Abstract
Even before the onslaught of COVID-19 pandemic could settle, the unprecedented rise in cases with COVID-19 associated mucormycosis pushed the medical health to the fringe. Hyperglycaemia and corticosteroids appear to be the most consistent associations leading to the commonest manifestation of mucormycosis, Rhino-Orbito-Cerebral Mucormycosis. To address challenges right from categorisation and staging of the disease to the management of relentless progression, a multi-disciplinary expert committee was formed to handle the task in an evidence-based format to enforce best practices. The report of the committee on one hand attempts to succinctly present the currently available evidence while at the other also attempts to bridge the evidence-deficient gaps with the specialty-specific virtuosity of experts.
Keywords: COVID-19, Mucormycosis, Rhino-orbital-cerebral mucormycosis, Anti-fungal, Amphotericin, Staging
With a waning 2nd wave, Rhino-Orbito-Cerebral Mucormycosis (ROCM) has emerged as another medical emergency where the data is sparse on all aspects, ranging from prevention to management. ROCM is a rapidly progressive disease where average mortality borders around 40%.1 In view of the emergency and the associated shortcomings, need for a multi-disciplinary committee of experts was observed to address the issues.
King George's Medical University (KGMU) is a tertiary-care referral Institution that caters to more than 100 million population of northern India. In the current COVID-19 pandemic, KGMU has been functioning as a dedicated Level-3 referral COVID-19 facility and has managed >5000 critically ill COVID-19 patients in its intensive care units. ‘KGMU - Mucormycosis Treatment & Coordination Committee’ was formed to provide an insight into the disease and help in prevention as well as effective management of Mucormycosis. The core committee was constituted by specialty experts with a minimum of 10 years of experience in their respective fields of Medicine (V.A), Infectious Diseases (H.D), Otorhinolaryngology (V·V), Oral and Maxillofacial Surgery (D.M), Ophthalmology (A.K·C), Neurology (H·S.M), Neurosurgery (B·K·O), Plastic and Reconstructive Surgery (B.M), Radiodiagnosis (N·K), Microbiology (P.G), and Pathology (R.J). The co-opted domains looked at Anaesthesia provisioning (M.S), data management (N·K and V·P) and Hospital Administration (N.D.B). The overall logistics, and issues arising thereof, were addressed by the Hon. Vice Chancellor (B·P).
The scope of the committee included all ‘COVID-19 positives’ or ‘post-COVID-19 patients’ suspected of or diagnosed with COVID-19 associated Mucormycosis, with a special focus on COVID-19 associated Rhino-Orbito-Cerebral Mucormycosis (C-ROCM).
C-ROCM included patients in the acute as well as in the post-COVID-19 state. Acute COVID-19 state was defined up to 4 weeks from the date of onset of first symptom; a state thereafter, as per Centers for Disease Control and Prevention, was defined as the post-COVID-19 state.2
The objectives were to critically appraise the available literature and formulate evidence-based guidelines to help in prevention, early detection and management of C-ROCM based on severity of disease and rational use of medications. At the time of submission of this report more than 400 admissions of C-ROCM had taken place and recent experiences were amalgamated into the existent evidence. Approval of the Institutional Ethics Committee was obtained prior to data analysis. The committee conducted a total of 8 meetings and more than 10 mortality audits to arrive at suggestions and recommendations mentioned in the paper.
1. Epidemiology
The estimated prevalence of mucormycosis is approximately 70-times higher in India when compared with the developed world.3,4 The current extrapolations done from data obtained from a series of publications estimate the prevalence to be around 14 cases per 100,000 population.3 There are definite indications that the prevalence of mucormycosis is rising in India and that seems to be strongly associated with the rising trend related with diabetes mellitus.5, 6, 7
2. Pathogenesis
The evidence on pathogenesis of mucormycosis was reviewed; it has been summarized as agent-related factors and host-related factors.
2.1. Agent-related factors
There are several genera and species in the order mucorales; Rhizopus, Mucor, Lichtheimia (formerly Absidia), Cunninghamella, Rhizomucor, Apophysomyces spp. and Saksenaea are the ones commonly associated with Mucormycosis. Species of genera Rhizopus, Mucor and Lichtheimia account for almost 3/4th of all cases with mucormycosis. Rhizopus spp. have been isolated most frequently in these cases, especially in the central nervous system (CNS) forms of Mucormycosis; Lichtheimia spp. are the second most prevalent ones but depending on the geographical region either of Mucor spp. or Lichtheimia spp. may be seen as the dominant form.4,8, 9, 10
The pathogenic mechanisms associated with the aggressive nature of Mucormycosis have been best studied in Rhizopus especially in diabetics. The important factors defined in this regard are CotH (spore coating) fungal protein, GRP78 (glucose-regulated protein, molecular chaperone belonging to the family of heat shock proteins) endothelial cell receptor upregulation and enzymes modulating the sequestration of iron. Other factors associated with germination of spores, cell wall and immune evasion have also been defined.11,12
Iron, in the free form, helps in the proliferation of invading fungi and has been shown to be a predisposing factor for Mucormycosis.13 Expression patterns studied in Lichtheimia in this regard, reveal the possible pathways involved in the utilisation of iron. These may be summarized as the reductive iron assimilation, a siderophore permease pathway and a heme oxygenase pathway.14
Hyperglycemia and ketone reductase: Uncontrolled sugars (hyperglycemia) in known diabetics, newly diagnosed diabetics and in cases secondary to corticosteroid usage demonstrate reduce capability of the host in combating fungi. This can range from impaired ciliary motility of nasal mucosa to ineffective phagocytosis of the invading organism besides providing them an excellent substrate for proliferation. Presence of ketone reductase in the fungi help them thrive through critical situations when there is ketoacidosis and metabolic acidosis.13
2.2. Host-related factors
Important host-related factors identified/proposed till date15,16 are summarized in Table 1.
Table 1.
Host-related factors (identified/proposed) that increase the susceptibility of patients to Mucormycosis.
| Enhanced relevance in the current pandemic | Apparently independent of COVID-19 |
|---|---|
|
|
3. Environment and agent-host interactions
Literature on associations obtained from previous Mucormycosis-related outbreaks was reviewed to look at interactions, beyond the host-related factors, that could have possibly led to a surge in the number of patients in the current pandemic.17 The shortlisted relevant associations were:
-
•
Dirty linen, contaminated linen shelves and dirty bins
-
•
Contaminated air handling units and ventilation ducts
-
•
Negative pressure isolation rooms
-
•
Water leak (wall dampness leading to accumulation of fungus)
-
•
Hospital construction; dust and moisture
-
•
Fungal contamination of nebulizer devices18
The committee recommends that the known associations be taken care of pre-emptively. The committee came across two more significant associations but were found irrelevant in the current scenario. These were:
-
•
Fungal contamination of medication (methylprednisolone)
-
•
Environmental disruptions (more relevant to cutaneous mucormycosis)
4. Does COVID-19 (SARS-CoV-2) contribute to the unprecedented rise in mucormycosis?
The committee deliberated at length on this issue and attempted to define the possibilities, especially COVID-19-associated hyperglycaemia, that have been summarized in Table 2. The table also attempts to highlight how COVID-19 associated mucormycosis might be different from non-COVID-19 associated Mucormycosis both in diabetics and non-diabetics.19, 20, 21, 22
Table 2.
Possible factors contributing to the development of COVID-19 associated Mucormycosis.
| Factors directly attributable to COVID-19 | Factors indirectly attributable to COVID-19 |
|---|---|
|
|
5. When to suspect COVID-19 associated mucormycosis?
Any patient, either in the acute phase of COVID-19 or in the post-COVID-19 phase, presenting with visual deterioration, periorbital swelling, proptosis, facial pain or numbness, headache, nasal obstruction or nasal bleed must be dealt with a high index of suspicion; neurological manifestations in the form of encephalopathy, focal neurological deficit or seizures may also be seen.16,23,24
6. How to diagnose mucormycosis?
6.1. Specimen
The first step in diagnosing C-ROCM is to conduct a diagnostic nasal endoscopy (DNE) and obtain nasal tissue or nasal scrapings from the affected area.25,26 Tissue biopsies constitute the best specimen for the diagnosis of mucormycosis (Fig. 1). If a biopsy is not possible, nasal scrapping and high nasal swabs should be used for direct examination; though high nasal swab and discharges are unreliable as the yield is low in microscopy and cultures.16 In case of sinusitis, sinus biopsies must be taken. It is prudent to collect the specimen before initiating antifungal therapy as the morphological features may be altered, reducing the ability to definitively differentiate mucorales species from other filamentous fungi in microscopy.27 Multiple biopsies may be needed if on DNE the infection appears patchy. It is emphasized that a repeat biopsy may be required if the microscopy or culture is negative, but the patient continues to have signs of progression.
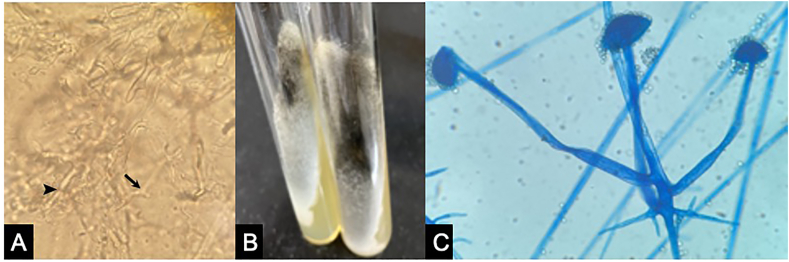
Fig. 1.
KOH mount (A) shows broad aseptate fungal hyphae with right angle branching (arrow) and ribbon like folding (arrowhead). White cotton candy growth on SDA media can be seen with older cultures showing greyish patches (B). LCB mount (C) of the culture of Rhizopus spp.
6.2. Transportation
Specimen such as tissue (whole, uncrushed) must be collected in at least 1 ml of sterile saline in a sterile leak-proof container and must be immediately transported (within 2 h) to the laboratory at room temperature as excessive cold and heat are detrimental to fungal viability. If the delay is inevitable, it is suggested that transport occurs in less than 24 h after collection. When immediate processing is not possible, the specimen should be held at ambient temperatures.28
6.3. Precautions to be taken with sample processing
Nasal tissue must not be crushed or minced in the laboratory before processing for culture as fungal hyphae are damaged by these procedures.27
6.4. Microscopy
Direct microscopy of clinical specimens, allows a rapid presumptive diagnosis of mucormycosis in 10–20% KOH (Potassium hydroxide) in the laboratory.28 Addition of Calcoflour white to KOH enables easy visibility even in the presence of digested human cells and other material. Mucorales hyphae are distinct refractile hyphae of 6–16 μm diameter, aseptate or partially septate, with right angle branching and ribbon-like folding.25 (Fig. 2A)
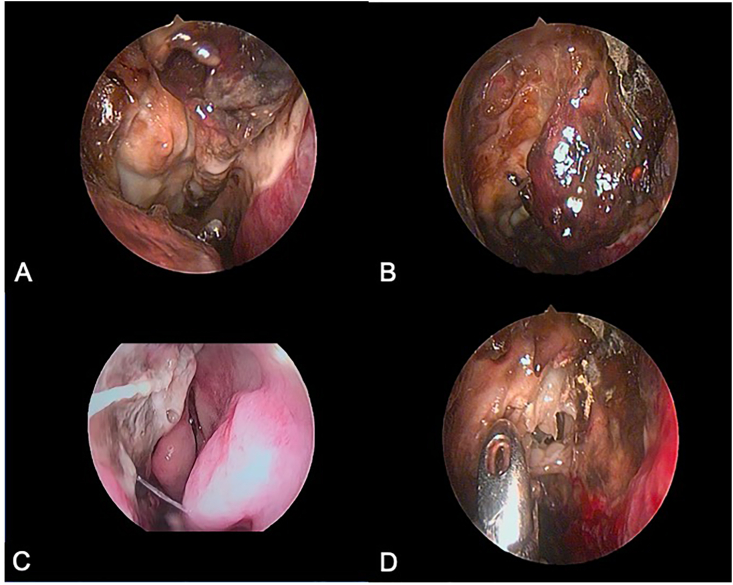
Fig. 2.
Nasal endoscopic images showing eschar over middle turbinate and slough over septum and medial wall of the maxillary sinus (A), eschar over middle turbinate and disease in cribriform area (B), diseased septum with normally appearing medial wall of the maxillary sinus (C) and obtaining biopsy from the necrotic area (D).
6.5. Culture
The material taken from biopsies or other specimen should be carefully managed so as not to be crushed because mucorales are fragile, and culture may, thus, come negative.25,26 Sabouraud dextrose agar with chloramphenicol and without cyclohexamide (to prevent inhibition) can be used for culture processing. Cultures should be done in two tubes that are incubated at 37 °C and at 22 °C.29,30 The growth of the mucorales tends to be rapid, with mycelial elements expanding to cover the entire culture media plate or tube in only a few (1–7) days (Fig. 2B).
Culture Identification: Culture identification is done either by conventional methods such as slide culture or lactophenol cotton blue preparations (Fig. 2C) or by Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF).31 Accurate species identification is important both for epidemiological purpose and antifungal susceptibility profile.
Antifungal Susceptibility Testing (AFS): AFS is done by broth micro dilution as per Clinical Laboratory Standard Institute (CLSI) guidelines (M 38-A2).32 AFS for mucormycosis with broth micro dilution, requires experience and should be performed only by personnel trained in mycology antifungal susceptibility testing. E-test has also been evaluated for AFS testing of mucorales. Overall agreement between E-test and the CLSI or EUCAST reference methods for AmB and posaconazole varies from ca. 70% to ca. 100%, according to the species tested.32
It is strongly recommended that AFS be done in all cases since antifungal susceptibility profiles differ between species.32,33 For example, R. oryzae, the most common isolate from specimen, may tend to exhibit in vitro resistance to posaconazole; Mucor circinelloides is less commonly isolated but shows greater susceptibility to posaconazole. Cunninghamella spp. tends to have a higher minimum inhibitory concentrations (MIC) to amphotericin B (AmB) and a higher overall mortality. Currently, AmB, posaconazole (POS) and isavuconazole (ISA) are considered to be the most active agents against Mucormycosis. Given the scarcity of antifungal agents, AFS can provide a window for the utilisation of other antifungals such as terbenafine and itraconazole that have also been found to have low MICs against some species of mucorales. In general, it has been seen that MIC values of Mucor species for posaconazole, isavuconazole, and itraconazole are higher than those for Rhizopus and the Lichtheimia.
6.6. Pathology
Histopathological examination complements the microbiological examination in diagnosing mucormycosis. Soft tissue invasion (mucosal penetration), destruction of cartilage or bone (nasal turbinate), necrosis and inflammation, are the observations that can help confirm clinical suspicion at an early stage of disease. The bulk of tissue received at this end provides an excellent substrate for further microbiological examination. These hyphae are fragile, and in some cases, culture may remain negative.27
It is recommended that the specimen is processed, embedded in paraffin blocks, cut as 4–6 μm sections, stained with Hematoxylin & Eosin stain and examined for fungal elements. Special stains like Periodic acid Schiff (PAS) and Gomori Methanamine Silver (MGS) are used to highlight the hyphae as dark magenta and black coloured structures, respectively. Rapid histological examination shows hyaline hyphae of 6–16 μm diameter, aseptate or partially septate, with ribbon like folding.25 Hyphal entrapment in necrotic tissue, angioinvasion, neutrophilic soft tissue infiltrate or granuloma with histiocytic giant cells, are the hallmark of mucormycosis.
7. What other investigations help in the diagnosis and management of ROCM?
7.1. Craniofacial imaging
Imaging of the face and cranium plays an important role in the early diagnosis, staging and follow up of patients with ROCM mucormycosis. Magnetic Resonance Imaging (MRI) with gadolinium (GAD) contrast is the overall modality of choice to assess such patients. Computed Tomography (CT), in addition or singularly, may be required to look specifically at bony involvement, surgical planning and three-dimensional reconstruction of the involved segments for rehabilitation purposes.
CT may show a nodular mucosal sinonasal thickening with absence of fluid levels and hyperdense content leading to erosions/remodelling of bony sinus walls. Presence of retroantral, facial and orbital fat stranding is indicative of an aggressive infection.34 In most patients, the extra-sinus involvement occurs with intact bones indicative of perineural/perivascular invasion; here, MRI scores over CT.35 In some patients, CT may show bone rarefaction, erosions, permeative destruction and septal perforation.
Because of a superior resolution, MRI provides a better visualization of the involved orbital soft tissue, infratemporal fossa, intracranial structures, perineural invasion and vascular compression or obstruction. MRI is also better than CT because iodinated contrast used in CT adds to kidney damage in these patients who are likely to be on nephrotoxic drugs such as amphotericin, etc.34 T2-weighted images show an isointense to mildly hypointense, heterogenous, or hyperintense sinonasal soft tissue lesions. Hyperintense mucosal thickening and intra-sinus hyperintense fluid may be seen; presence of a fluid level should always take into consideration the possibility of a secondary bacterial infection. Variable signal intensity, due to iron and manganese contained in the fungal elements, may be seen.36 Common sites of extra-sinus involvement are orbit and face, followed by orbital apex, masticator space, pterygopalatine fossa, skull base, cavernous sinus and brain parenchyma (with or without vascular involvement). These changes are more evident on T2 fat-suppressed images.
On GAD-contrast, patterns of enhancement may include either an intense homogenous, or heterogeneous, or complete central non-enhancement, with or without a thin irregular rim of peripheral enhancement. Enhancing nerves are a sign of perineural invasion. Extensive angioinvasion is considered as the main cause leading to vascular thrombosis and tissue necrosis. Gadolinium enhanced MRI is the radiological gold standard for the evaluation of orbital extension of disease. Enlargement of medial rectus muscle is an early sign, while fat stranding, loss of contrast enhancement, thickening and straightening of the optic nerve indicate significant involvement.
Restriction on DWI and corresponding hypointensity on ADC maps is noted in the involved areas, presumably a result of fungal angioinvasion, similar to that seen in other ischemic & necrotic lesions.37 For appropriate evaluation of infarcts or infarct-like lesions, MR angiography is advisable.
Two important signs have been noted in such patients. First, the “black turbinate sign” evident as non-enhancing dark and mottled turbinates38; second, the “guitar pick sign” (suggestive of an advanced disease) occurring as a result of tenting of posterior surface of globe from retrobulbar inflammatory soft tissue.39 (Fig. 3)
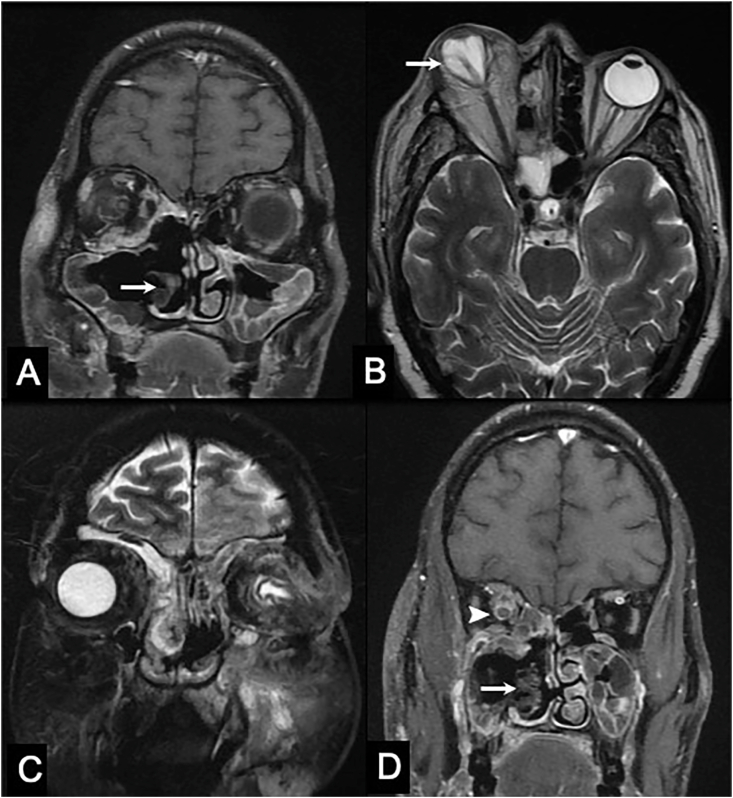
Fig. 3.
MRI of the brain showing mottled and non-enhancing right inferior turbinate, also known as the ‘black turbinate sign’ (A, coronal post-contrast T1-weighted image); tenting of posterior surface of right globe (the ‘Guitar Pick sign’) suggestive of an advanced disease (B, axial T2-weighted image); contiguous intracranial extension of infection into left frontal lobe via. cribriform plate, fovea ethmoidalis and orbital plate (C, coronal T2 fat-saturated image); peripheral enhancement of right optic nerve suggestive of perineural spread of the disease (D, coronal post-contrast T1-weighted image).
Once in a while, MR spectroscopy may aid in the diagnosis if the lesions bear semblance to demyelinating lesions.
7.2. Echocardiography
Cases with COVID-19 may be predisposed to thromboembolic phenomenon. It is, thus, advisable to get an echocardiography done in patients who are dyspnoeic (right-sided overload as a result of pulmonary complications) and those who are labelled having stroke or stroke-like lesions (cardioembolism).
7.3. Serum markers
There are no serum markers for the diagnosis of mucormycosis. However, in a few complicated cases or where the diagnosis is equivocal, galactomannan and 1,3 β D-glucan may be estimated to rule out concomitant or disguised Aspergillosis.
8. What are the diagnostic categories of ROCM?
The committee did not find an established scheme in this regard. A proposal reviewed in light of extant global guidelines for the diagnosis and management of Mucormycosis appeared suitable for application as it followed the most commonly used methods for diagnostic categorisation.16,25 The diagnostic categories may, therefore, be summarized as follows:
8.1. Possible ROCM
Typical symptoms and signs in appropriate clinical setting, but.
No supportive evidence on diagnostic nasal endoscopy and/or GAD-MRI/CT scan.
8.2. Probable ROCM
Clinical supportive evidence, plus.
Supportive diagnostic nasal endoscopy and/or GAD-MRI/CT scan, but.
No evidence on direct microscopy or culture or histopathology.
8.3. Definite ROCM
Clinical supportive evidence, plus.
Supportive diagnostic nasal endoscopy and/or GAD-MRI/CT scan, plus.
Confirmation on direct microscopy or culture or histopathology.
9. Can we stage ROCM?
An extensive review of literature was done to shortlist a method or scheme to stage ROCM. A 3-tiered staging system developed by Rupa et al.23 and a recently proposed 4-tiered system of staging16 were taken as currently available reference points for staging the disease.
In order to test the applicability of the available systems, 200 consecutive patients with C-ROCM were staged. Based on the analysis, the committee recommends that:
The 4-tiered system appears to be a practical and valid staging system and seems to have matured from the 3-tiered system of staging.
Major shortfalls of the 4-tiered staging noted by the committee were:
The highest stage does not necessarily imply that all other anatomical structures are involved; thus, the staging needs improvisation in suggesting the anatomical structures per se. This may lead to an alteration in the ordinal nature of staging but will pinpoint the relevant pathology.
A case with stage 4b (diffuse cavernous sinus involvement and/or cavernous sinus thrombosis) implies that the nasal mucosa, paranasal sinuses and orbits (all stages below the highest one listed) have been involved. On the contrary, rhino-cerebral forms (with orbital sparing) were seen in 7 patients; in addition, any cerebral parenchymal lesion (besides cavernous sinus) get ignored. Both staging systems, published previously, have this anomaly.
It was noted that the involvement of turbinates in more than 1/3rd patients did not necessarily follow the pattern of progression initiating from the middle turbinate.
A case with stage 3c implies that the involvement of orbital contents has occurred. While this is generally true isolated presentations of central retinal artery or ophthalmic artery occlusion were noted in 6 patients.
The committee recommends the use of a grid system for the precise localisation of disease process and staging. The application of this grid addresses the aforementioned deficiencies, especially where the highest listed stage may not necessarily mean involvement of all structures lower down, contiguous or non-contiguous (viz. rhinocerebral type).10,23 Revised and restructured staging system of ROCM has been shown in Table 3.
Table 3.
Revised and restructured staging of Rhino-Orbito-Cerebral Mucormycosis.
| Stage | Anatomical correlate | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Involvement of nasal mucosa | ||||||||||||||||||
|
|||||||||||||||||||
| Stage 2 | Involvement of paranasal sinuses | ||||||||||||||||||
|
|||||||||||||||||||
| Stage 3 | Involvement of the orbit | ||||||||||||||||||
|
|||||||||||||||||||
| Stage 4 | Involvement of the intracranial structures | ||||||||||||||||||
|
|||||||||||||||||||
| Suffix P | Involvement of Pterygopalatine fossa | ||||||||||||||||||
| Suffix I | Involvement of Infratemporal fossa | ||||||||||||||||||
| Suffix C | Cutaneous extension of local disease | ||||||||||||||||||
| Suffix D | Disseminated disease with isolation of mucormycosis from sites other than ROCM such as pulmonary, gastrointestinal, renal, cutaneous∗ | ||||||||||||||||||
| Prefix Lt/Rt | Aids in specifying the side of involvement and, if bilateral, whether one side is more involved than the other (Rt>LT, Lt>Rt, Lt = Rt) | ||||||||||||||||||
| ∗A differentiation should be made from cutaneous extension of the disease in ROCM versus pure cutaneous mucormycosis seen with contaminated cutaneous breaches | |||||||||||||||||||
| Staging Grid (mark the involved segment with a ‘X’) | |||||||||||||||||||
| 1a | Sup | Mid | Inf | 1b | Sup | Mid | Inf | 1c | Sup | Mid | Inf | 1d | Sup | Mid | Inf | ||||
| 2a | M | E | F | S | 2b | M | E | F | S | 2c | M | E | F | S | 2d | M | E | F | S |
| 3a | 3b | 3c | 3d | ||||||||||||||||
| 4a | 4b | 4c | 4d | ||||||||||||||||
| P | I | C | D | ||||||||||||||||
Other modifications recommended by the committee are:
The overall stage should be prefixed to denote the laterality (right or left/asymmetrical or symmetrical involvement).
The involved turbinates and sinuses should be named for better clarity.
Loosening of teeth is commonly seen. ‘Alveolar bone’, thus, should be incorporated in the stage 2c as an anatomic correlate.
Coexistent or newly detected mucormycosis may be seen in patients with ROCM at the same or another site. It is therefore recommended to have a category (Suffix D) to record other sites of mucormycosis.
The committee also recommends that the status of pterygopalatine fossa (Suffix P), being an important conduit for spread of inflammation, be listed in the staging-system to aid in defining the progression of the disease.23,40
Extension of mucormycosis in the infratemporal fossa was noticed to result in facial nerve deficits, temporomandibular involvements, vascular breaches, etc. The committee recommends that the same (Suffix I) should figure in the staging system.
Similarly, cutaneous extension of disease (Suffix C) must be mentioned. It may be noted that cutaneous extension is different from ‘cutaneous mucormycosis’ that is seen with contamination of cutaneous breaches.
The committee recommends that the ‘suffix’ be used independent of individual stages (as shown in the Table 3) since the denotations listed may get affected unrelated with the stage of disease.
10. Prevention of C-ROCM based on susceptibility and target associations
The recommendations of the committee have been summarized in Table 4.
Table 4.
Prevention of COVID-19 associated Rhino-Orbito-Cerebral Mucormycosis (C-ROCM) based on susceptibility and target associations.
| Summary of preventive aspects of C-ROCM |
|---|
|
|
|
|
11. Medical management
11.1. Specific management with anti-fungal agents
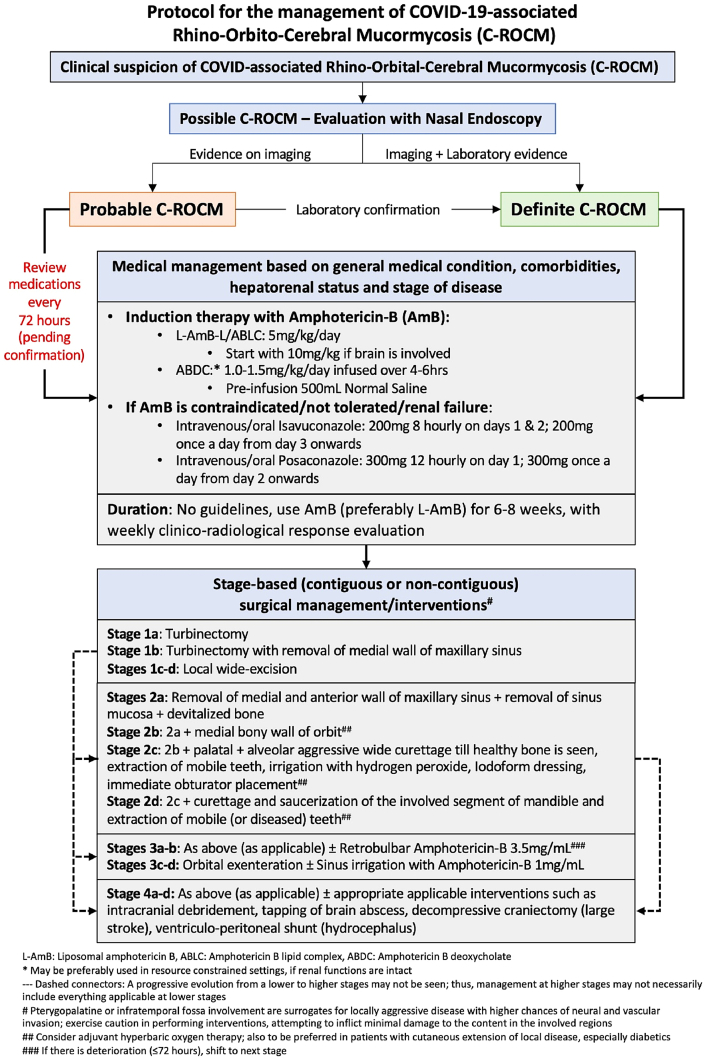
Early commencement of treatment in patients with ROCM is very important; it has been shown that the survival doubles (61%) if the treatment is initiated within the first 12 days of symptoms.1 The committee recommends an early treatment and advocates that it may be initiated in probable cases too, pending microbiological confirmation (Fig. 4).
Fig. 4.
Algorithm depicting the proposed protocol for the management of patients with C-ROCM.
The first-line drug of choice for the treatment of Mucormycosis is Liposomal-Amphotericin B (L-AmB).25 L-AmB is the preferred AmB due to a lower incidence of nephrotoxicity, higher tissue penetrability and higher tissue concentration.41,42 Other available formulations of Amphotericin-B, Amphotericin B lipid complex (ABLC) and Amphotericin B deoxycholate (ABDC, conventional AMB), may also be used in situations with either shortage or unavailability of L-AmB.41 Data on the use of amphotericin B colloidal dispersion is sparse; coupled with its poor availability or unavailability, its preference over the aforementioned forms of AmB cannot be recommended.
ABDC, on the other hand, has been used extensively, has better availability and is the cheapest of the lot. ABDC can be used in the absence of L-AmB but renal parameters and potassium levels need to be monitored. It can be used if there is < 1.5-fold rise in creatinine from baseline or the decrease in estimated GFR is <25% within 7 days of initiation of ABDC. If the tolerance is good, use it for a minimum for 2 weeks, up to 4–6 weeks, before stepping down. As a L-AmB sparing therapy, patients can be initiated directly on ABDC; only when there is a ≥1.5-fold rise in creatinine from baseline or the decrease in estimated GFR is >25% within 7 days of its initiation, a shift must be made.42
In the event that AmB preparations cannot be given as the first line of therapy due to contraindications or non-availability, either parenteral or oral forms of Isavuconazole are recommended as an alternative. This is an FDA approved agent for the treatment of Mucormycosis. Posaconazole has also been used for treatment though it is preferred as a prophylactic agent for mucormycosis in transplant patients.43
Early step-down therapy to Isavuconazole or Posaconazole (before 4–6 weeks of any AmB) should be considered if any form of amphotericin is not tolerated, or if there is > 2-fold rise in creatinine from baseline or if the decrease in estimated GFR is >50% suggestive of acute kidney injury.43 Isavuconazole, has many advantages compared to other azoles, as it has linear pharmacokinetics, has lesser drug-drug interactions and lesser toxicity. Hepatotoxicity, skin and ocular side-effects, or QT prolongations are not seen; since there is no cyclodextrin in the intravenous formulation, dose adjustment is usually not an issue in patients with renal or hepatic involvement. It also has an excellent oral bioavailability, unlike Posaconazole which has a better absorption in an acidic mileu.44 An overlap between the first line and the step-down therapy should be done so that therapeutic drug levels are achieved without loss of efficacy of the drug; this is especially true for Posaconazole where therapeutic drug levels need to be monitored.
A summary of the main antifungal agents is provided in Table 5.
Table 5.
Systemic antifungal medications for Mucormycosis.
| Medication | Indications | Dosage and delivery | Side effects | Remarks |
|---|---|---|---|---|
Amphotericin B
|
Initial therapy |
|
|
|
| Posaconazole | Step down or Salvage therapy |
|
|
|
| Isavuconazole | Step down or salvage therapy |
|
Nausea, vomiting, diarrhoea, headache QTc prolongation, hepatotoxicity, oedema and hypokalaemia |
|
AmB-Amphotericin B, IV-Intravenous.
The committee made a conscious note of L-AmB-based intermittent dosing regimen to treat invasive fungal infections but does not recommend its usage outside of a research setting.45
Other than these two novel azoles, Itraconazole and Terbinafine have also been tested for their efficacy. Besides being sensitive to Posaconazole, Lichtheimia and Syncephalastrum species have shown susceptibility to both Itraconazole and Terbinafine. On the other hand, Cunninghamella species have been reported to be resistant to Posaconazole and Itraconazole but sensitive to Terbinafine. In this era of precision medicine, these findings attest that species identification and AFS should be done, wherever feasible; besides providing a rationalised treatment, the high cost (associated with L-AmB, Isavuconazole and Posaconazole) and drug shortage can also be addressed in resource constrained setups.32,33,46
Combination therapy of other antifungals, triazoles and echinocandins, with polyenes have not shown to be of advantage. Similarly use of deferasirox, an iron chelator, with L-AmB has also not demonstrated much promise in vivo. The committee recommends against the use of combinations except its usage as a salvage therapy in the hands of an expert.25,33,47,48
11.2. Glycaemic control
The single most important issue in managing a patient with mucormycosis is appropriate blood glucose control.25,47,49
Knowledge of basic principles of preparing medications, such as use of dextrose solution for lyophilized L-AmB, may help in appropriate glucose monitoring.
Diabetic ketoacidosis is an independent risk factor and Insulin therapy is recommended for proper glycaemic control. Concomitant use of sodium bicarbonate has been suggested to help such patients irrespective of the severity of acidosis.47,50
11.3. Secondary bacterial infections
Secondary bacterial infections may occur in patients with mucormycosis. In view of COVID-19, one should be especially vigilant in evaluating lungs. Blood and urine cultures along with sampling of the respiratory tract, or samples from the endotracheal tubes, must be sent regularly if there is pyrexia, unexplained leucocytosis or features not logically explained by Mucormycosis per se. The treatment must be guided by drug-susceptiblility results and the local antibiotic policies.
Some mucormycosis species are known to have a symbiotic relationship with certain bacteria and eradication of these can be helpful in the treatment. Rhizopus microsporus, which is one of the common mucorales, is known to be colonized by bacterial endosymbionts of the genera Burkholderia and Ralstonia. Animal studies have shown that treating these symbionts with ciprofloxacin can decrease the growth of these fungi.51
11.4. Management of neurological complications
Neurological involvement in C-ROCM can manifest in several forms such as nerve palsies (optic, oculomotor complex, trigeminal, facial, eighth), cavernous sinus involvement, local meningeal reactions (basal regions), extra-axial collections, cerebritis, cerebral abscess, large artery stroke or multiple diffusion restricted lesions (infarcts or infarct-like), or hydrocephalus.
Being an infective process, that in addition is angioinvasive in nature, thrombolytic therapy is not indicated in patients presenting with stroke. Safety of mechanical thrombectomy also seems doubtful. Decompressive craniectomy may, however, be done in large artery infarcts or space-occupying lesions to reduce intracranial pressure (ICP).
The management of (septic) cerebral venous disease, especially cavernous sinus thrombosis, in patients with C-ROCM is fraught with complications. It has been proposed that thrombosis prevents the spread of infection; therefore, a school of thought advocates against the use of anticoagulants, that otherwise form the mainstay of management in aseptic states. In addition, risk of intracranial as well as systemic haemorrhage remains due to angioinvasive nature of the fungus. There are no trials; certain conclusions, however, can be drawn to aid in its management.52, 53, 54
Low molecular weight heparin seems to be better than conventional heparin in terms of tolerance, ease of administration and bleeding complications.
Prophylactic dose (1 I·U/kg body weight of enoxaparin or equivalent) of LMWH may be carried forward with caution in those patients of COVID-19 who had/have deranged coagulation parameters or are at an increased risk. One must be vigilant in patients with meningitis ± cerebritis where an increased risk of haemorrhage is expected.
Routine use of antibiotics is not indicated in such cases.
There appears to be no role of surgical drainage of the cavernous sinus.
Use of anti-epileptic medication.
Prophylactic use of anti-epileptic medication is not recommended; any out-of-setting usage should require a specialist's approval.
Either of Levetiracetam or Lacosamide may be used as they have minimal drug interactions and are available in intravenous as well as per-oral forms.
12. Surgical intervention/management
There are no randomized studies for the management of ROCM; the recommendations made are essentially based on expert's experience and consensus.16,23,25 A protocol for surgical interventions has been provided as an algorithm (Fig. 4). Overall, surgical debridement, depending on the site, continues to be an important part in the management of C-ROCM as a part of the standard of care. The scope of surgery may vary from local resection to radical resection of the involved segment/area.
12.1. Otorhinolaryngologist's perspective
Endoscopic approach is preferred in patients with early limited disease or those with significant comorbidities. Studies suggest that an open surgery be reserved for an extensive disease, particularly with involvement of CNS or orbits.25,55 Surgeries in such cases include maxillectomy, orbital exenteration and or craniofacial resection. Recent data, however, suggests that radical surgeries do not result in any statistically significant improvement in survival, especially in patients with limited life expectancy. Debridement of necrotic tissue in rapidly progressive disease, particularly in diabetics, may increase chances of survival.56
As a rule of thumb, the extent of debridement is best determined by the ooze of fresh blood from the region of involvement. This concept also holds weight in other regions involved with mucormycosis.
It is emphasized that mortality from ROCM in severely immunocompromised patients is still very high despite of surgical intervention.57
12.2. Oral and maxillofacial surgeon's perspective
Radical surgical debridement of the diseased tissue harbouring infectious fungal elements, is essential for the successful management of the disease, as it helps to prevent the systemic spread of infection by removal of the nidus. There may sometimes be a need for a repeated debridement based on the disease progression. Local irrigation with AmB or topical hydrogen peroxide helps. Iodoform pack allows secondary granulation and should be changed every 72 h after irrigation of the wound.58 The defect should be closed with a custom-made acrylic obturator to prevent local food enlargement and further infection.59
In cases with larger necrotic lesions, signifying an aggressive angio-invasive infection and an advanced spread of disease, more aggressive surgical extirpation may involve radical resection with partial or total maxillectomy or mandibulectomy, as required.
12.3. Ophthalmologist's perspective
Orbital involvement in ROCM occurs due to either a direct extension of the disease affecting the paranasal sinuses or through superior orbital fissure or pterygopalatine fossa. Angioinvasion of the Central Retinal artery may lead to Central Retinal artery occlusion (CRAO) while involvement of the cavernous sinus may result in impaired venous flow.
Orbital disease, in the initial stage, consists of unilateral retro-orbital pain (continuous or associated with ocular movements), pre-septal cellulitis, conjunctival chemosis and diplopia. An advanced disease is suggested by the presence of proptosis and total ophthalmoplegia, with or without eyelid discolouration and necrosis. Vision loss accompanies the spread of disease. Sudden onset painless loss of vision, as a result of CRAO, may also occur. A contralateral spread of disease usually indicates cavernous sinus involvement.3,60
Case specific therapy is guided by the extent of disease. Early disease, with preserved vision, may respond to transcutaneous retrobulbar Amphotericin (TRAMB) injection, 1 ml of 3.5 mg/ml Amphotericin for 3 days. This may also be given in patients undergoing sinus debridement where the orbital wall is breached. Transient orbital inflammation with amphotericin may be seen in recipients.61, 62, 63, 64
Orbital debridement is indicated for breach of medial wall with medial orbital disease. Disease beyond medial orbit warrants exenteration.63,64
Vision loss due to CRAO, in the absence of orbital disease, does not warrant any significant orbital debridement and may be dealt with along the sinus-interface.
12.4. Neurosurgeon's perspective
Neurosurgical interventions may be needed in case of raised ICP due to hemispheric stroke (decompressive craniectomy), hydrocephalus (CSF diversion procedure), abscess/granulomas (stereotactic/ultrasound guided aspiration/biopsy), helping ENT and Eye surgeons (maximal debridement specifically the contiguously spread fungal elements), and invasive elements in brain parenchyma (maximal safe resection).24
12.5. Reconstructive (plastic surgery) surgeon's perspective
Breathing pure oxygen under high pressure environment greatly increases the partial pressure of oxygen inhaled. Hyperbaric Oxygen Therapy (HBOT) works on every stage by elevating diffusion rate, diffusion distance, and solubility based on the principles of physics Dalton's law and Henry's law.65, 66, 67, 68, 69
Hyperbaric therapy has been tried in patients with ROCM with cerebral extension as an adjunctive therapy with promising results.68 The beneficial effects have been attributed to reduction in tissue hypoxia and acidosis that accompany vascular invasion by the fungus.69
Daily sessions of 45 min of HBOT at 1.5 ATM pressure for 2–3 weeks is recommended in patients undergoing extensive necrotic tissue debridement.
12.6. Anaesthesiologist's perspective
ROC mucormycosis in patients with COVID-19 poses a distinct challenge for anaesthesiologists. Anaesthetic considerations include patient's comorbidities, amphotericin-induced renal and cardiovascular changes, and COVID-19-related multi-organ derangements. As surgical debridement plays a very vital role in the prognosis of ROC mucormycosis, preoperative assessment and optimization is of paramount importance in perioperative management.70
It is advised that the anaesthesia providers should necessarily review the renal, electrolyte, coagulopathic, hemodynamic, and respiratory abnormalities when the patient in question is receiving amphotericin B therapy.71 The level of anxiety is these patients is usually high; care must be taken to provide them preoperative anxiolysis.72 COVID-19 essentially being a respiratory ailment, perioperative ABG analysis is mandatory.73
Basics of risk stratification (as per American society of Anaesthesiology) and appropriate risk consent, not specifically linked to COVID-19, should be followed. Besides the regular monitoring, invasive monitoring should be done for all patients with hemodynamic compromise. Difficult intubation cart should be ready and necessary precaution should be taken for a potential difficult airway caused by fungal debris and supraglottic oedema in patients with ROC mucormycosis.74 Use of video laryngoscope is preferable, if available.
Peroperatively, COVID-19 associated deranged lung compliance and pulmonary functions may necessitate close monitoring of plateau and peak pressures. Hypokalaemia due to amphotericin use may enhance effect of skeletal muscle relaxant. Use of amphotericin, especially with concurrent steroid use, provides a perfect milieu for the development of hypokalaemia that may enhance the effect of skeletal muscle relaxant; cardiac arrythmias (all categories) need to be monitored, in addition. Inhalational agents can be used in these patients.75
Postoperatively, patients with a respiratory rate of >12/minute, tidal volume of >5 ml/kg, Spo2 >95% and following verbal commands are the best candidates for extubation before shifting to post-operative care unit. Hemodynamically unstable patients, with no or minimal respiratory effort, should be shifted to post-operative care unit in an intubated condition. Depending on the postoperative status of the patient on the background of comorbidities and the nature of surgery performed, minimal versus extensive, the post-operative care unit may constitute either an ICU or a step-down unit.
12.7. Other surgical interventions
Presence of a pulmonary cavitary lesion (fungal ball) or extensive involvement of the lung, secondary to Mucormycosis, may necessitate resection of the lesion or lobectomy (with or without pneumonectomy) to improve the outcome.76 Overall, the prognosis of such patients is worse than that seen with pulmonary aspergillosis.76, 77, 78
It may be highlighted that dual-pathology (pulmonary aspergillosis in patients with C-ROCM) has been noted in at least 4 of our evaluated patients; thus, all lesions in the lung may not be related to Mucormycosis alone. A recent report has also noted dual-pathology, within the same site.79 Thus, all efforts ranging from serology for aspergillus to tissue diagnosis must be made to address the diagnostic dilemma. The medical management of pulmonary aspergillosis may be done as per recommendations.78
13. COVID-19 associated mucormycosis may be summarized as
ROCM is a rapidly progressive disease with an average mortality of around 40%.
The unprecedented rise in cases of C-ROCM may be related to either agent-related or host-related factors.
Diabetes, historically, and inadvertent use of corticosteroids, in the current scenario, appear to be the commonest predisposing factors for the development of C-ROCM.
Prevention of C-ROCM essentially revolves around judicious use of corticosteroids and managing hyperglycaemia effectively.
A high index of suspicion should be there to diagnose C-ROCM.
Diagnostic nasal endoscopy forms the first step in diagnosing C-ROCM. Prompt transport of the specimen obtained helps in initiating an early treatment.
All specimen should be subjected to species identification and AFST, if available.
A revised staging system is proposed to aid in site-and-side specific categorisation along with the extent of the disease.
The primary drug of choice in managing patients with C-ROCM is Amphotericin B, preferably liposomal, followed by isavuconazole and posaconazole. More data on itraconazole and terbinafine is required to recommend its usage.
Management of mucormycosis is incomplete without an effective debridement strategy depending on the region involved.
Follow up surgeries directed towards rehabilitation and reconstruction should be undertaken to improve long-term outcome.
Funding
None.
Declaration of competing interest
None of the authors have any competing interests.
Contributor Information
Hardeep Singh Malhotra, Email: drhsmalhotra@gmail.com.
Prashant Gupta, Email: prashantgupta46@hotmail.com.
Divya Mehrotra, Email: divyamehrotra@hotmail.com.
Himanshu Dandu, Email: dr.himanshu.reddy@gmail.com.
Neera Kohli, Email: drneerakohli@gmail.com.
Veerendra Verma, Email: drveerendraverma@rediffmail.com.
Apjit Kaur, Email: apjit@rediffmail.com.
Neeraj Kumar, Email: drneeraj2903@gmail.com.
Vikas Prabhu, Email: vikasneurokgmu@gmail.com.
Manish Kumar Singh, Email: manishsingh@kgmcindia.edu.
Riddhi Jaiswal, Email: riddhiadvay@gmail.com.
Brijesh Mishra, Email: drbrijeshmishra@gmail.com.
Bal Krishna Ojha, Email: balkrishnaojha@kgmcindia.edu.
Nitin Dutt Bhardwaj, Email: drnitindb@gmail.com.
Virendra Atam, Email: v_atam@yahoo.com.
Bipin Puri, Email: bipinpuri@gmail.com.
References
- 1.Vaughan C., Bartolo A., Vallabh N., Leong S.C. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin Otolaryngol. 2018;43(6):1454–1464. doi: 10.1111/coa.13175. Epub 2018 Aug 8. PMID: 29947167. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Post-COVID conditions. Updated Apr. 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html; last accessed on 27 June 2021.
- 3.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel) 2020;6(4):265. doi: 10.3390/jof6040265. PMID: 33147877; PMCID: PMC7711598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash H., Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523. doi: 10.3390/microorganisms9030523. PMID: 33806386; PMCID: PMC8000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarti A., Das A., Sharma A. Ten years' experience in zygomycosis at a tertiary care centre in India. J Infect. 2001;42(4):261–266. doi: 10.1053/jinf.2001.0831. PMID: 11545569. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A., Das A., Mandal J. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44(4):335–342. doi: 10.1080/13693780500464930. PMID: 16772227. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti A., Chatterjee S.S., Das A. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85(1009):573–581. doi: 10.1136/pgmj.2008.076463. PMID: 19892892. [DOI] [PubMed] [Google Scholar]
- 8.Roden M.M., Zaoutis T.E., Buchanan W.L. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. Epub 2005 Jul 29. PMID: 16080086. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez E., Sutton D.A., Cano J. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol. 2009;47(6):1650–1656. doi: 10.1128/JCM.00036-09. Epub 2009 Apr 22. PMID: 19386856; PMCID: PMC2691065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong W., Keighley C., Wolfe R. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. Epub 2018 Jul 21. PMID: 30036666. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Franco B., Nava-Villalba M., Medina-Guerrero E.O. Host-Pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in diabetes mellitus. Curr Trop Med Rep. 2021:1–12. doi: 10.1007/s40475-020-00222-1. Epub ahead of print. PMID: 33500877; PMCID: PMC7819772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamilos G., Lewis R.E., Lamaris G., Walsh T.J., Kontoyiannis D.P. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. 2008;52(2):722–724. doi: 10.1128/AAC.01136-07. Epub 2007 Nov 19. PMID: 18025115; PMCID: PMC2224765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim A.S., Spellberg B., Walsh T.J., Kontoyiannis D.P. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54:S16–S22. doi: 10.1093/cid/cir865. PMID: 22247441; PMCID: PMC3286196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford F.A., Matthies N., Cseresnyés Z., Figge M.T., Hassan M.I.A., Voigt K. Expression patterns in reductive iron assimilation and functional consequences during phagocytosis of Lichtheimia corymbifera, an emerging cause of mucormycosis. J Fungi (Basel) 2021;7(4):272. doi: 10.3390/jof7040272. PMID: 33916756; PMCID: PMC8065604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid G., Lynch J.P., 3rd, Fishbein M.C., Clark N.M. Mucormycosis. Semin Respir Crit Care Med. 2020;41(1):99–114. doi: 10.1055/s-0039-3401992. Epub 2020 Jan 30. PMID: 32000287. [DOI] [PubMed] [Google Scholar]
- 16.Honavar S.G. Code mucor: guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69(6):1361–1365. doi: 10.4103/ijo.IJO_1165_21. PMID: 34011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedict K., Richardson M., Vallabhaneni S., Jackson B.R., Chiller T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect Dis. 2017;17(12):e403–e411. doi: 10.1016/S1473-3099(17)30443-7. Epub 2017 Jul 31. PMID: 28774697; PMCID: PMC5712439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peckham D., Williams K., Wynne S., Denton M., Pollard K., Barton R. Fungal contamination of nebuliser devices used by people with cystic fibrosis. J Cyst Fibros. 2016;15(1):74–77. doi: 10.1016/j.jcf.2015.06.004. Epub 2015 Jun 20. PMID: 26104996. [DOI] [PubMed] [Google Scholar]
- 19.Ceriello A. Hyperglycemia and COVID-19: what was known and what is really new? Diabetes Res Clin Pract. 2020;167:108383. doi: 10.1016/j.diabres.2020.108383. Epub 2020 Aug 25. PMID: 32853690; PMCID: PMC7445137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 2021;7(4):298. doi: 10.3390/jof7040298. PMID: 33920755; PMCID: PMC8071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiterer M., Rajan M., Gómez-Banoy N. Hyperglycemia in acute COVID-19 is characterized by adipose tissue dysfunction and Insulin resistance. medRxiv [Preprint] 2021 doi: 10.1101/2021.03.21.21254072. Mar 26:2021.03.21.21254072. PMID: 33791724; PMCID: PMC8010756. [DOI] [Google Scholar]
- 22.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. Epub 2020 Apr 28. PMID: 32346093; PMCID: PMC7187672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupa V., Maheswaran S., Ebenezer J., Mathews S.S. Current therapeutic protocols for chronic granulomatous fungal sinusitis. Rhinology. 2015;53(2):181–186. doi: 10.4193/Rhino14.183. PMID: 26030043. [DOI] [PubMed] [Google Scholar]
- 24.Chikley A., Ben-Ami R., Kontoyiannis D.P. Mucormycosis of the central nervous system. J Fungi (Basel) 2019;5(3):59. doi: 10.3390/jof5030059. PMID: 31288475; PMCID: PMC6787740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Mucormycosis ECMM MSG Global Guideline Writing Group Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. Epub 2019 Nov 5. PMID: 31699664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh V.P., Bansal C., Kaintura M. Sinonasal mucormycosis: a to Z. Indian J Otolaryngol Head Neck Surg. 2019;71:1962–1971. doi: 10.1007/s12070-018-1384-6. Epub 2018 May 4. PMID: 31763277; PMCID: PMC6848679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh T.J., Gamaletsou M.N., McGinnis M.R., Hayden R.T., Kontoyiannis D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis) Clin Infect Dis. 2012;54(Suppl 1):S55–S60. doi: 10.1093/cid/cir868. PMID: 22247446. [DOI] [PubMed] [Google Scholar]
- 28.CLSI . 2nd ed. Vol. 54. Clinical Laboratory and Standard Institute; 2021. Principles and Procedures for Detection and Culture of Fungi in Clinical Specimens. (CLSI Guideline M). [Google Scholar]
- 29.Lackner M., Caramalho R., Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9(5):683–695. doi: 10.2217/fmb.14.23. PMID: 24957094. [DOI] [PubMed] [Google Scholar]
- 30.Bala K., Chander J., Handa U., Punia R.S., Attri A.K. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. 2015;53(3):248–257. doi: 10.1093/mmy/myu086. Epub 2015 Jan 13. PMID: 25587084. [DOI] [PubMed] [Google Scholar]
- 31.Sanguinetti M., Posteraro B. Identification of molds by matrix-assisted laser desorption ionization-time of Flight mass spectrometry. J Clin Microbiol. 2017;55(2):369–379. doi: 10.1128/JCM.01640-16. Epub 2016 Nov 2. PMID: 27807151; PMCID: PMC5277505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reference C.L.S.I. second ed. 38-A2. Clinical Laboratory and Standard Institute; Wayne PA: 2008. Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard. (CLSI Document M). [Google Scholar]
- 33.Dannaoui E. Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50(5):617–621. doi: 10.1016/j.ijantimicag.2017.08.010. Epub 2017 Aug 9. PMID: 28802855. [DOI] [PubMed] [Google Scholar]
- 34.Lone P.A., Wani N.A., Jehangir M. Rhino-orbito-cerebral mucormycosis: magnetic resonance imaging. Indian J Otol. 2015;21:215–218. [Google Scholar]
- 35.Silverman C.S., Mancuso A.A. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. AJNR Am J Neuroradiol. 1998;19(2):321–325. PMID: 9504486. [PMC free article] [PubMed] [Google Scholar]
- 36.Parsi K., Itgampalli R.K., Vittal R., Kumar A. Perineural spread of rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. Ann Indian Acad Neurol. 2013;16(3):414–417. doi: 10.4103/0972-2327.116921. PMID: 24101833; PMCID: PMC3788297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaviani P., Schwartz R.B., Hedley-Whyte E.T. Diffusion-weighted imaging of fungal cerebral infection. AJNR Am J Neuroradiol. 2005;26(5):1115–1121. PMID: 15891169; PMCID: PMC8158608. [PMC free article] [PubMed] [Google Scholar]
- 38.Safder S., Carpenter J.S., Roberts T.D., Bailey N. The "Black Turbinate" sign: an early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31(4):771–774. doi: 10.3174/ajnr.A1808. Epub 2009 Nov 26. PMID: 19942703; PMCID: PMC7964235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Indiran V. Guitar pick sign" on MRI. Indian J Ophthalmol. 2019;67(10):1737. doi: 10.4103/ijo.IJO_404_19. PMID: 31546546; PMCID: PMC6786219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tashi S., Purohit B.S., Becker M., Mundada P. The pterygopalatine fossa: imaging anatomy, communications, and pathology revisited. Insights Imaging. 2016;7(4):589–599. doi: 10.1007/s13244-016-0498-1. Epub 2016 May 26. PMID: 27230518; PMCID: PMC4956626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamill R.J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73(9):919–934. doi: 10.1007/s40265-013-0069-4. PMID: 23729001. [DOI] [PubMed] [Google Scholar]
- 42.Ricci Z., Cruz D., Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538–546. doi: 10.1038/sj.ki.5002743. Epub 2007 Dec 26. PMID: 18160961. [DOI] [PubMed] [Google Scholar]
- 43.Sipsas N.V., Gamaletsou M.N., Anastasopoulou A., Kontoyiannis D.P. Therapy of mucormycosis. J Fungi (Basel) 2018;4(3):90. doi: 10.3390/jof4030090. PMID: 30065232; PMCID: PMC6162664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spellberg B., Ibrahim A.S. Recent advances in the treatment of mucormycosis. Curr Infect Dis Rep. 2010;12(6):423–429. doi: 10.1007/s11908-010-0129-9. PMID: 21308550; PMCID: PMC2947016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Peppel R.J., Schauwvlieghe A., Van Daele R. Outpatient parenteral antifungal therapy (OPAT) for invasive fungal infections with intermittent dosing of liposomal amphotericin B. Med Mycol. 2020;58(7):874–880. doi: 10.1093/mmy/myz134. PMID: 31965178; PMCID: PMC7527269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomes M.Z., Lewis R.E., Kontoyiannis D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011;24(2):411–445. doi: 10.1128/CMR.00056-10. PMID: 21482731; PMCID: PMC3122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skiada A., Lass-Floerl C., Klimko N., Ibrahim A., Roilides E., Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93–101. doi: 10.1093/mmy/myx101. PMID: 29538730; PMCID: PMC6251532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong W., Keighley C., Wolfe R. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(5):589–597. doi: 10.1016/j.ijantimicag.2019.01.002. Epub 2019 Jan 10. PMID: 30639526. [DOI] [PubMed] [Google Scholar]
- 49.Corzo-León D.E., Chora-Hernández L.D., Rodríguez-Zulueta A.P., Walsh T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56(1):29–43. doi: 10.1093/mmy/myx017. PMID: 28431008. [DOI] [PubMed] [Google Scholar]
- 50.Gebremariam T., Lin L., Liu M. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest. 2016;126(6):2280–2294. doi: 10.1172/JCI82744. Epub 2016 May 9. PMID: 27159390; PMCID: PMC4887168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itabangi H., Sephton-Clark P.C., Zhou X. Environmental interactions with amoebae as drivers of bacterial-fungal endosymbiosis and pathogenicity. bioRxiv. 2020:584607. [Google Scholar]
- 52.Khatri I.A., Wasay M. Septic cerebral venous sinus thrombosis. J Neurol Sci. 2016;362:221–227. doi: 10.1016/j.jns.2016.01.035. Epub 2016 Jan 19. PMID: 26944152. [DOI] [PubMed] [Google Scholar]
- 53.Dinkin M., Patsalides A., Ertel M. Diagnosis and management of cerebral venous diseases in neuro-ophthalmology: ongoing controversies. Asia Pac J Ophthalmol (Phila). 2019;8(1):73–85. doi: 10.22608/APO.2018239. Epub 2019 Jan 23. PMID: 30672173. [DOI] [PubMed] [Google Scholar]
- 54.Caranfa J.T., Yoon M.K. Septic cavernous sinus thrombosis: a review. Surv Ophthalmol. 2021;(21) doi: 10.1016/j.survophthal.2021.03.009. Epub ahead of print. PMID: 33831391. [DOI] [PubMed] [Google Scholar]
- 55.Kasapoglu F., Coskun H., Ozmen O.A., Akalin H., Ener B. Acute invasive fungal rhinosinusitis: evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol Head Neck Surg. 2010;143(5):614–620. doi: 10.1016/j.otohns.2010.08.017. PMID: 20974328. [DOI] [PubMed] [Google Scholar]
- 56.Zuniga M.G., Turner J.H. Treatment outcomes in acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):242–248. doi: 10.1097/MOO.0000000000000048. PMID: 24756031. [DOI] [PubMed] [Google Scholar]
- 57.Davoudi S., Kumar V.A., Jiang Y., Kupferman M., Kontoyiannis D.P. Invasive mould sinusitis in patients with haematological malignancies: a 10 year single-centre study. J Antimicrob Chemother. 2015;70(10):2899–2905. doi: 10.1093/jac/dkv198. Epub 2015 Jul 17. PMID: 26188039. [DOI] [PubMed] [Google Scholar]
- 58.Mizokami F., Murasawa Y., Furuta K., Isogai Z. Iodoform gauze removes necrotic tissue from pressure ulcer wounds by fibrinolytic activity. Biol Pharm Bull. 2012;35(7):1048–1053. doi: 10.1248/bpb.b11-00016. PMID: 22791151. [DOI] [PubMed] [Google Scholar]
- 59.Ramadorai A., Ravi P., Narayanan V. Rhinocerebral mucormycosis: a prospective analysis of an effective treatment protocol. Ann Maxillofac Surg. 2019;9(1):192–196. doi: 10.4103/ams.ams_231_18. PMID: 31293952; PMCID: PMC6585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen M., Honavar S.G., Sharma N., Sachdev M.S. COVID-19 and Eye: a review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69(3):488–509. doi: 10.4103/ijo.IJO_297_21. PMID: 33595463; PMCID: PMC7942063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirabayashi K.E., Kalin-Hajdu E., Brodie F.L., Kersten R.C., Russell M.S., Vagefi M.R. Retrobulbar injection of amphotericin B for orbital mucormycosis. Ophthalmic Plast Reconstr Surg. 2017;33(4):e94–e97. doi: 10.1097/IOP.0000000000000806. PMID: 27768642. [DOI] [PubMed] [Google Scholar]
- 62.Kalin-Hajdu E., Hirabayashi K.E., Vagefi M.R., Kersten R.C. Invasive fungal sinusitis: treatment of the orbit. Curr Opin Ophthalmol. 2017;28(5):522–533. doi: 10.1097/ICU.0000000000000394. PMID: 28505035. [DOI] [PubMed] [Google Scholar]
- 63.Ashraf D.C., Idowu O.O., Hirabayashi K.E. Outcomes of a modified treatment ladder algorithm using retrobulbar amphotericin B for invasive fungal rhino-orbital sinusitis. Am J Ophthalmol. 2021 Jun 8;(21):S0002–S9394. doi: 10.1016/j.ajo.2021.05.025. Epub ahead of print. PMID: 34116011. [DOI] [PubMed] [Google Scholar]
- 64.Naik M. Transcutaneous retrobulbar amphotericin B and exenteration in rhino-orbital cerebral mucor mycosis: do we know it all yet? TNOA J Ophthalmic Sci Res. 2021;59:131–132. [Google Scholar]
- 65.John B.V., Chamilos G., Kontoyiannis D.P. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–517. doi: 10.1111/j.1469-0691.2005.01170.x. PMID: 15966968. [DOI] [PubMed] [Google Scholar]
- 66.Guo D., Pan S., Wang M., Guo Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: two case reports. Undersea Hyperb Med. 2020;47(2):181–187. PMID: 32574433. [PubMed] [Google Scholar]
- 67.Senniappan K., Jeyabalan S., Rangappa P., Kanchi M. Hyperbaric oxygen therapy: can it be a novel supportive therapy in COVID-19? Indian J Anaesth. 2020;64(10):835–841. doi: 10.4103/ija.IJA_613_20. Epub 2020 Oct 1. PMID: 33437070; PMCID: PMC7791429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couch L., Theilen F., Mader J.T. Rhinocerebral mucormycosis with cerebral extension successfully treated with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg. 1988;114(7):791–794. doi: 10.1001/archotol.1988.01860190095032. PMID: 3382536. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson B.J., Mitchell T.G., Moon R., Camporesi E.M., Farmer J. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis. 1988;10(3):551–559. doi: 10.1093/clinids/10.3.551. PMID: 3393782. [DOI] [PubMed] [Google Scholar]
- 70.Marchetti A., Jayachandran A., Guha A. Post-traumatic invasive mucormycosis. J Intensive Care Soc. 2011;12:143–144. [Google Scholar]
- 71.Choleva A.J. Anesthetic management for lobectomy in a patient with coccidioidomycosis: a case report. AANA J (Am Assoc Nurse Anesth) 2010 Aug;78(4):321–325. PMID: 20879633. [PubMed] [Google Scholar]
- 72.Wali U., Balkhair A., Al-Mujaini A. Cerebro-rhino orbital mucormycosis: an update. J Infect Public Health. 2012;5(2):116–126. doi: 10.1016/j.jiph.2012.01.003. Epub 2012 Mar 27. PMID: 22541257. [DOI] [PubMed] [Google Scholar]
- 73.Kulkarni P.K., Reddy N.B., Shrinivas B., Takkalki V.V. Anesthetic considerations in the management of mucormycosis. Int J Med Publ Health. 2015;5(4) [Google Scholar]
- 74.Karaaslan E. Anesthetic management of rhinoorbitocerebral mucormycosis; Focus on challenges. J Mycol Med. 2019;29(3):219–222. doi: 10.1016/j.mycmed.2019.07.001. Epub 2019 Jul 26. PMID: 31399350. [DOI] [PubMed] [Google Scholar]
- 75.Barodka V.M., Acheampong E., Powell G. Antimicrobial effects of liquid anesthetic isoflurane on Candida albicans. J Transl Med. 2006;4:46. doi: 10.1186/1479-5876-4-46. PMID: 17094810; PMCID: PMC1664588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilos G., Samonis G., Kontoyiannis D.P. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693–702. doi: 10.1055/s-0031-1295717. Epub 2011 Dec 13. PMID: 22167397. [DOI] [PubMed] [Google Scholar]
- 77.Gao Y., Soubani A. Advances in the diagnosis and management of pulmonary aspergillosis. Adv Respir Med. 2019;87(6):231–243. doi: 10.5603/ARM.2019.0061. PMID: 31970725. [DOI] [PubMed] [Google Scholar]
- 78.Koehler P., Bassetti M., Chakrabarti A. European confederation of medical mycology; international society for human animal mycology; asia fungal working group; INFOCUS LATAM/ISHAM working group; ISHAM Pan africa mycology working group; European society for clinical Microbiology; infectious diseases fungal infection study group; ESCMID study group for infections in critically ill patients; interregional association of clinical Microbiology and antimicrobial chemotherapy; medical mycology society of Nigeria; medical mycology society of China medicine education association; infectious diseases working party of the German society for haematology and medical oncology; association of medical Microbiology; infectious disease Canada. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–e162. doi: 10.1016/S1473-3099(20)30847-1. Epub 2020 Dec 14. PMID: 33333012; PMCID: PMC7833078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson A.K., Ghazarian Z., Cendrowski K.D., Persichino J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. Epub 2021 Apr 7. PMID: 33842203; PMCID: PMC8025540. [DOI] [PMC free article] [PubMed] [Google Scholar]