Abstract
Background
Some patients with COVID-19 who have recovered from the acute infection after experiencing only mild symptoms continue to exhibit persistent exertional limitation that often is unexplained by conventional investigative studies.
Research Question
What is the pathophysiologic mechanism of exercise intolerance that underlies the post-COVID-19 long-haul syndrome in patients without cardiopulmonary disease?
Study Design and Methods
This study examined the systemic and pulmonary hemodynamics, ventilation, and gas exchange in 10 patients who recovered from COVID-19 and were without cardiopulmonary disease during invasive cardiopulmonary exercise testing (iCPET) and compared the results with those from 10 age- and sex-matched control participants. These data then were used to define potential reasons for exertional limitation in the cohort of patients who had recovered from COVID-19.
Results
The patients who had recovered from COVID-19 exhibited markedly reduced peak exercise aerobic capacity (oxygen consumption [VO2]) compared with control participants (70 ± 11% predicted vs 131 ± 45% predicted; P < .0001). This reduction in peak VO2 was associated with impaired systemic oxygen extraction (ie, narrow arterial-mixed venous oxygen content difference to arterial oxygen content ratio) compared with control participants (0.49 ± 0.1 vs 0.78 ± 0.1; P < .0001), despite a preserved peak cardiac index (7.8 ± 3.1 L/min vs 8.4±2.3 L/min; P > .05). Additionally, patients who had recovered from COVID-19 demonstrated greater ventilatory inefficiency (ie, abnormal ventilatory efficiency [VE/VCO2] slope: 35 ± 5 vs 27 ± 5; P = .01) compared with control participants without an increase in dead space ventilation.
Interpretation
Patients who have recovered from COVID-19 without cardiopulmonary disease demonstrate a marked reduction in peak VO2 from a peripheral rather than a central cardiac limit, along with an exaggerated hyperventilatory response during exercise.
Key Words: cardiopulmonary exercise test, COVID-19, hemodynamics, iCPET, long haulers, post-COVID-19 syndrome
Abbreviations: CaO2, arterial oxygen content; CO, cardiac output; CPET, cardiopulmonary exercise testing; DO2, oxygen delivery; EO2, systemic oxygen extraction; iCPET, invasive cardiopulmonary exercise testing; SV, stroke volume; SVI, stroke volume index; VE/VCO2, ventilatory efficiency; VO2, oxygen consumption
Take-home Points.
Study Question: What is the pathophysiologic mechanism of exercise intolerance that underlies post-COVID-19 long-haul syndrome in patients with COVID-19 without cardiopulmonary disease?
Results: Patients who have recovered from COVID-19 demonstrate reduced peak exercise aerobic capacity with impaired systemic oxygen extraction and abnormal ventilatory efficiency slope.
Interpretation: Patients without cardiopulmonary disease who have recovered from COVID-19 demonstrate a marked reduction in peak oxygen consumption from a peripheral rather than a central cardiac limit, along with an exaggerated hyperventilatory response during exercise.
Globally, more than 100 million confirmed cases of COVID-19 caused by SARS-CoV-2 infection have been reported. The acute manifestations of SARS-CoV-2 infection can involve the pulmonary, cardiovascular, neurologic, hematologic, and GI systems.1 Persistent physical symptoms after acute COVID-19 are common and include fatigue, dyspnea, chest pain, cough, and neurocognitive symptoms.2, 3, 4, 5, 6 In one retrospective study of approximately 1,300 hospitalized patients with COVID-19 discharged to home, only 40% of patients were independent in all activities of daily living at 30 days,6 and almost 40% of patients were unable to return to normal activities at 60 days after hospital discharge.7 Several recent studies have reported persistent symptoms among patients who demonstrated mild COVID-19 months after recovery from the acute illness.8, 9, 10 Persistent cardiorespiratory symptoms in those who have survived COVID-19 can be categorized into two clinical entities: (1) those directly related to organ injury or iatrogenic consequences during the acute phase and (2) those with persistent symptoms, including a decrease in exercise capacity determined objectively by cardiopulmonary exercise testing (CPET), with normal findings from pulmonary function testing, resting echocardiography, and CT scan of the chest months after the onset of acute symptoms,11 , 12 the so-called post-COVID-19 long-haul syndrome.
In a recent study, Baratto and colleagues13 showed that during CPET performed at the time of hospital discharge, patients who have recovered from COVID-19 exhibited a hyperventilatory response and reduced exercise capacity. The latter was attributed primarily to underlying anemia resulting in both reduced systemic oxygen delivery and extraction. However, the pathophysiologic basis for the persistent exertional and functional limitation among patients who have had COVID-19 and who have long since recovered from mild acute illness remains unknown. Accordingly, in the current study, we aimed to further characterize the persistent exercise intolerance among patients who have recovered from COVID-19 without evidence of cardiopulmonary disease or anemia using invasive CPET (iCPET).
Methods
Study Population and Design
We consecutively enrolled all patients who had recovered from COVID-19 and were referred to the Brigham and Women’s Hospital Dyspnea Clinic (Boston, MA) and the Yale New Haven Hospital Pulmonary Vascular Disease Clinic (New Haven, CT) between February and June 2021 for unexplained exercise intolerance. The study protocol was approved by Partners Healthcare Human Research Committee (Identifier: 2011P000272) and Yale University Institutional Review Board (Identifier: IRB 2000024783). All patients signed informed consent and agreed to have their anonymized clinical and investigative data used for research purposes.
All patients underwent conventional investigative testing during outpatient clinic evaluation, including CT scan of the chest, pulmonary function test, and resting echocardiography. In none of the patients were test results deemed contributory to the persistent exertional limitation before iCPET referral. Specifically, no evidence was found of parenchymal lung disease on chest CT imaging, and all patients demonstrated left ventricle ejection fraction of > 50% with no evidence of moderate or severe valvular heart disease, no evidence of right-to-left intracardiac shunt defect on resting right heart catheterization and echocardiography, and no evidence of acute coronary syndrome defined by ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, unstable angina, or a combination thereof during exercise testing.
Invasive Cardiopulmonary Exercise Testing
Our method for invasive CPET was described previously.14, 15, 16, 17, 18 Right heart catheterization was performed in the supine position with a five-port pacing pulmonary artery catheter (Edwards LifeSciences) inserted percutaneously under fluoroscopic and ultrasound guidance into the internal jugular vein and a radial artery catheter concurrently placed in the radial artery. Patients underwent a symptom-limited incremental CPET using an upright cycle ergometer with a breath-by-breath assessment of gas exchange (ULTIMA CPX; Medical Graphics Corporation) along with continuous 12-lead electrocardiography monitoring. Patients underwent 2 min of rest followed by 2 min of unloaded cycling at 40 to 60 revolutions per minute. Work rate then was increased continuously using a ramp protocol at 5, 10, 15, or 20 W/min depending on the patient’s functional status, until peak exercise was achieved as evident either by peak respiratory exchange ratio of > 1.10 or peak heart rate of > 85% predicted. Pulmonary and systemic hemodynamics were monitored continuously and simultaneously during exercise (Xper Cardio Physiomonitoring System; Phillips). Pulmonary pressures were recorded at the end of passive exhalation. When respirophasic changes persisted, an electronic average over three respiratory cycles was used.19 Arterial and mixed venous blood gases and pH were collected during each minute of exercise, and the arterial-mixed venous oxygen content difference was calculated. Systemic oxygen extraction (EO2) was calculated as arterial oxygen content (CaO2) minus mixed venous oxygen content (CvO2) divided by CaO2. Fick cardiac output and stroke volume were determined every minute. Oxygen delivery (DO2) was calculated by multiplying cardiac output by the CaO2. Physiologic dead space was calculated as: VD/VT = (Paco 2 – PETCO2) / Paco 2, where VD is dead space volume, VT is tidal volume, Paco 2 is the Pco 2 in arterial blood, and PETCO2 is the mixed expired Pco 2.
Pulmonary vascular resistance was calculated as: mean pulmonary artery pressure minus pulmonary artery wedge pressure divided by cardiac output, expressed in Woods units. Stroke volume (SV) was calculated as cardiac output (CO) divided by the heart rate. CO and SV were indexed to body surface area to obtain both cardiac index and SV index. Pulmonary artery compliance was calculated as the ratio of SV to pulmonary artery pulse pressure and was expressed as milliliters per millimeter of mercury. Total pulmonary resistance was calculated as mean pulmonary artery pressure divided by CO as expressed in Woods units.
To investigate further the determinants of exercise limitation in patients who have recovered from COVID-19, we identified 10 age- and sex-matched control participants from our iCPET database. This cohort consisted of symptomatic patients without a prior history of COVID-19 who previously underwent iCPET for clinical investigation of exertional intolerance, but who exhibited a normal physiological limit to exercise defined by a peak oxygen uptake (peak oxygen consumption [VO2]) and peak CO of ≥ 80% predicted.
Statistical Analysis
Unless otherwise stated, values are presented as mean ± SD. Comparisons of baseline characteristics, resting hemodynamics, and CPET parameters between patients who have recovered from COVID-19 and control participants were performed using an independent t test for normally distributed data and the Wilcoxon rank-sum test for data nonnormally distributed data. The χ 2 test was used to analyze dichotomous variables. A P value of < .05 was considered significant. Statistical analyses were performed using GraphPad Prism version 9 software (GraphPad Software) and SAS version 9.4 software (SAS Institute, Inc.).
Results
Demographic and Clinical Characteristics
We included 10 patients who have recovered from COVID-19 who at the time of iCPET were demonstrated negative results by polymerase chain reaction for SARS-CoV-2. Nine patients previously had experienced mild, acute SARS-CoV-2 infection that did not require hospitalization,20 whereas one patient underwent a brief 2-day in-patient stay during which Remdesivir (Gilead Sciences) and corticosteroids were administered. Two patients were excluded during the enrollment period: one patient with long-standing history of fibrotic interstitial lung disease and another who exhibited iatrogenic chronotropic incompetence from B-adrenergic blocker therapy. The latter patient did not attain maximum exercise effort by either by peak respiratory exchange ratio of > 1.10 or peak heart rate of > 85% predicted.
No differences were found in age, hemoglobin concentration, BMI, medication use, or comorbidities between patients who had recovered from COVID-19 and control participants. Importantly, the average interval between onset of acute COVID-19 illness (ie, from the time of positive SARS-CoV-2 polymerase chain reaction results) to iCPET was 11 months (Table 1 ). Patients who had recovered from COVID-19 demonstrated normal resting right heart hemodynamic values. The baseline characteristics, comorbidities, resting right heart hemodynamics, and pulmonary function test results are summarized in Table 1.
Table 1.
Baseline Characteristics and Resting Cardiopulmonary Hemodynamics
| Variable | Patients Recovered From COVID-19 (n = 10) | Control Participants (n = 10) | P Value |
|---|---|---|---|
| Characteristics | |||
| Age, y | 48 ± 15 | 48 ± 8 | .87 |
| Female sex | 9 (90) | 8 (80) | .53 |
| BMI, kg/m2 | 28 ± 6 | 24 ± 6 | .11 |
| Hemoglobin, g/dL | 13.4 ± 1.1 | 14.2 ± 1.4 | .16 |
| Interval from acute COVID-19 infection to iCPET, mo | 11 ± 1 | Not applicable | ... |
| Comorbidities | |||
| Systemic hypertension | 2 (20) | 3 (30) | .61 |
| Diabetes | 0 | 1 (10) | .30 |
| Medications | |||
| β-Adrenergic receptor blocker | 1 (5) | 1 (5) | 1.00 |
| ACE inhibitor or ARB | 2 (20) | 0 | .13 |
| Diuretics | 0 | 1 (10) | .30 |
| Pulmonary function test | |||
| FEV1, % | 97 ± 1 | 100 ± 1 | .34 |
| FVC, % | 96 ± 1 | 104 ± 1 | .19 |
| FEV1 to FVC ratio, % | 101 ± 3 | 98 ± 5 | .18 |
| Resting upright right heart catheterization | |||
| SaO2, % | 98 (97-98) | 98 (97-98) | .64 |
| MvO2, % | 73 ± 3 | 66 ± 6 | .01 |
| Right atrial pressure, mm Hg | 0 (0-1) | 3 (0-4) | .35 |
| Stroke volume index, mL/m2 | 36.3 ± 10.3 | 40.3 ± 12.8 | .44 |
| Cardiac index, L/min/m2 | 3.2 ± 0.6 | 2.8 ± 0.5 | .13 |
| mPAP, mm Hg | 8 ± 1 | 12 ± 3 | .002 |
| PAWP, mm Hg | 2 ± 2 | 5 ± 3 | .01 |
| PVR, WU | 1.13 (0.87-1.52) | 1.26 (0.95-2.01) | .44 |
| PA compliance, mL/mm Hg | 5.6 ± 2.4 | 7.7 ± 3.3 | .13 |
| SVR index, dynes/s/cm5/m2 | 2,554 ± 880 | 2,924 ± 487 | .26 |
Data presented as No. (%),mean ± SD, or median (interquartile range). ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; iCPET = invasive cardiopulmonary test; mPAP = mean pulmonary artery pressure; MvO2 = mixed venous oxygen saturation; PA = pulmonary artery; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; SaO2 = oxygen saturation in arterial blood; SVR = systemic vascular resistance; WU = Woods unit.
Peak Exercise Hemodynamic Response
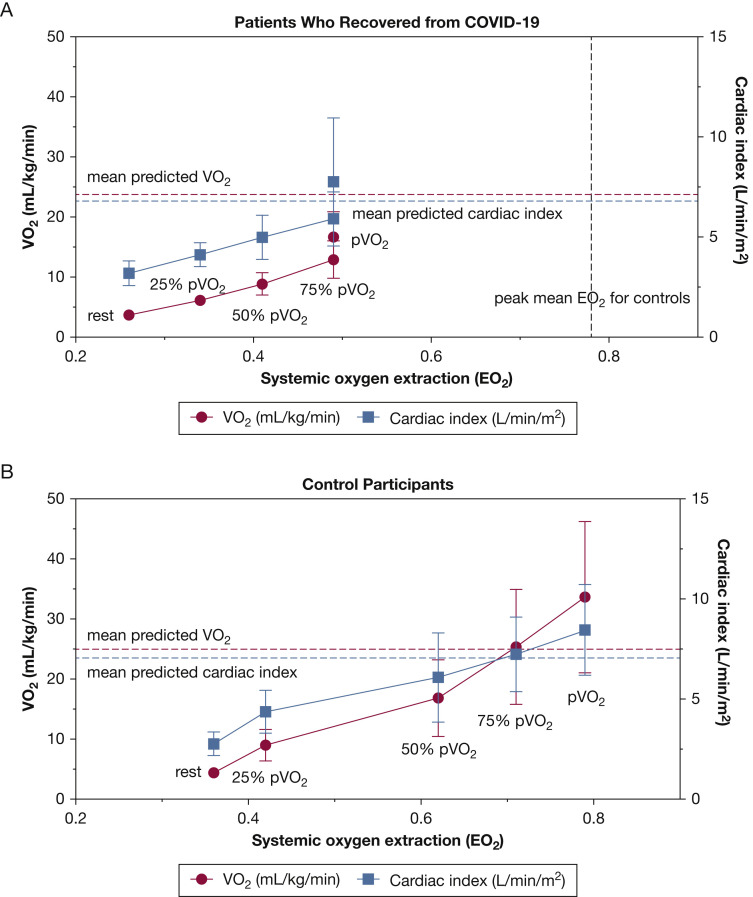
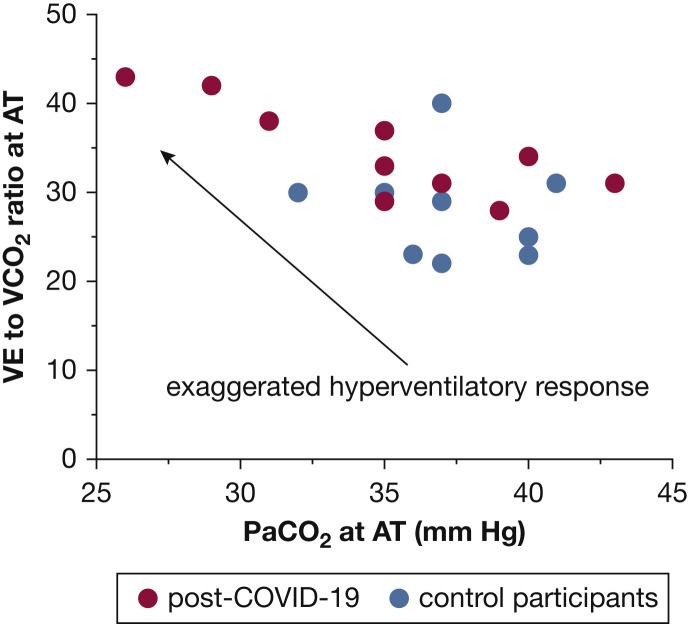
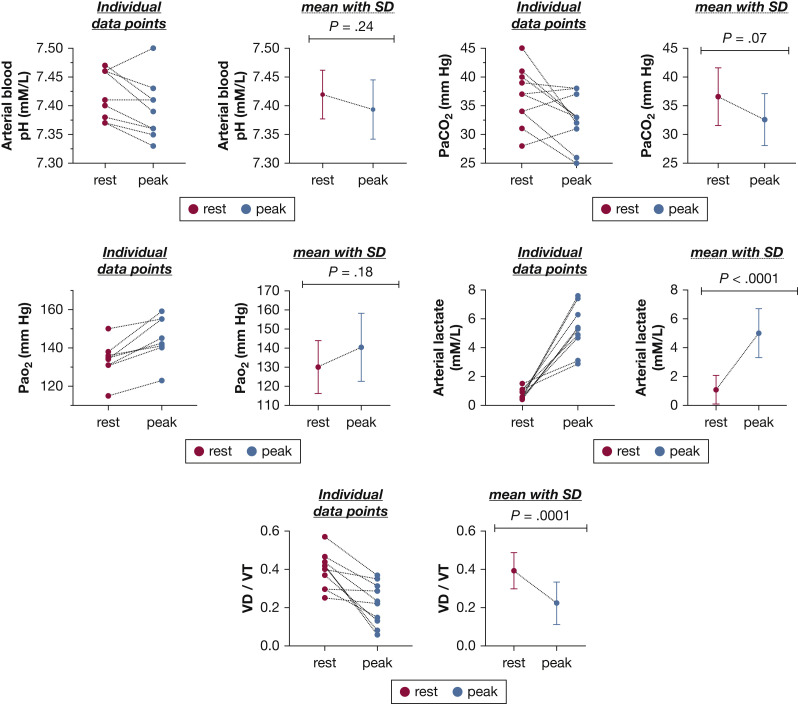
The maximum invasive CPET and cardiopulmonary hemodynamic data are summarized in Table 2 . At peak exercise, patients who had recovered from COVID-19 exhibited markedly reduced aerobic capacity (ie, peak VO2 < 80% predicted) with a normal peak DO2 and reduced EO2 compared with control participants (Fig 1 ). Patients who had recovered from COVID-19 showed greater peak exercise mixed venous oxygen saturation (50 ± 10% vs 22 ± 5%; P < .0001) and peak venous O2 content (33 ± 6 mm Hg vs 22 ± 2 mm Hg; P = .001) compared with control participants. Additionally, patients who had recovered from COVID-19 exhibited a greater degree of ventilatory inefficiency compared with control participants (ie, abnormal ventilatory efficiency [VE/VCO2] slope: 35 ± 5 vs 27 ± 5; P = .01) (Fig 2 ). Of the 10 patients who had recovered from COVID-19, only one patient demonstrated a VE/VCO2 slope of < 30 (the VE/VCO2 value of this patient was 28).21 In the patients who had recovered from COVID-19, a trend toward lower peak right atrial pressure (3 ± 4 mm Hg vs 6 ± 3 mm Hg; P = .08) was found, along with a significantly reduced left-side filling pressure (pulmonary artery wedge pressure, 8 ± 4 mm Hg vs 13 ± 3 mm Hg; P = .01). An appropriate decrease in dead space ventilation was found in patients who had recovered from COVID-19 from rest to peak exercise (0.39 ± 0.1 vs 0.22 ± 0.1; P = .001) (Fig 3 ). The total pulmonary resistance at peak exercise was normal in both groups (ie, peak total pulmonary resistance < 3 Woods units).
Table 2.
Maximum Exercise Cardiopulmonary Hemodynamics
| Variable | Patients Recovered From COVID-19 (n = 10) | Control Participants (n = 10) | P Value |
|---|---|---|---|
| Maximum CPET data | |||
| Peak VO2, % predicted | 70 ± 11 | 131 ± 45 | .001 |
| Peak VO2, mL/min/kg | 16.7 ± 4.2 | 33.5 ± 12.9 | .001 |
| Peak heart rate, % predicted | 84 ± 8 | 84 ± 2 | .85 |
| Delta ETCO2, mm Hg | –0.5 (–4 to 1) | –1 (–2 to 13) | .57 |
| Peak SaO2, % | 98 (98-98) | 97 (97-98) | .01 |
| Peak MvO2, % | 50 ± 10 | 22 ± 5 | < .0001 |
| Venous PO2, mm Hg | 33 ± 6 | 22 ± 2 | .001 |
| VE/VCO2 slope | 35 ± 5 | 27 ± 5 | .01 |
| CaO2, mL/dL | 18.6 ± 1.3 | 19.5 ± 2.3 | .29 |
| Peak DO2, mL/kg/min | 3.6 ± 1.4 | 4.2 ± 1.5 | .33 |
| Peak EO2 | 0.49 ± 0.1 | 0.78 ± 0.1 | < .0001 |
| Peak exercise hemodynamics | |||
| Cardiac output, % predicted | 115 ± 44 | 123 ± 34 | .64 |
| Cardiac index, L/min/m2 | 7.8 ± 3.1 | 8.4 ± 2.3 | .59 |
| Stroke volume index, mL/m2 | 54.1 ± 20.8 | 63.5 ± 22.2 | .34 |
| RA pressure, mm Hg | 3 ± 4 | 6 ± 3 | .08 |
| mPAP, mm Hg | 18 ± 5 | 30 ± 4 | < .0001 |
| PAWP, mm Hg | 8 ± 4 | 13 ± 3 | .01 |
| PVR, WU | 0.69 ± 0.44 | 0.99 ± 0.36 | .11 |
| TPR, WU | 1.2 ± 0.4 | 2.0 ± 0.4 | .002 |
| PA compliance, mL/mm Hg | 4.7 ± 2.3 | 4.3 ± 2.1 | .67 |
| SVR index, dynes/s/cm5/m2 | 1,272 ± 398 | 1,119 ± 283 | .33 |
Data are presented as No. (%), mean ± SD, or median (interquartile range). CaO2 = arterial oxygen content; CPET = cardiopulmonary exercise testing; DO2 = oxygen delivery; EO2 = systemic oxygen extraction; ETCO2 = end tidal CO2; mPAP = mean pulmonary artery pressure; MvO2 = mixed venous oxygen saturation; PA = pulmonary artery; PAWP = pulmonary artery wedge pressure; PVR = pulmonary vascular resistance; SaO2 = oxygen saturation in arterial blood; RA = right atrial; SVR = systemic vascular resistance; TPR = total pulmonary resistance; VE/VCO2 = ventilatory efficiency; VO2 = oxygen consumption; WU = Woods unit.
Figure 1.
A-B, Graphs showing the components of the Fick principle in the patients who recovered from COVID-19 (A) and control participants (B) during maximum incremental invasive cardiopulmonary testing at rest, 25% of pVO2, 50% of pVO2, 75% of pVO2, and at pVO2. Data presented as mean ± SD. EO2 = systemic oxygen extraction; pVO2 = peak oxygen consumption; VO2 = oxygen consumption.
Figure 2.
Graph showing abnormal ventilatory efficiency in patients who have recovered from COVID-19 at the AT. AT = anaerobic threshold; VE/VCO2 = ventilatory efficiency.
Figure 3.
Graphs showing blood gas data from patients who have recovered from COVID-19 at rest and peak exercise. Data are presented as individual data points for each patient and mean ± SD, with red dots representing data at rest and blue dots representing data at peak exercise. P value obtained using independent t test. VD/VT = ratio of dead space to tidal volume.
Discussion
In the current study, we demonstrate that nearly 1 year after recovery from mild disease, patients who experienced COVID-19 with decreased exercise tolerance, but no long-term cardiopulmonary disease sequelae, exhibited a peripheral, rather than a central, cardiac limit to aerobic exercise characterized by impaired systemic EO2 with resulting increased peak exercise mixed venous oxygen saturation and peak VO2 content. Additionally, they also demonstrated an exaggerated hyperventilatory response during exercise.
According to the Fick principle, in the absence of a pulmonary mechanical limitation, reduced peak VO2 is the result of a blunted CO and cardiac index response, impaired systemic EO2 (ie, arterial-mixed venous oxygen content difference), or both. In the current study, the depressed peak VO2 in patients who had recovered from COVID-19 was driven primarily by reduced systemic EO2 (Fig 1). In fact, the peak CO response was robust, representing on average 115% of the predicted value, and the DO2 was preserved. We also demonstrated that in both control participants and patients who have recovered from COVID-19, throughout incremental exercise testing, increases in VO2 were driven by increments in both EO2 and cardiac index (Fig 1). However, unlike control participants, at 75% of peak VO2 and at peak VO2, further increases in VO2 in patients who had recovered from COVID-19 were attenuated by limitations imposed by EO2, rather than cardiac index.
The delivery and subsequent use of oxygen is determined by convective and diffusive processes. Convective oxygen delivery involves alveolar ventilation and the transport of hemoglobin-bound oxygen by the heart and systemic vasculature to the peripheral microcirculation (ie, DO2). Diffusive oxygen delivery involves the diffusion of oxygen across the alveolar-pulmonary capillary membrane onto hemoglobin and the unloading of oxygen from hemoglobin in skeletal muscle capillaries where the process of aerobic mitochondrial respiration generates adenosine triphosphate. A study from Baratto and colleagues13 demonstrated that the reduced systemic EO2 among patients who have recovered from COVID-19 at time of hospital discharge was driven in part by reduced convective oxygen delivery from underlying anemia (ie, reduced CaO2 and DO2). Our findings differ from those of Baratto and colleagues for two main reasons. First, the current study examined patients who had recovered from COVID-19 with persistent exertional and functional limitation approximately 11 months after acute viral illness. Additionally, apart from one patient, these patients who had recovered from COVID-19 did not require in-patient care. Second, unlike the study from Baratto and colleagues, the current patients who recovered from COVID-19 did not have associated anemia or parenchymal lung disease. Importantly, we found that convective oxygen transport in the patients who recovered from COVID-19 was preserved (ie, normal DO2). Therefore, the impaired EO2 observed in the current study was attributed primarily to reduced oxygen diffusion in the peripheral microcirculation, resulting in increased peak exercise mixed venous oxygen saturation and peak venous O2 content (Table 2).
More recently, two noninvasive CPET studies in patients who have recovered from COVID-19 have been reported.22 , 23 The first study by Rinaldo and colleagues22 evaluated 75 patients 3 months after hospital discharge. Fifty-two percent and 24% of the patients who had recovered from COVID-19 were categorized as having critical and severe disease, respectively, whereas 63% of patients demonstrated residual parenchymal lung disease on chest CT imaging. The authors found that patients with reduced peak exercise capacity (defined by peak VO2 of < 85% predicted) attained anaerobic threshold early, but exhibited no pulmonary mechanical limit to exercise (ie, preserved breathing reserve index) with preserved ventilatory efficiency (ie, VE to VCO2 ratio slope of 28 ± 3). Also, no correlation was found between reduction in peak exercise capacity with reduced diffusing capacity on lung function test or parenchymal lung disease on chest CT imaging. Based on these findings, the authors concluded that the reduced peak exercise capacity seen in the patients who had recovered from COVID-19 is because of deconditioning. The second study by Skjorten and colleagues23 examined 189 patients also 3 months after hospital discharge, of whom 20% required ICU management.23 The peak VO2 (% predicted) was lower among patients who had recovered from COVID-19 and who required ICU management, but no difference was found in the breathing reserve and VE to VCO2 ratio slope between patients treated in the ICU and those who were not. Across the entire cohort, reduced peak VO2 (< 80% predicted) was observed in 31% of participants. When compared with a reference population, patients who recovered from COVID-19 exhibited preserved ventilatory efficiency (ie, VE to VCO2 ratio slope of 28 ± 5) and breathing reserve (30 ± 17%) along with preserved oxygen pulse (15 ± 4 mL/stroke). Accordingly, the authors concluded that deconditioning was the major cause of exercise limitation in the patients who had recovered from COVID-19. The hallmark of deconditioning is reduced peak CO.24 While patients who are deconditioned can exhibit impaired EO2 with exercise,25 the preserved peak exercise CO observed in our post-COVID-19 patients (115 ± 44 vs 123 ± 24 %predicted; P = .64) along with their normal peak heart rate response, makes the deconditioning hypothesis a less likely explanation for their exercise limitation. Furthermore, the patients presented herein who had recovered from COVID-19 demonstrated lower low biventricular filling pressures, rather than the higher pressures encountered in detrained individuals, which is attributable to cardiac atrophy and reduced ventricular compliance.26 , 27
During exercise, the greater need for local tissue metabolism coupled with reduced availability of tissue oxygen results in greater production of local vasodilatory substances in the skeletal muscles. This mechanism, along with sympathetic nervous system-mediated vasoconstriction to nonexercising areas, allows for increased tissue oxygen delivery during exercise.28 We recently demonstrated in a cohort of patients with chronic fatigue syndrome that systemic microcirculatory dysfunction with microvascular shunting (impaired systemic oxygen extraction) was prevalent particularly among patients who also exhibited small-fiber neuropathy on skin biopsy.29 Immunohistochemical studies have shown that these small fibers regulate microvascular tone through sympathetic and parasympathetic cholinergic synapses of perivascular myocytes.30 Although considerable overlap exists in the clinical presentation of patients with post-COVID-19 and chronic fatigue syndrome,12 whether a similar neuropathologic mechanism is seen in the patients who have recovered from COVID-19 remains to be determined.
The other important finding of the current study is the exaggerated hyperventilatory response among the patients who recovered from COVID-19, as evident by the abnormal VE to VCO2 ratio slope (Fig 2). Arterial CO2 set point is influenced by acidemia, hypoxemia, baroreceptors in the pulmonary vasculature, and sympathetic nervous system hyperactivity.31 , 32 VE to VCO2 ratio is measured at the anaerobic threshold before the onset of anaerobic metabolism and lactic acidosis generation. Additionally, no evidence was found of resting or exercise pulmonary hypertension or interstitial lung disease with the expectant decrease in dead space ventilation seen during exercise (Figure 2, Figure 3). The abnormal ventilatory efficiency in the patients who had recovered from COVID-19 thus can be attributed to enhanced peripheral mechanoergoreflex and metaboergoreflex sensitivity or enhanced chemosensitivity reflex, rather than a primary cardiopulmonary or central mediated hyperventilation process.33 In patients with heart failure, for example, skeletal muscle group III-IV afferents play an important role the exaggerated hyperventilatory response seen during exercise. These mechanoreceptors and metaboreceptors detect changes in muscle length, volume (ie, muscle loss or wasting), and by-products of muscle metabolism and stimulate group III-IV afferents of the spinal cord to the medullary respiratory centers to stimulate ventilation.34 , 35 Muscle weakness and fatigue are a common manifestation of post-COVID-19 syndrome,36 even among those who experienced mild COVID-19.37 It is possible that, in the patients who have recovered from COVID-19, similar to heart failure patients, a skeletal muscle myopathic process characterized by a shift in fiber type,38 reduced muscle aerobic enzyme activity with early dependance on anaerobic metabolism,39 or both culminate in overactivation of group III-IV skeletal muscle afferent activity with resulting exaggerated hyperventilation.
Results from the current study need to be interpreted in the context of limitations. Data for this study were drawn from a small number of patients who had recovered from COVID-19. However, the peripheral limitation to exercise intolerance exhibited by the patients who recovered from COVID-19 were striking compared with those of control participants, and the finding of ventilatory inefficiency (ie, abnormal VE to VCO2 ratio slope) is in keeping with a recent report.13 Additionally, by using iCPET, we provided a comprehensive and unparalleled insight into the long-term sequelae of SARS-CoV-2 infection that is otherwise not apparent on conventional investigative testing.
The control participants were derived from iCPET evaluation for unexplained exertional dyspnea, and therefore, may not be representative of a completely healthy population. However, the control participants were selected based on a preserved peak exercise capacity defined by a normal cardiac limit to exercise (peak VO2 and peak CO of ≥ 80% predicted). Therefore, they represent a studied population with a normal physiologic response to exercise and reflect so-called symptomatic normal individuals.
Interpretation
Exercise limitation is common manifestation of post-COVID-19 syndrome months after resolution of mild acute COVID-19 illness. A peripheral, rather than a central, cardiac limit to exercise characterized by diffusion defect in oxygen delivery (ie, impaired systemic EO2) contributes to patients who have recovered from COVID-19 demonstrating a depressed aerobic exercise capacity. Additionally, patients who have recovered from COVID-19 also exhibit an exaggerated hyperventilatory response during exercise. Further studies are warranted to investigative the pathobiologic basis of these mechanisms.
Acknowledgments
Author contributions: I. S., A. B. W., D. M. S., and P. M. H. contributed to conception and design of the work, interpretation of the data, and writing the manuscript. I. S., P. J., M. C., D. D. L., M. G., J. D. P., D. M. S., and A. B. W. contributed to data acquisition. I. S., P. M. H., and A. B. W. contributed to analysis. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. M. H. is a consuls for Philips and has a de-identified data sharing agreement with NTT Research. None declared (I. S., P. J., M. C., D. D. L., M. G., J. D. P., D. M. S., A. B. W.).
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Lutchmansingh D.D., Knauert M.P., Antin-Ozerkis D.E., et al. A clinic blueprint for post-Coronavirus disease 2019 recovery: learning from the past, looking to the Future. Chest. 2021;159(3):949–958. doi: 10.1016/j.chest.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfi A., Bernabei R., Landi F., Gemelli Against C-P-ACSG Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 5.Nehme M., Braillard O., Alcoba G., et al. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174(5):723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles K.H., McDonald M., Barron Y., Kennedy E., O’Connor M., Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havervall S., Rosell A., Phillipson M., et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin M., Schommers P., Stecher M., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motiejunaite J., Balagny P., Arnoult F., et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol. 2020;11:614590. doi: 10.3389/fphys.2020.614590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baratto C., Caravita S., Faini A., et al. Impact of COVID-19 on exercise pathophysiology. A combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. 2021;130:1470–1478. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maron B.A., Cockrill B.A., Waxman A.B., Systrom D.M. The invasive cardiopulmonary exercise test. Circulation. 2013;127(10):1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira R.K., Urbina M.F., Maran B.A., Santos M., Waxman A.B., Systrom D.M. Functional impact of exercise pulmonary hypertension with borderline resting pulmonary arterial pressure. Pulm Circ. 2017;7(3):1–12. doi: 10.1177/2045893217709025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh I., Oliveira R.K.F., Naeije R., et al. Pulmonary vascular distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest. 2019;156(4):724–732. doi: 10.1016/j.chest.2019.04.111. [DOI] [PubMed] [Google Scholar]
- 17.Singh I., Rahaghi F.N., Naeije R., Oliveira R.K.F., Systrom D.M., Waxman A.B. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest. 2019;156(5):933–943. doi: 10.1016/j.chest.2019.04.109. [DOI] [PubMed] [Google Scholar]
- 18.Singh I., Rahaghi F., Naeije R., et al. EXPRESS: dynamic right ventricular–pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm Circ. 2019;9(3):1–10. doi: 10.1177/2045894019862435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerrigter B.G., Waxman A.B., Westerhof N., Vonk-Noordegraaf A., Systrom D.M. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J. 2014;43(5):1316–1325. doi: 10.1183/09031936.00016913. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 21.Guazzi M., Adams V., Conraads V., et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldo R.F., Mondoni M., Parazzini E.M., et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58(2):2100870. doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skjorten I., Ankerstjerne O.A.W., Trebinjac D., et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58(2):2100996. doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltin B., Blomqvist G., Mitchell J.H., Johnson R.L., Jr., Wildenthal K., Chapman C.B. Response to exercise after bed rest and after training. Circulation. 1968;38(5 suppl):VII1–VII78. [PubMed] [Google Scholar]
- 25.Carrick-Ranson G., Hastings J.L., Bhella P.S., et al. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol. 2014;116(7):736–745. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stickland M.K., Welsh R.C., Petersen S.R., et al. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol. 2006;100(6):1895–1901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- 27.Perhonen M.A., Franco F., Lane L.D., et al. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91(2):645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- 28.Singh I., Oliveira R.K.F., Naeije R., et al. Systemic vascular distensibility relates to exercise capacity in connective tissue disease. Rheumatology. 2021;60(3):1429–1434. doi: 10.1093/rheumatology/keaa510. [DOI] [PubMed] [Google Scholar]
- 29.Joseph P., Arevalo C., Oliveira R.K.F., et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642–651. doi: 10.1016/j.chest.2021.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuller T.B., Hermann K., Baron R. Quantitative assessment and correlation of sympathetic, parasympathetic, and afferent small fiber function in peripheral neuropathy. J Neurol. 2000;247(4):267–272. doi: 10.1007/s004150050582. [DOI] [PubMed] [Google Scholar]
- 31.Weatherald J., Sattler C., Garcia G., Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J. 2018;51(2):1700860. doi: 10.1183/13993003.00860-2017. [DOI] [PubMed] [Google Scholar]
- 32.Naeije R., Faoro V. The great breathlessness of cardiopulmonary diseases. Eur Respir J. 2018;51(2):1702517. doi: 10.1183/13993003.02517-2017. [DOI] [PubMed] [Google Scholar]
- 33.Lalande S., Cross T.J., Keller-Ross M.L., Morris N.R., Johnson B.D., Taylor B.J. Exercise intolerance in heart failure: central role for the pulmonary system. Exerc Sport Sci Rev. 2020;48(1):11–19. doi: 10.1249/JES.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Hand G.A., Potts J.T., Wilson L.B., Mitchell J.H. c-Fos expression in the medulla induced by static muscle contraction in cats. Am J Physiol. 1997;272(1 Pt 2):H48–H56. doi: 10.1152/ajpheart.1997.272.1.H48. [DOI] [PubMed] [Google Scholar]
- 35.Iwamoto G.A., Waldrop T.G., Bauer R.M., Mitchell J.H. Pressor responses to muscular contraction in the cat: contributions by caudal and rostral ventrolateral medulla. Prog Brain Res. 1989;81:253–263. doi: 10.1016/s0079-6123(08)62015-4. [DOI] [PubMed] [Google Scholar]
- 36.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez B., Nansoz S., Cameron D.R., Z’Graggen W.J. Is myopathy part of long-Covid? Clin Neurophysiol. 2021;132(6):1241–1242. doi: 10.1016/j.clinph.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan M.J., Green H.J., Cobb F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan M.J., Green H.J., Cobb F.R. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84(4):1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]