Abstract
Leaf fungal endophytes (LFEs) contribute to plant growth and responses to stress. Fungi colonize leaves through maternal transmission, e.g. via the seed, and through environmental transmission, e.g. via aerial dispersal. The relative importance of these two pathways in assembly and function of the LFE community is poorly understood. We used amplicon sequencing to track switchgrass (Panicum virgatum) LFEs in a greenhouse and field experiment as communities assembled from seed endophytes and rain fungi (integration of wet and dry aerial dispersal) in germinating seeds, seedlings, and adult plants. Rain fungi varied temporally and hosted a greater portion of switchgrass LFE richness (greater than 65%) than were found in seed endophytes (greater than 25%). Exposure of germinating seeds to rain inoculum increased dissimilarity between LFE communities and seed endophytes, increasing the abundance of rain-derived taxa, but did not change diversity. In the field, seedling LFE composition changed more over time, with a decline in seed-derived taxa and an increase in richness, in response to environmental transmission than LFEs of adult plants. We show that environmental transmission is an important driver of LFE assembly, and likely plant growth, but its influence depends on both the conditions at the time of colonization and plant life stage.
Keywords: leaf fungal endophytes, community assembly, environmental transmission, maternal transmission, perennial grass, Panicum virgatum
1. Introduction
Globally, the leaf is one of the largest terrestrial biotic habitats for microbial communities, representing 6.4 × 108 km2 of the global surface area [1]. Within this habitat, leaf fungal endophyte (LFE) taxa are found in all plant species surveyed to date and contribute to plant host growth and survival. LFEs are taxa living asymptomatically within, or between, cells of host leaves for the majority of the fungus' life cycle [2]. These taxa can take on many roles in relation to their plant host, including mutualistic (e.g. increasing drought tolerance [3]), neutral (e.g. latent saprotrophs [4]), or pathogenic, both weak and latent [5]. Thus, LFEs are an important factor in determining plant community composition and productivity [6,7].
Despite the importance of LFEs to large-scale processes, the factors that determine the composition of these communities are, thus far, unresolved. Microbial community assembly is strongly shaped by selection or biotic filtering [8,9]. Selection can be observed when different plant species host different LFE communities, even at the same sites [10]. However, many studies now show that selection by the host plays a relatively minor part in assembly, compared with other processes, as indicated by LFE communities showing strong signatures of site [11,12]. This importance of site could be due to environmental selection (e.g. site's climate) or spatial dynamics (e.g. dispersal limitation within and between sites) outweighing host selection [13]. Historical and current climatic factors may filter regional pools of LFEs [14,15] (e.g. those in soil or air) affecting the kind of taxa that are available to colonize the leaf. Thus, while host selection no doubt plays a role in LFE assembly, predicting its assembly will require that we understand dispersal, transmission, and colonization.
LFE transmission can be broadly split into maternal, i.e. taxa transmitted directly or indirectly from maternal plants, and environmental, i.e. taxa transmitted from the surrounding environment. Our understanding of maternal transmission comes from studying systemic LFEs, i.e. those distributed throughout the host plant, while localized LFEs with restricted distributions within plants make up a higher proportion of global LFE diversity [2,16]. While environmental transmission may come from many sources (e.g. soil and other plants), here, we focus on aerial transmission since it may be especially important to LFEs, as leaf surfaces have high exposure to atmospheric deposition. Colonization of aerially transmitted fungi may be particularly successful during rain events when fungal communities become more active and release more spores and hyphae than during dry periods [17,18]. However, the importance of environmental transmission, relative to maternal transmission, is unknown for LFEs.
The contributions of maternal versus environmental transmission to LFE communities may alter the direction and intensity of interactions between host plants and LFEs. For instance, fungi originating from maternal transmission are predicted to form strong plant–fungal interactions because of LFE dependence on the host for survival and growth [19] giving rise to cross-generational mutualistic and/or parasitic interactions. Although less is known about the functional implications of environmental transmission, this mode is the dominant mode of transmission of pathogenic taxa [20], but also may be important in the spread of some mutualistic LFEs (e.g. [21]) and saprotrophs [4]. With few characterizations of aerial dispersal, and even fewer that contextualize its impact in combination with maternal transmission, it has been impossible to assess the relative importance of transmission mode, and the outcome on microbe–host interactions [2].
Plant life stage is also likely to interact with modes of transmission in the assembly of LFEs. Seedling LFEs are likely more variable and have higher beta diversity (i.e. more differences between individuals) than mature leaves due to the lesser selection by physical and chemical defences. Additionally, mature leaves have experienced longer exposure to the propagules coming from the environment likely stabilizing the community [22,23]. LFE communities increase in alpha diversity and abundance as leaves age [21]. Furthermore, the seedling LFE community is more strongly affected by seed endophytes (due to the proximity in time) and soil fungal community (due to its proximity in space) than the mature LFE community. These differences in the contribution of transmission modes between LFEs of seedling and mature leaves are of particular importance for perennial species since LFEs must either overwinter with their host or recolonize each growing season.
We quantified the importance of maternal and environmental transmission of fungal communities to the LFEs of seedlings and adult plants, testing three hypotheses. First, we hypothesize that (i) rain community shapes LFEs and reduces the relative contribution of seed endophytes. We predict exposure to the rain inoculum will alter the LFE composition and increase LFE diversity. We test this by manipulating seed exposure to live/sterile rain inoculum in Petri dishes (figure 1a). Second, we hypothesize that (ii) the importance of maternal versus environmental transmission in LFE assembly depends on plant life stage. We predict that seed endophytes will be abundant in the seedling LFEs, but replaced as seedlings are exposed to environmental transmission, and test this using seedling and adult plants in the field (figure 1b). Finally, we hypothesize (iii) that the mode of transmission will alter the putative function of LFE communities. Maternal contributions may result in more mutualistic LFE communities, while a high environmental contribution could increase pathogens. We infer putative functions using previously published effects of these taxa on host growth [3,24,25] and on the possible sources of LFEs [26].
Figure 1.
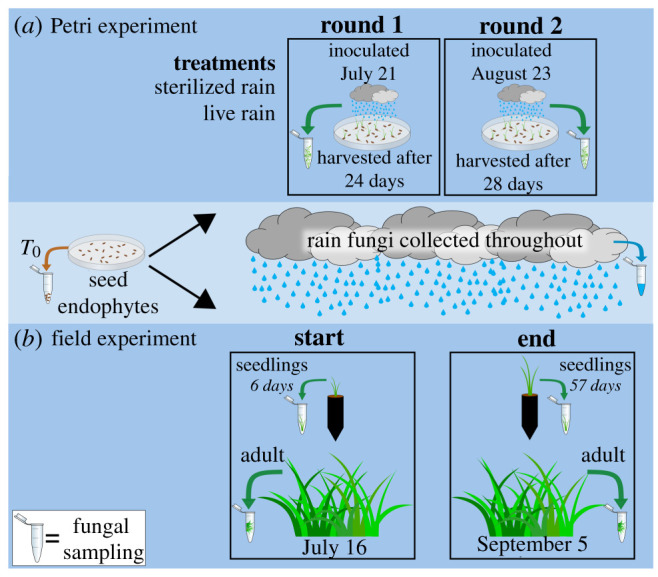
Two experiments were established to test the importance of maternal and environmental transmission in leaf fungal endophyte (LFE) community assembly. (a) The Petri experiment tested the effects of rain inoculum from two rain events (rounds) on germinating seedlings by inoculating seeds with autoclaved sterilized rain or live rain. (b) The field experiment tested the correlation between seedling and adult LFE communities to rain fungi by placing greenhouse germinated seedlings in a field monoculture of adult switchgrass. We collected seedling and adult samples at the start and end of the experiment to characterize the change in LFEs. Seed endophytes were used to characterize the maternally transmitted community while rain fungi were collected throughout the experiment, with events characterized separately, to capture the environmentally transmitted community. For full numbers of replicates, see electronic supplementary material, table S1. (Online version in colour.)
2. Material and methods
(a) . Site and focal host
We focused on the assembly of the LFE community of switchgrass (Panicum virgatum) because it is a perennial bioenergy crop of economic importance that hosts potentially beneficial LFEs [3,27,28], but little is known about the sources of these LFEs (but see [26]). All field samples were collected, and experiments were conducted, in a mature switchgrass monoculture established in 2009 at the Marshall Farms site of the Great Lakes Bioenergy Research Center Scale-up experiment (42.4475522 N, 85.3109636 W). For site and management descriptions, see [29].
(b) . Seed endophyte and rain fungal collection
Seeds used for both the Petri and field experiments were Cave-in-Rock switchgrass variety from 2007 lot SFD-07-F11 (United States Department of Agriculture (USDA) Elsberry Plant Materials Center). Seeds were surface sterilized and stratified at 4°C in Petri dishes with autoclaved filter paper soaked with nanopure water for approximately two months. On 10 July 2018, five random groups of 3–5 seeds were frozen at −80°C for sequence-based characterization of the maternal community (hereafter, ‘seed endophytes’) with this characterization used for both experiments.
At four field blocks, near trays of seedlings (see ‘Field experiment’), we set out rain collectors to capture the aerial dispersed, both dry and wet, fungal community that the seedlings and adult plants were exposed to over the course of the experiment (hereafter, ‘rain fungi’). Rain collectors were left in the field for the full 51 days of the experiment with samples collected within 6 h of each rain event (15 events and 60 total samples). In this way, we captured a realistic view of what the leaf sees, all air/wind deposition up to, and in, a rain event. Rain from two rain events were used to inoculate germinating seeds for the Petri experiment (hereafter, ‘rain inoculum’) and used in the characterization of rain fungi for both experiments. Rain was brought back to the laboratory, vacuum filtered, and stored at −80°C prior to characterization of rain fungi for each sample separately. For a full description of rain collectors and collection, see electronic supplementary material, methods.
(c) . Petri dish experiment
To test hypothesis i, we directly manipulated the presence/absence of rain inoculum on the LFE community of germinating seeds in Petri dishes (hereafter ‘Petri experiment’; figure 1a). In each 100 mm × 15 mm Petri dish, 20 seeds from the stratified batch described above were place on autoclaved Whatman no. 5 filter paper. Petri dishes received 5 ml of either autoclave sterilized rainwater or live rainwater (hereafter, ‘sterilized rain’ and ‘live rain’, respectively). Sterilized rain was autoclaved using a 30 min liquid cycle, then cooled at 4°C for at least 2 h. Petri dishes were sealed with parafilm and placed in the greenhouse. This experiment was conducted twice with rainwater collected (see ‘Rain collection’) on 21 July 2018 (round 1) and 21 August 2018 (round 2), seeds were allowed to germinate and grow for 24 and 28 days, respectively. For each rain event, two Petri dishes were inoculated from collections from three field blocks (total 24 Petri dishes). At harvest, fungal colonization was visually estimated by the number of seeds with fungal growth, germination was recorded, and germinated seedlings were pooled by Petri dish, then stored at −80°C.
(d) . Field experiment
To test hypothesis ii, we sowed 10 seeds per pot into autoclaved 50 : 50 sand and vermiculite in 107 ml containers (SC7 Stewe and Sons, Tangent, OR, USA) that were eventually placed in the field (hereafter, ‘Field experiment’; figure 1b). The 48 pots were blocked into four groups of 12 by tray to control for greenhouse effects and watered daily with nanopure water in an empty greenhouse for 6 days whereupon seedlings began to emerge from the soil (16 July 2018) and seedlings were transported to the field. Eight of the pots (two per block) transported to the field were haphazardly chosen for harvest. Five of these pots, with emerged seedlings, were used to characterize the initial LFE community and colonists from the greenhouse (hereafter, ‘start seedlings’). Of the remaining pots, 10 pots were randomly distributed in 98 cell trays at each of four locations along the southern and western edge of the field (hereafter, ‘field blocks’) surrounding the mature stand of switchgrass. Plants and pots were not allowed to touch the soil or adult plants; therefore, any environmental transmission of fungi occurred through aerial spread. Seedlings were fertilized at the beginning of the experiment and every week with 10 ml of 0.2 µm filtered half strength Miracle-Gro All Purpose Liquid Plant Food. After 52 days in the field (5 September 2018), leaves from the 17 emerged, and surviving, seedlings (4–5 seedlings per field block; hereafter, ‘end seedlings’). At the establishment and end of the experiment, leaves from three randomly chosen adult plants were harvested at each field block (four adult plant replicates; hereafter, ‘start adults’ and ‘end adults’, respectively). All plant material was stored at −80°C prior to sequencing.
(e) . Fungal community characterization
For the full description of community characterization, see electronic supplementary material, methods. Plant samples, seeds, and leaves, were surface sterilized then DNA was extracted using Plant DNeasy kits. Rain DNA was extracted from filters using PowerWater kits (Qiagen, Hilden, Germany). Communities were characterized using 250-bp paired-end MiSeq sequencing (MSU Genomics Core, East Lansing, MI, USA) of the ITS2 region [30]. Sequences were merged, quality checked, and clustered into zero-radius operational taxonomic units (hereafter, approximate sequence variants or ASVs) using unoise3 [31]. We used the level of 100% similarity to be conservative in our estimate of overlap between rain fungi and plant communities. We classified representative sequences against the UNITEv8.2 database [32] using CONSTAX [33]. We identified and removed possible contaminant taxa based on blank controls using microDecon [34]. Finally, we rarified the community to a depth of 1000 reads resulting in 2586 ASVs and 117 000 reads. In total, four plant samples were filtered out due to poor amplification and sequencing (electronic supplementary material, table S1).
To determine possible functional roles of LFEs, addressing hypothesis iii, we matched ASVs to previously published switchgrass LFEs at ≥97% sequence similarity. LFEs were classified into pathogens, mutualists, or context mutualists based on published effects of LFEs on switchgrass [14,15,24]. Putative sources of LFEs were classified based on significant plant community indicator taxa from [26]. For a full description of the functional classifications, see electronic supplementary material, methods.
(f) . Statistical analysis
We used an indicator value index [35], the product of taxon's specificity (i.e. uniqueness to a given habitat) and fidelity (i.e. frequency of occurrence in a given habitat), to classify the likely sources of LFEs, either rain fungi or seed endophytes. We weighted this value by taxon abundances to calculate the contribution of sources to the LFE community. We also calculated the abundance of significant indicator taxa (p < 0.05). We created PERMANOVA and mixed effects models to test the dissimilarity between, and diversity of, LFE communities, seed endophyte, and rain fungi (see electronic supplementary material, methods). Additionally, we tested whether community change was more driven by nestedness (i.e. loss of taxa with no replacement) or turnover (i.e. loss of taxa with replacement) [36] by calculating the ratio (nestedness:turnover; higher values indicate a larger role for nestedness). All PERMANOVA [37] and mixed effects models [38] included field block as a random grouping variable.
3. Results
Rain fungi showed tremendous taxonomic and functional variability over the course of the experiment (electronic supplementary material, figures S1 and S2). The richness and diversity of rain fungi was also consistently higher than the LFE community (electronic supplementary material, figures S3 and S4). Basidiomycota dominated rain fungi until the end of the experiment when Ascomycota reached equal abundance (electronic supplementary material, figure S2a). Overall, these rain fungi appear to be a significant source of LFE taxa; rain fungi made up greater than 65% of the richness and approximately 90% of the reads found in adult and seedling LFE communities.
(a) . Hyp i: rain inoculum alter LFEs
We found that live rain inoculum altered the LFEs of germinating seeds (electronic supplementary material, table S2 and figure S5); however, the strength of these effects differed across our two experimental rounds. Specifically, live rain increased similarity between LFE communities and rain fungi in round 2, but significantly increased dissimilarity between LFEs and seeds in round 1 (electronic supplementary material, table S3 and figure 2a), and only when taking account of abundance (i.e. Bray–Curtis distance). In both rounds, live rain increased rain-indicator taxa in LFE communities, without increasing LFE diversity (electronic supplementary material, table S4 and figure S3) or replacing seed indicator taxa (electronic supplementary material, table S5; figure 3a and electronic supplementary material, figure S6a). Though turnover explained much of the difference between LFE communities and each source (rain or seeds), nestedness explained more differences between LFE communities and rain fungi, presumably because LFEs were a subset of the highly diverse rain fungal community (electronic supplementary material, table S3; figure 2c and electronic supplementary material, figure S7a).
Figure 2.
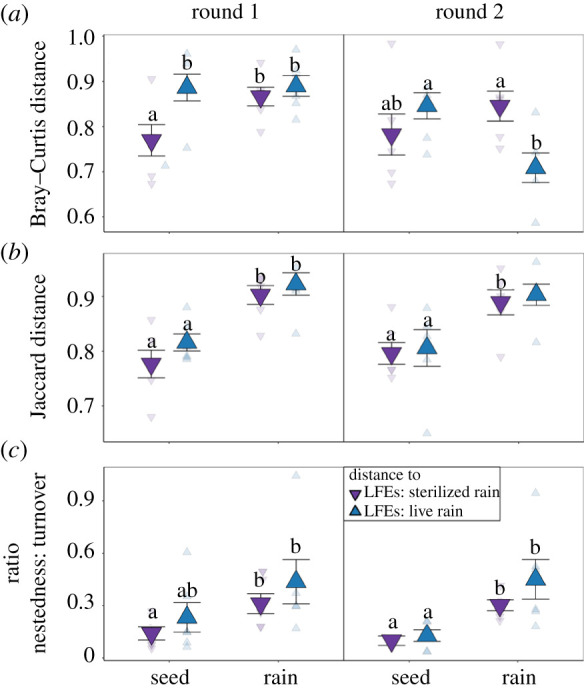
Pairwise community distance of LFE communities to seed endophytes (seed) or rain fungal (rain) communities using (a) Bray–Curtis, (b) Jaccard distance, and (c) the ratio of nestedness to turnover of seedlings receiving autoclave sterilized rain (dark purple triangle pointing down) or live rain (dark blue triangle pointing up). Round of inoculations are round 1 (July 21) and round 2 (August 21). Points represent means with s.e. Raw data are represented by transparent points. Within round post-hoc pairwise significances are false discovery rate adjusted represented by letters. (Online version in colour.)
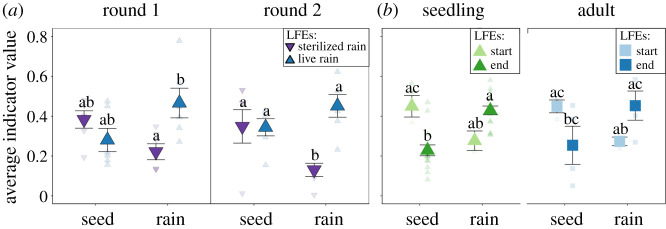
Figure 3.
Average seed endophyte and rain fungal indicator values in LFE communities of the (a) Petri experiment and (b) field experiment. (a) Germinating seedling LFE communities were inoculated in round 1 (July 21) and round 2 (August 21) with autoclave sterilized rain (dark purple triangle pointing down) or live rain (dark blue triangle pointing up). (b) Seedling LFEs were characterized at the start (July 16: light green triangle) or end (September 5: dark green triangle), and adult LFEs were characterized at the start (July 16: light blue square) or end (September 5: dark blue square). Points represent means with s.e. Raw data are represented by transparent points. False discovery rate adjusted post-hoc pairwise significances for (a) within round comparisons for the Petri experiment and (b) all sample comparisons for the field experiment are represented by letters. (Online version in colour.)
In general, rain fungi were highly distinct from both LFEs and seed endophytes (electronic supplementary material, figure S5), tended to have lower variance in terms of taxa presence/absence (i.e. Jaccard distance; electronic supplementary material, table S4 and figure S8b), and higher diversity (electronic supplementary material, figure S3). There were also significant differences in rain fungi used in round 1 and round 2 (pairwise PERMANOVA p < 0.03). Rain fungi in round 1, collected earlier in the summer, was more dominated by Basidiomycota, specifically Agaricomycetes, Exobasiomycetes, and Tremellomycetes, while round 2 was dominated by Ascomycota, specifically Dothideomycetes (electronic supplementary material, figure S9). Still, across both rounds, live rain exposure consistently increased the abundance of Dothideomycetes and Sordariomycetes while decreasing the abundance of Tremellomycetes, which dominated seeds and LFEs receiving sterilized rain (electronic supplementary material, figure S9b). Finally, inoculation with live rain did not significantly alter seed germination rate (electronic supplementary material, figure S10a) or visible fungal colonization (electronic supplementary material, table S6 and figure S10b).
(b) . Hyp ii: importance of seed endophytes and rain fungi across two life stages
LFE communities of both life stages, end seedling and adult plants, were significantly different from the starting LFE communities (electronic supplementary material, table S7 and figure S11) and gained rain indicators (Tukey honestly significant difference (HSD): p = 0.016; electronic supplementary material, table S8 and figure 3b) by the end of the experiment. Seedling LFE communities shifted more from start to end compared with adult plants (Jaccard-based composition; electronic supplementary material, table S9; figure 4b) and experienced a significant loss of seed indicator taxa (figure 3b). In addition, the richness of seedling LFEs more than doubled from start to end while there was no change in adult LFEs (electronic supplementary material, table S10 and figure S4a). Still, some patterns were similar across life stage. Seedling LFEs were no more similar to rain fungi than adults (figure 4a,b) supported by the fact that LFEs had similar contributions from rain fungi overall (figure 3b). While turnover dominated changes in fungal communities, when comparing relative importance between life stages, nestedness contributed more to the distance between rain fungi and end adult LFEs (figure 4c and electronic supplementary material, figure S12a).
Figure 4.
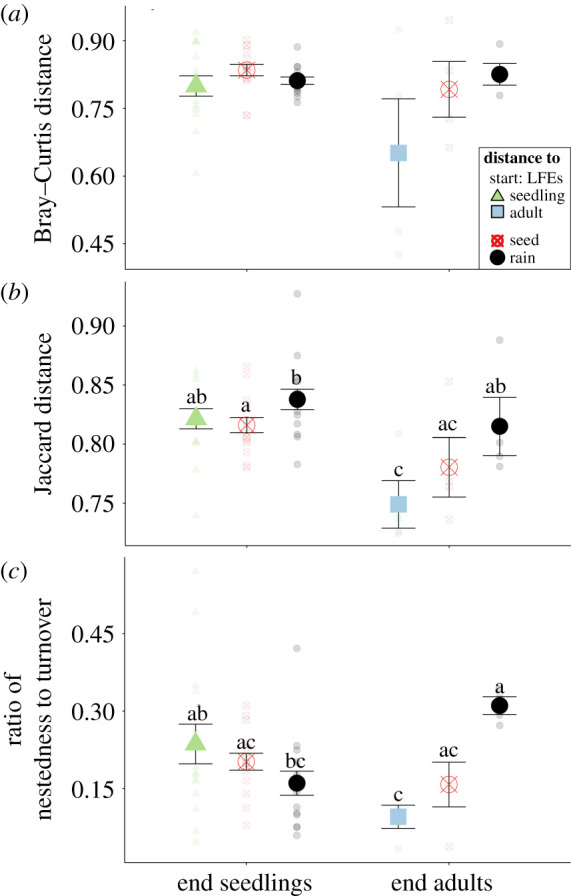
Pairwise community distance using (a) Bray–Curtis, (b) Jaccard distance, and (c) the ratio of nestedness to turnover from end (September 5) seedling and adult LFE communities to start LFEs (July 16; seedlings: green triangle or adults: blue square), seed endophytes (red circled x), or rain fungi (black circles). Points represent means with s.e. Raw data are represented by transparent points. Post-hoc pairwise significances are false discovery rate adjusted represented by letters, and no letters indicate no significant difference. (Online version in colour.)
The endophyte communities of the seeds, start seedlings and start adults were dominated by likely yeast from Basidiomycota, specifically Tremellomycetes, but, by the end of the experiment, both adult plants and seedlings were dominated by Ascomycota, specifically Dothideomycetes (electronic supplementary material, figure S13). There was no difference in beta-dispersion across endophyte communities (electronic supplementary material, table S10 and figure S14).
(c) . Hyp iii: mode of transmission alters the function of LFE communities
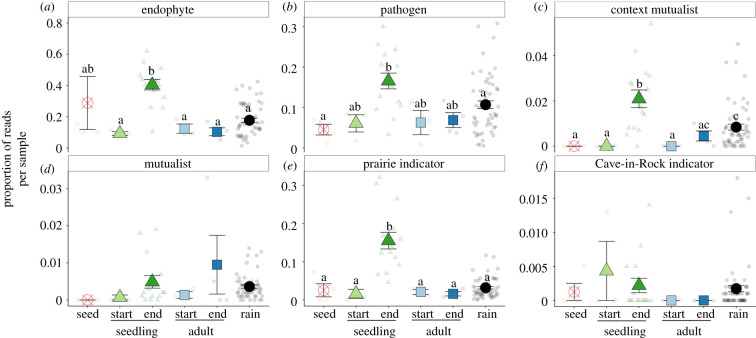
Switchgrass LFEs were common in rain fungi (approx. 18% of reads; figure 5a and electronic supplementary material, figure S1a). Pathogens made up the largest portion of the putative LFEs found in rain fungi (approx. 11% of reads; figure 5b and electronic supplementary material, figure S1b). In the field experiment, the relative proportion of pathogens in seedling LFEs increased from start to end (figure 5b) further supporting rain as the dominant pathway for pathogens. This was corroborated by the Petri experiment, in which LFEs originating from live rain were primarily pathogens (electronic supplementary material, figure S15b). We found no recorded mutualists in the seed endophyte community (figure 5c,d) instead likely pathogens made up approximately 16% of the putative LFEs found in seed endophytes (figure 5b). On the other hand, mutualists and context mutualists were found in rain fungi (figure 5c,d and electronic supplementary material, figure S1c,d). In general, functional attributes of LFEs changed more in seedlings than adults, consistent with the compositional data (figure 5).
Figure 5.
Proportion of fungal reads matching previously published endophytes. Points are seed endophytes (red circled x), rain fungi (black circles), seedling LFE collected at the start (July 16: light green triangle) or end (September 5: dark green triangle), adult LFEs collected at the start (July 16: light blue square) or end (September 5: dark blue square). Points represent means with s.e. Raw data are represented by transparent points. False discovery rate adjusted post-hoc pairwise significances are represented by letters, and no letters indicate no significant difference. (Online version in colour.)
4. Discussion
We show how maternal and environmental transmission contribute to short-term (post germination) and long-term (adult leaves) assembly of LFE communities. We found that rain (representing wet and dry aerial dispersal) is comprised of a rich community of fungi, many of which are found in LFE communities, and exposure to this environmental transmission changes LFE composition. Together, this suggests that rain is an important driver of LFE assembly, supporting our first hypothesis. This first look at the relative influence of seed versus rain communities in LFE assembly revealed three roles for environmental transmission. First, rain affects LFE assembly, but these effects are likely temporally dependent and not necessarily predictable. Depending on the characteristics of the rain event, rain inoculum seems to shift the germinating LFE community compositionally away from seed endophytes by enrichment of taxa and not by displacing seed endophytes. As LFEs continue to assemble under environmental transmission, and increase in richness, seed indicators are lost from the LFE community. Second, LFE communities of early life stages (seedlings) are most responsive to environmental transmission. We observed large shifts in LFE composition when seedlings were exposed to environmental transmission, and relatively little change in adult LFEs. Finally, wet and dry aerial dispersed fungi, integrated through rain, hosts a large temporally variable community of putative LFEs, with fungi able to colonize contributing unique functions to the LFE community.
(a) . Rain fungi shifts LFE community away from the maternal endophytes via enrichment
We show that environmentally transmitted taxa can alter the communities of germinating seedlings, reducing similarity to maternal communities, but not through displacement of seed endophytes by novel rain fungi. Rather, LFEs became more dissimilar to seed endophyte communities under the first round of inoculation (figure 2a) without a loss of seed endophytes (figure 3a). This lack of displacement of seed endophytes may be driven by rain inoculum enriching taxa that have overlapping presence in seed endophytes and rain fungi (greater than 55% of seed endophytes are found in rain fungi) which may be the result of historical environmental transmission. Even though all seeds were from the same USDA grown population, gamma diversity across seeds was high (greater than 150 ASVs; electronic supplementary material, table S1). This rich pool of maternal taxa may have originated from aerial dispersed microbes colonizing florets during fertilization and seed development (reviewed in [39]). Seed endophytes possibly originating from ‘historical’ environmental transmission makes separating environmental and maternal transmission challenging, but our study allows the separation of maternal contributions from contemporary environmental transmission. We primarily found that rain inoculum increased the abundance of Ascomycota and pathogens (electronic supplementary material, figures S9a and S15b), but effects of environmental transmission appeared temporally dependent.
High diversity and temporal variability in the rain fungi seems to have important implications for the assembly of LFEs. Despite the rich rain fungal community, inoculation with rain inoculum had no effect on LFE diversity (electronic supplementary material, figure S3). Many rain fungi may not have been able to colonize the plant due to strong leaf selection, making increasing abundance of extant endophytes the primary effect of rain inoculum, not introduction of new taxa. The increased similarity between LFEs and rain fungi only in round 2 (figure 2a) may be partially a result of the variability of the rain fungi over time. Specifically, rain inoculum used for round 2 had a higher portion of Ascomycota (electronic supplementary material, figure S9a), a high portion of previously documented LFEs, mostly putative pathogens (electronic supplementary material, figure S15a,b; [15,24]), and higher concentrations of fungal hyphae and spores (data not included). The higher concentration of putative LFEs, and fungi in general, may have increased colonization success and thus environmental transmission [40,41]. Variation in rain chemistry between rounds also could have driven the rain inoculum effect on LFEs, nitrate levels were higher the week that round 2 inoculant was collected (approx. 2× higher, Station MI26; http://nadp.slh.wisc.edu/ntn/). Regardless of the mechanism, our study highlights that LFE assembly may be sensitive to the changes in the composition, and colonization ability, of rain fungi, which appear highly temporally variable (electronic supplementary material, figure S1a). Additionally, this temporal dependency of colonization appears to continue into the adult life stage since rain indicators increased in the LFE community over time (figure 3b). Our results, as well as a recent study of switchgrass fungal epiphytes in our region [42], show seasonal succession in the leaf microbiome which may be driven by an active exchange between the leaf microbiome and the rain community.
(b) . Seedlings are more responsive to environmental transmission than adults
The persistence of seed endophytes and magnitude of temporal change in the LFE community differed by life stage, confirming our second hypothesis. The change in the presence and absence of taxa best captured the greater change in LFE communities, and increased dissimilarity from seed endophytes (figure 4b), in seedlings compared with adults, suggesting changes in rare taxa drove shifts in community composition. Our findings are consistent with other studies that have found a greater temporal change in seedling than adult LFE communities [22,43]. These rapid changes in seedling LFEs may be driven by a lack of physical and chemical defences making the leaves of seedlings more susceptible to colonization from external sources. Early life stages may be especially important in shaping the final LFE community, and we show that they are susceptible to environmental transmission, including of pathogens (figure 5b).
Though their importance declined with exposure to rain inoculum, seed endophytes had a surprisingly long-lasting presence in the switchgrass LFE community. Our finding that seed indicators persisted into seedling and even adult stages (making up approximately 14% of adult reads; electronic supplementary material, figure S6b) suggests that even though colonization of rain fungi reduces its importance, the seed community is still important for long-term LFE dynamics. To better understand the long-term effects of seed endophytes on LFE communities requires experimental manipulation such as knocking out the current seed endophyte community and monitoring LFE assembly. Importantly, though seed endophytes are a part of the long-term LFE community, two-month-old seedling LFE communities already greatly diverge from the seed endophyte community (electronic supplementary material, figure S11).
Though the drivers of the shift differed between the two life stages, both the seedling and adult LFE communities tended to show increased relative abundances of rain indicators (figure 3b). In contrast to the Petri experiment, the colonization of novel rain fungi played a role in seedling LFE community change and increased LFE richness (electronic supplementary material, figure S4a). Interestingly, the increase in richness with exposure to rain inoculum did not occur in the LFEs of seedlings in the Petri experiment (electronic supplementary material, figure S3a). It is possible that the relatively short duration, or the single inoculation under controlled conditions, of the Petri experiment reduced our ability to observe the effects of rain inoculum on LFE richness. Though turnover dominated the changes in the LFE communities, compared with seedlings, the adult LFE community was more of a taxonomic subset of the rain fungi (i.e. higher nestedness in figure 4c), suggesting that adult plants may exert higher selection on colonists from the diverse rain community.
(c) . Environmentally transmitted taxa contribute unique functions to LFEs
Our data also suggests that transmission pathway influences LFE functional diversity and LFE community impact on plant host, partially supporting our third hypothesis. Rain fungi hosted many taxa that have been identified as switchgrass LFEs from previous culture-based surveys (electronic supplementary material, figure S1a; [15,24,26]), allowing us to infer putative functions. The dominance of putative pathogens in rain fungi, and the fact that these groups became abundant in the seedling LFEs by the end of the experiment (figure 5b), suggests that rain is a significant source for pathogen dispersal [20,44,45]. Contrary to our predictions, seed endophytes hosted no recorded mutualists and instead hosted putative LFEs composed of possible pathogens (figure 5b). Furthermore, we found that rain hosted a small portion of LFE mutualists and context mutualists, beneficial under drought but antagonistic under other conditions [14]. These mutualists became somewhat enriched in both the adult and seedling LFE communities by the end of the experiment (figure 5c,d), highlighting that aerial transmission can introduce both beneficial as well as harmful taxa.
Rain also appears to be a major pathway for the transmission of taxa from surrounding plant communities. LFEs that have been found to be indicative of prairie ecosystems [26] were present at low frequency, but consistent levels across rain events. These same taxa became relatively dominant in the final seedling community compared to all other LFE communities (figure 5e). Since our field experiment was conducted near an experimental prairie restoration [29], these taxa may also be colonists from the prairie plant community in our experiment, highlighting the potential for spillover between cultivated and non-cultivated lands via aerial dispersal [46]. On the other hand, taxa indicative of the specific population from which our target switchgrass plants were derived (i.e. Cave-in-Rock variety) [26] were relatively rare in the rain fungal and LFE communities (figure 5f). This suggests that the selection of regional pools of potential LFEs by nearby plant communities may explain the ‘site signal’ found in many LFE studies [11,12].
5. Conclusion
Rain, as an integrator of wet and dry aerial dispersal, hosts a functionally diverse community of putative LFEs and alters community assembly and function. We found that seed endophytes remained in the LFE communities of seedlings and adult plants, but exposure to environmental transmission made LFE communities less similar to seed endophytes and increased the contribution of aerial transmitted taxa to the LFE community, demonstrating that environmental transmission is an important driver of LFE community assembly. This interaction is dynamic over time, with plant ontogeny and seasonal shifts in rain community composition and chemistry likely affecting assembly outcomes. Future work should test the seasonal LFE-rain interchange and its importance for long-term dynamics of the plant microbiome.
Supplementary Material
Acknowledgements
We thank Tayler Ulbrich, Corinn Rutkoski, Heather Kittredge, and Holly Vander Stel for help with field and laboratory work, and Kevin Dougherty for help with laboratory work and experimental design. We thank Nick Haddad and two anonymous reviewers for comments and improving the manuscript. This work took place on occupied Anishinaabe land where Hickory Corners, Michigan, is now located. We thank local communities and the state of Michigan for maintaining and allowing access to the field sites. This is Kellogg Biological Station contribution number 2286.
Data accessibility
Sequence data are available at NCBI SRA under Bio-Project no. PRJNA709151, and datasets, bioinformatics scripts, and metadata used in the current study are available at https://github.com/ldereske/Bell-Dereske_Evans_Fugal_Rain and archived at https://doi.org/10.5281/zenodo.4604699.
Authors' contributions
L.P.B.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft; S.E.: conceptualization, funding acquisition, resources, supervision, writing-review, & editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Support for this research was provided by the MMPRNT project (DOE BER Office of Science award DE-SC0014108), by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-SC0018409 and DE-FC02-07ER64494), and by the NSF Long-Term Ecological Research Program (DEB 1832042 and 1637653) at the Kellogg Biological Station.
References
- 1.Morris CE, Kinkel L. 2002Fifty years of phyllosphere microbiology: significant contributions to research in related fields. In Phyllosphere microbiology (eds Lindow SE, Hecht-Poinar EI, Elliott V), pp. 365-375. St. Paul, MI: APS Press. [Google Scholar]
- 2.Rodriguez RJ, White JF, Arnold AE, Redman RS. 2009Fungal endophytes: diversity and functional roles. New Phytol. 182, 314-330. ( 10.1111/j.1469-8137.2009.02773.x) [DOI] [PubMed] [Google Scholar]
- 3.Giauque H, Connor EW, Hawkes CV. 2019Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytol. 221, 2239-2249. ( 10.1111/nph.15504) [DOI] [PubMed] [Google Scholar]
- 4.Osono T. 2006Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can. J. Microbiol. 52, 701-716. ( 10.1139/w06-023) [DOI] [PubMed] [Google Scholar]
- 5.Lofgren LA, et al. 2018Fusarium graminearum: pathogen or endophyte of North American grasses? New Phytol. 217, 1203-1212. ( 10.1111/nph.14894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clay K, Holah J. 1999Fungal endophyte symbiosis and plant diversity in successional fields. Science 285, 1742-1744. ( 10.1126/science.285.5434.1742) [DOI] [PubMed] [Google Scholar]
- 7.Cheplick GP, Faeth SH. 2009Ecology and evolution of the grass-endophyte symbiosis. New York, NY: Oxford University Press. [Google Scholar]
- 8.Fu H, Uchimiya M, Gore J, Moran MA. 2020Ecological drivers of bacterial community assembly in synthetic phycospheres. Proc. Natl Acad. Sci. USA 117, 3656-3662. ( 10.1073/pnas.1917265117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langenheder S, Székely AJ. 2011Species sorting and neutral processes are both important during the initial assembly of bacterial communities. ISME J. 5, 1086. ( 10.1038/ismej.2010.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U'Ren JM, Lutzoni F, Miadlikowska J, Laetsch AD, Arnold AE. 2012Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Botany 99, 898-914. ( 10.3732/ajb.1100459) [DOI] [PubMed] [Google Scholar]
- 11.Seabloom EW, Condon B, Kinkel L, Komatsu KJ, Lumibao CY, May G, Mcculley RL, Borer ET. 2019Effects of nutrient supply, herbivory, and host community on fungal endophyte diversity. Ecology 100, e02758. ( 10.1002/ecy.2758) [DOI] [PubMed] [Google Scholar]
- 12.Lumibao CY, Borer ET, Condon B, Kinkel L, May G, Seabloom EW. 2019Site-specific responses of foliar fungal microbiomes to nutrient addition and herbivory at different spatial scales. Ecol. Evol. 9, 12 231-12 244. ( 10.1002/ece3.5711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins KL, Arnold AE, Coley PD, Kursar TA. 2014Communities of fungal endophytes in tropical forest grasses: highly diverse host- and habitat generalists characterized by strong spatial structure. Fungal Ecol. 8, 1-11. ( 10.1016/j.funeco.2013.12.005) [DOI] [Google Scholar]
- 14.Giauque H, Hawkes CV. 2016Historical and current climate drive spatial and temporal patterns in fungal endophyte diversity. Fungal Ecol. 20, 108-114. ( 10.1016/j.funeco.2015.12.005) [DOI] [Google Scholar]
- 15.Giauque H, Hawkes CV. 2013Climate affects symbiotic fungal endophyte diversity and performance. Am. J. Botany 100, 1435-1444. ( 10.3732/ajb.1200568) [DOI] [PubMed] [Google Scholar]
- 16.Newcombe G, Harding A, Ridout M, Busby PE. 2018A hypothetical bottleneck in the plant microbiome. Front. Microbiol. 9, 1645-1645. ( 10.3389/fmicb.2018.01645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crandall SG, Gilbert GS. 2017Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 162, 87-99. ( 10.1016/j.atmosenv.2017.05.018) [DOI] [Google Scholar]
- 18.Rathnayake CM, Metwali N, Jayarathne T, Kettler J, Huang Y, Thorne PS, O'Shaughnessy PT, Stone EA. 2017Influence of rain on the abundance of bioaerosols in fine and coarse particles. Atmos. Chem. Phys. 17, 2459-2475. ( 10.5194/acp-17-2459-2017) [DOI] [Google Scholar]
- 19.Gundel PE, Rudgers JA, Ghersa CM. 2011Incorporating the process of vertical transmission into understanding of host–symbiont dynamics. Oikos 120, 1121-1128. ( 10.1111/j.1600-0706.2011.19299.x) [DOI] [Google Scholar]
- 20.Kinkel LL. 1997Microbial population dynamics on leaves. Annu. Rev. Phytopathol. 35, 327-347. ( 10.1146/annurev.phyto.35.1.327) [DOI] [PubMed] [Google Scholar]
- 21.Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA. 2003Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl Acad. Sci. USA 100, 15 649-15 654. ( 10.1073/pnas.2533483100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oono R, Lefèvre E, Simha A, Lutzoni F. 2015A comparison of the community diversity of foliar fungal endophytes between seedling and adult loblolly pines (Pinus taeda). Fungal Biol. 119, 917-928. ( 10.1016/j.funbio.2015.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold AE, Herre EA. 2003Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95, 388-398. ( 10.1080/15572536.2004.11833083) [DOI] [PubMed] [Google Scholar]
- 24.Kleczewski NM, Bauer JT, Bever JD, Clay K, Reynolds HL. 2012A survey of endophytic fungi of switchgrass (Panicum virgatum) in the Midwest, and their putative roles in plant growth. Fungal Ecol. 5, 521-529. ( 10.1016/j.funeco.2011.12.006) [DOI] [Google Scholar]
- 25.Giauque H. 2016Hierarchical controls of endophyte-mediated drought tolerance: ecological, physiological, and molecular. UT Electronic Theses and Dissertations, University of Texas. https://repositories.lib.utexas.edu/handle/2152/68387.
- 26.Whitaker BK, Reynolds HL, Clay K. 2018Foliar fungal endophyte communities are structured by environment but not host ecotype in Panicum virgatum (switchgrass). Ecology 99, 2703-2711. ( 10.1002/ecy.2543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor EW, Sandy M, Hawkes CV. 2017Microbial tools in agriculture require an ecological context: stress-dependent non-additive symbiont interactions. Agron. J. 109, 917-926. ( 10.2134/agronj2016.10.0568) [DOI] [Google Scholar]
- 28.Hestrin R, Lee MR, Whitaker BK, Pett-Ridge J. 2021The switchgrass microbiome: a review of structure, function, and taxonomic distribution. Phytobiomes J. 5, 14-28. ( 10.1094/pbiomes-04-20-0029-fi) [DOI] [Google Scholar]
- 29.Abraha M, Gelfand I, Hamilton SK, Chen J, Robertson GP. 2018Legacy effects of land use on soil nitrous oxide emissions in annual crop and perennial grassland ecosystems. Ecol. Appl. 28, 1362-1369. ( 10.1002/eap.1745) [DOI] [PubMed] [Google Scholar]
- 30.Ihrmark K, et al. 2012New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 82, 666-677. ( 10.1111/j.1574-6941.2012.01437.x) [DOI] [PubMed] [Google Scholar]
- 31.Edgar RC. 2016UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 081257. ( 10.1101/081257) [DOI]
- 32.Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F, Nilsson RH, Kõljalg U. 2020UNITE USEARCH/UTAX release for eukaryotes. Version 04.02.2020. UNITE Community. ( 10.15156/BIO/786376) [DOI]
- 33.Gdanetz K, Benucci GMN, Vande Pol N, Bonito G. 2017CONSTAX: a tool for improved taxonomic resolution of environmental fungal ITS sequences. BMC Bioinf. 18, 538. ( 10.1186/s12859-017-1952-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mcknight DT, Huerlimann R, Bower DS, Schwarzkopf L, Alford RA, Zenger KR. 2019microDecon: a highly accurate read-subtraction tool for the post-sequencing removal of contamination in metabarcoding studies. Environmental DNA 1, 14-25. ( 10.1002/edn3.11) [DOI] [Google Scholar]
- 35.Dufrêne M, Legendre P. 1997Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345-366. [Google Scholar]
- 36.Baselga A, Orme CDL. 2012betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808-812. ( 10.1111/j.2041-210X.2012.00224.x) [DOI] [Google Scholar]
- 37.Clarke K, Gorley R. 2007Primer, Version 6.1.10: User Manual and Tutorial. Primer-E, Plymouth. Albany, Auckland, New Zealand. [Google Scholar]
- 38.Bates D, Mächler M, Bolker B, Walker S. 2015Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 39.Shade A, Jacques M-A, Barret M. 2017Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin Microbiol. 37, 15-22. ( 10.1016/j.mib.2017.03.010) [DOI] [PubMed] [Google Scholar]
- 40.Ownley BH, Griffin MR, Klingeman WE, Gwinn KD, Moulton JK, Pereira RM. 2008Beauveria bassiana: endophytic colonization and plant disease control. J. Invertebr. Pathol. 98, 267-270. ( 10.1016/j.jip.2008.01.010) [DOI] [PubMed] [Google Scholar]
- 41.Bamisile BS, Dash CK, Akutse KS, Keppanan R, Afolabi OG, Hussain M, Qasim M, Wang L. 2018Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: an insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 217, 34-50. ( 10.1016/j.micres.2018.08.016) [DOI] [PubMed] [Google Scholar]
- 42.Bowsher AW, Benucci GMN, Bonito G, Shade A. 2021Seasonal dynamics of core fungi in the switchgrass phyllosphere, and co-occurrence with leaf bacteria. Phytobiomes J. 5, 60-68. ( 10.1094/pbiomes-07-20-0051-r) [DOI] [Google Scholar]
- 43.Maignien L, Deforce EA, Chafee ME, Eren AM, Simmons SL. 2014Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. mBio 5, e00682-13. ( 10.1128/mBio.00682-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H, Kim S, Gruszewski HA, Schmale DG, Boreyko JB, Jung S. 2020Dynamics of splashed droplets impacting wheat leaves treated with a fungicide. J. R. Soc. Interface 17, 20200337. ( 10.1098/rsif.2020.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S, Park H, Gruszewski HA, Schmale DG, Jung S. 2019Vortex-induced dispersal of a plant pathogen by raindrop impact. Proc. Natl Acad. Sci. USA 116, 4917-4922. ( 10.1073/pnas.1820318116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell T, Tylianakis JM. 2016Microbes in the Anthropocene: spillover of agriculturally selected bacteria and their impact on natural ecosystems. Proc. R. Soc. B 283, 20160896. ( 10.1098/rspb.2016.0896) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data are available at NCBI SRA under Bio-Project no. PRJNA709151, and datasets, bioinformatics scripts, and metadata used in the current study are available at https://github.com/ldereske/Bell-Dereske_Evans_Fugal_Rain and archived at https://doi.org/10.5281/zenodo.4604699.