Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by a disturbed redox balance and increased production of reactive oxygen species (ROS), which is believed to contribute to epithelial injury and fibrotic lung scarring. The main pulmonary sources of ROS include mitochondria and NADPH oxidases (NOXs), of which the NOX4 isoform has been implicated in IPF. Non-receptor SRC tyrosine kinases (SFK) are important for cellular homeostasis and are often dysregulated in lung diseases. SFK activation by the profibrotic transforming growth factor-β (TGF-β) is thought to contribute to pulmonary fibrosis, but the relevant SFK isoform and its relationship to NOX4 and/or mitochondrial ROS in the context of profibrotic TGF-β signaling is not known. Here, we demonstrate that TGF-β1 can rapidly activate the SRC kinase FYN in human bronchial epithelial cells, which subsequently induces mitochondrial ROS (mtROS) production, genetic damage shown by the DNA damage marker γH2AX, and increased expression of profibrotic genes. Moreover, TGF-β1–induced activation of FYN involves initial activation of NOX4 and direct cysteine oxidation of FYN, and both FYN and mtROS contribute to TGF-β–induced induction of NOX4. NOX4 expression in lung tissues of IPF patients is positively correlated with disease severity, although FYN expression is down-regulated in IPF and does not correlate with disease severity. Collectively, our findings highlight a critical role for FYN in TGF-β1–induced mtROS production, DNA damage response, and induction of profibrotic genes in bronchial epithelial cells, and suggest that altered expression and activation of NOX4 and FYN may contribute to the pathogenesis of pulmonary fibrosis.
Keywords: idiopathic pulmonary fibrosis, mitochondria, NOX4, redox signaling, SRC
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive interstitial lung disease with a median survival of 3–5 years, and its pathology is believed to be driven by continuous epithelial injury and altered fibroblast biology causing aberrant wound healing and scarring of the lung tissue, eventually leading to death from respiratory failure (1, 2). The growth factor transforming growth factor (TGF)-β is widely recognized as a main driver in the development of IPF (3). Although TGF-β is important in wound healing responses, chronic TGF-β production due to repetitive injury of the lung epithelium contributes to epithelial injury as well as features of epithelial-to-mesenchymal transition (EMT), thereby leading to aberrant wound healing responses. A key process in the pathology of IPF is augmented production of reactive oxygen species (ROS) (4, 5), which are believed to contribute to epithelial cell death as well as differentiation of pulmonary fibroblasts, two key processes in IPF development (6). Chronic ROS overload can cause progressive oxidative damage to DNA, proteins, and lipids, and increased chromosomal DNA damage, in association with telomere shortening, are recognized features of IPF (7–9). Nevertheless, therapeutic approaches to mitigate the effects of ROS, such as N-acetylcysteine, have so far not been successful (10), although alternative redox-based approaches have shown promise (11).
TGF-β1 is capable of inducing ROS production by increasing NADPH oxidase (NOX) 4 expression (12), as well as by promoting mitochondrial dysfunction (13, 14), thereby contributing to a redox imbalance in IPF (15). Indeed, NOX4 is highly up-regulated in the lungs of IPF patients (16–18) and NOX4 knockout mice are protected from the development of bleomycin-induced pulmonary fibrosis (18). Other studies have also implicated mitochondria-derived ROS in IPF (13, 19) and emerging studies suggest the importance of cross talk mechanisms between NOX4 and mitochondria (13, 20), although specific mechanisms are still largely unclear. In addition, whereas ROS can cause cellular damage, they can also function as second messenger(s) by regulating protein function by redox signaling (21).
SRC family kinases (SFKs) represent a family of non-receptor tyrosine kinases that are involved in various signaling pathways important for cellular homeostasis, including cell differentiation and proliferation (22, 23), processes that are often dysregulated in the pathogenesis of IPF (24). SFKs have also been implicated in EMT (25, 26), an important feature of aberrant wound healing. Moreover, general pharmacological inhibition of SFKs can inhibit collagen deposition and fibrotic lesions in experimental models of IPF (27–29). Normally, SFKs are primarily regulated by (de)phosphorylation events (30, 31), but their activation can also be regulated by redox-dependent mechanisms, due to the presence of cysteine residues (32, 33), independent of the dephosphorylation of Tyr-527 (34–36). However, the specific SFK isoform, and oxidative mechanisms involved in SFK activation in the context of epithelial TGF-β signaling are not clear.
Here, we show increased TGF-β1–mediated SFK activation as well as increased NOX4 expression in lung epithelial cells from IPF patients. Additionally, we describe a key function of the SFK FYN in NOX4-mitochondria cross talk as an important mechanism in profibrotic epithelial TGF-β1 responses as well as DNA damage responses, as potential contributors to IPF pathogenesis.
MATERIALS AND METHODS
Cell Culture and Treatments
Primary human bronchial epithelial (HBE) cells were obtained from endobronchial lining fluid (ELF) of IPF patients and non-IPF controls by minimally invasive bronchoscopic microsampling (BMS) during bronchoscopy from subsegmental airways as previously described (37). In total, 7 patients were enrolled in this study via recruitment at the Center for Interstitial and Rare Lung Diseases, Thoraxklinik at Heidelberg University Hospital (patient characteristics are presented in Supplemental Table S1 (all Supplemental material is available at: https://doi.org/10.6084/m9.figshare.13028138). All patients gave their written informed consent and the study was approved by the ethics committee of the Medical Faculty Heidelberg, Germany (S-538/2012). Primary HBE cells were centrifuged for 5 min at room temperature and 300 × g to elute sampled cells from sponges. Afterwards, cells were resuspended and expanded in DMEM/F12 media (Gibco, Carlsbad, CA) supplemented with bovine pituitary extract (0.004 mL/mL), epidermal growth factor (10 ng/mL), insulin (5 µg/mL), hydrocortisone (0.5 µg/mL), triiodo-l-thyronine (6.7 ng/mL), and transferrin (10 µg/mL) (PromoCell, Heidelberg, Germany), sodium selenite (30 nM, Sigma), ethanolamine (10 µM, Sigma), phosphorylethanolamine (10 µM, Sigma), sodium pyruvate (0.5 µM, Gibco), adenine (0.18 mM, Sigma), Hepes (15 mM, Gibco), 1 × GlutaMAX (Gibco) and in the presence of 10 µM Rock-inhibitor (StemCell, Cologne, Germany), as described previously (38). Cells were seeded in 24-well–plates and after reaching 90% confluence, starved in EGF-free media for 24 h before experiments.
Immortalized human bronchial epithelial (HBE1) cells were maintained in DMEM/F12 media (Invitrogen, Carlsbad, CA) supplemented with 1 ng/mL of cholera toxin (List Biological Laboratories, Campbell, CA), 10 ng/mL of epidermal growth factor (Calbiochem, San Diego, CA), 5 µg/mL of insulin (Sigma, St. Louis, MO), 5 µg/mL of transferin (Sigma), 0.1 µM dexamethasone (Sigma), 15 µg/mL of bovine pituitary extract (Invitrogen), 0.5 mg/mL of bovine serum albumin (Invitrogen), and 50 U/50 μg/mL of penicillin/streptomycin (Invitrogen), as described previously (39). Cells were seeded in 24-well–plates or chamber slides unless otherwise indicated and grown to confluence and starved (EGF-free media) 24 h before experiments.
Cells were stimulated with 10 ng/mL of TGF-β1 (R&D Systems, Minneapolis, MN) harvested at appropriate time points for analysis of SRC activation, ROS production, DNA damage response, and mRNA expression of NADPH oxidases and pro-fibrotic genes. Where indicated, pharmacological SFK inhibitors PP2 (1 µM, Sigma) or AZD0530 (saracatinib; 1 µM, Selleck Chemicals), the mitochondria-targeted antioxidant MitoQ (1 µM, Sigma), or the NADPH oxidase inhibitor diphenylene iodonium (DPI; 1 µM, Sigma) were administered 20 min before cell stimulation.
Small Interfering RNA Silencing
At 60% to 70% confluence, HBE1 cells were transfected with ON-TARGETplus SMARTpool small interfering RNAs (siRNAs) targeted against SRC, FYN, NOX4, or non-targeting siRNA Pool#1 as a control with DharmaFECT transfection reagent (Dharmacon, Lafayette, CO), according to the manufacturer’s instructions, 72 h before experimentation.
RNA Isolation and Semiquantitative Reverse Transcription (RT)-PCR
RNA was isolated and purified using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA concentration was determined using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA). cDNA was synthesized from 500 ng of isolated RNA using IScript (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. RT-PCR was performed using SYBR Green PCR Supermix (Bio-Rad) with 1 μL of cDNA and 0.5 μmol/L of predesigned primers and PCR amplifications were carried out for up to 40 cycles of denaturation (95°C for 15 s), annealing (57°C for 15 s), and extension (60°C for 45 s) for selected genes (Supplemental Table S2). The gene expression was normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and quantified using the ΔΔ cycle threshold (CT) method.
Analysis of Cellular ROS Production
Intracellular ROS production was assessed by cell preloading with 10 µM 2,7-dichlorofluorescein diacetate (DCF-DA; Sigma), and analysis of DCF fluorescence in a 96-well–plate reader. Extracellular H2O2 production was measured in cell culture supernatants using Amplex Red (Invitrogen) according to the manufacturer’s instructions. Mitochondrial ROS production was assessed by live-cell imaging, after 20-min preloading with the mitochondria-targeted fluorescent probe MitoSOX (5 µM, Invitrogen), using a Zeiss LSM 510 META confocal scanning laser microscope (Carl Zeiss Microimaging, Thornwood, NY).
Analysis of Protein Cysteine Oxidation
For analysis of protein cysteine oxidation to sulfenic acids (–S-OH), cells were lysed in the presence of 1 mM sulfenic acid-specific probe DCP-Bio1 (Kerafast, Boston, MA), after which biotin-tagged proteins were analyzed by Western blot (39).
Analysis of DNA Damage by Immunofluorescence Staining
Cells plated on chamber slides (Merck Millipore, Billerica, MA) were exposed to TGF-β1 for 4 h at 37°C. Slides were probed with an antibody targeted at γH2AX (Cell Signaling) followed by 4′-6-diamidino-2-phenylindole dihydrochloride staining (DAPI, Invitrogen). Images were captured with a 40× objective using a Zeiss LSM 510 META confocal scanning laser microscope and quantification of images was performed on a minimum of 200 cells per condition using MetaMorph (MetaMorph Inc., Nashville, TN).
IPF Gene Expression Data
Microarray data for NOX4 and FYN were obtained from the Lung Tissue Research Consortium data set GSE47460 (www.ncbi.nlm.nih.gov/geo), which includes lung tissue RNA samples collected during thoracic surgery. In total, 105 healthy control patients and 159 patients diagnosed with IPF by clinical history, CT scan, and surgical pathology were included upon removal of outliers by hierarchical clustering. Microarray data were normalized using a cyclic loss approach as previously described (40). Tissue expression of genes were also correlated with available lung function data (DLCO).
Statistical Analysis
All quantitative data are represented as means ± SE. Statistical differences between groups were evaluated by 2-way ANOVA with Bonferroni’s post hoc analysis or by Student’s t-test, depending on appropriate datasets, using GraphPad Prism software (version 7.3; GraphPad Software, La Jolla, CA), and considered significant at a P value less than 0.05.
RESULTS
Epithelial SFK Activation Is Increased in IPF and Contributes to Profibrotic Responses
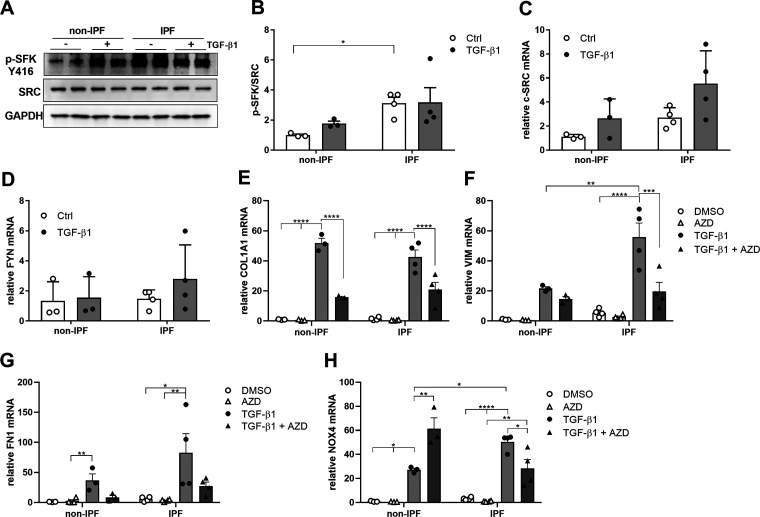
We obtained bronchial epithelial cells from subjects with IPF and non-IPF control subjects, and evaluated the activation of SFK in response to TGF-β1, indicated by (auto)phosphorylation at Tyr-416.1 As shown in Fig. 1, A and B, SFK activation was markedly enhanced in HBE cells from IPF patients compared to non-IPF control patients and increased in response to TGF-β1. However, no significant changes in mRNA expression of SRC and FYN were observed (Fig. 1, C and D). Stimulation of HBE cells from both IPF and non-IPF subjects with TGF-β1–induced mRNA expression of various pro-fibrotic genes, such as COL1A1, VIM, FN1, and NOX4 (Fig. 1, E–H), and TGF-β1–induced VIM and NOX4 was significantly higher in HBE cells from IPF subjects compared to non-IPF controls. Inhibition of SFK activity with the inhibitor AZD0530 (Supplemental Fig. S1) significantly reduced TGF-β1–induced mRNA induction of COL1A1 and VIM (Fig. 1, E and F) and tended to reduce FN1 induction, although this was not statistically significant (Fig. 1G). AZD pretreatment also appeared to suppress basal NOX4 expression and significantly suppressed TGF-β1-induced NOX4 expression in HBE cells from IPF patients, but surprisingly further enhanced TGF-induced NOX4 expression in HBE cells from non-IPF patients (Fig. 1H). Collectively, these findings further point to an enhanced role for SFK and NOX4 in profibrotic responses in HBE cells from subjects with IPF.
Figure 1.
IPF patient have an increased activation of SRC kinases which is linked to fibrotic gene expression. A: Western blots indicating phosphorylation of SRC kinases at tyrosine 416 in HBE cells from non-IPF and IPF patients before and after stimulation with TGF-β1 (10 ng/mL). B: densitometry analysis of SFK activation in HBE cells from non-IPF (n = 3) and IPF patients (n = 4). Gene expression of SRC (C) and FYN (D) after stimulation with TGF-β1 (10 ng/mL) for 24 h, determined by RT-PCR (non-IPF n = 3, IPF n = 4). Gene expression of (E) COL1A1, (F) VIM, (G) FN1, and (H) NOX4 after stimulation with TGF-β1 (10 ng/mL) for 24 h, in the absence or presence of AZD, determined by RT-PCR in non-IPF (n = 3) and IPF patients (n = 4). Results are expressed as means ± SE (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
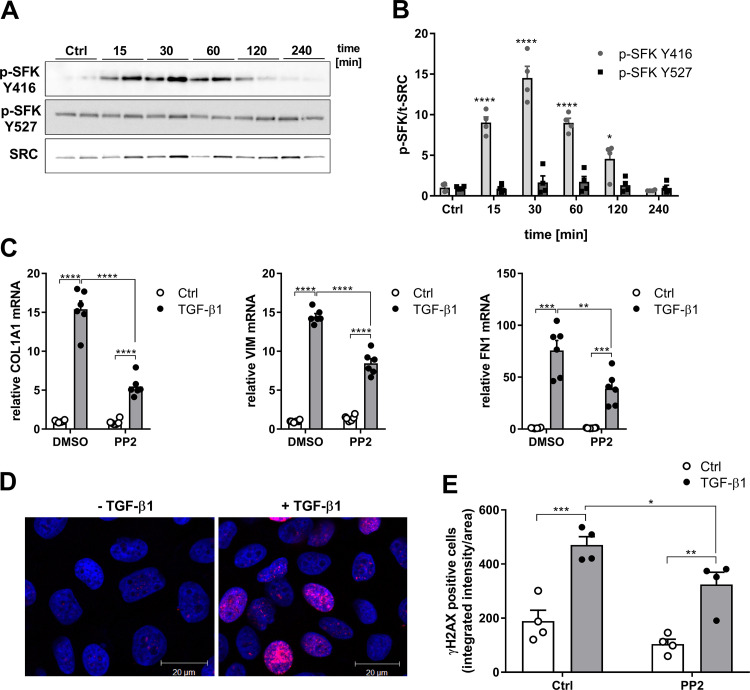
We used the bronchial epithelial cell line HBE1 to further explore the importance of TGF-β1–induced SFK activation (Fig. 2). As expected, TGF-β1 (10 ng/mL) induced a rapid increase in SFK activity in HBE1 cells, peaking at around 30 min (Fig. 2, A and B). Activation of SFKs typically involves dephosphorylation at Tyr-5277 (41), but TGF-β1 did not significantly alter overall Tyr-527 phosphorylation, suggesting that SFK is activated through a non-canonical pathway (Fig. 2, A and B). To evaluate the effect of SFK inhibition on induction of profibrotic genes by TGF-β1, cells were stimulated for 24 h with TGF-β1 (10 ng/mL) in the absence or presence of SFK inhibitors PP2 or AZD0530. As expected, TGF-β1–stimulated induction of profibrotic genes COL1A1, VIM, and FN1 was in each case partially reduced by PP2 (Fig. 2C) or AZD0530 (Supplemental Fig. S2), indicating that SFKs contribute to TGF-β1–mediated profibrotic responses in epithelial cells. Since DNA damage responses represent another important epithelial feature in IPF (7, 8, 42), we determined the DNA damage response induced by TGF-β1 by assessing phosphorylation of the histone variant H2AX, referred to as γH2AX. Indeed, TGF-β1 induced significant DNA damage response in HBE1 cells, which was significantly reduced after pretreatment with the SFK inhibitor PP2 (Fig. 2, D and E).
Figure 2.
TGF-β1 induces SFK-mediated profibrotic gene expression and DNA damage response. A: representative Western blots indicating phosphorylation of SRC kinases at tyrosine 416 and 527 after stimulation with TGF-β1 (10 ng/mL). B: densitometry analysis of 4 replicates from 2 independent experiments. C: gene expression of COL1A1, VIM, and FN1 after stimulation with TGF-β1 (10 ng/mL) for 24 h, in the absence or presence of PP2, determined by RT-PCR (n = 6). D: representative immunofluorescence imaging of γH2AX (red) and 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI; blue) in unstimulated or TGF-β1–stimulated (4 h) HBE1 cells. E: quantitative analysis of nuclear γH2AX intensity in HBE1 cells in the absence or presence of PP2 (1 µM) with and without TGF-β1 (10 ng/mL) stimulation for 4 h. For each experimental condition, a minimum of 200 cells from a total of 2 independent experiments with 2 replicates were analyzed. Results are expressed as means ± SE (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
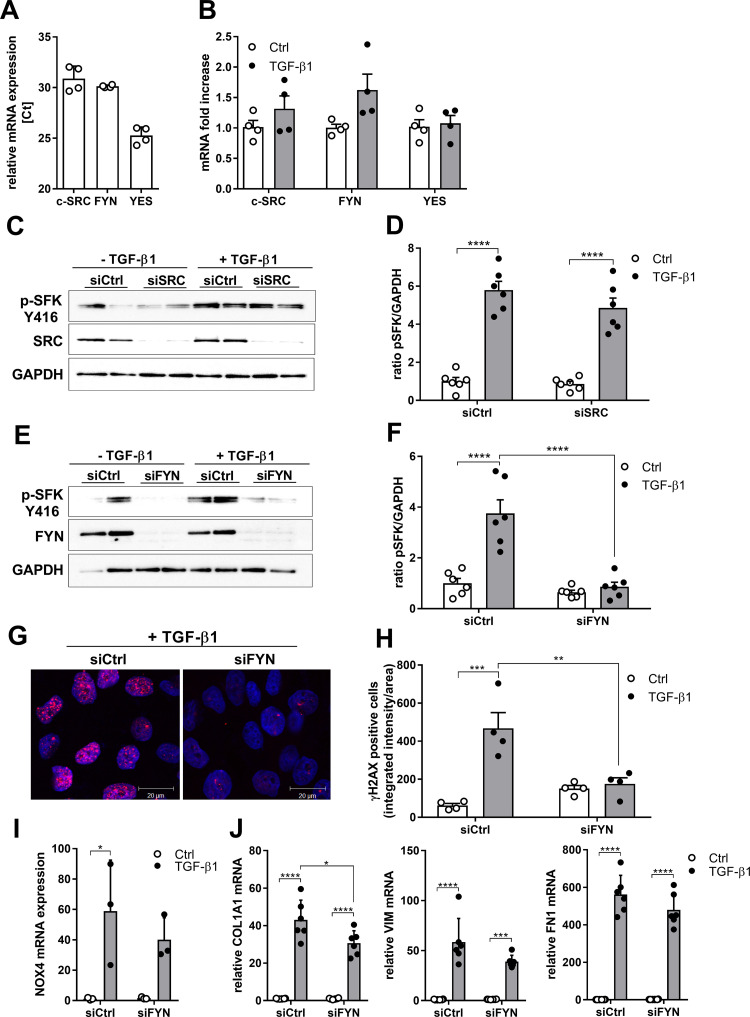
The SFK family includes nine members, of which SRC, FYN, and YES are ubiquitously expressed (35). All three SFKs are also expressed in HBE1 cells (Fig. 3A) and their expression was not significantly altered after TGF-β1 stimulation (Fig. 3B). To determine the involvement of individual SFK members in TGF-β–mediated SFK activation and profibrotic responses, we used specific small interference RNA to silence them individually. TGF-β1-induced SFK activation in HBE1 cells, measured as phosphorylation of Tyr-416, was not affected by knockdown of SRC (Fig. 3, C and D) but was significantly reduced upon silencing of FYN (Fig. 3, E and F). FYN siRNA silencing also significantly attenuated the TGF-β–induced DNA damage response, assessed as γH2AX (Fig. 3, G and H), but only modestly affected TGF-β1–mediated induction of NOX4 or the profibrotic genes COL1A1, VIM, and FN1, which in most cases was not statistically significant (Fig. 3, I and J). Overall, these studies highlight the importance of the SFK FYN in TGF-β1–induced epithelial responses associated with fibrosis, particularly with respect to the DNA damage response and to a lesser extent profibrotic gene expression.
Figure 3.
TGF-β1-induced SFK activation and profibrotic responses depend on FYN. A: relative gene expression of c-SRC, FYN, and YES analyzed by qRT-PCR (n = 4). B: gene expression of the SRC kinases c-SRC, FYN, and YES after stimulation with TGF-β1 (10 ng/mL) for 4 h analyzed by qRT-PCR (n = 4). C: knockdown of SRC does not reduce phosphorylation at Tyr-416 after stimulation with TGF-β1 (10 ng/mL). D: densitometry analysis of 3 independent experiments (n = 6). E: phosphorylation at Tyr-416 is decreased in FYN-silenced cells after 30 min of TGF-β1 (10 ng/mL) stimulation. F: densitometry analysis of 3 independent experiments (n = 6). G: representive image of γH2AX (red) and DAPI (blue) stained control (Ctrl) and FYN-silenced HBE1 cells stimulated with TGF-β1 for 4 h. H: analysis of nuclear γH2AX intensity in HBE1 cells in Ctrl and FYN-silenced cells with and without TGF-β1 stimulation for 4 h. For each experimental condition, a minimum of 200 cells from in total 2 independent experiments with 2 replicates were analyzed. I: NOX4 expression in FYN silenced cells after 24 h stimulation with TGF-β1 (n = 3). J: COL1A1, VIM and FN1 expression in FYN-silenced cells after 24 h stimulation with TGF-β1 (n = 6). Results are expressed as means ± SE (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
TGF-β1 Activates FYN by a NOX4-dependent Redox Mechanism
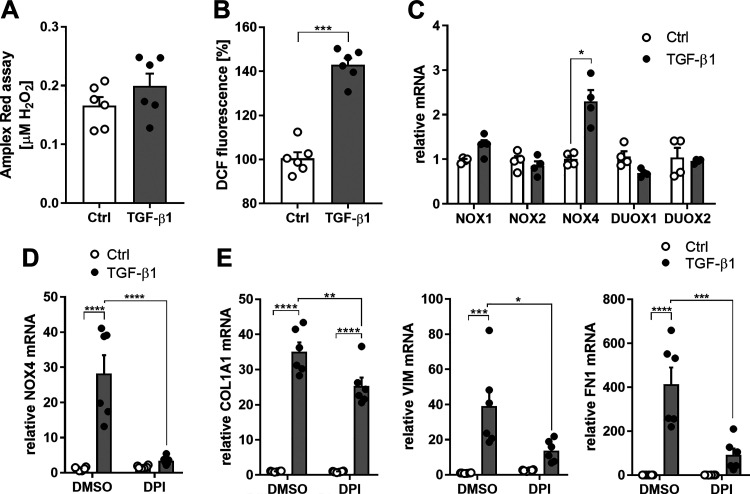
TGF-β1 can enhance cellular ROS production from several sources (43), and SFKs are also subject to redox regulation through reversible oxidation of conserved cysteines (33, 35). To assess the importance of such redox-based mechanisms, we first determined whether TGF-β1 induced extracellular or intracellular production of ROS in HBE1 cells. Indeed, TGF-β1 significantly enhanced intracellular but not extracellular ROS (Fig. 4, A and B). To determine the possible source of TGF-β1–induced intracellular ROS production, we assessed potential changes in expression of diverse NOX isoforms. As expected (12, 16, 18), TGF-β1 stimulation of HBE1 cells caused a rapid induction of NOX4 mRNA within 4 h, but did not affect expression of other NOX isoforms (Fig. 4C). To investigate the involvement of NOXs in the induction of TGF-induced profibrotic gene expression, we utilized the general NOX inhibitor DPI (1 µM) and observed that pretreatment with DPI significantly attenuated the TGF-β–induced increase in NOX4 mRNA (Fig. 4D) as well as COL1A1, VIM, and FN1 (Fig. 4E).
Figure 4.
TGF-β1 induced intracellular ROS production and NOX4 expression. A: effect of TGF-β1 (10 ng/mL; 30 min) on extracellular H2O2 production, measured using Amplex Red (n = 6). B: analysis of intracellular ROS production using H2DCF-DA, in the absence or presence of TGF-β1 (10 ng/mL; 30 min). Fluorescence intensity is expressed relative to unstimulated controls (n = 6). C: gene expression of NOX1, NOX2, NOX4, DUOX1, and DUOX2 after stimulation with TGF-β1 (10 ng/mL) for 4 h (n = 4). D: NOX4 expression in HBE after stimulation with TGF-β1 (10 ng/mL) for 24 h, in the absence or presence of DPI, determined by RT-PCR (n = 6). E: COL1A1, VIM, and FN1 expression in HBE after stimulation with TGF-β1 (10 ng/mL) for 24 h, in the absence or presence of DPI, determined by RT-PCR (n = 6). Results are expressed as means ± SE (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
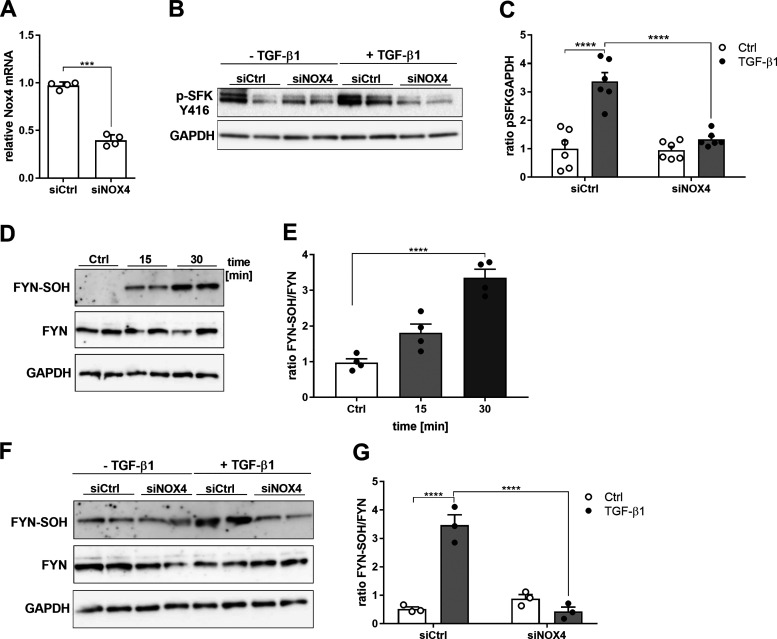
To examine the contribution of NOX4 to TGF-β–induced ROS production and FYN activation, it was silenced by siRNA approaches (Fig. 5A). NOX4 silencing attenuated DCF fluorescence in both unstimulated and TGF-β–stimulated HBE1 cells (not shown), so we cannot firmly conclude whether TGF-β–induced ROS originated from NOX4. NOX4 silencing markedly reduced TGF-β1–induced (auto)phosphorylation of FYN, compared to control-transfected cells (Fig. 5, B and C). We used a dimedone trapping strategy to detect oxidation of cysteines within FYN to a sulfenic acid. Indeed, TGF-β1 stimulation was found to induce cysteine sulfenylation within FYN, at time points corresponding with its autophosphorylation (Fig. 2, A and B, and Fig. 5, D and E). Moreover, TGF-β1–induced sulfenylation of FYN was suppressed in NOX4-silenced cells compared to controls (Fig. 5, F and G), indicating a critical role for NOX4. Collectively, these findings indicate that TGF-β1–induced activation of FYN were initiated by NOX4-mediated redox signaling and sulfenylation of FYN. Curiously, although NOX4 siRNA silencing tended to attenuate TGF-induced gene expression in some cases, these changes were not statistically significant (Supplemental Fig. S3). In addition to NOX4, other NOX enzymes could conceivably contribute to TGF-β–induced profibrotic responses, such as, e.g., DUOX1 (44). However, siRNA silencing of DUOX1 did not affect TGF-β–induced profibrotic gene expression (not shown). In aggregate, whereas NOX4 appears to contribute to TGF-β–induced activation of FYN, its contribution to profibrotic gene induction is relatively minor, and other NOXs likely contribute to these latter outcomes.
Figure 5.
TGF-β1 activates FYN by NOX4-dependent redox signaling. A: NOX4 gene expression in HBE1 cells transfected with siCtrl (Ctrl) and siNOX4 determined using qRT-PCR (n = 4). B: phosphorylation of SFKs (Tyr-416) in NOX4-silenced cells compared to controls after 30 min of stimulation with TGF-β1 (10 ng/mL). C: densitometry analysis of 3 independent experiments (n = 6). D: sulfenylation of FYN and total FYN analyzed by Western blotting. E: quantification of sulfenylated cysteine levels of FYN in 3 independent experiments (n = 6). F: FYN sulfenylation in NOX4-silenced cells. G: quantification of sulfenylated cysteine levels of FYN in 2 independent experiments (n = 3). Results are expressed as mean ± SEM (***P < 0.001, ****P < 0.0001).
TGF-β1–mediated Profibrotic Responses Involve FYN-mediated Mitochondrial ROS Production
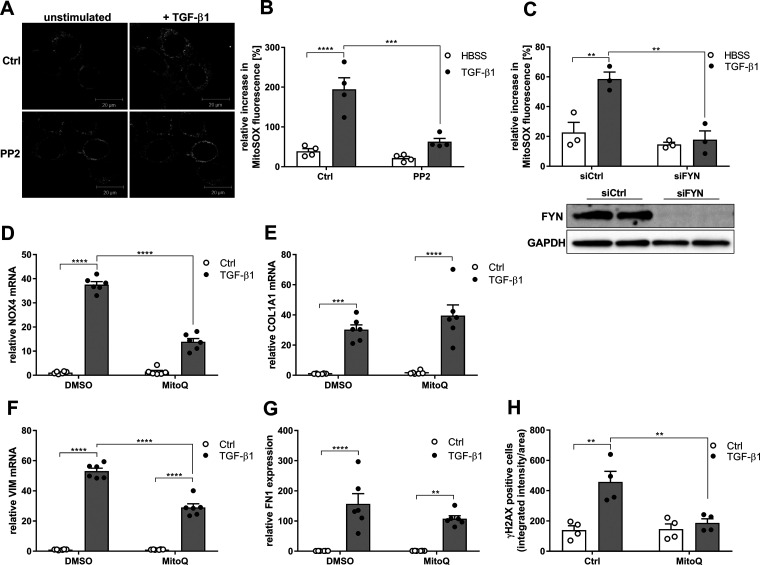
IPF development has also associated with mitochondrial dysfunction and mitochondrial ROS (mtROS) production (45, 46), and TGF-β1 has been reported to enhance mtROS production (13), although the upstream mechanism in this is unclear. To investigate a potential role for SFK activation in TGF-β1–induced generation of mtROS, we used live-cell imaging analysis with the mtROS indicator MitoSOX. Indeed, HBE1 cell stimulation with TGF-β1 markedly enhanced mtROS production within 20 min, and this was markedly attenuated by the SFK inhibitor PP2 (Fig. 6, A and B) and was also attenuated after silencing of FYN (Fig. 6C). To determine whether mtROS production contributes TGF-β1–induced profibrotic responses, we pretreated HBE1 cells with the mitochondria-targeted antioxidant MitoQ. Interestingly, MitoQ pretreatment attenuated the TGF-β–induced increase in NOX4 mRNA expression, and also attenuated TGF-β1–induced expression of the profibrotic genes VIM and FN1 but not COL1A1 (Fig. 6, E–G). Last, MitoQ pretreatment also attenuated TGF-β–induced DNA damage response, shown by the reduced presence of γH2AX foci (Fig. 6H). Collectively, these results indicate complex and reciprocal interactions between NOX4 and mtROS in TGF-β–induced profibrotic responses, which is a strong role for FYN activation (perhaps by NOX4) in mtROS production and, conversely, a role for mtROS in TGF-β–induced induction of NOX4 and profibrotic gene expression.
Figure 6.
Mitochondrial ROS production mediates TGF-β1–mediated fibrotic responses. A: representative image of mtROS analysis using MitoSOX in unstimulated or TGF-β1–stimulated (10 ng/mL; 20 min) HBE1 cells. B: quantification of MitoSOX fluorescence increase over 20 min following TGF-β1 stimulation in the absence or presence of PP2 (1 µM). Fluorescence increase was quantified in 10-15 individual cells from 3 independent experiments (n = 3) and expressed as means ± SE. C: quantification of TGF-β1–stimulated MitoSOX fluorescence increase in siCtrl (control) and siFYN cells. Increase in fluorescence was analyzed in 10-15 individual cells from 4 independent experiments (n = 4), and expressed as means ± SE. D: NOX4 expression after 24 h stimulation with TGF-β1 with or without pretreatment with the mitochondrial antioxidant MitoQ (n = 6). (E) COL1A1, (F) VIM and (G) FN1 expression after 24 h stimulation with TGF-β1 with or without pretreatment with the mitochondrial antioxidant MitoQ (n = 6). H: analysis of nuclear γH2AX immunofluorescence in TGF-β1-stimulated HBE1 cells (4 h) pretreated with MitoQ. For each experimental condition, a minimum of 200 cells from a total of 2 independent experiments with 2 replicates were analyzed. Results are expressed as means ± SE (**P < 0.01, ***P < 0.001, ****P < 0.0001).
Gene Expression of NOX4 and FYN Is Altered in IPF in Association with Disease Severity
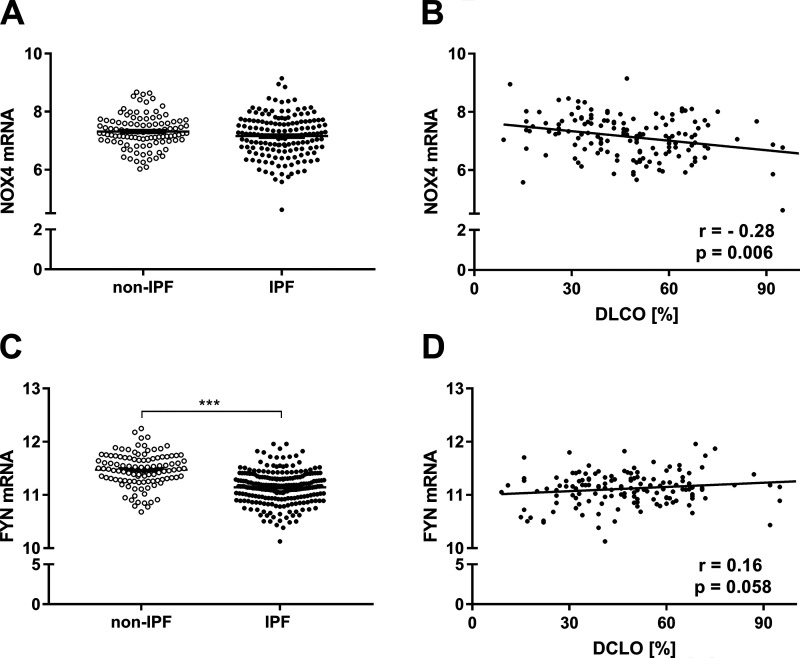
To assess the importance of NOX4 and FYN in human IPF, we examined their expression profiles in lung tissues from patients with IPF available through the Lung Tissue Research Consortium. Somewhat surprisingly, NOX4 gene expression was not significantly altered between control subjects and IPF patients (Fig. 7A), although NOX4 expression levels correlated inversely with the predicted diffusion capacity for carbon monoxide (DLCO), indicating that NOX4 expression is associated with disease severity (Fig. 7B). Interestingly, lung tissue expression of FYN was found to be significantly decreased in patients with IPF (Fig. 7C), although FYN expression levels did not correlate with disease severity (Fig. 7D). These observations suggest that dysregulation of NOX4/FYN expression in IPF may lead to altered regulation of these signaling pathways and contribute to disease severity.
Figure 7.
NOX4 and FYN mRNA expression in controls (n = 105) and IPF patients (n = 159). A: NOX4 expression levels in lung tissues from IPF patients and controls. B: correlation of NOX4 expression with DLCO (%). C: FYN expression in lungs of control subject and IPF patients. D: correlation of FYN expression with DLCO (%). Results are expressed as means ± SD. Differences between IPF patients and controls were determined by Student’s t-test and correlation between NOX4/FYN and DLCO was analyzed by Pearson’s correlation (***P < 0.001).
DISCUSSION
The present studies addressed the mechanisms by which the TGF-β1 induces fibrotic responses within the lung epithelium and demonstrate that TGF-β1 induces DNA damage responses as well as profibrotic gene expression in human epithelial cells, both relevant features of IPF, and establish a role for SFKs herein. Recent studies have implicated SFKs in the pathophysiology of IPF (47), due to their ability to promote myofibroblast differentiation and inducing features of EMT (27, 28). Since these observations were based primarily on pharmacological SFK inhibitors, such as saracatinib (AZD0530) or dasatinib, which lack isoform specificity and also inhibit other tyrosine kinases (48), we explored the SFK isoform(s) involved in TGF-β1–mediated SFK activation in lung epithelial cells, and identified FYN as the main isoform that mediates SFK activation and subsequent mtROS production and induction of profibrotic genes, including NOX4. Conversely, we also identified an acute redox-based mechanism involved in TGF-β1–mediated activation of FYN, which appeared to involve NOX4. Our studies are based on bronchial epithelial cells, including primary cells obtained from IPF subjects, whereas IPF is thought to primarily involve alveolar epithelial cells. However, our studies suggest an enhanced role for NOX4/SFK in primary bronchial epithelial cells from IPF subjects, which may be reflective of similar changes within the alveolar epithelium. In addition, recent studies have suggested a role for the airway epithelium in the development of IPF as well but research is limited (49, 50).
Our findings indicate that FYN contributed strongly to TGF-β–induced mtROS production and DNA damage, as measured by γH2AX, but had a relatively modest effect on fibrotic gene expression. This likely suggests that these processes may be largely independent via diverse signaling mechanisms. TGF-β–induced profibrotic signaling largely involves signaling through Smad2/3, but activation of SFK appears to be largely independent of Smad activation (51, 52). It is also worth noting that the contribution of mtROS in profibrotic responses in fibroblasts was also found to be Smad-independent (13). DNA damage responses have been linked to profibrotic signaling and have been demonstrated in pulmonary epithelial cells during IPF (53), and bronchial epithelial senescence can be accelerated by DNA damage (54). The shared involvement of FYN and mtROS in both DNA damage responses and profibrotic gene expression by TGF-β would suggest that these pathways may be somewhat related, although their contributions to profibrotic gene expression may be relatively modest compared to the more prominent role of Smad2/3 signaling. It was recently suggested that the initial fibrotic changes in IPF are actually not located in the alveolar epithelium but rather in the distal airways instead (55) and TGF-induced DNA damage responses in the airway epithelium, mediated by FYN, may be an important early event in disease development.
Activation of SFKs is regulated largely by dephosphorylation of Tyr-527 and autophosphorylation at Tyr-416 within the kinase domain (41), but redox-based mechanisms can also contribute to the activation of SFK and may relate to oxidation of one or more conserved cysteine residues within its SH2 or kinase domains (33, 36, 56, 57). Indeed, our observation that TGF-β1 enhances SFK phosphorylation at Tyr-416 without significant dephosphorylation at Tyr-527 suggests an alternative redox-dependent mechanism, as suggested previously (34, 36). Furthermore, TGF-β1–mediated SFK activation, primarily associated with FYN, was accompanied by sulfenylation of one or more of cysteine residues within FYN, and was found to be mediated by NOX4. We recently reported that redox-dependent activation of SRC involves sulfenylation of two of its cysteine residues (Cys-185 and Cys-277) (56). Curiously, neither of these cysteines are present within FYN, which indicates that oxidation of alternative cysteines may be mediating FYN activation, such as Cys-488, which is conserved among all SFKs and has been implicated in its redox-dependent activation (58) or Cys-404, which was recently identified as redox-sensitive in the OxiMouse data set (59). More detailed future studies will be needed to clarify the significance of FYN sulfenylation for regulating its activity.
Our observations implicating NOX4 in acute activation of FYN in responses to TGF-β1 would suggest that TGF-β1 causes activation of NOX4, which contrasts with the common notion that NOX4 function is primarily regulated transcriptionally and is constitutively active (60). However, some emerging studies have indicated that NOX4 activity can also be regulated through transcription-independent mechanisms, such as phosphorylation events (61) or in the context of Toll-like receptor (TLR) 5 signaling (62). NOX4 was also recently described to be subject to negative allosteric regulation by ATP (63) and could therefore be potentially activated by localized ATP degradation in response to TGF-β. Induction of NOX4 expression during chronic TGF-β1 stimulation or in IPF, combined with metabolic alterations and ATP degradation (64), could therefore lead to augmented NOX4 activation and enhanced disease pathology.
NOX4 has been strongly implicated in IPF pathology based on its involvement in fibroblast differentiation and myofibroblast proliferation (16, 18). NOX4 was also found to contribute to bleomycin-induced alveolar epithelial cell death in mice, thus representing an alternative mechanism by which NOX4 activation promotes pulmonary fibrosis (65). Indeed, NOX4-deficient mice are protected from developing bleomycin-induced pulmonary fibrosis indicating a key role for NOX4 in the development of IPF (18, 66). Although our findings indicate that NOX4 promotes redox-dependent activation of FYN within the bronchial epithelium, its role in fibrotic remodeling remains unclear as silencing of NOX4 did not affect fibrotic gene expression.
Age-related lung diseases such as IPF are also characterized by mitochondrial dysfunction and enhanced mtROS production, which contributes to epithelial cell death as well as profibrotic gene expression (13, 67). Our present findings demonstrate that FYN is required for TGF-β1–mediated mtROS production. Intriguingly, both NOX4 and FYN may be present in mitochondria, as observed, e.g., cardiomyocytes (61, 68). SRC family kinases are recognized as important metabolic regulators in mitochondria (69), and mitochondria-localized FYN can promote phosphorylation of complexes I, II, and IV subunits as well as pyruvate dehydrogenase, thereby regulating their function (70, 71). Moreover, FYN is also involved in the regulation of mitochondrial protein synthesis and oxidative phosphorylation indicating its importance in regulating mitochondrial function as well as mtROS production (72).
It has been reported that NOX4 is able to interact with mitochondrial complex I, thereby negatively regulating its activity (73). Overexpression of NOX4 might have a role in mediating mitochondrial dysfunction in lung fibroblasts (20). This brings up the interesting question whether our observations of mtROS production actually originates from components of the electron transport chain (e.g., complexes I or III) or from mitochondria-localized NOX4. Previous studies indicated that TGF-β–induced mtROS production indeed originates from complex III (13), although the upstream mechanisms by which TGF-β induces this response were not defined. However, recent efforts to manipulate mitochondrial NOX4 expression have suggested its importance in vascular ECM remodeling and fibrosis (74), thus suggesting that some of the apparent mtROS production could also originate from NOX4. Further complicating this issue are observations that mtROS can also regulate NOX4 mRNA expression (13), and thereby potentially enhance mitochondria-localized NOX4. Furthermore, whereas our data with MitoQ suggest a role for mtROS in regulating NOX4 gene expression and profibrotic responses, we cannot fully rule out the possibility that MitoQ also impaired responses to ROS generated by NOX4 that was localized to mitochondria.
Our findings indicate that TGF-β1–induced activation of NOX4-FYN promotes production of mtROS as an important mechanism for induction of DNA damage and changes in gene expression associated with fibrotic remodeling within the epithelium. Moreover, TGF-β1 signaling also induces NOX4 expression, which appears to involve initial activation of FYN and mtROS, and this may result in amplified profibrotic responses in the context of chronic TGF-β1 activation as seen in IPF. Conversely, it is worth noting that FYN can negatively regulate NOX4 activity by phosphorylating Tyr-566 in its C-terminus (61). Hence, NOX4-mediated activation of FYN could also serve to regulate NOX4 in a negative feedback mechanism. In this light, our findings indicating down-regulation of FYN in IPF lung tissues could suggest that such negative feedback is impaired in the context of IPF, although such observations should be interpreted with caution, because global tissue mRNA analyses does not offer insights into cell-specific alterations, and analysis of gene expression may not reflect the degree to which FYN is activated. Indeed, our studies of bronchial epithelial cells suggest increased SFK activation in IPF, although it is possible that SFK are also suppressed in other lung cell types.
In summary, the present results demonstrate that TGF-β1–induced fibrotic responses in lung epithelial cells are mediated by NOX4-induced activation of the SRC kinase FYN, which in turn induces mtROS production and epithelial DNA damage responses as well as induction of profibrotic genes. It is important to note that our studies only addressed acute TGF-β1 signaling in epithelial cells, whereas biological effects of TGF-β may be altered in chronic conditions, such as IPF, due to alterations in expression and activity of NOX4 and FYN, as well as mitochondrial dysfunction and altered metabolic status.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Figs. S1, S2, and S3: https://doi.org/10.6084/m9.figshare.13028138.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL085646, R01 HL138708, and R21 AG055325 (to A.vdV.). C.V. was supported by the Nutrim Graduate Programme from Maastricht University. This study was also supported by Lung Foundation Netherlands Grant 9.2.17.214FE (to C.V.), and the German Center for Lung Research Grant 82DZL00402. C.M.D. was supported by an F31 Predoctoral Fellowship from NIH (HL142221).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.V., F.J.vS., A.W.B., and A.vdV. conceived and designed research; C.V., M.H., K.D., A.H., C.M.D., M.K., M.A.S., and N.K. performed experiments; C.V., J.E.M., and B.M.V. analyzed data; A.W.B. and A.vdV. interpreted results of experiments; C.V. prepared figures; C.V. drafted manuscript; F.J.vS., A.W.B., and A.vdV. edited and revised manuscript; C.V., M.H., K.D., A.H., C.M.D., J.E.D., B.M.V., M.K., M.A.S., N.K., F.J.vS., A.W.B., and A.vdV. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nicole Bishop for assistance with confocal microscopy imaging, which was performed on a Zeiss 510 META laser scanning confocal microscope supported by National Institutes of Health Award 1S10RR019246 from the National Center for Research Resources.
Footnotes
Amino acid numbering of SRC is commonly based on the avian SRC sequence, which is different for human SRC (which includes 3 additional amino acids) and FYN.
REFERENCES
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis, , et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Dai H, Wang C. Epithelium-dependent profibrotic milieu in the pathogenesis of idiopathic pulmonary fibrosis: current status and future directions. Clin Respir J 10: 133–141, 2016. doi: 10.1111/crj.12190. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thoracic Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 4.Kuwano K, Nakashima N, Inoshima I, Hagimoto N, Fujita M, Yoshimi M, Maeyama T, Hamada N, Watanabe K, Hara N. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J 21: 232–240, 2003. doi: 10.1183/09031936.03.00063203. [DOI] [PubMed] [Google Scholar]
- 5.Liepelt A, Tacke F. Healing the scars of life-targeting redox imbalance in fibrotic disorders of the elderly. Ann Transl Med 3: S13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 49: 707–717, 2010. doi: 10.1016/j.freeradbiomed.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 7.McDonough JE, Martens DS, Tanabe N, Ahangari F, Verleden SE, Maes K, Verleden GM, Kaminski N, Hogg JC, Nawrot TS, Wuyts WA, Vanaudenaerde BM. A role for telomere length and chromosomal damage in idiopathic pulmonary fibrosis. Respir Res 19: 132, 2018. doi: 10.1186/s12931-018-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Povedano JM, Martinez P, Flores JM, Mulero F, Blasco MA. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep 12: 286–299, 2015. doi: 10.1016/j.celrep.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest 36: 362–367, 2006. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FJ, de Andrade JA, Anstrom KJ, King TE Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. New Engl J Med 370: 2093–2101, 2014. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, , et al. Reducing protein oxidation reverses lung fibrosis. Nat Med 24: 1128–1135, 2018. doi: 10.1038/s41591-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53: 1489–1499, 2012. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem 288: 770–777, 2013. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGFβ1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 24: 1895–1903, 2005. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 15.Liu RM, Desai LP. Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol 6: 565–577, 2015. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crestani B, Besnard V, Boczkowski J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 43: 1086–1089, 2011. doi: 10.1016/j.biocel.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Cheresh P, Jablonski RP, Morales-Nebreda L, Cheng Y, Hogan E, Yeldandi A, Chi M, Piseaux R, Ridge K, Hart MC, Chandel N, Budinger GRS, Kamp DW. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic Biol Med 101: 482–490, 2016. doi: 10.1016/j.freeradbiomed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM, Thannickal VJ. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J Biol Chem 292: 3029–3038, 2017. doi: 10.1074/jbc.M116.752261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5: 47–62, 2010. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roskoski R. Jr.Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res 94: 9–25, 2015. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 24.Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 45: 1426–1433, 2015. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 25.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFβ-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res 314: 143–152, 2008. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Xie YG, Yu Y, Hou LK, Wang X, Zhang B, Cao XC. FYN promotes breast cancer progression through epithelial-mesenchymal transition. Oncol Rep 36: 1000–1006, 2016. doi: 10.3892/or.2016.4894. [DOI] [PubMed] [Google Scholar]
- 27.Abdalla M, Thompson L, Gurley E, Burke S, Ujjin J, Newsome R, Somanath PR. Dasatinib inhibits TGFβ-induced myofibroblast differentiation through Src-SRF pathway. Eur J Pharmacol 769: 134–142, 2015. doi: 10.1016/j.ejphar.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, Luckhardt T, Siegal GP, Zhou Y, Liu RM, Desai LP, O'Reilly PJ, Thannickal VJ, Ding Q. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther 351: 87–95, 2014. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu YY, Zhao XK, Yu L, Qi F, Zhai B, Gao CQ, Ding Q. Interaction of Src and α-V integrin regulates fibroblast migration and modulates lung fibrosis in a preclinical model of lung fibrosis. Sci Rep 7: 46357, 2017. doi: 10.1038/srep46357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison SC. Variation on an Src-like theme. Cell 112: 737–740, 2003. doi: 10.1016/S0092-8674(03)00196-X. [DOI] [PubMed] [Google Scholar]
- 31.Roskoski R. Jr.Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun 324: 1155–1164, 2004. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 32.Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, Vincenzini MT. Redox regulation of platelet-derived growth factor receptor: role of NADPH-oxidase and c-Src tyrosine kinase. Biochim Biophys Acta 1745: 166–175, 2005. doi: 10.1016/j.bbamcr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 25: 6391–6403, 2005. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem 274: 25821–25826, 1999. doi: 10.1074/jbc.274.36.25821. [DOI] [PubMed] [Google Scholar]
- 35.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med 49: 516–527, 2010. doi: 10.1016/j.freeradbiomed.2010.04.025. . [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Davies KJ, Forman HJ. TGFβ1 rapidly activates Src through a non-canonical redox signaling mechanism. Arch Biochem Biophys 568: 1–7, 2015. doi: 10.1016/j.abb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn N, Kuner R, Eberhardt R, Meister M, Muley T, Winteroll S, Schnabel PA, Ishizaka A, Herth FJ, Poustka A, Sultmann H, Hoffmann H. Gene expression analysis of endobronchial epithelial lining fluid in the evaluation of indeterminate pulmonary nodules. J Thorac Cardiovasc Surg 138: 474–479, 2009. doi: 10.1016/j.jtcvs.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Wu R. Growth of human lung tumor cells in culture. In: Culture of Human Tumor Cells. Edited by Pfragner R, Freshney RI, Hoboken, NJ, Wiley-Liss Inc.: 2004, pp. 1–21. doi: 10.1002/0471722782. [DOI] [Google Scholar]
- 39.Hristova M, Habibovic A, Veith C, Janssen-Heininger YM, Dixon AE, Geiszt M, van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol 137: 1545–1556e11, 2016. doi: 10.1016/j.jaci.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonough JE, Kaminski N, Thienpont B, Hogg JC, Vanaudenaerde BM, Wuyts WA. Gene correlation network analysis to identify regulatory factors in idiopathic pulmonary fibrosis. Thorax 74: 132–140, 2019. doi: 10.1136/thoraxjnl-2018-211929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene 23: 7918–7927, 2004. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 42.Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, Rock JR, Looney MR, Wolters PJ. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: e86704, 2016. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal 31: 1092–1115, 2019. doi: 10.1089/ars.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louzada RA, Corre R, El Hassani RA, Meziani L, Jaillet M, Cazes A, Crestani B, Deutsch E, Dupuy C. NADPH oxidase DUOX1 sustains TGF-β1 signalling and promotes lung fibrosis. Eur Respir J 57: 01949, 2020. doi: 10.1183/13993003.01949-2019. [DOI] [PubMed] [Google Scholar]
- 45.Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. The role of mitochondrial DNA in mediating alveolar epithelial cell apoptosis and pulmonary fibrosis. Int J Mol Sci 16: 21486–21519, 2015. doi: 10.3390/ijms160921486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest 127: 405–414, 2017. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aschner Y, Downey GP. The importance of tyrosine phosphorylation control of cellular signaling pathways in respiratory disease: pY and pY not. Am J Respir Cell Mol Biol 59: 535–547, 2018. doi: 10.1165/rcmb.2018-0049TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, Elvin P, Boyer B, Carragher N, Plé PA, Bermingham A, Holdgate GA, Ward WH, Hennequin LF, Davies BR, Costello GF. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol 3: 248–261, 2009. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habiel DM, Espindola MS, Jones IC, Coelho AL, Stripp B, Hogaboam CM. CCR10+ epithelial cells from idiopathic pulmonary fibrosis lungs drive remodeling. JCI Insight 3, 2018. doi: 10.1172/jci.insight.122211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1: e90558, 2016. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dubon MJ, Yu J, Choi S, Park KS. Transforming growth factor β induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J Cell Physiol 233: 201–213, 2018. doi: 10.1002/jcp.25863. [DOI] [PubMed] [Google Scholar]
- 52.Seo HY, Jeon JH, Jung YA, Jung GS, Lee EJ, Choi YK, Park KG, Choe MS, Jang BK, Kim MK, Lee IK. Fyn deficiency attenuates renal fibrosis by inhibition of phospho-STAT3. Kidney Int 90: 1285–1297, 2016. doi: 10.1016/j.kint.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 53.Gunther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev 21: 152–160, 2012. doi: 10.1183/09059180.00001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–401, 2011. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jonsdottir HR, Arason AJ, Palsson R, Franzdottir SR, Gudbjartsson T, Isaksson HJ, Gudmundsson G, Gudjonsson T, Magnusson MK. Basal cells of the human airways acquire mesenchymal traits in idiopathic pulmonary fibrosis and in culture. Lab Invest 95: 1418–1428, 2015. doi: 10.1038/labinvest.2015.114. [DOI] [PubMed] [Google Scholar]
- 56.Heppner DE, Dustin CM, Liao C, Hristova M, Veith C, Little AC, Ahlers BA, White SL, Deng B, Lam YW, Li J, van der Vliet A. Direct cysteine sulfenylation drives activation of the Src kinase. Nat Commun 9: 4522, 2018. doi: 10.1038/s41467-018-06790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480: 109–112, 2011. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JE, Roh E, Lee MH, Yu DH, Kim DJ, Lim TG, Jung SK, Peng C, Cho YY, Dickinson S, Alberts D, Bowden GT, Einspahr J, Stratton SP, Curiel-Lewandrowski C, Bode AM, Lee KW, Dong Z. Fyn is a redox sensor involved in solar ultraviolet light-induced signal transduction in skin carcinogenesis. Oncogene 35: 4091–4101, 2016. doi: 10.1038/onc.2015.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, Li J, Long J, Mills EL, Szpyt J, He Z, Du G, Garrity R, Reddy A, Vaites LP, Paulo JA, Zhang T, Gray NS, Gygi SP, Chouchani ET. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180: 968–983e24, 2020. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Matsushima S, Kuroda J, Zhai P, Liu T, Ikeda S, Nagarajan N, Oka S, Yokota T, Kinugawa S, Hsu CP, Li H, Tsutsui H, Sadoshima J. Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J Clin Invest 126: 3403–3416, 2016. doi: 10.1172/JCI85624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J, Yoo JY, Suh JM, Park S, Kang D, Jo H, Bae YS. The flagellin-TLR5-Nox4 axis promotes the migration of smooth muscle cells in atherosclerosis. Exp Mol Med 51: 78, 2019. doi: 10.1038/s12276-019-0275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D, Rodriguez R, Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun 8: 997, 2017. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, Lee WJ, Choi CW, Shin HK, Kim DJ, Koh ES, Park CS, Kwon SW, Park SW. Metabolic profiling regarding pathogenesis of idiopathic pulmonary fibrosis. J Proteome Res 15: 1717–1724, 2016. doi: 10.1021/acs.jproteome.6b00156. [DOI] [PubMed] [Google Scholar]
- 65.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarman ER, Khambata VS, Cope C, Jones P, Roger J, Ye LY, Duggan N, Head D, Pearce A, Press NJ, Bellenie B, Sohal B, Jarai G. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am J Respir Cell Mol Biol 50: 158–169, 2013. doi: 10.1165/rcmb.2013-0174OC. [DOI] [PubMed] [Google Scholar]
- 67.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AM, Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-β1 in pulmonary fibrosis. PloS One 10: e0121246, 2015. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernard K, Hecker L, Luckhardt TR, Cheng G, Thannickal VJ. NADPH oxidases in lung health and disease. Antioxid Redox Signal 20: 2838–2853, 2014. doi: 10.1089/ars.2013.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogura M, Yamaki J, Homma MK, Homma Y. Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochem J 447: 281–289, 2012. doi: 10.1042/BJ20120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salvi M. Receptor tyrosine kinases take a direct route to mitochondria: an overview. Curr Protein Pept Sci 14: 635–640, 2013. doi: 10.2174/13892037113146660087. [DOI] [PubMed] [Google Scholar]
- 71.Salvi M, Brunati AM, Toninello A. Tyrosine phosphorylation in mitochondria: a new frontier in mitochondrial signaling. Free Radic Biol Med 38: 1267–1277, 2005. doi: 10.1016/j.freeradbiomed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Koc EC, Miller-Lee JL, Koc H. Fyn kinase regulates translation in mammalian mitochondria. Biochim Biophys Acta 1861: 533–540, 2017. doi: 10.1016/j.bbagen.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozieł R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, Jansen-Dürr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452: 231–239, 2013. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 74.Canugovi C, Stevenson MD, Vendrov AE, Hayami T, Robidoux J, Xiao H, Zhang YY, Eitzman DT, Runge MS, Madamanchi NR. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol 26: 101288, 2019. doi: 10.1016/j.redox.2019.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]