Abstract
Deployment of the BNT162b2 mRNA Covid-19 Vaccine in Israel began in December 2020.
This is a retrospective analysis of serological data, showing SARS-CoV-2 anti-S IgG kinetics in 116 Israeli health care workers receiving BNT162b2.
Sero-conversion occurred in 14 days in all study participants, with IgG levels peaking approximately 30 days after initiation of the vaccination series.
A statistically significant difference was observed in IgG levels between subjects younger than 50 years and older participants, although in all cases, IgG levels were well above the level considered reactive by the test's manufacturer.
The importance of this difference needs to be studied further, but a potential difference in vaccine efficacy and vaccine effect length could possibly be present between these two groups.
Keywords: SARS-CoV-2, BNT162b2, IgG kinetics, Serology
1. Introduction
Deployment of the BNT162b2 mRNA Covid-19 Vaccine in Israel began on December 19th 2020, following high efficacy shown in a randomized placebo-controlled trial (RCT) [1].
Recently, analyses based on real world data have been published [2], [3], as different approaches attempt to overcome the difficulty in demonstrating vaccine efficacy in real world non-controlled settings.
The ability to infer immunological protection by serologic tests, as assessed in other infectious conditions, is yet unknown, as data pertaining to antibody levels in relation to clinical outcomes remains limited [4]. This possible correlation (or lack thereof) has significant implications on healthcare policy making.
This descriptive real-world study aims to assess antibody response of vaccinated individuals, by analyzing data collected from healthcare workers in Israel, among the first worldwide to be vaccinated with BNT162b2 in two-dose course 21 days apart. Though analyses of antibody kinetics in response to COVID-19 have been examined rather extensively [5], [6], [7], data regarding antibody kinetics after vaccine administration is emerging but not yet complete: While data presented by Danese et al. [8] shows early kinetics, it is based on 3 subjects. A Larger cohort of 155 subjects is described by Favresse [9], with some sero-positive individuals, but without stating the effects of age on the response. A more comprehensive antibody panel is reported by Jalkanen [10], including IgA and IgM, but the data is less granular, and is tested at weeks 3 and 6 post vaccination. We present data highlighting the initial time period after vaccination in healthcare workers.
2. Methods
2.1. Setting and data collection
Maccabi Healthcare Services (MHS) is the second largest state-mandated, not-for-profit, healthcare provider in Israel with over 2.5 million members. Vaccine rollout in MHS began in December 2020.
As part of the ongoing follow-up and quality control efforts by MHS' Health Division, serologic tests were made available to its employees in MHS’ central laboratory (MegaLab). Healthcare workers over the age of 18 were serially tested for SARS-CoV-2 specific anti-s antibodies between January 14, 2020 and February 24, 2021 (six weeks).
At the time of vaccine roll-out in Israel, previously COVID-19 infected individuals were not eligible to be vaccinated, thus only employees without documented history of COVID-19 infection participated in serology collection.
The serology data was retrospectively analyzed, examining vaccine exposure (calculated as days from the first dose of vaccine administration), additional information collected was age and sex. The study was approved by the MHS's institutional review board.
3. Laboratory methods
A commercial assay to detect IgG antibodies against SARS-CoV-2 RBD portion of the spike protein was used - Quant II IgG anti-Spike 2-CoV-SARS by Abbott (Illinois, U.S.A.) and reported as AU/mL (Arbitrary units). The cutoff for serology reactivity is 50 AU/mL according to the manufacturer's instructions.
Values below 21 AU/mL were truncated to 21 AU/mL and similarly for values above 40,000 AU/mL.
4. Statistical analyses
Geometric mean concentrations, along with associated 95% confidence intervals, were computed for each week and age group. Mann–Whitney U test was used to ascertain the difference in serological response between age groups using SPSS version 23 (IBM inc.). Two-sided confidence interval for weighted mean of data was calculated using Python. Categorical variables were compared using Chi-squared test or Fisher's exact test.
5. Results
A total of 382 serology tests were performed to 116 MHS' personnel. For each individual, there was no more than 1 test per week. Baseline characteristics are presented in Table 1 . The mean age of individuals was 50.3 with 48 younger than 50 years of age and 68 were older.
Table 1.
Baseline characteristics.
| All (n = 116) |
Age ≥ 50 (n = 68) |
Age < 50 (n = 48) |
|
|---|---|---|---|
| Age y (mean [±SD]) | 50.3 (11.0) | 57.9 (5.0) | 39.6 (7.6) |
| Sex, male (%) | 9 (7.8%) | 5 (7.4%) | 4 (8.3%) |
| Serology tests, n (mean [±SD]) | 3.3 (1.7) | 3.3 (1.7) | 3.3 (1.7) |
| Days between vaccine doses (mean [±SD]) | 21.5 (1.0) | 21.3 (0.8) | 21.7 (1.1) |
3 individuals had not yet been eligible to receive the second dose of BNT162b2 by the time of data analysis. Two of these are older than 50.
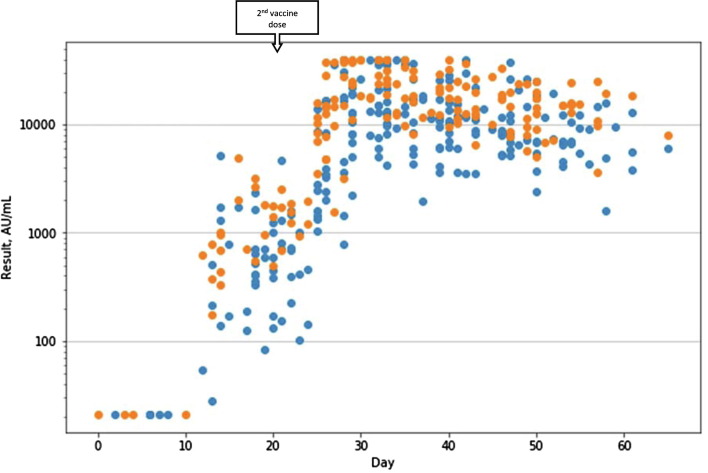
Seroconversion occurred in all individuals in the studied cohort.
All samples taken after day 14 were reactive (i.e. - more than 50 AU/mL), no samples were reactive before day 13.
In 16 individuals, AU values higher than the upper limit of the assay were observed. The mean day for these occurrences was 30 (SD 9.81).
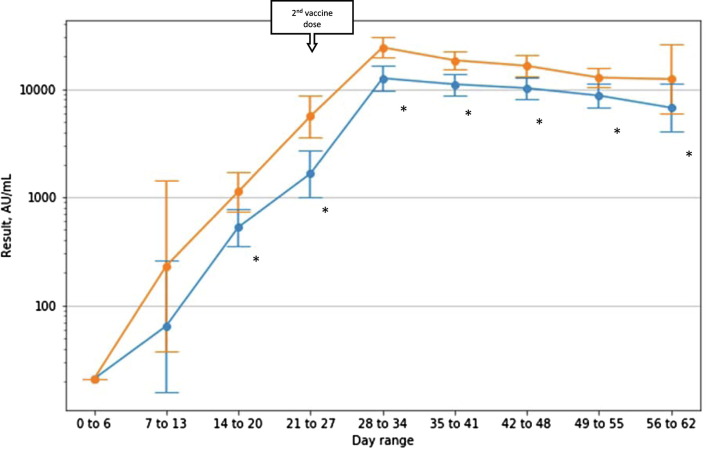
Differences were observed in the anti-S IgG levels in members under 50 and over 50. Median peak IgG levels in the under 50 group were 23,801 AU/ml (IQR 14773–38471) and 13,031 (IQR 7427–21856) in the over 50 group (P = 0.00045). These peak values occurred in the 5th week for both groups – day 31.0 (SD 8.3) and 32.4 (SD 8.3) respectively.
Individual IgG levels for each test are presented in Fig. 1 , divided by age group.
Fig. 1.
Serology results by days from first vaccine (age ≥ 50 in blue, age < 50 in orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Analysis on a per-week basis shows a lasting and statistically significant difference starting at day 15, with the population under 50 having higher IgG levels. It is important to note that while differences do exist, the values are well beyond the cutoff for reactivity. At the last week of follow-up geometric mean IgG titers for the under 50 group was 12336 (95% CI 5907-25765) and 6726 (95% CI 4015-11268) for the over 50 group.
Fig. 2 shows serology results per week (with day 0 being the day of the first BNT162b2 dose).
Fig. 2.
Mean (95% CI) serology result per range of days from first vaccine (age ≥ 50 in blue, age < 50 in orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
6. Discussion
In our cohort of Israeli healthcare workers, a robust antibody response is demonstrated, starting roughly two weeks after the first dose of BNT162b2 and peaking around day 30 (approximately 10 days after the second dose).
Decline is slow after peaking, though data was limited to six weeks.
A statistically significant difference in IgG levels was observed between employees over the age of 50 and younger individuals, nevertheless, levels remained well above the threshold for serological reactivity. Although our results are not in line with a previous work evaluating a single time point after natural infection, and showed higher IgG titers with advancing age [11], a non linear correlation to age was shown by Yang et al and very low levels in young adults – alluding to a possible confounder in these results. Our data are in accordance with not-yet peer reviewed data from a study evaluating BNT162b2-induced antibody responses [12].
The importance of this difference needs to be studied further, but a potential difference in vaccine efficacy and vaccine effect length could possibly be present between these two groups.
This study has several limitations. The available cohort is small and not representative of the general population (especially male to female ratio).
Since baseline serology was available for only a single individual, previous infection in other subjects can not be completely ruled out. Had such an infection occurred, a rapid increase in IgG [13], [14], [15] would have been observed, skewing our results. However, we believe previous infection to be unreasonable since vaccine eligibility at the time of the study solely included people with no documented past infection and while our data demonstrated marked heterogeneity in the 7–14 days period, none of the subjects reached levels similar to the peak levels at this point (as would have been expected with a previous exposure [9]).
7. Conclusion
This study demonstrated the kinetics of the antibody response post vaccination with BNT162b2. The robustness of seroconversion was observed, alongside a significant age dependent difference in antibody response. Further research is required in order to examine whether the difference persists over time.
More importantly, further investigations should be conducted to evaluate the correlation between seroconversion and clinical course of COVID-19, as well as examining other factors that might affect magnitude of seroconversion as larger datasets become available.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 1–12 (2021). doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed]
- 3.Amit S., Regev-yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;6736:18–19. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SF Lumley et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2020:1–8. doi:10.1056/nejmoa2034545. [DOI] [PMC free article] [PubMed]
- 5.Guo L., et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okba N.M.A., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long Q.X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 8.Danese E et al. Comprehensive assessment of humoral response after Pfizer BNT162b2 mRNA Covid-19 vaccination: a three-case series. Clin Chem Lab Med 2021. doi:10.1515/CCLM-2021-0339. [DOI] [PubMed]
- 9.Favresse J et al. Antibody titres decline 3-month post-vaccination with BNT162b2. https://doi.org/10.1080/22221751.2021.1953403 10, 1495–1498 (2021). [DOI] [PMC free article] [PubMed]
- 10.Jalkanen P., et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;121(12):1–11. doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HS et al. Association of age With SARS-CoV-2 antibody response. JAMA Netw Open 4, e214302 (2021). [DOI] [PMC free article] [PubMed]
- 12.Müller L et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv 2021.03.03.21251066 (2021). doi: 10.1101/2021.03.03.21251066.
- 13.Ebinger JE et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 1–4 (2021). doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed]
- 14.Lombardi A., et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health. 2021;14:1120–1122. doi: 10.1016/j.jiph.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraley E et al. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med 2021;191(19):1–12. [DOI] [PMC free article] [PubMed]