Abstract
Volumetric muscle loss (VML) is the traumatic loss of muscle tissue that results in long-term functional impairments. Despite the loss of myofibers, there remains an unexplained significant decline in muscle function. VML injury likely extends beyond the defect area, causing negative secondary outcomes to the neuromuscular system, including the neuromuscular junctions (NMJs), yet the extent to which VML induces denervation is unclear. This study systematically examined NMJs surrounding the VML injury, hypothesizing that the sequela of VML includes denervation. The VML injury removed ∼20% of the tibialis anterior (TA) muscle in adult male inbred Lewis rats (n = 43), the noninjured leg served as an intra-animal control. Muscles were harvested up to 48 days post-VML. Synaptic terminals were identified immunohistochemically, and quantitative confocal microscopy evaluated 2,613 individual NMJ. Significant denervation was apparent by 21 and 48 days post-VML. Initially, denervation increased ∼10% within 3 days of injury; with time, denervation further increased to ∼22% and 32% by 21 and 48 days post-VML, respectively, suggesting significant secondary denervation. The appearance of terminal axon sprouting and polyinnervation were observed as early as 7 days post-VML, increasing in number and complexity throughout 48 days. There was no evidence of VML-induced NMJ size alteration, which may be beneficial for interventions aimed at restoring muscle function. This work recognizes VML-induced secondary denervation and poor remodeling of the NMJ as part of the sequela of VML injury; moreover, secondary denervation is a possible contributing factor to the chronic functional impairments and potentially an overlooked treatment target.
NEW & NOTEWORTHY This work advances our understanding of the pathophysiologic complexity of volumetric muscle loss injury. Specifically, we identified secondary denervation in the muscle remaining after volumetric muscle loss injuries as a novel aspect of the injury sequela. Denervation increased chronically, in parallel with the appearance of irregular morphological characteristics and destabilization of the neuromuscular junction, which is expected to further confound chronic functional impairments.
Keywords: innervation, neuromuscular junction, polyinnervation, skeletal muscle, sprouting
INTRODUCTION
Volumetric muscle loss (VML) injuries are defined as the traumatic or surgical removal of skeletal muscle tissue that outstrips endogenous regenerative capacity and causes chronic functional deficits (1). A variety of etiologies may directly or indirectly lead to VML injury, such as type III open tibia fracture, crush injury, compartment syndrome, infectious myositis, and sarcoma (2–4). The relative incidence of VML is particularly high among battlefield injuries. For instance, recent studies evaluating ∼14,500 US and ∼2,000 UK injured and evacuated service members between 2001 and 2014 revealed more than three-quarters had traumatic musculoskeletal injures, nearly all of which involved VML (1, 5–7). Following necessary surgical repair, physical therapy is the primary clinical treatment option; however, little functional improvement following intensive therapy has been observed in cohorts of VML-injured active-duty service members (8). Regenerative medicine therapies are under various stages of development, although while generally effective at improving force production in preclinical animal models they are yet to realize their full potential (9). Given the difficulties experienced for improving functional outcomes, a more in-depth understanding of the mechanisms of force loss after VML injury is needed (10–12).
Chronic limb dysfunction after VML may be attributed to injury sequelae both intrinsic (e.g., permanent contractile tissue loss) and extrinsic (e.g., nonunion, musculocutaneous tethering) to the traumatized musculature that manifest as impairments of active force production, weight-bearing capacity, and/or joint range of motion. With focus on intrinsic impairment of active force production, models of VML often present a greater magnitude of active force deficit than estimated contractile tissue loss, inferring that the residual portion of muscle is producing force suboptimally (11, 13). In support, a recent study that investigated alterations in rat tibialis anterior (TA) muscle architecture 4 wk after VML injury observed an ∼50% isometric peak tetanic force deficit despite not observing a significant decrease in physiological cross-sectional area (14), the primary determinant of isometric force-producing capacity (15). Given the use of direct, maximal peripheral nerve stimulation to elicit active force production in this study (14), suboptimal force produced by the residual muscle tissue after VML injury may be broadly attributed to failure to generate (e.g., denervation and excitation-contraction uncoupling) and/or transmit force (16).
Due to the destructive nature of VML, disruption of myofiber innervation secondary to intramuscular neural damage is a plausible significant determinant of prolonged active force deficits. In support, seven of eight patients with VML injuries displayed signs of chronic mononeuropathy in clinical nerve conduction and EMG studies (17). Additionally, two recent animal studies have observed a reduced number of motor endplates (18) and sustained motoneuron axotomy (19) up to 14–28 days after VML injury in the mouse quadriceps muscle and rat TA muscle, respectively. Collectively, these studies support the presence of prolonged neuromuscular damage after VML injury; however, an in-depth analysis of neuromuscular junction (NMJ) homeostasis is currently lacking from VML studies. Therefore, the purpose of the current study was to systematically and quantitatively evaluate individual en face NMJs in the residual muscle tissue after VML injury using a well-established rat TA muscle model. The hypothesis that VML injury causes prolonged denervation and irregular NMJ morphological characteristics associated with other neuromuscular pathologies was tested.
MATERIALS AND METHODS
Animals and Ethical Statements
Adult male (∼4 mo of age) inbred Lewis rats were purchased (Charles River, n = 43) and maintained on a 14–10-h light–dark schedule with ad libitum food and water in a vivarium accredited by the American Association for the Accreditation of Laboratory Animal Care. All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee at the University of Minnesota (1811-36513 A) in compliance with the National Institute of Health Guidelines. All experiments were conducted in compliance with the Animal Welfare Act, the Implementing Animal Welfare Regulations, and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals.
Volumetric Muscle Loss
Rats were given at least 1 wk to acclimate before any experimentation and randomly assigned to experimental groups (n = 8 per time point). All rats underwent a full thickness VML surgery to the left TA muscle, and the noninjured contralateral limb was used as an intra-animal control. Rats were administered a subcutaneous injection (buprenorphine-SR: 1.2 mg/kg) ∼2 h before surgery. Surgeries were performed using aseptic techniques under anesthesia (1–3% isoflurane in oxygen) as previously described (20–24). Briefly, a longitudinal incision was made along the anterior compartment of the left hind leg to expose the TA muscle. A metal spatula was inserted between the TA muscle and the anterior portion of the tibia, and a 6-mm full thickness muscle punch biopsy was used to remove ∼20% of the middle third of the TA. In a subset of animals, the removed muscle defect was saved for histologic analysis. Proline sutures were placed around the removed area, for reference during harvest and tissue processing. Lastly, the fascia and skin were closed in layers.
Terminally, rats were kept under anesthesia (1–3% isoflurane in oxygen) during tissue harvest, and while still anesthetized, euthanized with an overdose intraperitoneal injection of sodium pentobarbital (≥100 mg/kg). A subset of rats were included in an acute biopsy study to elucidate the magnitude of direct frank removal of NMJs in which the TA muscle was harvested immediately after the VML surgery (day 0) before skin closure (nonsurvivable surgery). These evaluations were done in combination with the removed muscle defects noted above. Otherwise TA muscles were harvested at terminal time points of 3, 7, 14, 21, and 48 days post-VML. Samples were weighed, and the TA muscle was then split using the Proline sutures for reference. The proximal half of the TA was further partitioned into six longitudinal sections and used for fresh labeling of the NMJs. The distal half of the TA muscle was mounted and frozen in isopentane cooled in liquid nitrogen and stored at −80°C for subsequent histological identification of myofiber cross-sections. The total number of myofibers were counted in a subset of the control and VML-injured TAs at day 0, immediately following surgery, and the VML defects. The distal section of the TA muscles and the removed VML defect area were sectioned at 10 µm and stained with Masson’s trichrome, and subsequently imaged using a 20× objective (0.75 NA, 0.5 µm/pixel resolution) on the Bright-field Huron TissueScope LE slide scanner (Huron Digital Pathology, St. Jacobs, ON, Canada).
Neuromuscular Junction Histology
Immediately following dissection, the proximal TA muscle was stained for the assessment of pre- and postsynaptic NMJ structures as previously described and validated (25–29). Briefly, the proximal portion of the TA muscle was fixed in 4% paraformaldehyde, cut into six longitudinal segments, and pinned to a black Sylgard bottom petri dish. Sampling of NMJs for analysis was across all six segments, within all TA muscles. Muscles were incubated in α-bungarotoxin to stain the postsynaptic motor endplate (Alexa Fluor 488 conjugated, 0.1 μg/mL; B13422, Invitrogen). Samples were washed, blocked, and left to incubate 18 h at 4°C in anti-neurofilament (0.04 µg/ml; 2H3) and synaptic vesicle (0.8 µg/ml; SV2) primary antibodies, labeling the axon and presynaptic endplate of the NMJ, respectively. Anti-neurofilament and SV2 were obtained from the Developmental Studies Hybridoma Bank. Samples were incubated in a goat anti-mouse IgG1 secondary antibody (Alexa Fluor 546 conjugated, 10 µg/mL; A-21123, Thermo Fisher). All TA muscles were washed and stored at 4°C in TBS until confocal imaging, which occurred no more than 3 days following the staining procedure.
Confocal Imaging
Imaging of NMJs in whole muscle mounts using confocal microscopy have been previously described (25–31). All efforts were made to minimize bias and blind investigators to injured versus control samples. To do this, previous studies have shown that the postsynaptic endplate stays mostly intact despite possible retraction of the terminal axon. Therefore, individual NMJs were identified in the eyepiece using only the 488 filter (postsynaptic motor endplate), blinding the investigator to any presynaptic outcomes (i.e., denervation). Identified and imaged NMJs were no more than 60 μm deep within individual muscle sections, to minimize possible effects of antibody penetration or optical distortion. Laser intensity and gain settings were established during the initial imaging session and kept within a limited range (±5 and 10 units, respectively) throughout the study to maintain imaging consistency. In total 47 ± 15 en face NMJs per TA muscle were identified and imaged, representing a total of 2,613 individual NMJs evaluated across this study. Imaging was conducted using a Nikon C2 automated upright laser scanning confocal microscope equipped with an NIR Apo 40x 0.80 W DIC N2 water dipping objective and dual GaSP detectors (Nikon Instruments Inc., Melville, NY). Image sampling was determined using nyquist calculations with a pixel size set to 0.16 µm and 512 × 512 dimensions. Step size was set to match the empirically determined Z-axis resolution (0.8 μm), as determined previously (25, 27–29). In all cases, the expected staining patterns in normal skeletal muscle were observed and the specificity of antilabeling was confirmed by the absence of staining outside expected neural structures, consistent with manufacturer’s technical information, and previous experience. For display purposes only, images were down-converted, without introducing any changes in brightness or contrast and produced in Adobe Photoshop (Adobe Systems Inc.).
Neuromuscular Junction Morphology Analysis
Analysis of all 2,613 individual pre- and postsynaptic NMJ structures (Fig. 1) was conducted with confocal image stacks consisting of 20–60 optical slices (each a two-channel 8-bit multi-TIFF file) as previously described (25, 32). All analyses were conducted using Fiji (33). Briefly, the 2-D planar area of the motor endplate was obtained from a maximum intensity projection image where the pre- and postsynaptic channels were separated and the image intensity threshold was determined with the Otsu algorithm. If parts of the NMJ were still visible, the threshold was manually adjusted. All NMJ images were cropped to fit tightly within the image borders, allowing for measurement of relative planar area, calculated by dividing the postsynaptic 2-D planar area by the area defined by the main orthogonal axes of the endplate; this measure reflects the complexity of the motor endplate (i.e., fragmentation). Additionally, the colocalization area of the pre- and postsynaptic terminals, which represent NMJ innervation, was performed using the “colocalization.” Colocalization area was measured and calculated as a percentage of the postsynaptic area. The volume of the pre- and postsynaptic terminals was determined using the “3 D object counter” feature, wherein the threshold was set manually based on marker visibility. Lastly, NMJs were categorized by a size distribution frequency to evaluate potential differences in area and/or volume.
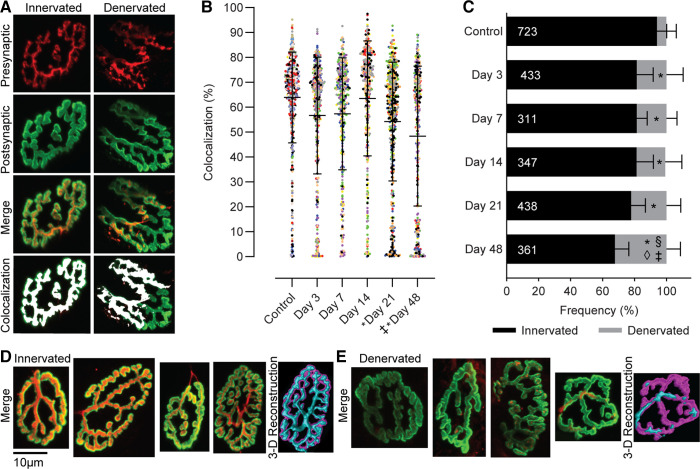
Figure 1.
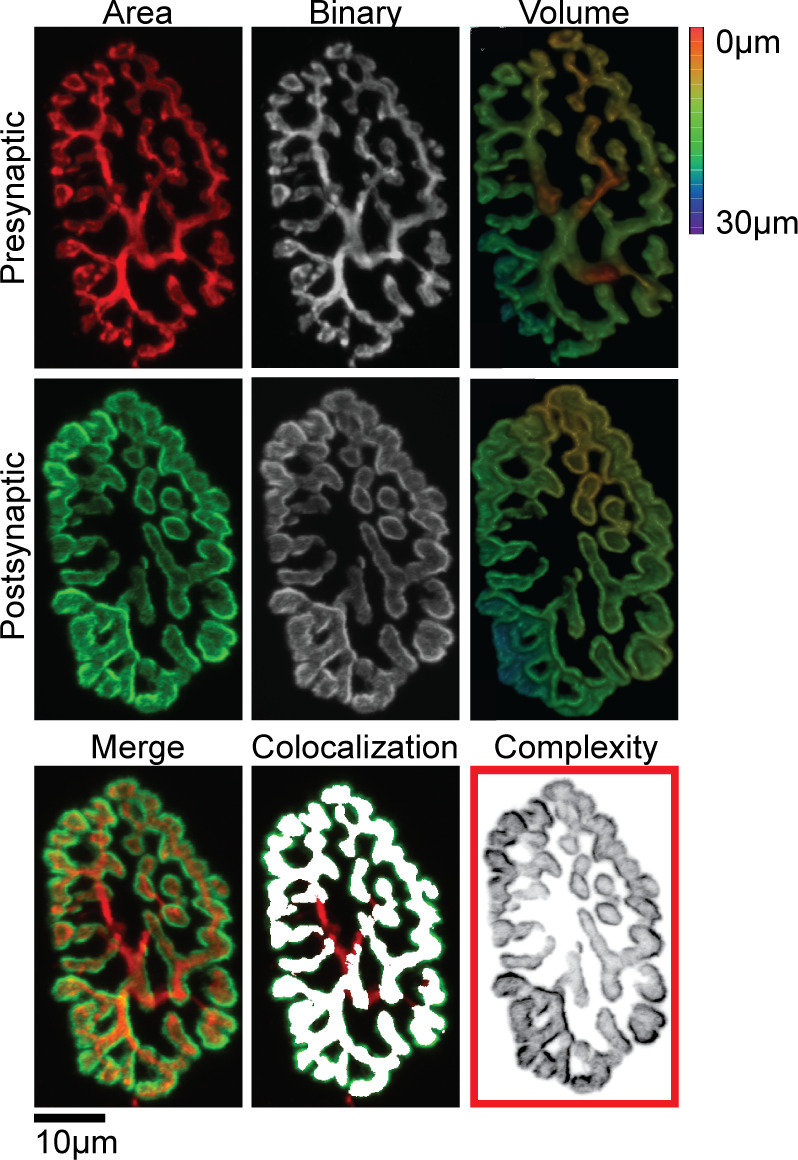
Individual presynaptic (red) and postsynaptic (green) NMJ structures were stained, imaged, binarized, and used to quantitatively measure two-dimensional (2-D) planar area, three-dimensional (3-D) volume, colocalization, and complexity. Images were also used for visual analysis of NMJ denervation and the appearance of irregular morphological characteristics. Scale bar 10 µm and 3-D color depth scale range 0–30 µm. NMJ, neuromuscular junctions.
Innervation is determined by identification of three main structures of the motor unit: the terminal axon branches that extend from the motoneuron, and the NMJ pre- and postsynaptic terminals (32); NMJs are best identified for in depth analysis in the longitudinal myofiber orientation (34, 35). Qualitative assessment of NMJ innervation and morphology was performed using maximal image projections by two investigators blinded to experimental groups. The intrareliability between investigators was 97% and 99% for visual assessment of innervation and morphological characteristics, respectively. Classification of denervation of an individual NMJ was determined if there was a partial or complete lack of colocalization between the pre- and postsynaptic terminals. Morphological characteristics such as fragmentation, sprouting, and/or polyinnervation were determined as previously illustrated (34). Irregular morphological characteristics can be identified when the axon terminal sprouts, forming extensions that do not correspond with the postsynaptic endplate, or by evaluation of polyinnervation, when a single postsynaptic site is innervated by two or more axons (25, 31, 34). As the morphological characteristics that were identified are mostly dependent on axon innervation, data are calculated as a percentage of innervated NMJs (n = 2,395), excluding all denervated NMJs (n = 218).
Statistical Analyses
Individual NMJs were analyzed for 2-D planar area, 3-D volume, complexity, and colocalization, whereas innervation and morphology were calculated as a percentage for each animal. Outcome measures were analyzed using a mixed linear model to correct for intra-“animal” variability as a random effect when comparing control and VML responses at 3, 7, 14, 21, and 48 days postinjury using a fixed effect of “group.” In all analyses, the animal random effect was statistically significant (P ≤ 0.001). If the main effect of group was significant, a Tukey HSD post hoc comparison of means was performed to further delineate differences. The control group comprised pooled data from at least two contralateral muscles per time point (3, 7, 14, 21, and 48 days), as well as the biopsied and contralateral muscles from the acute biopsy study (0 days); the biopsied muscles were statistically similar to the contralateral muscles included in the control group (P ≥ 0.166) for all neuromuscular variables. NMJ area and volume distribution analysis was performed using a chi-square statistical model, where each day was compared independently to the control group. Analyses were performed using JMP Pro (v. 14.2, SAS Institute, Cary, NC). Data are presented as mean ± SD. Results were considered statistically significant at P < 0.05.
RESULTS
Body and TA Muscle Weights
Following surgery, all rats recovered promptly and without complications. The average VML defect weight was 84.5 ± 12.8 mg. As expected, body weight steadily increased after VML surgery, such that by 48 days post-VML rats were ∼15% heavier. The mass of the VML-injured TA muscles increased initially (day 3), likely due to edema, before decreasing to values lesser than controls after 7 days post-VML (P < 0.001).
Acute Frank Loss of Myofibers and NMJs
Immediately following the biopsy to create the VML defect (day 0), total myofiber number per TA muscle cross-section was similar between controls (9,285 ± 414) and the sum of residual (5,199 ± 581) and excised (3,866 ± 869; sum = 9,065 myofibers; P = 0.757) tissues of the biopsied muscle; creation of the VML defect resulted in an immediate loss of ∼42% of the TA myofibers in cross-section (P < 0.001). The NMJs within rodent TA muscle distribute in an inverted V shape configuration on the proximal third of the muscle (36); thus, the VML defect is unlikely to directly remove a substantial number of NMJs. In confirmation, only 75 ± 24 NMJs were detected in the removed VML defect, indicating only an estimated 1% of NMJs in the entire muscle are abruptly removed in this VML model.
Denervation of the Neuromuscular Junction
Serving as a quantitative index of innervation status, the average magnitude of colocalization of pre- and postsynaptic terminals was significantly reduced after VML injury (Fig. 2; main effect for group P < 0.001). Specifically, compared with controls the percentage of colocalization was ∼10% and 15% less at 21 and 48 days post-VML. Additionally, qualitative assessment of each NMJ demonstrated an increased percentage with a partial or complete lack of colocalization (i.e., denervated) following VML (P < 0.001). Specifically, there were ∼13% more denervated NMJs at 3 days post-VML compared with control, which remained unchanged through 14 days before increasing an additional ∼3% and 13% by 21 and 48 days post-VML, respectively.
Figure 2.
A: representative images showing both individual and merged presynaptic (red) and postsynaptic (green) terminals along with the overlap area (colocalization; white) for an innervated and a partially denervated NMJ. B: the percentage of colocalization was determined by comparing the colocalization area to the postsynaptic area. Colocalization data were analyzed as a mixed model where individual animals were used as a random effect (P < 0.001). Data are presented as overall group means ± SD; additionally each dot represents an individual NMJ and each color represents an individual muscle. C: evidence of denervation was significantly greater in VML injured muscle, particularly at later time points. The numbers within the bars represent the total number of NMJs analyzed. Data are analyzed by one-way-ANOVA and presented as individual animal means ± SD. Significant difference (P < 0.05) compared to *controls; §day 3; ◊day 7; ‡day 14. Representative images of innervated (D) and denervated (E) NMJs. For visualization, confocal z-stacks were rendered into 3-D stacks using Volume View in Nikon NIS-Elements and displayed as binary reconstructions. Denervation was defined as the partial or complete absence of the presynaptic terminal based on maximum intensity projections of en face NMJs. NMJ, neuromuscular junctions; VML, volumetric muscle loss.
Volumetric Muscle Loss Effects on Neuromuscular Junction Size
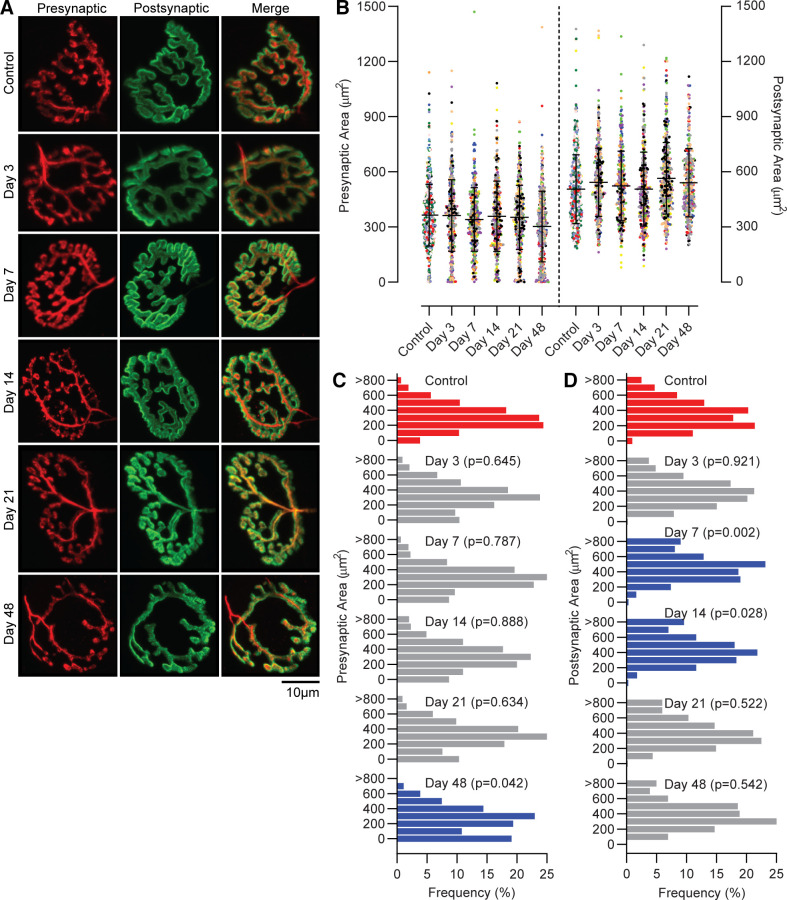
The mean presynaptic NMJ area was 351 ± 180 µm2 across groups and was not significantly different at any time point after VML compared with controls (Fig. 3; P = 0.089). However, analysis of the distribution of presynaptic areas indicated a shift toward smaller presynaptic terminals by 48 days post-VML (P = 0.042). Specifically, 19% of presynaptic terminals were within 0–100 µm2 at 48 days post-VML, compared with only 3% in the control. The mean postsynaptic NMJ area was 508 ± 187 µm2 across groups and also was not significantly different at any time point after VML compared with controls, despite initially observing a significant main effect of group (P = 0.048). Distribution analysis demonstrated a shift toward larger postsynaptic terminals, wherein ∼20% more terminals were in the larger (≥401 µm2) versus smaller half (0–400 µm2) of postsynaptic areas at 7 and 14 days post-VML compared with control (P ≤ 0.028).
Figure 3.
A: representative maximum projection of confocal images of control and VML-injured NMJs, labeled with synaptic vesicle/neurofilament (red; presynaptic terminal and axon) and α-bungarotoxin (green; postsynaptic motor end-plate). The merged image displays overlap of pre- and postsynaptic structures. B: two-dimensional (2-D) planar area of the presynaptic and postsynaptic terminals were similar in control and VML-injured NMJs. Data were analyzed using a mixed linear model where the random effect of animal was significant (presynaptic P = 0.004; postsynaptic P = 0.007). Data are presented as overall group means ± SD; additionally each dot represents an individual NMJ and each color represents an individual muscle. There was a significant shift in NMJ size distributions for both pre- (C) and post-synaptic (D) area as determined by chi-square analysis (P < 0.05) compared with control. Red bars represent controls, blue bars indicate significantly different from controls, and gray bars indicate no difference from control; individual P values are noted at each group. NMJ, neuromuscular junctions; VML, volumetric muscle loss.
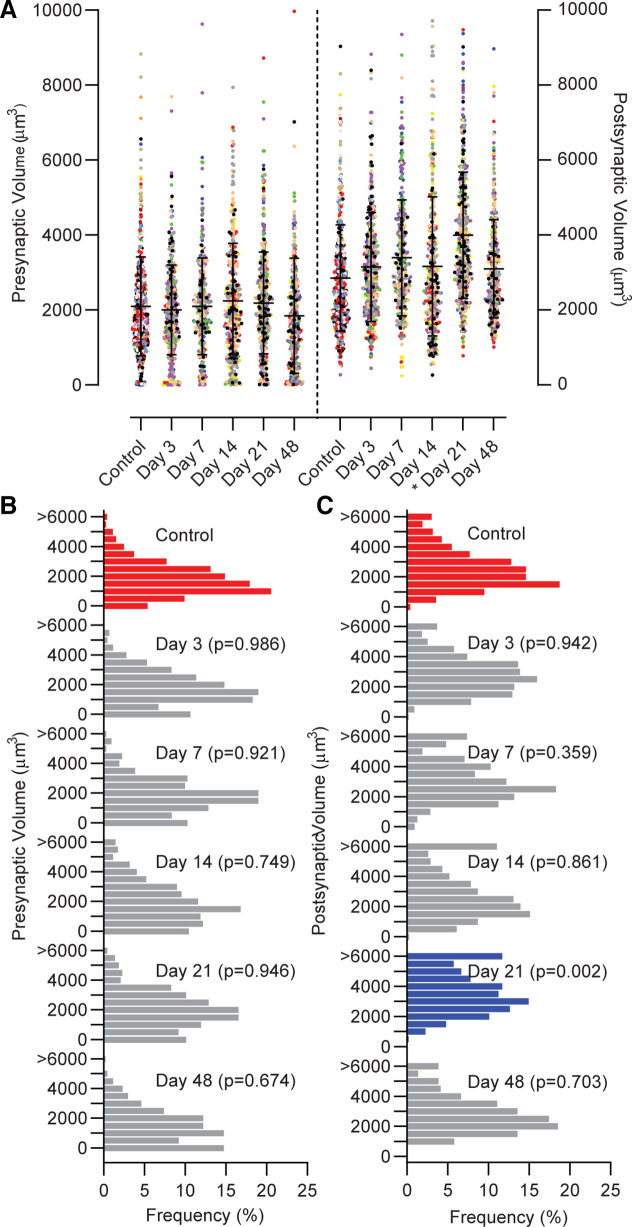
The presynaptic volume was not different among groups (Fig. 4; P = 0.663), and a distribution of presynaptic volumes was similar between control and VML at all time points (P ≥ 0.673). An increase in postsynaptic volume (P = 0.005) at 21 days post-VML was observed compared with control and all other time points. Supporting this finding, ∼33% more postsynaptic terminals reside in the larger half (≥3,001 µm3) of the volume distributions at 21 days post-VML compared with control (P = 0.002). Distributions of postsynaptic volumes were similar between control and VML at all other time points (P ≥ 0.359).
Figure 4.
A: the volume of the presynaptic terminal was similar in control and VML-injured NMJs (P = 0.667). The postsynaptic 3-D volume was significantly larger at 21 days post-VML compared with control (noted by *P = 0.005). Data were analyzed using a mixed linear model where the random effect of animal was significant (presynaptic P < 0.001; postsynaptic P < 0.001). Data are presented as overall group means ± SD; additionally each dot represents an individual NMJ and each color represents an individual muscle. B: the presynaptic volume distribution was similar for all groups, and there was a shift toward larger (C) postsynaptic volume distributions at day 21 as determined by chi-square analysis compared with control (P < 0.05). Red bars represent controls, blue bars indicate significantly different from controls, and gray bars indicate no difference from control; individual P values are noted at each group. NMJ, neuromuscular junctions; VML, volumetric muscle loss.
Pathologic Morphology of the Neuromuscular Junction
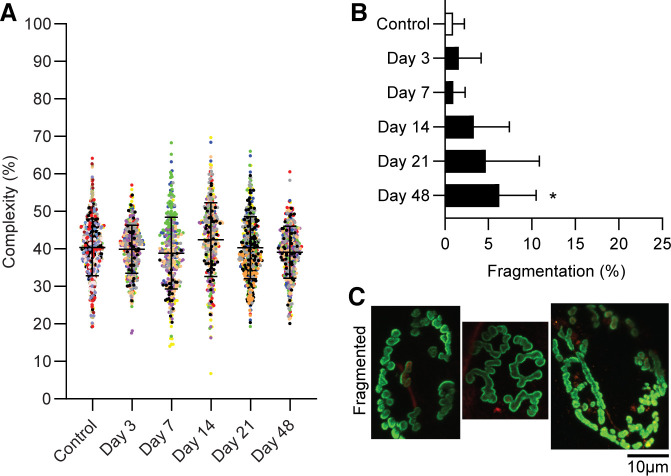
Irregular morphological characteristics of NMJs appeared more frequently following VML. There were less singly innervated NMJs in the TA muscle at time points studied following VML (Fig. 5; P < 0.001). The appearance of sprouting or polyinnervation alone were significantly increased at all times after day 3 (P ≤ 0.001), with the exception of polyinnervation at 14 days post-VML. The combination of both sprouting and polyinnervation of NMJs was most frequently observed at 48 day post-VML. Complexity remained unchanged across groups (P = 0.103); however, qualitatively greater fragmentation of the postsynaptic terminals was apparent 48 days post-VML (Fig. 6; P = 0.013).
Figure 5.
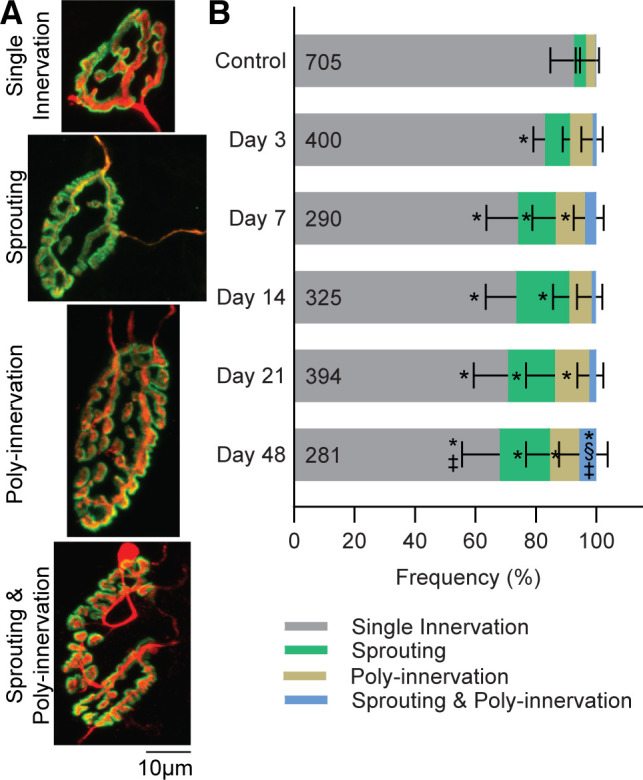
A: representative images of a single innervated NMJ compared with NMJs with irregular morphology, including sprouting (the motor axon terminal forms extensions that do not correspond with the postsynapse), polyinnervation (a single postsynaptic site is innervated by two or more motor axons), or a combination of sprouting and polyinnervation. B: irregular presynaptic morphological alterations appeared more frequently in VML-injured NMJs. The numbers within the bars represent the number of innervated NMJs analyzed. Data are analyzed by one-way-ANOVA and presented as individual muscle means ± SD. Significant (P < 0.05) differences compared with *controls; §day 3; ‡day 14. NMJ, neuromuscular junctions; VML, volumetric muscle loss.
Figure 6.
A: complexity of NMJs was measured by comparing the two-dimensional area of the postsynaptic terminal to the total orthogonal area of the image. Data were analyzed as a mixed linear model where individual animals were used as a random effect (P < 0.001). Complexity was similar between groups (P = 0.103). Data are presented as means ± SD; additionally each dot represents an individual NMJ and each color represents an individual muscle. B: fragmentation was qualitatively analyzed and significantly greater by 48 days compared with controls (noted by *P = 0.013). Data are analyzed by one-way-ANOVA and presented as individual animal means ± SD; total NMJ n = 2,606. C: representative images of fragmented NMJs displaying the postsynaptic terminal separating into a series of islands. NMJ, neuromuscular junction; VML, volumetric muscle loss.
DISCUSSION
The most salient finding herein is that VML injury resulted in acute denervation of myofibers within the residual muscle tissue that progressively worsened through the ensuing 6 wk. In synchrony, the presence of irregular NMJ morphological characteristics, mainly terminal axon sprouting, polyinnervation, and fragmentation, also progressively increased. It is important to emphasize that the denervation observed was not explained by blunt excision of NMJs, as a small fraction (<1%) of the estimated total number of postsynaptic endplates in the TA muscle were detected within the removed muscle tissue. Therefore, the progressive disruption of NMJ homeostasis observed appears to be a secondary degenerative process initiated by the primary injury. Collectively, these pathophysiological changes presumably contribute to the significant and irrecoverable loss of muscle function following VML.
The most overt consequence of VML injury is the blatant removal of myofibers. In this study ∼42% of TA myofibers were removed during the VML surgery, which is in line with previous reports (19, 37, 38). The magnitude of strength loss after VML injury is not fully explained by myofiber or contractile protein loss and architectural changes (4, 11, 19, 39); prolonged denervation of the remaining fibers has been postulated as an exacerbating factor (11). In support, spinal cord and peripheral nerve injury studies have shown that innervation is intimately tied to muscle contractility and the excitation-contraction coupling (40, 41), such that successful restoration of muscle strength is dependent on axonal and myofiber regeneration, as well as re-establishment of functional NMJs. The pathological NMJ features observed in this study bear similarities to other degenerative conditions (35, 42–45), although unfortunately adds to the already stark regenerative challenges encountered after VML injuries. Notwithstanding, denervation is a long overlooked component of the VML pathophysiology and may help explain why muscle function following VML fails to fully recover.
Acute denervation and altered NMJ morphology are not unexpected discoveries following VML. In fact, these characteristics are a common pathophysiologic consequence of skeletal muscle injury and indicative of the endogenous neuromuscular remodeling response (44, 46, 47). Perhaps the most concerning and unique aspect of the pathology observed is that secondary denervation, sprouting, polyinnervation, and fragmentation become progressively greater. Indicating that a more chronic and progressive pathological response similar to that found in aging (i.e., sarcopenia) and muscle wasting diseases (e.g., amyotrophic lateral sclerosis, ALS; muscular dystrophy) may follow VML injuries (42, 43, 48–50). However, secondary denervation is strongly supported by a recent study using this VML model, which showed significant axotomy without motoneuron death, with no signs of improved axon reinnervation by 3 wk postinjury (19). Furthermore, the trajectory and mapping of axons within the rodent TA muscle (51), though variable, supports a prominent role of direct intramuscular axon damage in the progressive disruption of NMJ homeostasis observed in this study.
Polyinnervation and axon sprouting are commonly seen during development and at acute stages of injury-induced neuromuscular remodeling (52–56). Polyinnervation is a process where terminal axons compete for ownership of a single motor end plate (57, 58), whereas axon sprouting is thought to be a compensatory mechanism for providing stimulation to adjacent denervated fibers (59). These characteristics are thought to be involved in NMJ remodeling (60, 61), but may also present complications for contraction and force generation. Thus, an interesting possibility is that the early recovery in muscle strength (i.e., between 0–14 days post-VML), polyinnervation, and axon sprouting observed following VML may be partially attributed to an attempted reinnervation process before additional recovery in strength is blunted. This may also explain why the chronic appearance of these traits correspond with the increase of secondary denervation. Conversely, these irregularities may also reveal dysfunction within the neuromuscular system (59, 62). In fact, many of the NMJs evaluated had the appearance of both polyinnervation and sprouting on individual NMJs at 48 days post-VML. Notably, many of these NMJs also presented a component of fragmentation, which was significantly increased at 48 days post-VML. Degenerating NMJs or fragmentation of the postsynaptic terminal is indicated when the terminal breaks into a series of islands, which is also known to contribute to declines in muscle function (63–65) and destabilization of the NMJ. Thus, it seems likely that the increasingly complex appearance of NMJ morphology may carry negative consequences for the overall health and functionality of the myofibers. Additional investigations are needed to determine if these are attempts to reinnervate the myofibers or markers of instability in the neuromuscular remodeling process.
A potentially promising discovery from this study was the overall maintenance of NMJ size (area and volume), which are altered in other muscle wasting conditions (25, 31, 45, 63, 64, 66, 67). This finding suggests that the NMJs may be more readily accessible to reinnervation if presented with the appropriate cues. Taken together with the remaining, although axotomized, motoneurons, the potential for axon regeneration and NMJ reinnervation is apparent (19). In support of this possibility, previous work has identified NMJ structures on newly forming myofibers in and around the VML defect area following various regenerative treatments (68–70). However, these studies do not sufficiently examine innervation within the remaining tissue, as this was not their purpose. It is recommended that future studies examining innervation take a similar approach to what has been done herein, using longitudinal sections to identify the pre- and postsynaptic structures of the NMJ with any corresponding axons within the defect area and throughout the remaining tissue.
Targeting the obvious loss of contractile tissue, therapies have focused primarily on interventions aimed at myofiber regeneration and/or strengthening the remaining tissue, including rehabilitation, implantation of acellular biomaterials, cell transplants, drug administration, or any combination of approaches. Collectively these therapies have shown promising yet underwhelming results (9). For example, rehabilitation shows poor hypertrophic and metabolic adaptability in the remaining tissue (71, 72). Due to the added structural support, acellular biomaterials generally show some improvement in force transmission but do not contribute to force generation (22, 24, 73). Stem cell transplants show potential to regenerate pockets of de novo myofibers, but are still unable to fully restore function to the tissue (23, 37, 74). Antifibrotic drugs have successfully prevented the overwhelming formation of pathologic fibrosis but likely contribute to impaired force transmission (75, 76). Fundamentally, innervation is necessary for any existing or de novo myofiber to survive, maturate, and activate the excitation-contraction coupling apparatus to generating force. Although myofiber regeneration can initially occur without neural influence, prolonged denervation inhibits growth and leads to fiber atrophy, and poor conduction velocity, all in addition to the loss of function (77). If the ultimate goal of treatment is to restore muscle function, current therapies alone have not been sufficient and will likely require the addition of other innovative and integrated therapies (10). The VML pathophysiology presents a poor environment for neuromuscular remodeling with a greater need to apply treatments specifically targeting innervation. It is possible that innervation may be the supplemental link that connects rehabilitation and regenerative therapies together to successfully restore function to VML injured muscle (78).
It is important to note that despite the maintenance of NMJ size and complexity throughout the course of this study, the overall impact of VML injury on innervation appears to progressively worsen with time. If the current trajectory continues, fragmenting and degradation of the synaptic terminals could increase, perhaps similar to the delayed response seen in denervation injuries (35). Therefore, any rehabilitative or regenerative medicine intervention may be more impactful if administered subacutely after injury. Other studies support this claim, indicating there is likely a window for optimized success when applying rehabilitative and regenerative therapies (71, 79). Although not investigated in this work, VML-induced disruption of sympathetic and sensory aspects of the neuromuscular system are probable, and important to consider in future studies evaluating rehabilitative approaches.
The mechanisms driving secondary denervation and destabilization of the NMJ after VML injury are currently unknown. Age-related changes at the NMJ, such as denervation, fragmentation, and axonal sprouting, have been linked to decreased metabolic function (49, 80–82). With sarcopenia, it is also well understood that there is a downregulation of the PGC1α pathway (80), in parallel with the loss of muscle size and function. In fact, the age-related destabilization of the NMJ has been corrected with exercise (66) and direct overexpression of PGC1α (55). Recent investigations of the remaining muscle after VML support similar physiologic impairment in the metabolic function (39, 71, 83), and a lack of metabolic gene response specifically of PGC1α (39, 72), suggesting a possible similar mechanism. Unfortunately, following VML there is a resistance to the expected beneficial adaptation of progressive muscle overload, or exercise, including the absence of the expected increase in AMPK-PGC1α signaling (39, 71, 72).
The ongoing maintenance and repair of the NMJs is dependent on various cellular interactions within the terminal axons, pre- and postsynaptic terminals, and myofibers. These interactions bring into play both resident cell populations of satellite, perisynaptic Schwann, and endothelial cells and infiltrating cell populations, such as immune cells and macrophages (84–86). In models of ALS, in which the NMJ is progressively destabilized, the cellular interactions of the macrophages and satellite cells are well understood (87). Although persistent macrophage activation plays a role in the progression of ALS, satellite cells contribute to the stability of the NMJ (88). Unfortunately the cellular interactions at the NMJ following VML are unclear and appear to have a conflicting response between macrophages and satellite cells that could negatively impact the NMJ. Globally following VML injury, the residual muscle tissue transitions to a nonregenerative phenotype with persistent inflammatory and fibrotic signaling that suppresses regeneration (11, 21, 24, 89). This does not transition to an anti-inflammatory phenotype, which is ultimately needed to support regeneration. The lack of regenerative response following VML is expected to suppress the proliferative capacity of the satellite cells, which has been proposed yet not fully understood. There is a significant and robust infiltration of macrophages following VML, but a possible lack of support for prompting satellite cell proliferation and differentiation. Future work is needed to systematically evaluate the synaptic-related cells and their interactions, to elucidate the mechanisms of denervation and destabilization of the NMJ following VML.
Understanding that denervation increases chronically, in parallel with the appearance of irregular morphological characteristics, highlights the pathophysiologic complexity of VML injuries. This work identifies secondary denervation as a novel pathophysiologic aspect of the sequela of VML injury. Although other muscle pathologies show NMJ degeneration, there was no evidence of a VML-induced impact on NMJ area or volume, particularly at the presynaptic terminal. It is possible that secondary denervation following VML is contributing to both the chronic loss of muscle function and limited efficacy for regenerative and rehabilitative treatments currently being developed and evaluated. Future research should examine long-term innervation outcomes to see if the unique pathology observed herein continues chronically. Future work is needed to elucidate the exact mechanisms responsible for VML-induced denervation and destabilization of the NMJ. Studies aimed at restoring function to VML-injured muscle should consider targeting NMJs in the remaining muscle, alone or in combination with regenerative medicine and rehabilitation interventions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee at the University of Minnesota (1811-36513 A).
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are primarily presented in the current manuscript and are available from the corresponding author on reasonable request.
GRANTS
This work was supported by funding from the Department of Defense (W81XWH-19-1-0075 to S. M. Greising).
DISCLAIMERS
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.T.C. and S.M.G. conceived and designed research; J.R.S., D.B.H., and S.M.G. performed experiments; J.R.S., D.B.H., B.T.C., and S.M.G. analyzed data; J.R.S., D.B.H., B.T.C., and S.M.G. interpreted results of experiments; J.R.S., D.B.H., and S.M.G. prepared figures; J.R.S. and S.M.G. drafted manuscript; J.R.S., D.B.H., B.T.C., and S.M.G. edited and revised manuscript; J.R.S., D.B.H., B.T.C., and S.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Alicia Strtak for assistance with depth and 3-D reconstruction images and Trevor Johnson for assistance in completion of this work. This work was completed using the TissueScope LE slide scanner and the C2 Nikon Confocal microscope at the University of Minnesota–University Imaging Centers.
REFERENCES
- 1.Grogan BF, Hsu JR; Consortium STR. Volumetric muscle loss. J Am Acad Orthop Surg 19: S35–S37, 2011. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev 52: 785–792, 2015. doi: 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- 3.Criswell TL, Corona BT, Ward CL, Miller M, Patel M, Wang Z, Christ GJ, Soker S. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol 180: 787–797, 2012. doi: 10.1016/j.ajpath.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Garg K, Ward CL, Hurtgen BJ, Wilken JM, Stinner DJ, Wenke JC, Owens GJ, Corona BT. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res 33: 40–46, 2015. doi: 10.1002/jor.22730. [DOI] [PubMed] [Google Scholar]
- 5.Armed Forces Health Surveillance Center (AFHSC). Medical evacuations from Operation Iraqi Freedom/Operation New Dawn, active and reserve components, U.S. Armed Forces, 2003–2011. MSMR 19: 18–21, 2012. [PubMed] [Google Scholar]
- 6.Armed Forces Health Surveillance Center (AFHSC). Medical evacuations from Afghanistan during Operation Enduring Freedom, active and reserve components, U.S. Armed Forces, 7 October 2001-31 December 2012. MSMR 20: 2–8, 2013. [PubMed] [Google Scholar]
- 7.Spear AM, Lawton G, Staruch RMT, Rickard RF. Regenerative medicine and war: a front-line focus for UK defence. NPJ Regen Med 3: 13, 2018. doi: 10.1038/s41536-018-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera JC, Corona BT. Muscle-related disability following combat injury increases with time. US Army Med Dep J: 30–34, 2016. [PubMed] [Google Scholar]
- 9.Greising SM, Corona BT, McGann C, Frankum JK, Warren GL. Therapeutic approaches for volumetric muscle loss injury: a systematic review and meta-analysis. Tissue Eng Part B Rev 25: 510–525, 2019. doi: 10.1089/ten.teb.2019.0207. [DOI] [PubMed] [Google Scholar]
- 10.Greising SM, Dearth CL, Corona BT. Regenerative and rehabilitative medicine: a necessary synergy for functional recovery from volumetric muscle loss injury. Cells Tissues Organs 202: 237–249, 2016. doi: 10.1159/000444673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corona BT, Wenke JC, Ward CL. Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs 202: 180–188, 2016. doi: 10.1159/000443925. [DOI] [PubMed] [Google Scholar]
- 12.Corona BT, Greising SM. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials 104: 238–246, 2016. doi: 10.1016/j.biomaterials.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Westman AM, Dyer SE, Remer JD, Hu X, Christ GJ, Blemker SS. A coupled framework of in situ and in silico analysis reveals the role of lateral force transmission in force production in volumetric muscle loss injuries. J Biomech 85: 118–125, 2019. doi: 10.1016/j.jbiomech.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Goldman SM, Feng JP, Corona BT. Volumetric muscle loss disrupts length-dependent architectural and functional characteristics of skeletal muscle. Connect Tissue Res 62: 72–82, 2021. doi: 10.1080/03008207.2020.1789608. [DOI] [PubMed] [Google Scholar]
- 15.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol 57: 1715–1721, 1984. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- 16.Warren GL, Ingalls CP, Lowe DA, Armstrong RB. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc Sport Sci Rev 29: 82–87, 2001. doi: 10.1097/00003677-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Han N, Yabroudi MA, Stearns-Reider K, Helkowski W, Sicari BM, Rubin JP, Badylak SF, Boninger ML, Ambrosio F. Electrodiagnostic evaluation of individuals implanted with extracellular matrix for the treatment of volumetric muscle injury: case series. Phys Ther 96: 540–549, 2016. doi: 10.2522/ptj.20150133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson SE, Han WM, Srinivasa V, Mohiuddin M, Ruehle MA, Moon JY, Shin E, Emeterio CLS, Ogle ME, Botchwey EA, Willett NJ, Jang YC. Determination of a critical size threshold for volumetric muscle loss in the mouse quadriceps. Tissue Eng Part C Methods 25: 59–70, 2019. doi: 10.1089/ten.TEC.2018.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corona BT, Flanagan KE, Brininger CM, Goldman SM, Call JA, Greising SM. Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle Nerve 57: 799–807, 2018. doi: 10.1002/mus.26016. [DOI] [PubMed] [Google Scholar]
- 20.Pollot BE, Corona BT. Volumetric muscle loss. Methods Mol Biol 1460: 19–31, 2016. doi: 10.1007/978-1-4939-3810-0_2. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar CA, Greising SM, Watts A, Goldman SM, Peragallo C, Zook C, Larouche J, Corona BT. Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov 4: 33, 2018. doi: 10.1038/s41420-018-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 34: 3324–3335, 2013. doi: 10.1016/j.biomaterials.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Corona BT, Garg K, Ward CL, McDaniel JS, Walters TJ, Rathbone CR. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. Am J Physiol Cell Physiol 305: C761–C775, 2013. doi: 10.1152/ajpcell.00189.2013. [DOI] [PubMed] [Google Scholar]
- 24.Greising SM, Rivera JC, Goldman SM, Watts A, Aguilar CA, Corona BT. Unwavering pathobiology of volumetric muscle loss injury. Sci Rep 7: 13179, 2017. doi: 10.1038/s41598-017-13306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greising SM, Stowe JM, Sieck GC, Mantilla CB. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp Gerontol 72: 184–191, 2015. doi: 10.1016/j.exger.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol (1985) 117: 910–920, 2014. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience 146: 178–189, 2007. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 28.Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve 30: 774–783, 2004. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- 29.Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol 180: 88–96, 2012. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieck GC, Prakash YS. Morphological adaptations of neuromuscular junctions depend on fiber type. Can J Appl Physiol 22: 197–230, 1997. doi: 10.1139/h97-014. [DOI] [PubMed] [Google Scholar]
- 31.Pratt SJP, Iyer SR, Shah SB, Lovering RM. Imaging analysis of the neuromuscular junction in dystrophic muscle. Methods Mol Biol 1687: 57–72, 2018. doi: 10.1007/978-1-4939-7374-3_5. [DOI] [PubMed] [Google Scholar]
- 32.Tse N, Morsch M, Ghazanfari N, Cole L, Visvanathan A, Leamey C, Phillips WD. The neuromuscular junction: measuring synapse size, fragmentation and changes in synaptic protein density using confocal fluorescence microscopy. J Vis Exp 94: 52220, 2014. doi: 10.3791/52220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taetzsch T, Valdez G. NMJ maintenance and repair in aging. Curr Opin Physiol 4: 57–64, 2018. doi: 10.1016/j.cophys.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannucci B, Santosa KB, Keane AM, Jablonka-Shariff A, Lu CY, Yan Y, MacEwan M, Synder-Warwick AK. What is normal? Neuromuscular junction reinnervation after nerve injury. Muscle Nerve 60: 604–612, 2019. doi: 10.1002/mus.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohan R, Tosolini AP, Morris R. Segmental distribution of the motor neuron columns that supply the rat hindlimb: a muscle/motor neuron tract-tracing analysis targeting the motor end plates. Neuroscience 307: 98–108, 2015. doi: 10.1016/j.neuroscience.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Corona BT, Henderson BE, Ward CL, Greising SM. Contribution of minced muscle graft progenitor cells to muscle fiber formation after volumetric muscle loss injury in wild-type and immune deficient mice. Physiol Rep 5: e13249, 2017. doi: 10.14814/phy2.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward CL, Ji L, Corona BT. An autologous muscle tissue expansion approach for the treatment of volumetric muscle loss. Biores Open Access 4: 198–208, 2015. doi: 10.1089/biores.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Southern WM, Nichenko AS, Tehrani KF, McGranahan MJ, Krishnan L, Qualls AE, Jenkins NT, Mortensen LJ, Yin H, Lin A, Guldberg RE, Greising SM, Call JA . PGC-1α overexpression partially rescues impaired oxidative and contractile pathophysiology following volumetric muscle loss injury. Sci Rep 9: 4079, 2019. doi: 10.1038/s41598-019-40606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J 13: 100–108, 2013. [PMC free article] [PubMed] [Google Scholar]
- 41.Kern H, Boncompagni S, Rossini K, Mayr W, Fanò G, Zanin ME, Okolow MP, Protasi F, Carraro U. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol Exp Neurol 63: 919–931, 2004. doi: 10.1093/jnen/63.9.919. [DOI] [PubMed] [Google Scholar]
- 42.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One 6: e28090, 2011. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol (1985) 89: 2235–2240, 2000. doi: 10.1152/jappl.2000.89.6.2235. [DOI] [PubMed] [Google Scholar]
- 44.Mohiuddin M, Lee NH, Moon JY, Han WM, Anderson SE, Choi JJ, Shin E, Nakhai SA, Tran T, Aliya B, Kim DY, Gerold A, Hensen LM, Tyalor WR, Jang YC. Critical limb ischemia induces remodeling of skeletal muscle motor unit, myonuclear and mitochondrial-domains. Sci Rep 9: 9551, 2019. doi: 10.1038/s41598-019-45923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu H, Zhang D, Corrick RM, Muelleman RL, Wadman MC, Li Y-L. Morphological regeneration and functional recovery of neuromuscular junctions after tourniquet-induced injuries in mouse hindlimb. Front Physiol 8: 207, 2017. doi: 10.3389/fphys.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta R, Chan JP, Uong J, Palispis WA, Wright DJ, Shah SB, Ward SR, Lee TQ, Steward O. Human motor endplate remodeling after traumatic nerve injury. J Neurosurg 18: 1–8, 2020. doi: 10.3171/2020.8.JNS201461. [DOI] [PubMed] [Google Scholar]
- 47.Vasilaki A, Pollock N, Giakoumaki I, Goljanek-Whysall K, Sakellariou GK, Pearson T, Kayani A, Jackson MJ, McArdell A. The effect of lengthening contractions on neuromuscular junction structure in adult and old mice. Age (Dordr) 38: 259–272, 2016. doi: 10.1007/s11357-016-9937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Pollack MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185: 232–240, 2004. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Jang YC, Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp Gerontol 46: 193–198, 2011. doi: 10.1016/j.exger.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park KH. Mechanisms of muscle denervation in aging: insights from a mouse model of amyotrophic lateral sclerosis. Aging Dis 6: 380–389, 2015. doi: 10.14336/AD.2015.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Xu J, Zhu J, Yu T, Zhu D. Three-dimensional visualization of intramuscular innervation in intact adult skeletal muscle by a modified iDISCO method. Neurophotonics 7: 015003, 2020. doi: 10.1117/1.NPh.7.1.015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wernig A, Herrera AA. Sprouting and remodelling at the nerve-muscle junction. Prog Neurobiol 27: 251–291, 1986. doi: 10.1016/0301-0082(86)90023-7. [DOI] [PubMed] [Google Scholar]
- 53.Son YJ, Trachtenberg JT, Thompson WJ. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci 19: 280–285, 1996. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- 54.van Mier P, Lichtman JW. Regenerating muscle fibers induce directional sprouting from nearby nerve terminals: studies in living mice. J Neurosci 14: 5672–5686, 1994. doi: 10.1523/JNEUROSCI.14-09-05672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold A-S, Gill J, Christe M, Ruiz R, McGuirk S, St-Pierre J, Tabares L, Handschin C. Morphological and functional remodeling of the neuromuscular junction by skeletal muscle PGC-1α. Nat Commun 5: 3569, 2014. doi: 10.1038/ncomms4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grinnell AD. Dynamics of nerve-muscle interaction in developing and mature neuromuscular junctions. Physiol Rev 75: 789–834, 1995. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- 57.Favero M, Cangiano A, Busetto G. Lesson from the neuromuscular junction: role of pattern and timing of nerve activity in synaptic development. Neural Regen Res 10: 686–688, 2015. doi: 10.4103/1673-5374.156944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloch-Gallego E. Mechanisms controlling neuromuscular junction stability. Cell Mol Life Sci 72: 1029–1043, 2015. doi: 10.1007/s00018-014-1768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res 26: 174–185, 2004. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- 60.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45: 389–393, 2010. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson MH, Deschenes MR. The neuromuscular junction: anatomical features and adaptations to various forms of increased, or decreased neuromuscular activity. Int J Neurosci 115: 803–828, 2005. doi: 10.1080/00207450590882172. [DOI] [PubMed] [Google Scholar]
- 62.Sleigh JN, Grice SJ, Burgess RW, Talbot K, Cader MZ. Neuromuscular junction maturation defects precede impaired lower motor neuron connectivity in Charcot-Marie-Tooth type 2D mice. Hum Mol Genet 23: 2639–2650, 2014. doi: 10.1093/hmg/ddt659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pratt SJP, Valencia AP, Le GK, Shah SB, Lovering RM. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front Physiol 6: 252, 2015. doi: 10.3389/fphys.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratt SJP, Shah SB, Ward CW, Kerr JP, Stains JP, Lovering RM. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell Mol Life Sci 72: 153–164, 2015. doi: 10.1007/s00018-014-1663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudolf R, Khan MM, Labeit S, Deschenes M. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci 6: 99, 2014. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA 107: 14863–14868, 2010. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wokke JH, Jennekens FG, van den Oord CJ, Veldman H, Smit LM, Leppink GJ. Morphological changes in the human end plate with age. J Neurol Sci 95: 291–310, 1990. doi: 10.1016/0022-510X(90)90076-Y. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Matthias N, Bhalla S, Darabi R. Evaluation of the therapeutic potential of human iPSCs in a murine model of VML. Mol Ther 29: 121–131, 2020. doi: 10.1016/j.ymthe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilbert-Honick J, Iyer SR, Somers SM, Takasuka H, Lovering RM, Wagner KR, Mao HQ, Garyson WL. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials 255: 120154, 2020. doi: 10.1016/j.biomaterials.2020.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez BL, Vega-Soto EE, Kennedy CS, Nguyen MH, Cederna PS, Larkin LM. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLoS One 15: e0239152, 2020. doi: 10.1371/journal.pone.0239152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greising SM, Warren GL, Southern WM, Nichenko AS, Qualls AE, Corona BT, Call JA. Early rehabilitation for volumetric muscle loss injury augments endogenous regenerative aspects of muscle strength and oxidative capacity. BMC Musculoskelet Disord 19: 173, 2018. doi: 10.1186/s12891-018-2095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aurora A, Garg K, Corona BT, Walters TJ. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci Med Rehabil 6: 41, 2014. doi: 10.1186/2052-1847-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen XK, Walters TJ. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J Plast Reconstr Aesthet Surg 66: 1750–1758, 2013. doi: 10.1016/j.bjps.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 74.Ward CL, Pollot BE, Goldman SM, Greising SM, Wenke JC, Corona BT. Autologous minced muscle grafts improve muscle strength in a porcine model of volumetric muscle loss injury. J Orthop Trauma 30: e396–e403, 2016. doi: 10.1097/BOT.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 75.Garg K, Corona BT, Walters TJ. Losartan administration reduces fibrosis but hinders functional recovery after volumetric muscle loss injury. J Appl Physiol (1985) 117: 1120–1131, 2014. doi: 10.1152/japplphysiol.00689.2014. [DOI] [PubMed] [Google Scholar]
- 76.Corona BT, Rivera JC, Dalske KA, Wenke JC, Greising SM. Pharmacological mitigation of fibrosis in a porcine model of volumetric muscle loss injury. Tissue Eng Part A 26: 636–646, 2020. doi: 10.1089/ten.tea.2019.0272. [DOI] [PubMed] [Google Scholar]
- 77.Wu P, Chawla A, Spinner RJ, Yu C, Yaszemski MJ, Windebank AJ, Wang H. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen Res 9: 1796–1809, 2014. doi: 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das S, Gordián-Vélez WJ, Ledebur HC, Mourkioti F, Rompolas P, Chen HI, Serruya MD, Kullen DK. Innervation: the missing link for biofabricated tissues and organs. NPJ Regen Med 5: 11, 2020. doi: 10.1038/s41536-020-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saunders D, Rose L. Regenerative rehabilitation of catastrophic extremity injury in military conflicts and a review of recent developmental efforts. Connect Tissue Res 62: 83–98, 2021. doi: 10.1080/03008207.2020.1776707. [DOI] [PubMed] [Google Scholar]
- 80.Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, Cruz J, Fournier B, Brachat S, Gutzwiller S, Ma QC, Markovits J, Broome M, Steinkrauss M, Skuba E, Galarneau JR, Gygi SP, Glass DJ. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol 33: 194–212, 2013. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stockinger J, Maxwell N, Shapiro D, deCabo R, Valdez G. Caloric restriction mimetics slow aging of neuromuscular synapses and muscle fibers. J Gerontol A Biol Sci Med Sci 73: 21–28, 2017. doi: 10.1093/gerona/glx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MP, Camargo W, Naves LA, Prado VF, Prado MAM, Guatimosim C, Valdez G. VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle 6: 31, 2016. doi: 10.1186/s13395-016-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chao T, Burmeister DM, Corona BT, Greising SM. Oxidative pathophysiology following volumetric muscle loss injury in a porcine model. J Appl Physiol (1985) 126: 1541–1549, 2019. doi: 10.1152/japplphysiol.00026.2019. [DOI] [PubMed] [Google Scholar]
- 84.Lu CY, Santosa KB, Jablonka-Shariff A, Vannucci B, Fuchs A, Turnbull I, Pan D, Wood MD, Synder-Warwick AK. Macrophage-derived vascular endothelial growth factor-A is integral to neuromuscular junction reinnervation after nerve injury. J Neurosci 40: 9602–9616, 2020. doi: 10.1523/JNEUROSCI.1736-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Llyod AC. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 162: 1127–1139, 2015. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castro R, Taetzsch T, Vaughan SK, Godbe K, Chappell J, Settlage RE, Valdez G. Specific labeling of synaptic Schwann cells reveals unique cellular and molecular features. eLife 9: e56935, 2020. doi: 10.7554/eLife.56935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One 7: e34640, 2012. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. eLife 4: e09221, 2015. doi: 10.7554/eLife.09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoffman DB, Raymond-Pope CJ, Sorensen JR, Corona BT, Greising SM. Temporal changes in the muscle extracellular matrix due to volumetric muscle loss injury. Connect Tissue Res 1–14, 2021. doi: 10.1080/03008207.2021.1886285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are primarily presented in the current manuscript and are available from the corresponding author on reasonable request.