Abstract
Purpose
This review provides an updated overview of observational and intervention studies investigating the effect of a low-FODMAP (fermentable oligo-, di- and monosaccharides, and polyols) diet (LFD) on gastrointestinal (GI) symptoms, quality of life (QoL), nutritional adequacy, and gut microbiome in irritable bowel syndrome (IBS) patients.
Methods
We systematically searched available literature until October 2020 for studies that investigated the effect of LFDs on GI symptoms, QoL, nutritional adequacy, and the gut microbiome in IBS patients. The data were represented as standardized mean differences (SMD) for IBS severity, and as mean differences (MD) for IBS-QoL. Meta-analyses were performed for the quantitative analyses using random effects models with inverse variance weighing.
Results
Twelve papers (nine parallel trials, three crossover studies) were included for the meta-analysis. The LFD reduced IBS severity by a moderate-to-large extent as compared to a control diet (SMD − 0.66, 95% CI − 0.88, − 0.44, I2 = 54%). When analyzing only studies that used the validated IBS-SSS questionnaire, a mean reduction of 45 points (95% CI − 77, − 14; I2 = 89%) was observed. Subgroup analyses on adherence, age, intervention duration, IBS subtype, outcome measure, and risk of bias revealed no significantly different results. The LFD also increased IBS-QoL scores, when compared with a control diet (MD 4.93; 95% CI 1.77, 8.08; I2 = 42%).
Conclusions
The low-FODMAP diet reduces GI symptoms and improves quality of life in IBS subjects as compared to control diets. Future work is required to obtain definitive answers regarding potential long-term effects of such diets on nutritional adequacy and the gut microbiome.
PROSPERO registration number
CRD42020175157.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-020-02473-0.
Keywords: Low-FODMAP diet, Irritable bowel syndrome, Exclusion diet, Gastrointestinal symptoms
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder that is characterized by abdominal pain, bloating, and altered bowel habits [1]. It is the most commonly diagnosed GI disorder, estimated to affect approximately 11% of the global population [2], with an increased prevalence in women as compared to men [3]. IBS has repeatedly been demonstrated to both reduce quality of life (QoL) [4–6] and increase health care utilization [7–9], leading to a significant economic burden [8–10].
The complex pathophysiology of IBS is not yet fully understood, but is suggested to involve visceral hypersensitivity, low-grade digestive tract inflammation, changes in GI motility, gut microbiota, and the gut–brain axis [1, 11–15]. As a result of this, IBS treatments currently rely on multifactorial approaches that are primarily focused on treating symptoms [13, 14, 16, 17]. Both IBS patients and gastroenterologists have reported a strong association between consumption of specific foods and IBS-related symptoms [4, 18, 19], indicating the need for an effective dietary treatment strategy. As each IBS subtype presents itself with different symptoms, treatment should be based on IBS subtype and symptom severity [1]. The goal of treatment for IBS with predominantly diarrhea (IBS-D) is to reduce the excessive bowel movements, while treatment for IBS with predominantly constipation (IBS-C) will aim for regular bowel movements, each requiring different nutritional approaches [1]. Besides, general advice to IBS patients comprises eating healthily and in small portions, limiting intakes of gas-producing and fermentable foods, alcohol, fat, and spicy foods [1, 20]. Many patients also try diets like the gluten-free and lactose-free diet to relieve symptoms [19]. Yet, there is little evidence for the efficacy of these elimination diets in the absence of specific conditions like lactose or gluten intolerance or celiac disease, and therefore these diets are not generally recommended [19, 21, 22].
However, there is a growing body of evidence for the effectiveness of the low fermentable oligo-, di- and monosaccharides, and polyols (FODMAP) diet (LFD) in managing IBS symptoms [22, 23]. Currently, advisory bodies like the American College of Gastroenterology and the British Dietetic Association advise the LFD to be respectively first- and second-line treatment for IBS [24, 25]. The underlying hypothesis suggests that reducing the intake of these small, indigestible and often fermentable carbohydrates, reduces intestinal osmolarity and gas production; hence, helping to reduce GI symptoms [26, 27]. The LFD starts with a general phase that aims to eliminate all FODMAPs. If symptoms are successfully reduced within 6–8 weeks, specific groups of FODMAPs are reintroduced into the diet. This serves to identify which FODMAPs cause symptoms, so that patients can adapt a personalized long-term diet that effectively reduces IBS symptoms. Owing to its restrictive nature, however, there are concerns about the effect of the LFD on nutritional adequacy, intestinal microbiota, and health-related quality of life [28–31]. Therefore, the LFD should only be followed in consultation with a specialized dietitian.
Since the two most recent meta-analyses that were performed on the effect of an LFD on GI symptoms in IBS patients [22, 23], four new RCTs and two new cross-sectional studies have been published. The purpose of the current work is to provide an updated systematic review and meta-analysis of both observational and intervention studies that investigates the effect of a low-FODMAP diet, as compared to a control diet, on GI symptoms and quality of life in IBS patients.
Methods
The protocol for this systematic review and meta-analysis was registered in the international prospective register of systematic reviews (PROSPERO, registration number: CRD42020175157), and conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [32].
Search strategy
We systematically searched the electronic databases PubMed/Medline, SCOPUS, and Web of Science until October 1st 2020 for English language records. Titles, abstracts, and keywords were searched for variations and combinations of the following terms: FODMAP(s), saccharides, oligosaccharide, disaccharide, monosaccharide, galacto-oligosaccharides, fructan(s), fructose, galactans, lactose, polyol(s), sorbitol, mannitol, xylitol, maltitol, sweetener(s), sweetening agent, IBS, irritable bowel syndrome, and irritable colon. Separate searches including additional terms related to gut microbiome and nutritional adequacy were also performed (full PubMed search syntaxes in the Supplementary Materials). Intervention and observational studies were included when they respectively examined the effect of the LFD or assessed the association between FODMAP content in the diet and GI complaints or IBS prevalence in adult human subjects with IBS diagnosed according to the Rome III or IV criteria [11, 33].
Papers were excluded when they had an unsuitable intervention (e.g., a co-intervention from which the effects of an LFD could not be distinguished) or control diet, were conducted in children, non-IBS patients or IBS patients with significant clinical co-morbidities, were conference abstracts, or when English text was unavailable. In the case of multiple papers referencing the same study, relevant data were extracted from both papers and included as a single study in the analysis.
Screening and selection of trials
The systematic search was followed by a two-step screening and selection process. During the first step, titles, abstracts, and keywords of publications were screened separately by two of the authors (ASL and AG) to identify potentially eligible studies. During the second step, the full texts of these publications were examined to gauge eligibility based on the stated inclusion criteria. In cases of inter-reviewer disagreement, questions on study eligibility were resolved through consensus and consultation with the other co-author (AB).
Outcome assessment
The primary outcome of interest was IBS symptom severity, preferably assessed by the widely used and validated IBS Severity Scoring System (IBS-SSS) [34]. The IBS-SSS questionnaire assesses the intensity of GI symptoms during a 10-day period and focuses on abdominal pain, distension, stool frequency and consistency, and interference with daily life. Each of these items is scored on a 0–100 visual analog scale, adding up to a total sum score of 0–500, with higher scores indicating more severe symptoms [34]. Studies using other measures of symptom severity, both validated measures and nonvalidated VAS and Likert scales, were included as well. When no assessment of the overall symptom severity was reported, abdominal pain was used as an outcome of interest [22].
The secondary outcomes of interest were quality of life, gut microbiome effects and impact on measures of nutritional adequacy. Quality of life was measured by the validated IBS-QoL questionnaire [35]. The IBS-QoL questionnaire consists of 34 questions regarding dysphoria, interference, body image, health worry, food avoidance, social reaction, sexual, relationships. The results are averaged and transformed to a 0–100 scale, with increasing scores indicating a better QoL [35]. Owing to heterogeneity in methodology and reporting of data, it was deemed inappropriate to conduct meta-analyses of the gut microbiome and nutritional adequacy data. These outcomes were therefore included as part of the qualitative analysis.
Data extraction and quantification
Data extraction was performed by two authors (ASL, AG) and consisted of information on the year of publication, country of origin, study design, duration, intervention diet, control diet, adherence to the diets, number of cases, number of controls, total sample size, IBS diagnostic criteria, mean age and gender, and IBS subtype distribution. The means (mean value at the end of the intervention and end of control period, respectively) and standard deviations between symptom severity measures and IBS-QoL before and after intervention were collected. If no means and standard deviations were reported in the text, the data were extracted from tables or graphs (using a web-based plot digitizing tool [36]). When these data were not available and whenever possible, the 95% CIs and P values were used to calculate means and standard deviations [37]. Where median values and ranges were reported, they were converted to mean values and standard deviations according to the conversion formulas of Wan et al. and Luo et al. [38, 39]. This was done in one case [40]. Where no end values were reported, change from baseline data were used instead [41, 42]. Where insufficient data were available to calculate or extract the mean and standard deviation, the study was excluded from analysis [43].
Data synthesis and statistical analysis
For the primary outcome, standardized mean differences (SMD) were calculated to allow comparison between the variety of outcome measures used in the studies, and to prevent unnecessary exclusion of study data. The SMD is a unitless measure that can be interpreted as a small, moderate or large magnitude of effect [44]. Meta-analyses were conducted using a random effects model with inverse variance weighing [45]. Where enough data were available (minimum of four studies per subgroup), the potential effects of predefined covariates (IBS subtype, intervention duration, sex, age) on the change in IBS severity measures were examined by means of subgroup analyses. The I2 statistic was inspected to assess the extent of possible heterogeneity with I2 values of 25, 50, and 75% considered to be low-, moderate-, and high-level heterogeneity respectively [46]. Data analysis was performed using Review Manager 5 (Version 5.4, Cochrane).
Risk of bias assessment
Publication bias was investigated through visual inspection of funnel plots and Egger’s regression test (with P < 0.1 indicating asymmetry) [47]. The risk of bias in the included studies was assessed using the Cochrane Collaboration’s tool for assessing risk of bias [48]. For this purpose, seven different domains were considered: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. For cross-sectional studies, we used an adapted version of the Newcastle–Ottawa quality assessment scale [49]. The assessments were carried out independently by two authors (ASL and AG), and differences resolved by consensus.
Results
Quantitative analysis
Included trial characteristics
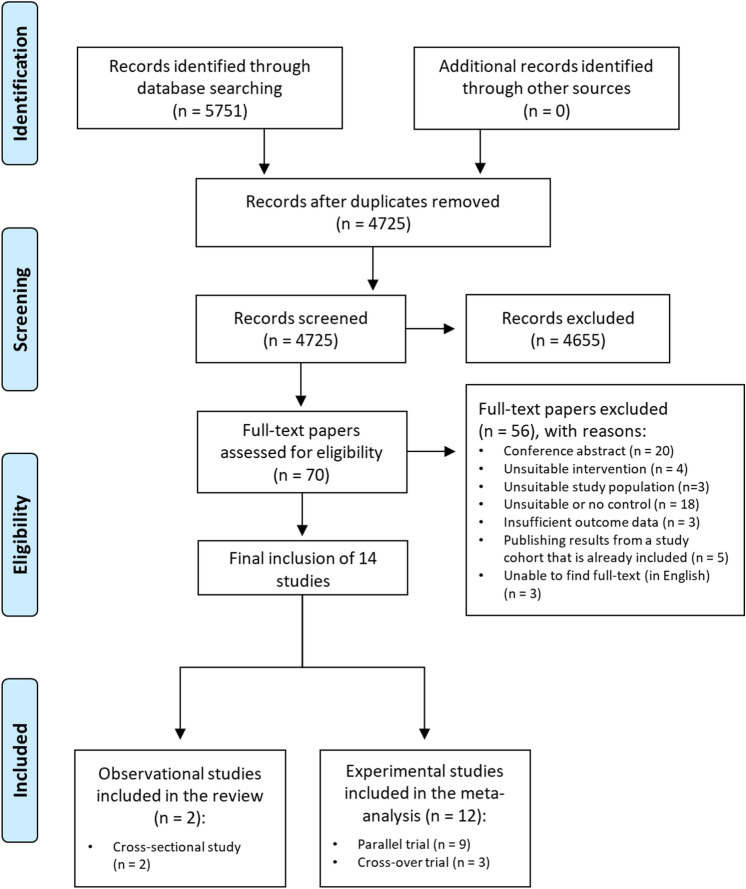
A total of 5751 records was identified through database searching. After duplicate removal, 4725 records were screened, leading a full-text assessment of 70 studies. After exclusion of 56 studies, 14 original studies were included in the review (Fig. 1). Of these, 12 original parallel or crossover trials reported on IBS symptom severity outcomes (Table 1) and were included in the meta-analysis. The remaining two cross-sectional studies are described in Table 2. One post hoc analysis reported quality of life data from the same study population as a study that was already included. Relevant data were extracted, and the paper was excluded [50].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the study selection procedure
Table 1.
Characteristics of experimental studies included in the meta-analysis
| First author, year (country) | Study duration | Total case/controlsa | Type of treatment: intervention vs. control | Age (years)b | Female (%)b | Predominant IBS subtype (%)b | Results |
|---|---|---|---|---|---|---|---|
| Bohn, 2015 [63] (Sweden) | 4 weeks | 33/34 | Dietary education: LFD vs. traditional IBS diet (NICE and BDA) | 42.5 | 81.3 | IBS-M (47) | No significant difference in IBS-SSS was observed between the LFD and control group (246 vs. 23, P = 0.62) |
| Eswaran, 2016 [70] (USA) | 4 weeks | 43/39 | Dietary education: LFD vs. traditional IBS diet (NICE) | 42.6 | 70.7 | IBS-D (100) | The mean abdominal pain score decreased to 3.4 in the LFD group vs. 4.4 in the mNICE group (P = 0.005), and the IBS- QoL score increased to 69.3 for the LFD group vs. 59.4 for the mNICE group (P value not reported) |
| Halmos, 2014 [61] (Australia) | 42 days | 30/30 | Provided diets: LFD vs. typical Australian diet (4.4 g oligosaccharides and 2.6 g polyols/day) | 41.0 | 71.1 | IBS-C (43) | Subjects reported lower mean VAS-scores (0–100) for GI symptoms when on an LFD compared to control: 22.8 vs. 44.9 (P < 0.001) |
| Harvie, 2017 [52] (New Zealand) | 3 months | 23/27 | Dietary education on LFD vs. no dietary education | 41.8 | 86 | IBS-D (64) | Subjects on the LFD had a lower mean IBS-SSS (128 vs. 206) and higher mean IBS-QoL (81 vs. 73) compared to control, after 3 months (P < 0.05 in both for improvement) |
| McIntosh, 2017 [64] (Canada) | 21 days | 18/19 | Dietary education: LFD vs. HFD | 50.9 | 86.5 | IBS-M (62) | Mean IBS-SSS decreased to 208 in the LFD group vs. 290 in the control group (P = 0.01) |
| Ong, 2010 [40] (Australia) | 4 days | 15/15 | Provided diets: LFD (9 g FODMAPs/day) vs. HFD (50 g/day) | 40.8 | 73.3 | IBS-C (47) | IBS symptom severity assessed by a self-rating Likert scale was reported to be lower during the LFD (median 2; range 0–7) than during HFD (6; 2–9) |
| Paduano, 2019 [53] (Italy) | 12 weeks | 34/28 | Dietary education: LFD vs. balanced Mediterranean diet | 28.6 | 83.3 | IBS-D (52) | No significant differences were found between the LFD and control diet when looking at mean IBS-SSS (16 vs. 17, P = 0.44) and IBS-QoL (83 vs. 81, P = 0.27) |
| Patcharatrakul, 2019 [62] (Thailand) | 4 weeks | 30/32 | Dietary education: personalized LFD vs. commonly recommended diet to reduce IBS symptoms | 51.0 | 75.8 | IBS-C (53) | The mean global IBS symptom severity score (VAS 0–100) after intervention was lower in the LFD group than the control group (38.5 ± 20 vs. 53.5 ± 19, P < 0.01) |
| Pedersen, 2014 [41] (Denmark) | 6 weeks | 42/40 | Dietary education: LFD including personalized reintroduction vs. unchanged Danish/Western diet | 34.6 | 76.8 | IBS-D (45) | There was a significantly greater reduction in mean IBS-SSS in the LFD group than in the control group (133 vs. 34, P < 0.01). Mean IBS-QoL was not altered significantly (LFD: 8 vs. control: 0.1, P = 0.13) |
| Staudacher, 2012 [66] (UK) | 4 weeks | 16/19 | Dietary education: LFD vs. habitual diet | 35.1 | 35.1 | NR | The mean overall symptom severity score (0–3 scale) after intervention was lower in the LFD group than in the control group (1.1 vs. 1.7, P < 0.002) |
| Staudacher, 2017 [69] (UK) | 4 weeks | 51/53 | Dietary education: LFD vs. sham exclusion diet (comparable in number of restricted foods and difficulty) | 34.4 | 68.6 | IBS-D (67) | Mean IBS-SSS was significantly lower for patients on the LFD than the sham diet (173 vs. 224, P = 0.001). No significant difference was observed between the groups for IBS-QoL (72.4 vs. 70.6, P = 0.057) |
| Zahedi, 2018 [42] (Iran) | 6 weeks | 50/51 | Dietary education: LFD (< 0.5 g of FODMAPs per meal) vs. traditional IBS diet (BDA) | 37.5 | 50.5 | IBS-D (100) | Mean IBS-SSS decreased to a greater extent in the LFD group compared to control (108 vs. 149.8, P = 0.002). No significant difference was observed between the groups for IBS-QOL (− 7.3 vs. − 5.35, P = 0.332) |
The data are represented as mean value unless indicated otherwise
BDA British Dietetic Association; FODMAP fermentable oligo-, di-, monosaccharides and polyols; HFD high-FODMAP diet; IBS-C irritable bowel syndrome with constipation; IBS-D irritable bowel syndrome with diarrhea; IBS-M irritable bowel syndrome with mixed stool pattern; IBS-QoL irritable bowel syndrome-associated quality of life; IBS-SSS irritable bowel syndrome severity scoring system; LFD low-FODMAP diet; NICE National Institute for Health and Care Excellence; NR not reported
aNumbers are retrieved from per-protocol data
bNumbers are retrieved from intention-to-treat data
Table 2.
Characteristics of observational studies included in the qualitative synthesis
| First author, year (country) | Study design | Number of subjects | Diagnostic criteria | Age (years) | Female (%) | Predominant IBS subtype (%) | Quality assessmenta (number of starsb) | Results |
|---|---|---|---|---|---|---|---|---|
| Lee, 2019 [18] (South Korea) | Cross-sectional | 393 | Validated modified Korean Rome III | 49.4 | 61.8 | IBS-D (43.6) | Poor (3) | High-FODMAP foods were reported by 43.5% of controlsc and 63.4% of IBS subjects to induce GI symptoms |
| Pourmand, 2018 [51] (Iran) | Cross-sectional | 3362 (number of confirmed IBS cases NR) | Unvalidated modified Persian Rome III | NR | NR | NR | Good (7) | No significant association was found between adherence to the LFD and IBS prevalence |
The data are represented as mean value unless indicated otherwise
FODMAP fermentable oligo-, di-, monosaccharides, and polyols; IBS-D irritable bowel syndrome with diarrhea; LFD low-FODMAP diet; NR not reported
aAccording to an adapted Newcastle–Ottawa scale for cross-sectional studies [48]
bOn a scale from 0 to 10
cThe control group comprised of symptomatic and nonsymptomatic subjects
A total of 772 subjects took part in the nine parallel and three crossover trials that investigated the effect of an LFD on GI symptoms in IBS patients. The number of participants per study ranged from 30 to 104. The study duration ranged from 4 days to 3 months. The mean age ranged from 29 to 51 years. Two studies were controlled diet interventions that provided almost all food to subjects during the intervention. Subjects in the remaining ten studies received dietary education as an intervention. The control diets, provided or prescribed, comprised a traditional IBS diet (n = 4), the subjects habitual diet (n = 2), typical diet for the country where the study was carried out (n = 2), high-FODMAP diet (n = 2), balanced Mediterranean diet (n = 1), or a sham exclusion diet specifically designed for the study (n = 1).
Effect of LFD on GI symptoms in IBS patients
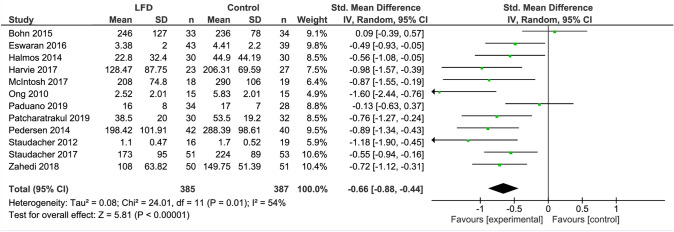
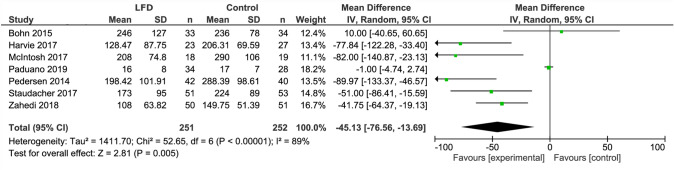
The LFD was found to reduce IBS severity by a moderate to large extent as compared to a control diet (SMD − 0.66, 95% CI − 0.88, − 0.44, I2 = 54%) (Fig. 2). When analyzing studies that used IBS-SSS only, a mean reduction of 45 points (95% CI − 76.56, − 13.69; I2 = 89%) was observed (Fig. 3).
Fig. 2.
Forest plot showing standardized mean differences for IBS severity outcome measures
Fig. 3.
Forest plot showing mean IBS-SSS scores for studies that used IBS-SSS as outcome
One of the observational studies included in the qualitative analysis observed a larger proportion of IBS subjects to report high-FODMAP foods to induce GI symptoms, as compared to control subjects (63.4% vs. 43.5% respectively) [18] (Table 2). The other observational study reported no association between adherence to the LFD and IBS prevalence [51] (Table 2).
Effect of LFD on QoL in IBS patients
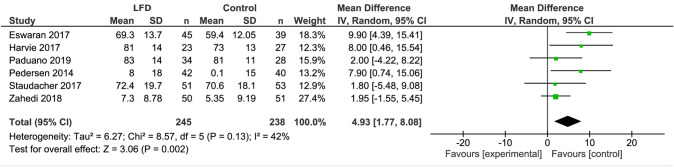
The LFD was associated with higher IBS-QoL scores when compared with a control diet (MD 4.93; 95% CI 1.77, 8.08; I2 = 42%) (Fig. 4).
Fig. 4.
Forest plot showing mean IBS-QoL values
Subgroup analyses
Subgroup analyses for age, outcome measure, and adherence revealed no statistically significant differences between subgroups (Table 3, Supplementary Figures 1–6). In all studied subgroups, the change in IBS symptom severity scores remained statistically significant (Table 3, Supplementary Figures 1–6).
Table 3.
Results of subgroup analyses for different covariates
| Covariate | Number of studies | Standardized mean difference | LL | UL | P value within group | P value between subgroups | I2 (%) |
|---|---|---|---|---|---|---|---|
| Adherence | – | – | – | – | – | 0.77 | 54 |
| Reported adherencea | 6 | − 0.63 | − 1.01 | − 0.24 | 0.001 | – | 66 |
| Adherence not reported | 6 | − 0.70 | − 0.96 | − 0.43 | 0.001 | – | 42 |
| Age | – | – | – | – | – | 0.40 | 54 |
| Below medianb | 6 | − 0.76 | − 1.09 | − 0.43 | 0.001 | – | 59 |
| Above medianb | 6 | − 0.56 | − 0.87 | − 0.25 | 0.001 | – | 52 |
| Duration | – | – | – | – | – | 0.59 | 50 |
| Medianc | 5 | − 0.53 | − 0.88 | − 0.18 | 0.003 | – | 61 |
| Above medianc | 5 | − 0.65 | − 0.93 | − 0.37 | 0.001 | – | 39 |
| IBS subtype | – | – | – | – | – | – | – |
| Majority IBS-D | 6 | − 0.62 | − 0.84 | − 0.39 | 0.001 | – | 30 |
| Outcome measure | – | – | – | – | – | 0.28 | 13.9 |
| IBS-SSS | 6 | − 0.56 | − 0.85 | − 0.27 | 0.001 | – | 61 |
| Non-IBS-SSS | 6 | − 0.81 | − 1.16 | − 0.46 | 0.001 | – | 44 |
| Risk of bias | – | – | – | – | – | – | – |
| Low risk of bias | 9 | − 0.66 | − 0.92 | − 0.40 | 0.001 | – | 55 |
IBS irritable bowel syndrome; IBS-D irritable bowel syndrome with diarrhea; IBS-SSS IBS symptom severity score; LL lower level of 95% confidence interval; UL upper level of 95% confidence interval
aAdherence was good in all studies that reported adherence
bMedian age was 40.9 years
cMedian duration was 4 weeks
Sensitivity analysis, assessment of potential biases, and heterogeneity
Sensitivity analyses, conducted by omitting every study from the meta-analysis, were carried out and did not significantly affect the results (Supplementary Tables 1 and 2). Overall, all included studies had some risk of bias, most notably assessed unclear in allocation concealment and blinding of participants, personnel and of outcome assessment (Supplementary Table 3). Three studies were judged to have a high risk of bias in at least two out of seven areas [41, 52, 53], which all at least include blinding of participants, personnel, and of outcome assessment. Excluding these studies in a subgroup analysis did not affect the SMD (Table 3).
Visual inspection of the funnel plot suggested some publication bias (Fig. 5), which was confirmed by Egger’s regression test (P = 0.087). The pooled IBS severity measure differences showed moderate heterogeneity (I2 = 54%) between studies.
Fig. 5.
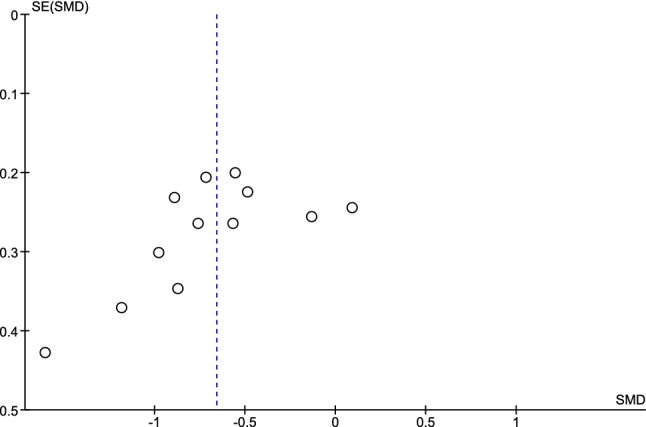
Funnel plot used to assess risk of publication bias for IBS severity outcome measures
Qualitative analysis
Overviews of the systematic searches for studies investigating the effects of the LFD on gut microbiome and nutritional adequacy are presented in Supplementary Figures 7 and 8. For both outcomes, seven studies met the inclusion criteria and were included in the qualitative analysis.
Gut microbiome effects
The methodology employed for fecal microbial analyses varied across studies and included fluorescence in situ hybridization (FISH), quantitative real-time PCR and 16 s rRNA sequencing or combinations thereof.
Five of the included studies reported measures of microbial diversity, six studies reported absolute or relative abundances of total bacteria and/or specific taxa and two studies determined a “dysbiosis index”. In all five studies that measured it, no influence of the LFD measures on microbial diversity was found. However, in most studies, abundances of bifidobacteria and/or their overarching phylum, actinobacteria were reduced following LFD interventions (Table 4).
Table 4.
Overview of studies assessing the effect of the LFD on gut microbiome
| First author, year (country) | Study design | Number of IBS subjects | Intervention | Study duration | Methodology | Results |
|---|---|---|---|---|---|---|
| Halmos, 2015 [31] (Australia) | Crossover | 27 | LFD vs. baseline habitual diet vs. Australian diet | 6 weeks | qPCR |
↓ Total bacterial abundance ↓ A. muciniphila, Bifidobacteria absolute abundance ↓ A. muciniphila, relative abundance ↓ Clostridium cluster IV and XIVa absolute and relative abundance |
| Harvie, 2017 [52] (New Zealand) | Parallel | 45 | LFD vs. habitual diet | 12 weeks | 16S rRNA sequencing |
↔ α- and β-diversity ↔ In any of 244 observed OTUs |
| Hustoft, 2017 [71] (Norway) | Crossover | 27 | LFD (maltodextrin supplement) vs. HFD (FOS) | 20 days | GA-map™ Dysbiosis Test |
vs. baseline: ↓ Actinobacteria abundance ↓ Bifidobacterium abundance ↓ Clostridium, F. prausnitzii, Megasphaera, Pediococcus abundance ↑ Dorea abundance |
| Bennet, 2018 [72] (Sweden) | Parallel | 67 | LFD vs. traditional IBS diet | 4 weeks | GA-map™ Dysbiosis Test |
↑ Dysbiosis Index ↓ Actinobacteria abundance ↓ Bifidobacteria abundance |
| McIntosh, 2017 [64] (Canada) | Parallel | 37 | LFD vs. HFD diet | 3 weeks | 16S rRNA sequencing |
↔ α- and β-diversity ↑ Acintobacteria richness and diversity ↑ Firmicutes-, clostridiales richness (IBS-D and IBS-M only) ↓ Bifidobacterial relative abundance |
| Staudacher, 2012 [66] (UK) | Parallel | 41 | LFD vs. habitual diet | 4 weeks | FISH |
↔ Concentrations and proportions of total bacteria, Bacteroides–Prevotella, E. rectale–C. coccoides, F. prausnitzii, and Lactobacillus–Enterococcus ↓ Concentrations and proportions of bifidobacteria |
| Staudacher, 2017 [69] (UK) | Parallel | 104 | LFD vs. sham diet | 4 weeks | qPCR and 16S rRNA sequencing |
↔ α- and β-diversity ↓ Absolute- and relative abundance of bifidobacteria ↔ Relative abundance of lactobacilli and streptococci |
| Wilson, 2020 [73] (UK) | Parallel | 41 | LFD vs. sham diet | 4 weeks | FISH and 16S rRNA sequencing |
↔ α- and β-diversity ↓ Actinobacteria abundance ↔ Bifidobacteria abundance |
All reported changes are for LFD vs. respective control situations
CTRL controls; FISH fluorescence in situ hybridization; FODMAP fermentable, oligo-, di-, mono-saccharides and polyols; GOS Galacto-oligosaccharides; HFD high-FODMAP diet; IBS irritable bowel syndrome; LFD low-FODMAP diet; OTUs operational taxonomic units; qPCR quantitative polymerase chain reaction; ↑ increase; ↓ decrease; ↔ no change
Nutritional adequacy
Studies reporting on the effects of the LFD on nutrient intake consisted of two post hoc analyses of previous RCTs, three observational studies and two RCTs that only analyzed changes in macronutrient intakes (Table 5).
Table 5.
Studies included to assess nutritional adequacy of the LFD
| First author, year (country) | Study design | Number of IBS subjects | Intervention | Study duration | Methodology | Results |
|---|---|---|---|---|---|---|
| Eswaran, 2019 [56] (USA) | Parallel | 78 | LFD vs. traditional IBS diet (NICE) | 4 weeks | 3-day food diary (at baseline and last week of intervention period). Post hoc analysis of [70] | Reduction in energy-adjusted carbohydrate (− 31.6 g/day), total sugar (− 17.4 g/day), sodium (− 0.5 g/day) (all P < 0.01) and riboflavin (− 0.2 mg/day) intake (P < 0.05) vs. baseline, compared to no changes in traditional IBS diet; increase in energy-adjusted niacin (0.7 mg/day, P < 0.05) and vit B6 (0.3 mg/day, P < 0.01) intake vs. baseline, compared to no changes in traditional IBS diet; fewer patients met the DRIs for thiamin and iron in the LFD group, vs. fewer patients meeting the DRIs for calcium and copper in the control group |
| O’Keeffe, 2018 [54] (UK) | Prospective follow-up study | 103 | LFD vs. habitual diet | 6–18 month follow-up after initial 6-week LFD | Semi-quantitative FFQ (at follow-up) | No statistically significant differences between groups at long-term follow-up for energy and (micro)nutrient intakes, except for higher folate (398 µg/day vs. 318 µg/day, P = 0.02) and vitamin A (2147 µg/day vs. 1429 µg/day, P = 0.045) compared to habitual diet |
| Ostgaard, 2012 [55] (Norway) | Prospective follow-up study | 114 | LFD advice vs. no advice vs. healthy controls | 2-year follow-up after LFD advice | Semi-quantitative FFQ (at follow-up) | No difference in calories or macronutrients between LFD guided patients, unguided patients and healthy controls; no difference in micronutrients between LFD guided and unguided patients; lower intakes of riboflavin (1.9 mg/day vs. 2.1 mg/day) and calcium (1065 mg/day vs. 1184 mg/day) and higher intakes of β-carotene (3.9 mg/day vs. 3.6 mg/day) and vitamin B6 (1.7 mg/day vs. 1.6 mg/day) for LFD guided patients vs. healthy controls |
| Pourmand, 2018 [51] (Iran) | Cross-sectional | 3362 (number of confirmed IBS cases NR) | Quintiles of FODMAP intake | – | 106-item semi-quantitative food frequency questionnaire | Individuals with the highest adherence to the low FODMAP diet had lower dietary intakes of all measured foods groups and (micro)nutrients (P < 0.001) |
| Staudacher, 2019 [57] (UK) | Parallel | 130 | LFD vs. habitual diet; LFD vs. sham exclusion diet | 4 weeks |
7-day food record (at baseline and last week of intervention period); diet quality according to Healthy Diet Indicator and Healthy Diet Score; Diet Diversity according to Diet Quality Index-Revised Dietary Diversity and Dietary Diversity Score |
Lower intake of starch vs. habitual control diet (109 g/day vs. 128 g/day, P = 0.03); no difference in micronutrient intakes except for higher intake of vitamin B-12 vs. habitual and sham control diets (6.1 μg/day vs. 3.9 μg/day and 4.7 μg/day respectively, P < 0.01) and higher intake of selenium vs. sham control diet (52 μg/day vs. 42 μg/day, P = 0.03); no difference in proportion of patients meeting micronutrient DRIs; overall scores for diet quality were lower after low FODMAP advice vs. habitual control diet (P < 0.01) |
| Only macronutrient data | ||||||
| Böhn, 2015 [63] (Sweden) | Parallel | 67 | LFD vs. traditional IBS diet (NICE and BDA) | 4 weeks | 4-day food diary (at screening and during last week of intervention period) | Reduced mean intake of carbohydrates (159.1 g/day vs. 193.1 g/day, P = 0.007) and dietary fiber (15.1 g vs. 20.2 g, P = 0.003) vs. traditional IBS diet |
| Zahedi, 2018 [42] (Iran) | Parallel | 101 | LFD vs. traditional IBS diet (BDA) | 6 weeks | 3-day food diary (at baseline and last week of intervention period) | Reduced mean intake of carbohydrates (266.1 g/day vs. 360.9 g/day, P < 0.001) and increased mean intake of fat (65.2 g/day vs. 51.4 g/day, P = 0.04) vs. traditional IBS diet |
BDA British Dietetic Association; DRI dietary reference intakes; FFQ food frequency questionnaire; FODMAP fermentable oligo-, di-, monosaccharides and polyols; GI gastrointestinal; IBS irritable bowel; LFD low-FODMAP diet; NICE National Institute for Health and Care Excellence; QoL quality of life
In most studies, no differences in the majority of analyzed micronutrient intakes were found. Exceptions were small increases in intakes of vitamin A [54], β-carotene [55], B-vitamins [54–57], and selenium [57] after the LFD as compared to control or habitual diets. Conversely, small decreases in riboflavin [55, 56] and calcium [55] intake were also found.
One RCT found that an LFD intervention resulted in a lower proportion of patients meeting the DRIs for thiamin and iron as compared to control [56], whereas a post hoc analysis of two RCTs found no difference in the proportion of subjects meeting micronutrient DRIs when comparing LFD to control diets [57].
One cross-sectional study reported lower intakes of energy, and all measured food groups, macro- and micronutrients across all quintiles of increasing adherence to an LFD [51].
Discussion
This updated meta-analysis of 12 controlled human intervention studies found that the LFD reduced IBS severity by a moderate to large extent as compared to a control diet (SMD − 0.66, 95% CI − 0.88, − 0.44, I2 = 54%). Furthermore, the LFD also resulted in higher IBS-QoL scores when compared with a control diet (mean difference 4.93; 95% CI 1.77, 8.08; I2 = 42%). It should be noted that we used standardized mean differences to include studies that did not use the standard IBS-SSS as an outcome measure. As the SMD can only be interpreted in terms of a small, moderate, or large effect, it limits the extent to which conclusions can be derived about clinical relevance of the demonstrated effect. However, when analyzing only studies that used the IBS-SSS as an outcome measure, a mean reduction of 45 points was found (95% CI − 77, − 14) when comparing subjects on the LFD to a control diet. A 50-point reduction in IBS-SSS score is typically considered to be associated with a clinically meaningful improvement [58]. Nevertheless, the LFD was found to have a moderate to high efficacy in reducing GI symptoms in IBS patients. Our findings are in line with the previous meta-analyses [22, 23, 59, 60], and conclusions are more substantiated due to the higher number of controlled intervention studies that could be included in our analyses (12 controlled intervention studies). The two most recent meta-analyses [22, 23] included only one study and four studies, respectively, to assess the effect of the LFD on QoL. Our review includes six controlled intervention studies that assessed QoL and found a statistically significant 5-point improvement when comparing subjects on an LFD to those on a control diet. Whether this reflects a meaningful change in health-related QoL is unclear, as a 10-point change has previously been considered clinically relevant [35].
In subgroup analyses, we found that the demonstrated improvements in IBS symptom severity were consistent between subgroups with different levels of adherence, age, intervention duration, IBS subtype, outcome measure, and risk of bias. Regarding intervention duration, the longest intervention duration was three months, therefore persistence of symptom reduction may need to be researched further. For IBS subtypes, we only had data to perform a subgroup analysis on IBS with predominantly diarrhea (IBS-D), which revealed outcomes similar to the main analysis. Individual studies with a majority of subjects with IBS with predominantly constipation (IBS-C) [40, 61, 62] or IBS with a mixed stool pattern (IBS-M) [63, 64] generally demonstrated similar improvements in IBS symptom severity, although this was not consistent among all studies [41]. More studies are needed to determine whether the efficacy of the LFD is consistent among these different subtypes. It should be noted that all the subgroups in the current meta-analysis were relatively small and as such the outcomes should be interpreted with caution. Future studies with larger sample sizes and clear reporting on adherence assessment, IBS-QoL assessment, IBS subtype, age, sex, and ethnicity are needed to inform in this regard. Furthermore, there are also no studies that investigated a potential dose–response relationship between FODMAP intake and IBS symptom severity in a controlled systematic fashion, indicating a gap in currently available evidence. However, as the threshold for tolerance of FODMAPs and type of FODMAP varies between individuals, carrying out such study would be very complex. This would likely require a large number of patients recruited in a multicenter setting over a prolonged period of time in a collaborated fashion to be feasible.
All studies had some risk of bias, most notably performance bias due to the lack of blinding of participants, personnel, or outcome assessment. Blinding remains a methodological factor in dietary intervention studies that is very difficult to address, especially in LFD trials where IBS subjects may already be familiar with the LFD due to its increasing popularity. However, a subgroup analysis including only studies with the lowest risk of bias (n = 9) did not result in a different SMD as compared to the overall analysis. Furthermore, we found indications of publication bias and visual inspection of the funnel plot suggested an absence of studies reporting a low or no effect on IBS symptom severity.
Owing to the LFDs restrictive nature, concerns have been raised over the long-term nutritional adequacy of the LFD [28, 29, 65, 66], as well as its effects on the gut microbiome [28, 31, 67]. As such, we also examined these aspects as part of the qualitative synthesis of this review (Tables 4 and 5). However, it is difficult to draw definitive conclusions regarding these two outcomes. In both cases, there were only a limited number of studies. Along with heterogeneity in analytical measures and outcome reporting, this precluded meta-analyses or direct comparisons of the available data.
In general, different studies demonstrated that substantial nutritional inadequacies do not occur, both during short-term interventions and at long-term follow-up after initial LFD advice [54–57], and may in some cases even lead to small increases in micronutrient intake. Conversely, a cross-sectional study of a large Iranian cohort did find lower intakes of energy, and all measured food groups, macro- and micronutrients across quintiles of increasing adherence to an LFD [51]. However, it is not clear whether the analyses were corrected for energy intake or other potential confounders.
It is important to note that in most of the included studies, subjects received personalized diets and/or nutritional advice under specialist dietetic or nutritionist guidance, which would have helped to maintain a balanced diet. This underscores the importance of specialist counseling where food items are also reintroduced on a timely basis for IBS patients when following an LFD [26]. Furthermore, although the outcomes of the two included long-term follow-up studies [54, 55] are promising, more work is required to conclusively determine the nutritional impact of LFD in individuals that follow it without seeking specialist advice.
The gut microbiome composition is hypothesized to undergo detrimental changes on an LFD, mainly due to decreased fiber intake and availability of prebiotic fructans, causing a reduction in the substrate available for colonic fermentation [66, 68]. Generally, the LFD did not seem to affect measures of overall microbial diversity, but absolute or relative abundances of actinobacteria were reduced in many cases. Owing to differences in the methodology employed for fecal microbial analyses, it is difficult to compare outcomes between studies. It should also be noted that, since the natural interpersonal variation in gut microbiome composition can result in potentially larger differences than the effect of a dietary intervention, large sample sizes are required to enable robust investigations in this regard. As such, none of the included studies were sufficiently powered to allow for firm conclusions to be drawn. It must also be noted that very few studies have investigated the sustained effects of the LFD on the gut microbiome effects of an LFD (the longest study duration included here was 12 weeks). More work is therefore needed in this regard.
There are some limitations to the current study. First, there was a large variation between studies in control diets, ranging from subjects maintaining their habitual diet without dietary advice to high-FODMAP diets and sham exclusion diets. The FODMAP content of these control diets was often unclear or not reported. The high variety in control diets is also a possible explanation for the moderate heterogeneity observed between studies included in this meta-analysis. Second, half of the included studies did not assess subject adherence to the diet [41, 42, 52, 53, 66, 69]. Other studies assessed adherence via food diaries [40, 62–64, 70] or breath hydrogen tests [61] and reported good adherence. Since adherence is crucial to symptom relief [65], proper reporting in this regard is important to be able to determine the efficacy of an LFD intervention. Also, from a practical point of view, reporting adherence explores the feasibility of following an LFD for IBS patients. Nevertheless, subgroup analyses did not reveal significant differences in effect between studies that reported adherence and studies that did not.
In conclusion, this up-to-date systematic review and meta-analysis found that the low-FODMAP diet reduces gastrointestinal symptoms and improves quality of life in IBS subjects when compared to a control diet. Future research is recommended to obtain definitive answers regarding potential long-term effects of such diets on nutritional adequacy and the gut microbiome. This will require larger RCTs with appropriate controls that report on gut microbiome effects, dietary adherence, IBS-QoL and dose–response effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Hongeun Choi for her help with investigating the effect of the LFD on gut microbiome, and dr.ir. Nicole de Roos and Simone Pyle for their feedback on the manuscript.
Abbreviations
- CI
Confidence interval
- FODMAP
Fermentable oligo-, di- and monosaccharides, and polyols
- GI
Gastrointestinal
- GRADE
Grading of recommendations, assessment, development and evaluations
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- IBS-C
Irritable bowel syndrome with constipation
- IBS-D
Irritable bowel syndrome with diarrhea
- IBS-M
Irritable bowel syndrome with mixed stool pattern
- IBS-U
Unspecified irritable bowel syndrome
- IBS-SSS
Irritable bowel syndrome severity scoring system
- MD
Mean difference
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- QoL
Quality of life
- RCT
Randomized controlled trial
- SD
Standard deviation
- SE
Standard error
- SMD
Standardized mean difference
- NR
Not reported
Author contributions
The authors’ responsibilities were as follows—ASL and AG: conceived and designed the study, conducted the literature review, conducted the statistical analysis, and drafted the manuscript; AB: amended and approved the protocol, provided critical revision and important intellectual content. All authors made significant contributions to this manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by Unilever, a commercial food and beverage manufacturer. ASL, AG and AB were employees of Unilever at the time of the study. ASL and AG were involved in the design, implementation, analysis, and interpretation of the data.
Data availability
Data will remain available for 5 years.
Compliance with ethical standards
Conflict of interest
Unilever is a company that manufactures food and beverages, of which some may be considered low-FODMAP.
References
- 1.Defrees DN, Bailey J. Irritable bowel syndrome: epidemiology, pathophysiology, diagnosis, and treatment. Prim Care Clin Off Pract. 2017;44(4):655–671. doi: 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(7):712–721.e714. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107(7):991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 4.Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simren M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 5.Monnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45(Suppl):S98–101. doi: 10.1097/MCG.0b013e31821fbf44. [DOI] [PubMed] [Google Scholar]
- 6.Wang YT, Lim HY, Tai D, Krishnamoorthy TL, Tan T, Barbier S, Thumboo J. The impact of irritable bowel syndrome on health-related quality of life: a Singapore perspective. BMC Gastroenterol. 2012;12:104–104. doi: 10.1186/1471-230X-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, Frech F, Ofman JJ. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98(3):600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 8.Doshi JA, Cai Q, Buono JL, Spalding WM, Sarocco P, Tan H, Stephenson JJ, Carson RT. Economic burden of irritable bowel syndrome with constipation: a retrospective analysis of health care costs in a commercially insured population. J Manag Care Spec Pharm. 2014;20(4):382–390. doi: 10.18553/jmcp.2014.20.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buono JL, Mathur K, Averitt AJ, Andrae DA. Economic burden of irritable bowel syndrome with diarrhea: retrospective analysis of a U.S. commercially insured population. J Manag Care Spec Pharm. 2017;23(4):453–460. doi: 10.18553/jmcp.2016.16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tack J, Stanghellini V, Mearin F, Yiannakou Y, Layer P, Coffin B, Simren M, Mackinnon J, Wiseman A, Marciniak A, On Behalf of the IBIS-C Study Group Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol. 2019;19:69. doi: 10.1186/s12876-019-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drossman DA. Functional gastrointestinal disorders: what's new for Rome IV? Lancet Gastroenterol Hepatol. 2016;1(1):6–8. doi: 10.1016/s2468-1253(16)30022-x. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA, Hasler WL. Rome IV—functional GI disorders: disorders of gut–brain interaction. Gastroenterology. 2016;150(6):1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Holtmann G, Walker MM. Therapeutic strategies for functional dyspepsia and irritable bowel syndrome based on pathophysiology. J Gastroenterol. 2015;50(6):601–613. doi: 10.1007/s00535-015-1076-x. [DOI] [PubMed] [Google Scholar]
- 14.Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1(2):133–146. doi: 10.1016/s2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 15.Moayyedi P, Simrén M, Bercik P. Evidence-based and mechanistic insights into exclusion diets for IBS. Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1038/s41575-020-0270-3. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Ford AC. Pharmacotherapy for irritable bowel syndrome. J Clin Med. 2017;6(11):101. doi: 10.3390/jcm6110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares RLS. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20(34):12144–12160. doi: 10.3748/wjg.v20.i34.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Kim HJ, Kang EH, Jung KW, Myung SJ, Min YW, Choi CH, Ryu HS, Choi JK, Kwon JG, Hong KS, Park KS. Self-reported food intolerance in Korean patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2019;25(2):222–232. doi: 10.5056/jnm18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenhart A, Ferch C, Shaw M, Chey WD. Use of dietary management in irritable bowel syndrome: results of a survey of over 1500 United States gastroenterologists. J Neurogastroenterol Motil. 2018;24(3):437–451. doi: 10.5056/jnm17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O'Sullivan NA, Pettitt C, Reeves LB, Seamark L, Williams M, Thompson J, Lomer MC. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(5):549–575. doi: 10.1111/jhn.12385. [DOI] [PubMed] [Google Scholar]
- 21.Cozma-Petrut A, Loghin F, Miere D, Dumitrascu DL. Diet in irritable bowel syndrome: what to recommend, not what to forbid to patients! World J Gastroenterol. 2017;23(21):3771–3783. doi: 10.3748/wjg.v23.i21.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionne J, Ford AC, Yuan Y, Chey WD, Lacy BE, Saito YA, Quigley EMM, Moayyedi P. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol. 2018;113(9):1290–1300. doi: 10.1038/s41395-018-0195-4. [DOI] [PubMed] [Google Scholar]
- 23.Schumann D, Klose P, Lauche R, Dobos G, Langhorst J, Cramer H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24–31. doi: 10.1016/j.nut.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Ford AC, Moayyedi P, Chey WD, Harris LA, Lacy BE, Saito YA, Quigley EMM. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113(Suppl 2):1–18. doi: 10.1038/s41395-018-0084-x. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O'Sullivan NA, Pettitt C, Reeves LB, Seamark L, Williams M, Thompson J, Lomer MCE, British Dietetic A. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update) J Hum Nutr Diet. 2016;29(5):549–575. doi: 10.1111/jhn.12385. [DOI] [PubMed] [Google Scholar]
- 26.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31(8):874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 27.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25(2):252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 28.Bellini M, Rossi A. Is a low FODMAP diet dangerous? Tech Coloproctol. 2018;22(8):569–571. doi: 10.1007/s10151-018-1835-9. [DOI] [PubMed] [Google Scholar]
- 29.Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32(Suppl 1):16–19. doi: 10.1111/jgh.13688. [DOI] [PubMed] [Google Scholar]
- 30.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8):1517–1527. doi: 10.1136/gutjnl-2017-313750. [DOI] [PubMed] [Google Scholar]
- 31.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res Ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointest Liver Dis. 2006;15(3):237–241. [PubMed] [Google Scholar]
- 34.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 35.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 36.Rohatgi A (2015) https://apps.automeris.io/wpd/. Accessed 1 May 2020
- 37.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 38.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 40.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen N, Andersen NN, Vegh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20(43):16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. 2018;33(6):1192–1199. doi: 10.1111/jgh.14051. [DOI] [PubMed] [Google Scholar]
- 43.Guerreiro MM, Santos Z, Carolino E, Correa J, Cravo M, Augusto F, Chagas C, Guerreiro CS. Effectiveness of two dietary approaches on the quality of life and gastrointestinal symptoms of individuals with irritable bowel syndrome. J Clin Med. 2020;9(1):125. doi: 10.3390/jcm9010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. Pharm Ther. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- 45.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res Ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, Perruolo E, Parati G, Settings ESHWGoCRiLR Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eswaran S, Chey WD, Jackson K, Pillai S, Chey SW, Han-Markey T. A diet low in fermentable oligo-, di-, and monosaccharides and polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2017;15(12):1890–1899.e1893. doi: 10.1016/j.cgh.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Pourmand H, Keshteli AH, Saneei P, Daghaghzadeh H, Esmaillzadeh A, Adibi P. Adherence to a low FODMAP diet in relation to symptoms of irritable bowel syndrome in Iranian adults. Dig Dis Sci. 2018;63(5):1261–1269. doi: 10.1007/s10620-018-4986-7. [DOI] [PubMed] [Google Scholar]
- 52.Harvie RM, Chisholm AW, Bisanz JE, Burton JP, Herbison P, Schultz K, Schultz M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol. 2017;23(25):4632–4643. doi: 10.3748/wjg.v23.i25.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paduano D, Cingolani A, Tanda E, Usai P. Effect of three diets (low-FODMAP, gluten-free and balanced) on irritable bowel syndrome symptoms and health-related quality of life. Nutrients. 2019 doi: 10.3390/nu11071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Keeffe M, Jansen C, Martin L, Williams M, Seamark L, Staudacher HM, Irving PM, Whelan K, Lomer MC. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2018 doi: 10.1111/nmo.13154. [DOI] [PubMed] [Google Scholar]
- 55.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5(6):1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 56.Eswaran S, Dolan RD, Ball SC, Jackson K, Chey W. The impact of a 4-week low-FODMAP and mNICE diet on nutrient intake in a sample of US adults with irritable bowel syndrome with diarrhea. J Acad Nutr Diet. 2019 doi: 10.1016/j.jand.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Staudacher HM, Ralph FSE, Irving PM, Whelan K, Lomer MCE. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. J Acad Nutr Diet. 2019 doi: 10.1016/j.jand.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Singh P, Ballou S, Katon J, Takazawa E, Rangan V, Mukherjee R, Iturrino J, Nee J, Lembo A. Symptom severity, mood, and healthcare use are associated with satisfaction in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2020;18:2945–2951.e1. doi: 10.1016/j.cgh.2020.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varju P, Farkas N, Hegyi P, Garami A, Szabo I, Illes A, Solymar M, Vincze A, Balasko M, Par G, Bajor J, Szucs A, Huszar O, Pecsi D, Czimmer J. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: a metaanalysis of clinical studies. PLoS ONE. 2017 doi: 10.1371/journal.pone.0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55(3):897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 61.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75.e65. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 62.Patcharatrakul T, Juntrapirat A, Lakananurak N, Gonlachanvit S. Effect of structural individual low-FODMAP dietary advice vs. brief advice on a commonly recommended diet on IBS symptoms and intestinal gas production. Nutrients. 2019 doi: 10.3390/nu11122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Böhn L, Storsrud S, Liljebo T, Collin L, Lindfors P, Tornblom H, Simren M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407.e1392. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 64.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–1251. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 65.Nawawi KNM, Belov M, Goulding C. Low FODMAP diet significantly improves IBS symptoms: an Irish retrospective cohort study. Eur J Nutr. 2019 doi: 10.1007/s00394-019-02074-6. [DOI] [PubMed] [Google Scholar]
- 66.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 67.Reddel S, Putignani L, Del Chierico F. The impact of low-FODMAPs, gluten-free, and ketogenic diets on gut microbiota modulation in pathological conditions. Nutrients. 2019 doi: 10.3390/nu11020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut. 2016;65(1):169–178. doi: 10.1136/gutjnl-2015-309757. [DOI] [PubMed] [Google Scholar]
- 69.Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, Scholz M, Tuohy KM, Lindsay JO, Irving PM, Whelan K. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936–947. doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111(12):1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 71.Hustoft TN, Hausken T, Ystad SO, Valeur J, Brokstad K, Hatlebakk JG, Lied GA. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2017 doi: 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- 72.Bennet SMP, Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Öhman L, Simrén M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–881. doi: 10.1136/gutjnl-2016-313128. [DOI] [PubMed] [Google Scholar]
- 73.Wilson B, Rossi M, Kanno T, Parkes GC, Anderson S, Mason AJ, Irving PM, Lomer MC, Whelan K. β-Galactooligosaccharide in conjunction with low FODMAP diet improves irritable bowel syndrome symptoms but reduces fecal bifidobacteria. Am J Gastroenterol. 2020;115(6):906–915. doi: 10.14309/ajg.0000000000000641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will remain available for 5 years.