Graphical abstract
Keywords: Covid-19, SARS-CoV-2, GS-441524, Antiviral, Nucleoside, Prodrug, Isobutyrate
Abstract
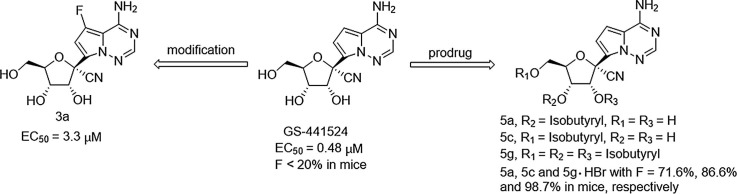
The nucleoside metabolite of remdesivir, GS-441524 displays potent anti-SARS-CoV-2 efficacy, and is being evaluated in clinical as an oral antiviral therapeutic for COVID-19. However, this nucleoside has a poor oral bioavailability in non-human primates, which may affect its therapeutic efficacy. Herein, we reported a variety of GS-441524 analogs with modifications on the base or the sugar moiety, as well as some prodrug forms, including five isobutyryl esters, two l-valine esters, and one carbamate. Among the new nucleosides, only the 7-fluoro analog 3c had moderate anti-SARS-CoV-2 activity, and its phosphoramidate prodrug 7 exhibited reduced activity in Vero E6 cells. As for the prodrugs, the 3′-isobutyryl ester 5a, the 5′-isobutyryl ester 5c, and the tri-isobutyryl ester 5g hydrobromide showed excellent oral bioavailabilities (F = 71.6%, 86.6% and 98.7%, respectively) in mice, which provided good insight into the pharmacokinetic optimization of GS-441524.
1. Introduction
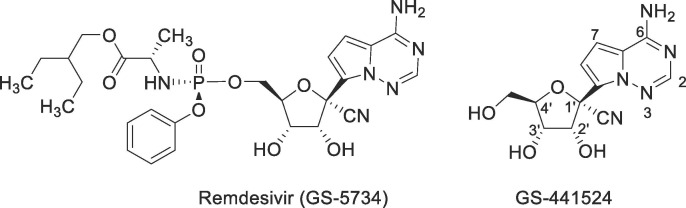
The coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was firstly identified in December of 2019.1 The disease has developed into a global pandemic, leading to more than 182 million confirmed cases and 3.9 million deaths till July 2, 2021. SARS-CoV-2 is a novel positive-sense single-stranded RNA virus, sharing 79.5% genetic sequence identity with SARS-CoV.2 Evidences have indicated that this new virus is more contagious than SARS-CoV, and has a long incubation period, which poses a great challenge to control the pandemic.3 Among the medications under investigation, only remdesivir (Fig. 1 ) received the U.S. Food and Drug Administration (FDA) approval for the treatment of COVID-19 patients who require hospitalization.4, 5, 6
Fig. 1.
The structures of remdesivir and its parent nucleoside GS-441524.
Structurally, remdesivir is a phosphoramidate prodrug that is designed to improve the intracellular nucleoside triphosphate conversion efficiency.7 This kind of nucleoside prodrugs shows liver-targeting properties and has been widely applied in the development of antiviral drugs against hepatitis virus infections.8 For the treatment of COVID-19, RDV is administered by intravenous (IV) injection mainly due to its specific pharmacokinetic (PK) properties. After IV administration in human, RDV is found to be rapidly metabolized to its predominant metabolite GS-441524 (Fig. 1) that persists in the circulation with t 1/2 of about 27 h.9 GS-441524 is a 1′-cyano substituted pyrrolotriazine C-glycoside with broad-spectrum antiviral activities.10 This nucleoside also displays potent anti-SARS-CoV-2 activity in different cells, especially in the primary human epithelial cells.11 Of note, it was recently reported that GS-441524 could effectively inhibit SARS-CoV-2 infection in mouse models,12 and has a synergic antiviral efficacy with the 3CL protease inhibitor, GC376.13 Currently, GS-441524 is deemed as a promising anti-SARS-CoV-2 therapeutic, and is being investigated under preclinical study.
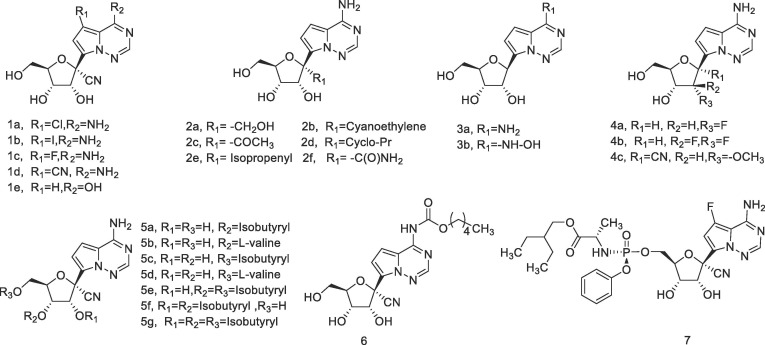
In addition to GS-441524, some other pyrrolotriazine C-nucleosides have been previously reported, and several of them proved to be potent antiviral agents. It was also indicated that the substituents on the sugar or the base moiety could significantly affect the antiviral activities.7, 10, 14 Given the high demand of effective anti-SARS-CoV-2 agents, in this work, we synthesized a variety of GS-441524 analogs with modifications at the sugar 1′, 2′ position or the base 6, 7 position, and investigated their anti-SARS-CoV-2 activities in Vero E6 cells. In another aspect, in order to improve the oral bioavailability of GS-441524, some prodrugs of this nucleoside were prepared (Fig. 2 ), and the isobutyryl ester prodrugs exhibited favorable oral bioavailability in mice.
Fig. 2.
GS-441524 derivatives in the present study.
2. Result and discussion
2.1. Chemistry
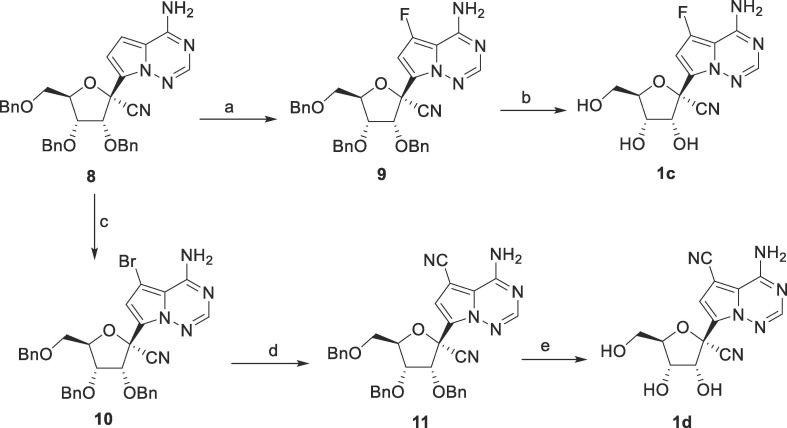
Compounds 1a and 1b were prepared from GS-441524 by halogenation using N-chlorosuccinimide and N-iodosuccinimide in DMF, respectively. Synthesis of compounds 1c and 1d was shown in Scheme 1 . Treatment of 8 with Selectfluor in acetonitrile at room temperature afforded 9, which was then debenzylated with BCl3 to afford the desired 7-fluoro derivative 1c. The 7-CN derivative 1d was synthesized in three steps from 8, bromination, cyano-substitution and deprotection.
Scheme 1.
Reagents and conditions: a) Selectfluor, NaHCO3, CH3CN, 28%; b) BCl3, CH2Cl2, −30 °C, 37%; c) NBS, DMF, 88%; d) Zn, Zn(CN)2, Pd2(dba)3, Ni(dppf)Cl2, DMAC, 120 °C, 74%; e) BCl3, CH2Cl2, −30 °C, 28%.
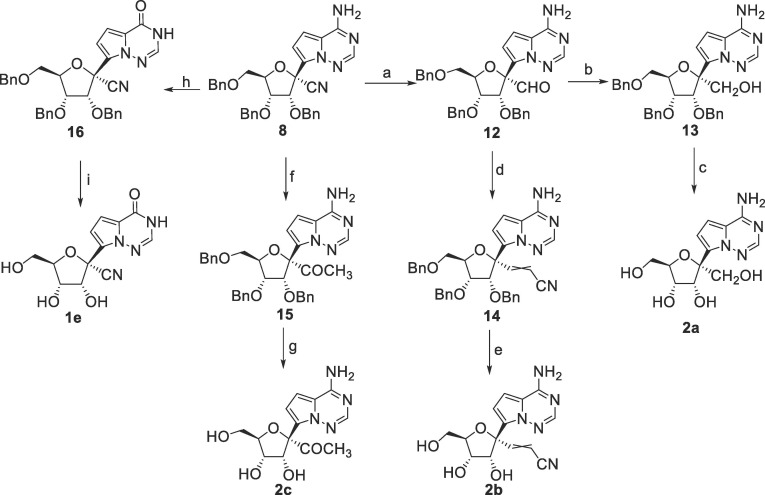
Synthesis of 1e and 2a–2c was shown in Scheme 2 . Reaction of 8 with NaNO2 in 80% AcOH under heating condition afforded 16, which was debenzylated to give 1e. The 1′-CN of 8 could be converted to the aldehyde by reduction with diisobutylaluminum hydride (DIBAL), and the obtained intermediate 12 was readily converted to the 1′-hydroxymethyl intermediate 13 and the 1′-cyanovinyl intermediate 14 by NaBH4 reduction and Wittig reaction, respectively. The nucleophilic addition of methylmagnesium bromide to 8 smoothly gave the 1′-acetyl substituted intermediate 15. Removal of the benzyl groups of 13, 14 and 15 afforded 2a–2c, respectively.
Scheme 2.
Reagents and conditions: a) DIBAL, CH2Cl2, −78 °C, 52%; b) NaBH4, ethanol, 0 °C to rt, 80%; c) Pd/C, H2, HCOOH, CH3OH, 20%; d) diethyl cyanomethylphosphonate, NaH, THF, 0 °C to rt; e) BCl3, CH2Cl2, −30 °C, 36% over two steps; f) methyl magnesium bromide, THF, 0 °C to 60 °C, 66%; g) BCl3, CH2Cl2, −30 °C, 46%; h) NaNO2, 80% AcOH, rt to 90 °C, 98%; i) BCl3, CH2Cl2, −30 °C, 48%.
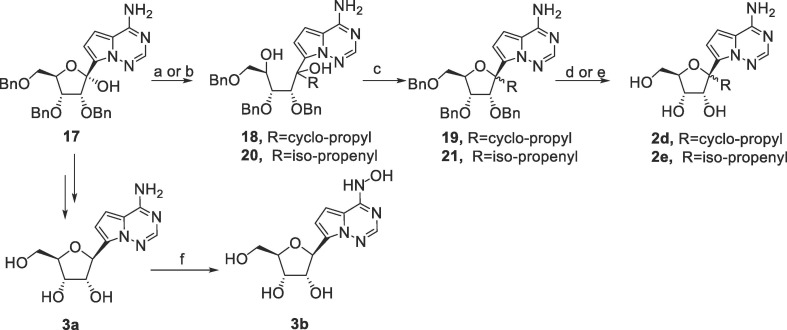
Synthesis of 2d, 2e, 3a and 3b was shown in Scheme 3 . The nucleophilic addition of cyclopropyl magnesium bromide and isopropenyl magnesium bromide to the hemiketal 17, a commercial intermediate of RDV, provided 18 and 20, respectively. The two intermediates were treated with methanesulfonic acid to give the cyclized products (19 and 21) followed by debenzylation to give 2d (two stereoisomers in a ratio of 5:1) and 2e (two stereoisomers in a ratio of 1:1). 2f derived from the debenzylation of 8 as a byproduct, and 3a was prepared according to the reported method.15
Scheme 3.
Reagents and conditions: a) cyclopropyl magnesium bromide, THF, 0 °C to rt, 37% of 18; b) isopropenyl magnesium bromide, THF, 0 °C to rt, 68% of 20; c) methanesulfonic acid, CH2Cl2, 50% of 19, 45% of 21; d) Pd/C, H2, HCOOH/CH3OH, 47% of 2d; e) BCl3, CH2Cl2, −30 °C, 14% of 2e; f) NH2OH.HCl, H2O, 40 °C, 71%.
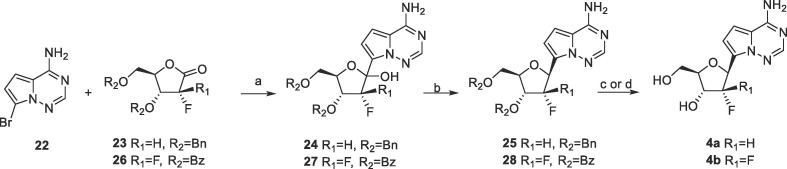
4a and 4b were synthesized starting from 22 and the lactones (23 and 26) according to reported methods (Scheme 4 ). Both of the benzyl and the benzoyl group were tolerated in the Grignard addition reaction. The subsequent anomeric reduction was achieved with triethylsilane and boron trifluoride etherate. Removal of the protecting groups of 25 and 28 afforded 4a and 4b, respectively.
Scheme 4.
Reagents and conditions: a) n-BuLi, TMSCl, THF, −78 °C, 30% of 24, 14% of 27; b) Et3SiH, BF3.Et2O, CH2Cl2, 0 °C, 62% of 25, 41% of 28; c) Pd/C, H2, HCOOH/CH3OH, 46% of 4a; d) 7 M NH3/CH3OH, 17% of 4b.
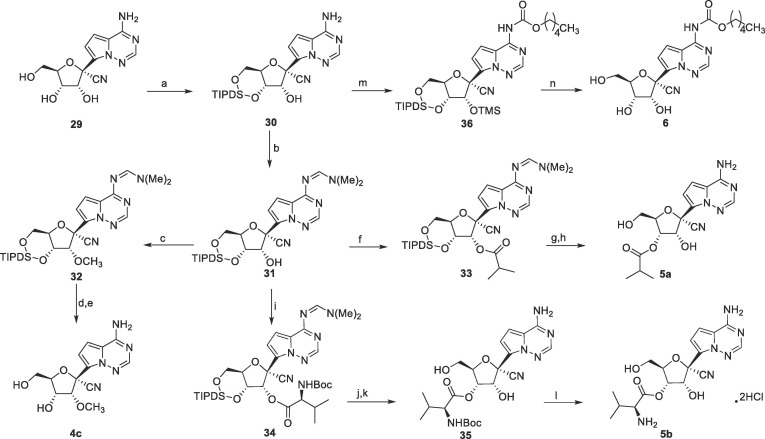
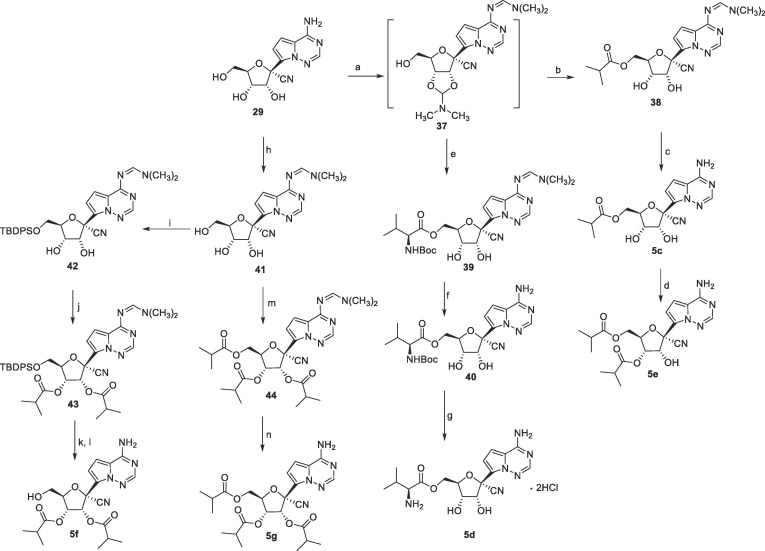
Synthesis of 4c, 5a, 5b and 6 was shown in Scheme 5 . Selective 3′, 5′-hydroxy group protection of 29 with 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (TIPDSCl) followed by exocyclic amino protection with N, N-dimethylformamide dimethyl acetal (DMF-DMA) afforded 31. The intermediate 31 was used for the synthesis of compounds 4c, 5a and 5b. Methylation of 31 with methyl iodide in DMF afforded 32, and the subsequent removal of the amino and the hydroxyl protecting groups afforded 4c. Condensation of 31 with isobutyryl chloride and Boc-l-Val under general reaction conditions gave 33 and 34, respectively. After removal of the TIPDS group with TBAF, the acyl groups were completely migrated from the 2′-position to the 3′-position, which was unambiguously confirmed by the single crystal X-ray crystallography (Fig. S43 X-ray single-crystal structure of 35). The similar migration had been reported in previous literature.16, 17 6 was synthesized from 30 in three steps, TMS protection of the 2′-hydroxyl group, amidation with pentyl chloroformate, and deprotection with TBAF.
Scheme 5.
Reagents and conditions: a) TIPDSCl, imidazole, DMF, 79%; b) DMF-DMA, toluene, 60 °C, 91%; c) MeI, NaH, DMF, 0 °C; d) 85% hydrazine hydrate, CH3CN, 30% over two steps; e) TBAF, THF, 0 °C to rt, 50%; f) isobutyryl chloride, TEA, DMAP, CH2Cl2, 72%; g) TBAF, THF, RT; h) TFA, THF, 61% over two steps; i) N-Boc-l-Valine, HOBT, EDCI, DMAP, CH2Cl2, 75%; j) 85% hydrazine hydrate, CH3CN; k) TBAF, AcOH, THF, 64% over two steps; l) 4 M HCl/CH3OH, 75%; m) TMSCl, pyridine, then pentyl chloroformate, CH2Cl2, 0 °C; n) TBAF, THF, RT, 48% over two steps.
N, N-Dimethylformamide dimethyl acetal (DMF-DMA) served as a versatile protecting reagent that facilitated the synthesis of the 5′-isobutyryl ester 5c, the 5′-valine ester 5d, and the tri-isobutyrate 5g (Scheme 6 ). Because of the easy migration of acyl group from 2′-position to 3′-position, reaction of 5c with trimethyl orthoisobutyrate followed by acidic hydrolysis exclusively gave the 3′, 5′-di-isobutyryl ester 5e. As for the synthesis of the 2′, 3′-di-isobutyryl ester 5f, three steps were required, selective protection of the 5′-hydroxyl group with TBDPSCl, acylation with isobutyryl chloride, and deprotection.
Scheme 6.
Reagents and conditions: a) DMF-DMA, pyridine; b) isobutyric anhydride, TEA, DMAP, CH2Cl2; c) AcOH, ethanol, 50 °C, 66% over three steps; d) trimethyl orthoisobutyrate, pTsOH·H2O, CH2Cl2, then 1 M HCl, THF 76%; e) N-Boc-l-Valine, HOBT, EDCI, DMAP, CH2Cl2; f) 85% hydrazine hydrate, CH3CN; g) 4 M HCl/CH3OH, 17% over four steps; h) DMF-DMA, toluene, 60 °C, 84%; i) TBDPSCl, imidazole, DMF, 75%; j) isobutyric anhydride, TEA, DMAP, CH2Cl2; k) TBAF, THF; l) AcOH, ethanol, 50 °C, 40% over four steps; m) isobutyric chloride, TEA, DMAP, CH2Cl2, 80%; n) 85% hydrazine hydrate, CH3CN, 85%.
2.2. Antiviral screening
All of the synthesized GS-441524 derivatives were screened for antiviral activity against SARS-CoV-2 in Vero E6 cells. Inhibition of viral replication was determined by quantification of viral copy numbers in the cell supernatant via real-time fluorescence quantitative PCR (qRT-PCR), and conducted in a biosafety level 3 laboratory. Among compounds 1a-1e that were differed by substituents at the 7-position, only the fluorinated analog 1c was able to inhibit the viral replication at an initial screening concentration of 5.0 μM, shown in Table 1 . The subsequent EC50 determination indicated that 1c was about 10-fold less potent than GS-441524 (EC50 values of 3.3 μM and 0.48 μM, respectively). A previous study involving the discovery of anti-HCV pyrrolotriazine C-nucleosides revealed that introducing a chloro or a cyano group at the C7 position of the pyrrolotriazine base enhanced the antiviral activity.14 However, such kind of modifications at the C7 position of GS-441524 led to the loss of the anti-SARS-CoV-2 activity (1a and 1d). Moreover, we designed the phosphoramidate prodrug of 1c, but it exhibited decreased antiviral activity (7, 82% inhibition at 10 µM). RDV also had a much weaker antiviral activity (EC50 = 2.0 µM) than GS-441524, which was mainly due to the limited formation of the active nucleoside triphosphate form in Vero E6 cells.18
Table 1.
Antiviral activity of GS-441524 derivatives against SARS-CoV-2 in Vero E6 cells.
| Compound | Inhibition ratea | EC50 (μM) |
|---|---|---|
| 1a | <10% | / |
| 1b | <10% | / |
| 1c | 80%, 97 %b | 3.3 |
| 1d | <10% | / |
| 1e | <10% | / |
| 2a | <10% | / |
| 2b | <10% | / |
| 2c | <10% | / |
| 2d | <10% | / |
| 2e | <10% | / |
| 2f | <10% | / |
| 3a | cytotoxic | / |
| 3b | <10% | / |
| 4a | <10% | / |
| 4b | <10% | / |
| 4c | <10% | / |
| 7 | 82 %b | |
| GS-441524 | 99% | 0.48 |
| Remdesivir | 65% | 2.0 |
Inhibition rate at 5 µM unless other specified.
Inhibition rate at 10 µM.
With respect to the 1′-modifications, all the six derivatives (2a-2f) did not show any inhibition of SARS-CoV-2 replication at the concentration of 5.0 µM. The C1′-cyano group was very important for the anti-SARS-CoV-2 activities, as evidenced by a previous research.18 Recently, it was revealed that the C1′-cyano group of RDV or GS-441524 nucleoside monophosphate could cause a translocation barrier, which played a crucial role in the termination of viral RNA elongation.19 3a derived by the removal of the C1′-cyano group of GS-441524 was highly toxic to Vero E6 cells even at low concentrations, and its antiviral activity was hard to be determined. 3b bearing a hydroxyamino group was designed according to the structure of β-D-N4-hydroxycytidine, a highly potent anti-SARS-CoV-2 nucleoside, but this nucleoside did not show any antiviral activity.20 For the other three compounds (4a-4c), to our disappointment, they were all inactive against SARS-CoV-2 (Table 1).
2.3. Pharmacokinetic studies
Adsorption of nucleosides in the gastrointestinal tract largely depended on the transporters, and a number of approved antiviral nucleoside drugs are given by oral route in the parent nucleoside form.21 As for GS-441524, its oral bioavailability varies greatly in different species with F values of 33% in rats, 85% in dogs and 8.3% in cynomolgus monkeys.22 Recently, a PK study of GS-441524 in a volunteer showed that a high oral dose (750 mg) for three times a day could achieve the exposure that was required to completely suppress the replication of SARS-CoV-2 in vivo.23 GS-441524 contained multiple hydrophilic groups, but it was very slightly soluble in water (pH = 6.0) with a solubility of 0.105 mg/ml at 37 °C determined in our lab. Based on these evidences, we concluded that the oral bioavailability of GS-441524 in human would be low, and a suitable prodrug of GS-441524 may serve as a better candidate for clinical development. In order to improve the oral bioavailability of GS-441524, at first, we designed and synthesized five prodrugs, including two mono-isobutyric acid esters (5a and 5c), two l-valine esters (5b and 5d), and one carbamate 6. Compounds 5a-5d were found to exhibit stronger anti-SARS-CoV-2 activities than GS-441524 (Table 2 ), which likely resulted from their improved cellular permeability. The carbamate 6 showed a significant decrease in the anti-SARS-CoV-2 activity with only 65% inhibition rate at the concentration of 10 µM. This kind of prodrug was previously applied for the modification of R1479, an anti-HCV nucleoside, and proved to be unable to efficiently release the parent nucleoside.24 The low antiviral activity of 6 also indicated that this prodrug could not be efficiently converted to the nucleoside in Vero E6 cells.
Table 2.
Antiviral activity of GS-441524 prodrugs against SARS-CoV-2 in Vero E6 cells.
| Compound | Inhibition ratea | EC50 (μM) |
|---|---|---|
| 5a | 99% | 0.25 |
| 5b | 99% | 0.11 |
| 5c | 99% | 0.23 |
| 5d | / | / |
| 5e | 99% | / |
| 5f | 99% | / |
| 5g | / | / |
| 6 | 65 %b | / |
| GS-441524 | 99% | 0.48 |
| Remdesivir | 65% | 2.0 |
Inhibition rate at 5 µM unless other specified.
Inhibition rate at 10 µM.
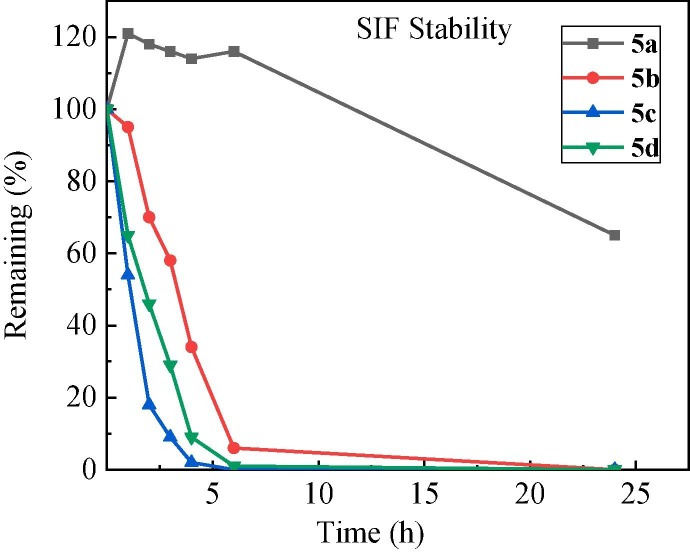
Then, we investigated the stability of four ester prodrugs (5a-5d) in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF). All of them were found to be stable in SGF with pepsin, but only the 3′-isobutyryl ester 5a showed good stability in SIF with pancreatin (Figure 3 ). At 4 h post incubation in SIF, there remained 100%, 34%, 2% and 9% of the parent esters for 5a, 5b, 5c and 5d, respectively. It seemed that the two 3′-esters exhibited a higher resistance to in vitro hydrolysis in SIF than the corresponding 5′-esters.
Fig. 3.
The in vitro stability of four GS-441524 ester prodrugs in SIF.
Next, we evaluated the PK properties of three esters (5a, 5b and 5c) in mice. Due to the hepatic-first pass metabolism, and the potential ester hydrolysis in mouse plasma that contained high level of esterases, all the parent esters were hardly detected in mouse plasma. As shown in Table 3 , 5a and 5c had excellent oral bioavailabilities in mice with F values of 71.6% and 86.6%, respectively, which were much higher than that of GS-441524 (F = 15.7% reported by us recently),25 and that of 5b (F = 27.5%). There was little difference between the bioavailabilities of 5a and 5c, even though 5a had a better stability in SIF. This was likely due to the rapid oral adsorption of 5a and 5c in view of the short Tmax of the nucleoside metabolite (0.33 h and 0.42 h, respectively). Considering the low oral bioavailability of 5b, the valine ester prodrug may be not a good choice for GS-441524 modification. Besides 5a and 5c, we also synthesized two di-isobutyrates (5e and 5f) and one tri-isobutyrate (5g). Among them, 5g was most easily synthesized, but it was obtained as a white foam. Salt formationwas then applied to improve the solid state properties of 5g. Among the salts formed by hydrochloric acid, sulphuric acid or hydrobromic acid, only hydrobromide salt (5g·HBr) was obtained as a well-crystallized salt. In addition, 5g·HBr had low hygrogscopicity and good chemical stability. Afterwards, the PK study of 5g·HBr was conducted in mice. To our delight, 5g·HBr also had a good oral bioavailability with the F value of 98.7%. Indeed, oral administration of 5g·HBr afforded a relatively lower (calculated by the molar dose) plasma exposure of GS-441524 compared with that of 5a and 5c. For the three GS-441524 ester prodrugs, further PK studies especially in monkeys would be necessary to determine the optimal candidate.
Table 3.
Single-dose PK parameters for GS-441524 in mice. Calculation of PK parameters for GS441524 following oral (50 mg/Kg) and intravenous (25 mg/Kg) administration of the ester prodrugs (5a, 5b, 5c and 5g·HBr) in CD-1 mice (N = 3 per group).
| Compd. (Route) | T1/2 | Tmax | Cmax | AUC_last | CL_obs | MRTINF_obs | Vss_obs | F |
|---|---|---|---|---|---|---|---|---|
| (h) | (h) | (ng/mL) | (h*ng/mL) | (mL/min/kg) | (h) | (L/kg) | (%) | |
| 5a (p.o.) | 1.11 | 0.33 | 7879 | 14,565 | – | 1.82 | – | 71.6 |
| 5a (i.v.) | 0.83 | – | – | 10,174 | 40.9 ± 9.1 | 1.07 | 2.59 | |
| 5b (p.o.) | 1.72 | 0.83 | 1506 | 2984 | – | 2.76 | 27.5 | |
| 5b (i.v.) | 0.61 | – | – | 5420 | 82.0 ± 24.3 | 0.78 | 3.86 | |
| 5c (p.o.) | 2.46 | 0.42 | 6677 | 13,817 | – | 2.16 | 86.6 | |
| 5c (i.v.) | 4.77 | – | – | 7981 | 52.5 ± 6.13 | 1.33 | 4.17 | |
| 5g·HBr (p.o.) | 2.71 | 0.25 | 3613 | 7112 | – | 3.00 | – | 98.7 |
| 5g·HBr (i.v.) | 2.48 | – | – | 3603 | 118 ± 128 | 2.23 | 15.6 |
2.4. Acute toxicity studies
An acute toxicity study of compound 5c was conducted with ICR mice. The animals were fasted for 16 h, and then administered a single oral gavage dose of the test compound at 50 mg/kg, 100 mg/kg, 200 mg/kg, 500 mg/kg and 1000 mg/kg (formulated with 5% DMSO, 5% Solution Hs15 and 90% saline). No mortality occurred during the 7-day observation period. No significant changes in body weight, organ weight, or tissue histology were observed after animal sacrifice. This acute toxicity study indicated that compound 5c was well tolerated in ICR mice at an oral dosage of up to 1000 mg/kg.
3. Conclusion
In summary, a series of pyrrolotriazine C-nucleosides and several GS-441524 prodrugs were synthesized and evaluated for their anti-SARS-CoV-2 activity in Vero E6 cell line. Among these novel nucleosides, only the fluoro-substituted GS-441524 analog showed moderate anti-SARS-CoV-2 activity. GS-441524 has been considered a promising oral anti-SARS-CoV-2 candidate currently evaluated in early-stage clinical trials, but its therapeutic potential may be limited due to the poor oral adsorption. Our study showed that the oral bioavailability of GS-441524 could be significantly improved by the means of the ester prodrug strategy. With respect to the three ester prodrugs (5a, 5c and 5g·HBr) that had high oral bioavailability in mice, further PK studies especially in monkeys would be necessary to determine the optimal prodrug candidate.
4. Experimental section
4.1. Synthesis
All commercially available chemicals and solvents were directly used without further purification. All reactions were monitored by thin layer chromatography (TLC) on silica gel plates (GF-254). Low-resolution mass spectra (LRMS) were measured on a Thermo Fisher FINNIGAN LTQ spectrometer. 1H, 13C and 19F NMR data were all recorded on a Bruker AVANCE III instrument (500 MHz or 600 MHz). The specific rotation was measured on a Rudolph Autopol VI automatic polarimeter at the sodium D line at 20 ± 0.35 °C.
Analytical analysis for purity was determined by three different methods denoted as Method a, Method b and Method c. Method a:Thermo U3000 equipped with Waters XSelect CSH C18, 3.5 µm, 150 × 4.6 mm using a mobile phase (A: 0.02 M NH4H2PO4 pH = 5.0, B = CH3CN), the gradient elution started with 2% B at 0 min, held for 3 min, then ramped to 20% at 15 min, next increased to 85% at 23 min, held for 7 min, finally returned to 2% at 30.1 min until end of the run, at a flow rate of 1.0 mL/min, with a wavelength of 240 nm and column oven temperature at 30 °C. Method b: Agilent1100 equipped with Waters SunFire C18, 5 μm, 150 × 4.6 mm using a mobile phase (A: 20 mM sodium 1-octanesulfonate, B: CH3CN), gradient of 10–70% B over 20 min, held at 70% until end of the run at 30 min, at a flow rate of 1.0 mL/min, with a wavelength of 210 nm and column oven temperature at 30 °C. Method c: Thermo U3000 equipped with Agilent ZORBAX SB C18, 5 μm, 150 × 4.6 mm using a mobile phase (A: 0.1% H3PO4, B: CH3CN), gradient of 20–90% B over 20 min, then ramped to 95% for 10 min, at a flow rate of 1.0 mL/min, with a wavelength of 230 nm and column oven temperature at 30 °C.
4.1.1. (2R,3R,4S,5R)-2-(4-amino-5-chloropyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile (1a)
Compound 1a and 1b were synthesized starting from GS-441254 with reference to reported method.26 1H NMR (500 MHz, DMSO‑d 6) δ 8.26 (br, 1H), 7.96 (s, 1H), 7.10 (br, 1H), 6.99 (s, 1H), 6.25 (d, J = 6.1 Hz, 1H), 5.19 (d, J = 5.7 Hz, 1H), 4.93 (t, J = 5.7 Hz, 1H), 4.53 (t, J = 5.5 Hz, 1H), 4.06–4.00 (m, 1H), 3.96–3.88 (m, 1H), 3.69–3.61 (m, 1H), 3.54–3.45 (m, 1H). 13C NMR (126 MHz, DMSO‑d 6) δ 154.91, 148.42, 124.07, 116.77, 112.09, 110.30, 102.84, 84.87, 78.01, 74.68, 69.60, 60.25.MS m/z = 326.0 [M+1]+. Optical rotation [α] 20 D = −15.6 (c 0.25, DMSO).
4.1.2. (2R,3R,4S,5R)-2-(4-amino-5-iodopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile (1b)
1H NMR (500 MHz, DMSO‑d 6) δ 7.98 (s, 1H), 7.12 (s, 1H), 6.21 (br, 1H), 5.20 (br, 1H), 4.91 (s, 1H), 4.52 (d, J = 4.6 Hz, 1H), 4.11–3.99 (m, 1H), 3.97–3.85 (m, 1H), 3.64 (d, J = 11.3 Hz, 1H), 3.49 (d, J = 10.9 Hz, 1H). 13C NMR (126 MHz, DMSO‑d 6) δ 155.49, 147.91, 126.62, 118.55, 116.86, 115.58, 84.97, 77.95, 74.61, 69.69, 60.37, 52.64. MS m/z = 418.0 [M+1]+. Optical rotation [α] 20 D = −11.2 (c 0.25, DMSO).
4.1.3. (2R,3R,4S,5R)-2-(4-amino-5-fluoropyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile (1c)
To a solution of 8 (1.5 g, 2.67 mmol) in acetonitrile (30 mL) was added 1-chloromethyl-4-fluoro-1, 4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (1.13 g, 3.2 mmol, 1.2 eq.) and NaHCO3 (670 mg, 8.0 mmol, 3.0 eq.). The mixture was stirred at rt for 3 h, then poured into water and extracted with ethyl acetate. The organic extract was washed with brine, dried over Na2SO4 and concentrated. The residue was purified on silica gel to give 9 (430 mg, 28%) as an off-white solid. 1H NMR (500 MHz, DMSO‑d 6) δ 8.12 (s, 2H), 7.87 (s, 1H), 7.42–7.14 (m, 15H), 6.59 (s, 1H), 4.92 (d, J = 11.7 Hz, 1H), 4.86–4.76 (m, 2H), 4.57–4.46 (m, 4H), 4.39 (m, 1H), 4.12 (t, J = 5.7 Hz, 1H), 3.73 (dd, J = 11.2, 3.2 Hz, 1H), 3.60 (dd, J = 11.2, 4.3 Hz, 1H). MS m/z = 580.2 [M+H]+. Compound 9 (250 mg, 0.43 mmol, 1.0 eq.) was deprotected with 1 M BCl3 dichloromethane in CH2Cl2 by reference to the reported method to afford 1c (49 mg, 37%) as a white solid.27 1H NMR (500 MHz, DMSO‑d 6) δ 8.11 (br, 1H), 7.88(s, 1H), 7.41 (br, 1H), 6.80 (s, 1H), 6.23 (d, J = 6.1 Hz, 1H), 5.20 (d, J = 5.7 Hz,1H), 4.93 (t, J = 5.7 Hz, 1H), 4.54 (t, J = 5.5 Hz, 1H), 4.08–4.01(m, 1H), 3.96–3.90 (m, 1H), 3.71–3.64 (m, 1H), 3.55–3.48 (m, 1H). 13C NMR (126 MHz, DMSO‑d 6) 13C NMR (126 MHz, DMSO‑d 6) δ 154.02, 148.32, 142.71, 140.75, 120.69, 116.83, 102.28 (d, J C-F = 22.40 Hz), 97.12 (d, J C-F = 14.91 Hz), 84.90, 78.09, 74.71, 69.63, 60.32. 19F NMR (471 MHz, DMSO‑d 6) δ 158.83. MS m/z = 310.1 [M+H]+. Optical rotation [α] 20 D = −17.2 (c 0.25, DMSO).
4.1.4. 4-amino-7-((2R,3R,4S,5R)-2-cyano-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrrolo[2,1-f][1,2,4]triazine-5-carbonitrile (1d)
A mixture of 8 (500 mg, 0.89 mmol, 1.0 eq.) and N-bromosuccinimide (158 mg, 0.89 mmol, 1.0 eq.) in DMF (10 mL) was stirred at rt for 4 h. After workup, the residue was purified on silica gel to give 10 (500 mg, 88%) as a yellow foam. 1H NMR (500 MHz, DMSO‑d 6) δ 8.31 (s, 1H), 7.99 (s, 1H), 7.44–7.24 (m, 15H), 6.97 (d, J = 22.6 Hz, 1H), 6.81 (s, 1H), 4.94 (d, J = 11.6 Hz, 1H), 4.87–4.79 (m, 2H), 4.51 (q, J = 12.0 Hz, 4H), 4.40 (dt, J = 7.0, 3.6 Hz, 1H), 4.12 (dd, J = 6.7, 4.8 Hz, 1H), 3.74 (dd, J = 11.2, 3.1 Hz, 1H), 3.61 (dd, J = 11.2, 4.2 Hz, 1H). A mixture of 10 (150 mg, 0.23 mmol, 1.0 eq.), Pd2(dba)3 (20 mg, 0.02 mmol, 0.1 eq.), zinc cyanide (60 mg, 0.51 mmol, 2.2 eq.), zinc power (2 mg, 0.03 mmol, 0.13 eq.) and Ni(dppf)Cl2 (31 mg, 0.05 mmol, 0.2 eq.) in N,N-dimethylacetamide (5 mL) was stirred under nitrogen atmosphere at 120 °C for 2 h. After cooling to rt, the mixture was filtered through celite, and the filtrate was concentrated. The residue was purified on silica gel to give 11 (100 mg, 74%) as a white solid. 1H NMR (600 MHz, DMSO‑d 6) δ 8.20 (s, 1H), 7.40–7.25 (m, 15H), 7.23 (s, 1H), 4.93 (d, J = 11.7 Hz, 1H), 4.86–4.79 (m, 2H), 4.54–4.45 (m, 4H), 4.41 (dt, J = 7.0, 3.7 Hz, 1H), 4.15–4.07 (m, 1H), 3.74 (dd, J = 11.3, 3.1 Hz, 1H), 3.61 (dd, J = 11.2, 4.2 Hz, 1H). The debenzylation of 11 (100 mg, 0.17 mmol) produced 1d (15 mg, 28%) following the same procedure as described for 1c. 1H NMR (500 MHz, DMSO‑d 6) δ 8.23 (s, 1H), 7.50 (s, 1H), 6.30 (d, J = 6.1 Hz, 1H), 5.22 (d, J = 6.0 Hz, 1H), 4.93 (t, J = 5.7 Hz, 1H), 4.55 (t, J = 5.5 Hz, 1H), 4.11–4.03 (m, 1H), 3.94 (q, J = 5.9 Hz, 1H), 3.73–3.65 (m, 1H), 3.57–3.49 (m, 1H). 13C NMR (126 MHz, DMSO‑d 6) δ 155.49, 147.91, 126.63, 118.56, 116.86, 115.58, 84.96, 77.94, 74.61, 69.69, 60.37, 52.66. MS m/z = 317.1 [M+1]+.
4.1.5. (2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-2-(4-oxo-3,4-dihydropyrrolo[2,1-f][1,2,4]triazin-7-yl)tetrahydrofuran-2-carbonitrile (1e)
To a solution of 8 (350 mg, 0.62 mmol) in 80% aqueous acetic acid solution (10 mL) was added NaNO2 (856 mg, 12.4 mmol, 20 eq.) in portions at 0 °C. The mixture was stirred at rt for 30 min, and then stirred at 90 °C for 4 h. After cooling to rt, the reaction mixture was partitioned between water and toluene. The organic layer was washed with saturated aqueous NaHCO3 solution, dried and concentrated. The residue was purified on silica gel to give 16 (345 mg, 98%) as a light yellow solid. The debenzylation of 16 (345 mg, 0.61 mmol) with BCl3 produced 1e (85 mg, 48%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 11.91 (d, J = 4.1 Hz, 1H), 7.98 (d, J = 4.1 Hz, 1H), 6.91 (d, J = 4.4 Hz, 1H), 6.81 (d, J = 4.4 Hz, 1H), 6.14 (br, 1H), 5.22 (br, 1H), 4.55 (d, J = 5.1 Hz, 1H), 4.06–4.03 (m, 1H), 3.95 (t, J = 5.4 Hz, 1H), 3.62 (dd, J = 12.2, 3.4 Hz, 1H), 3.49 (dd, J = 12.2, 4.6 Hz, 1H). MS m/z = 293.0 [M+H]+.
4.1.6. (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2,5-bis(hydroxymethyl)tetrahydrofuran-3,4-diol (2a)
To a solution of 8 (4.0 g, 7.12 mmol, 1.0 eq.) in anhydrous dichloromethane (40 mL), diisobutylaluminium hydride (1.2 M in toluene, 30 mL, 35.6 mmol, 5.0 eq.) was added dropwise at −78 °C. The mixture was stirred for 3 h, and quenched with methanol (1.5 mL). The mixture was allowed to warm to rt, and 20% aqueous potassium sodium tartrate solution (50 mL) was added. The resulting solution was extracted with dichloromethane, dried over Na2SO4 and concentrated. The residue was purified on silica gel to give 12 (2.1 g, 52%) as a white solid. To a solution of 12 (600 mg, 1.06 mmol, 1.0 eq.) in ethanol (10 mL) was added sodium borohydride (104 mg, 2.76 mmol, 2.6 eq.) at 0 °C. After stirring for 2 h at rt, the reaction was quenched with acetic acid. The resulting solution was concentrated, and the residue was partitioned between water and ethyl acetate. The organic layer was dried, concentrated, and purified on silica gel to afford 13 (480 mg, 80%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 7.83 (s, 1H), 7.60 (s, 2H), 7.42–7.20 (m, 15H), 6.78 (d, J = 4.4 Hz, 1H), 6.67 (d, J = 4.4 Hz, 1H), 4.77 (s, 2H), 4.69 (d, J = 4.4 Hz, 1H), 4.60–4.52 (m, 2H), 4.47 (d, J = 11.7 Hz, 1H), 4.41 (dd, J = 11.3, 7.3 Hz, 1H), 4.36 (d, J = 11.8 Hz, 1H), 4.29 (dd, J = 7.3, 5.0 Hz, 1H), 4.20–4.14 (m, 1H), 4.01 (dd, J = 11.3, 5.0 Hz, 1H), 3.85 (dd, J = 8.8, 4.4 Hz, 1H), 3.73 (dd, J = 10.8, 2.7 Hz, 1H), 3.61 (dd, J = 10.8, 5.2 Hz, 1H).
A mixture of 13 (480 mg, 0.85 mmol, 1.0 eq.), 10% Pd/C (60 mg) and formic acid (2 mL) in methanol (6 mL) was stirred under hydrogen atmosphere overnight. The mixture was filtered, and the filtrate was concentrated to give a crude product, which was purified on reverse silica gel to give 2a (50 mg, 20%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 7.80 (s, 1H), 7.61 (s, 2H), 6.81 (d, J = 4.4 Hz, 1H), 6.73 (d, J = 4.4 Hz, 1H), 5.18 (d, J = 4.7 Hz, 1H), 4.89 (d, J = 7.1 Hz, 1H), 4.79 (t, J = 5.7 Hz, 1H), 4.51–4.38 (m, 2H), 4.16 (dd, J = 11.6, 6.7 Hz, 1H), 3.99 (dd, J = 11.6, 4.6 Hz, 1H), 3.94–3.83 (m, 1H), 3.78–3.69 (m, 1H), 3.70–3.59 (m, 1H), 3.56–3.47 (m, 1H). 13C NMR (126 MHz, DMSO‑d6) δ 155.71, 147.17, 131.72, 114.78, 110.92, 100.44, 85.50, 82.48, 74.11, 71.24, 62.48, 62.10. MS m/z = 297.1 [M+1]+. Optical rotation [α] 20 D = −22.4 (c 0.25, DMSO).
4.1.7. 3-((2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)acrylonitrile (2b)
To a solution of 12 (200 mg, 0.35 mmol) and diethyl cyanomethylphosphonate (310 mg, 1.75 mmol, 5.0 eq) in tetrahydrofuran (4 mL) was added 60% NaH (70 mg, 1.8 mmol, 5.0 eq.) at 0 °C. The mixture was stirred at rt for 4 h, and quenched with saturated aqueous NH4Cl solution. After workup, the residue was purified on silica gel to give 14 as an oil. Debenzylation of 14 with BCl3 afforded 2b (40 mg, 36% over two steps) as a white solid. 1H NMR (500 MHz, Methanol‑d 4) δ 7.84 (s, 0.4H), 7.82 (s, 0.6H), 7.63 (d, J = 16.3 Hz, 0.4H), 7.36 (d, J = 11.9 Hz, 0.6H), 6.92–6.82 (m, 1.6H), 6.78 (d, J = 4.5 Hz, 0.4H), 5.89 (d, J = 16.3 Hz, 0.4H), 5.69 (d, J = 11.8 Hz, 0.6H), 4.81 (t, J = 5.4 Hz, 1H), 4.25 (dt, J = 6.7, 3.4 Hz, 0.6H), 4.15–4.04 (m, 1H), 3.99 (dd, J = 7.1, 4.9 Hz, 0.4H), 3.93 (td, J = 12.7, 2.8 Hz, 1H), 3.82–3.69 (m, 1H). MS m/z = 318.1 [M+H]+.
4.1.8. 1-((2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)ethan-1-one (2c)
A mixture of 8 (560 mg, 1.0 mmol) and methyl magnesium bromide (3.0 M in 2-methyltetrahydrofuran, 1.67 mL, 5.0 mmol, 5.0 eq.) in tetrahydrofuran (5 mL) was stirred at 60 °C for 2 h. The mixture was cooled to rt, and quenched with saturated aqueous NH4Cl solution at 0 °C. The resulting solution was concentrated, and the residue was partitioned between water and ethyl acetate. The organic layer was died and concentrated. The residue was purified on silica gel to give 15 (380 mg, 66%) as a white solid. 1H NMR (500 MHz, DMSO‑d 6) δ 7.77 (s, 1H), δ 7.73 (br, 2H), 7.39–7.25 (m, 10H), 7.24–7.17 (m, 3H), 6.99–6.93 (m, 2H), 6.83 (d, J = 4.5 Hz, 1H), 6.72 (d, J = 4.5 Hz, 1H), 5.10 (d, J = 4.1 Hz, 1H), 4.76 (dd, J = 11.6, 4.0 Hz, 2H), 4.67 (d, J = 11.8 Hz, 1H), 4.60 (d, J = 11.3 Hz, 1H), 4.44–4.37 (m, 1H), 4.33–4.27 (m, 2H), 4.24 (d, J = 12.3 Hz, 1H), 3.52 (dd, J = 11.3, 2.7 Hz, 1H), 3.39–3.36 (m, 1H), 2.37 (s, 3H). The debenzylation of 15 (200 mg, 0.35 mmol) with BCl3 afforded 2c (50 mg, 46%) as a white solid. 1H NMR (500 MHz, DMSO‑d 6) 7.80–7.51 (m, 3H), 6.80 (d, J = 4.5 Hz, 1H), 6.63 (d, J = 4.5 Hz, 1H),5.51 (d, J = 5.3 Hz, 1H), 5.00 (d, J = 6.9 Hz, 1H), 4.74 (t, J = 4.9 Hz, 1H), 4.54 (t, J = 5.6 Hz, 1H), 4.10–3.95 (m, 2H), 3.55–3.44(m, 1H), 3.27–3.18 (m, 1H), 2.32 (s, 3H). 13C NMR (126 MHz, DMSO‑d 6) δ 208.17, 155.51, 147.13, 129.92, 114.62, 109.06, 100.37, 87.65, 83.73, 75.80, 71.75, 61.74, 28.54. MS m/z = 309.2 [M+1]+. Optical rotation [α] 20 D = −180.8 (c 0.25, DMSO).
4.1.9. (3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-cyclopropyl-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (2d)
To a solution of 17 (1.0 g, 1.81 mmol) in anhydrous tetrahydrofuran (10 mL) at 0 °C was added cyclopropyl magnesium bromide (0.5 M in tetrahydrofuran, 36.2 mL, 18.1 mmol, 10 eq.) dropwise under nitrogen atmosphere. The mixture was stirred at rt for 10 h, and quenched with saturated aqueous NH4Cl solution at 0 °C. The resulting solution was concentrated, and the residue was partitioned between water and ethyl acetate. The organic layer was dried and concentrated. The residue was purified on silica gel to give 18 (400 mg, 37%) as a white solid. MS m/z = 595.3 [M+1]+. To a solution of 18 (300 mg, 0.50 mmol, 1.0 eq.) in anhydrous dichloromethane (3 mL) at 0 °C was added methane sulfonic acid (144 mg, 1.5 mmol, 3.0 eq.). The mixture was allowed to warm to rt and stirred for 10 h, then neutralized with saturated aqueous NaHCO3 solution. After workup, the residue was purified on silica gel to give 19 (144 mg, 50%) as a white solid. MS m/z = 577.2 [M+1]+. The debenzylation of 19 (240 mg, 0.42 mmol) with H2 and Pd/C afforded two diastereoisomers. One diastereoisomer (Rf = 0.17 in CH2Cl2:CH3OH = 15:1, 10 mg, 8%): 1H NMR (500 MHz, Methanol‑d 4) δ 7.78 (s, 1H), 6.85 (d, J = 4.5 Hz, 1H), 6.79 (d, J = 4.5 Hz, 1H), 4.78 (d, J = 5.1 Hz, 1H), 4.00–3.95 (m, 1H), 3.95–3.88 (m, 1H), 3.81 (dd, J = 11.9, 2.8 Hz, 1H), 3.68 (dd, J = 11.8, 5.5 Hz, 1H), 2.02–1.91 (m, 1H), 0.69–0.51 (m, 2H), 0.28–0.19 (m, 1H), 0.19–0.08 (m, 1H). MS m/z = 307.1 [M+1]+. The other diastereoisomer (Rf = 0.14 in CH2Cl2:CH3OH = 15:1, 50 mg, 39%): 1H NMR (500 MHz, Methanol‑d 4) δ 7.78 (s, 1H), 6.86 (d, J = 4.5 Hz, 1H), 6.69 (d, J = 4.4 Hz, 1H), 4.65 (d, J = 5.0 Hz, 1H), 4.10 (dd, J = 9.3, 5.1 Hz, 1H), 3.97–3.92 (m, 1H), 3.90 (dd, J = 12.0, 2.3 Hz, 1H), 3.69 (dd, J = 12.0, 5.8 Hz, 1H), 1.96–1.89 (m, 1H), 0.76–0.68 (m, 1H),0.48–0.41 (m, 1H), 0.35–0.28 (m, 1H), 0.18–0.11 (m, 1H). MS m/z = 307.1 [M+1]+, optical rotation [α] 20 D = +12.8 (c 0.25, DMSO).
4.1.10. (3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-(hydroxymethyl)-2-(prop-1-en-2-yl)tetrahydrofuran-3,4-diol (2e)
The title compound 2e was prepared following the similar procedure as described for 2d except that BCl3 was used for the debenzylation. Reaction of 17 (2.2 g, 3.98 mmol) with isopropenyl magnesium bromide gave 20 (1.6 g, 68%) that was treated with methane sulfonic acid to afford the cyclized product 21 (700 mg, 45%). The subsequent debenzylation with BCl3 produced two diastereoisomers. One diastereoisomer (Rf = 0.13 in CH2Cl2:CH3OH = 15:1, 25 mg, 7%): 1H NMR (600 MHz, DMSO‑d6) δ 7.74 (s, 1H), 7.55 (s, 2H), 6.82 (d, J = 4.5 Hz, 1H), 6.58 (d, J = 4.4 Hz, 1H), 5.03 (s, 1H), 4.88–4.73 (m, 3H), 4.72–4.58 (m, 2H), 4.09–3.89 (m, 1H), 3.90–3.71 (m, 1H), 3.70–3.56 (m, 1H), 3.54–3.42 (m, 1H), 1.59 (s, 3H). MS m/z = 307.2 [M+1]+.The other diastereoisomer (Rf = 0.09 in CH2Cl2:CH3OH = 15:1, 25 mg, 7%): 1H NMR (600 MHz, DMSO‑d 6) δ 7.77 (s, 1H), 7.59 (s, 2H), 6.82 (d, J = 4.4 Hz, 1H), 6.64 (d, J = 4.5 Hz, 1H), 5.12 (t, J = 5.0 Hz, 1H), 5.01 (dd, J = 2.4, 1.0 Hz, 1H), 4.84 (d, J = 5.4 Hz, 1H), 4.81–4.76 (m, 1H), 4.73 (d, J = 7.1 Hz, 1H), 4.47 (t, J = 5.7 Hz, 1H), 3.89–3.83 (m, 1H), 3.79–3.72 (m, 1H), 3.47–3.40 (m, 1H), 3.30–3.24 (m, 1H), 1.69 (s, 3H). MS m/z = 307.2 [M+1]+, optical rotation [α] 20 D = −145.6 (c 0.25, DMSO).
4.1.11. (2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carboxamide (2f)
Compound 2f was obtained as a byproduct in the debenzylation of intermediate 8 with BCl3. 1H NMR (500 MHz, DMSO‑d6) δ 7.77 (s, 1H), 7.72 (br, 2H), 7.49 (s, 1H), 7.44 (s, 1H), 6.83 (d, J = 4.5 Hz, 1H), 6.62 (d, J = 4.5 Hz, 1H), 5.58 (s, 1H), 4.93 (s, 1H), 4.85 (d, J = 4.4 Hz, 1H), 4.68 (s, 1H), 4.08–3.98 (m, 2H), 3.52 (d, J = 12.0 Hz, 1H), 3.34 – 3.29 (m, 1H). 13C NMR (126 MHz, DMSO‑d6) δ 173.01, 155.64, 147.16, 128.55, 115.22, 110.45, 100.47, 84.31, 82.90, 74.89, 71.71, 61.99. MS m/z = 310.1 [M+1]+. Optical rotation [α] 20 D = −133.2 (c 0.25, DMSO).
4.1.12. (2S,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (3a)
3a was synthesized according to the literature procedure.15 1H NMR (500 MHz, DMSO‑d 6) δ 7.82 (s, 1H), 7.67 (s, 2H), 6.84 (d, J = 4.4 Hz, 1H), 6.68 (d, J = 4.4 Hz, 1H), 5.11 (d, J = 6.5 Hz, 1H), 4.95 (d, J = 6.3 Hz, 1H), 4.86 (d, J = 5.3 Hz, 1H), 4.76 (t, J = 5.7 Hz, 1H), 4.24 (q, J = 5.9 Hz, 1H), 3.95 (q, J = 4.9 Hz, 1H), 3.79 (q, J = 4.4 Hz, 1H), 3.55 (dt, J = 11.7, 4.5 Hz, 1H), 3.46 (dt, J = 11.3, 5.3 Hz, 1H). 13C NMR (126 MHz, DMSO‑d 6) δ 156.06, 148.10, 129.41, 115.34, 110.20, 101.22, 84.98, 75.87, 74.21, 71.75, 62.56. MS m/z = 267.2 [M+1]+. Optical rotation [α] 20 D = −49.6 (c 0.25, DMSO).
4.1.13. (2S,3R,4S,5R)-2-(4-(hydroxyamino)pyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (3b)
A solution of hydroxylamine hydrochloride (540 mg, 7.8 mmol, 30 eq.) in water (3 mL) was prepared, and adjusted to pH = 6 with 10% aqueous NaOH solution. Then 3a (69 mg, 0.26 mmol, 1.0 eq.) was added and the mixture was stirred at 40 °C overnight. After evaporation, the residue was purified on reverse silica gel to afford 3b (52 mg, 71%) as an off-white solid. 1H NMR (500 MHz, DMSO‑d6) 1H NMR (500 MHz, DMSO‑d 6) δ 10.61 (d, J = 4.0 Hz, 1H), 10.06 (s, 1H), 7.39 (d, J = 4.0 Hz, 1H), 6.38–6.33 (m, 2H), 4.96 (d, J = 6.6 Hz, 1H), 4.93 (d, J = 6.3 Hz, 1H), 4.86 (d, J = 5.1 Hz, 1H), 4.74–4.68 (m, 2H), 4.15 (q, J = 6.1 Hz, 1H), 3.91 (q, J = 4.9 Hz, 1H), 3.74 (q, J = 4.5 Hz, 1H), 3.55–3.49 (m, 1H), 3.47–3.43 (m, 1H). MS m/z = 283.0 [M+1]+.
4.1.14. (2R,3R,4R,5S)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-4-fluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol (4a)
To a suspension of 22 (852 mg, 4.0 mmol) and trimethylchlorosilane (956 mg, 8.8 mmol, 2.2 eq.) in anhydrous tetrahydrofuran (20 mL) was added n-BuLi (1.6 M in hexane, 10 mL, 16.0 mmol, 4.0 eq.) dropwise at −78 °C. After stirring for 0.5 h, a solution of 23 (1.32 g, 4.0 mmol, 1.0 eq.) in anhydrous tetrahydrofuran (10 mL) was added, and stirred for extra 1 h at −78 °C. The resulting solution was poured into aqueous 1 M HCl solution (20 mL) and extracted with ethyl acetate. The organic extract was washed with saturated aqueous NaHCO3 solution, then dried and concentrated. The residue was purified on silica gel to give 24 (560 mg, 30%). A mixture of 24 (405 mg, 0.87 mmol) and triethylsilane (404 mg, 3.48 mmol, 4.0 eq.) in dichloromethane (30 mL) was cooled to 0 °C, followed by the addition of boron trifluoride diethyl etherate (494 mg, 3.48 mmol, 4.0 eq.). The mixture was stirred at rt for 2 h, and then quenched with saturated aqueous NaHCO3 solution (5 mL). After workup, the residue was purified on silica gel to give 25 (241 mg, 62%). 25 (240 mg, 0.54 mmol, 1.0 eq.) was debenzylated by the same method as described for 2a to give 4a (67 mg, 46%) as a white solid. 1H NMR (500 MHz, DMSO‑d 6) δ 7.87 (s, 1H), 7.76 (br, 2H), 6.86 (d, J = 4.5 Hz, 1H), 6.76 (d, J = 4.4 Hz, 1H), 5.49 (d, J = 2.6 Hz, 0.5H), 5.47–5.41 (m, 1.5H), 5.10–5.07 (m, 0.5H), 4.97 (dd, J = 4.4, 2.7 Hz, 0.5H), 4.86 (t, J = 5.8 Hz, 1H), 4.18–4.10 (m, 1H), 3.85–3.80 (m, 1H), 3.76–3.68 (m, 1H), 3.57–3.49 (m, 1H). MS m/z = 269.2 [M+1]+.
4.1.15. (2R,3R,5S)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-4,4-difluoro-2-(hydroxymethyl)tetrahydrofuran-3-ol (4b)
Compound 4b was prepared following the same procedure as described for 4a. The glycosylation between 22 (1.77 g, 8.31 mmol) and 26 (3.76 g, 10.0 mmol, 1.2 eq.) produced 27 (600 mg, 14%). 27 (500 mg, 0.98 mmol) was reduced with triethylsilane to yield 28 (200 mg, 41%) which was subjected to debenzoylation by methanolic ammonia (7 M, 1 mL) to give 4b (20 mg, 17%) as a white solid. 1H NMR (500 MHz, DMSO‑d 6) δ 7.89 (s, 1H), 7.82 (br, 2H), 6.91 (d, J = 4.4 Hz, 1H), 6.76 (dd, J = 4.5, 1.4 Hz, 1H), 6.14 (d, J = 6.3 Hz, 1H), 5.58 (dd, J = 14.3, 10.7 Hz, 1H), 4.99 (t, J = 5.8 Hz, 1H), 4.13 (dq, J = 13.2, 6.5 Hz, 1H), 3.84–3.78 (m, 1H), 3.72–3.64 (m, 1H), 3.59 (dt, J = 11.8, 5.6 Hz, 1H). MS m/z = 287.2 [M+1]+.
4.1.16. (2R,3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-4-hydroxy-5-(hydroxymethyl)-3-methoxytetrahydrofuran-2-carbonitrile (4c)
To a solution of 29 (2.2 g, 7.55 mmol) and imidazole (3.08 g, 45.30 mmol, 6.0 eq.) in DMF (20 mL) was added 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (2.86 g, 9.06 mmol, 1.2 eq.) dropwise at 0 °C. The mixture was stirred at rt for 3 h, and then partitioned between water and ethyl acetate. The organic layer was concentrated and the residue was purified on silica gel to give 30 (3.2 g, 79%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 7.99–7.82 (m, 3H), 6.88 (d, J = 4.5 Hz, 1H), 6.79 (d, J = 4.4 Hz, 1H), 6.45 (d, J = 5.7 Hz, 1H), 4.56 (t, J = 5.0 Hz, 1H), 4.22–4.09 (m, 3H), 3.95–3.86 (m, 1H), 1.09–0.76 (m, 28H)
A mixture of 30 (1.2 g, 2.25 mmol) and DMF-DMA (520 mg, 4.50 mmol, 2.0 eq.) in toluene (20 mL) was stirred at 60 °C for 2 h. The solvent was removed to give 31 (1.2 g, 91%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 8.96 (s, 1H), 8.16 (s, 1H), 6.91 (d, J = 4.5 Hz, 1H) 6.81 (d, J = 4.4 Hz, 1H), 6.49 (d, J = 5.8 Hz, 1H), 4.59 (t, J = 5.1 Hz, 1H), 4.26–4.11 (m, 3H), 3.93 (dd, J = 13.3, 2.4 Hz, 1H), 3.265 (s, 3H), 3.20 (s, 3H), 1.09–0.87 (m, 28H). Compound 31 (1.2 g, 2.04 mmol) and methyl iodide (580 mg, 4.08 mmol, 2.0 eq.) were dissolved in DMF (10 mL), followed by the addition of 60% NaH (163 mg, 4.08 mmol, 2.0 eq.) at 0 °C. The mixture was stirred for 15 min at 0 °C, and quenched with saturated aqueous NH4Cl solution. The resulting solution was extracted with ethyl acetate, and the extract was concentrated to afford crude 32. To a solution of 32 in acetonitrile (5 mL) was added 85% hydrazine (480 mg, 8.16 mmol, 4.0 eq.) at rt, and the mixture was stirred at rt for 1 h. After workup, the residue was purified on silica gel to give the desired intermediate (330 mg, 30% over two steps) as a white solid. Then, the intermediate was dissolved in tetrahydrofuran (4 mL), and TBAF (1 M in tetrahydrofuran, 0.66 mL, 0.66 mmol, 1.1 eq.) was added at 0 °C. The mixture was stirred at rt for 2 h, then poured into water, and extracted with ethyl acetate. The organic layer was concentrated, the residue was purified on silica gel to give 4c (92 mg, 50%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 8.07–7.78 (m, 3H), 6.92 (d, J = 4.5, 1H), 6.87 (d, J = 4.4, 1H), 5.30 (d, J = 5.4 Hz, 1H), 4.94 (t, J = 5.8 Hz, 1H), 4.37 (d, J = 4.9 Hz, 1H), 4.11 (q, J = 5.4 Hz, 1H), 4.08–4.02 (m, 1H), 3.71–3.62 (m, 1H), 3.55 (s, 3H), 3.54–3.48 (m, 1H). 13C NMR (126 MHz, DMSO‑d 6) δ 155.62, 147.98, 123.45, 117.02, 116.62, 110.58, 100.87, 85.67, 83.33, 76.97, 68.87, 60.63, 58.93. MS m/z = 306.0 [M+1]+. Optical rotation [α] 20 D = +2.8 (c 0.25, DMSO).
4.1.17. (2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl isobutyrate (5a)
To a solution of 31 (294 mg, 0.5 mmol), triethylamine (101 mg, 1.0 mmol, 2.0 eq.) and DMAP (12 mg, 0.10 mmol, 0.2 eq.) in dichloromethane (5 mL) was added isobutyryl chloride (85 mg, 0.8 mmol, 1.6 eq.) at 0 °C. The mixture was stirred at rt for 2 h, then diluted with dichloromethane (10 mL), and washed successively with aqueous 1 M HCl solution and saturated aqueous NaHCO3 solution. The organic layer was concentrated, and the residue was subjected to silica gel column chromatography to afford 33 (240 mg, 72%) as a white foam. A solution of compound 33 (240 mg, 0.36 mmol) and TBAF (1 M in tetrahydrofuran, 0.72 mL, 0.72 mmol, 2.0 eq.) in tetrahydrofuran (2 mL) was stirred at rt for 2 h. After workup, the desired intermediate was dissolved in THF (6 mL), then a solution of trifluoroacetic acid (205 mg, 1.8 mmol, 5.0 eq.) in water (0.5 mL) was added and stirred overnight. The mixture was neutralized, then partitioned between water and ethyl acetate. The organic layer was concentrated, and the residue was purified on silica gel to give 5a (80 mg, 61% over two steps) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 8.09–7.84 (m, 3H), 6.94 (d, J = 4.6 Hz, 1H), 6.90 (d, J = 4.6 Hz, 1H), 6.43 (d, J = 6.5 Hz, 1H), 5.22 (dd, J = 5.7, 3.3 Hz, 1H), 5.07 (t, J = 5.8 Hz, 1H), 5.01 (t, J = 6.1 Hz, 1H), 4.31–4.25 (m, 1H), 3.66–3.52 (m, 2H),2.68–2.58 (m, 1H), 1.19 (d, J = 7.0 Hz, 3H), 1.17 (d, J = 7.0 Hz, 3H). 13C NMR (126 MHz, DMSO‑d6) δ 175.46, 155.67, 148.00, 122.79, 117.04, 116.87, 111.23, 100.88, 84.12, 77.78, 72.68, 72.23, 60.77, 33.35, 18.73, 18.62. MS m/z = 362.0 [M+H]+. Optical rotation [α] 20 D = −58.2 (c 0.50, CH3CN).
4.1.18. (2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl L-valinate (dihydrochloride salt) (5b)
To a solution of 31 (260 mg, 0.44 mmol) in dichloromethane (10 mL) was sequentially added N-Boc-l-valine (115 mg, 0.53 mmol, 1.2 eq.), HOBT (89 mg, 0.66 mmol, 1.5 eq.), EDCI (169 mg, 0.88 mmol, 2.0 eq.) and DMAP (215 mg, 1.76 mmol, 4 eq.). The mixture was stirred at rt overnight, then poured into water, and extracted with dichloromethane. The organic layer was dried and concentrated. The residue was purified on silica gel to give 34 (260 mg, 75%) as a white solid. 1H NMR (500 MHz, DMSO‑d6) δ 8.98 (s, 1H), 8.15 (s, 1H), 7.23 (d, J = 9.0 Hz, 1H), 6.96 (d, J = 4.5 Hz, 1H), 6.85 (d, J = 4.6 Hz, 1H), 5.90 (d, J = 5.0 Hz, 1H), 4.57 (dd, J = 9.2, 4.9 Hz, 1H), 4.27–4.17 (m, 3H), 3.95 (dd, J = 13.8, 2.8 Hz, 1H), 3.27 (s, 3H), 3.21 (s, 3H), 2.30–2.19 (m, 1H), 1.45–1.34 (m, 9H), 1.10–0.86 (m, 34H). 34 (260 mg, 0.33 mmol) was subjected to deprotection to give 35 (103 mg, 64%) following the same procedure as described for 32. 1H NMR (500 MHz, DMSO‑d6) δ 8.08–7.87 (m, 3H), 7.06 (d, J = 8.6 Hz, 1H), 6.94 (d, J = 4.5 Hz, 1H), 6.91 (d, J = 4.6 Hz, 1H), 6.50 (d, J = 6.7 Hz, 1H), 5.19–5.14 (m, 1H), 5.10–5.05 (m, 1H), 5.03–4.98 (m, 1H), 4.26–4.20 (m, 1H), 4.10 (dd, J = 8.7, 5.5 Hz, 1H), 3.66–3.53 (m, 2H), 2.31–2.22 (m, 1H), 1.45–1.35 (m, 9H), 0.93 (d, J = 6.8 Hz, 3H), 0.90 (d, J = 6.8 Hz, 3H). 35 (103 mg, 0.21 mmol) was added in 4 M HCl/CH3OH (2 mL) and stirred at rt for 2 h. The solvent was evaporated, and the residue was slurried in MTBE (5 mL) to give 5b (73 mg, 75%) as a white solid. 1H NMR (500 MHz, Methanol‑d4) δ 8.17 (s, 1H), 7.50 (d, J = 4.8 Hz, 1H), 7.21 (d, J = 4.8 Hz, 1H), 5.52 (dd, J = 5.8, 3.2 Hz, 1H), 5.19 (d, J = 5.8 Hz, 1H), 4.54–4.48 (m, 1H), 4.16–4.10 (m, 1H), 3.83 (d, J = 3.7 Hz, 2H), 2.58–2.48 (m, 1H), 1.18 (d, J = 4.2 Hz, 3H), 1.17 (d, J = 4.2 Hz, 3H). 13C NMR (126 MHz, Methanol‑d4) δ 167.83, 149.05, 135.63, 128.54, 115.34, 113.74, 113.30, 108.72, 84.02, 77.83, 73.85, 73.56, 60.26, 57.85, 28.67, 16.95, 15.67. MS m/z = 391.1 [M+1]+.
4.1.19. ((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methyl isobutyrate (5c)
29 (1.0 g, 3.43 mmol) was coevaporated with anhydrous pyridine. Afterwards, pyridine (10 mL) and DMF-DMA (1.63 g, 13.72 mmol, 4.0 eq.) were added. The mixture was stirred at rt overnight, and concentrated to give crude 37 as an oil. A mixture of the crude, isobutyric anhydride (1.08 g, 6.86 mmol, 2.0 eq.), triethylamine (1.04 g, 10.29 mmol, 3.0 eq.) and DMAP (420 mg, 3.43 mmol, 1.0 eq.) in dichloromethane (10 mL) was stirred at rt overnight. After workup, 38 was obtained as an oil which was treated with a mixture of ethanol (20 mL) and acetic acid (6.17 g, 102.90 mmol, 30 eq.) at 50 °C overnight. The resulting solution was concentrated and the residue was partitioned between water and ethyl acetate. The organic layer was concentrated, and the residue was purified on silica gel to give 5c (818 mg, 66% over three steps) as a white solid. 1H NMR (500 MHz, DMSO‑d6) 8.00–7.80 (m, 3H),6.92 (d, J = 4.5 Hz, 1H), 6.82 (d, J = 4.6 Hz, 1H), 6.33 (d, J = 6.0 Hz, 1H), 5.39 (d, J = 5.8 Hz, 1H), 4.70 (dd, J = 6.0, 5.0 Hz, 1H), 4.32 (dd, J = 12.0, 2.9 Hz, 1H), 4.27–4.21 (m, 1H), 4.18 (dd, J = 12.0, 5.3 Hz, 1H), 4.00–3.92 (m, 1H), 2.58–2.52 (m, 1H), 1.07 (d, J = 2.4 Hz, 3H), 1.06 (d, J = 2.4 Hz, 3H). 13C NMR (126 MHz, DMSO‑d6) δ 175.91, 155.60, 147.95, 123.54, 116.95, 116.60, 110.25, 100.81, 81.29, 79.03, 74.03, 70.18, 62.94, 33.17, 18.74, 18.66. MS m/z = 362.0 [M+1]+. Optical rotation [α] 20 D = −23.8 (c 0.50, CH3CN).
4.1.20. ((2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methyl L-valinate (dihydrochloride salt) (5d)
Following the procedure for the synthesis of 5b, 5d (80 mg, 17% over four steps) was obtained as a white solid from 29 (290 mg, 1 mmol). 1H NMR (500 MHz, Methanol‑d4) δ 8.15 (s, 1H), 7.49 (d, J = 4.8 Hz, 1H), 7.12(d, J = 4.8 Hz, 1H), 4.74 (d, J = 5.2 Hz, 1H), 4.62 (dd, J = 12.1, 7.4 Hz, 1H), 4.54 (dd, J = 12.1, 2.8 Hz, 1H), 4.45 (td, J = 7.5, 2.7 Hz,1H), 4.05–4.00 (m, 2H), 2.35–2.27 (m, 1H), 1.08 (d, J = 1.9 Hz, 3H), 1.07 (d, J = 1.8 Hz, 3H). 13C NMR (126 MHz, Methanol‑d4) δ 168.17, 148.63, 135.04, 129.81, 114.73, 113.37, 112.72, 109.33, 80.81, 79.54, 74.44, 70.18, 64.81, 57.59, 29.21, 16.51, 16.34. MS m/z = 391.1 [M+1]+.
4.1.21. (2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-4-hydroxy-2-((isobutyryloxy)methyl)tetrahydrofuran-3-yl isobutyrate (5e)
To a solution of 5c (360 mg, 1 mmol) and trimethyl orthoisobutyrate (370 mg, 2.5 mmol, 2.5 eq.) in acetonitrile (5 mL) was added p-toluenesulfonic acid monohydrate (230 mg, 1.2 mmol, 1.2 eq.). The mixture was stirred at rt for 4 h, and then neutralized with saturated NaHCO3 solution. The resulting solution was partitioned between water and ethyl acetate. The organic layer was evaporated, and the residue was dissolved in tetrahydrofuran (5 mL). Then, aqueous 1 M HCl solution (1 mL) was added, and the resulting mixture was stirred at rt for 1 h. After workup, the residue was purified on silica gel to give 5e (328 mg, 76%) as a white solid. 1H NMR (600 MHz, DMSO‑d6) δ 8.05–7.83 (m, 3H), 6.92(d, J = 4.6 Hz, 1H), 6.85 (d, J = 4.6 Hz, 1H), 6.56 (d, J = 6.5 Hz, 1H), 5.16 (dd, J = 5.6, 4.1 Hz, 1H), 5.09 (t, J = 6.1 Hz, 1H), 4.46 (q, J = 4.3 Hz, 1H), 4.27 (dd, J = 12.2, 4.0 Hz, 1H), 4.23 (dd, J = 12.2, 4.8 Hz, 1H), 2.67–2.58 (m, 1H), 2.55–2.49 (m, 1H), 1.16 (d, J = 7.0 Hz, 3H), 1.15 (d, J = 7.0 Hz, 3H), 1.05 (d, J = 7.0 Hz, 3H), 1.03 (d, J = 7.2 Hz, 3H). MS m/z = 432.2 [M+1]+.
4.1.22. (2R,3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-cyano-5-(hydroxymethyl)tetrahydrofuran-3,4-diyl bis(2-methylpropanoate) (5f)
A solution of 29 (2 g, 6.87 mmol) and DMF-DMA (5.5 g, 46.03 mmol, 6.7 eq.) in DMF (20 mL) was stirred at 60 °C for 1 h. The resulting solution was co-evaporated with ethanol and toluene, successively. Then, the obtained oil was treated with isopropanol to give 41 (2.0 g, 84%) as a white solid. To a solution of 41 (2.0 g, 5.77 mmol) and imidazole (1.57 g, 23.08 mmol, 4.0 eq.) in DMF (20 mL) was added tert-butyldiphenylsilyl chloride (2.38 g, 8.66 mmol, 1.5 eq.) dropwise in an ice bath. The mixture was stirred at rt for 3–4 h, then poured into water and extracted with ethyl acetate. The organic extract was dried and evaporated to give crude 42 which was then subjected to three sequential reactions to give 5f (1.0 g, 40% over four steps) following the same procedure as described for 5a. 1H NMR (500 MHz, DMSO‑d6) δ 8.15–7.89 (m, 3H), 6.94(d, J = 4.73,1H), 6.79 (d, J = 4.65,1H), 6.01 (d, J = 5.7 Hz, 1H), 5.44 (dd, J = 5.7, 3.1 Hz, 1H), 5.18 (dd, J = 6.1, 5.2 Hz, 1H), 4.41 (q, J = 3.4 Hz, 1H), 3.71–3.60 (m, 2H), 2.69–2.54 (m, 2H), 1.19 (d, J = 7.0 Hz, 3H), 1.16 (d, J = 7.0 Hz, 3H), 1.11 (d, J = 2.4 Hz, 3H), 1.09 (d, J = 2.4 Hz, 3H). MS m/z = 432.2 [M+1]+.
4.1.23. (2R,3R,4R,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-cyano-5-((isobutyryloxy)methyl)tetrahydrofuran-3,4-diyl bis(2-methylpropanoate) (5g)
41 (173 mg, 0.5 mmol), triethylamine (405 mg, 4.0 mmol, 8.0 eq.) and DMAP (30 mg, 0.25 mmol, 0.5 eq.) were suspended in dichloromethane (5 mL), and then isobutyryl chloride (240 mg, 2.25 mmol, 4.5 eq.) was added dropwise in an ice bath. The mixture was stirred at rt overnight. The resulting solution was washed aqueous 1 M HCl solution, saturated aqueous NaHCO3 solution, then dried, and concentrated to afford 44 (223 mg, 80 %) as a white solid. A mixture of 44 (223 mg, 0.4 mmol), 85% hydrazine hydrate (94 mg, 1.6 mmol, 4.0 eq.) in acetonitrile (10 mL) was stirred at rt for 1 h. After workup, the residue was purified on silica gel to give 5g (170 mg, 85%) as a white foam. 1H NMR (500 MHz, DMSO‑d 6) δ 8.04 (s, 1H), 7.98 (s, 2H), 7.94 (s, 1H), 6.94 (d, J = 4.6 Hz, 1H), 6.77 (d, J = 4.6 Hz, 1H), 6.09 (d, J = 5.7 Hz, 1H), 5.45 (dd, J = 5.8, 3.7 Hz, 1H), 4.64 (q, J = 3.7 Hz, 1H), 4.34 (dd, J = 12.4, 3.4 Hz, 1H), 4.29 (dd, J = 12.4, 4.1 Hz, 1H), 2.68–2.57 (m, 2H), 2.50–2.46 (m, 1H), 1.17 (d, J = 7.0 Hz, 3H), 1.15 (d, J = 6.9 Hz, 3H), 1.12–1.09 (m, 6H), 1.05 (d, J = 7.0 Hz, 3H), 1.02 (d, J = 7.0 Hz, 3H). 13C NMR (126 MHz, DMSO‑d 6) δ 175.59, 174.96, 174.18, 155.59, 148.18, 121.02, 117.22, 115.49, 110.41, 101.14, 81.31, 75.73, 72.11, 70.33, 62.51, 33.22, 33.18, 33.10, 18.63, 18.54, 18.49, 18.42, 18.26. MS m/z = 502.0 [M+1]+. Optical rotation [α] 20 D = +11.2 (c 0.50, CH3CN).
4.1.24. Pentyl (7-((2R,3R,4S,5R)-2-cyano-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)carbamate (6)
To a solution of 30 (150 mg, 0.28 mmol) in anhydrous dichloromethane (2 mL) was added pyridine (265 mg, 3.36 mmol, 12 eq.) and trimethylchlorosilane (91 mg, 0.84 mmol, 3.0 eq.) at 0 °C. The mixture was stirred for 1 h, and then pentyl chloroformate (128 mg, 0.84 mmol, 3.0 eq.) was added. After stirring for 2 h at rt, the resulting solution was poured into water, and extracted with dichloromethane. The organic layer was washed with saturated aqueous NaHCO3 solution and brine, dried and concentrated to afford 36 as an oil. To a solution of 36 in tetrahydrofuran (2 mL) was added TBAF (1 M in tetrahydrofuran, 0.56 mL, 0.56 mmol, 2.0 eq.) at rt, and the mixture was stirred at rt for 2 h. After workup, the residue was purified on silica gel to give 6 (55 mg, 48% over two steps) as a white solid. 1H NMR (600 MHz, DMSO‑d6) δ 10.88 (s, 1H), 8.37 (s, 1H), 7.30 (d, J = 4.8 Hz, 1H), 7.11 (d, J = 4.8 Hz, 1H), 6.21 (d, J = 6.2 Hz, 1H), 5.23 (d, J = 5.5 Hz, 1H), 4.90 (t, J = 5.6 Hz, 1H), 4.61 (t, J = 5.7 Hz, 1H), 4.18 (t, J = 6.7 Hz, 2H), 4.10–4.04 (m, 1H), 3.95 (q, J = 5.5 Hz, 1H), 3.68–3.61 (m, 1H), 3.54–3.47 (m, 1H), 1.71–1.61 (p, J = 6.8 Hz, 2H), 1.39–1.29 (m, 4H), 0.89 (t, J = 7.0 Hz, 3H). MS m/z = 406.0 [M+1]+.
4.1.25. 2-ethylbutyl ((S)-(((2R,3S,4R,5R)-5-(4-amino-5-fluoropyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)-L-alaninate (7)
Compound 7 was synthesized starting from 1c by reference to the reported method.27 1H NMR (500 MHz, DMSO‑d6) δ 8.10 (br, 1H), 7.88 (s, 1H), 7.429(br, 1H), 7.36 (t, J = 7.7 Hz, 2H), 7.24–7.14 (m, 3H), 6.73 (s, 1H), 6.45 (d, J = 6.0 Hz, 1H), 6.12–6.02 (m, 1H), 5.39 (d, J = 6.0 Hz, 1H), 4.56 (t, J = 5.2 Hz, 1H), 4.31–4.19 (m, 2H), 4.16–4.06 (m, 1H), 4.01–3.77 (m, 4H), 1.29–1.20 (m, 8H), 0.80 (t, J = 7.4 Hz, 6H). MS m/z = 621.22 [M+1]+.
4.2. X-ray crystallographic data of 35 (intermediate used for the synthesis of 5b)
The colorless crystal was grown by slow evaporation in ethyl acetate solution. Diffraction intensity data were acquired with a CCD area detector with graphite-monochromated Cu Kα radiation (λ = 1.54178 Å). Crystal data of 35: C22H30N6O7 (M = 490.52); block crystal (0.15 × 0.08 × 0.06 mm); space group P212121; unit cell dimensions a = 8.8319(4) Å, b = 12.7389(5) Å, c = 22.7753(9) Å, V = 2562.42(18) Å3; Z = 4; α = 90°; β = 90°; γ = 90°, F (0 0 0) = 1040. The final refinement gave R1 = 0.0361 and wR 2 = 0.0865 [I > 2σ (I)]. The X-ray single-crystal structure of 35 was presented in the Supporting Information, and the crystallographic data was deposited in the Cambridge Crystallographic Data Center with deposition number CCDC2101800.
4.3. Methods to determine stability in artificial gastric and intestinal juice
The artificial gastric and intestinal juice were prepared according to Pharmacopeia of USA. The artificial gastric juice was prepared by dissolving 2.0 g of sodium chloride and 3.2 g of pepsin (3.2 g) into 600 mL pure water, then adding 7.0 mL of concentrated hydrochloric acid and diluted with water to 1 L, which was finally adjusted to pH 1.2. The artificial intestinal juice was prepared by firstly dissolving 6.8 g potassium dihydrogen phosphate in 250 mL water, then mixing with 77 mL sodium hydroxide solution (0.2 M) and 500 mL water with 10 g pancreatin, which was finally adjusted to pH 6.8 and diluted with water to 1 L. The compounds (5a-5d) were dissolved with DMF to make stock solutions (2 μM). Then 2 μL the above solution was added to a 1.7 mL microcentrifuge tube, followed by the addition of 398 μL artificial gastric or intestinal juice. The mixture was incubated for 24 h at 37 °C. At the predetermined time point (0, 1, 2, 3, 4, 6 and 24 h) incubations were terminated by adding 450 μL ice-cold acetonitrile. The resulting mixtures were centrifuged and the supernatants were subjected to HPLC analysis.
4.4. Methods to determine antiviral activities and cytotoxicity
The pre-seeded Vero E6 cells (5 × 104 cells/well) were treated with compound at indicated doses for 1 h, then infected with SARS-CoV-2(nCoV-2019BetaCoV/Wuhan/WIV04/2019) for 2 h. After removal of the supernatant of the mixture, the infected cells were washed with phosphate buffered saline (PBS), and cultured in fresh compound containing medium for 24 h. Then the supernatant of the mixture was collected and subjected to real-time fluorescence quantitative PCR (qRT-PCR) analysis for quantification of viral copy number which was used for the calculation of inhibition rate of tested compounds. The half-maximal effective concentration (EC50) was calculated with Graphpad Prism software 8.0. The cytotoxicities of compounds to the Vero E6 cells were determined using the cell counting kit-8 (CCK-8) colorimetric assay.
4.5. Methods for pharmacokinetic study
The PK studies were conducted at SIMM-Servier Joint Laboratory. CD-1 mice (N=3 per group) were fasted for 12 h before the administration of tested compounds. Each compound dissolved in DMSO-enthanol-PEG300-saline (5/5/40/50, v/v/v/v) was administered orally at 50 mg/Kg and intravenously at 25 mg/Kg, respectively. Blood samples were collected from the femoral vein into heparinized tubes at various time points post-dose. Serum samples were obtained following general procedures and the concentrations of analytes in the supernatant were analyzed by LC-MS/MS system.
4.6. Methods for acute toxicity study
For the acute toxicity study, 15 male ICR mice (28–36 g) were randomized into five treatment groups, with three animals per group. The animals were dosed once with 5c at 50 mg/kg, 100 mg/kg, 200 mg/kg, 500 mg/kg and 1000 mg/kg by oral gavage, respectively (formulated with 5% DMSO, 5% Solution Hs15 and 90% saline). After administration, the mice were observed for 7 days (general clinical observation, body weight, and food consumption) and sacrificed at day 8.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the grant from the Shanghai Science and Technology Committee in China (Number: 21S11903100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmc.2021.116364.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanche S., Lin Y.T., Xu C., et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infect Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA approval of remdesivir — a step in the right direction. N Engl J Med. 2020;383 doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 5.Lamb Y.N. Remdesivir: first approval. Drugs. 2020;80:1355–1363. doi: 10.1007/s40265-020-01378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eastman R.T., Roth J.S., Brimacombe K.R., et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel D., Hui H.C., Doerffler E., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo2,1-ftriazin-4-amino adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 8.Mehellou Y., Rattan H.S., Balzarini J. The ProTide prodrug technology: from the concept to the clinic. J Med Chem. 2018;61:2211–2226. doi: 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humeniuk R., Mathias A., Cao H., et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho A., Saunders O.L., Butler T., et al. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg Med Chem Lett. 2012;22:2705–2707. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do N-D-T, Chatterjee A, Gallay P, et al., A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents; 2021. [DOI] [PMC free article] [PubMed]
- 12.Li Y., Cao L., Li G., Cong F., Zhang X. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mice models. J Med Chem. 2020 doi: 10.1021/acs.jmedchem.0c01929. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y., Shuai L., Wen Z., et al. The preclinical inhibitor GS441524 in combination with GC376 efficaciously inhibited the proliferation of SARS-CoV-2 in the mouse respiratory tract. Emerg Microbes Infect. 2021;10:481–492. doi: 10.1080/22221751.2021.1899770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draffan A.G., Frey B., Fraser B.H., et al. Derivatives of imidazotriazine and pyrrolotriazine C-nucleosides as potential new anti-HCV agents. Bioorg Med Chem Lett. 2014;24:4984–4988. doi: 10.1016/j.bmcl.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Metobo S.E., Xu J., Saunders O.L., et al. Practical synthesis of 1′-substituted Tubercidin C-nucleoside analogs. Tetrahedron Lett. 2012;53:484–486. [Google Scholar]
- 16.Kaczmarek O, Brodersen N, Bunge A, et al. Synthesis of nucleosides with 2′-fixed lipid anchors and their behavior in phospholipid membranes. 2008;2008:1917–28.
- 17.Zhu L., dos Santos O., Seeman N.C., Canary J.W. Reaction of N3-benzoyl-3',5'-O-(di-tert-butylsilanediyl)uridine with hindered electrophiles: intermolecular N3 to 2'-O protecting group transfer. Nucleosides Nucleotides Nucleic Acids. 2002;21:723–735. doi: 10.1081/NCN-120015728. [DOI] [PubMed] [Google Scholar]
- 18.Pruijssers A.J., George A.S., Schäfer A., et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020;32:107940. doi: 10.1016/j.celrep.2020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Zhang D., Wang X.W., et al. 1 '-Ribose cyano substitution allows Remdesivir to effectively inhibit nucleotide addition and proofreading during SARS-CoV-2 viral RNA replication. Phys Chem Chem Phys. 2021;23:5852–5863. doi: 10.1039/d0cp05948j. [DOI] [PubMed] [Google Scholar]
- 20.Sheahan T.P., Sims A.C., Zhou S., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12:eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright N.J., Lee S.-Y. Toward a molecular basis of cellular nucleoside transport in humans. Chem Rev. 2021;121:5336–5358. doi: 10.1021/acs.chemrev.0c00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GS-441524 PK Property Overview. Can be found under https://opendata.ncats.nih.gov/covid19/GS-441524.
- 23.First-in-Human Study of Orally Administered GS-441524 for COVID-19 can be found under https://clinicaltrials.gov/ct2/show/NCT04859244.
- 24.Klumpp K., Smith D.B. Discovery and clinical evaluation of the nucleoside analog balapiravir (R1626) for the treatment of HCV infection. Antiviral Drugs. 2011:287–304. [Google Scholar]
- 25.Xie Y., Guo X., Hu T., et al. Significant inhibition of porcine epidemic diarrhea virus in vitro by remdesivir, its parent nucleoside and β-D-N(4)-hydroxycytidine. Virol Sin. 2021;1–9 doi: 10.1007/s12250-021-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Groaz E., Rocha-Pereira J., Neyts J., Herdewijn P. Anti-norovirus activity of C7-modified 4-amino-pyrrolo2,1-f1,2,4triazine C-nucleosides. Eur J Med Chem. 2020;195 doi: 10.1016/j.ejmech.2020.112198. [DOI] [PubMed] [Google Scholar]
- 27.Warren T.K., Jordan R., Lo M.K., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.