Abstract
Background
Early in the coronavirus disease 2019 (COVID-19) pandemic, before severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines became available, it was hypothesized that BCG (Bacillus Calmette–Guérin), which stimulates innate immunity, could provide protection against SARS-CoV-2. Numerous ecological studies, plagued by methodological deficiencies, revealed a country-level association between BCG use and lower COVID-19 incidence and mortality. We aimed to determine whether BCG administered in early life decreased the risk of SARS-CoV-2 infection in adulthood and the severity of COVID-19.
Methods
This case-control study was conducted in Quebec, Canada. Cases were patients with a positive SARS-CoV-2 nucleic acid amplification test performed at two hospitals between March–October 2020. Controls were identified among patients with non-COVID-19 samples processed by the same microbiology laboratories during the same period. Enrolment was limited to individuals born in Quebec between 1956 and 1976, whose vaccine status was accessible in a computerized registry of 4.2 million BCG vaccinations.
Results
We recruited 920 cases and 2123 controls. Fifty-four percent of cases (n = 424) and 53% of controls (n = 1127) had received BCG during childhood (OR: 1.03; 95% CI: 0.89–1.21), while 12% of cases (n = 114) and 11% of controls (n = 235) had received two or more BCG doses (OR: 1.14; 95% CI: 0.88–1.46). After adjusting for age, sex, material deprivation, recruiting hospital and occupation there was no evidence of protection conferred by BCG against SARS-CoV-2 (AOR: 1.01; 95% CI: 0.84–1.21). Among cases, 77 (8.4%) needed hospitalization and 18 (2.0%) died. The vaccinated were as likely as the unvaccinated to require hospitalization (AOR: 1.01, 95% CI: 0.62–1.67) or to die (AOR: 0.85, 95% CI: 0.32–2.39).
Conclusions
BCG does not provide long-term protection against symptomatic COVID-19 or severe forms of the disease.
Keywords: BCG, SARS-CoV-2, COVID-19, Vaccine effectiveness
1. Introduction
One hundred years ago, Albert Calmette and Camille Guérin initiated the first clinical trial of their vaccine against tuberculosis, Bacillus Calmette–Guérin (BCG). Its efficacy against pulmonary tuberculosis is ≈50%, a protection which persists for up to 40 years [1], [2]. Furthermore, BCG stimulates innate immunity, which becomes ‘trained’, leading to non-specific effects against a broad range of viruses in humans (influenza, herpes simplex, respiratory syncytial virus, human papillomavirus, and the yellow fever vaccinal strain) and animals [3]. BCG enhances the response against subsequent triggering agents through an epigenetic long-term reprogramming of innate immune cells, some of which (macrophages, monocytes, and NK cells) display intrinsic memory [4], [5], [6], [7].
Non-specific, ‘off-target’, effects of BCG were first described by Naeslund in 1932 and termed ‘para-specific immunity’ by Calmette who, with great foresight, attributed this to an ‘excitation of phagocytic cells’ [8], [9]. Their maximal duration remains unknown. In Spain, neonatal BCG was associated, at the population level, with a lower risk of hospitalization for pneumonia or other severe infections until at least an age of 14 years [10]. In Kenya, BCG vaccination in infancy led to a lower risk of pneumonia in adulthood [11]. In Denmark, among individuals followed for a median of 32 years, those who had received BCG at school entry experienced a reduction in mortality from natural causes, after adjusting for socioeconomic status [12].
Thus, BCG may theoretically provide some protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection for years after immunisation. Numerous ecological studies published or in preprint, have reported associations between a universal BCG policy and a low incidence of or mortality due to coronavirus disease 2019 (COVID-19) at the country level [13], [14], [15], [16], [17]. Ongoing placebo-controlled trials of BCG in healthcare workers will address a putative short-term protection [18].
To determine whether BCG administered during infancy/childhood decreases the risk of SARS-CoV-2 infection in adulthood and/or the severity of COVID-19, the current case-control study exploited a unique opportunity provided by the combination of three factors in the province of Quebec: a population-wide yet non-mandatory BCG program wherein about 50% of children born between 1949 and 1976 were vaccinated; a computerized registry of BCG vaccination going as far back as 1956; and a high incidence of SARS-CoV-2 infection during the first wave of the pandemic [19], [20]. We hypothesized that the BCG may exert a protective effect, which would likely be stronger among individuals vaccinated more recently.
2. Material and methods
Cases and controls were identified through the microbiology laboratories of the Hôpital Maisonneuve-Rosemont (HMR) in Montreal and the Centre Hospitalier Universitaire in Sherbrooke (CHUS), Canada. Only individuals born in the province of Quebec between 1 January 1956 and 31 December 1976 (aged 43–64 years) were enrolled as they could be linked to the BCG registry. The estimated catchment populations of HMR and CHUS are 540,000 and 492,000 inhabitants, respectively, and the former covers the eastern part of the island of Montreal while the latter covers adjacent regions (Estrie and parts of Montérégie) east of Montreal.
Cases were patients with a positive SARS-CoV-2 nucleic acid amplification test (NAAT) at one of the two participating hospitals between 17 March 2020 and 22 October 2020. We initially aimed to select three controls per case, with frequency matching on sex and year of birth, but eventually reduced this to two controls per case for the HMR site, given the high number of cases and a relative paucity of suitable controls. Potential controls were identified through the databases of patients who had a sample other than a SARS-CoV-2 NAAT processed by the same microbiology laboratory during the same period and belonged to the same birth cohorts. Thus, controls were recruited from the same population as cases, as they had access to the same laboratories for an investigation when they got sick.
To avoid misclassification of case-control status in the context of a relatively high rate of false negativity of the SARS-CoV-2 NAAT due to pre-analytical issues [21], we excluded as potential controls patients who had a negative or indeterminate SARS-CoV-2 NAAT and those who underwent cultures of blood or respiratory specimens. To obtain controls who were relatively representative of the catchment population at large rather than its sickest fraction, we excluded as controls patients who: had been hospitalized or had attended the emergency room during the study period; underwent tests for detection of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, or multi-resistant Gram-negative bacilli (generally associated with hospitalization); or were likely to have some degree of immunosuppression (attending outpatient clinics for haematology, oncology, radio-oncology, rheumatology, immunology, HIV, renal transplants, or dialysis). Patients whose samples had been sent from a mental health facility or who lived in long-term care settings were excluded as they were deemed unable to give informed consent. To decrease the workload of interviewers, we excluded potential cases and controls whose patronyms indicated that they were very unlikely to have been born in Quebec during 1956–1976, based on the history of immigration into the province [22]. We then randomly selected the controls to obtain the desired numbers in each of the 22 strata based on sex and year of birth (e.g. 1956–57, 1958–59, etc.).
Potential participants were contacted by phone. After explaining the study’s goals and procedures, verbal consent was sought to administer the questionnaire and link up the person’s data with the BCG registry at INRS after verifying whether they were indeed born in Quebec. Demographic information was collected, including the six-digit postal code which was used to obtain a census-based material deprivation index [23]. We asked questions about occupation (healthcare or other frontline workers with exposure to the public during the lockdown), and whether participants remembered having received the BCG vaccine or having a vaccine scar. For the controls, we asked two additional questions to assess whether they might in fact have been an undiagnosed case: whether they had close contact with a COVID-19 case and/or experienced a recent episode of anosmia or dysgeusia [24]. For cases, we determined whether they had required hospitalization for COVID-19 or had died, as per hospital records. For the deceased participants, the institutional review boards waived the requirement of informed consent from the next of kin, and we collected data from hospital records.
The Quebec BCG vaccination program targeting newborns and children began in 1949, and was gradually phased out in the mid-1970s. BCG was prepared at the Institute of Microbiology and Hygiene of the University of Montreal using daughter strains 450–51 (until 1956) and 568–571 (from 1957 onward), and delivered in capillary tubes at a concentration of 60 mg/cc. It was administered by scarifications: two on each deltoid for newborns, three on each deltoid for children. To decide whether previously immunised school-age children needed to be revaccinated, scarifications with killed BCG ('CutiBCG') were performed on the lower back. A further BCG dose was administered to non-reactive subjects [25].
Each participant’s data were linked to a computerized BCG registry at INRS which holds information on all 4.2 million BCG vaccinations performed in Quebec from 1956 to 1992 [19], [20]. Using the surname, given name, sex, date of birth, and father’s given name, we proceeded to identify whether each participant had received BCG and the age at vaccination. The registry was designed to store information on vaccinees and has been verified as highly complete and accurate; individuals not found in the registry were considered unvaccinated [20]. Probabilistic data linkage was performed with the fastLink package in R (R Foundation, Vienna, Austria) [26]. Manual verification of matches below a predefined threshold was done to look for spelling and other data-entry errors. Ninety-five percent of linkages were qualified as definite; the remainder was considered as probable.
At the design stage, we aimed to recruit 900 cases and 2700 controls. This corresponded to 80% power (with alpha error = 0.05) to detect a vaccine effectiveness of 20%. When this study was initiated in the spring of 2020, no SARS-CoV-2 vaccine was on the horizon, and we believed that a vaccine effectiveness of at least 20% would be a useful contribution.
Data analyses were performed with R version 4.0.2 [27]. Unconditional logistic regression was used to assess associations between BCG vaccination and SARS-CoV-2 infection. Apart from the main analysis (vaccinated vs. unvaccinated), secondary analyses further categorized vaccination according to the number of doses received, and age at first vaccination. Analyses were performed for all subjects, and then stratified into four age categories to look for effect modification and potential waning of immunity. Analyses were carried out for both sexes combined, then for males and females separately. In multivariate models, adjustments were made for age, sex, hospital, occupation (healthcare setting, essential worker or contact with public, and all others), urban vs. rural residence, and material deprivation (in quintiles) as potential confounders. A sensitivity analysis excluded controls that had close contact with a person infected with SARS-CoV-2 or had reported an episode of anosmia or dysgeusia (strongly associated with SARS-CoV-2). Another analysis examined whether BCG immunisation had an impact on the severity of COVID-19 as determined by the need for hospital admission or mortality.
3. Results
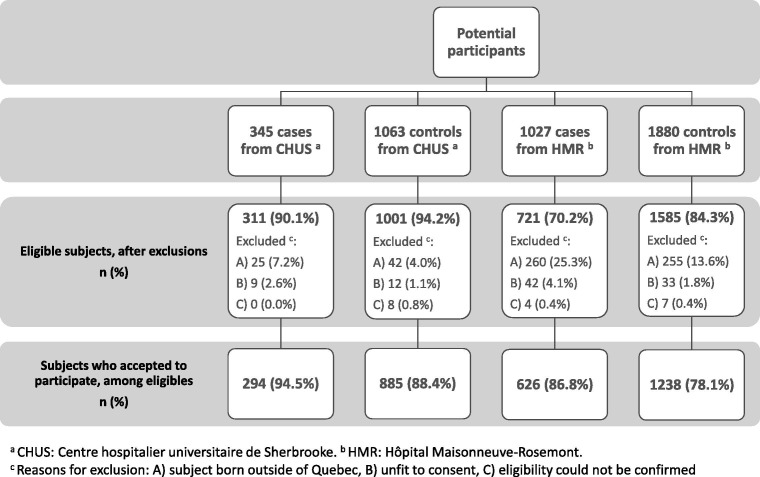
The study sample profile is shown in Fig. 1 . Exclusions for birth outside Quebec and consent refusals were more common at HMR compared to CHUS. At HMR, the proportion of exclusions for birth outside Quebec was lower in controls than in cases, due to a more stringent pre-selection based on patronyms amongst the former. Participation rates were high, 95% and 88% among eligible cases and controls at CHUS, and 86% and 78% at HMR, respectively.
Fig. 1.
Study sample profile.
We recruited 920 cases and 2123 controls. Out of 3043 participants, 1545 (51%) were considered to have been definitely vaccinated, 78 (3%) were probably vaccinated, while 1420 (47%) were unvaccinated. Given the small number of participants with a probable rather than definite match with the BCG registry, analyses did not differ whether the former were excluded or not, and we will present results based on definite and probable matches. Compared with the registry data, self-reported BCG vaccination was considered unreliable as was self-report of a vaccine scar (data not shown), many of which were presumably provoked by smallpox vaccine rather than BCG, and these were not analysed further.
Table 1 displays sociodemographic characteristics of cases and controls. Sex and years of birth were similar because of the frequency matching process, although there was a slight imbalance for birth year. Differences between hospitals reflected the 2:1 controls-to-cases ratio at HMR vs. 3:1 ratio at CHUS. Due to occupational infections with SARS-CoV-2, healthcare workers were overrepresented among cases.
Table 1.
Sociodemographic characteristics of cases and controls.
| Characteristics | Cases (%) n = 920 |
Controls (%) n = 2123 |
|---|---|---|
| Sex | ||
| Male | 354 (38.5) | 821 (38.7) |
| Female | 566 (61.5) | 1302 (61.3) |
| Birth year | ||
| 1956–1960 | 218 (23.7) | 544 (25.6) |
| 1961–1965 | 263 (28.6) | 580 (27.3) |
| 1966–1970 | 216 (23.5) | 467 (22.0) |
| 1971–1976 | 223 (24.2) | 532 (25.1) |
| Hospital | ||
| HMR | 626 (68.0) | 1238 (58.3) |
| CHUS | 294 (32.0) | 885 (41.7) |
| Residence | ||
| Montreal/Laval | 450 (48.9) | 1053 (49.6) |
| Other urban areas | 349 (37.9) | 781 (36.8) |
| Rural areas | 103 (11.2) | 279 (13.1) |
| Missing | 18 (2.0) | 10 (0.5) |
| Material deprivation | ||
| Lowest | 153 (16.6) | 293 (18.7) |
| Low | 161 (17.5) | 388 (22.0) |
| Middle | 171 (18.6) | 444 (20.9) |
| High | 205 (22.3) | 466 (18.3) |
| Highest | 158 (17.2) | 396 (13.8) |
| Missing | 72 (7.8) | 136 (6.4) |
| Work | ||
| Healthcare settings | 434 (47.2) | 233 (11.0) |
| Essential/contact with public | 143 (15.5) | 422 (19.9) |
| All others | 327 (35.5) | 1465 (69.0) |
| Missing | 16 (1.7) | 3 (0.1) |
Abbreviations: CHUS: Centre hospitalier universitaire de Sherbrooke; HMR: Hôpital Maisonneuve-Rosemont
Table 2 presents the comparison of cases and controls for BCG status. There was no evidence of a protective effect of BCG against COVID-19 when BCG was evaluated as a dichotomous variable, nor when number of doses or age at first dose were examined. As it was plausible that a protective effect existed only among the younger participants, for whom the interval between BCG and COVID-19 was shorter, Supplementary Table S1 presents the same data after stratifying for year of birth. The lack of protection provided by BCG was uniform across all groups, including those born in 1971–1976 (p-value for BCG-age interaction = 0.84). The proportion of immunised individuals, both in cases and controls, decreased from 69% among participants born in 1956–1960 to 34% for those born in 1971–1976. Among the latter birth cohort, very few participants received more than one dose or were immunised beyond an age of 1 year. Supplementary Table S2 presents stratified analyses for female and male participants whereas Supplementary Table S3 displays analyses stratified by hospital. Again, no effect was seen in any of the strata (p-value for BCG-sex interaction = 0.35; for BCG-hospital interaction = 0.86). No changes were seen either in a sensitivity analysis that excluded 106 controls who had close contact with a person infected with SARS-CoV-2 or reported an episode of anosmia or dysgeusia (data not shown).
Table 2.
BCG status and COVID-19, descriptive statistics and logistic regression.
| Cases (%) n = 920 |
Controls (%) n = 2123 |
Crude odds ratio (95% CI) |
Adjusted odds ratio (95% CI) a |
|
|---|---|---|---|---|
| BCG received | ||||
| Never | 424 (46.1) | 996 (46.9) | 1.00 | 1.00 |
| Ever | 496 (53.9) | 1127 (53.1) | 1.03 (0.89–1.21) | 1.01 (0.84–1.21) |
| Number of BCG doses | ||||
| 0 | 424 (46.1) | 996 (46.9) | 1.00 | 1.00 |
| 1 | 382 (41.5) | 892 (42.0) | 1.01 (0.85–1.19) | 0.97 (0.80–1.18) |
| ≥2 | 114 (12.4) | 235 (11.1) | 1.14 (0.88–1.46) | 1.17 (0.86–1.57) |
| Age at first BCG, years | ||||
| Unvaccinated | 424 (46.1) | 996 (46.9) | 1.00 | 1.00 |
| 0–1 | 340 (37.0) | 726 (34.2) | 1.10 (0.93–1.31) | 1.04 (0.85–1.27) |
| 2–9 | 119 (12.9) | 313 (14.7) | 0.89 (0.70–1.13) | 0.95 (0.71–1.27) |
| ≥10 | 37 (4.0) | 88 (4.1) | 0.99 (0.65–1.46) | 0.80 (0.48–1.29) |
Abbreviations: BCG: Bacillus Calmette-Guérin; CI: confidence interval; COVID-19: coronavirus disease 2019.
Adjusted for sex, age, recruitment hospital, material deprivation, occupation (healthcare worker vs other), and residence area (rural vs urban).
Table 3 displays the frequency of hospital admission, and Table 4 shows the case-fatality ratio according to BCG status. For the latter, the small number of deaths precluded full adjustment for confounders. As COVID-19 mortality is strongly related to age, and the vaccinated were older than the unvaccinated (mean 55.7 vs. 52.9 years), we present age- and sex-adjusted odds ratios. There was no evidence that BCG conferred any protection against more severe forms of COVID-19.
Table 3.
BCG status and hospitalization among cases of COVID-19.
| Hospitalized cases/Total cases (%) n = 77/920 |
Crude odds ratio (95% CI) |
Adjusted odds ratio (95% CI) a |
|
|---|---|---|---|
| BCG received | |||
| Never | 31/424 (7.3) | 1.00 | 1.00 |
| Ever | 46/496 (9.3) | 1.30 (0.81–2.10) | 1.04 (0.57–1.90) |
| Number of BCG doses | |||
| 0 | 31/424 (7.3) | 1.00 | 1.00 |
| 1 | 35/382 (9.2) | 1.28 (0.77–2.13) | 1.04 (0.55–1.95) |
| ≥2 | 11/114 (9.5) | 1.35 (0.63–2.71) | 1.05 (0.42–2.45) |
| Age at BCG, years | |||
| Unvaccinated | 31/424 (7.3) | 1.00 | 1.00 |
| 0–1 | 29/340 (8.5) | 1.18 (0.69–2.01) | 1.14 (0.60–2.16) |
| 2–9 | 12/119 (10.1) | 1.42 (0.68–2.80) | 0.83 (0.32–2.01) |
| ≥10 | 5/37 (13.5) | 1.98 (0.64–5.06) | 0.83 (0.12–3.36) |
Abbreviations: BCG: Bacillus Calmette-Guérin; CI: confidence interval; COVID-19: coronavirus disease 2019.
Adjusted for sex, age, recruitment hospital, material deprivation quintile, occupation (healthcare worker vs other), residence area (rural vs urban).
Table 4.
BCG status and mortality among cases of COVID-19.
| Deaths/Total cases (%) n = 18/920 |
Crude odds ratio (95% CI) |
Adjusted odds ratio (95% CI) a |
|
|---|---|---|---|
| BCG received | |||
| Never | 7/424 (1.7) | 1.00 | 1.00 |
| Ever | 11/496 (2.2) | 1.35 (0.53–3.70) | 0.87 (0.33–2.44) |
| Number of BCG doses | |||
| 0 | 7/424 (1.7) | 1.00 | 1.00 |
| 1 | 9/382 (2.4) | 1.44 (0.53–4.06) | 1.03 (0.37–3.01) |
| ≥2 | 2/114 (1.8) | 1.06 (0.16–4.47) | 0.49 (0.07–2.17) |
| Age at BCG, years | |||
| Unvaccinated | 7/424 (1.7) | 1.00 | 1.00 |
| 0–1 | 4/340 (1.2) | 0.71 (0.18–2.37) | 0.56 (0.14–1.94) |
| 2–9 | 5/119 (4.2) | 2.61 (0.76–8.34) | 1.20 (0.33–3.98) |
| ≥10 | 2/37 (5.4) | 3.40 (0.49–14.73) | 1.46 (0.20–6.74) |
Abbreviations: BCG: Bacillus Calmette-Guérin; CI: confidence interval; COVID-19: coronavirus disease 2019.
Adjusted for sex and age.
4. Discussion
This large case-control study showed that BCG vaccination in infancy or childhood does not provide long-term protection against COVID-19 or lessen illness severity. When it was designed in May 2020, experts predicted that developing a specific vaccine would require at least 12–18 months. The availability less than a year later of several marketed vaccines with efficacy ranging between 70% and 95% [28], [29], [30] was beyond the most optimistic expectations of a North American expert panel [31]. Access to COVID-19 vaccines remains uneven across and within countries and identifying all potential prevention tools and measures remains valuable.
In that context, there has been much interest in the hypothesis that BCG might confer some protection against COVID-19, due to its non-specific effect on innate immunity. More than twenty ecological studies were deposited as preprints on MedRxiv or published after peer review with most claiming that countries using BCG in infancy or childhood experienced a lower incidence of COVID-19 or a lower mortality [13], [14], [15], [16], [17]. Ecological studies are useful for the generation and preliminary testing of hypotheses. With the power of the internet and publicly available data, they can now be carried out in a matter of days. However, nothing comes easily in science, and ecological studies are plagued with multiple deficiencies. In this case, a major flaw is that access to a diagnosis of laboratory-confirmed SARS-CoV-2 infection is limited in low- and middle-income countries that still use BCG. Neither do ecological studies allow for adjustment for potential confounders, which are many here (age, socioeconomic status, ethnicity, etc.). Further, they are subject to the ecological fallacy, which consists in falsely interpreting at the individual level results observed at the group or country level.
For BCG in infancy or childhood to protect against COVID-19 in adulthood, two postulates must be met. First, trained immunity triggered by BCG should provide at least a short-term protection against SARS-CoV-2 infection, as it does for several other viruses [3]. We could not address this question in the current study, as BCG was infrequently used in Quebec after 1976. Ongoing randomized trials, mostly in healthcare workers and the elderly, will evaluate putative short-term protection. Second, this trained immunity should persist for a very long period. For how many years does the BCG-induced trained immunity last, providing a protection against respiratory pathogens? A case in point is its effect against the pathogen for which BCG was originally developed, Mycobacterium tuberculosis. Long-assumed to be reflective of cell-mediated adaptive immunity, recent work suggests that some of the BCG-induced protection is derived from innate trained immunity [32]. The protection against tuberculosis persists for at least 15 years and possibly up to 40 years [1], [33]. BCG also provides very long-term protection against leprosy [2]. While most studies in Africa have supported BCG-induced non-specific protection against respiratory pathogens during the first two years of life [34], evidence for long-term beneficial effects is sparse. An ecological study in Spain, wherein Basque Country (using BCG) was compared to other regions (not using BCG), suggested a lower frequency of hospitalizations for respiratory infections at least until an age of 14 years in Basque Country–but the design made it impossible to take confounding factors into account [10]. In a case-control study of adults in Kenya, having a BCG scar was associated with a lower risk of pneumonia, more strongly so in males than in females [11].
Recent studies on COVID-19 and BCG have investigated this question using various methods. In Israel, there was no difference in COVID-19 incidence between the 1979–1981 (assumed to be all vaccinated) and the 1983–1985 (assumed to be all non-vaccinated) birth cohorts [35]. However, this rather crude ecological design implied misclassification of exposure in both cohorts (immigrants), some of which was non-random (the Hasidim were less likely to be vaccinated with BCG and, at the time, at a high risk of COVID-19). Of higher methodological quality was another ecological study that used data from a ‘natural experiment’ in Sweden, where a change in policy in April 1975 led to the abrupt discontinuation of BCG for neonates, such that coverage was 92% before that date and 2% thereafter. In a regression discontinuity analysis that compared cohorts born before or after this pivotal date, the incidence of COVID-19 was identical in both groups, suggesting the absence of a long-term protection [36]. Among healthcare workers in California, self-reported history of BCG vaccination was associated with a 24% lower odds of SARS-CoV-2 seropositivity after adjustment for age and sex, but not ethnicity [37]. In Italy, BCG was not associated with lower severity of COVID-19 after adjustment for confounders; however, only 63/2548 participants had received the vaccine [38].
Our study, the first that was specifically designed to address this issue with individual-level data, demonstrates the absence of effectiveness of BCG against COVID-19 on the very long term (40 years or more). We could not document a protective effect neither in all participants, nor in pre-determined subgroups based on age or sex. Neither could we demonstrate that BCG reduces the severity of COVID-19.
Some methodological considerations deserve attention. The test-negative design (TND) has been extensively used for assessing the effectiveness of vaccines [39], including inactivated influenza [40] and COVID-19 vaccines [41], [42], [43], [44], [45], [46], [47], [48]. In TND studies, clinical specimens from oral or nasopharyngeal swabs (NPS) are tested by multiplex NAAT and/or cultures and results classified into 3 categories: (i) positive for the virus targeted by the vaccine, (ii) negative for this virus but positive for another or other viruses, and (iii) negative for all viruses tested. A basic assumption of the TND is that the risk of disease caused by viruses not targeted by the vaccine under investigation is not modified by the vaccination status. This is usually the case for protection resulting from the adaptive immunity when cross-reactivity between different viral species is minimal. When the protection generated by a vaccine results from the activation of unspecific innate immune mechanisms (trained immunity) as it may be the case for BCG against COVID-19, the above-mentioned condition is not met as the protection may extend in a quite uniform way against all respiratory viruses. As a consequence, the proportion of cases caused by the pathogen under investigation among all tests or tests positive for any pathogen in the TND will be similar among vaccinated and unvaccinated individuals, and any effect of the vaccine will be missed. Conversely, the classic case-control design using diseases targeted by the vaccine as cases and healthy controls is appropriate for testing the hypothesis of a protection generated by unspecific innate mechanisms.
Recruitment of a suitable control group requires great care, and this requirement was complexified by the pandemic context. Since cases were identified through hospital microbiology laboratories, controls were selected from the same source. Given the lockdown, it was necessary to ensure that selected controls were not much sicker than the general population, which led us to make some exclusions (persons hospitalized, with an emergency room visit, with infections highly related to hospitalizations, or who were likely immunosuppressed). The decision to exclude potential controls who had a negative or indeterminate SARS-CoV-2 NAAT was due to a concern about false-negative results. Indeed, during the study period, SARS-CoV-2 testing in the general population was indicated only for symptomatic individuals or contacts of infected persons. The overall sensitivity of SARS-CoV-2 NAATs from a nasopharyngeal aspirate, NPS, or throat swab, compared to other clinical tools such as radiology and serology, was initially estimated at 73% (95% CI: 0.68–0.78) [49]. This clinical sensitivity is influenced by the anatomical site swabbed, the sampling technique, the types of swab and transport media, the analytical sensitivity [50] of the available assay and the protocol used in the laboratory (e.g., with or without chemical extraction, pooling of samples) and timing after symptom onset or contact with a COVID-19 case. The negative predictive value of a single NPS NAAT in symptomatic patients was estimated at 0.80 [51]. Consequently, participants with a negative or indeterminate NAAT result were excluded from potential controls to prevent misclassification of disease status. There is a legitimate concern that this may have introduced selection bias.
We attempted to assess the extent of selection bias that could have resulted from these exclusions. In an analysis of the association between occupational status and SARS-CoV-2 infection, healthcare workers were 8.4 times more likely than other participants to have had a positive SARS-CoV-2 NAAT result (Table S4). This is congruent with the relative risk estimated in the Quebec population during the first COVID-19 wave (RR = 9) in the province of Quebec [52], during which the vast majority of cases and controls were recruited into our study. Although it is not possible to entirely rule out selection bias, this argues toward the lack of a sizeable bias due to the selection of the control group.
A study limitation is that, given time and budgetary constraints, we elected not to collect data on chronic co-morbidities. We believed that chronic diseases were very unlikely to be confounders, given that this would require them to be associated with exposure to BCG several decades earlier. Halfway into the study, we had to reduce the controls-to-cases ratio to 2:1 for the HMR site for practical reasons. Fortunately, the association between BCG exposure and SARS-CoV-2 did not differ by recruitment site. The higher refusal rate at HMR compared to CHUS might reflect a large-city effect where people are more suspicious of phone calls from unknown persons.
Another limitation of the study is that there was substantial evolution of BCG strains, propagated in culture media in several laboratories between 1921 and 1961, when laboratories started using −80 °C freezers to store seed lots and standardize their products. For some parameters reflecting trained innate immunity, variations between BCG strains have been documented [53], [54]. The original Montréal (or Frappier) strain, obtained from Institut Pasteur in 1933, was lost in 1957 when it was replaced with strain 568–571, also from Paris, which was subsequently used for almost all of our participants [25], [54].
Further studies could examine whether administering BCG a few weeks prior to a SARS-CoV-2 vaccine enhances immune response, as it does with other vaccines, including those against the 2009 H1N1 pandemic influenza [55]. We could not address whether BCG provides short-term protection against SARS-CoV-2 and this remains a relevant question, especially for low-income countries. In the meantime, there is unfortunately no evidence that BCG can play a role in the global fight against COVID-19.
CRediT authorship contribution statement
Jacques Pépin: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing. Annie-Claude Labbé: Funding acquisition, Investigation, Resources, Supervision, Writing - review & editing. Alex Carignan: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing - review & editing. Marie-Elise Paren: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing - review & editing. Jennifer Yu: Data curation, Formal analysis, Investigation, Validation, Writing - review & editing. Cynthia Grenier: Investigation, Supervision, Validation, Writing - review & editing. Stéphanie Beauchemin: Investigation, Supervision, Validation, Writing - review & editing. Philippe De Wals: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. Louis Valiquette: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. Marie-Claude Rousseau: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The following persons contributed to data collection (alphabetical order): Karim Abdul Baki, David Ag Bazet, Marc-Alexandre Binette, Jonathan Boisvert, Marie-Pier Boisvert, Jeremy Bourget, Vincent Choinière, Aya Delimi, Antoine Deneault, Véronique Dumont, Loai Duquette-Laplante, Rodrigo Escobar Careaga, Karen Farag, Lisa Foudil, Stéphany Gélin, Kim Gendron, Louis-Antoine Gervais, Olivier Grimard, Rhita Harti, Raphael Lachance, Anais Marcil-Héguy, Noémie Métayer, Sophie Payeur, Jane-Carole Pellerin, Catherine Simard, Rachel Thibeault, Ann-Sophie Thiffault, and Kim Vettese. We are also grateful to Mendy Malachy, Ju-Hong Lee, and Nathalie Frappier for their assistance with hospital databases, to Nicolas Gagnon for his help in setting up the data entry interface, and to Geneviève Deceuninck for helpful suggestions.
Author agreement
All authors have seen and approved the final version of the manuscript to be submitted. The article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Data sharing statement
The anonymized datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the ethics research committees of CIUSSS de l'Estrie-CHUS (Project MP-31-2021-3730), CIUSSS de l'Est-de-l’Île-de-Montréal (Project MEO-31-2021-2274) and Institut National de la Recherche Scientifique (Project CÉR-20-566), and all study procedures were conducted in conformance with the principles for biomedical research that were expounded in the Declaration of Helsinki, 1964 and its subsequent amendments.
Funding
This work was supported by the Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke through a special COVID-19 emergency funding provided by the Fondation du Centre Hospitalier Universitaire de Sherbrooke. Funding for computerization of the BCG vaccination registry was provided by a grant from the Canada Foundation for Innovation and the Québec Ministry of Education, Leisure and Sports [grant number 12532, to M.C.R.]. The funder had no role in study design, in collection, analysis and interpretation of data, in the writing of the report nor in the decision to submit.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.08.019.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nguipdop-Djomo P., Heldal E., Rodrigues L.C., Abubakar I., Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis. 2016;16(2):219–226. doi: 10.1016/S1473-3099(15)00400-4. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Report on BCG vaccine use for protection against mycobacterial infections including tuberculosis, leprosy, and other nontuberculous mycobacteria infections. https://www.who.int/immunization/sage/meetings/2017/october/1_BCG_report_revised_version_online.pdf. Accessed 10 May 2021.
- 3.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki-Nakashimada M.A., Unzueta A., Berenise Gámez-González L., González-Saldaña N., Sorensen R.U. BCG: a vaccine with multiple faces. Hum Vaccin Immunother. 2020;16(8):1841–1850. doi: 10.1080/21645515.2019.1706930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohrabi Y., Dos Santos J.C., Dorenkamp M., Findeisen H., Godfrey R., Netea M.G., et al. Trained immunity as a novel approach against COVID-19 with a focus on Bacillus Calmette-Guérin vaccine: mechanisms, challenges and perspectives. Clin Transl Immunol. 2020;9(12):e1228. doi: 10.1002/cti2.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik Y.S., Ansari M.I., Ganesh B., Sircar S., Bhat S., Pande T., et al. BCG vaccine: a hope to control COVID-19 pandemic amid crisis. Hum Vaccin Immunother. 2020;16(12):2954–2962. doi: 10.1080/21645515.2020.1818522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naeslund C. Résultats des expériences de vaccination par le BCG poursuivies dans le Norrbotten (Suède) (Septembre 1927-Décembre 1931) [Results of BCG vaccination experiments conducted in Norrbotten (Sweden) (September 1927-December 1931)]. In: Vaccination préventive de la tuberculose de l'homme et des animaux par le BCG. Rapports et documents provenant de divers pays (la France exceptée) transmis à l'Institut Pasteur en 1932 [BCG preventive vaccination against tuberculosis in humans and animals. Reports and documents from various countries (France excepted) transmitted to the Institut Pasteur in 1932]. Paris: Masson et Cie, 1932:274−81.
- 9.Calmette A., Saenz A. Sur l'immunité para-spécifique conférée par le BCG [About para-specific immunity conferred by BCG] Ann Inst Pasteur. 1933;50:433–445. [Google Scholar]
- 10.de Castro M.J., Pardo-Seco J., Martinón-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60:1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 11.Muthumbi E., Lowe B.S., Muyodi C., Getambu E., Gleeson F., Scott J.A.G. Risk factors for community-acquired pneumonia among adults in Kenya: a case-control study. Pneumonia (Nathan) 2017;9:17. doi: 10.1186/s41479-017-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieckmann A., Villumsen M., Sørup S., Haugaard L.K., Ravn H., Roth A., et al. Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971–2010. Int J Epidemiol. 2017;46(2):695–705. doi: 10.1093/ije/dyw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020;6(32):eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolgikh S. Further evidence of a possible correlation between the severity of COVID-19 and BCG immunization. J Infect Dis Epidemiol. 2020;6:120. doi: 10.23937/2474-3658/1510120. [DOI] [Google Scholar]
- 15.Urashima M., Otani K., Hasegawa Y., Akutsu T. BCG Vaccination and Mortality of COVID-19 across 173 countries: an ecological study. Int J Environ Res Public Health. 2020;17(15):5589. doi: 10.3390/ijerph17155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar L.E., Molina-Cruz A., Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joy M., Malavika B., Asirvatham E.S., Sudarsanam T.D., Jeyaseelan L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clin Epidemiol Glob Health. 2021;9:202–203. doi: 10.1016/j.cegh.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wals P., Menzies D., Divangahi M. Can BCG be useful to mitigate the COVID-19 pandemic? A Canadian perspective. Can J Public Health. 2020;111(6):939–944. doi: 10.17269/s41997-020-00439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousseau M.-C., Conus F., Kâ K., El-Zein M., Parent M.-É., Menzies D. Bacillus Calmette-Guérin (BCG) vaccination patterns in the province of Québec, Canada, 1956–1974. Vaccine. 2017;35(36):4777–4784. doi: 10.1016/j.vaccine.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau M.C., Conus F., Li J., Parent M.É., El-Zein M. The Québec BCG Vaccination Registry (1956–1992): assessing data quality and linkage with administrative health databases. BMC Med Inform Decis Mak. 2014;14:2. doi: 10.1186/1472-6947-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woloshin S., Patel N., Kesselheim A.S. False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications. N Engl J Med. 2020;383(6):e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 22.Piché V. In: La démographie québécoise. Enjeux du XXIe siècle [Quebec demography. 21st century challenges] Le Bourdais C, Piché V, editors. Presses de l’Université de Montréal; Montréal: 2003. Un siècle d’immigration au Québec: de la peur à l’ouverture [A century of immigration to Quebec: from fear to openness] pp. 225–263.https://books.openedition.org/pum/23988?lang=en [Google Scholar]
- 23.Institut National de Santé Publique du Québec. Indice de défavorisation matérielle et sociale. https://www.inspq.qc.ca/defavorisation/indice-de-defavorisation-materielle-et-sociale. Accessed 27 July 2021.
- 24.Carignan A., Valiquette L., Grenier C., Musonera J.B., Nkengurutse D., Marcil-Héguy A., et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ. 2020;192(26):E702–E707. doi: 10.1503/cmaj.200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frappier A. Presses de l'Université du Québec; Sillery: 1992. Un rêve, une lutte [One dream, one struggle]https://www.puq.ca/catalogue/livres/reve-une-lutte-715.html [Google Scholar]
- 26.Enamorado T.E.D., Fifield B., Imai K. Using a probabilistic model to assist merging of large-scale administrative records. Am Polit Sci Rev. 2019;113(2):353–371. doi: 10.1017/S0003055418000783. [DOI] [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/.
- 28.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane P.B., Moyer H., MacPherson A., Papenburg J., Ward B.J., Broomell S.B., et al. Expert forecasts of COVID-19 vaccine development timelines. J Gen Intern Med. 2020;35(12):3753–3755. doi: 10.1007/s11606-020-06244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann E., Sanz J., Dunn J.L., Khan N., Mendonça L.E., Pacis A., et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1-2):176–190.e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Abubakar I., Pimpin L., Ariti C., Beynon R., Mangtani P., Sterne J.A., et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):1–372. doi: 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth A., Garly M.L., Jensen H., Nielsen J., Aaby P. Bacillus Calmette-Guérin vaccination and infant mortality. Expert Rev Vaccines. 2006;5(2):277–293. doi: 10.1586/14760584.5.2.277. [DOI] [PubMed] [Google Scholar]
- 35.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Chaisemartin C., de Chaisemartin L. BCG vaccination in infancy does not protect against COVID-19. Evidence from a natural experiment in Sweden. Clin Infect Dis. 2021;72(10):e501–e505. doi: 10.1093/cid/ciaa1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131(2):e145157. doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi M., Miglietta A., Romeo G., Bartolacci S., Ariani F., Cipriani F., et al. Letter in response to article in journal of infection: impact of routine infant BCG vaccination on COVID-19. J Infect. 2021;82(1):e41–e43. doi: 10.1016/j.jinf.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 40.De Serres G., Skowronski D.M., Wu X.W., Ambrose C.S. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Eurosurveillance. 2013;18(37):20585. doi: 10.2807/1560-7917.ES2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 41.Kissling E., Hooiveld M., Sandonis Martín V., Martínez-Baz I., William N., Vilcu A.M., et al. Vaccine effectiveness against symptomatic SARS-CoV-2 infection in adults aged 65 years and older in primary care: I-MOVE-COVID-19 project, Europe, December 2020 to May 2021. Euro Surveill. 2021;26(29):2100670. doi: 10.2807/1560-7917.Es.2021.26.29.2100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butt A.A., Omer S.B., Yan P., Shaikh O.S., Mayr F.B. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann Intern Med. 2021 doi: 10.7326/M21-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrejko K.L., Pry J., Myers J.F., Jewell N.P., Openshaw J., Watt J., et al. Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skowronski D.M., Setayeshgar S., Zou M., Prystajecky N., Tyson J.R., Galanis E., et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia, Canada. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021 doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 48.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Böger B., Fachi M.M., Vilhena R.O., Cobre A.F., Tonin F.S., Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2021;49(1):21–29. doi: 10.1016/j.ajic.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mostafa H.H., Hardick J., Morehead E., Miller J.-A., Gaydos C.A., Manabe Y.C. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J Clin Virol. 2020;130:104578. doi: 10.1016/j.jcv.2020.104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George B., McGee J., Giangrasso E., Finkelstein S., Wu S., Glatt A.E. What Is the Predictive Value of a Single Nasopharyngeal SARS-CoV-2 PCR Swab Test in a Patient With COVID-Like Symptoms and/or Significant COVID-19 Exposure? Open Forum. Infectious Diseases. 2020;7(10):ofaa399. doi: 10.1093/ofid/ofaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Serres G, Carazo S, Villeneuve J, Laliberte D, Martin R, Denis G, et al. Enquête épidémiologique sur les travailleurs de la santé atteints par la COVID-19 : rapport d’étape pour la période du 12 juillet 2020 au 16 janvier 2021. Institut national de santé publique du Québec; 2021. https://www.inspq.qc.ca/publications/3137-enquete-epidemiologique-travailleurs-sante-atteints-covid19 Accessed 27 July 2021.
- 53.Ponte C., Hacker M., Moraes M., Castello-Branco L., Silva F., Antas P. The patterns of in vitro cell-death and inflammatory cytokines induced by distinct BCG vaccine strains are differentially induced in human mononuclear cells. Hum Vaccin Immunother. 2018;14(1):28–35. doi: 10.1080/21645515.2017.1382788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdallah A.M., Behr M.A. Evolution and strain variation in BCG. Adv Exp Med Biol. 2017;1019:155–169. doi: 10.1007/978-3-319-64371-7_8. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann P., Curtis N. The influence of BCG on vaccine responses - a systematic review. Expert Rev Vaccines. 2018;17(6):547–554. doi: 10.1080/14760584.2018.1483727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.