Abstract
Background
In a 12 month period, three Irish-born adult cases with pulmonary TB were initially diagnosed by Xpert® MTB/RIF Ultra assay, which detected a rifampicin resistance-conferring mutation prompting treatment as potential MDR cases.
Methods
Further laboratory investigations on the cultured isolates included GenoType MTBDRplus assay, phenotypic drug susceptibility tests using the BD BACTEC MGIT culture system and MIC broth microdilution tests. Sequencing of the rpoB gene was performed using Sanger sequencing and WGS.
Results
Phenotypic drug susceptibility tests determined the isolates to be rifampicin susceptible. Molecular investigations identified an A451V (codon 532) mutation in the Mycobacterium tuberculosis rpoB gene that has not previously been found to cause rifampicin resistance. Genome sequencing revealed that the three isolates’ genomes differed by ≤5 SNPs, indicating a high likelihood of recent transmission events. Furthermore, a cluster of six related M. tuberculosis isolates from our in-house typing database showed four were highly related; all were rifampicin susceptible and lacked this mutation.
Conclusions
False detection of rifampicin resistance, albeit rare, should be considered possible with Xpert® MTB/RIF Ultra assay, particularly in low TB incidence settings. Confirmatory sequencing methods should be performed to prevent the unnecessary use of second-line anti-tuberculous drugs.
Introduction
Subsequent to its endorsement by WHO in 2010 the Xpert® MTB/RIF assay, succeeded by the Xpert® MTB/RIF Ultra (Cepheid, Sunnyvale, CA, USA), became an established test for rapid diagnosis of pulmonary TB and detection of mutations within the 81 bp rpoB gene rifampicin-resistance determining region (RRDR).1,2 When an RRDR mutation is detected using the Xpert® MTB/RIF Ultra assay, WHO guidelines recommend starting treatment with an MDR-TB regimen.3 However, in cases where the rpoB mutation is not associated with phenotypic rifampicin resistance, this could result in overuse of second-line anti-tuberculous drugs .
We report three cases of pulmonary TB where the Xpert® MTB/RIF Ultra assay result for rifampicin resistance mutation(s) led to initial therapy with an MDR-TB drug regimen. Subsequent confirmation of rifampicin susceptibility enabled a change in regimen to include rifampicin in two cases with successful completion of therapy. Treatment in the third case did not include rifampicin because of the patient’s underlying immunocompromise and lack of clarity at the time on the clinical relevance of the A451V mutation. Genome sequencing of a further six isolates associated with this cluster showed that four were closely related to the index isolates but lacked an A451V mutation. Further epidemiological investigations failed to identify a link between any of these cases .
Materials and methods
Three sputum samples that were processed as part of routine diagnostic testing in the Irish Mycobacteria Reference Laboratory (IMRL) in a 12 month period between 2018 and 2019 were included in this study .
The Xpert® MTB/RIF Ultra assay was performed as per manufacturer’s instructions (Cepheid). The Xpert® MTB/RIF Ultra v2 assay was used for sample 1 whereas Xpert® MTB/RIF Ultra v3 assay was used for samples 2 and 3.
All three sputum samples were cultured using the BACTEC MGIT 960 culture system according to the manufacturer’s instructions (Becton Dickinson and Company, NJ, USA).
A WHO-endorsed line-probe assay (LPA) for determining rifampicin and isoniazid resistance, GenoType MTBDRplus v2.0 (Bruker-Hain Diagnostics, Germany),4,5 was performed post-culture (median time to positivity 4 days, range 3–7 days). Species identification was performed with the GenoType MTBC assay (Bruker-Hain Diagnostics).
Phenotypic drug susceptibility testing (pDST) was performed using the BD BACTEC MGIT 960 culture system according to the manufacturer’s instructions. The Mycobacterium tuberculosis isolates were tested for susceptibility to first-line and second-line anti-tuberculous drugs at WHO-defined critical concentrations: rifampicin (1.0 mg/L), isoniazid (0.1 mg/L), ethambutol (5 mg/L), pyrazinamide (100 mg/L), moxifloxacin (0.25 mg/L), amikacin (1.0 mg/L), linezolid (1.0 mg/L) and clofazimine (1.0 mg/L).3 Additional testing to determine rifampicin MICs for each isolate was performed using a broth microdilution method, the Sensititre MYCOTB MIC Plate (TREK Diagnostic Systems, Cleveland, OH, USA) according to the manufacturer’s instructions. Identification of the rifampicin resistance mutation was performed using rpoB gene Sanger sequencing.6 Epidemiological typing was performed in house using a 24 locus MIRU-VNTR typing kit (GenoScreen, Lille, France).7 Phylogenetic lineages were assigned to each isolate using the MIRU-VNTRplus online tool.8,9 There were a further six M. tuberculosis isolates from the IMRL database, dating from 2010 to the present investigation, with an indistinguishable MIRU-VNTR genotype. WGS was performed on the nine M. tuberculosis isolates using an Illumina high output MiniSeq kit (Illumina®, San Diego, CA, USA) according to the manufacturer’s instructions. SNP-based analysis was performed using the MTBseq v1.0.4 pipeline.10
Ethics statement
This outbreak was investigated by the local Departments of Public Health under statutory legislation and did not require ethics approval. Legal duties, organizational policies and good practices were observed in data handling and data processing for the study which was conducted by the authors to inform the statutory function of the Health Services Executive in Ireland to improve, promote and protect the health and welfare of the public (Section 7, Health Act 2004), in line with the General Data Protection Regulations and their application in Ireland. This report was approved, prior to submission, by the Data Protection Officer at St James’s Hospital.
Data availability
Raw sequence reads of all nine sequenced M. tuberculosis genomes in this study were submitted to the European Nucleotide Archive database under project accession number PRJEB43194.
Results
The laboratory data for the three M. tuberculosis isolates are shown in Table 1.
Table 1.
Comparison of genotypic and phenotypic laboratory test results for M. tuberculosis isolates harbouring the A451V mutation
| Sample no. | Sample type | Xpert® MTB/ RIF Ultraa, SMB probe with Tm shift | GenoType MTBDRplusv2.0b |
Rifampicin pDST |
rpoB mutatione | ||
|---|---|---|---|---|---|---|---|
| rpoB WT | rpoB MUT | BACTEC MGIT liquid culturec | broth microdilution MIC (mg/L)d | ||||
| 1 | sputum | rpoB4A | WT1-8 present | not detected | S | <0.12 | A451V (A532V) |
| 2 | sputum | rpoB2, rpoB4A | WT8 absent | not detected | S | <0.12 | A451V (A532V) |
| 3 | sputum | rpoB2, rpoB4A | WT8 absent | not detected | S | 0.25 | A451V (A532V) |
MUT, mutation/mutant; SMB, sloppy molecular beacon; Tm, melting temperature; S, susceptible .
Xpert® MTB/RIF Ultra v2 assay used for sample 1; Ultra v3 assay used for samples 2 and 3.
Assay performed on M. tuberculosis recovered from each sample.
pDST performed using the BD BACTEC MGIT 960 culture system and tested at WHO-defined critical concentration of 1 mg/L rifampicin.
MIC determined by broth microdilution using TREK® Sensititre MYCOTB MIC Plate.
rpoB mutation identified using Sanger sequencing, confirmed with WGS analysis.
Xpert® MTB/RIF Ultra detected M. tuberculosis complex DNA and a rifampicin resistance-associated mutation in the three respiratory samples. Sample 1 displayed slightly different Xpert® results to Samples 2 and 3. The raw data from this test showed that probe rpoB4A was detected in all samples, with a second probe (rpoB2) observed in samples 2 and 3 (both WT and mutation melt curves detected). GenoType MTBDRplus LPA results were similar for samples 2 and 3 where rifampicin resistance was inferred from the banding pattern obtained due to lack of signal to one rpoB WT probe (WT8 probe not developed; no mutation probe developed).5 However, there was no indication of rifampicin resistance from the banding pattern obtained for sample 1 (all WT probes developed, no mutation probe developed). All three samples were confirmed as M. tuberculosis and pDST results showed susceptibility to all first- and second-line anti-TB drugs when tested at WHO-defined critical concentrations.3 Additional tests showed rifampicin susceptible MICs (shown in Table 1) for each isolate using a broth microdilution method. Sanger sequencing revealed an A451V (codon 532) rpoB gene mutation in all three isolates and was confirmed with WGS analysis.
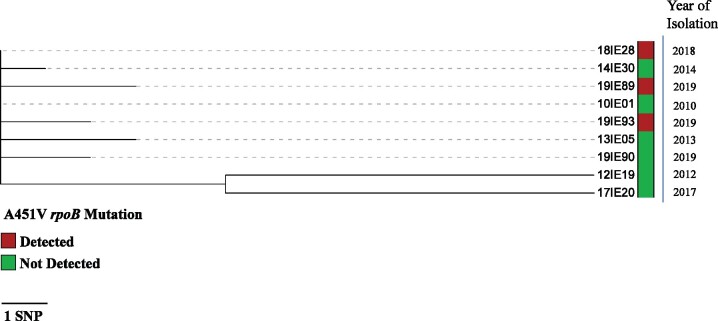
MIRU-VNTR typing showed the three M. tuberculosis isolates were indistinguishable and belonged to Euro-American lineage 4 (MtbC 15-9 code: 3199-15). SNP based WGS analysis using the MTBseq pipeline showed that these three isolates’ genomes differed from each other by ≤5 SNPs (Figure 1), which indicated a high likelihood of recent transmission events involving these cases due to the low mutation rate (estimated at 0.5 SNPs per genome per year) of M. tuberculosis.10,11 An analysis of all nine sequenced M. tuberculosis isolates showed that 16 SNPs separated the most distant of the isolates in this cohort while the majority (7/9), including the isolates from the three index cases, were no more than 6 SNPs apart from each other. Despite extensive Public Health investigations and review of each case and their contacts, no epidemiological links were identified. Two of the three TB cases with the A451V mutation lived in the same town (population 30 000), while the third case lived in the same province as these cases.
Figure 1.
SNP-based analysis performed using the MTBseq v1.0.4 pipeline with minimum stringency threshold confirmed the frequency of the mutant allele at 58% in sample 1 with 88× coverage depth. The frequency of the mutation in samples 2 and 3 was 100% in all instances with coverage in that region 172× and 85×, respectively. A maximum likelihood tree was constructed with the RAxML GUI v2.0.0. The analysis parameters selected were ML + rapid bootstrap with 1000 reps and compute branch lengths selected. The resulting output file was visualized on the interactive tree of life website.
Discussion
WGS has recently become the gold standard for detecting TB transmission and identifying TB outbreaks.12 But in order to effectively translate WGS data, real-time epidemiological analysis is required. While this A451V mutation appears to be rare, no epidemiological links were identified between the three Irish-born adult cases harbouring this mutation. Furthermore, no epidemiological links could be established between the seven cases that were ≤6 SNPs apart.
WHO recommends Xpert® MTB/RIF Ultra and GenoType MTBDRplus v2.0 as initial tests to detect drug resistance prior to the initiation of appropriate therapeutic regimens.1,4 While these tests are rapid and highly sensitive, they are not considered highly specific for the diagnosis of rifampicin resistance in M. tuberculosis, so pDST remains the gold standard.3 However, phenotypic methods are time-consuming and are not without limitations. Therefore, WHO now recommends sequencing the entire rpoB gene to identify rifampicin resistance-associated mutations.3
The difference in Xpert® results between sample 1 and samples 2 and 3 was most likely due to the difference between Ultra v2 and Ultra v3 assays. The rpoB4 probe detected in all three samples covers codons 525–536, which would include the A451V mutation (codon 532). The presence of the second probe, rpoB2, which covers codons 512–525 in samples 2 and 3 suggests a mixed population that was not borne out by sequencing data.2 Sanger sequencing and WGS were re-analysed, but no further variants (silent or otherwise) could be found to explain a melting curve change in this region. Neither could the one mutation account for both melt curve changes since their regions do not overlap. Each of the tests was performed using a kit with a different lot number. Also, the WT probe (WT8) that was absent on the LPA covers codons 531–533, which again correlates with A451V and not any other variant.
The A451V mutation being associated with an Xpert® MTB/RIF Ultra report of rifampicin resistance in a clinical sample has not been reported previously. There is one report of an M. tuberculosis isolate with an A451V mutation that was phenotypically susceptible to rifampicin, but its clinical relevance was not assessed.13 In previous reports where this mutation has been found in rifampicin-resistant strains there was an accompanying high-confidence rpoB gene mutation.14 Not all rpoB mutations have the same effect on rifampicin susceptibility and some have been described as ‘disputed mutations’.15 Recently, WHO has revised the rifampicin critical concentration for pDST using the BACTEC MGIT 960 system to capture these ‘disputed mutations’. The rifampicin critical concentration was lowered from 1.0 mg/L to 0.5 mg/L.16
In recognizing the potential for discordance between identified rpoB gene mutations and phenotypic rifampicin susceptibility results a list of confidence-graded mutations associated with rifampicin resistance has been proposed; this does not include A451V.17,18 A publicly available list of mutations that do not confer phenotypic rifampicin resistance in M. tuberculosis is required and would be helpful to guide therapeutic decision-making.
The fact that novel, disputed or unknown variants could alter the melting curves of the Xpert® assay indicates that users should be aware of the possibility of false detection of rifampicin resistance, even though this is most likely a rare occurrence.19 In high TB burden countries, it may be appropriate to treat with an MDR regimen in cases where the Xpert® indicates rifampicin resistance. However, in countries where the TB burden is relatively low, the impact of false positive rifampicin resistance is more prominent and waiting for a confirmatory pDST result might be preferable. The significance of disputed or novel mutations is still debatable as there is little knowledge of the consequences of infection with M. tuberculosis strains harbouring them.20 Furthermore, the frequency of these mutations is unknown as they are likely missed when only pDST is performed.
Acknowledgements
We wish to acknowledge Paolo Miotto from the Supra-National TB Reference Laboratory based in Milan (Emerging Pathogens Unit, IRCCS Ospedale San Raffaele) for contributing rpoB primer sequences. We would also like to thank the clinicians, nursing staff and laboratory scientists of the Irish Mycobacteria Reference Laboratory and Microbiology Department, Laboratory Medicine Directorate, St James’s Hospital, Dublin, Ireland for their respective contributions to this investigation.
Contributor Information
Margaret M Fitzgibbon, Irish Mycobacteria Reference Laboratory, St James’s Hospital, Dublin, Ireland; Department of Clinical Microbiology, Trinity College Dublin, Ireland.
Emma Roycroft, Irish Mycobacteria Reference Laboratory, St James’s Hospital, Dublin, Ireland; Department of Clinical Microbiology, Trinity College Dublin, Ireland.
Gerard Sheehan, Mater Misericordiae University Hospital, Dublin, Ireland; School of Medicine, University College Dublin, Ireland.
Anne-Marie Mc Laughlin, Department of Respiratory Medicine, St James’s Hospital, Dublin, Ireland; Clinical Medicine, Trinity College Dublin, Ireland.
Keith Ian Quintyne, Department of Public Health North-East, Meath, Ireland.
Elaine Brabazon, Department of Public Health North-East, Meath, Ireland.
Mary O’Meara, Department of Public Health East, Health Service Executive, Dublin, Ireland.
Peter R Flanagan, Irish Mycobacteria Reference Laboratory, St James’s Hospital, Dublin, Ireland; Department of Clinical Microbiology, Trinity College Dublin, Ireland.
A -Louise Seagar, Scottish Mycobacteria Reference Laboratory, Edinburgh, Scotland.
Ian F Laurenson, Scottish Mycobacteria Reference Laboratory, Edinburgh, Scotland.
Joseph Keane, Department of Respiratory Medicine, St James’s Hospital, Dublin, Ireland; Clinical Medicine, Trinity College Dublin, Ireland.
Thomas R Rogers, Irish Mycobacteria Reference Laboratory, St James’s Hospital, Dublin, Ireland; Department of Clinical Microbiology, Trinity College Dublin, Ireland.
Funding
This work was supported by the IMRL and partly funded by the Department of Clinical Microbiology, Trinity College Dublin, Ireland.
Transparency declarations
Thomas R. Rogers reports personal fees from Pfizer Healthcare Ireland; grant and personal fees from Gilead Sciences; advisory board fees and educational support from Menarini Pharma; and advisory board fees from Mundi Pharma and Inserm, outside of the submitted work. All other authors: none to declare.
References
- 1.WHO. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert® MTB/RIF System: Policy Statement. 2011. https://apps.who.int/iris/handle/10665/44586. [PubMed]
- 2.Chakravorty S, Simmons AM, Rowneki M. et al. The new Xpert® MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017; 8: e00812–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. 2018. https://apps.who.int/iris/handle/10665/275469.
- 4.WHO. The Use of Molecular Line Probe Assay for the Detection of Resistance to Isoniazid and Rifampicin: Policy Update. 2016. https://apps.who.int/iris/handle/10665/250586.
- 5.Global Laboratory Initiative. Line Probe Assays for Drug Resistant TB Detection: Interpretation and Reporting Guide for Laboratory Staff and Clinicians. 2018. http://www.stoptb.org/wg/gli/assets/documents/LPA_test_web_ready.pdf.
- 6.Miotto P, Piana F, Penati V. et al. Use of genotype MTBDR assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis clinical strains isolated in Italy. J Clin Microbiol 2006; 44: 2485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Supply P, Allix C, Lesjean S. et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 2006; 44: 4498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weniger T, Krawczyk J, Supply P. et al. MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res 2010; 38: W326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allix-Béguec C, Harmsen D, Weniger T. et al. Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. J Clin Microbiol 2008; 46: 2692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohl TA, Utpatel C, Schleusener V. et al. MTBseq: a comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ 2018; 6: e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker TM, Ip CL, Harrell RH. et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis 2013; 13: 137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meehan CJ, Moig GA, Kohl TA. et al. Whole genome sequencing of Mycobacterium tuberculosis: current standards and open issues. Nat Rev Microbiol 2019; 17: 533–45. [DOI] [PubMed] [Google Scholar]
- 13.Wang H-Y, Young U, Kim S. et al. Detection of rifampicin-and isoniazid-resistant Mycobacterium tuberculosis using the Quantamatrix multiplexed assay platform system. Ann Lab Med 2018; 38: 569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson DA, Roberts SA, Bower JE. et al. Clinical failures associated with rpoB mutations in phenotypically occult multi-drug resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2012; 16: 216–20. [DOI] [PubMed] [Google Scholar]
- 15.Miotto P, Cabibbe AM, Borroni E. et al. Role of disputed mutations in the rpoB gene in interpretation of automated liquid MGIT culture results for rifampin susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2018; 56: e01599–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Technical Report on Critical Concentrations for Drug Susceptibility Testing of Isoniazid and the Rifamycins (Rifampicin, Rifabutin and Rifapentine). 2021. https://apps.who.int/iris/handle/10665/339275.
- 17.Miotto P, Tessema B, Tagliani E. et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J 2017; 50: 1701354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allix-Béguec C, Arandjelovic I, Bi L. et al. ; CRyPTIC Consortium and the 100,000 Genomes Project. Prediction of susceptibility to first line tuberculosis drugs by DNA sequencing. N Engl J Med 2018; 379: 1403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omar SV, Hillemann D, Pandey S. et al. Systematic rifampicin resistance errors with Xpert® MTB/RIF Ultra: implications for regulation of genotypic assays. Int J Tuberc Lung Dis 2020; 24: 1307–11. [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Zhang H, Fleming J. et al. Retrospective analysis of false-positive and disputed rifampin resistance Xpert® MTB/RIF assay results in clinical samples from a referral hospital in Hunan, China. J Clin Microbiol 2019; 57: e11707-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw sequence reads of all nine sequenced M. tuberculosis genomes in this study were submitted to the European Nucleotide Archive database under project accession number PRJEB43194.