Abstract
Background:
Early-onset Parkinson’s disease (EOPD), occurring between ages 40 and 55, carries social, societal, and personal consequences and may progress, with fewer comorbidities than typical, later-onset disease.
Objective:
To examine the incidence and survival of EOPD and other Parkinsonism occurring before age 55 in the population-based cohort of residents in seven Minnesota counties.
Methods:
A movement-disorder specialist reviewed all the medical records in a 2010–2015 Parkinsonism-incident cohort to confirm diagnosis and subtypes.
Results:
We identified 27 patients diagnosed at <50 years with incident Parkinsonism 2010–15: 11 (41%) cases of EOPD, 13 (48%) drug-induced Parkinsonism, and 3 (11%) other Parkinsonism and 69 incident cases of Parkinsonism <55 years of age. Overall incidence for Parkinsonism <50 years was 1.98/100,000 person-years, and for EOPD was 0.81/100,000 person-years. In patients <55 years, Parkinsonism incidence was 5.05/100,000 person-years: in EOPD, 2.05/100,000 person-years. Levodopa-induced dyskinesia was present in 45% of EOPD (both <50 years and <55 years). Onset of cardinal motor symptoms was proximate to the diagnosis of EOPD, except for impaired postural reflexes, which occurred later in the course of EOPD. Among the 69 Parkinsonism cases <55 years, 9 (13%; all male) were deceased (only 1 case of EOPD). Men had a higher mortality risk compared to women (p= 0.049).
Conclusions:
The incidence of EOPD <50 years was 0.81/100,000 person-years (1.98 in Parkinsonism all type); prior to 55 years was 2.05/100,000 person-years (5.05 in Parkinsonism all type) with higher incidence in men than women. Men with Parkinsonism, all type, had higher mortality compared to women.
Keywords: Early-onset Parkinson’s disease (EOPD), incident cohort, Rochester Epidemiology Project (REP), prevalence
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, after Alzheimer’s dementia [1]. The prevalence of PD is estimated to be around 0.3% across all ages [2; 3], and 2% after the age of 70 [4; 5]. The incidence for all ages is estimated at 8 to 18 cases per 100,000 person-years [2; 6; 7]. Prevalence and incidence of PD increase with age; however, the onset of the disease is typically around 65 to 70 years of age [8; 9].
The causes of PD are still unknown, but genetic and environmental factors have been suggested to contribute to the development of the disease [8; 10]. Unfortunately, despite major efforts to study PD genetics [10; 11], gene mutations explain a minority of patients with PD. However, an earlier onset of PD seems to be associated to a strong genetic predisposition and family history of PD [12; 13; 14].
A number of studies have used different age cut-offs to better define early-onset Parkinson’s Disease (EOPD), varying between 40 and 55 years of age [4; 14; 15; 16; 17]. Because EOPD affects people in the years of employment and fertility, it carries an additional set of social, societal and personal consequences, like early retirement [4; 18; 19; 20], sexual dysfunction [21], and psychosocial disruption. On the other hand, EOPD seemed to have a slower progression and lesser number of comorbidities, when compared to the later-onset PD (LOPD) [4].
When considering early-onset Parkinsonism of all type, there are basically no data available. Indeed, not only the clinical definition and age cut-off are unknown, but most of the studies available are mainly focused on genetic etiology [10; 22].
Because of the relative rarity of this condition, few epidemiological studies are available and the incidence and survival of EOPD is unknown in the population [4; 23]. Thus, we used the medical records-linkage system of the expanded Rochester Epidemiology Project (eREP) to study the incidence and survival of EOPD between 2010 and 2015 in a population of ~366,124 individuals that live in Olmsted County, MN, and six surrounding counties. Given the different age cut-offs for EOPD used in the literature, we used two definitions to report our results: ≤50 years of age and ≤55 years of age. In addition, as secondary goal of the study, we explored the clinical features, medications usage, and frequency of motor complications in our cohort.
METHODS
Case ascertainment
We used the eREP medical records-linkage system to identify the cases of early-onset Parkinsonism (≤55 years) among individuals who reside in Olmsted, Dodge, Freeborn, Mower, Steele, Wabasha and Waseca Counties of Minnesota [24; 25; 26; 27]. This system provides the infrastructure for indexing and linking essentially all medical information of the counties’ population. All medical diagnoses, surgical interventions, and other procedures are entered into computerized indexes using codes from the Hospital Adaptation of the International Classification of Diseases–9th Revision (H-ICDA) or the International Classification of Diseases–10th Revision (ICD-9). The eREP includes 366,124 individuals, according to the last census. The eREP is able to enlarge our radius of catchment from one county (Olmsted County: 150,000 inhabitants) to include the six additional surrounding counties (~160,000 inhabitants with 93% catchment), providing a unique resource to expand our study population without major reduction of our clinical accuracy [28].
We ascertained potential cases of Parkinsonism using a computerized screening phase and a clinical confirmation phase. In phase 1, we searched the indexes for 38 diagnostic codes potentially indicative of Parkinsonism including 5 codes for PD, 14 for Parkinsonism, 7 for tremor, 2 for extrapyramidal disorders, 5 for non-specific neurodegenerative diseases, 2 for Multiple System Atrophy, and 3 for Progressive Supranuclear Palsy. These 38 codes (see Savica et al. [29], eTable 1) were the smallest subset of codes that completely captured all cases of Parkinsonism in a previous study of the incidence of Parkinsonism performed in the Olmsted County population from 1976 to 1990 [30]. This list of 38 codes was designed to yield maximum sensitivity at the cost of low specificity.
In phase 2, a physician (E.C.) reviewed the complete records of the 7,220 patients who had a code of interest during the incidence study period or in the following 3 years using a specifically designed data abstraction computerized form for direct data entry. We extended the search for incident cases of early-onset Parkinsonism for 3 years after the incidence study period (2010–2015) to capture persons with delayed diagnoses. The physician defined the likeliness of diagnosis, the onset date and the type of Parkinsonism. A movement disorders specialist (R.S.) reviewed the complete records of all the cases that had been initially identified as possible Parkinsonism and who received at least one diagnostic code during the 6-year incidence study period or in the following 3 years, using a specifically designed data-abstraction form that was computerized for direct-data entry (clinical confirmation phase). Onset of PD was defined as the approximate date in which 1 of the 4 cardinal signs of PD was first noted by the patient, by family members, or by a care provider (as documented in the medical record). The validity of this approach is discussed and reported elsewhere [29].
We also examined the time of occurrence and clustering of the cardinal clinical features of Parkinsonism such as rest tremor, bradykinesia, rigidity, asymmetry, and impaired postural reflexes. We estimated the development of the different symptoms at any time of the disease course (we treated them as ever/never variables) and created an individual timeline of symptoms for each patient with Parkinsonism and EOPD.
Lastly, we evaluated the use of one or more of the following medications: dopamine agonists (DA), amantadine, trihexyphenidyl, and levodopa. We also investigated the temporal association between Levodopa use and the development of levodopa-induced dyskinesia (LID).
Diagnostic criteria
Our diagnosis criteria were consisting of two steps: the definition of Parkinsonism as a syndrome and the definition of types of Parkinsonism within the syndrome. Parkinsonism was defined as the presence of at least two of four cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. Among the persons fulfilling the criteria for Parkinsonism, we applied the following diagnostic criteria to classify the type of Parkinsonism. PD was diagnosed when all of the three following were present: no other causes; no documentation of unresponsiveness to levodopa at doses of at least 1 gm/day in combination of carbidopa; no prominent or early signs of more extensive nervous system involvement [29]. Dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD) were combined following the McKeith consensus guidelines [31]. Multiple system atrophy (MSA) was defined with one between: early (<1 year) and/or prominent cerebellar syndrome; early and/or prominent autonomic syndrome. Exclusion criteria were considered a marked sustained levodopa response [29; 32]. Progressive Supranuclear Palsy (PSP) was defined according to the criteria by Collins et al. [33]. Corticobasal Syndrome (CBS) was diagnosed according to the criteria by Maraganore et al. [29]. Criteria for drug-induced Parkinsonism (DIP) included all three of the following: onset within 6 months of treatment with neuroleptic or dopaminergic-depleting drug; no symptoms before treatment; resolution of symptoms within withdrawal of treatment [29]. Criteria for vascular Parkinsonism included these three criteria: abrupt onset, non-progressive or stepwise, infarct in appropriate basal ganglia on CT or MRI [29].
Our diagnostic criteria have been deemed valid as reported elsewhere [29].
Statistical analysis
We included all individuals who met criteria for Parkinsonism with symptom onset between January 1, 2010, through December 31, 2015, residing in one of the aforementioned counties at the time of symptom onset, and who provided authorization to use their medical records for research.[26] We calculated incidence rates using incident cases as the numerator and population counts from the Rochester Epidemiology Project Census as the denominator [26]. When calculating incidence of Parkinsonism and EOPD occurring before 50 years of age, eREP population was restricted to those with less than 51 years of age; whereas when calculating incidence of Parkinsonism and EOPD before 55 years of age, we restricted eREP only to those with less than 56 years of age.
Continuous features are summarized with medians and interquartile ranges (IQR); categorical features are summarized with frequency counts and percentages. We computed age- and sex-specific incidence rates for Parkinsonism overall, for specific subtypes of Parkinsonism, and for specific proteinopathies. Because our study was descriptive and involved the entire eREP population, no sampling procedures were involved [34]. A log-rank test independently compared the continuous risk of dyskinesia and mortality relative to the onset of Parkinsonism between males and females. Patients were censored at the time of last follow-up for both comparisons, with an additional censored at the time of death for the dyskinesia analysis. As the log-rank test is a univariate test, no adjustment covariates were included. The overall prevalence of dyskinesia was compared between men and women using a Fisher’s exact test. All tests were two-sided, and p-values <0.05 were considered significant. Statistical analyses were performed using R version 3.6.2.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Participating patients (or their legally authorized representatives) provided informed written consent for use of their medical information for research.
RESULTS
Demographics and incidence
From January 1, 2010, through December 31, 2015, we identified 27 (16 men, 11 women) incident cases of Parkinsonism, all types, among residents ≤50 years from the seven counties. We identified 11 (41%) cases of EOPD (6 men, 5 women), 13 (48%) of DIP (7 men, 6 women), 3 (11%) of “other Parkinsonism” (including 2 DLB/PDD and 1 case of PSP; no cases of vascular Parkinsonism, MSA, or unspecified Parkinsonism). Overall, we observed an incidence of Parkinsonism ≤50 years, all types, of 1.98 per 100,000 person-years (2.41 in men, 1.57 in women). The incidence of EOPD before 50 years of age was 0.81 per 100,000 person-years (0.90 men, 0.71 women); the incidence of DIP was 0.95 (1.05 men, 0.85 women), whereas it was 0.22 in other Parkinsonism.
When we defined early-onset Parkinsonism as ≤55 years of age, we identified 69 (50 men, 19 women) incident cases of Parkinsonism, all types. In particular, we observed 28 (41%) cases of EOPD (19 men, 9 women), 28 (41%) cases of DIP (19 men, 9 women), and 13 (19%) of “other Parkinsonism” (including 6 cases of DLB/PDD, 1 case of vascular Parkinsonism, 2 cases of MSA, 3 cases of PSP, and 1 case unspecified Parkinsonism). Overall, we observed an incidence of 5.05 per 100,000 person-years (7.53 in men, 2.71 in women) of Parkinsonism, all types, before 55 years of age. The incidence of EOPD and DIP were identical and were 2.05 per 100,000 person-years (2.86 in men, 1.28 in women). Incidence of other Parkinsonism was 0.95 per 100,000 person-years.
Medications
Among the 11 EOPD (≤50 years) patients, 10 (91%) used Levodopa (Table 1. LID occurred in 5 (50%) patients with a median time lag of 3.54 years (Q1: 1.92; Q3: 5.62) after the initiation of Levodopa (men 1.92 [Q1: 1.16; Q3: 3.77], women 5.01 [Q1: 4.27; Q3: 5.74]).
Table 1:
Usage of Levodopa
| ≤ 50 years | Females (N=5) | Males (N= 6) | Overall (N=11) |
|---|---|---|---|
| Levodopa | |||
| Yes | 5 (100%) | 5 (83%) | 10 (91%) |
| No | 0 (0%) | 1 (17%) | 1 (9%) |
| Levodopa Response* | |||
| None | 0 (0%) | 0 (0%) | 0 (0%) |
| Poor | 1 (20%) | 0 (0%) | 1 (10%) |
| Modest | 2 (40%) | 1 (20%) | 3 (30%) |
| Robust | 2 (40%) | 4 (80%) | 6 (60%) |
| ≤ 55 years | Females (N= 9) | Males (N= 19) | Overall (N= 28) |
| Levodopa | |||
| Yes | 9 (100%) | 16 (84%) | 25 (89%) |
| No | 0 (0%) | 3 (16%) | 3 (11%) |
| Levodopa response | |||
| None | 0 (0.0%) | 0 (0%) | 0 (0%) |
| Poor | 1 (11%) | 2 (13%) | 3 (12%) |
| Modest | 2 (22%) | 2 (13%) | 4 (16%) |
| Robust | 6 (67%) | 12 (75%) | 18 (72%) |
As reported in medical records by the neurologist
For EOPD (≤55 years), we observed that 25/28 (89%) patients used Levodopa (Table 1). LID occurred in 13 (52%) patients 4.57 years (Q1: 3.54; Q3: 5.30) after the initiation of Levodopa (men 4.57 (Q1: 2.82; Q3: 5.18), women 5.01 (Q1: 4.72; Q3: 5.74)).
Among the 11 EOPD (≤50 years), DAs were used by 6 (55%) patients (3 men, 3 women), amantadine was used by 1 patient (9%), and trihexyphenidyl usage was not observed.
In EOPD (≤55 years), DAs were used by 13 (46%) patients (8 men, 5 women), amantadine was taken by 5 (18%) cases (all men), and trihexyphenidyl usage was not observed.
Timeline of clinical symptoms
Cardinal motor symptoms
We reported cardinal motor symptoms whenever they were indicated at any point in the clinical notes of our patients, both during the incidence study period (2010–2015) and in any following visit to the most current date. All 11 EOPD (≤50 years) patients had a rest tremor and it preceded EOPD diagnosis by 0.66 years (Q1: 0.06; Q3: 1.35). Also bradykinesia was present in all cases; whereas rigidity was observed in 10 (91%) cases (5 men, 5 women). Impaired postural reflexes were present in 5 (45%) cases (4 men, 1 woman), and they followed EOPD diagnosis by 4.33 years (Q1: 0.99; Q3: 6.17).
Of the 28 EOPD ≤55 years: 26 (93%) had a rest tremor (18 men, 8 women) that occurred at the time of EOPD diagnosis (0.20 years; Q1: 0.00; Q3: 0.85). Bradykinesia and rigidity were reported in 25 (89%) patients (17 men, 8 women). Impaired postural reflexes were present in 17 (61%) cases, and they followed EOPD diagnosis by 1.32 years (Q1: 0.00; Q3: 5.66).
Table 2 shows frequency of each motor symptom and their time to EOPD diagnosis (in the two separate cohorts: ≤50 years and ≤55 years). Figures 1a and 1b represent individual timeline of cardinal motor symptoms in individuals with EOPD diagnosis before 50 and 55 years of age.
Table 2:
Frequency of motor symptoms, levodopa-induced dyskinesia, and time to EOPD diagnosis
| EOPD ≤ 50 years | EOPD ≤ 55 years | |||||
|---|---|---|---|---|---|---|
| Females (N= 5) | Males (N= 6) | Overall (N= 11) | Females (N= 9) | Males (N= 19) | Overall (N= 28) | |
| Tremor | ||||||
| No | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 1 (5%) | 2 (7%) |
| Minimal | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 2 (11%) | 3 (11%) |
| Yes | 5 (100%) | 6 (100%) | 11 (100%) | 7 (78%) | 16 (84%) | 23 (82%) |
| Years to EOPD diagnosis | −1.82 [−2.00; −0.75] | −0.06 [−0.40; 0.00] | −0.66 [−1.35; −0.06] | −1.02 [−1.87; −0.68] | −0.02 [−0.40; 0.00] | −0.20 [−0.85; 0.00] |
| Bradykinesia | ||||||
| No | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11%) | 2 (11%) | 3 (11%) |
| Minimal | 1 (20%) | 0 (0%) | 1 (9%) | 1 (11%) | 2 (11%) | 3 (11%) |
| Yes | 4 (80%) | 6 (100%) | 10 (91%) | 7 (78%) | 15 (79%) | 22 (79%) |
| Years to EOPD diagnosis | 0.00 [−0.62; 2.57] | 0.00 [−0.02; 0.03] | 0.00 [−0.07; 0.51] | 0.00 [−0.63; 1.53] | 0.00 [−0.02; 0.08] | 0.00 [−0.06; 0.25] |
| Rigidity | ||||||
| No | 0 (0%) | 1 (17%) | 1 (9%) | 1 (11%) | 2 (11%) | 3 (11%) |
| Minimal | 2 (40%) | 0 (0%) | 2 (18%) | 2 (22%) | 0 (0%) | 2 (7%) |
| Yes | 3 (60%) | 5 (83%) | 8 (73%) | 6 (67%) | 17 (90%) | 23 (82%) |
| Years to EOPD diagnosis | 0.00 [−0.04; 0.00] | 0.00 [−0.02; 0.02] | 0.00 [−0.04; 0.02] | 0.00 [−0.02; 0.64] | 0.00 [−0.02; 0.00] | 0.00 [−0.02; 0.00] |
| Impaired postural reflexes | ||||||
| No | 4 (80%) | 2 (33%) | 6 (55%) | 5 (56%) | 6 (32%) | 11 (39%) |
| Minimal | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Yes | 1 (20%) | 4 (67%) | 5 (45%) | 4 (44%) | 13 (68%) | 17 (61%) |
| Years to EOPD diagnosis | −0.11 [−0.11; −0.11] | 5.25 [3.50; 6.22] | 4.33 [0.99; 6.17] | −0.06 [−0.26; 0.38] | 4.33 [0.99; 5.88] | 1.32 [0.00; 5.66] |
| Asymmetry | ||||||
| No | 1 (20%) | 0 (0%) | 1 (9%) | 4 (44%) | 3 (16%) | 7 (25%) |
| Minimal | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Yes | 4 (40%) | 6 (100%) | 10 (91%) | 5 (56%) | 16 (84%) | 21 (75%) |
| Years to EOPD diagnosis | −1.29 [−1.87; −0.56] | −0.06 [−0.40; 0.00] | −0.31 [−0.85; 0.00] | −0.75 [−1.82; −0.69] | −0.08 [−0.56; 0.00] | −0.28 [−0.75; 0.00] |
| LID | ||||||
| No | 3 (60%) | 3 (50%) | 6 (55%) | 6 (67%) | 6 (32%) | 12 (43%) |
| Minimal | 1 (20%) | 0 (0%) | 1 (9%) | 1 (11%) | 1 (5%) | 2 (7%) |
| Yes | 1 (20%) | 3 (50%) | 4 (36%) | 2 (22%) | 12 (63%) | 14 (50%) |
| Years to EOPD diagnosis | 6.69 [6.65; 6.73] | 1.8 [1.12; 3.99] | 6.17 [1.80; 6.61] | 6.69 [6.65; 6.73] | 4.96 [4.14; 5.76] | 5.18 [4.57; 6.17] |
Positive values indicate that symptoms followed EOPD, whereas negative values indicate that they preceded EOPD diagnosis. EOPD= Early-onset Parkinson’s disease; LID=levodopa-induced dyskinesia
Figure 1.


a: Timeline of onset of cardinal motor symptoms in patients with EOPD diagnosis before 50 years of age; b: Patient’s timeline of cardinal motor symptoms onset relative to EOPD diagnosis before 55 years of age. EOPD=Early-onset Parkinson’s disease
Levodopa-induced Dyskinesia (LID)
In the 10 EOPD ≤50 years that were on Levodopa treatment, LID was present in 5 (50%) cases (3 men, 2 women) and followed EOPD diagnosis by 6.17 years (Q1: 1.80; Q3: 6.61), with 1.8 in men (Q1: 1.12; Q3: 3.99) and 6.69 in women (Q1: 6.65; Q3: 6.73).
Among the 25 EOPD ≤55 years using Levodopa, 13 (52%) developed LID (13 men, 3 women [p= 0.11]). Interestingly, the median time of LID after EOPD diagnosis was 5.18 (Q1: 4.57; Q3: 6.17) years. Despite there was a difference between men and women for the median time of LID after the diagnosis of EOPD (men: 4.96 years (Q1: 4.14; Q3: 5.76); women: 6.69 years (Q1: 6.65; Q3: 6.73)), the difference was non-significant (p= 0.10).
Five (18%) EOPD patients underwent deep brain stimulation (DBS) as results of motor fluctuations.
Table 2 reports LID frequency and onset relative to EOPD ≤50 years diagnosis and relative to EOPD ≤55 years diagnosis.
Survival
At the time of data extraction, 2/27 (7%) patients in the Parkinsonism cohort diagnosed before 50 years of age had died. Time from Parkinsonism to death was 5.0 and 5.5 years, and both were men. They were 1 case of DIP and 1 of PSP.
When expanding to the cohort of people with a diagnosis of Parkinsonism at ≤55 years of age, 9 (13%) patients were deceased. Death occurred 2.6 years after the diagnosis of a Parkinsonism. Median age at death was 55, and only one case was EOPD (a man who died 0.6 years following the onset of EOPD). The remaining were 5 DIP, 2 PSP, and 1 DLB/PDD. Importantly, 9/50 (18%) of the men had died, whereas no women had died. We observed that men with Parkinsonism, all types, had a greater risk of mortality than women (p= 0.049). Due to the small sample size in the EOPD cohort, no comparison could be made. Cumulative incidence of mortality and sex difference is shown in the Figure 2.
Figure 2:
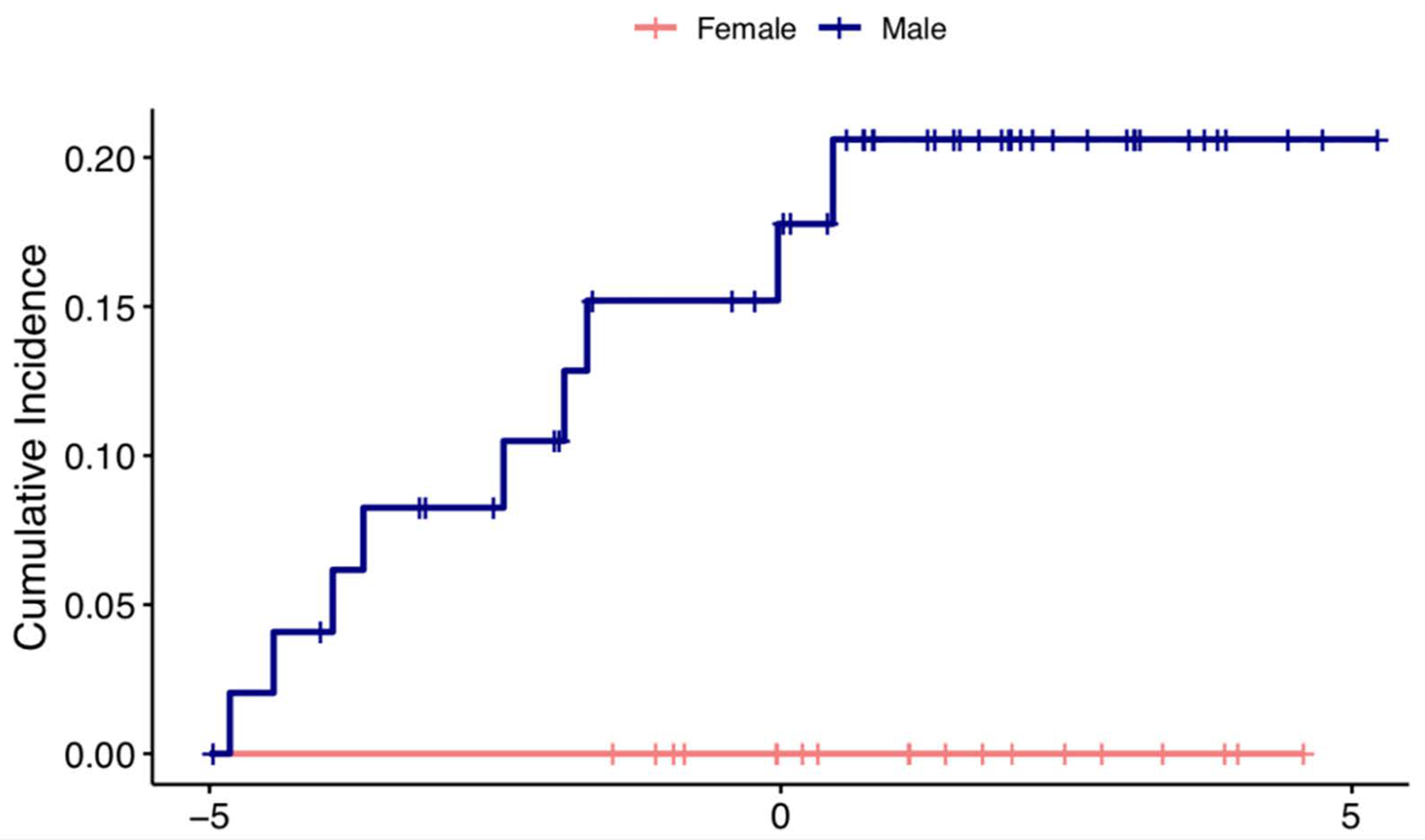
Cumulative incidence of patient mortality and sex difference in Parkinsonism, all types.
Table 3 reports the causes of death, as reported in the death certificate.
Table 3:
Causes of death, as listed in the death certificate
| De-identification number | Diagnosis | Cause of death |
|---|---|---|
| 1 | PSP | Complications of neurodegenerative disorder |
| 2 | DIP | Huntington’s disease |
| 3 | DIP | Complication of alcoholic cirrhosis |
| 4 | PSP | Respiratory failure |
| 5 | DIP | Seizure; motor vehicle accident |
| 6 | EOPD | Esophagus adenocarcinoma |
| 7 | DLB/PDD | Aspiration pneumonia; dysphagia |
| 8 | DIP | Complication of dementia (AD) |
| 9 | DIP | Panhypopituitarism following surgery |
DIP=Drug-induced Parkinsonisn; DLB=Dementia with Lewy bodies; EOPD=Early-onset Parkinson’s disease; PDD=Parkinson’s disease dementia; PSP=Progressive supranuclear palsy
DISCUSSION
In our study using the eREP (2010–2015), we observed an incidence of Parkinsonism all type (≤50 years) of 1.98 per 100,000 person-years; in the same cohort, incidence of EOPD (≤50 years) was 0.81 per 100,000 person-years. When expanding the cohort to those with an age at diagnosis below 55, incidence of Parkinsonism, all types, was 5.05 per 100,000 person-years; whereas incidence of EOPD was 2.05 per 100,000 person-years. The vast majority of the non-EOPD cases in our cohort (81% in ≤50 years and 68% in ≤55) were DIP. This result can be explained considering that DIP has a peak incidence before 40 years of age, with a decreasing incidence of 32% every 10 years [35].
Epidemiological studies addressing EOPD only are quite rare [23]. Most of the available data reporting incidence of EOPD come indirectly from PD incidence studies [29; 36; 37]. The methodologies used in these studies are quite different and not necessarily comparable to ours. One study [23] aimed to calculate incidence of EOPD only.
Incidence of EOPD (0–54 years) in the Finnish study [23] was 3.3 cases per 100,000 person-years; in a Russian study [36] it was 0.29 cases per 100,000 person-years in the 0–49 years group and 0.66 cases per 100,000 person-years in the 0–54 years group; in a Norwegian study [37] it was 1.10 cases per 100,000 person-years in the 0–49 years group.
In our study, the incidence reported was 0.81 (0–50 years) and 2.05 per 100,000 person-years (0–55 years). The discrepancy in the results we observed can be explained when considering the different methodologies, different time intervals, and the intrinsic characteristics of the populations. Our study is retrospective and based on diagnostic codes identified through the medical records-linkage system for early-onset Parkinsonism and then a manual standardized review from a physician and a movement disorders specialist. Furthermore, we included the whole population and not a clinical series/convenience series from a tertiary center[23], [36] [37]; thus, our study is not affected by selection bias. Indeed, the enrollment from a tertiary center of EOPD cases may skew the estimation of frequency because the cases will be more likely to be collected in facility with a higher ability to diagnose a rare condition such EOPD; whereas exploring the whole population greatly limits such occurrence.
When comparing our results with a previous population-based cohort of Parkinsonism collected in Olmsted County (1991–2005) with the same methodology [29], (Table 4) we observed that incidence of EOPD (and Parkinsonism, both ≤50 and ≤55 years) increased in the more recent cohort, when compared to the Parkinson cohort of Olmsted 1991–2005 (Table 4). This observation is in agreement with our previous study reporting an increased incidence of both Parkinsonism and PD in men in Olmsted county 1976–2005 [38]. The increased number of younger cases in the more recent era can be explained by a number of reasons. It is possible that we are observing a true increase of the incidence of early-onset degenerative diseases due to major changes in environmental and genetic risk factors [8]. However, it is also possible that both physicians and patients are able detect signs and symptoms of Parkinsonism earlier than in the past. Another reasons for an earlier diagnosis may be associated more inclusive diagnostic criteria and more advanced diagnostic imaging techniques [38].
Table 4.
Comparison of incidence, disease duration, and death rate in Parkinsonism in Olmsted County (1991–2005) vs Olmsted + 6 surrounding counties (2010–2015).
| 1991–2005 vs 2010–2015 | 1991–2005 vs 2010–2015 | 1991–2005 vs 2010–2015 | |
|---|---|---|---|
| Incidence (cases per 100,000 person-years) | Median disease duration (years) | Death rate | |
| Parkinsonism (≤50 years) | 1.64 vs 1.98 | 19.5 vs 5.3 | 21% vs 7% |
| Parkinsonism (≤55 years) | 3.07 vs 5.05 | 13.7 vs 2.6 | 31% vs 13% |
| EOPD (≤50 years) | 0.55 vs 0.81 | 19.6 vs NA | 38% vs 0% |
| EOPD (≤55 years) | 1.60 vs 2.05 | 19.5 vs 0.6 | 36% vs 3.6% |
Our study also identifies a number of clinical characteristics of the symptom timeline of EOPD. Around 90% of EOPD cases used Levodopa at any time of their diseases, regardless of the age cut-off considered. Although this finding reports a higher frequency of use than what previously reported [14], it can be considered in line with current guidelines of PD management and reflects the local practice of using Levodopa when needed, without delaying it in favor of other medications. Importantly, we observed a median onset of LID of 5.18 years after the diagnosis in EOPD group (≤55 years), and a one-year longer interval in EOPD (≤50 years) (6.17 years post-EOPD diagnosis), with a similar prevalence (around 50%). These findings support our previous study reporting LID in 30% of PD patients of any age but with an increased frequency in younger onset.[39] Importantly, not all the patients with EOPD developed LID, and in some cases, the severity was minimal. Interpretation of our results should take into account recent evidence [40] that LID is not influenced by the medication per se but rather by the duration of the disease at the time of Levodopa initiation. EOPD typically has a longer course than the later-onset type, which may explain the higher prevalence of LID found in younger patients [14; 39].
Five of the 28 EOPD patients (18%) with disease onset ≤55 years underwent deep brain stimulation (DBS). DBS has been used more frequently in the younger patients, due to evidence of better outcomes if performed at a younger age [41]. Particularly, it showed superiority, compared with medical therapy, in a wide subset of categories including quality of life, motor disability, activities of daily living (ADL), levodopa-induced motor complications, and time with good mobility without dyskinesia [41]. Because of the opportunity to control motor fluctuations and motor disability, DBS can substantially help patients with EOPD; however, the relative low percentage of DBS in EOPD in our cohort may be secondary to our specific practice patterns but also the fact that not all EOPD patients progress to poor motor control.
In the clinical practice, it is common to withhold Levodopa treatment when EOPD is diagnosed, due to the higher number of Levodopa-induced complications in the younger patients [4; 42; 43] and the slower neurological deterioration of this specific subgroup of PD patients [43]. In such cases, a clinician can either “watch and wait”, or can prescribe other dopaminergic medications such as DAs. In our cohort, roughly 1 out of 2 EOPD cases used such medications.
Another important clinical observation is that many of the cardinal motor symptoms (rest tremor, bradykinesia, rigidity, and impaired postural reflexes) were already occurring proximate to the diagnosis date. However, impaired postural reflexes followed EOPD diagnosis by a median of 4.33 years in EOPD (≤50 years) and 1.32 years in EOPD (≤55 years). These findings support previous reports, showing that EOPD patients do not have major balance disturbances at the onset of the disease but may develop falls relatively later in their disease course [4; 14]. Conversely, we observed a higher-than-previously-reported percentage of EOPD patients with rest tremor (all EOPD ≤50 years and 93% of EOPD ≤55 years). Previous studies reported a lower frequency of tremor in EOPD, ranging around 62% [4; 44] or lower [45; 46]. This may be due to the fact that other studies evaluated presenting or predominant motor symptoms, whereas we counted motor symptoms as ever/never reported and that many studies were based on observations from tertiary centers. On the other hand, the frequency of the number of cases reporting bradykinesia or rigidity at any time in EOPD was around 90% (except bradykinesia in EOPD ≤50 years, which was present in all the cases), similar to those previously reported [47].
At the time of data extraction, most of the patients were alive. In the ≤50 years cohort, only 2/27 (7%) were deceased. Both were men and none of them was EOPD. When expanding to all types of Parkinsonism ≤55 years, 87% were alive. Notably, all but one reported deaths were non-EOPD cases (time from Parkinsonism diagnosis to death, 2.6 years). This observation is not unexpected given that EOPD reportedly has a milder course than LOPD, characterized by a survival between 6.9 and 14.9 years after the diagnosis [48]. Stratifying by the onset of PD, age of diagnosis prior to 50 years has shown a 32-year median survival; whereas a PD diagnosis between 50 and 69 years of age has a median death 18.5 years after diagnosis. PD onset after age 70 is associated with 9.3-year disease duration [14]. Moreover, causes of death for EOPD and LOPD also differ [49].
A comparison of our cohort to previous studies is limited due to the low number of deaths. Also, when comparing our cohort with the one in Olmsted 1991–2005 (Table 4), we noticed a substantial difference in median disease duration and death rate, for both EOPD and Parkinsonism. EOPD cases in the older cohort (1991–2005) had, at time of clinical data collection (2020), a much longer follow up, compared to the newer cohort (2010–2015).
Nonetheless, all 5 DIP deaths were unrelated to Parkinsonism (Table 3), whereas among EOPD, DLB/PDD, and PSP, 3/4 (75%) parkinsonism was indicated as the main cause of death, with respiratory complication as co-leading cause (50%), which was not surprising [50; 51].
Importantly, we did not have any missing data at censoring/death time; we were able to have follow-up information of all the cases included in the survival analyses. On the other hand, unfortunately, because the relatively small sample size we could not explore any additional possible effect modifiers (for example race, socio-economic status, etc.) due to the lack of power.
Regarding sex differences, we observed a statistically significant higher risk of death in men only in Parkinsonism, all types group (p= 0.049). When we further analyze the sex difference between groups, we observed that men had a greater incidence of Parkinsonism of any kind, regardless of the age cut-off. Our results do not differ from the available data showing an increased number of PD and Parkinsonism in men compared to women [38; 52; 53; 54], with the exception of DIP [35].
Overall, we did not observe any significant and/or clinically relevant discrepancy in prevalence of rest tremor and bradykinesia between men and women; however, although not significant, men had more frequently rigidity, asymmetry and impaired postural reflexes compared to women. Notably, women with EOPD had rest tremor for a slightly longer period before EOPD diagnosis, whereas the presence of impaired postural reflexes was at time of diagnosis for women and delayed a few years for men. No sex difference was noted for dyskinesia among EOPD (≤55 years) cases (p= 0.11).
Our study has a number of strengths. First, we used the medical records-linkage system of the eREP that provides the infrastructure for storing and linking all medical information of the population of Olmsted County and the six surrounding counties. Second, all the medical records were reviewed by a movement disorder specialist (R.S.) to confirm the final diagnosis and the presence of the various subtypes of Parkinsonism and the diagnostic procedure has been validated [29]. Third, the standardized codes used to screen the seven counties’ population allowed us to detect all the incident cases of Parkinsonism during the period of interest. Fourth, we extended our extraction to 3 years following the 6-year incidence period, in order to capture all the cases with an onset in the 6-years interval that were visited and reported in the subsequent 3 years.
We also acknowledge a number of limitations. First, medical records are not standardized for research purposes; therefore, it is possible that not all the clinical information is systematically available. Second, due to the rarity of Parkinsonism before the age of 50 and particularly of EOPD, we found a low number of cases; however, with our high clinical catchment of 93%, it is unlikely that a patient with early-onset Parkinsonism would not be seen in our medical system. Third, none of these patients had a pathological confirmation of the diagnosis due to the fact that the vast majority is still alive. Fourth, imaging techniques were not consistently used for diagnosis and/or follow-up. Fifth, some cases may not yet have been recognized because of the possible unusual presentation of EOPD, and the clinical diagnosis has been delayed; however, we extended our extraction to 3 years following the 6-year incidence period, in order to catch all the cases with an onset in the 6-year interval that were visited and reported in the subsequent 3 years.
In conclusion, our results indicate that EOPD is rare disease with an incidence of 0.81 per 100,000 person-years in EOPD ≤50 years and 2.05 per 100,000 person-years in EOPD ≤55 years. Men affected by early-onset Parkinsonism all type (≤55 years) have a higher mortality than women (p= 0.049), but no survival comparisons between sexes could be made in EOPD cohort due to the low number of deaths. No significant sex differences regarding clinical symptoms have been observed in EOPD patients. LID was reported in half of EOPD, 6.17 years after diagnosis in the EOPD ≤50 group and 5.18 years after diagnosis in the EOPD ≤55 years group. Additional studies with a longer observation period and larger population are needed to confirm our findings and further elucidate the impact of EOPD in the population. In addition, a consensus definition of the age cut-off of EOPD is needed to harmonize the available and future studies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lea Dacy, Mayo Clinic Department of Neurology, for proofreading and formatting of this article. This study was partially funded by Acadia Pharmaceuticals, Inc. The funder had no role in the conception, design, or writing of this study.
Disclosures/Conflicts of Interest:
Dr. Savica received unrestricted research grant support from Acadia Pharmaceuticals, Inc. during the research and preparation of this manuscript.
Funding Source:
This study was supported in part by Acadia Pharmaceuticals, Inc.
FINANCIAL DISCLOSURES
Messrs. Stang, Mullan, and Martin and Drs. Camerucci, Hajeb, and Turcano report no disclosures. Dr. Mielke receives funding from the National Institutes of Health, and unrestricted research grants from Biogen and Lundbeck; she has consulted for Lysosomal Therapeutics, Inc, and Eli Lilly. Dr. Ross receives support from the National Institute of Neurological Disorders and Stroke, the U.S. Department of Defense, and the Michael J. Fox Foundation for Parkinson’s Research. Dr. Bower receives support from the Parkinson’s Disease Foundation. Dr. Savica receives support from the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, the Parkinson’s Disease Foundation, and Acadia Pharmaceuticals.
REFERENCES
- [1].Erkkinen MG, Kim MO, Geschwind MD (2018) Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].de Lau LML, Breteler MMB (2006) Epidemiology of Parkinson’s disease. The Lancet Neurology, 5 525–535. [DOI] [PubMed] [Google Scholar]
- [3].Wirdefeldt K, Adami H-O, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. European Journal of Epidemiology, 26 1. [DOI] [PubMed] [Google Scholar]
- [4].Mehanna R, Jankovic J (2019) Young-onset Parkinson’s disease: Its unique features and their impact on quality of life. Parkinsonism Relat Disord, 65 39–48. [DOI] [PubMed] [Google Scholar]
- [5].Schrag A, Ben-Shlomo Y, Quinn NP (2000) Cross sectional prevalence survey of idiopathic Parkinson’s disease and Parkinsonism in London. BMJ, 321 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kalia LV, Lang AE (2015) Parkinson’s disease. The Lancet, 386 896–912. [DOI] [PubMed] [Google Scholar]
- [7].Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol, 157 1015–1022. [DOI] [PubMed] [Google Scholar]
- [8].Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol, 15 1257–1272. [DOI] [PubMed] [Google Scholar]
- [9].Bower JH, Maraganore DM, McDonnell SK, Rocca WA (2000) Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson’s disease. Mov Disord, 15 819–825. [DOI] [PubMed] [Google Scholar]
- [10].Al-Rumayyan A, Klein C, Alfadhel M (2017) Early-Onset Parkinsonism: Case Report and Review of the Literature. Pediatr Neurol, 67 102–106 e101. [DOI] [PubMed] [Google Scholar]
- [11].Puschmann A (2017) New Genes Causing Hereditary Parkinson’s Disease or Parkinsonism. Curr Neurol Neurosci Rep, 17 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deng H, Wang P, Jankovic J (2018) The genetics of Parkinson disease. Ageing Res Rev, 42 72–85. [DOI] [PubMed] [Google Scholar]
- [13].Xiao B, Deng X, Ng EY, Allen JC Jr., Lim SY, Ahmad-Annuar A, Tan EK (2018) Association of LRRK2 Haplotype With Age at Onset in Parkinson Disease. JAMA neurology, 75 127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC (2014) Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord, 20 530–534. [DOI] [PubMed] [Google Scholar]
- [15].Schrag A, Schott JM (2006) Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. The Lancet Neurology, 5 355–363. [DOI] [PubMed] [Google Scholar]
- [16].Schrag A, Jahanshahi M, Quinn N (2000) What contributes to quality of life in patients with Parkinson’s disease? Journal of neurology, neurosurgery, and psychiatry, 69 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rana AQ, Siddiqui I, Yousuf MS (2012) Challenges in diagnosis of young onset Parkinson’s disease. J Neurol Sci, 323 113–116. [DOI] [PubMed] [Google Scholar]
- [18].Schrag A, Banks P (2006) Time of loss of employment in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society, 21 1839–1843. [DOI] [PubMed] [Google Scholar]
- [19].Murphy R, Tubridy N, Kevelighan H, O’Riordan S (2013) Parkinson’s disease: how is employment affected? Ir J Med Sci, 182 415–419. [DOI] [PubMed] [Google Scholar]
- [20].Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M (2003) Young- versus older-onset Parkinson’s disease: impact of disease and psychosocial consequences. Movement disorders : official journal of the Movement Disorder Society, 18 1250–1256. [DOI] [PubMed] [Google Scholar]
- [21].Bhattacharyya KB, Rosa-Grilo M (2017) Sexual Dysfunctions in Parkinson’s Disease: An Underrated Problem in a Much Discussed Disorder. Int Rev Neurobiol, 134 859–876. [DOI] [PubMed] [Google Scholar]
- [22].Rohe CF, Montagna P, Breedveld G, Cortelli P, Oostra BA, Bonifati V (2004) Homozygous PINK1 C-terminus mutation causing early-onset parkinsonism. Annals of neurology, 56 427–431. [DOI] [PubMed] [Google Scholar]
- [23].Ylikotila P, Tiirikka T, Moilanen JS, Kääriäinen H, Marttila R, Majamaa K (2015) Epidemiology of early-onset Parkinson’s disease in Finland. Parkinsonism & Related Disorders, 21 938–942. [DOI] [PubMed] [Google Scholar]
- [24].St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA (2012) Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc, 87 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA (2012) Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol, 41 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA (2011) Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol, 173 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd (2012) History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc, 87 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rocca WA, Grossardt BR, Brue SM, Bock-Goodner CM, Chamberlain AM, Wilson PM, Finney Rutten LJ, St Sauver JL (2018) Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol, 47 368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013) Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol, 70 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bower JH, Maraganore DM, McDonnell SK, Rocca WA (1999) Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology, 52 1214–1214. [DOI] [PubMed] [Google Scholar]
- [31].McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology, 47 1113–1124. [DOI] [PubMed] [Google Scholar]
- [32].Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P, Litvan I, Mathias C, Oliver E, Robertson D, Schatz I, Wenning G (1998) Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin Auton Res, 8 359–362. [DOI] [PubMed] [Google Scholar]
- [33].Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM (1995) Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry, 58 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anderson DW, Mantel N (1983) On epidemiologic surveys. Am J Epidemiol, 118 613–619. [DOI] [PubMed] [Google Scholar]
- [35].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Mielke MM, Rocca WA (2017) Incidence and time trends of drug-induced parkinsonism: A 30-year population-based study. Mov Disord, 32 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Winter Y, Bezdolnyy Y, Katunina E, Avakjan G, Reese JP, Klotsche J, Oertel WH, Dodel R, Gusev E (2010) Incidence of Parkinson’s disease and atypical parkinsonism: Russian population-based study. Mov Disord, 25 349–356. [DOI] [PubMed] [Google Scholar]
- [37].Alves G, Muller B, Herlofson K, HogenEsch I, Telstad W, Aarsland D, Tysnes OB, Larsen JP, Norwegian ParkWest study g (2009) Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry, 80 851–857. [DOI] [PubMed] [Google Scholar]
- [38].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2016) Time Trends in the Incidence of Parkinson Disease. JAMA Neurol, 73 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Turcano P, Mielke MM, Bower JH, Parisi JE, Cutsforth-Gregory JK, Ahlskog JE, Savica R (2018) Levodopa-induced dyskinesia in Parkinson disease: A population-based cohort study. Neurology, 91 e2238–e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, Cereda E, Fabbri M, Adjei P, Akassi J, Bonetti A, Pezzoli G (2014) The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain, 137 2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, Group ES (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med, 368 610–622. [DOI] [PubMed] [Google Scholar]
- [42].Jankovic J (2000) Parkinson’s disease therapy: tailoring choices for early and late disease, young and old patients. Clin Neuropharmacol, 23 252–261. [DOI] [PubMed] [Google Scholar]
- [43].Kostic VS (2009) Treatment of young-onset Parkinson’s disease: role of dopamine receptor agonists. Parkinsonism Relat Disord, 15 Suppl 4 S71–75. [DOI] [PubMed] [Google Scholar]
- [44].Wickremaratchi MM, Knipe MD, Sastry BS, Morgan E, Jones A, Salmon R, Weiser R, Moran M, Davies D, Ebenezer L, Raha S, Robertson NP, Butler CC, Ben-Shlomo Y, Morris HR (2011) The motor phenotype of Parkinson’s disease in relation to age at onset. Movement disorders : official journal of the Movement Disorder Society, 26 457–463. [DOI] [PubMed] [Google Scholar]
- [45].Friedman A (1994) Old-onset Parkinson’s disease compared with young-onset disease: clinical differences and similarities. Acta neurologica Scandinavica, 89 258–261. [DOI] [PubMed] [Google Scholar]
- [46].Giovannini P, Piccolo I, Genitrini S, Soliveri P, Girotti F, Geminiani G, Scigliano G, Caraceni T (1991) Early-onset Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society, 6 36–42. [DOI] [PubMed] [Google Scholar]
- [47].Pagano G, Ferrara N, Brooks DJ, Pavese N (2016) Age at onset and Parkinson disease phenotype. Neurology, 86 1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Macleod AD, Taylor KS, Counsell CE (2014) Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord, 29 1615–1622. [DOI] [PubMed] [Google Scholar]
- [49].Hoogland J, Post B, de Bie RMA (2019) Overall and Disease Related Mortality in Parkinson’s Disease - a Longitudinal Cohort Study. J Parkinsons Dis, 9 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hobson P, Meara J (2018) Mortality and quality of death certification in a cohort of patients with Parkinson’s disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open, 8 e018969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pinter B, Diem-Zangerl A, Wenning GK, Scherfler C, Oberaigner W, Seppi K, Poewe W (2015) Mortality in Parkinson’s disease: a 38-year follow-up study. Mov Disord, 30 266–269. [DOI] [PubMed] [Google Scholar]
- [52].Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA (2013) Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA neurology, 70 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Boeve BF, Graff-Radford J, Rocca WA, Mielke MM (2017) Survival and Causes of Death Among People With Clinically Diagnosed Synucleinopathies With Parkinsonism: A Population-Based Study. JAMA neurology, 74 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stang CD, Turcano P, Mielke MM, Josephs KA, Bower JH, Ahlskog JE, Boeve BF, Martin PR, Upadhyaya SG, Savica R (2020) Incidence and Trends of Progressive Supranuclear Palsy and Corticobasal Syndrome: A Population-Based Study. J Parkinsons Dis, 10 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


