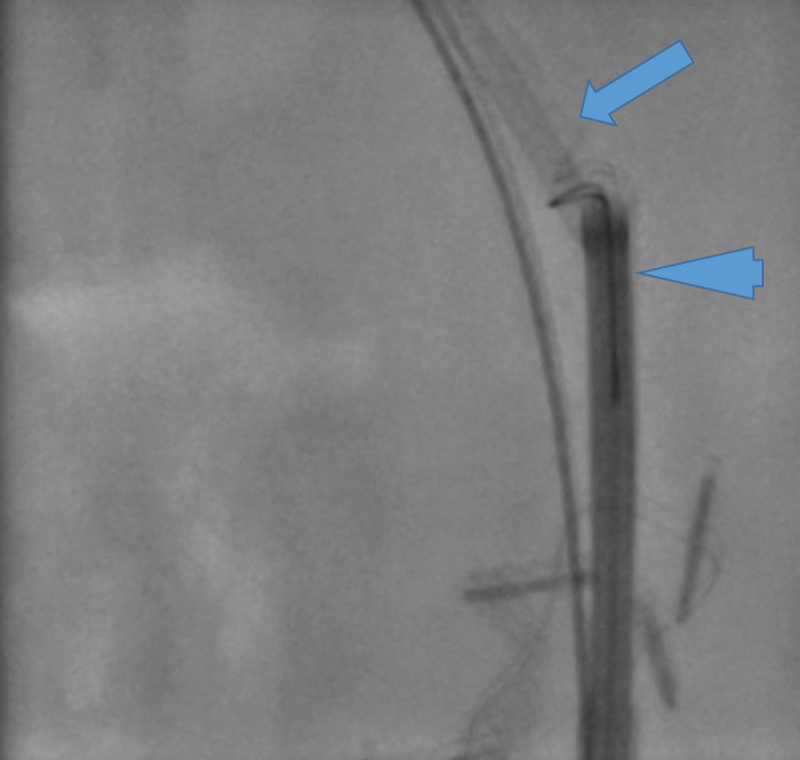
A 10-mm goose neck snare around the Cotton-Leung stent (arrow) through the SpyGlass Discover (arrow head). This access is through a modified Hutson loop to remove stents.
Biliary interventions using PTJA is successful in 88 to 95% of cases. 33 36 40 In a study by Riaz et al, successful modified Hutson loop access was performed 61 times in 21 patients. 32 There were 3 out of 26 (12%) modified Hutson loop retrograde biliary intervention failures before introduction of endoscopy and no failures (0/38; 0%) following the use of endoscopy by interventional radiologist ( p = 0.06). 33 There was a low major complication rate (1.6%), which is similar to previous observations with surgically affixed bowel loops. 36 There was no procedure-related mortality.
The advantages of PTJA include (1) cannulation of multiple biliary systems/anastomoses from a single PTJA; (2) decreased hepatic injury especially in patients with nondilated biliary ducts; (3) repeat biliary interventions can be safely performed; and (4) improved quality of life as PTJA and interventions allow the majority of patients to live a life without a biliary drain.
Interventional radiology practice has evolved with the use of an endoscope to guide procedures with direct visualization of the biliary-enteric anastomosis and the biliary system. 41 The failure rate of accessing and intervening on the biliary system through the modified Hutson loop has significantly decreased with the introduction of endoscopy/cholangioscopy. Endoscopy allows for wire advancement into the biliary system under direct visualization of the ostium and the ability to view and address pathologies such as choledocholithiasis (lithotripsy).
Complications of Biliary Endoscopy
In our practice, biliary endoscopy is well tolerated with complications rarely encountered and often mild. Close monitoring of saline infusion through the endoscope during the procedure minimizes the potential for over-distension of the biliary. Mild intraprocedural vasovagal reactions have been previously reported. 41 This can be managed with fluids and, if needed, atropine. 16 Acute cholangitis is a potential complication of any biliary intervention; this can be mitigated with pre- and postprocedure administration of antibiotics. Hypothermia and electrolyte abnormalities can be infrequently encountered secondary to protracted saline infusion. Enteric and rectal tubes can be considered if prolonged intervention is anticipated. Adequate hemodynamic and temperature monitoring intraoperatively can prevent these issues from manifesting. Any manipulation of the gallbladder carries the risk of subsequent biliary peritonitis. Clinical signs of peritonitis after the procedure could indicate bile leakage, which must be managed appropriately.
Conclusions
Interventional radiology–operated endoscopy is an underutilized tool that has the potential to improve patient care by providing new and innovative treatments for a variety of conditions involving the biliary system. Its use has the potential to expand the practice of interventional radiology as an adjunct to image-guided interventions.
Note
All the authors have read and contributed to this manuscript. The authors have no relevant disclosures. All individuals shown in the photographs have given their permission for inclusion in this manuscript and for publication.
References
- 1.Yamakawa T, Mieno K, Nogucki T, Shikata J. An improved choledochofiberscope and non-surgical removal of retained biliary calculi under direct visual control. Gastrointest Endosc. 1976;22(03):160–164. doi: 10.1016/s0016-5107(76)73733-7. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasa R N, Chick J FB. Endoscopy for the interventional radiologist: an introduction. Tech Vasc Interv Radiol. 2019;22(03):117–118. doi: 10.1053/j.tvir.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Allescher H D, Fedorov E D, Hochberger J, Schreiber F, Seewald S, Siersema P D. Interdisciplinary endoscopy. Visc Med. 2016;32(01):59–62. doi: 10.1159/000444205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chick J FB, Romano N, Gemmete J J, Srinivasa R N. Disposable single-use ureteroscopy-guided nephroureteral stent placement in a patient with pyelovesicostomy stricture and failed prior nephroureteral stent placement. J Vasc Interv Radiol. 2017;28(09):1319–1321. doi: 10.1016/j.jvir.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Ray D M, Srinivasan I, Tang S J. Complementary roles of interventional radiology and therapeutic endoscopy in gastroenterology. World J Radiol. 2017;9(03):97–111. doi: 10.4329/wjr.v9.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasa R N, Osher M L, Murrey D A. Percutaneous transgastric interventional radiology-operated duodenoscopy for the identification of duodenal perforation and Graham patch dehiscence. Radiol Case Rep. 2017;12(04):790–793. doi: 10.1016/j.radcr.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raymond C J, Khayat M, Chick J FB, Srinivasa R N. Endoscopy as an adjunct to image-guided interventions: a new frontier in interventional radiology. Tech Vasc Interv Radiol. 2019;22(03):119–124. doi: 10.1053/j.tvir.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Halldestam I, Enell E L, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004;91(06):734–738. doi: 10.1002/bjs.4547. [DOI] [PubMed] [Google Scholar]
- 9.Halldestam I, Kullman E, Borch K. Incidence of and potential risk factors for gallstone disease in a general population sample. Br J Surg. 2009;96(11):1315–1322. doi: 10.1002/bjs.6687. [DOI] [PubMed] [Google Scholar]
- 10.Caddy G R, Tham T C. Gallstone disease: symptoms, diagnosis and endoscopic management of common bile duct stones. Best Pract Res Clin Gastroenterol. 2006;20(06):1085–1101. doi: 10.1016/j.bpg.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee J Y, Kim J S, Moon J M. Incidence of cholangiocarcinoma with or without previous resection of liver for hepatolithiasis. Gut Liver. 2013;7(04):475–479. doi: 10.5009/gnl.2013.7.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J S, Jeong S, Lee D H. Risk factors for long-term outcomes after initial treatment in hepatolithiasis. J Korean Med Sci. 2013;28(11):1627–1631. doi: 10.3346/jkms.2013.28.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh Y C, Chen C K, Su C W. Outcome after percutaneous cholecystostomy for acute cholecystitis: a single-center experience. J Gastrointest Surg. 2012;16(10):1860–1868. doi: 10.1007/s11605-012-1965-8. [DOI] [PubMed] [Google Scholar]
- 14.Arnaud J P, Pessaux P. Percutaneous cholecystostomy for high-risk acute cholecystitis patients. South Med J. 2008;101(06):577. doi: 10.1097/SMJ.0b013e31817308bd. [DOI] [PubMed] [Google Scholar]
- 15.Kim H J, Lee S K, Kim M H. Safety and usefulness of percutaneous transhepatic cholecystoscopy examination in high-risk surgical patients with acute cholecystitis. Gastrointest Endosc. 2000;52(05):645–649. doi: 10.1067/mge.2000.107286. [DOI] [PubMed] [Google Scholar]
- 16.Herr A, Collins D, White M. Percutaneous biliary endoscopy for stones. Tech Vasc Interv Radiol. 2019;22(03):127–134. doi: 10.1053/j.tvir.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Chiverton S G, Inglis J A, Hudd C, Kellett M J, Russell R C, Wickham J E.Percutaneous cholecystolithotomy: the first 60 patients BMJ 1990300(6735):1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillams A, Donald J J, Russell R C, Hatfield A R, Lees W R. The percutaneous rotary lithotrite: a new approach to the treatment of symptomatic cholecystolithiasis. Gut. 1993;34(06):837–842. doi: 10.1136/gut.34.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picus D, Hicks M E, Darcy M D. Percutaneous cholecystolithotomy: analysis of results and complications in 58 consecutive patients. Radiology. 1992;183(03):779–784. doi: 10.1148/radiology.183.3.1533946. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi S. Percutaneous transhepatic cholecystoscopic lithotomy in the management of acute cholecystitis caused by gallbladder stones. Diagn Ther Endosc. 1998;5(01):19–29. doi: 10.1155/DTE.5.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver (EASL). Electronic Address: easloffice@easloffice.eu . EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146–181. doi: 10.1016/j.jhep.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Patel N, Chick J FB, Gemmete J J. Interventional radiology-operated cholecystoscopy for the management of symptomatic cholelithiasis: approach, technical success, safety, and clinical outcomes. AJR Am J Roentgenol. 2018;210(05):1164–1171. doi: 10.2214/AJR.17.18690. [DOI] [PubMed] [Google Scholar]
- 23.Judah J R, Draganov P V. Endoscopic therapy of benign biliary strictures. World J Gastroenterol. 2007;13(26):3531–3539. doi: 10.3748/wjg.v13.i26.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman A, Zangan S M. Benign biliary strictures. Semin Intervent Radiol. 2016;33(04):297–306. doi: 10.1055/s-0036-1592325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tummala P, Munigala S, Eloubeidi M A, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47(06):532–537. doi: 10.1097/MCG.0b013e3182745d9f. [DOI] [PubMed] [Google Scholar]
- 26.Makary M S, Farrell J J, Khayat M, Chick J FB, Srinivasa R N. Biliary endoscopy for benign and malignant biliary strictures. Tech Vasc Interv Radiol. 2019;22(03):135–138. doi: 10.1053/j.tvir.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan A M, Kundu S, Sacks D. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol. 2010;21(11):1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Srinivasa R N, Gemmete J J, Chick J FB. Disposable single-use choledochoscopy may facilitate recanalization of occlusive biliary anastomotic strictures. Radiol Case Rep. 2017;13(01):135–138. doi: 10.1016/j.radcr.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mer J, Chick J FB, Healey T L, Johnson E J, Srinivasa R N. Billing, coding, and credentialing for interventional radiology-operated endoscopy. Tech Vasc Interv Radiol. 2019;22(03):162–164. doi: 10.1053/j.tvir.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 30.American Society for Laser Medicine and Surgery (ASLMS) . Accessed May, 30, 2021 at:http://www.aslms.org/for-professionals/professional-resources/standards-of-practice/standards-of-training-for-physicians-for-the-use-of-lasers-in-medicine-and-surgery
- 31.SIR—Practice resources—Coding update 2019Accessed May, 30, 2021 at:https://www.sirweb.org/practice-resources/
- 32.Riaz A, Entezari P, Ganger D. Percutaneous access of the modified Hutson loop for retrograde cholangiography, endoscopy, and biliary interventions. J Vasc Interv Radiol. 2020;31(12):2113–21200. doi: 10.1016/j.jvir.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 33.McPherson S J, Gibson R N, Collier N A, Speer T G, Sherson N D. Percutaneous transjejunal biliary intervention: 10-year experience with access via Roux-en-Y loops. Radiology. 1998;206(03):665–672. doi: 10.1148/radiology.206.3.9494484. [DOI] [PubMed] [Google Scholar]
- 34.Riaz A, Pinkard J P, Salem R, Lewandowski R J. Percutaneous management of malignant biliary disease. J Surg Oncol. 2019;120(01):45–56. doi: 10.1002/jso.25471. [DOI] [PubMed] [Google Scholar]
- 35.Severini A, Cozzi G, Salvetti M, Mazzaferro V, Doci R. Management of complications from hepatobiliary surgery using the percutaneous transjejunal approach. Tumori. 1997;83(06):912–917. doi: 10.1177/030089169708300608. [DOI] [PubMed] [Google Scholar]
- 36.Kim D, Bolus C, Iqbal S I. Percutaneous transjejunal biliary access in 60 patients with bilioenteric anastomoses. J Vasc Interv Radiol. 2019;30(01):76–810. doi: 10.1016/j.jvir.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Fontein D B, Gibson R N, Collier N A. Two decades of percutaneous transjejunal biliary intervention for benign biliary disease: a review of the intervention nature and complications. Insights Imaging. 2011;2(05):557–565. doi: 10.1007/s13244-011-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amitha Vikrama K S, Keshava S N, Surendrababu N R. Jejunal access loop cholangiogram and intervention using image guided access. J Med Imaging Radiat Oncol. 2010;54(01):5–8. doi: 10.1111/j.1754-9485.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 39.Perry L J, Stokes K R, Lewis W D, Jenkins R L, Clouse M E. Biliary intervention by means of percutaneous puncture of the antecolic jejunal loop. Radiology. 1995;195(01):163–167. doi: 10.1148/radiology.195.1.7892460. [DOI] [PubMed] [Google Scholar]
- 40.Sandhu J, Swersky A, Salsamendi J. Utilization of a modified Roux-en-Y anastomosis as an access point for percutaneous transjejunal cholangioplasty of recurrent biliary strictures. Cardiovasc Intervent Radiol. 2019;42(12):1745–1750. doi: 10.1007/s00270-019-02335-1. [DOI] [PubMed] [Google Scholar]
- 41.Venbrux A C, McCormick C D. Percutaneous endoscopy for biliary radiologic interventions. Tech Vasc Interv Radiol. 2001;4(03):186–192. doi: 10.1016/s1089-2516(01)90024-1. [DOI] [PubMed] [Google Scholar]


