Abstract
Serotonin (5-HT) is one of the principal neurotransmitters in the nervous system of vertebrates. It is initially synthesized by hydroxylation of tryptophan (Trp) by means of tryptophan hydroxylase or TPH which is the rate-limiting enzyme in the production of 5-HT. In most vertebrates, there are two isoforms of TPH present, TPH1 and TPH2, which exhibit different catalytic or substrate specificity as well as different expression domains. Studies carried out in mammals show that only tph2 is expressed in the brain whereas tph1-mRNA is primarily localized in the enterochromaffin cells and pineal gland. A large number of neurons are also considered to be serotonergic or “pseudo-serotonergic” as they accumulate and release 5-HT yet do not produce it as no amine-synthetic enzymes are expressed, yet a combination of 5-HT transporters is observed. Therefore, tph expression is considered to be the only specific marker of 5-HT-producing neurons that can discriminate true 5-HT from pseudo-serotonergic neurons. This work examined in situ hybridization to study the mRNA distribution of one paralogue for tph1 and tph2 in the central nervous system of rainbow trout. Results show a segregated expression for both paralogues that predominantly match previous immunocytochemical studies. This study thus adds valuable information to the scarce analyses focusing on the central distribution of the expression of serotonergic markers, particularly tphs, in the vertebrate brain thus characterizing the true serotonergic brain territories.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00429-021-02322-8.
Keywords: Trout, Teleost fish, Brain, Serotonin, TPH, In situ hybridization
Introduction
The monoamine serotonin or 5-hydroxytryptamine (5-HT) is one of the major neurotransmitters of the central nervous system (CNS). The 5-HT metabolic pathway is initiated by tryptophan (Trp) being hydroxylated to the intermediate metabolite 5-hydroxytryptophan (5-HTP) by the action of the tryptophan hydroxylase (TPH). 5-HTP is subsequently decarboxylated to become 5-HT by the aromatic L-amino acid decarboxylase (AADH) enzyme. Due to the rapid activity of AADH, 5-HTP levels are usually low, making TPH the rate-limiting enzyme in the 5-HT production (Höglund et al. 2019). In the pineal gland, 5-HT is further processed by the serial action of the aryl-alkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (HIOMT) to produce melatonin (Falcón et al. 2010). Following synthesis, 5-HT is accumulated in intracellular organelles, the synaptic vesicles in neurons, by the vesicular monoamine transporters (VMAT1 and VMAT2) (Gaspar and Lillesaar 2012). The 5-HT transporter SERT or SLC6A4 also mediates the reuptake from the synaptic cleft back into the presynaptic boutons thus finalizing 5-HT effects and allowing neurotransmitter recycling by presynaptic neurons (Rudnick and Sandtner 2019; Bader 2020). Catabolism of 5-HT to 5-hydroxyindol acetic acid (5-HIAA) is mediated by the consecutive action of the monoamine oxidases (MAO-A and MAO-B) and the aldehyde dehydrogenase (ALDH2) (Höglund et al. 2019). A large number of cells/neurons could also be regarded as serotonergic or “pseudo-serotonergic” as they accumulate and release 5-HT yet do not produce it as no amine-synthetic enzymes are expressed, yet a combination of 5-HT transport is observed (Gaspar and Lillesaar 2012).
Therefore, tph expression is specific for the 5-HT producing cells/neurons and the only specific marker of 5-HT-producing neurons as aadh and mao are also expressed by other monoaminergic neurons, many cells use SERT/SLC6A4 for 5-HT reuptake without undergoing synthesis (Norton et al. 2008; Lillesaar 2011). TPH appears in two isoforms, TPH1 and TPH2. In mammals, peripheral organs such as the enterochromaffin cells, mammary and pineal glands, placenta and pancreatic beta cells predominantly use TPH1 for 5-HT synthesis. On the contrary, TPH2 is basically a central isoform, yet some cells in the periphery also use it for 5-HT synthesis, such as the serotonergic myenteric cells (Panula et al. 2010; Gaspar and Lillesaar 2012).
Neurons containing 5-HT have been identified in all major metazoan groups suggesting an early appearance of the system during animal evolution (Cornide-Petronio et al. 2013). The central serotonergic system has been characterized in depth in all vertebrate classes (Sako et al. 1986; Van Mier et al. 1986; Adrio et al. 1999; Hay-Schmidt 2000; Manger et al. 2002; Lillesaar and Gaspar 2018; Lozano et al. 2020) yet most studies have used specific antibodies to map serotonergic neurons. In mammalian species, central 5-HT is confined within the raphe nuclei brainstem where tph2 is expressed. On the contrary, in non-placental vertebrates the central serotonergic system disseminates to other brain structures of the forebrain which include pretectal and hypothalamic areas (Lillesaar 2011; Gaspar and Lillesaar 2012; Lozano et al. 2020; Timothy and Forlano 2020). However, the absence of studies using reliable serotonergic markers, tph1 and tph2 expression (Lillesaar 2011), makes it unclear whether extra raphe 5-HT neuronal populations are truly serotonergic (producing 5-HT) or pseudo-serotonergic (storing 5-HT).
Distribution of 5-HT immunoreactivity in the diencephalon and mesencephalon of rainbow trout (Onchorhynchus mykiss) was reported in the early 80’s revealing six 5-HT immunoreactive areas (Frankenhuis-van den Heuvel and Nieuwenhuys 1984) yet expression studies to elucidate the true 5-HT neurons in the trout brain have never been conducted. Similar to mammalian models and other teleost fish, the involvement of central serotonergic pathways in key aspects of the trout physiology and behaviour, including the regulation of food intake (Ruibal et al. 2002; Pérez-Maceira et al. 2014), stress response and coping styles (Lepage et al. 2002; Øverli et al. 2005; Schjolden et al. 2006; Gesto et al. 2015), cognitive function (Carpenter and Summers 2009; Vindas et al. 2014), and aggression (Winberg et al. 2001; Øverli et al. 2004; Lepage et al. 2005) has been reported. Studies carried out in a variety of fish species have also demonstrated the involvement of the central serotonergic system in reproduction (Prasad et al. 2015), sleep regulation (Oikonomou et al. 2019), locomotion (Gabriel et al. 2009), fear and anxiety (Egan et al. 2009), neurogenesis (Kuscha et al. 2012; Pérez et al. 2013) and neuronal regeneration (Sobrino-Cameán et al. 2019). Therefore, the study of serotonergic neurons in rainbow trout gains a general importance in understanding this wide array of roles played by 5-HT. This paper characterizes the expression of tph1 and tph2 in the brain of rainbow trout showing a segregated expression for both isoforms that greatly match previous immunocytochemical studies (Frankenhuis-van den Heuvel and Nieuwenhuys 1984). Our experiments add valuable information to the scarce analyses focusing on serotonergic marker expression, particularly tphs in vertebrates, which are basically limited to pigeons and zebrafish (Gaspar and Lillesaar 2012).
Materials and methods
Phylogenetic analysis
Inference of tph evolutionary relationships was performed using protein data sets of complete coding sequences, which excluded the hagfish Eptatretus burgueri (111 amino acid sequences), from 39 sequenced genomes (Esembl, http://www.ensembl.org/index.html). Multiple sequence alignments were generated using ClustalX 2.1 on the whole number of species, only fish species or exclusively salmonid sequences and the evolutionary history was inferred using maximum likelihood, minimum evolution, maximum parsimony and neighbour-joining methods on the JTT matrix-based model using MEGA. A phylogenetic view of thp evolutionary relationships was obtained by MEGA 7.02.21, moreover, cladograms and robustness were estimated at each branching node by 100 random bootstrap replications.
Fish and tissue processing
Two-year-old rainbow trout (n = 10; ≈ 200 g) were obtained from a local fish farm (Aigua Natura dels Ports, Tarragona). The specimens were anesthetized in 2-phenoxyethanol 0.02% v/v (Sigma), transcardial perfusion was carried out using 50 ml of physiological saline solution (NaCl 0.65%), and subsequently specimens were perfused with the same volume of fixative containing paraformaldehyde (PAF; 4%) in phosphate buffer (PB; 0.1 M, pH = 7.4). Following decapitation, the brains were removed, postfixed overnight in the same fixative at 4 °C, dehydrated, and embedded in Paraplast (Sherwood, St. Louis, MO, USA). Serial 6-µm cross sections were cut using a rotary microtome. One section every 200-µm was mounted on 3-aminopropyltriethoxylane-treated (TESPA) slides and then air-dried at room temperature overnight. Six consecutive series, covering the length of the rainbow trout brain, were made, one of these series was stained with cresyl-violet 0.1% (Cerdá-Reverter et al. 2001) for detailed identification of brain nuclei and the remaining series were used for hybridization with sense and antisense probes. The sections were stored at 4 °C under dry conditions and used for hybridization within 2 weeks.
Synthesis of riboprobes
Total mRNA was extracted from the fish brain using Trizol reagent (Life Technologies, Grand Island, NY, USA) and treated with RQ1-DNAse (Promega, Madison, WI, USA). One μg of the total RNA was reverse transcribed using Superscript II reverse transcriptase (Promega) and random hexamer primers (Promega) in 20 μl final volume. A pull of the cDNA obtained was subsequently used as a template for PCR amplification with Taq DNA polymerase (Promega) using specific oligoprimers for tph1a (fw: 5′-CAAGATCGACGAGAACAAGGACA-3′; rv: 5′-GTGAACTCGATATGCGGAATTGG-3′; 528 bp) and tph2 (fw: 5′-CCTGTTCTTGAAAGAGACGTCTG-3′; rv: 5′-CCAGGGTCAAACATCTTCACTGAG-3′; 417 bp). PCR fragments were separated onto 1% agarose gel, then purified using NucleoSpin® Gel and PCR Clean-up (Machery-Nagel). Subsequently, the fragments were cloned using pGEM-T easy vector (Promega). Plasmid DNA were obtained using QIAprep Spin Miniprep Kit (Quiagen) and fragments were sequenced on both strands to verify their identity. Clones were linearized with Sal I and Sac II, respectively, and transcribed for the riboprobes using SP6/T7 RNA polymerase (Promega) and digoxigenin (DIG)-labelled UTPs (Roche). The probes were then treated with RQ1-DNAse-RNAse free (Promega) for 15 min at 37 °C to remove the DNA template. Finally, the probes were purified using Micro Bio-Spin Chromatography Columns (BioRad) and quantified in a Thermo Scientific Nanodrop 2000c spectrophotometer.
In situ hybridization
Brain slides were deparaffinized, re-hydrated, post-fixed (PAF 4%), and then treated with Proteinase-K solution (20 μg/ml in 50 mM Tris–HCl, 5 mM EDTA at pH 8) for 6 min at RT. Brain slides were washed in PB, post-fixed again in PAF4% for 5 min, rinsed in sterile water, and acetylated in a triethanolamine (0.1 M, pH 8)/acetic anhydride solution for 15 min in constant agitation. Anti-sense or sense cRNA probes of tph1a or tph2 were preheated at 75 °C for 7 min and diluted in hybridization buffer [50% formamide, 300 mM NaCl, 20 mM Tris–HCl (pH 8), 5 mM EDTA (pH 8), 10% dextran sulphate (Sigma), and 1× Denhardt’s solution (Sigma)] at a concentration of 3 ng/µl. Sections were covered with 80–100 μl of hybridization solution and incubated in a humidification chamber at 65 °C O/N. The optimal probe concentration, wash times and hybridization temperature were determined in previous pilot experiments.
Slides were incubated in 5× standard saline citrate buffer (SSC, 150 mM NaCl, 15 mM sodium citrate at pH = 7) for 30 min at 55 °C to remove coverslips. The slides were then rinsed in 2× SSC and 50% formamide for 15 min at 65 °C and immersed in NTE buffer (500 mM NaCl, 10 mM Tris–HCl, 5 mM EDTA, pH 7.5) three times for 5 min at 37 °C. Following the ribonuclease A treatment (40 μg/ml ribonuclease A in NTE) for 15 min at 37 °C, slides were incubated in NTE buffer for 5 min at 37 °C, once in 2× SSC and 50% formamide for 10 min at 65 °C, once in 2× SSC for 10 min at RT and twice in 0.1× SSC for 10 min at RT. Before being incubated with anti-DIG antibody, slides were rinsed 3 times in MAB (150 mM NaCl, 100 mM maleic acid, pH 7.5) containing 40 mg/ml Tween 20 (Sigma) for 5 min at RT and incubated in blocking buffer (150 ml MAB, 150 µl Tween 20, 750 µl normal goat serum (NGS), 75 mg levamisol and 3 g blocking reagent (Roche Diagnostic)) for 3 h. Slides were incubated at 4 °C O/N with primary antibody 1:2000 anti-digoxigenin in MAB plus TWEEN 20. The antibody was removed by washing 6 times in MABT and twice in developing buffer (100 mM Tris, 100 mM NaCl, 50 mM MgCl2, pH 9.5) for 10 min at RT. Subsequently, the slides were incubated with chromogen substrates NBT/BCIP (Roche Diagnostic) to develop the staining. Sections were mounted with a mount quick aqueous medium (Bio-Optica) and visualized on an Olympus BX41. Serial sections were stained with 0.1% cresyl violet (Sigma) for cytoarchitectonic analysis. Nissl staining permanently dyes genetic material (DNA and RNA) therefore it is not restricted to neurons exclusively. Anatomical locations were confirmed by reference to a brain atlas of rainbow trout (Billard and Peter 1982) but nomenclature followed (Wullimann et al. 1996).
Results
Phylogenetic analysis
Maximum likelihood phylogenetic trees showing the evolutionary relationship of tph proteins for salmonid species, fish species and animal species are shown in Supplementary Fig. 1. The different phylogenetic methods efficiently determine the segregation of vertebrate tph sequences into two main clades for tph1 and tph2, respectively (Supplementary Fig. 1A–C). The genome of most basal phyla has only one tph gene, this also occurs in the lamprey (Petromyzon marinus) and myxine (Eptatretus burgueri) species. However, the genome of the chondrichthyes (Callorhinchus milii) displays both tph1 and tph2 thus suggesting that the duplication of tph genes took place in the gnathostomata following the divergence of the cyclostomata species (Supplementary Fig. 1A, B). Accordingly, tph2 sequence of the chondrichthyes is basal to all tph2 sequences. Tetrapod tph2 sequences (except Xenopus tropicalis) are grouped in a common clade which also includes the coelacanth sequence. The second clade includes all tph2 from teleost species with the non-teleost spotted gar (Lepisosteus oculatus) as a basal sequence. Lamprey and myxine tph seem to be more similar to tph1 than tph2 thus suggesting tph1 was used as a template for gene duplication following the gnathostome divergence (Supplementary Fig. 1A).
Bootstrap values indicated that the phylogenetic methods applied cannot discriminate tph1 sequences as consistently as for tph2 gene (Supplementary Fig. 1A, B). Significantly, chondrichthyes, lepisosteiforms, coelacanthiforms and tetrapod genomes exhibit only a tph1 gene suggesting that the presence of tph1 and tph2 genes is the ancestral vertebrate condition. As a result of teleost genome-specific duplication (TGSD), most teleost fish exhibit two tph1 genes that had initially been labelled as tph1a and tph1b yet tph2 duplication is not found in any species (Xu et al. 2019). tph1a and tph1b do not form monophyletic groups thus making the evolutionary inferences challenging (Supplementary Fig. 1A, B). Salmonids have undergone an additional duplication by reaching a tetraploid condition, therefore more gene copies are expected including tph1a1/tph1a2, tph1b1/tph1b2, and tph2a/tph2b. In silico data from genome sequencing projects demonstrate that salmonid species exhibit 4 tph genes yet a single copy of tph2 gene (Supplementary Fig. 1A–C). All three other copies are grouped together with fish tph1a yet low bootstrap values validate such association (Supplementary Fig. 1A, B). Independent analysis of salmonid tph sequences displays segregated clades for thp1 and tph2 (Supplementary Fig. 1C). Furthermore, tph1 sequences are arranged in two subclades suggesting that tph1 forms can be split up into tph1a/tph1b as well as tph1a1/tph1a2. Tph1b/tph1a2 subclade also exhibits two subdivisions/clades which seldom include one sequence of each species, suggesting that two loci for tph1b/tph1a2 are identifiable.
Tph1 expression in the rainbow trout brain
Hybridization with sense tph1a-cRNA and tph2-cRNA probes never generated specific signals in the rainbow trout brain (data not shown) supporting the probe specificity. It should be considered that in situ hybridization cannot discriminate the cell type expressing the specific mRNA, however, up to our knowledge, tph expression in the brain is restricted to neuronal cell bodies as no expression has been reported in glia cells including ependymal cells and tanycytes (Perez et al. 2013). Therefore, tph-expressing cells in the brain will be referred as neuron from here on. More detailed studies would involve double labelling experiments with glial fibrillary acidic protein (GFAP) to identify potential glia cells expressing tph.
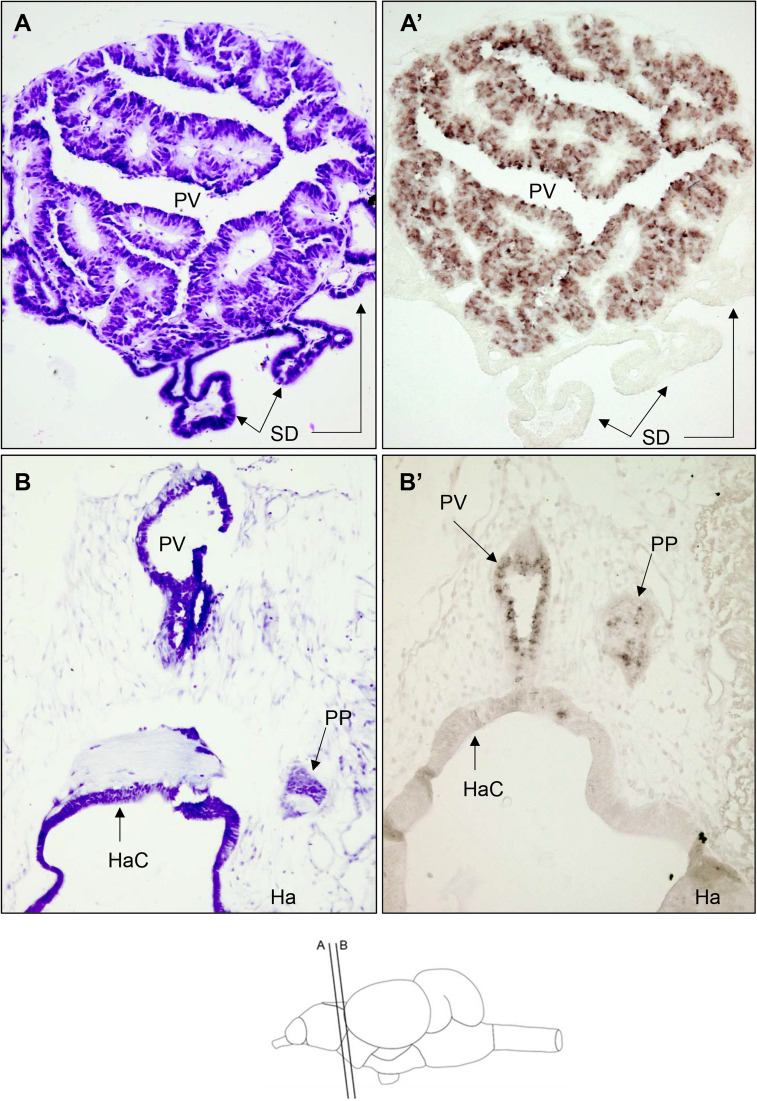
tph1 mRNA was massively expressed in the pineal vesicle (PV) of rainbow trout which rostrally appears on the top of olfactory bulbs progressing to the intersection between the telencephalic hemispheres and optic tectum where epithalamus habenula is located (Fig. 1A). At the caudal end of the PV, just dorsal to the habenula and located on only one hemisphere, a conspicuous group of cells conforming to the parapineal organ (PP) also produce tph1 mRNA (Fig. 1B). Our experiments cannot discriminate the cell type expressing tph mRNA in the PV and PP but as discussed later (see “Discussion”) these cells should be photoreceptors.
Fig. 1.
Bright-field photomicrographs of transverse sections of the rainbow trout brain at the level of rostral diencephalon showing tph1-expressing cells. Section levels are shown in the schematic drawing at the bottom of the figure. A and B are Nissl staining with cresyl violet of transverse sections at similar rostrocaudal level of those shown in A’ and B’, respectively. A’ displays a high expression level in most cells of the pineal vesicle (PV), presumably photoreceptors (see “Discussion” for details), whereas B’ shows lower expression levels in the parapineal organ (PP). The identity of thp-expressing cells in the PP remains unknown (see “Discussion” for details). Arrows indicate the saccus dorsalis (SD) A and A’, PP, PV and habenular commissure (HaC) in B and B’. Ha habenula. Scale bar = 50 μm
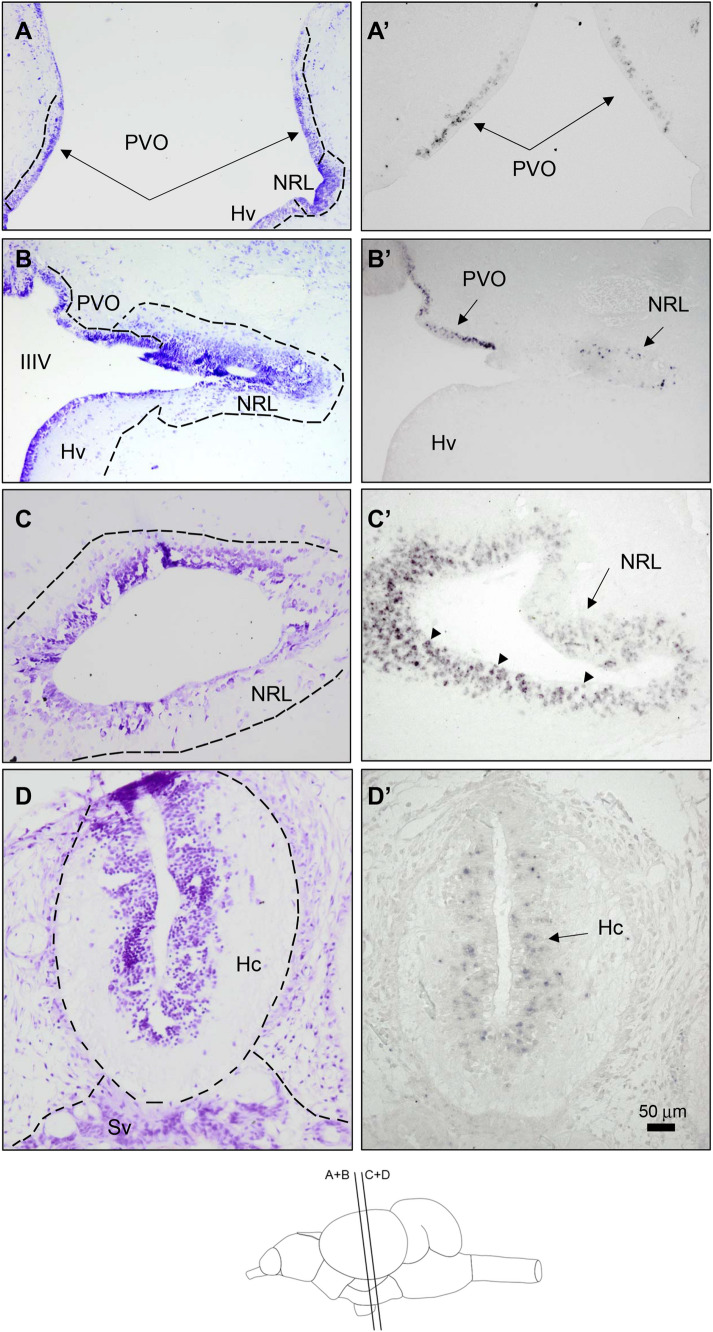
Closer to the caudal, tph1 expression in the paraventricular organ (PVO) was found. Tph1-expressing neurons in the rostral PVO line the third ventricle accurately coinciding with the lateral aperture of the hypothalamic medial tuberal ventricle (Fig. 2A) which being slightly more caudal will come in contact with the lateral recesses in the inferior hypothalamic lobe. Such neurons seem to migrate initially into the dorsal region of the lateral recesses (Fig. 2B) and subsequently coat the entire perimeter of the hypothalamic lateral recess (Fig. 2C). Also concurring with the lateral expansion of the third ventricle, some tph1-expressing neurons coat the ventral hypothalamic region of the third ventricle (Hv) (data not shown). At the caudal pole on the tuberal hypothalamus, the thp1-expressing neurons coat the caudal region of the third ventricle in the caudal hypothalamus (Hc, Fig. 2D). Some tph1-expressing periventricular neurons in the PVO and NRL (see arrowheads in Fig. 2A, C) appeared to make contact with the ventricular wall thus suggesting a physical link with the cerebrospinal fluid (CSF).
Fig. 2.
Bright field photomicrographs of transverse sections of the rainbow trout brain showing tph1-expressing neurons at the level of rostral (A, A’, B, B’) and caudal hypothalamus (C, C’, D, D’). Rostro caudal levels of the sections are shown in the schematic drawing at the bottom of the figure. A–D Provide morphological details by Nissl staining with cresyl violet of transverse sections at a similar level of those shown in A’–D’, respectively. A’ Positive tph1-expressing neurons in the paraventricular organ of the posterior tubercle (PVO). Arrows in A and A’ show cell (A) and tph1-expressing neurons in PVO, respectively. B’ tph-mRNA expressing neurons are also present in the lateral opening of the III ventricle (IIIV) at the lateral recess nucleus (NRL) according to Cerdá-Reverter’s (2000) nomenclature or Hd according to Wullimann et al. (1996). Arrows in B’ show tph1-expressing neurons in the PVO and NRL. C’ Numerous small and rounded positive cells coating the lateral recess. Arrows in C’ indicate tph1-expressions neurons in the NRL, respectively. Arrowheads in C’ indicate neurons contacting the ventricular wall in the NRL. D’ Positive cells in the most caudal region of the tuberal hypothalamus (Hc). Arrows in D’ show positive neurons in the caudal hypothalamus. Scale bar = 50 μm
Tph2 expression in the rainbow trout brain
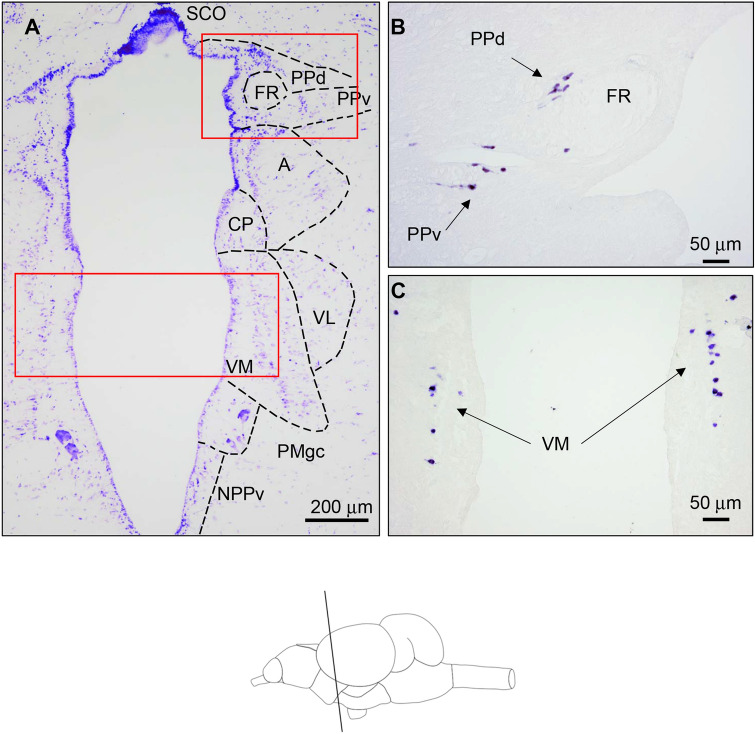
tph2-expressing neurons were confined to the pretectal area, ventral thalamus and posterior brain (Figs. 3, 4). In the pretectal area, tph2 expression was found in two adjacent neuronal populations dorsally and ventrally surrounding the fasciculus retroflexus (FR), in the so-called dorsal (PPd) and ventral (PPv) periventricular pretectal nucleus (Fig. 3B). The PPd lies immediately caudal to habenula, dorsal to the anterior thalamic nucleus (A), and lateroventral to the subcommissural organ SCO (Fig. 3A). Slightly caudal and around the medioventral and ventral areas of the FR, the PPv begins. At the caudal end of the posterior commissure, both PPd and PPv move laterally away from the ventricle. Tph-mRNA-expressing neurons in the PPd and PPv exhibit fusiform shape and show laterally directed dendritic processes (Fig. 3B). Slightly ventral, yet found at the same rostro-caudal level, a prominent tph2-expressing neuronal population is localized in the ventromedial nucleus of the ventral thalamus (VM). thp2-mRNA neurons in the VM are disposed in parallel to the medial ventricular wall and arranged into a single dorsoventral 1-cell thick column (Fig. 3C). tph2-expressing neurons are found in the superior raphe (SRa) caudal to the level of the interpeduncular nucleus (Fig. 4). Rostrally, tph2-expressing large fusiform and highly stained round neurons are intermingled with ventrally localized smaller round neurons which are disposed along the medial line (Fig. 4A). Slightly more caudal, the dorsal population of larger tph2-expressing neurons disappears and only smaller neurons placed on the midline remain (Fig. 4B). However, both populations coexist along the rostral pole of the SRa suggesting that they could form differentiated parts of the SRa. In the most caudal region where tph2 expression was detected, some sparse and minute tph2-expressing neurons are found in the midline at the level of the inferior raphe (IRa). In the same section, some scattered neurons in the superior part of the reticular formation (RF) are densely stained. Neuronal bodies expressing tph2 in the RF display an ovoid or fusiform shape with long dendritic processes.
Fig. 3.
Bright-field photomicrographs of transverse sections of the rainbow trout brain showing tph2-expressing neurons at the level of ventral thalamus and pretectal area. Rostro caudal levels of the sections are shown in the schematic drawing at the bottom of the figure. A Transverse section stained with cresyl violet that shows anatomical details of a similar section level as in B and C. Dashed lines in A set the limits of different diencephalic and pretectal nuclei after Nissl staining. Red rectangular frames indicate equivalent regions shown in B (opposite hemisphere) and C (both hemispheres). B tph2-expressing neurons in the rostral pretectal area at the level of retroflexus fascicle (FR) in the dorsal (d) and ventral (v) parts of the pretectal periventricular nucleus (PPd and PPv). C Positive neurons in the ventromedial nucleus of the ventral thalamus (VM), note that these cells are organized into a single vertical column. Arrows in B (pretectal/synencephalic) and C (thalamic) indicate neuronal cell bodies expressing tph2 mRNA. tph expression in the brain is restricted to neuronal cell bodies as no expression has been reported in glia cells including ependymal cells and tanycytes (Perez et al. 2013). A (anterior nucleus of the ventral thalamus), CP (central posterior nucleus on the ventral thalamus), NPPv (posterior periventricular nucleus), PMgc, gigantocellular part of the magnocellular preoptic nucleus, SCO subcommissural organ. Scale bar = 50 μm (B) and = 200 μm (A, C)
Fig. 4.
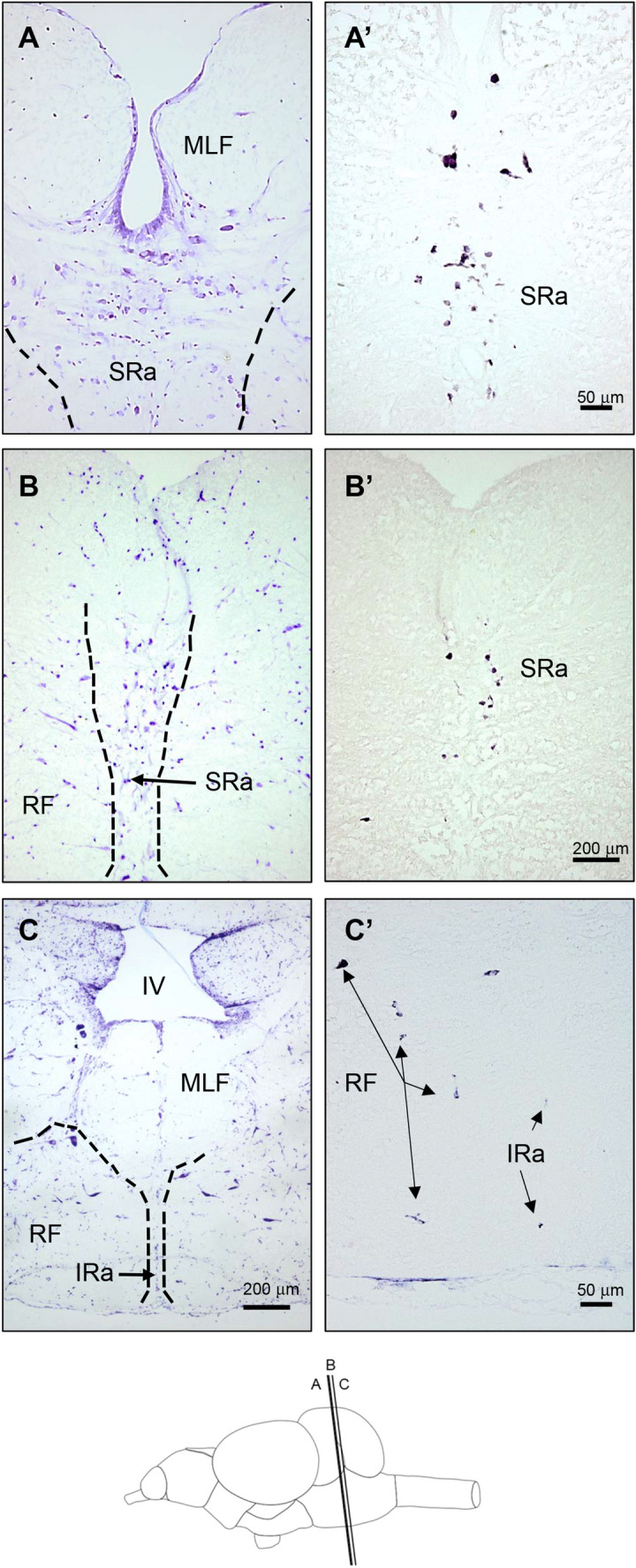
Bright-field photomicrographs of transverse sections of the rainbow trout brain showing tph2-expressing neurons at the level of raphe. Rostro caudal levels of the sections are shown in the schematic drawing at the bottom of the figure. A–C Transverse sections stained with cresyl violet showing anatomical details of a similar section level as in A’–C’. Dashed lines in A–C demarcate different nuclei in the posterior brain after Nissl staining. tph2 expression in the rostral (A’) and more caudal (B’) superior raphe (SRa). C’ Arrows indicate disperse positive neurons weakly stained in the inferior raphe (IRa) and the reticular formation (RF). MLF Medial longitudinal fascicle, IV fourth ventricle. Scale bar = 50 μm (A, A’, C’) and = 200 μm (B, B’, C)
Discussion
Teleost fish have undergone an extra genome duplication commonly known as teleost-specific genome duplication (TSGD) resulting in an extra-duplication of all their genes (Amores et al. 1998). Following this event, many duplicated genes became pseudogenes (loss of function) yet others experienced a process of neofunctionalization, thus acquiring new functions or subfunctionalization in which both copies share the original function. This is also true for the tph system. Most vertebrates exhibit two paralogues called tph1 and tph2. However, a single gene copy is found in invertebrates and cyclostomata species thus suggesting that the duplication of ancestral tph took place following the divergence of the Gnathostomata (Cornide-Petronio et al. 2013). Lamprey tph seems to exhibit more homology to the tph1 than tph2 genes, it is thus conceivable that the tph1 gene was the substrate for gene duplication. As a result of TSGD, teleost additional tph copies are expected, at least tph1a and b and tph2a and b, but only a single copy of tph2 is present in all fish species. This suggests that the second form of tph2 was rapidly pseudogenized following TSGD. On the contrary, most teleost fish exhibit paralogues of thp1, such as tph1a and tph1b (Xu et al. 2019) despite the fact that the conventional phylogenetic analysis could not group them into monophyletic groups. By increasing the genome complexity of teleost fish, some species, including salmonids and cyprinids, have experienced an additional genome duplication resulting in an extra genome tetraploidization. Therefore, salmonids are expected to show two tph2 paralogues (thp2a and tph2b) and four paralogues for tph1 (tph1a1, tph1a2, tph1b1 and tph1b2). A second form of tph2 could not be found in any tetraploidized teleost species suggesting additional pseudogenization events. From a functional point of view, it is extraordinary how the evolutionary process has systematically deleted any paralogue of the tph2 gene. Remarkably, the thp1 system seems to be much more permissive to the presence of additional copies. The salmonid species also exhibit additional copies of tph1 gene. All salmonid genomes show three tph1 genes. Phylogenetic relationships (Xu et al. 2019; and present results) and synteny studies (Xu et al. 2019) suggest the presence of tph1a, tph1b1 and tph1b2 in salmonid fish, therefore, the paralogue of tph1a was once more pseudogenized during the evolutionary process. Alignment and phylogenetic studies indicate that the cloned sequences matched tph2 and tph1b2 (data not shown), however, henceforth we will use the nomenclature tph1 and tph2 to name the cloned rainbow tph sequences used in our in situ hybridization studies.
Both members of the TPH family (TPH1 and TPH2) exhibit different catalytic or substrate specificity (Walther and Bader 2003) yet distinct expression domains. In non-tetrapod vertebrates, both isoforms are expressed in specific areas of the CNS. However, in mammalian species, tph1 expression is restricted to peripheral tissues whereas tph2 is expressed primarily in the CNS. The lamprey genome only exhibits a tph copy which is phylogenetically more related to tph1 than tph2. Since lamprey tph1 is expressed in both the diencephalic nuclei and pineal gland, the restricted expression of tph1 in mammalian species seems to be a derived condition (Cornide-Petronio et al. 2013).
The main tph1 expression levels are found predominantly in the pineal gland and the results observed in rainbow trout effectively verify data reported in other vertebrate species (Bellipanni et al. 2002; Teraoka et al. 2004; Gaspar and Lillesaar 2012). Fish pineal complex consists of the pineal and parapineal organ and the saccus dorsalis. The pineal organ consists of the pineal vesicle dorsally located to the telencephalic hemispheres and connected to the brain by a slim pineal stalk (Birba et al. 2014; Rincón Camacho et al. 2016). In rainbow trout, tph1 is mainly expressed in the pineal vesicle that exhibits three type of cells, i.e. photoreceptor, projection neurons and interstitial cells (Shainer et al. 2017). Only photoreceptor express aralkylamine N-acetyltransferase (AANAT), the step-limiting enzyme in the melatonin biosynthetic pathway, therefore, they should produce tph1 to be able to synthesize melatonin as reported in zebrafish (Teraoka et al. 2004). However, it is also plausible that some projection neurons can synthesize 5-HT but this assumption requires further investigation using double labelling with FoxD3/HuC for pineal neurons and GFAP for glia cells. Our results also revealed some expression level in the parapineal organ. The function of this organ remains unknown but it has been shown to project unilaterally to the left habenula in zebrafish (Turner et al. 2016). Some authors have suggested the presence of photoreceptors (García-Fernández et al. 1997) although others were unable to detect cone or rod opsin immunoreactivity (Rincón Camacho et al. 2016). The potential photosensitivity of the parapineal organ cannot be neglected as some other types of photoreceptors could be present (Birba et al. 2014). Our data suggest that the parapineal organ exhibits true serotonergic cells that are potentially able to synthesize melatonin thus further suggesting that it could participate in the regulation of circadian functions through melatonin secretion. Studies showing aralkylamine N-acetyltransferase (AANAT) expression, which is the step-limiting enzyme in the melatonin biosynthetic pathway in the parapineal organ, could help to elucidate its participation in the melatonin synthesis.
Previous studies reported 5-HT immunoreactivity in six different areas of the rainbow trout brain (Frankenhuis-van den Heuvel and Niewenhuys, (1984). Our findings complete these earlier studies by characterizing the type of tph expressed in the different serotonergic areas. Studies using 5-HT antibodies cannot entirely discriminate between 5-HT accumulating and/or synthesizing neurons (Gaspar and Lillesaar 2012) as tph expression is the only specific marker of 5-HT-producing neurons (see “Introduction”). Therefore, our results complement studies by Frankenhuis-van den Heuvel and Niewenhuys, (1984) by discriminating the areas of tph-expressing neurons among those showing immunoreactivity to 5-HT. The neurons expressing tph will be able to synthesize the amine whereas pseudo-serotonergic neurons will only accumulate the neurotransmitter.
The phenotype of these pseudo-serotonergic neurons is regulated by the transitory expression of 5-HT transporters (SERT and/or VMAT) that promote 5-HT capture which can be retrogradely transported to neuronal perikarya. These neurons do not synthesize the amine, only take it up, thus explaining the low levels of 5-HT that occasionally make difficult their visualization. However, the low staining levels can establish also morphological differences between both serotonergic phenotypes (Lebrand et al. 1996). The functional implications of these pseudo-serotonergic neurons remains uncertain. It has been shown in rodents that cortical fibers with thalamic origin transiently express SERT and VMAT2 to capture 5-HT synthesized in the raphe (Lebrand et al. 1996). This transient pseudo-serotonergic phenotype is only perceptible at postnatal day 1 (P1) in mice pups and abruptly disappears at P10 (Fujimiya et al. 1986; D’amato et al. 1987) coinciding with the absence of SERT expression. The captured 5-HT could serve as an intracellular signal-regulating gene expression in the thalamic neurons or alternatively could regulate thalamic neurotransmission preventing receptor overstimulation during some developmental phases by controlling extracellular levels of the amine. Finally, thalamic neurons could capture and release themselves 5-HT as a borrowed neurotransmitter (Lebrand et al. 1996; Hansson et al. 1998). Therefore, 5-HT of the brain stem could take advantage of existing neuronal networks during particular developmental phases without the need to establish a new neuronal pattern to regulate, for example, the ingrowth and/or axon arborisation.
In the rainbow trout brain, tph1 expression was detected in the paraventricular organ of the posterior tubercle (PVO). tph1-expressing neurons in PVO emerge from the medial region just above the lateral tuberal nucleus and migrate laterally on the dorsal region of the lateral recess to entirely coat the perimeter of the ventricle. Tph1-mRNA neurons also cover the medial region on the third ventricle in its most caudal area, also called posterior recess nucleus or caudal hypothalamus. Some authors denominate all these areas of the posterior tubercle as PVO (Pérez et al. 2013; Lozano et al. 2020) by observing three rostro-caudal regions such as anterior, intermediate and posterior (Pérez et al. 2013) regions whereas others also include hypothalamic subdivisions as dorsal and caudal hypothalamus (Timothy and Forlano 2020). Regardless, 5-HT immunoreactivity in this area has been reported in all fish species examined (Lillesaar 2011; Gaspar and Lillesaar 2012) including rainbow trout (Frankenhuis-van den Heuvel and Nieuwenhuys 1984). Positive neurons expressing tph1 in the PVO of the rainbow trout correspond to the 5-HT immunoreactive neurons previously described in “area 2” by Frankenhuis-van den Heuvel and Niewenhuys (1984), who also described a conspicuous group of immunoreactive neurons in the ventral hypothalamus or nucleus tuberis inferior (nti) (according to Niewenhuys’ nomenclature) denominated “area 3” (Frankenhuis-van den Heuvel and Nieuwenhuys 1984). Only some tph1-mRNA expressing neurons were labelled in this “area 3”. Serotonergic neurons coating the posterior hypothalamic recess would correspond to the “area 4” of 5-HT immunoreactive neurons previously described by Frankenhuis-van den Heuvel and Niewenhuys (1984). Remarkably, authors reported only a few neurons surrounding the lateral recess in the inferior hypothalamic lobe, however, results show a profuse tph1 expression predominantly in the rostral extension of the recess. There is no explanation for this discrepancy other than the fact that the 5-HT synthesized in the lateral recess nucleus is rapidly transported to other areas of the brain or into the ventricular CSF. PVO is a region which is rich in radial glial cells (RGCs) expressing brain aromatase that give birth to 5-HT neurons that come in contact with CSF but also migrate to other regions of the zebrafish brain. The somata of the 5-HT neurons in the PVO are located closer to the ventricle than those of RGCs that extend processes to form a continuous barrier along the ventricular surface. In turn, 5-HT neurons contact the CSF via processes that cross this barrier through small pores (Pérez et al. 2013). In adult zebrafish treated with TPH inhibitors, the number of proliferating cells in the PVO decrease yet this does not occur in other hypothalamic areas thus suggesting that 5-HT promotes the genesis of 5-HTergic neurons specifically in the PVO that will be spread along the brain ventricles (Pérez et al. 2013). The PVO also displays a prominent population of dopaminergic cells (Yamamoto et al. 2010) but none show double phenotype (Sallinen et al. 2009). The PVO seems to take part in the ascending dopaminergic midbrain system of fish which integrates three subsystems in tetrapods such as the mesolimbic (reward response), mesocortical (learning and memory) and mesostriatal (sensorimotor) (Rink and Wullimann 2001; Yamamoto et al. 2010) systems. Therefore, hypothalamic 5-HT could be involved in the regulation of several behavioural responses. In fact, 5-HT is a well-known anxiolytic agent in vertebrates which also regulates feeding behaviour in fish (Rubio et al. 2006; Ceinos et al. 2008; Nowicki et al. 2014; Soares et al. 2018; Ziegler et al. 2020).
Tph2-expressing neurons first appear in two neuronal populations of the pretectal area, such as the dorsal and ventral part of the periventricular pretectal nucleus (PPd and PPv). Both populations surround the dorsal and ventral aspects of the fasciculus retroflexus (FR) placed in the most dorsal pole of the third ventricle, respectively. These populations correspond to the “area 1” of 5-HT immunoreactive neurons previously described by Frankenhuis-van den Heuvel and Niewenhuys (1984). These 5-HT neurons have no homologues in tetrapod species suggesting that it is a fish-specific characteristic. In fact, pretectal serotonergic neurons have been reported in most studies on fish (reviewed in Lillesaar 2011; Gaspar and Lillesaar 2012) with the exception of flatfish Senegalese sole (Rodríguez-Gómez et al. 2000). It has been suggested in tilapia that periventricular pretectal nucleus conveys sensory information from visual and lateral line pathways into the cerebellum (Xue et al. 2007). The presence of tph2-expressing neurons in the VM is more controversial as serotonergic studies using specific antibodies in many species have not reported 5-HT immunoreactive neurons in the VM (Lillesaar 2011). However, studies in trout showed some immunoreactive neurons stretching dorsally along the median thalamic line within the “area 2” (Fig. 5c in Frankenhuis-van den Heuvel and Niewenhuys 1984). Accordingly, studies in lamprey reported a tph1-expressing neuronal population in the thalamus at the same level in which pretectal 5-HT neurons were located (Fig. 1F in Rincón-Camacho et al. 2016). Tph2-expressing neurons were also found downstream in the raphe and reticular formation of the hindbrain of the rainbow trout CNS. A conspicuous population of tph2-expressing neurons in the SRa was detected yet scattered tph2-labelled neurons in the more caudal IRa were also observed. Serotonergic neurons in the raphe are characterized by the expression of the ETS-domain transcription factor-encoding gen pet1 which is essential for the development of the brainstem 5-HT system (Lillesaar et al. 2007). Using a transgenic zebrafish overexpressing green fluorescent protein (GFP) under the control of pet1 proximal promoter, Lillesaar et al. (2009) initially characterized two serotonergic populations in the SRa, such as the 5-HT neurons located in the midline of the hindbrain and a second overlooked serotonergic population found in the ventrolateral hindbrain of zebrafish. The latter population projects to the migrated nuclei of the posterior tuberculum. Tracing studies combined with pet1-directed GFP expression have demonstrated that cells in and along the SRa midline projecting to the hypothalamus tend to be more ventrally localized and exhibit larger neuronal bodies than those projecting to olfactory bulbs and the telencephalon. Projections coming from dorsal and ventral populations are arranged into different clusters thus revealing some functional organization within the SRa (Lillesaar et al. 2009). Morphological studies have also suggested a functional subdivision of the SRa in plainfin midshipman (Porichthys notatus) (Timothy and Forlano 2020). Our in situ hybridization experiments in rainbow trout only detected the midline serotonergic population. However, two serotonergic subpopulations, dorsal and ventral, could be differentiated in the most rostral midline region of the SRa thus suggesting some kind of functional organization, but this assumption is only based on morphology and location of the tph2-expressing neuronal bodies in the SRa. Such neuronal population would correspond to the “area 5” from Frankenhuis-van den Heuvel and Niewenhuys (1984). Our expression studies were unable to locate the 5-HT ventrolateral neurons described in the zebrafish and stickleback hindbrain (Ekström and Van Veen 1984) yet 5-HT immunoreactive neurons were described in the dorsolateral position to the fasciculus longitudinalis medialis, at the level of the superior raphe (“area 6” in Frankenhuis-van den Heuvel and Niewenhuys 1984). These neurons could correspond to those described in zebrafish and stickleback yet could also be a distinct population of pseudoserotonergic cells of the trout CNS. Alternatively, it cannot be discarded that other rainbow trout tph1 paralogues were expressed in this location.
Serotonergic neurons in the IRa of the brainstem of trout lie more caudally in the ventral region of the midline. The presence of true serotonergic neurons in the IRa is a constant characteristic of the vertebrate brain (Lillesaar 2011; Gaspar and Lillesaar 2012; Timothy and Forlano 2020). Even in lamprey two rhombencephalic populations (isthmic and caudal) have been characterized presumably as homologues of the raphe nuclei (Barreiro-Iglesias et al. 2008; Cornide-Petronio et al. 2013). Studies in mammalian species demonstrated that SRa and LRa 5-HT neurons project to the rostral and caudal regions, respectively. This polarization in the projection patterns was also verified in zebrafish (McLean and Fetcho 2004). However, studies by Lillesaar et al. (2009) using pet1:eGFP transgenic line zebrafish showed that a minor population of 5-HT neurons in the SRa project caudally into the hindbrain but not further than the spinal cord.
In summary, the expression distribution of one tph1 paralogue (out of three) was reported, such as the tph1b2 and tph2 gene in rainbow trout. The tph1 and tph2-expression distribution is compared to the reported 5-HT immunoreactive neurons previously described thus showing the true serotonergic territories in the trout brain. Results show that segregated expression for both isoforms primarily match immunocytochemical studies but some relevant variations were found to be predominantly localized in the ventral thalamus, hypothalamic lateral recess and rostral hindbrain populations. Our research provides further insight into the very few and restricted studies addressing the serotonergic marker expression to characterize the true serotonergic brain territories.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 Figure S1. Phylogeny of tph proteins. Multiple sequence alignments of tph amino acid sequences for all taxons (A), fish species (B) and salmonid species (C) were generated using ClustalX 2.1 and the evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model [1]. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. The analysis involved 111 amino acid sequences. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 362 positions in the final dataset. Evolutionary analyses were conducted in MEGA7. (PDF 102 KB)
Acknowledgements
We are very grateful to José Monfort and Lucinda Rodríguez for their assistance in the histological sectioning of brain samples. This research was funded by the Spanish State Agency of Research (AEI), Grant Number AGL2016-74857-C3-3-R and PID2019-103969RB-C33 to JMCR and PID2019-103969RB-C31 to JMM. M.C. was recipient of a predoctoral fellowship (Program FPI) from Spanish Ministerio de Ciencia e Innovación (BES-2017-079708).
Authors’ contributions
Conceptualization, JMC-R and JMM; methodology, MC and EL; data curation, MC and JMC-R, writing—original draft preparation, JMC-R; writing—review and editing, JMC-R, EL, MC, JMM project administration, JMC-R and JMM; funding acquisition, JMC-R and JMM. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Spanish State Agency of Research (AEI), grant number AGL2016-74857-C3-3-R and PID2019-103969RB-C33 to JMCR and PID2019-103969RB-C31 to JMM. M.C. was recipient of a predoctoral fellowship (Program FPI) from Spanish Ministerio de Ciencia e Innovación (BES-2017-079708).
Availability of data and materials
Data are available on reasonable request.
Code availability
No applicable.
Declarations
Conflict of interest
Authors have nothing to declare.
Ethical approval
All experiments were carried out in accordance with the principles published in the European animal directive (86/609/EEC) for the protection of experimental animals and approved by the Consejo Superior de Investigaciones Científicas (CSIC) ethics committee (project number AGL2016-74857-C3-3-R and PID2019-103969RB-C33).
Studies involving humans and/or animals
No applicable.
Consent to participate
No applicable.
Consent for publication
No applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adrio F, Anadón R, Rodríguez-Moldes I. Distribution of serotonin (5HT)-immunoreactive structures in the central nervous system of two chondrostean species (Acipenser baeri and Huso huso) J Comp Neurol. 1999;407:333–348. doi: 10.1002/(SICI)1096-9861(19990510)407:3<333::AID-CNE3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, et al. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Bader M. Inhibition of serotonin synthesis: a novel therapeutic paradigm. Pharmacol Ther. 2020;205:107423. doi: 10.1016/j.pharmthera.2019.107423. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cerviño V, Anadón R, Rodicio MC. Development and organization of the descending serotonergic brainstem-spinal projections in the sea lamprey. J Chem Neuroanat. 2008;36:77–84. doi: 10.1016/j.jchemneu.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Mech Dev. 2002;119:215–220. doi: 10.1016/S0925-4773(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Billard R, Peter RE. A stereotaxic atlas and technique for nuclei of the diencephalon of rainbow trout (Salmo gairdneri) Reprod Nutr Dev. 1982;22:1–25. doi: 10.1051/rnd:19820101. [DOI] [PubMed] [Google Scholar]
- Birba A, Ramallo MR, Morandini L, et al. The pineal complex in the cichlid Cichlasoma dimerus: effect of different photoperiods on its cell morphology. J Fish Biol. 2014;85:605–620. doi: 10.1111/jfb.12446. [DOI] [PubMed] [Google Scholar]
- Carpenter RE, Summers CH. Learning strategies during fear conditioning. Neurobiol Learn Mem. 2009;91:415–423. doi: 10.1016/j.nlm.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceinos RM, Polakof S, Illamola AR, et al. Food deprivation and refeeding effects on pineal indoles metabolism and melatonin synthesis in the rainbow trout Oncorhynchus mykiss. Gen Comp Endocrinol. 2008;156:410–417. doi: 10.1016/j.ygcen.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Cerdá-Reverter JMZ, S., Muñoz-Cuento JA. Cytoarchitectonic study of the brain of a perciform species, the Sea Bass (Dicentrarchus labrax). II. The Diencephalon. J Morphol. 2001;247:229–251. doi: 10.1002/1097-4687(200103)247:3<229::AID-JMOR1014>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Cornide-Petronio ME, Anadón R, Rodicio MC, Barreiro-Iglesias A. The sea lamprey tryptophan hydroxylase: new insight into the evolution of the serotonergic system of vertebrates. Brain Struct Funct. 2013;218:587–593. doi: 10.1007/s00429-012-0412-7. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci USA. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekström P, Van Veen T. Distribution of 5-hydroxytryptamine (serotonin) in the brain of the teleost Gasterosteus aculeatus L. J Comp Neurol. 1984;226:307–320. doi: 10.1002/cne.902260302. [DOI] [PubMed] [Google Scholar]
- Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol. 2010;1:165. doi: 10.1016/j.ygcen.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Frankenhuis-van den Heuvel THM, Nieuwenhuys R. Distribution of serotonin-immunoreactivity in the diencephalon and mesencephalon of the trout, Salmo gairdneri—Cellbodies, fibres and terminals. Anat Embryol (berl) 1984;169:193–204. doi: 10.1007/BF00303149. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Kimura H, Maeda T. Postnatal development of serotonin nerve fibers in the somatosensory cortex of mice studied by immunohistochemistry. J Comp Neurol. 1986;246:191–201. doi: 10.1002/cne.902460205. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Kyriakatos A, et al. Serotonergic modulation of locomotion in zebrafish—Endogenous release and synaptic mechanisms. J Neurosci. 2009;29:10387–10395. doi: 10.1523/JNEUROSCI.1978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fernández JM, Jiménez AJ, González B, et al. An immunocytochemical study of encephalic photoreceptors in three species of lamprey. Cell Tissue Res. 1997;288:267–278. doi: 10.1007/s004410050812. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Lillesaar C. Probing the diversity of serotonin neurons. Philos Trans R Soc B Biol Sci. 2012;367:2382–2394. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesto M, López-Patiño MA, Hernández J, et al. Gradation of the stress response in rainbow trout exposed to stressors of different severity: the role of brain serotonergic and dopaminergic systems. J Neuroendocrinol. 2015;27:131–141. doi: 10.1111/jne.12248. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience. 1998;89:243–265. doi: 10.1016/s0306-4522(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Hay-Schmidt A. The evolution of the serotonergic nervous system. Proc R Soc B Biol Sci. 2000;267:1071–1079. doi: 10.1098/rspb.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol (lausanne) 2019;10:158. doi: 10.3389/fendo.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscha V, Barreiro-Iglesias A, Becker CG, Becker T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J Comp Neurol. 2012;520:933–951. doi: 10.1002/cne.22739. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lepage O, Larson ET, Mayer I, Winberg S. Serotonin, but not melatonin, plays a role in shaping dominant-subordinate relationships and aggression in rainbow trout. Horm Behav. 2005;48:233–242. doi: 10.1016/j.yhbeh.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Lepage O, Tottmar O, Winberg S. Elevated dietary intake of l-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2002;205:3679–3687. doi: 10.1242/jeb.205.23.3679. [DOI] [PubMed] [Google Scholar]
- Lillesaar C. The serotonergic system in fish. J Chem Neuroanat. 2011;44:294–308. doi: 10.1016/j.jchemneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Gaspar P (2018) Serotonergic neurons in vertebrate and invertebrate model organisms (Rodents, zebrafish, drosophila melanogaster, aplysia californica, caenorhabditis elegans). In: Serotonin: The Mediator that Spans Evolution. pp 49–80
- Lillesaar C, Stigloher C, Tannhäuser B, et al. Axonal projections originating from raphe serotonergic neurons in the developing and adult Zebrafish, Danio Rerio, using transgenics to visualize Raphe-specific pet1 expression. J Comp Neurol. 2009;512:158–182. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Tannhäuser B, Stigloher C, et al. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn. 2007;236:1072–1084. doi: 10.1002/dvdy.21095. [DOI] [PubMed] [Google Scholar]
- Lozano D, González A, López JM. Neuroanatomical distribution of the serotonergic system in the brain and retina of holostean fishes, the sister group to teleosts. Brain Behav Evol. 2020;95:25–44. doi: 10.1159/000505473. [DOI] [PubMed] [Google Scholar]
- Manger PR, Fahringer HM, Pettigrew JD, Siegel JM. The distribution and morphological characteristics of serotonergic cells in the brain of monotremes. Brain Behav Evol. 2002;60:315–332. doi: 10.1159/000067194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Norton WHJ, Folchert A, Bally-Cuif L (2008) Comparative analysis of serotonin receptor (HTR1A/HTR1B Families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J Com Neurol 511:521-542 [DOI] [PubMed]
- Nowicki M, Tran S, Muraleetharan A, et al. Serotonin antagonists induce anxiolytic and anxiogenic-like behavior in zebrafish in a receptor-subtype dependent manner. Pharmacol Biochem Behav. 2014;126:170–180. doi: 10.1016/j.pbb.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Oikonomou G, Altermatt M, Zhang R, wei, , et al. The serotonergic raphe promote sleep in zebrafish and mice. Neuron. 2019;103:686–701. doi: 10.1016/j.neuron.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øverli Ø, Korzan WJ, Höglund E, et al. Stress coping style predicts aggression and social dominance in rainbow trout. Horm Behav. 2004;45:235–241. doi: 10.1016/j.yhbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Winberg S, Pottinger TG. Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—a review. Integr Comp Biol. 2005;45:463–474. doi: 10.1093/icb/45.3.463. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Pérez-Maceira JJ, Mancebo MJ, Aldegunde M. The involvement of 5-HT-like receptors in the regulation of food intake in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol C Toxicol Pharmacol. 2014;161:1–6. doi: 10.1016/j.cbpc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Pérez MR, Pellegrini E, Cano-Nicolau J, et al. Relationships between radial glial progenitors and 5-HT neurons in the paraventricular organ of adult zebrafish—potential effects of serotonin on adult neurogenesis. Eur J Neurosci. 2013;38:3292–3301. doi: 10.1111/ejn.12348. [DOI] [PubMed] [Google Scholar]
- Prasad P, Ogawa S, Parhar IS. Serotonin reuptake inhibitor citalopram inhibits gnrh synthesis and spermatogenesis in the male zebrafish. Biol Reprod. 2015;93(102):1–10. doi: 10.1095/biolreprod.115.129965. [DOI] [PubMed] [Google Scholar]
- Rincón Camacho L, Morandini L, Birba A, et al. The pineal complex: a morphological and immunohistochemical comparison between a tropical (Paracheirodon axelrodi) and a subtropical (Aphyocharax anisitsi) characid species. J Morphol. 2016;277:1355–1367. doi: 10.1002/jmor.20581. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/S0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Gómez FJ, Rendón-Unceta MC, Sarasquete C, Muñoz-Cueto JA. Distribution of serotonin in the brain of the Senegalese sole, Solea senegalensis: an immunohistochemical study. J Chem Neuroanat. 2000;18:103–115. doi: 10.1016/S0891-0618(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Rubio VC, Sánchez-Vázquez FJ, Madrid JA. Oral serotonin administration affects the quantity and the quality of macronutrients selection in European sea bass Dicentrarchus labrax L. Physiol Behav. 2006;87:7–15. doi: 10.1016/j.physbeh.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Sandtner W. Serotonin transport in the 21st century. J Gen Physiol. 2019;151:1248–1264. doi: 10.1085/JGP.201812066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Soengas JL, Aldegunde M. Brain serotonin and the control of food intake in rainbow trout (Oncorhynchus mykiss): effects of changes in plasma glucose levels. J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol. 2002;188:479–484. doi: 10.1007/s00359-002-0320-z. [DOI] [PubMed] [Google Scholar]
- Sako H, Kojima T, Okado N. Immunohistochemical study on the development of serotoninergic neurons in the chick: I. Distribution of cell bodies and fibers in the brain. J Comp Neurol. 1986;253:61–78. doi: 10.1002/cne.902530106. [DOI] [PubMed] [Google Scholar]
- Sallinen V, Torkko V, Sundvik M, et al. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem. 2009;108:719–731. doi: 10.1111/j.1471-4159.2008.05793.x. [DOI] [PubMed] [Google Scholar]
- Schjolden J, Pulman KGT, Pottinger TG, et al. Serotonergic characteristics of rainbow trout divergent in stress responsiveness. Physiol Behav. 2006;87:938–947. doi: 10.1016/j.physbeh.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Shainer I, Buchshtab A, Hawkins TA, Wilson SW, Cone RD, Gothilf Y. Novel hypophysiotropic AgRP2 neurons and pineal cells revealed by BAC transgenesis in zebrafish. Sci Rep. 2017;7:44777. doi: 10.1038/srep44777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MC, Gerlai R, Maximino C. The integration of sociality, monoamines and stress neuroendocrinology in fish models: applications in the neurosciences. J Fish Biol. 2018;93:170–191. doi: 10.1111/jfb.13757. [DOI] [PubMed] [Google Scholar]
- Sobrido-Cameán D, Robledo D, Sánchez L, Rodicio MC, Barreiro-Iglesias A (2019) Serotonin inhibits axonal regeneration of identifiable descending neurons after a complete spinal cord injury in lampreys. Dis Model Mech 12(2):dmm037085 [DOI] [PMC free article] [PubMed]
- Teraoka H, Russell C, Regan J, et al. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J Neurobiol. 2004;60:275–288. doi: 10.1002/neu.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timothy M, Forlano PM. Serotonin distribution in the brain of the plainfin midshipman: Substrates for vocal-acoustic modulation and a reevaluation of the serotonergic system in teleost fishes. J Comp Neurol. 2020;528:3451–3478. doi: 10.1002/cne.24938. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Hawkins TA, Yáñez J, et al. Afferent connectivity of the zebrafish habenulae. Front Neural Circuits. 2016;10:30. doi: 10.3389/fncir.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mier P, Joosten HWJ, Van Rheden R, Donkelaar HJT. The development of serotonergic raphespinal projections in Xenopus laevis. Int J Dev Neurosci. 1986;4:465–475. doi: 10.1016/0736-5748(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Vindas MA, Sørensen C, Johansen IB, et al. Coping with unpredictability: Dopaminergic and neurotrophic responses to omission of expected reward in Atlantic salmon (Salmo salar L) PLoS ONE. 2014;9:e85543. doi: 10.1371/journal.pone.0085543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/S0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Winberg S, Øverli Ø, Lepage O. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary l-tryptophan. J Exp Biol. 2001;204:3867–3876. doi: 10.1242/jeb.204.22.3867. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H (1996) Neuroanatomy of the Zebrafish Brain [DOI] [PubMed]
- Xu J, Li Y, Lv Y, et al. Molecular evolution of tryptophan hydroxylases in vertebrates: a comparative genomic survey. Genes (basel) 2019;10:203. doi: 10.3390/genes10030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue HG, Yang CY, Yamamoto N, Ozawa H. Fiber connections of the periventricular pretectal nucleus in a teleost, tilapia (Oreochromis niloticus) Neurosci Res. 2007;57:184–193. doi: 10.1016/j.neures.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P. Two tyrosine hydroxylase genes in vertebrates. New dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci. 2010;43:394–402. doi: 10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Ziegler M, Knoll S, Köhler HR, et al. Impact of the antidepressant citalopram on the behaviour of two different life stages of brown trout. PeerJ. 2020;8:e8765. doi: 10.7717/peerj.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1 Figure S1. Phylogeny of tph proteins. Multiple sequence alignments of tph amino acid sequences for all taxons (A), fish species (B) and salmonid species (C) were generated using ClustalX 2.1 and the evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model [1]. The tree with the highest log likelihood is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. The analysis involved 111 amino acid sequences. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 362 positions in the final dataset. Evolutionary analyses were conducted in MEGA7. (PDF 102 KB)
Data Availability Statement
Data are available on reasonable request.
No applicable.