Abstract
Food limitation is a universal stressor for wildlife populations and is increasingly exacerbated by human activities. Anthropogenic environmental change can significantly alter the availability and quality of food resources for reservoir hosts and impact host–pathogen interactions in the wild. The state of the host's nutritional reserves at the time of infection is a key factor influencing infection outcomes by altering host resistance. Combining experimental and model-based approaches, we investigate how an environmental stressor affects host resistance to West Nile virus (WNV). Using American robins (Turdus migratorius), a species considered a superspreader of WNV, we tested the effect of acute food deprivation immediately prior to infection on host viraemia. Here, we show that robins food deprived for 48 h prior to infection, developed higher virus titres and were infectious longer than robins fed normally. To gain an understanding about the epidemiological significance of food-stressed hosts, we developed an agent-based model that simulates transmission dynamics of WNV between an avian host and the mosquito vector. When simulating a nutritionally stressed host population, the mosquito infection rate rose significantly, reaching levels that represent an epidemiological risk. An understanding of the infection disease dynamics in wild populations is critical to predict and mitigate zoonotic disease outbreaks.
Keywords: zoonotic disease, nutritional stress, bird, West Nile virus, agent-based models, host resistance
1. Introduction
The emergence and transmission of zoonotic pathogens is increasing at an alarming rate and is expected to continue with globalization and anthropogenic land-use changes [1]. The risk of zoonotic pathogens to public health cannot be overstated, as evident from the pandemic from a novel coronavirus, 2019-nCoV, a zoonotic pathogen that originated in animals and spilled over into human populations. In fact, the most devastating disease outbreaks in humans are caused by zoonotic pathogens [1]. Our ability to predict the emergence and spillover of zoonotic pathogens into human populations and mitigate the impact of disease outbreaks relies on an understanding of the factors underlying infection and transmission dynamics in wildlife populations [2]. Land-use change can mediate the abundance and distribution of reservoir hosts and their capacity to maintain, move and transmit pathogens [3]. Hence, understanding the drivers of disease dynamics within the wild reservoir host populations is critically important to mitigating the health risk to wildlife, domestic animal and human populations [4].
Food limitation is a dominant source of stress for wild populations, and the availability of food is increasingly threated by human activities. Habitat alterations are almost always followed by changing availability and quality of food resources for wildlife which can alter host–parasite interactions and the frequency and intensity of disease outbreaks. The mechanism(s) by which changing food resources may affect disease (transmission) dynamics through altering host–pathogen interactions include (i) behavioural changes that alter host's contact with other hosts or vectors [5,6], (ii) changes in host abundance and density through demographic shifts (increase and decrease in birth and death rates) and (iii) changing host physiology and immune defence [3,7,8]. Here, we examine the latter—the effect of food deprivation on host resistance to a viral pathogen.
A host's ability to maintain and engage their immune system depends on their ability to meet the energetic and nutrient requirements of immune responses [9], and a negative energy balance and/or malnutrition can significantly impair their immune function [10,11]. In particular, food deprivation may reduce cytokine production, cause immune organs to atrophy and reduce populations of immune cells [12]. In the absence of a properly functioning immune system, pathogen replication may be unchecked leading to higher pathogen loads and delayed recovery rates, and lengthening the time the host is infectious [8,13]. The duration and intensity (i.e. pathogen load) of this infectious state is one of the critical factors influencing transmission dynamics [14]; yet, despite the positive association between host nutrition and immune function [15], there are few studies on how the scarcity of food can affect a host's ability to defend itself against parasites [16,17].
The factors underlying variation in host infectiousness at the level of the population and individual are not well understood. The variation may be driven by the host's life history [18], host genetics, host–pathogen interactions and/or extrinsic factors [19,20]. In multi-host systems, interspecific variation in infectiousness has received the most attention due to its role in community-level disease dynamics by either diluting or amplifying pathogen transmission [21]. This interspecific variation in infectiousness is one determinant of a host's reservoir competency for arthropod-transmitted pathogen; another is the likelihood of the host being a source of an arthropod vector's blood meal and susceptibility to becoming infected [22]. More recently, attention has been focused on within-species variation in infectiousness [23–25], in which pathogen loads exhibit patterns consistent with the Pareto distribution with the top most infectious individuals being responsible for 80% of the total pathogen load [24]. If these highly infectious or ‘supershedders’ also make contact with susceptible hosts or vectors, they can alter disease dynamics and trigger superspreading events [26].
In this study, we use West Nile virus (WNV) and a common reservoir host, American Robin (Turdus migratorius; hereafter ‘robin’), to advance our understanding of how an environmental stressor, food availability, affects within-species variation in host's infectiousness. In the 1990s, WNV became the most geographically widespread arbovirus globally with outbreaks occurring in Eastern Europe and Northern Africa, and its invasion and the subsequent human and bird outbreaks in the USA [27]. Human risk of WNV increases with enzootic transmission between birds and mosquitoes, which is driven by the reservoir competence of the amplifying hosts (i.e. birds). Robins are a ubiquitous and largely migratory landbird throughout much of North America. While they may not exhibit exceptionally high viral titres relative to other bird species [28–30], robins do develop moderately high viraemia and serve as a primary source of Culex mosquito blood meals, particularly in the eastern USA [31–33]; and as such, they have been classified as ‘superspreaders’ of WNV [34,35].
Here we investigate an environmental determinant of host infectiousness—the availability of food. We tested the hypothesis that short-term food deprivation would make robins less resistant to WNV than birds not food stressed. Specifically, we predicted that robins deprived of food for a couple days prior to exposure to WNV would exhibit (i) higher viral titres, (ii) longer viraemia and (iii) higher morbidity and/or mortality than non-food-stressed individuals. We then use an agent-based model (ABM) approach to explore how changes in individual host infectiousness may impact population-level transmission dynamics of WNV.
Specifically, we developed an ABM to simulate enzootic transmission of WNV in an avian host (American robin)–mosquito vector (Culex spp.) system during the fall when migratory populations of robins are migrating south to their non-breeding grounds. The energetic cost of migration is high and migratory landbirds, including robins, rely on finding suitable stopover sites where they can rest and replenish depleted fat stores [36]. Yet, for myriad reasons, the availability of food can be unpredictable and/or insufficient [37,38], which not only has fitness consequences for the migrant [36], it can have implications for seasonal transmission dynamics of pathogens. Using empirical and experimental infection data, we use the ABM to test the effect of food stress on the population prevalence of WNV-infected Culex mosquitoes, termed the mosquito infection rate (MIR), which is a predictor of human risk [39]. Understanding the basis for this variation is needed to prevent disease outbreaks and minimize the global threat that emerging infectious diseases pose to human, animal and ecosystem health.
2. Methods
(a) . Study species and location
First-year (hatch year, HY) robins (n = 42) were captured during fall migration from 12 to 22 October 2018 using mist nets (36 mm mesh; 12 m × 2.6 m) in Laingsburg, MI (42.82, −84.38). Upon capture, we assessed bird's condition, body mass (±0.1 g), sex, and wing length, and presence of ectoparasites.
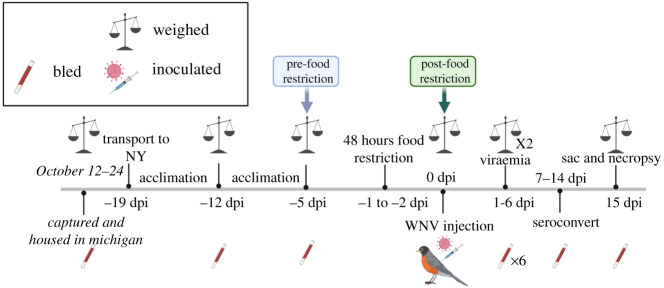
We transported birds to Michigan State University's Research Containment Facility's (URCF) and housed them in one of two identical rooms. Initially, we placed birds in individual wire cages (30 × 38 × 38 cm) until they acclimated, at which point we moved them to small aviaries (6″H × 2″W × 9″D) with two to four robins in each. Room temperature was maintained at 68–70oF on average and the photoperiod that mimicked natural conditions of central Michigan (13 L : 11 D). Birds were provided ad libitum access to water throughout the entire experimental period. All the birds were fed a mixed diet appropriate for the species (see [23,40]). The nutrient composition of the diet was constant across the experiment. The birds were fed a modified version of the semisynthetic diet described by Johnston et al. [40] that all had the same macronutrient composition (49% carbohydrate : 30% protein : 17% fat). The amount of food was determined in previous pilot experiments as being sufficient for maintaining their condition/mass.
On 25 October 2019, robins were placed into bird holding boxes and transported via car from East Lansing, MI to Albany, NY, a 12 h trip. They were housed in one ABSL3 room at the New York State Health Department Wadsworth Center's Griffin Lab. Upon arrival, all birds were placed into individual wire cages identical to the ones described above and provided food (as described above) and water, ad libitum. After one week in captivity in NY, we switched to feeding birds a fixed amount of food (30 g) to maintain their body mass (figure 1). The light schedule was identical to Michigan, and ambient room conditions were similar with average temperature and humidity of 70°F and 60%, respectively.
Figure 1.
The experimental timeline. American robins were captured between 10 and 22 October and then transported to NY on 25 October, which, for purposes of describing the experiment, is denoted as −19 days post-inoculation (dpi). See ‘Methods’ for details. Created with Biorender.com. (Online version in colour.)
(b) . Experimental treatments
Prior to group assignment, we tested all robins for previous exposure to WNV by the plaque reduction neutralization test (PRNT; see below for methods). One robin tested positive for WNV antibodies (greater than 1 : 10), and two birds were inconclusive (titres of 1 : 10). Aside from these three individuals, we randomly assigned robins, stratified by sex and capture date to four treatment groups: two food restriction groups (i) ‘FR_WNV’ (n = 10; food deprived and infected with WNV) and (ii) ‘FR_Sham’ (n = 11; food restricted and inoculated with saline) and two normal-fed groups (iii) ‘Norm_WNV’ (n = 10; normal fed and WNV infected and (iv) ‘Norm_Sham’ (n = 12; normal fed and uninfected). The three robins that may have been previously exposed to WNV were randomly assigned to one of the two sham groups.
Forty-eight hours (−2 dpi; figure 1) prior to experimental infection, we stopped feeding the FR_WNV and FR_Sham groups and then resumed feeding birds on the day of inoculation (0 dpi). The Norm WNV and Norm_Sham birds were fed normally, and all groups received water ad libitum. We weighed birds prior to and after food deprivation as well as throughout the entire experimental period (figure 1).
On 0 dpi (figure 1), birds in FR_WNV and Norm_WNV were inoculated subcutaneously in the cervical region with 0.1 ml of 105 log PFU/ml of infectious WNV (strain WN02, 1986 in PBS diluent). The two sham groups were similarly inoculated with the PBS.
To assess viral titres, we collected whole blood (0.05 ml) daily through 6 dpi from the ulnar vein using a 25-gauge needle [41]. Blood was dispensed in BA-1 (M199 medium with Hank's salts, 1% bovine albumin, TRIS base (tris [hydroxymethyl] aminomethane), sodium bicarbonate, 2% fetal bovine serum and antibiotics) and stored at −80°C. Within a week, we quantified viral titres using the Vero cell plaque assay [42].
Additionally, to measure WNV antibodies, we collected whole blood (0.350 ml) prior to the experiment and on 14 dpi. The blood sample was stored at 4°C until antibody titres were assayed via PRNTs within two weeks of collection. Briefly, sera were diluted in BA-1 and heat inactivated at 56°C for 30 min. Sera were screened at a 1 : 10 dilution against WNV. Antibody titre was expressed as the inverse dilution of blood that neutralized 90% of the virus inoculum as measured by the virus-only control (no antibody; [43]) well. At 14 dpi, all the WNV-infected birds (control birds were held for a subsequent experiment not described here) were euthanized via CO2 asphyxiation.
(c) . Agent-based model
The ABM, AMRO_WNV_CULEX [44], was coded in the high-level language NetLogo [45]. AMRO_WNV_CULEX advances using daily time steps and simulates the transmission of WNV between the American robin (avian host) and the mosquito vector (Culex pipiens; hereafter ‘Culex’). Both these entities are modelled as individuals occurring in a hypothetical landscape, and interactions between the robin and Culex are simulated for 92 days (1 August to 31 October), when robins are actively migrating in mid-Michigan. The ABM approach facilitates the incorporation of heterogeneous and stochastic processes that influence WNV transmission in the real world, specifically robin migration events, host viraemia levels, mosquito population dynamics and Culex–robin interactions that underpin WNV transmission dynamics.
The purpose of the model is to provide a tool to run virtual experiments by implementing scenarios with varying levels of acute food stress experienced by robins during fall migration when there are large influxes of robins, particularly susceptible, HY birds and quantify the resultant proportion of WNV-infected Culex (or MIR) in the model landscape [39].
Here we provide the brief framework of the model, state variables, model parameters and model implementation. The ABM's full description following the ODD protocol [46] is shared in the electronic supplementary material.
(i) . Avian host (American robin)
In the model, robins have multiple states—age (HY; after-hatching year, AHY), infection status (susceptible, infected and infectious (i.e. virus titres ≥4.0 log/pfu 0.1 ml)), infected and not infectious (virus titres less than 4.0 log/pfu 0.1 ml), or recovered with immunity, and stress condition (food stressed: Y or N). The robin abundance, migration phenology and age ratio are based on 10+ years of banding data near the location where robins were captured for the study. At the start, robins (n = 500) are randomly scattered in patches with an initial age ratio of 1 : 4 AHY : HY. The robin population increases on days 50 and 71 with the total robin population set at 1000 (2 : 3 AHY : HY) and 2500 (3 : 7 AHY : HY) birds, respectively.
Each week 95% of the robins, excluding individuals who are both food stressed and have viral titres exceeding 6 log pfu/0.1 ml (i.e. ‘sick’ robins), depart and are replaced by a new cohort of uninfected robins. If the ‘sick’ robins survive, they will depart the following week. Each susceptible robin has a probability of being bitten and progressing to an infectious state or non-infectious state as determined by the results of our experimental challenge in the current study. The per cent of infectious robins on 2, 3, 4 and 5 dpi is 90%, 70%, 50% and 30% for stressed robins and 70%, 30%, 10% and 10% for non-stressed robins, respectively. Mortality only occurs in 10% of the infectious, stressed birds on 5 dpi. The proportion of robins immune is age-dependent (values derived from the current study and WNV serosurveillance efforts in NY; AP Dupuis II, 2007 unpublished data).
(ii) . Mosquito
The simulation starts with 5000 mosquitoes (representing an initial 10 : 1 ratio of mosquitoes to birds). Mosquito mortality is simulated using the daily natural mortality probability derived from the literature [47]. The starting abundance of Culex is constant throughout the run; every Culex that dies, one adult uninfected female Culex is introduced into the population. Mosquitoes are susceptible, infected and non-infectious (extrinsic incubation period, EIP, 7–11 days) [48] or infected and infectious.
(iii) . Mosquito–host interaction
Here we assume a mosquito will take a blood meal from one (minimum)–five (maximum) robins with a bite rate of 0.17 per bird per day [47,49]. Mosquitoes will only become infected by biting a viraemic robin (transmission probability of 0.05–0.30) [50–52] and once infectious can only transmit virus (transmission probability of 0.8) [47] to robins after EIP.
(d) . Data analysis
All variables were tested for normality of distribution (Kolmogorov–Smirnov test) and the equality of variance (Bartlett's tests), and the α level was set for 0.05. When data were not normally distributed, we used the equivalent non-parametric test if available. Otherwise, we transformed the data to meet assumptions of normality, or when transformations did not normalize data, we report non-normality in our results below. To examine food and viral treatment effects on body mass and viral titres, we used two-way repeated-measures ANOVAs to examine between and within-group differences and their interactions using SigmaPlot (Systat Software, San Jose, CA).
Analyses and sample sizes varied with different comparisons. Prior to inoculation, we compared changes in body mass of the two groups according to food restriction treatment (food restriction, [FR], n = 21 and normal fed [Norm], n = 21). Post-inoculation, we compared viraemia response of the two WNV-inoculated groups (FR-WNV, n = 10 and Norm_WNV, n = 11). In each case, we used a repeated-measure, mixed model ANOVA to test for differences between and within groups. For significant main effects, we used the Bonferroni-corrected pair-wise multiple comparison procedure. We calculated an infectious index for each bird by calculating the area under the curve for their viraemia profile above the 4 log pfu/0.1 ml (or commonly reported as 5 log pfu/ml) blood threshold. While this threshold of infectiousness varies with different mosquitoes [50,52,53], viral titres of 4 log pfu/0.1 ml and above are likely to infect blood-feeding C. pipiens, the primary vector in the northeastern and midwestern USA [54]. Differences in the AUC according to food treatment were analysed using a one-way ANOVA.
Using the ABM, we tested the effect of changing proportion (0.0, 0.10, 0.25 and 0.50) of stressed robins in the population on MIR from 1 August to 31 October. Each scenario yielded the number of WNV-infected Culex per 1000 mosquitoes (i.e. MIR; [39]) per time step based on 100 replicate simulations for each scenario. We analysed the data in two distinct phases that address two different aspects of WNV transmission dynamics: early in the migration season (referred to as Phase 1; 1 August–19 September) when the virus is locally amplifying in the simulation and Phase 2 (20 September–31 October) when there are large influxes of robins during migration.
The analysis approach for the model output was determined through visualization of the data by day and week for each phase, which revealed linear trend in Phase 1 and quadratic trend in Phase 2 (see the electronic supplementary material). Using the AIC model selection criterion, we determined the best model, which we report in this study. All ABM analysis was conducted using the R statistical software [55,56], with the glmmTMB [56] package used to account for heteroscedasticity.
3. Results
All the WNV-inoculated birds were successfully infected as evident by viraemia and production of WNV-specific antibodies by 14 dpi. None of the birds died during the experimental infection period; however, two of the birds in the FR_WNV group exhibited clinical signs of WNV—including lethargy and anorexia (lack of appetite). These overt symptoms became notable on 3 dpi and disappeared by 6 dpi, with both birds making a full recovery as evident by normal behaviour and mass gain.
(a) . Food treatment and mass changes
We examined the effect of food restriction on change in body mass (between −5 and 0 dpi; figure 1), prior to virus inoculation for both FR (n = 21) and Norm (n = 21) groups. There was a significant main effect between groups (FR and Norm; F1,82 = 13.92, p < 0.001; figure 2) and across time within groups (F1,82 = 181.23, p < 0.001) with a statistically significant interaction (F1,82 = 106.24, P < 0.001). Body mass on −5 dpi did not differ between groups (p = 0.339), but it did differ post-food restriction (0 dpi; p < 0.001). Within groups, body mass significantly dropped between −5 and 0 dpi for both groups (Tukey's test both p < 0.001); however, the mean drop in mass was 1.76 g (±2.62 s.d.) for Norm and 12.32 g (±3.81 s.d.) for FR birds. When we examined mass changes for the four treatment groups for −5, 0, 2 and 4 dpi, there was a significant main effect of group (F3,152 = 18.61, p < 0.001) and date (F3,152 = 9.049, p < 0.001) and no significant interaction effect. Post hoc analyses reveal no changes in body mass over time for the non-food-restricted groups (Norm_CTRL and Norm_WNV). Food-restricted birds all regained mass post-food restriction, regardless of infection status (see the electronic supplementary material for additional results on body mass).
Figure 2.
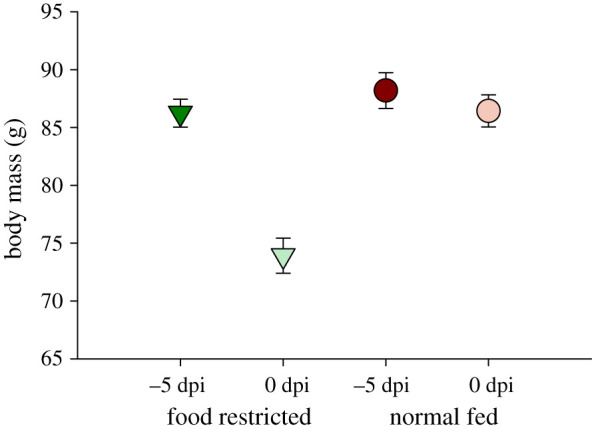
Average (±1 s.e.) change in American robin body mass between −5 and 0 dpi, following 48 h (−2 and −1 dpi) of food restriction for the FR groups. Food-restricted birds (n = 21) are denoted by green down triangles and normal-fed birds (n = 21) by red circles. (Online version in colour.)
(b) . Food treatment and viral infection
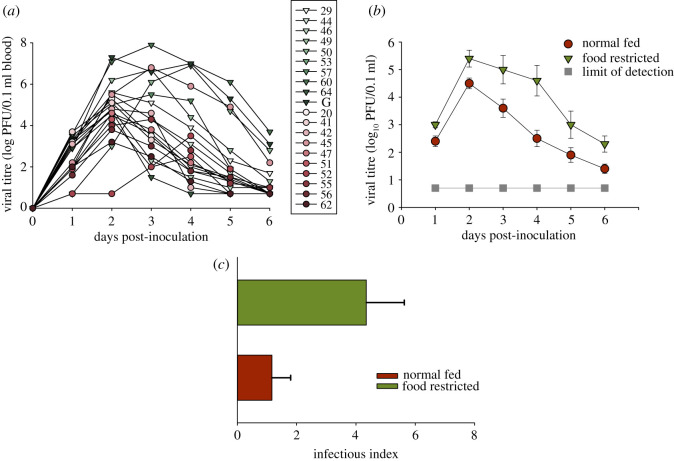
Birds that were food deprived for the 48 h preceding infection (FR_WNV) had higher and prolonged viraemia compared with Norm_WNV birds. All the FR_WNV (10/10) became infectious (i.e. 4.0 log/0.1 ml) for at least 1 day compared with only seven of the 10 Norm_WNV birds (figure 3a). Additionally, FR_WNV had consistently higher viral titres (figure 3b; H1,108 = 20.66, p < 0.001), were infectious longer (200% more infectious days, 24 days for FR_WNV and 12 days for Norm_WNV; t = −2.48, d.f. = 18, p = 0.023) and had a higher infectious index (i.e. AUC) than the Norm_WNV group (figure 3c; U = 18.0, N = 10, p = 0.017).
Figure 3.
(a) WNV titres (log PFU/0.1 ml blood) for experimentally infected American robins fed normally (red circles; n = 11) and robins food-deprived for 48 h prior to inoculation (green down triangles; n = 10). (b) Average (±1 s.e.) virus titre per log PFU/0.1 ml blood for normal (red circles) and food-restricted (green down triangles) robins. Virus titres below 0.7 log pfu/0.1 ml are undetectable (grey squares) via Vero cell plaque assay. (c) Mean (±1 s.d.) infectious (i.e. capable of infecting a biting mosquito) index for the two groups. (Online version in colour.)
All WNV-infected birds, regardless of the treatment group, seroconverted by 14 dpi, with titres of WNV-specific neutralizing antibody (PRNT90) ranging from 80 to 5120. While there was no significant difference in antibody titre by food restriction treatment (ANOVA on ranks; H = 3.171, p = 0.075), there was a positive association between virus titre and PRNT90 titres (R2 = 0.54, N = 20, p < 0.001), a relationship driven mainly by two FR birds with the highest viral titres.
(c) . Agent-based modelling results
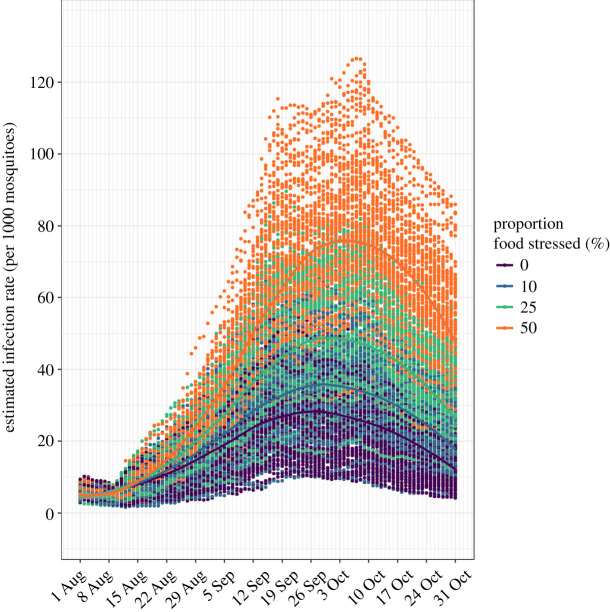
Changing the proportion of the American robin population experiencing food stress significantly affected the prevalence of infected Culex spp. (figure 4). From the models compared using the AIC in Phase 1, the best model was a model of weekly aggregated data considering unequal variances and a spline function over time. The results of this model show that the food stress level (F1,11188 = 5601.37, p < 0.001), week (F1,11188 = 46.86, p < 0.001) and their interaction (F3,11188 = 221.10, p < 0.001) were all significant, indicating that MIR varied with time and food stress level. Further pair-wise comparisons with Bonferroni correction show that the four food stress levels have a significant effect on MIR (electronic supplementary material, table S2).
Figure 4.
Daily estimated infection rate (no. of infected mosquitoes per 1000 mosquitoes) for four different scenarios with 0, 10, 25 and 50% of the American robin population experiencing food stress (100 simulations per scenario). (Online version in colour.)
From the models compared using the AIC in Phase 2, the best model was a model of daily data that included a linear term of food stress levels as a main predictor, linear term of day as a covariate, quadratic term of day and their interactions. The model found significant results of all terms in the model (day, F1,11188 = 116.81, p < 0.001; food stress, F3,11188 = 5827.85, p < 0.001; Day2, F1,11188 = 121.55, p < 0.001; day × food stress, F3,11188 = 13.23, p < 0.001; food stress × day2, F3,11188 = 10.77, p < 0.001). Pair-wise comparisons with Bonferroni correction show that all four stress levels are significantly different with each other (electronic supplementary material, table S2).
4. Discussion
Food restriction, even when for short duration and prior to infection, has a significant effect on the duration and magnitude of infectiousness of WNV-infected American robins. When deprived of food for 48 h, robins developed higher viral titres for longer duration than normal-fed birds. Moreover, the increase was epidemiologically significant with the total number of infectious days in food-deprived birds being 200% greater than the normal group. Its significance is further illustrated by the ABM, showing the positive association between food-stressed robins and the infection rate of mosquitoes. Even when only 10% of the population was ‘food stressed’, the infection rates for mosquitoes significantly increased. Furthermore, the MIR in the simulation reached levels associated with previous WNV epidemics [57]. American robins have already been considered one of the superspreaders of WNV [32,34,58,59], and here we show that limiting food resources during the migratory period further magnifies their role in the amplification of WNV transmission. Our experimental results support theoretical models that suggest food scarcity may lead to superspreading events due to within-host effects [60].
The availability of food is a universal stressor and, regardless of the factors driving that availability, it is an important limiting factor acting on wildlife populations. Nonetheless, while it is well understood that food deprivation and malnutrition can alter host defences to pathogens [61–63], few studies have investigated the effect of an acute lack of food prior to exposure to a pathogen, particularly in a wildlife reservoir. The state of the host's nutritional reserves at the time of infection is a key factor influencing viral infection, as is the duration and severity of the food restriction [64]. Our findings are consistent with other studies that show a link between short-term food deprivation and reduced immune function [16,65]. In laboratory mice, food deprivation, prior to and during the early part of the infection had large effects on host resistance to a fungal pathogen [16]. Moreover, in that same study, they found that the biggest effect on host resistance was when deprivation began prior to infection and continued through the first-day post-infection [16].
The relationship between food stress, immunity and resistance to pathogens is inherently complex and can vary with pathogen or host-related factors such as mode of transmission and/or site of binding and replication or the nature of the food stress (e.g. duration, nutrient or caloric modifications and restriction/supplementation). In mallards (Anas platyrynchous) infected with low-pathogenic avian influenza virus (LPAIV) chronic (30 days) food restriction affected viral shedding but, contrary to the current study, pathogen load progressively decreased with the severity of food limitation [17]. In swine influenza-infected mice, mice exhibited a cyclic susceptibility to viral infection relative to the duration of protein restriction, with mice on low protein diets for two and eight weeks showing the highest susceptibility, while mice restricted for moderate duration (four and six weeks) showing the lowest viral titres [64]. This oscillation of viral titres may be associated with different stages of gluconeogenesis [64]. In the current study, we just altered access to food; however, the lack of some nutrients, such as protein, which is an important modulator of host immunity, can have a greater effect on host resistance to pathogens (e.g. [62,66,67]). Furthermore, where a pathogen invades and colonizes the host tissues can affect the relationship between host resistance and the quantity and quality of food. In the two aforementioned studies [17,64], the influenza virus binds and replicates within the intestinal tract. Dietary changes can strongly influence the physiological and metabolic processes of tissues in the gastrointestinal tract [68], which in turn could affect the availability of tissues expressing receptors for the pathogen.
Too little food is just one form of poor nutrition. Overnutrition or obesity may cause delayed or suppressed antiviral responses, as seen in humans in which obesity as a pre-existing condition is shown to lead to worse disease outcomes [69]. While obesity is rarely a problem for free-living wild populations of birds, there are examples of augmented food resources leading to higher infection rates. For instance, food provisioning, such as supplemental feeding with bird feeders, is associated with higher infection rates, an outcome most likely a consequence of increased in contact rates as individuals congregate at feeding stations [7,70,71]. However, once infected, the negative effect of food provisioning may be mitigated by the positive effects of food resources on host immunity [72], thereby suppressing pathogen replication [7,70].
American robins are considered one of the most important avian reservoirs for WNV [31,35,58] and have been the focus of several previous experimental infection studies [29,73]. The viraemia profiles of the normal-fed robins in our study are similar to what has been observed previously ([73,74]; unpublished data by JC Owen & AP Dupuis II 2019). Furthermore, these experimental infection studies document low WNV-associated mortality, with birds only dying when their virus titres exceed 7.5 log pfu/0.1 ml [73]. While none of the robins succumbed to the virus in this study, some food-deprived robins did exhibit significant morbidity. Two FR_WNV robins (60 and G), with the highest titres in the whole study, did exhibit lethargy, anorexia, and were ‘puffed’ up, from 3–6 dpi, all common signs of diseased birds. While they ultimately recovered and survived the infection, their fate in the wild would be less certain. In the Norm_WNV, one bird (45) did have high viral titres similar ‘G’ and ‘60’ but never exhibited any overt signs of disease. Similarly, in a previous study with WNV-infected northern cardinals (Cardinalis cardinalis), corticosterone-implanted birds had higher morbidity and mortality, but similar viraemia profiles as the empty-implanted (control) birds [23].
A limitation of challenge experiments is knowing whether the treatment effects observed in captive experiments translate into epidemiologically important outcomes in nature. ABMs are being increasingly used to investigate complex host–pathogen systems to better understand the spread and persistence of pathogens in host populations and are particularly useful for elucidating complex causal effects. To illustrate the significance of food deprivation on disease dynamics of WNV, we developed an ABM to explore how changing individual-level infection dynamics in avian hosts affects infection rates of Culex mosquito (vector), which is a predictor of human infection risk. The model demonstrates that increasing the proportion of the robin population experiencing ‘food stress’ leads to an increase in the prevalence of infected Culex mosquitoes, with average weekly infection rates from six to 78 infected individuals per 1000 mosquitoes. Furthermore, with the weekly turnover in robin population during migration coupled with the larger movements of robins in mid to late September and early October, the transmission cycle stays elevated and peaks when robin numbers are highest (figure 4). The migration phenology depicted in this model characterizes the timing of robin movements in mid-Michigan and may not be applicable across its entire range. However, the relationship between food stress in the wildlife reservoir and the infection rate in the mosquito vector may reflect the patterns observed in other regions and even other host–vector–parasite interactions.
While it is difficult to link a specific population infection rate of mosquitoes ‘entomological risk’ [75] to a level of risk to humans, there is evidence that higher infection rates in mosquitoes correspond positively with the number of human cases of WNV [39,76]. The infection rate of Culex in the simulation, while offset temporally, does reflect the range observed in nature [77–79]. Here we are illustrating the relative increase and not interpreting the absolute value of the MIR. Entomological risk is a function of mosquito population infection rate, abundance and host-feeding preferences [34]. The contact rate between mosquitoes and avian hosts is not random; blood-seeking females will preferentially feed on some species more than others [31] and this variation in vector–host-feeding preferences is a key factor driving transmission dynamics of WNV [34]. In the northeast and midwestern USA, American robins are consistently the most common source of avian-derived blood meals for C. pipiens, regardless of their relative abundance within the community [31,33,35,58]. Given these host-feeding preferences for robins, our bird-to-mosquito contact rate is likely conservative.
Here we ask whether having food-stressed avian hosts in a community affects WNV transmission. To gain insights, we created a model depicting a simple system with WNV transmission occurring in a community with only two species—the American robin and a Culex mosquito. In nature, WNV transmission occurs within the context of a multi-host–multi-vector community, and within that community are a myriad of bird and mosquito species that vary in their reservoir and vector competence, respectively [57]. A suite of other factors can influence host–vector–pathogen interactions. For instance, host behaviour can influence vector–host contact rates, with highly viraemic birds becoming lethargic, as shown in our experiments with food-stressed robins, and exhibit fewer anti-mosquito defensive behaviours allowing mosquitoes to successfully complete their blood meal [80]. The empirical data and theoretical model collected and developed in this study further support the role of robins as WNV superspreaders, which is further amplified when migrating robins experience an environmental stressor, such as limited and unpredictable food availability.
Humans have dramatically altered the environment through technological advances and a population that has more than doubled in the last 50 years, moving us into a new geological period, the Anthropocene. The emergence and spread of zoonotic diseases are just one of the many unintended consequences of globalization and land-use change. Using both empirical and theoretical approaches, we demonstrate the effect of resource availability on within-host–parasite processes and how a change in host infectivity can alter community transmission dynamics and risk to human health.
Supplementary Material
Acknowledgements
We thank Z. Spodek, A. Cole, A. Shoffner and D. Owen who all helped make this research possible.
Ethics
All procedures and methods were approved by IACUC at Michigan State University (AUF 12-16-211-0) and Wadsworth (18-412) and Federal and State Scientific Collection Permits to J.C.O. (SC1386, MB194270) and Master Banding Permit (no. 23269).
Data accessibility
Data are available from the Dryad Digital Repository. American robin West Nile viral titre data and R script and for agent-based modelling data: https://doi.org/10.5061/dryad.ksn02v74s [81]. American robin banding data: https://doi.org/10.5061/dryad.3xsj3txgq [82].
Authors' contributions
J.C.O.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing-original draft, writing-review & editing; H.R.L.: data curation, investigation, methodology, writing-review & editing; D.B.S.: formal analysis, methodology, visualization, writing-original draft, writing-review & editing; S.W.: formal analysis, methodology, visualization, writing-original draft, writing-review & editing; A.T.C.: methodology, resources, supervision, writing-review & editing; L.D.K.: resources, supervision; A.P.D.: data curation, investigation, methodology, writing-review & editing; A.V.B.: conceptualization, data curation, formal analysis, methodology, writing-review & editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by NSF IOS-1350772 to J.C.O., MSU AgBioResearch and NYS Department of Health.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008Global trends in emerging infectious diseases. Nature 451, 990-993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothenburger JL, Himsworth CH, Nemeth NM, Pearl DL, Jardine CM. 2017Environmental factors and zoonotic pathogen ecology in urban exploiter species. EcoHealth 14, 630-641. ( 10.1007/s10393-017-1258-5) [DOI] [PubMed] [Google Scholar]
- 3.Gottdenker NL, Streicker DG, Faust CL, Carroll C. 2014Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth 11, 619-632. ( 10.1007/s10393-014-0941-z) [DOI] [PubMed] [Google Scholar]
- 4.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O. 2016Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Negl. Trop. Dis. 10, e0004796. ( 10.1371/journal.pntd.0004796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapan DD, Bennett SN, Ellis BN, Fox J, Lewis ND, Spencer JH, Saksena S, Wilcox BA. 2006Avian influenza (H5N1) and the evolutionary and social ecology of infectious disease emergence. EcoHealth 3, 187-194. ( 10.1007/s10393-006-0044-6) [DOI] [Google Scholar]
- 6.Nunes H, Rocha FL, Cordeiro-Estrela P. 2017Bats in urban areas of Brazil: roosts, food resources and parasites in disturbed environments. Urban Ecosyst. 20, 953-969. ( 10.1007/s11252-016-0632-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker DJ, Streicker DG, Altizer S. 2015Linking anthropogenic resources to wildlife–pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483-495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries DL, Scott ME, Vermund SH. 2021Pathways linking nutritional status and infectious disease: causal and conceptual frameworks. In Nutrition and infectious diseases, nutrition and health (eds Humphries DL, Scott ME, Vermund SH), pp. 3-22. Cham, Switzerland: Humana. ( 10.1007/978-3-030-56913-6_1) [DOI] [Google Scholar]
- 9.Pammi M, Abrams SA, Vallejo JG (eds). 2014. Nutrition-infection interactions and impacts on human health, 1st edn. Boca Raton, FL: CRC Press. ( 10.1201/b17311) [DOI] [Google Scholar]
- 10.Killpack TL, Carrel E, Karasov WH. 2015Impacts of short-term food restriction on immune development in altricial house sparrow nestlings. Physiol. Biochem. Zool. 88, 195-207. ( 10.1086/680168) [DOI] [PubMed] [Google Scholar]
- 11.Alonso-Alvarez C, Tella JL. 2001Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. J. Zool. (Lond.) 79, 101-105. ( 10.1139/z00-190) [DOI] [Google Scholar]
- 12.Ben-Nathan B, Drabkin N, Heller D. 1981The effect of starvation on the immune response of chickens. Avian Dis. 25, 214-217. ( 10.2307/1589845) [DOI] [PubMed] [Google Scholar]
- 13.Beldomenico PM, Begon M. 2010Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 25, 21-27. (doi: 10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 14.Woolhouse MEJ, et al. 1997Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338-342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershwin ME, Beach RS, Hurley LS. 1985Nutrition and immunity. San Diego, CA: Academic Press, Inc. [Google Scholar]
- 16.Oarada M, Nikawa T, Kurita N. 2002Effect of timing of food deprivation on host resistance to fungal infection in mice. Br. J. Nutr. 88, 151-158. ( 10.1079/BJN2002600) [DOI] [PubMed] [Google Scholar]
- 17.Arsnoe DM, Ip HS, Owen JC. 2011Influence of body condition on influenza A virus infection in mallard ducks: experimental infection data. PLos ONE 6, e22633. ( 10.1371/journal.pone.0022633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F. 2014Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS ONE 9, e107387. ( 10.1371/journal.pone.0107387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley DM, Altizer SM. 2011Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48-60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 20.Hoye BJ, Fouchier RA, Klaassen M. 2012Host behaviour and physiology underpin individual variation in avian influenza virus infection in migratory Bewick's swans. Proc. R. Soc. B 279, 529-534. ( 10.1098/rspb.2011.0958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allan BF, et al. 2009Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia 158, 699-708. ( 10.1007/s00442-008-1169-9) [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. 2005West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 11, 425-429. ( 10.3201/eid1103.040364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen JC, Nakamura A, Coon CAC, Martin LB. 2012The effect of exogenous corticosterone on West Nile virus infection in northern cardinals (Cardinalis cardinalis). Vet. Res. 43, 34-42. ( 10.1186/1297-9716-43-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowski MD, Williams CJ, Fair JM, Owen JC. 2013Birds shed RNA-viruses according to the Pareto Principle. PLos ONE 8, e0072611. ( 10.1371/journal.pone.0072611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gervasi SS, Civitello DJ, Kilvitis HJ, Martin LB. 2015The context of host competence: a role for plasticity in host–parasite dynamics. Trends Parasitol. 31, 419-425. ( 10.1016/j.pt.2015.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews L, McKendrick I, Ternent H, Gunn G, Synge B, Woolhouse M. 2006Super-shedding cattle and the transmission dynamics of Escherichia coli O157. Epidemiol. Infect. 134, 131-142. ( 10.1017/S0950268805004590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes EB, Gubler DJ. 2005West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 57, 181-194. ( 10.1146/annurev.med.57.121304.131418) [DOI] [PubMed] [Google Scholar]
- 28.Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. 2004Differential virulence of West Nile strains for American crows. Emerg. Infect. Dis. 10, 2161-2168. ( 10.3201/eid1012.040486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komar N, et al. 2003Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 9, 311-322. ( 10.3201/eid0903.020628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilpatrick AM, et al. 2013Predicted and observed mortality from vector-borne disease in wildlife: West Nile virus and small songbirds. Biol. Conserv. 165, 79-85. ( 10.1016/j.biocon.2013.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apperson CS, et al. 2004Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 4, 71-82. ( 10.1089/153036604773083013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilpatrick AM. 2011Globalization, land use, and the invasion of West Nile Virus. Science 334, 323-327. ( 10.1126/science.1201010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. 2008Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J. Med. Entomol. 45, 125-128. ( 10.1093/jmedent/45.1.125) [DOI] [PubMed] [Google Scholar]
- 34.Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA. 2012Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B 279, 925-933. ( 10.1098/rspb.2011.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, e82. ( 10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore FR, Smith RJ, Sandberg R. 2005Stopover ecology of intercontinental migrants: en route problems and consequences for reproductive performance. In Birds of two worlds. The ecology and evolution of migration, pp. 251-261. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 37.Oguchi Y, Smith RJ, Owen JC. 2017Fruits and migrant health: consequences of stopping over in exotic vs. native-dominated shrublands on immune and antioxidant status of Swainson's thrushes and gray catbirds. Condor Ornithol. Appl. 119, 800-816. ( 10.1650/CONDOR-17-28.1) [DOI] [Google Scholar]
- 38.Carey C. 2009The impacts of climate change on the annual cycles of birds. Phil. Trans. R. Soc. B 364, 3321-3330. ( 10.1098/rstb.2009.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeFelice NB, Little E, Campbell SR, Shaman J. 2017Ensemble forecast of human West Nile virus cases and mosquito infection rates. Nat. Commun. 8, 1-6. ( 10.1038/ncomms14592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnston E, Tsao JI, Muñoz JD, Owen J. 2013Anaplasma phagocytophilum infection in American robins and gray catbirds: an assessment of reservoir competence and disease in captive wildlife. J. Med. Entomol. 50, 163-170. ( 10.1603/ME12141) [DOI] [PubMed] [Google Scholar]
- 41.Owen JC. 2011Collecting, processing, and storing avian blood: a review. J. Field Ornithol. 82, 339-354. ( 10.1111/j.1557-9263.2011.00338.x) [DOI] [Google Scholar]
- 42.Blitvich BJ, Bowen RA, Marlenee NL, Hall RA, Bunning ML, Beaty BJ. 2003Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J. Clin. Microbiol. 41, 2676-2679. ( 10.1128/JCM.41.6.2676-2679.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsey HS, Calisher CH, Mathews JH. 1976Serum dilution neutralization test for California group virus identification and serology. J. Clin. Microbiol. 4, 503-510. ( 10.1128/jcm.4.6.503-510.1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belsare A, Owen JC. 2021AMRO_CULEX_WNV’ (version 1.0.1). CoMSES Computational Model Library. ( 10.25937/hfcr-bt18) [DOI] [Google Scholar]
- 45.Wilensky U. 1999NetLogo (and NetLogo User Manual). Center for Connected Learning and Computer-Based Modeling, Evanston, IL: Northwestern University. [Google Scholar]
- 46.Grimm V, et al. 2006A standard protocol for describing individual-based and agent-based models. Ecol. Model. 198, 115-126. ( 10.1016/j.ecolmodel.2006.04.023) [DOI] [Google Scholar]
- 47.Vogels CB, Hartemink N, Koenraadt CJ. 2017Modelling West Nile virus transmission risk in Europe: effect of temperature and mosquito biotypes on the basic reproduction number. Sci. Rep. 7, 1-11. ( 10.1038/s41598-017-05185-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. 2008Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 4, e1000092. ( 10.1371/journal.ppat.1000092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wonham MJ, de-Camino-Beck T, Lewis MA. 2004An epidemiological model for West Nile virus: invasion analysis and control applications. Proc. R. Soc. B 271, 501-507. ( 10.1098/rspb.2003.2608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. 2005An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile Virus. J. Med. Entomol. 42, 57-62. ( 10.1093/jmedent/42.1.57) [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. 2010Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg. 83, 607-613. ( 10.4269/ajtmh.2010.10-0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turell MJ, O'Guinn M, Dohm DJ, Jones JW. 2001Vector competence of North American mosquitos (Diptera: Culcidae) for West Nile virus. J. Med. Entomol. 38, 130-134. ( 10.1603/0022-2585-38.2.130) [DOI] [PubMed] [Google Scholar]
- 53.Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. 2001Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg. Infect. Dis. 7, 1018-1022. ( 10.3201/eid0706.010617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komar N. 2003West Nile virus: epidemiology and ecology in North America. Adv. Virus Res. 61, 185-234. ( 10.1016/S0065-3527(03)61005-5) [DOI] [PubMed] [Google Scholar]
- 55.R Core Team. 2019R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- 56.Brooks ME, et al. 2017glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 57.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. 2005Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11, 1167-1173. ( 10.3201/eid1108.050289a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. 2009Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 80, 268-278. ( 10.4269/ajtmh.2009.80.268) [DOI] [PubMed] [Google Scholar]
- 59.Diuk-Wasser MA, Molaei G, Simpson JE, Folsom-O'Keefe CM, Armstrong PM, Andreadis TG. 2010Avian communal roosts as amplification foci for West Nile virus in urban areas in northeastern United States. Am. J. Trop. Med. Hyg. 82, 337-343. ( 10.4269/ajtmh.2010.09-0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall RJ. 2019Modeling the effects of resource-driven immune defense on parasite transmission in heterogeneous host populations. Integr. Comp. Biol. 59, 1253-1263. ( 10.1093/icb/icz074) [DOI] [PubMed] [Google Scholar]
- 61.Field CJ, Johnson IR, Schley PD. 2002Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 71, 16-32. [PubMed] [Google Scholar]
- 62.Ritz BW, Aktan I, Nogusa S, Gardner EM. 2008Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J. Nutr. 138, 2269-2275. ( 10.3945/jn.108.093633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith VH, Jones TP, Smith MS. 2005Host nutrition and infectious disease: an ecological view. Front. Ecol. Environ. 3, 268-274. ( 10.1890/1540-9295(2005)003[0268:HNAIDA]2.0.CO;2) [DOI] [Google Scholar]
- 64.Sprunt DH, Flanigan CC. 1956The effect of malnutrition on the susceptibility of the host to viral infection. J. Exp. Med. 104, 687-706. ( 10.1084/jem.104.5.687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siva-Jothy MT, Thompson JJ. 2002Short-term nutrient deprivation affects immune function. Physiol. Entomol. 27, 206-212. ( 10.1046/j.1365-3032.2002.00286.x) [DOI] [Google Scholar]
- 66.Ritz BW, Gardner EM. 2006Malnutrition and energy restriction differentially affect viral immunity. J. Nutr. 136, 1141. ( 10.1093/jn/136.5.1141) [DOI] [PubMed] [Google Scholar]
- 67.Pollett M, Mackenzie JS, Turner KJ. 1979The effect of protein-deprivation on the susceptibility to influenza virus infection: a murine model system. Aust. J. Exp. Biol. Med. Sci. 57, 151-160. ( 10.1038/icb.1979.16) [DOI] [PubMed] [Google Scholar]
- 68.Genton L, Cani PD, Schrenzel J. 2015Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 34, 341-349. ( 10.1016/j.clnu.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 69.Honce R, Schultz-Cherry S. 2019Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front. Immunol. 10, 1071. ( 10.3389/fimmu.2019.01071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker DJ, Hall RJ. 2014Too much of a good thing: resource provisioning alters infectious disease dynamics in wildlife. Biol. Lett. 10, 20140309. ( 10.1098/rsbl.2014.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorensen A, van Beest FM, Brook RK. 2014Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: a synthesis of knowledge. Prev. Vet. Med. 113, 356-363. ( 10.1016/j.prevetmed.2013.11.010) [DOI] [PubMed] [Google Scholar]
- 72.Cornelius Ruhs E, Vézina F, Karasov WH. 2019Physiological and immune responses of free-living temperate birds provided a gradient of food supplementation. Physiol. Biochem. Zool. 92, 106-114. ( 10.1086/701389) [DOI] [PubMed] [Google Scholar]
- 73.VanDalen KK, Hall JS, Clark L, McLean RG, Smeraski C. 2013West Nile virus infection in American robins: new insights on dose response. PLoS ONE 8, e68537. ( 10.1371/journal.pone.0068537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kilpatrick AM, Dupuis AP, Chang G-JJ, Kramer LD. 2010DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonot. Dis. 10, 377-380. ( 10.1089/vbz.2009.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kilpatrick AM, Pape WJ. 2013Predicting human West Nile virus infections with mosquito surveillance data. Am. J. Epidemiol. 178, 829-835. ( 10.1093/aje/kwt046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bustamante DM, Lord CC. 2010Sources of error in the estimation of mosquito infection rates used to assess risk of arbovirus transmission. Am. J. Trop. Med. Hyg. 82, 1172-1184. ( 10.4269/ajtmh.2010.09-0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernard KA, et al. 2001West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg. Infect. Dis. 7, 679-685. ( 10.3201/eid0704.017415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kulasekera VL, Kramer L, Nasci RS, Mostashari F, Cherry B, Trock SC, Glaser C, Miller JR. 2001West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000. Emerg. Infect. Dis. 7, 722. ( 10.3201/eid0704.017421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dennett JA, et al. 2007Associations between two mosquito populations and West Nile virus in Harris County, Texas, 2003–06. J. Am. Mosq. Control Assoc. 23, 264. ( 10.2987/8756-971X(2007)23[264:ABTMPA]2.0.CO;2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darbro JM, Dhondt AA, Vermeylen FM, Harrington LC. 2007Mycoplasma gallisepticum infection in house finches (Carpodacus mexicanus) affects mosquito blood feeding patterns. Am. J. Trop. Med. Hyg. 77, 488-494. ( 10.4269/ajtmh.2007.77.488) [DOI] [PubMed] [Google Scholar]
- 81.Owen JC, Landwerlen HR, Dupuis AP II, Belsare AV, Sharma DB, Wang S, Ciota AT, Kramer LD. 2021Data from: Reservoir hosts experiencing food stress alter transmission dynamics for a zoonotic pathogen. Dryad Digital Repository. ( 10.5061/dryad.ksn02v74s) [DOI] [PMC free article] [PubMed]
- 82.Owen JC. 2021Data from: Capture data for American robins (Turdus migratorius) at a fall migration banding station in Mid-Michigan from 2012–2019. Dryad Digital Repository. ( 10.5061/dryad.3xsj3txgq) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Owen JC, Landwerlen HR, Dupuis AP II, Belsare AV, Sharma DB, Wang S, Ciota AT, Kramer LD. 2021Data from: Reservoir hosts experiencing food stress alter transmission dynamics for a zoonotic pathogen. Dryad Digital Repository. ( 10.5061/dryad.ksn02v74s) [DOI] [PMC free article] [PubMed]
- Owen JC. 2021Data from: Capture data for American robins (Turdus migratorius) at a fall migration banding station in Mid-Michigan from 2012–2019. Dryad Digital Repository. ( 10.5061/dryad.3xsj3txgq) [DOI]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository. American robin West Nile viral titre data and R script and for agent-based modelling data: https://doi.org/10.5061/dryad.ksn02v74s [81]. American robin banding data: https://doi.org/10.5061/dryad.3xsj3txgq [82].