Abstract
A putative biomarker of anxiety risk, the startle response is typically enhanced by negative compared to neutral emotion modulation in adults, but remains understudied in children. To determine the extent to which neutral, negative, and positively valenced emotional conditions modulate startle response in early life, a child-friendly film paradigm was used to vary emotion across these conditions during startle induction in sixty-four 4- to 7-year-old children. Association of emotion-modulated startle with parent-reported anxiety symptom severity and child behavioral inhibition, a risk factor for anxiety problems, were assessed. Analyses revealed no difference in startle magnitude during negative compared to neutral film clips. By contrast, startle during both negative and neutral conditions was greater than startle during the positive condition. Larger startle magnitude during the neutral condition associated with higher levels of child behavioral inhibition (BI). These results are consistent with possible immaturity of startle response in young children, and suggest that startle amplitude in more emotionally ambiguous, neutral conditions could serve as an early biomarker for anxiety risk.
Keywords: anxiety, behavioral inhibition, early childhood, startle response
1 |. INTRODUCTION
The startle reflex, a defensive reaction in animals and humans, is measured by recording the magnitude of orbicularis oculi muscle contraction in response to sudden aversive stimulation (e.g. electric shock, white noise burst). It is mediated by well-established neural circuitry underlying fear processing and captures individual differences in the excitability of the amygdala, a region of the brain implicated in anxiety (Davis, 2006). In adults and adolescents, larger startle magnitude associates with heightened threat aversion and negative emotional state and has been suggested as a biomarker of fear disorders (Vaidyanathan et al., 2012), but less is known about startle in young children.
Larger startle reflexes are elicited in fear-inducing, aversive contexts (e.g. angry face, violent scene) relative to positive (e.g. happy face, happy scene) or neutral (e.g. neutral face, neutral scene) contexts in healthy adults (Cuthbert et al., 1996; Grillon, 2002) and adolescents (Grillon et al., 1998, 1999); however, this phenomenon, known as fear-potentiated startle, may be disrupted by clinically significant anxiety. Higher levels of anxiety associate with larger startle during both threatening and neutral relative to positive conditions (Grillon et al., 1994; Morgan et al., 1995). In clinically anxious adults, selective failure to inhibit startle response during neutral conditions has been observed (Grillon, 2002; Lissek et al., 2006)—a finding that may reflect heightened threat reactivity that is only inhibited by overtly positive cues (Davis et al., 2010) or disturbances in fear modulation because neutral contexts are more difficult to interpret than negative or positive contexts (Denefrio et al., 2019; Grillon, 2002). Indeed, interpretation of neutral/ambiguous information as negative or threatening has been demonstrated in anxious adolescents and adults (Miers et al., 2008; Peschard & Philippot, 2017; Yoon & Zinbarg, 2008), and elevated startle response to safety stimuli in adolescence predicts the onset of anxiety disorders in adults (Craske et al., 2012).
Findings from the study of startle in anxious or fearful children are less consistent. Startle response across negative, positive, and neutral pictures has been found to be greater in children with anxiety disorders relative to non-anxious 9- to 12-year-old children (Waters et al., 2005). In a community sample of children 8 to 13 years, larger startle magnitude during a safety condition was found to predict greater anxiety symptoms (Jovanovic et al., 2014). However, other recent work found no relation between affectively modulated startle and anxiety disorder status in similarly aged, 8- to 10-year-old children (Meyer et al., 2017). In younger children, 4- to 8-year-old, larger startle magnitude during both neutral and negative faces distinguished those with an anxiety disorder compared to those without (Waters et al., 2008).
These divergent results may reflect developmental differences in neural systems for threat processing across different ages (Thomas et al., 2001), but could also stem from differences in startle paradigms. Traditional paradigms used to study emotion-modulated startle in adults and adolescents use threat of electric shock, aversive sounds, air blasts to the larynx, and darkness to create aversive conditions (Grillon & Ameli, 1998; Grillon et al., 1999). These same paradigms are widely held to be developmentally inappropriate and too averse to parse differences in response conditions in very young children (e.g. highly averse negative conditions could contaminate other conditions for some children, Grillon & Baas, 2003), prompting the use of more developmentally appropriate, picture-based emotional stimuli (e.g. Waters et al., 2005, 2008). However, most picture stimuli have been developed for the study of adults, and some images may be overly aversive, while others are insufficiently arousing for children (McManis et al., 2001; Waters et al., 2005). In sum, these methodological limitations have hindered attempts to determine whether emotion-modulated startle tracks anxiety and/or anxiety risk in children.
In response, Quevedo et al., (2010) designed a child- and adult-friendly, affectively valenced film paradigm to modulate emotion in a community sample of children ages 3, 5, 7, and 9 and adults. Across all ages, magnitude of startle was significantly higher in the negative relative to neutral, and neutral relative to positive conditions. These findings are in contrast to earlier studies that found no affective modulation of startle response in healthy children ages 7 to 12 (Cook et al., 1995; McManis et al., 1995; Waters et al., 2005, 2008). Self and/or parent-reported anxiety severity in Quevedo et al.,'s (2010) sample was related to larger startle magnitude, but only in the baseline condition. In addition, the relation was present in older children (ages 7 and 9) and adults, but not younger children (3 and 5 years). Adapting this paradigm, Lo et al., (2015) found that healthy children ages 4 to 7 years demonstrated larger startle magnitude in both negative and neutral relative to positive film clip conditions, but did not test if startle was related to anxiety symptoms. Thus, it remains unclear precisely how startle is affectively modulated in young children, and if affective startle modulation is related to anxiety severity.
Temperamental risk for anxiety may further influence startle characteristics in children. For example, behavioral inhibition (BI), a pattern of behavioral fear or avoidance of novel stimuli and early risk marker for anxiety disorders (Blackford et al., 2011; Kagan et al., 1988) has been shown to influence startle in children, with those higher in BI showing greater magnitude of startle to safety cues than those low in BI (Barker et al., 2014). However, studies of preschool aged children with the film clip paradigm (Lo et al., 2015; Quevedo et al., 2010) did not examine startle in relation to BI—an especially relevant risk marker for anxiety in children under 5 years who may be biologically prone to anxiety disorders but not yet exhibiting symptoms. Additional studies employing engaging, affectively-valenced film clips that concurrently measure anxiety severity and BI are needed to advance understanding of startle as a potential biomarker of anxiety and/or anxiety risk in young children.
Thus, the current study employed the film clip paradigm to examine startle during three affectively valenced conditions in children ages 4 to 7 years old. In addition, parent report was used to assess BI and anxiety symptoms in children. Prior studies of startle in young children during the film paradigm have produced inconsistent results (Lo et al., 2015; Quevedo et al., 2010). We predicted larger startle magnitude would associate with both negative and neutral compared to positive film clips, consistent with potentially less mature capacity for contextual differentiation of threat response in children and some prior work (Lo et al., 2015, but see Quevedo et al., 2010). To assess the viability of startle as a biomarker of either early risk or impairment, we explored the relation of startle response magnitude with concurrently measured anxiety problems and BI.
2 |. METHODS
Eighty-eight 4- to 7-year-old children were sampled from the community and a Child Psychiatry clinic to capture a spectrum of anxiety symptom severity. Children could have no history of neurodevelopmental delay, autism spectrum disorder, mental retardation, head injury, or serious medical illness, and take no medications affecting central nervous system functioning. Caregivers consented to the study and children were given an age-appropriate task overview (IRB waived assent due to young child age). While children completed the startle procedure, caregivers completed the Behavioral Inhibition Questionnaire (BIQ) and the Child Behavior Checklist (CBCL). Of 88 who attempted the startle procedure, 64 children (34 female) with analyzable startle data (see criteria below) ranged from 3.75 to 7.92 years old (M = 5.91, SD = 1.11); 64.1% were Caucasian, 10.9% African American, 21.9% bi-racial and 3.1% Asian/Pacific Islander. Of the 64, 12 were missing BIQ (measure added after study initiation), and 2 were missing CBCL. These data were found to be missing completely at random (Little's MCAR p =0.78), and multiple imputation was used to impute missing values (Expectation-Maximization algorithm, 10 iterations).
2.1 |. Measures
The Behavioral Inhibition Questionnaire (Bishop et al., 2003) assesses BI in children ages 2 through adolescence (Broeren & Muris, 2010). Parents rated frequency of their children's behavior on a 7-point scale from 1 (hardly ever) to 7 (almost always). Analyses considered raw scores on the BIQ which capture the full range of variance and because population-normed T-scores are not available for this instrument.
Child anxiety symptoms were measured by parent report on the DSM-oriented Anxiety Problems subscale of the Child Behavior Checklist (CBCL/1–5 years and CBCL/6–18 years; Achenbach & Rescorla, 2001). The CBCL is commonly used to measure child psychopathology and is considered more accurate than self-report in young children who struggle to provide accurate self-ratings (Tandon et al., 2009). Because the age range of our subjects required use of both the younger and older versions of the CBCL, population-normed T-scores, rather than raw scores, were used to collapse across subjects for analyses.
2.2 |. Startle paradigm
The startle paradigm developed by Lo et al., (2015) was used to test the effect of emotion modulation on startle response. Emotion modulation was induced using 12 unique video clips, selected for child-friendly, emotionally evocative content. Ratings by 11 early childhood development experts confirmed the clips were both child-appropriate and likely to induce positive, negative and neutral emotions in 4- to 7-year olds (Table S1). Clips ranged from 0.82 to 1.35 min in duration, and included four positive clips from “The Incredibles,” “Harry Potter & the Sorcerer's Stone,” “Hoosiers,” and “Unaccompanied Minors,” four negative clips from “The Fox & the Hound,” “Jumanji,” “Monster House,” and “Harry Potter & the Chamber of Secrets,” and four neutral clips from “Secret Garden,” “Goonies,” and “Matilda”. Between each clip, a blue screen was presented for 10 s to serve as an inter-trial interval (ITI) to reduce carry-over effects and quantify general startle reactivity. Each participant viewed one of three possible orders of film clip presentations, pseudo-randomized to ensure that films of the same valence were never presented consecutively.
During the film clip paradigm, startle probes were 100 db SPL(A) broadband white noise bursts (50 ms duration) presented binaurally at intermittent times throughout each video clip. Prior to beginning the experiment, probes were presented on 3 occasions during a neutral film clip with a nature scene for startle habituation. During the paradigm, probes were administered randomly (7–12 s intervals, 3 per film clip), following a 13 s acclimation period at the beginning of each clip. Probes were also presented randomly 2, 4, or 5 s into the ITI trials. A total of 52 startle probes were administered (3 during habituation, 3 during each film clip, 1 during each of 13 ITIs). After excluding habituation, 49 trials were analyzed.
2.3 |. Data collection and processing
Startle was measured as the electromyographic (EMG) response to startle probes, recorded using a Biosemi ActiveTwo System (BioSemi). Two Ag-AgCl active electrodes placed under the left eye, along the orbicularis oculi muscle (Blumenthal et al., 2005), and grounded by BioSemi's Common Mode Sense active and Driven Right Leg passive electrodes. Raw EMG was digitized by ActiveTwo's analog-to-digital converter (ADC) at a sampling rate of 1,024 Hz using a low-pass 5th order sinc response filter with a half power point of 1/5th the sampling rate (204.8 Hz), processed offline in BrainVision Analyzer 2 (BrainProducts) using a bandpass filter (28–256 Hz), rectified, and baseline-corrected using the −100 to 0 ms window preceding the startle probe. Peak amplitude was scored as the largest rectified value 20–220 ms following startle probe onset by a trained coder blinded to clip valence. Non-response trials were assigned a value of zero and were included in calculations of magnitude. Our scoring window exceeded the 20–150 ms window recommended by Blumenthal et al., (2005) but was consistent with scoring methods used on this task previously in young children (Lo et al., 2015). Individual trials were considered valid if signal from the beginning of the baseline through the end of the scoring window (−100 to 220 ms) was free of artifact (Blumenthal et al., 2005). For inclusion in analyses, subjects were required to meet two criteria. First, each subject was required to have at least 4 ITI trials and 2 trials from each affective condition. The average number of trials per condition was 10 in each condition for our included subjects. Baseline, probe and scoring windows had to be free from artifact. Second, subjects had to be classified as a startle responder, defined as having a startle response during at least 2 ITI trials (Blumenthal et al., 2005). Based on these criteria, data were analyzable for 64 of 88 participants. Of the 24 participants excluded from analyses, 6 had an insufficient number of non-artifactual trials during positive, negative, neutral conditions and/or ITIs and 18 were startle non-responders.
2.4 |. Startle magnitude quantification
Among those with analyzable startle data, startle magnitude was summarized using within-subjects T-scores, computed for each condition (positive, negative, and neutral) relative to all other valid trials. This method of quantifying startle magnitude was used to account for observed wide individual variation in absolute blink magnitude (e.g. disproportionately large blinks) that is unrelated to experimental phenomena (Blumenthal et al., 2005).
3 |. RESULTS
Included and excluded children did not differ by age, gender, or anxiety problems, though the excluded group demonstrated lower behavioral inhibition (Table S2).
To test the modulation of startle magnitude by affective condition, a repeated-measures ANOVA compared startle T-scores across conditions in all subjects with analyzable startle data. A significant effect of condition on startle emerged, Wilkes’ lambda = 0.490, F(2,62) = 32.24, p <0.001, ηp2 = 0.51. Post hoc Bonferroni-corrected paired samples t tests (see Table 1) indicated that startle was larger during both neutral and negative conditions compared to the positive condition, ps < 0.001. Startle during the neutral condition was not significantly different from the negative condition, p = 0.63. Age did not interact with the effect of valence on startle magnitude, F(6,118) = 0.973, p =0.447.
TABLE 1.
Descriptive statistics and post hoc pairwise comparisons of startle magnitude by condition
|
Descriptive statistics
|
|||||
| Condition | Mean | SD | Range | ||
| Neutral | 52.09 | 3.48 | 41.82–58.38 | ||
| Negative | 51.79 | 3.28 | 45.21–61.17 | ||
| Positive | 47.94 | 2.86 | 41.60–56.70 | ||
|
Pairwise comparisons
|
|||||
| Condition | Mean diff. | SE | 95% CI (LL, UL) | t-test | df |
| Neutral vs. positive | 4.15 | 0.575 | 2.74, 5.57 | 7.21*** | 63 |
| Negative vs. positive | 3.85 | 0.604 | 2.36, 5.33 | 6.37*** | 63 |
| Neutral vs. negative | 0.30 | 0.634 | −1.26, 1.86 | 0.48 | 63 |
Note: Table includes all subjects with analyzable startle response, n = 64. One-way ANOVA is presented in text. Post-hoc pairwise comparisons were Bonferroni corrected to consider a familywise error rate of 0.05. Mean diff = mean difference. CI = confidence interval. LL = lower limit, UL = upper limit. Unit of startle magnitude is the mean of within-subjects t-scores of a condition relative to all other valid trials in the experiment.
p <0.001.
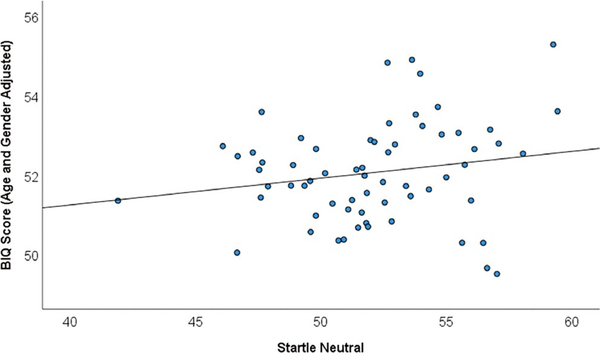
Correlational analyses were conducted to examine how BI, anxiety, age, and gender may relate to startle response (see Table 2). Partial correlations revealed that after controlling for age and gender, larger startle magnitude during the neutral condition related to greater BI (Figure 1), r(60) = 0.340, p = 0.007 (startle during negative and positive conditions was not associated with BI, ps > 0.56) and BI positively related to anxiety, r(60) = 0.259, p = 0.042, but startle (in any condition) was not associated with anxiety (ps > 0.56; see Figure S1). Furthermore, startle did not moderate BI-anxiety associations (ps > 0.10). Neither age nor gender related to startle magnitude (any condition), BI or anxiety, all ps > 0.26.
TABLE 2.
Descriptive statistics and correlations between primary variables
| Measure | Mean (SD) | Range | t | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Startle (neutral) | 52.09 (3.48) | 41.82–58.38 | 0.40 | – | ||||||
| 2. Startle (positive) | 47.94 (2.86) | 45.21–61.17 | −0.49 | −0.05 | – | |||||
| 3. Startle (negative) | 51.79 (3.28) | 41.60–56.70 | 0.63 | −0.13 | −0.24† | – | ||||
| 4. Behavioral inhibition | 93.81 (28.73) | 43.90–173.57 | −0.54 | 0.32* | −0.05 | 0.03 | – | |||
| 5. CBCL anxiety problems | 53.46 (5.61) | 50.00–73.00 | −0.29 | 0.10 | −0.06 | −0.08 | 0.25† | – | ||
| 6. Child age (years) | 5.91 (1.11) | 3.75–7.92 | −0.75 | −0.12 | −0.08 | −0.09 | −0.13 | −0.14 | – | |
| 7. Child gender | 34F; 30 M | – | – | −0.05 | 0.06 | −0.08 | 0.08 | 0.03 | 0.10 | – |
Note: n = 64. Descriptive statistics are computed from a mean of ten imputed datasets; correlations and t-tests are derived from a pooling of imputations. Startle refers to the mean of within-subjects t-scores of a condition relative to all other valid trials in the experiment. CBCL anxiety problems referenced are T-scores. t = independent-samples t tests of gender differences. SD = standard deviation.
p < 0.05
p < 0.10.
FIGURE 1.
Scatterplot of behavioral inhibition questionnaire (BIQ) by startle in the neutral condition, n = 64
4 |. DISCUSSION
Startle response magnitude in three emotionally valenced film clip conditions was examined in a sample of children 4 to 7 years old. Children demonstrated larger startle in both neutral and negative conditions, relative to the positive condition, standing in contrast to research in adult samples. Startle response in the neutral condition, but not other conditions, was associated with BI, consistent with prior work suggesting that startle during a “safety” context may identify risk for future anxiety disorders (Craske et al., 2012; Lissek et al., 2006).
Lack of difference in startle response across neutral and negative emotions in our child sample may stem from immaturity of neural mechanism underlying the startle response. In adults, prefrontal cortical inhibition of amygdala reactivity to threat is believed to impact variation in startle response across threating and non-threatening contexts (Vaidyanathan et al., 2009). Given that cortical-amygdala interactions develop during adolescence into early adulthood (Swartz & Monk, 2014), it is possible that children have less neural capacity for startle modulation than adults. This possibility is consistent with recent work in children 3–7 years old using the same child-friendly film paradigm as in our study (Lo et al., 2015; but see Quevedo et al., 2010) and prior research in older, school-aged children (Cook et al., 1995; McManis et al., 1995; Waters et al., 2005, 2008). In our sample, however, age did not interact with valence to modulate startle response. By sampling across a wider age range, ideally within a longitudinal design, future studies may be able to more fully address developmental factors when examining startle reactivity and emotion processing in children. Such work should consider the possibility that greater sensitivity to the laboratory environment (e.g. novel equipment, interactions with unfamiliar lab personnel and parental separation) could differentially influence neural systems underlying startle response in younger compared to older children and adults.
While negative and neutral conditions were not found to differentially modulate startle in our sample, larger startle during negative and neutral relative to positive conditions was observed. This pattern has been previously demonstrated in clinically anxious adults, and suggested to reflect increased perception of neutral stimuli as threatening (Lissek et al., 2006; Miers et al., 2008; Peschard & Philippot, 2017; Yoon & Zinbarg, 2008). In theory, immature capacity of amygdala-based neural networks for differentiating neutral from negative conditions may confer vulnerability for anxiety (i.e. anxiety risk) in children, even in the absence of clinically severe anxiety. This notion is supported by our finding that greater BI, a well-documented, early childhood risk marker of later anxiety disorders, significantly associated with larger startle in the neutral condition. Alternatively, children high in BI are likely those most susceptible to experiencing the lab environment as threatening which, in turn, could enhance startle reactivity (i.e. across neutral and negative conditions), leading to larger startle response unless dampened by overtly positive cues.
Prior work shows that children high in BI demonstrate increased startle response, but only during a neutral “safety” condition (Barker et al., 2014), and that startle during affectively neutral conditions predicts future anxiety risk (Craske et al., 2012). Indeed, preschoolers who are fearful in situations that are relatively low in threat are likely to develop anxiety by kindergarten (Buss, 2011). More generally, anxious individuals of all ages are biased to interpret neutral stimuli in more negative ways (Miers et al., 2008; Peschard & Philippot, 2017; Yoon & Zinbarg, 2008). Thus, a “neutral,” “safety,” or even baseline condition may not truly be affectively neutral due to interpretation bias in individuals at risk for anxiety (Denefrio et al., 2019; Grillon & Baas, 2003).
Consistent with prior research, BI and anxiety were positively correlated in our sample, but the significance of this finding in the context of startle association with BI, but not anxiety, deserves consideration. Our work and others (Meyer et al., 2017; Quevedo et al., 2010) suggests that startle magnitude may not directly relate to anxiety symptoms at young ages, contrasting with studies in adults showing direct associations between startle and anxiety symptoms when measured concurrently (Vaidyanathan et al., 2009). Rather, in children, larger magnitude startle during a neutral baseline condition at age 7 has been found to moderate the relationship between greater early-life BI and later anxiety severity (age 9, Barker et al., 2015). This finding is consistent with other work showing that impaired threat processing when measured behaviorally (e.g. attention-based towards threat) moderates the relation between early BI and later anxiety (Lonigan et al., 2004; Pérez-Edgar et al., 2011). In our sample, startle was measured concurrently with BI and anxiety; although startle associated with BI and BI associated with anxiety, no effects of startle on anxiety or moderation of BI-anxiety association by startle were observed. Collectively, these findings may suggest that, in children, larger startle response during neutral contexts could serve as a potentially useful indicator for future anxiety problems, rather than current anxiety symptoms.
Importantly, although we observed the same effect of emotional film clips on startle magnitude in children (i.e. Neg = Neu > Pos) as shown previously by Lo et al. (2015), our findings contrast with the adult-like pattern of emotion-modulated startle (Neg > Neu > Pos) demonstrated in children using the original version of the film clip paradigm (Quevedo et al., 2010). The reason for the discrepancy between studies is unclear. More startle probes per valence were delivered in our paradigm, relative to the original, which should have increased power to detect startle modulation by emotion valence. Thus, the possible effect of subtle technical differences must be considered. For example, if hardware used in our work accentuated the greater variation in noise level (e.g. background music) that tends to accompany negative compared to neutral films (Juslin & Timmer, 2010), then differences in startle magnitude between these conditions could have been obscured. This potential confound reflects an inherent disadvantage of startle induction via noise burst probes during any paradigm involving sound (e.g. film audio) for emotion modulation. Given that other, existing paradigms for emotion modulation involve images (e.g. International Affective Picture System), darkness or threat of shock that may be too aversive to be ethically used in the very young (Quevedo et al., 2010), efforts to generate age-appropriate and effective paradigms (e.g. preschool-appropriate emotional image sets) for the study of emotion-modulated startle are still needed.
There are several other important limitations to consider. The number of children with analyzable startle response was limited by non-response and motion artifact. We attempted to balance the competing challenges of retaining participants with ample amounts of data to compute averages in each condition, while retaining as many participants as possible. While the average number of trials retained per condition was 10, our inclusion of participants with as few as 2 trials per condition could have contributed to difficulties identifying potential differences between neutral and negative conditions. We also utilized filtering parameters based on similar studies (Lo et al., 2015), but which were more stringent than those applied to other datasets (Glenn et al., 2012; Kujawa et al., 2015). This could have contributed to difficulties detecting responses and inability to detect differences in startle magnitude between conditions. Future studies should consider ways to optimize filtering settings (Khemka et al., 2017). Few studies have examined startle in young children and best practices for eliciting startle, and processing startle data, in preschoolers requires further development (Quevedo et al., 2010). CBCL anxiety T-scores indicate only 6 children scored at or above borderline clinical range and children without analyzable startle data exhibited lower BIQ scores; future work should include children across a wider spectrum of anxiety severity and BIQ scores to further test for startle-anxiety associations and moderation of anxiety-BI associations by startle. Moreover replication of startle-BI associations in a larger sample will be important to confirm the findings presented here. Although trait anxiety was assessed, children's state-level anxiety was not despite its possible effects on startle response (Grillon et al., 1993; Smith et al., 2005); future work should ask children to report real-time anxiety levels using a simple, pictorial self-report. Further, BI was measured solely via parent-report, whereas laboratory-measured BI would be ideal.
In conclusion, we examined startle response across affectively valenced conditions in relation to BI and anxiety symptoms in early childhood. Findings are consistent with prior work suggesting that physiological response to threat in neutral and negative conditions may not differ in young children. Startle during the neutral condition was uniquely associated with BI, suggesting that larger startle during ambiguous cues may be most relevant for indexing anxiety risk in young children.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Jessica Hruschak and Faith Horbatch for their assistance with data collection and management.
Funding information
National Institute of Mental Health, Grant/Award Number: R03 MH102648 (KLR, KDF) and R33 MH121641 (KDF, JSM, KLR); University of Michigan Kickstart Award (KDF); One Mind/AIM Sullivan Family Rising Star Award (KDF)
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the National Institute of Mental Health Data Archive (NDA) at https://nda.nih.gov.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Achenbach TM, & Rescorla L (2001). Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Aseba. [Google Scholar]
- Barker TV, Reeb-Sutherland B, Degnan KA, Walker OL, Chronis-Tuscano A, Henderson HA, Pine DS, & Fox NA (2015). Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology, 52(11), 1544–1549. 10.1111/psyp.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland BC, & Fox NA (2014). Individual differences in fear potentiated startle in behaviorally inhibited children. Developmental Psychobiology, 56(1), 133–141. 10.1002/dev.21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G, Spence SH, & McDonald C (2003). Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Development, 74(6), 1899–1917. 10.1046/j.1467-8624.2003.00645.x [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, & Zald DH (2011). Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience, 6(5), 621–629. 10.1093/scan/nsq073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, & Van Boxtel A (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42(1), 1–15. 10.1111/j.1469-8986.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Broeren S, & Muris P (2010). A psychometric valuation of the behavioral inhibition questionnaire in a non-clinical sample of Dutch children and adolescents. Child Psychiatry & Human Development, 41(2), 214–229. 10.1007/s10578-009-0162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA (2011). Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology, 47(3), 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EW, Hawk LW, Hawk TM, & Hummer K (1995, August). Affective modulation of startle in children. Psychophysiology, 32, S25–S25. [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek-Schallhorn S, Epstein A, Naliboff B, & Ornitz E (2012). Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: Evidence from a longitudinal investigation. Journal of Abnormal Psychology, 121(2), 315. 10.1037/a0025738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, & Lang PJ (1996). Probing picture perception: Activation and emotion.Psychophysiology, 33(2), 103–111. 10.1111/j.1469-8986.1996.tb02114.x [DOI] [PubMed] [Google Scholar]
- Davis M (2006). Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist, 61(8), 741. 10.1037/0003-006X.61.8.741 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denefrio S, Myruski S, Mennin D, & Dennis-Tiwary TA (2019). When neutral is not neutral: Neurophysiological evidence for reduced discrimination between aversive and non-aversive information in generalized anxiety disorder. Motivation and Emotion, 43(2), 325–338. 10.1007/s11031-018-9732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, & Hajcak G (2012). The development of fear learning and generalization in 8 to 13 year-olds. Developmental Psychobiology, 54(7), 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry, 52(10), 958–975. 10.1016/S0006-3223(02)01665-7 [DOI] [PubMed] [Google Scholar]
- Grillon C, & Ameli R (1998). Effects of threat of shock, shock electrode placement, and darkness on startle. International Journal of Psychophysiology, 28(3), 223–231. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, & Davis M (1993). Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry, 33(8–9), 566–574. 10.1016/0006-3223(93)90094-T [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, & Davis M (1994). Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry, 35(7), 431–439. 10.1016/0006-3223(94)90040-X [DOI] [PubMed] [Google Scholar]
- Grillon C, & Baas J (2003). A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology, 114(9), 1557–1579. 10.1016/S1388-2457(03)00202-5 [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, & Merikangas KR (1998). Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry, 44(10), 990–997. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, Kagan J, Bonny Donzella B, Dikel T, & Nelson C (1999). Startle potentiation by threat of aversive stimuli and darkness in adolescents: A multi-site study. International Journal of Psychophysiology, 32(1), 63–73. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, & Bradley B (2014). Development of fear acquisition and extinction in children: effects of age and anxiety. Neurobiology of Learning and Memory, 113, 135–142. 10.1016/j.nlm.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juslin PN, & Timmers R (2010). Expression and communication of emotion in music performance. In Juslin PN & Sloboda JA (Eds.), Handbook of music and emotion: Theory, research, applications (pp. 453–489). New York: Oxford University Press. [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, & Johnson MO (1988). Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development, 59, 1580–1589. 10.2307/1130672 [DOI] [PubMed] [Google Scholar]
- Khemka S, Tzovara A, Gerster S, Quednow BB, & Bach DR (2017). Modeling startle eyeblink electromyogram to assess fear learning. Psychophysiology, 54, 204–214. 10.2307/1130672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Glenn CR, Hajcak G, & Klein DN (2015). Affective modulation of the startle response among children at high and low risk for anxiety disorders. Psychological Medicine, 45(12), 2647–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, & Grillon C (2006). The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology, 72(3), 265–270. 10.1016/j.biopsycho.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Lo SL, Schroder HS, Moran TP, Durbin CE, & Moser JS (2015). Neurophysiological evidence of an association between cognitive control and defensive reactivity processes in young children. Developmental Cognitive Neuroscience, 15, 35–47. 10.1016/j.dcn.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW, Phillips BM, & Hazen RA (2004). Temperament, anxiety, and the processing of threat-relevant stimuli. Journal of Clinical Child and Adolescent Psychology, 33(1), 8–20. 10.1207/S15374424JCCP3301_2 [DOI] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, & Lang PJ (2001). Emotional reactions in children: Verbal, physiological, and behavioral responses to affective pictures. Psychophysiology, 38(2), 222–231. 10.1111/1469-8986.3820222 [DOI] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Cuthbert BN, & Lang PJ (1995). Kids have feelings too: children’s physiological responses to affective pictures. Psychophysiology, 33, S53. [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Glenn CR, Kujawa AJ, & Klein DN (2017). Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion, 17(3), 487. 10.1037/emo0000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers AC, Blöte AW, Bögels SM, & Westenberg PM (2008). Interpretation bias and social anxiety in adolescents. Journal of Anxiety Disorders, 22(8), 1462–1471. 10.1016/j.janxdis.2008.02.010 [DOI] [PubMed] [Google Scholar]
- Morgan C, Grillon C, Southwick SM, Davis M, & Charney DS (1995). Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry, 38(6), 378–385. 10.1016/0006-3223(94)00321-S [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, Hane AA, Pine DS, & Fox NA (2011). Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology, 39(6), 885–895. 10.1007/s10802-011-9495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschard V, & Philippot P (2017). Overestimation of threat from neutral faces and voices in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 57, 206–211. 10.1016/j.jbtep.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Quevedo K, Smith T, Donzella B, Schunk E, & Gunnar M (2010). The startle response: Developmental effects and a paradigm for children and adults. Developmental Psychobiology, 52(1), 78–89. 10.1002/dev.20415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Bradley MM, & Lang PJ (2005). State anxiety and affective physiology: effects of sustained exposure to affective pictures. Biological Psychology, 69(3), 247–260. 10.1016/j.biopsycho.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Swartz JR, & Monk CS (2014). The role of corticolimbic circuitry in the development of anxiety disorders in children and adolescents. Current Topics in Behavioral Neurosciences, 16, 133–148. [DOI] [PubMed] [Google Scholar]
- Tandon M, Cardeli E, & Luby J (2009). Internalizing disorders in early childhood: A review of depressive and anxiety disorders. Child and Adolescent Psychiatric Clinics, 18(3), 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, & Casey BJ (2001). Amygdala response to facial expressions in children and adults. Biological Psychiatry, 49(4), 309–316. 10.1016/S0006-3223(00)01066-0 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, & Patrick CJ (2012). Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine, 42(3), 447–459. 10.1017/S0033291711001528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, & Cuthbert BN (2009). Linking dimensional models of internalizing psychopathology to neurobiological systems: Affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin, 135(6), 909. 10.1037/a0017222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Lipp OV, & Spence SH (2005). The effects of affective picture stimuli on blink modulation in adults and children. Biological Psychology, 68(3), 257–281. 10.1016/j.biopsycho.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, & Ornitz EM (2008). Baseline and affective startle modulation by angry and neutral faces in 4–8-year-old anxious and non-anxious children. Biological Psychology, 78(1), 10–19. 10.1016/j.biopsycho.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Yoon KL, & Zinbarg RE (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117(3), 680. 10.1037/0021-843X.117.3.680 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.