Abstract
Background:
Prognostic tools, such as risk calculators, improve the patient-physician informed decision making process. These tools are limited for breast cancer patients when assessing surgical complication risk pre-operatively. Here we aimed to assess predictors associated with acute postoperative complications for breast cancer patients and then develop a predictive model that calculates a complication probability using patient risk factors.
Methods:
We performed a retrospective cohort study using the NSQIP database from 2005–2017. Women diagnosed with ductal carcinoma in situ or invasive breast cancer who underwent either breast conservation or mastectomy procedures were included in this predictive modeling scheme. Four models were built using logistic regression methods to predict the following composite outcomes: overall, infectious, hematologic, and internal organ complications. Model performance, accuracy and calibration measures during internal/external validation included area under the curve, the brier score and Hosmer-Lemeshow statistic; respectively.
Results:
A total of 163,613 women met inclusion criteria. Area under the curve for each model was: Overall 0.70, Infectious 0.67, Hematologic 0.84, and Internal Organ 0.74. Brier scores were all between 0.04–0.003. Model calibration using the Hosmer- Lemeshow statistic found all p-values >0.05. Using model coefficients, individualized risk can be calculated on the web-based breast cancer surgical risk calculator (BCSRc) platform; www.breastcalc.org.
Conclusion:
We developed an internally and externally-validated risk calculator that estimates a breast cancer patient’s unique risk of acute complications following each surgical intervention. Preoperative use of the BCSRc can potentially help stratify patients with an increased complication risk and improve expectations during the decision making process.
INTRODUCTION
Breast surgery is one of the most common general surgical procedures performed in the United States (U.S.) and breast cancer is the most common cancer diagnosis in women (1, 2). Over the last two decades, surgery to treat patients with breast cancer in the U.S. has changed as traditional breast procedures, such as partial mastectomy and mastectomy procedures without reconstruction, have been decreasing while the rate of breast cancer reconstructive procedures have been increasing (3–9). Despite the shift in surgical options, complications persist in breast surgery and can markedly vary according to the type of reconstruction, risk profiles and duration of follow-up (10–13). Fortunately, mortality in breast surgery remains very low (< 1%) regardless of the type of surgery offered (14). There are several established risk factors associated with post-operative complications for general surgery procedure which include smoking, prior radiation, obesity, diabetes, and higher ASA class (American Society of Anesthesiologists classification) (11, 13). Among women undergoing breast surgery, many patient factors and surgical predictors thought to influence acute postoperative complications are unknown or controversial (11, 13, 15, 16). Breast surgery is a sub-specialty of surgery, with unique differences that include patient demographics (i.e. majority women), external vs. internal surgery, semi-elective surgery, anesthesia options, length of stay and lower re-operation rates (17–19). Thus, constructing a predictive model for patients undergoing breast cancer surgery is unique to the types of complications encountered by these patients rather than those undergoing non-breast surgical procedures. A predictive model could support the surgical decision making process which can be overwhelming and raise anxiety in an already vulnerable patient population psychological stressed with a diagnosis of cancer.
A number of institutional studies describe complications following breast surgery and benchmarked guidelines that surgeons use today (3, 7, 10, 13, 15, 16, 20–25). However, there is no prediction model available that estimates post-operative complications that are specific for breast cancer patients that can calculate an individual’s risk probability. Current predictive models lack generalizability to patients with breast cancer by focusing on individual complications, targeting one surgical group, such as the BRA (breast reconstruction risk assessment) score, or only encompassing one diagnostic patient cohort (26–30). In addition, many studies have suffered from small sample size, lack the power to adequately analyze the multiple covariates influencing acute complications, or have applied appropriate model performance measures (12, 26–31). Surgeons are in need of a risk calculator to provide objective estimates based on individual risk profiles to support shared decision making. Using data from the National Surgical Quality Improvement Program (NSQIP), we constructed an internally and externally validated a series of prediction models (The Breast Cancer Surgery Risk Calculator (BCSRc)) to estimate risk of four categories of post-operative complications for women undergoing five common breast cancer surgical procedures.
METHODS
Study Design:
We assembled a retrospective cohort from the American College of Surgeons NSQIP including all available participant user files (PUF) from 2005 to 2017. In November, 2019 we acquired the 2018 PUF for external validation purposes. We identified all patients who underwent breast interventions based on our inclusion and exclusion criteria (Table I). The NSQIP database collects prospective patient data for 30-days post-operatively and post-operative complications with a primary focus of improving surgical outcomes, thus if a complication arises within 30 days it was recorded. The Institutional Review Board’s authorization at Tufts Medical Center was obtained in August, 2018 prior to use of the database.
Table I:
Criteria for Model Development and Validation
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
| |
| • Patients who undergo surgery from 2005 to 2017 • Age: Over 18 • Female Sex • Diagnosis of breast cancer • Admitted under General or Plastic Surgery |
• Unknown or Male sex • Diagnosis: - Cosmetic Surgery, - Benign Breast Disease • Incorrect diagnosis - Not breast related or uncertain diagnosis • Diagnosis of previous breast surgery with current complication |
Patient Cohort from NSQIP:
Post-operative diagnoses were classified according to International Classification of Diseases Ninth Revision (ICD-9) and ICD-10 codes for invasive breast cancer (IvBC) and ductal carcinoma in situ (DCIS). We excluded patients with a diagnosis of benign breast disease or cosmetic surgery. All five surgical intervention groups were categorized using Current Procedural Terminology (CPT) codes. These five surgical groups were: a partial mastectomy, oncoplastic surgery, mastectomy alone, mastectomy with implant or tissue expander reconstruction, or mastectomy with autologous tissue reconstruction. Supplemental Appendix (SA) Table 1 provides the CPT and ICD codes used in our analysis. Patient categorization and outcomes were conducted in the same manner for the development and validation cohort.
Complications:
We identified 16 acute complications in the NSQIP database that were collected prospectively during a 30-day post-operative period. We categorized complications into three composites based on their medical similarities. This was done to minimize bias associated with competing risk of complications associated between composite groups. SA-Table 2 shows categorization of complications into composite outcome groups.
Statistical Analysis:
Predictor selection and missing data:
Patient baseline demographics and surgical predictors were collected based on practicality for a preoperative predictive model; shown in Table IIa-b. The entire cohort had 5% missing data, thus 10 imputed datasets were constructed using multiple imputation techniques. A logistic regression model was fit to each of the imputed datasets. For comparison, two methods were used for bidirectional variable selection, considering 23 predictor variables and 8 interaction terms (SA: Variable Selection). Five a priori covariates were forced into the model, aside from surgery type, that are known to be associated with surgical complications including: smoking, body mass index (BMI), diabetes mellitus and glucocorticoid use. In addition, the following interaction terms were considered: surgery type with admission status, diagnosis, surgeon specialty, patient age, current smoking status; diagnosis with axillary surgery or stage 4 metastatic cancer; and lastly chronic obstructive pulmonary disease (COPD) with dyspnea. Variance inflation factor tested for variable multicollinearity after a final logistic regression model was fit to each imputed dataset.
Table IIa:
Baseline Study Population Characteristics in Model Development and Validation
| Breast Cancer Patients Cohorts | Development | Validation | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | Female | 163613 | 100 | 28584 | 100 |
| Age (Years) | Mean (s.d.) | 46 | 12.8 | 44 | 12.4 |
| Race | White | 117378 | 71.7 | 19262 | 67.4 |
| AA/Black | 17488 | 10.7 | 2914 | 10.2 | |
| Native | 702 | 0.4 | 110 | 0.4 | |
| Asian | 8031 | 4.9 | 1589 | 5.6 | |
| Unknown | 20014 | 12.2 | 4709 | 16.5 | |
| Ethnicity | Hispanic | 9750 | 6.0 | 1794 | 6.3 |
| Unknown | 18272 | 11.1 | 4261 | 15.0 | |
| Body mass index | Mean (s.d.) | 29.0 | 7.13 | 29.4 | 7.18 |
| Smoking Status | Yes | 18989 | 11.6 | 2890 | 10.1 |
| Adjuvant Chemotherapy | Yes | 50499 | 26.5 | n/a | n/a |
| No | 2649 | 1.4 | n/a | n/a | |
| Unknown | 137339 | 72.1 | n/a | n/a | |
| Glucocorticoid Use | Yes | 3713 | 2.3 | 630 | 2.2 |
| Recent Unintentional Weight Loss | Yes | 652 | 0.4 | 125 | 0.4 |
| Functional Status | Independent | 161768 | 98.9 | 28138 | 98.4 |
| Partially Dependent | 1640 | 1.0 | 229 | 0.8 | |
| Fully Dependent | 205 | 0.1 | 35 | 0.1 | |
| Unknown | 0 | 0 | 182 | 0.6 | |
| Diabetes | Yes – Insulin | 5973 | 3.7 | 1043 | 3.6 |
| Yes – Oral | 14571 | 8.9 | 2769 | 9.7 | |
| No | 143069 | 87.4 | 24772 | 86.7 | |
| Hypertension | Yes | 69783 | 42.7 | 12241 | 42.8 |
| Dyspnea | At Rest | 400 | 0.2 | 79 | 0.3 |
| Moderate Exertion | 4893 | 4.8 | 1161 | 4.1 | |
| None | 155320 | 94.9 | 27344 | 95.7 | |
| Chronic obstructive pulmonary disease | Yes | 4600 | 2.8 | 785 | 2.7 |
| Chronic Heart Failure | Yes | 486 | 0.3 | 82 | 0.3 |
| On Anticoagulation Medication | Yes | 2532 | 1.5 | 416 | 1.5 |
| Diagnosis | In Situ Breast Cancer | 31000 | 18.9 | 6130 | 21.4 |
| Malignant Breast Cancer | 132613 | 81.1 | 22454 | 78.5 | |
| Metastatic Stage 4 Cancer | Yes | 3230 | 2.0 | 514 | 1.8 |
| Admission Status | Inpatient | 51739 | 31.6 | 6158 | 21.5 |
| Outpatient | 111874 | 68.4 | 22426 | 78.5 | |
| Admission to hospital from: transfer status | Directly from home | 162915 | 99.6 | 28394 | 99.3 |
| Nursing or Intermediate care | 599 | 0.4 | 71 | 0.3 | |
| Other | 99 | 0.1 | 25 | 0.1 | |
| Unknown | 0 | 0 | 94 | 0.3 | |
Table IIb:
Baseline Study Population Characteristics in Model Development and Validation
| Breast Cancer Patients Cohorts | Development | Validation | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Admitting Primary Surgeon | General Surgeon | 147780 | 90.3 | 25600 | 89.6 |
| Plastic Surgeon | 15833 | 9.7 | 2984 | 10.4 | |
| Admission Quarter | January 1 - March 31 | 41442 | 25.3 | 7499 | 26.2 |
| April 1 - June 30 | 39059 | 23.9 | 6901 | 24.1 | |
| July 1 - September 30 | 41824 | 25.6 | 6639 | 23.2 | |
| October 1 - December 31 | 41288 | 25.2 | 7545 | 26.4 | |
| Surgery Type | Partial Mastectomy | 67922 | 41.5 | 13677 | 47.8 |
| Oncoplastic Surgery | 10162 | 6.2 | 1399 | 4.9 | |
| Mastectomy Alone | 45690 | 20.7 | 7111 | 24.9 | |
| Mastectomy with Implant | 33865 | 20.7 | 5602 | 19.6 | |
| Mastectomy with Muscular Flap | 5974 | 3.7 | 795 | 2.0 | |
| Axillary Lymph Node Management | None SLNBx |
61343 76128 |
37.5 46.5 |
11253 14288 |
39.3 50.0 |
| ALNDx | 26142 | 16.0 | 3063 | 10.7 | |
| Anesthesia Type | General | 153020 | 93.5 | 26750 | 93.6 |
| Monitored Anesthesia Care | 9569 | 5.8 | 1722 | 6.0 | |
| Other: Spinal, Local or Regional block | 1024 | 0.6 | 112 | 0.4 | |
| Foreign Body Placement | Yes | 954 | 0.6 | 110 | 0.4 |
| Any Complication | Yes | 8797 | 5.4 | 1217 | 4.3 |
| Infectious Complication | Yes | 6239 | 3.8 | 927 | 3.2 |
| Hematologic Complication | Yes | 2107 | 1.3 | 256 | 0.9 |
| Internal Organ Complication | Yes | 639 | 0.4 | 81 | 0.3 |
Risk-Model Performance and Validation:
Each model was internally-validated by bootstrapping techniques, 300 times, on each imputed dataset and estimates were averaged. Optimism-corrected c-statistic was used to adjust and recalibrate the models for any estimated deterioration when fit to a new cohort of patients. Each bootstrapped model was externally validated on the 2018 NSQIP PUF breast cancer cohort. On each step, the area under the curve (AUC) assessed discrimination and Hosmer-Lemeshow test (HLT) statistic computed model calibration. In addition, a calibration plot of observed versus expected complications, visually demonstrated each models calibration. The Brier Score assessed model accuracy and ranges from 0–1 (0 for an ideal model) (32).
Risk Calculator Development:
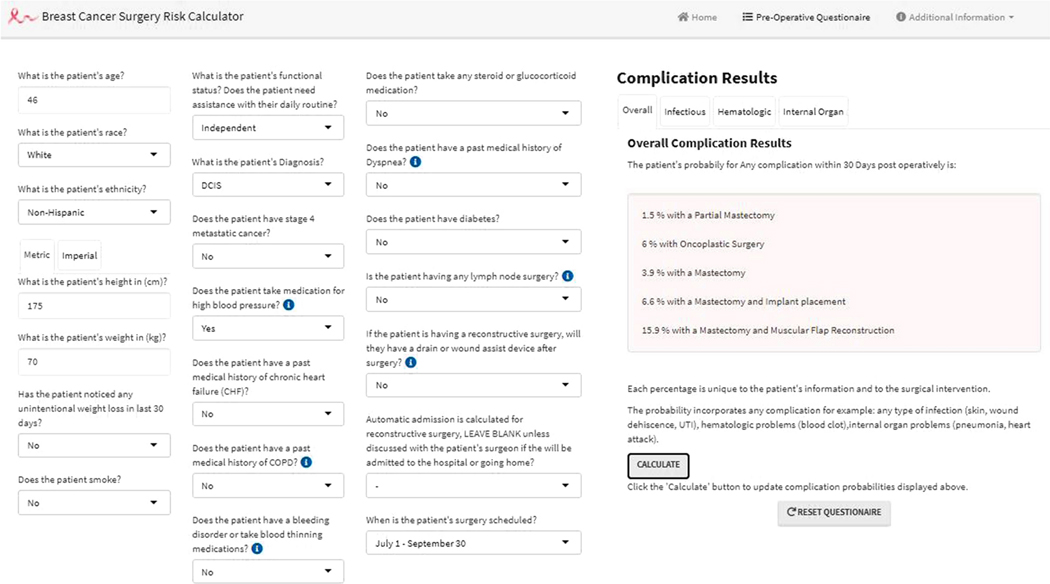
The four validated models were used to construct a risk calculator that returns an estimated probability for acquiring a complication for each surgery type. An individualized predicted probability of acquiring a complication can be calculated using the inverse logit function: probability = 1/ (1+e−B), where B, Beta, is the y-intercept and all the covariates unique to the patient. Using individualized patient- and surgery- specific risk factors, the interactive calculator inputs five Betas; one for each intervention and complication subgroup (Figure I). BCSRc is available at www.breastcalc.org. R studio version 3.5 was used to perform all analyses. Additional, statistical methods and R Studio related information is shown in the SA.
Figure I:
A screen shot of the BCSRc online platform that uses individual patient risk factors inputs on the left and after pressing the calculate button the results will be shown on the right side; hypothetical patient example shown. Scrolling between complication tabs will save patient information and show complication subgroup results.
RESULTS
Study Population Characteristics:
A total of 163,613 patients were identified in the NSQIP database that met our pre-defined inclusion criteria and were used to develop the four models; SA-Figure 1 attrition diagram. The 2018 cohort utilized to externally validate the model included 28,584 patients. Patient demographics, comorbidities and surgical characteristics were very similar between the development and validation cohorts; Table IIa-b. The observed complication incidence in the development cohort were as follows, overall 5.4%, infectious 3.8%, hematologic 1.3%, and internal organ complication 0.4%.
Model Development:
Amongst all the data fields in the entire cohort, there was 5% missing data. The variables with the most extensive missingness were race (27%) and adjuvant chemotherapy (72%). Adjuvant chemotherapy was excluded from our analysis due to >50% missing data. The entire dataset was imputed 10 times using multiple imputation to fill in missing data. A multivariable logistic regression model was developed after variable selection and concatenated from the 10 imputed datasets. The covariates used in variable selection included predictors that could be assessed preoperatively: age, race, ethnicity, BMI, smoking status, glucocorticoid or anticoagulation use, unintentional weight loss, diabetes mellitus (DM), hypertension (HTN), dyspnea, COPD, chronic heart failure (CHF), diagnosis, stage 4 metastatic cancer, surgeon specialty, type of anesthesia, axillary lymph node management, pre-operative functional status, anesthesia type, transfer status, admission status, and admission quarter. The covariates including, the y-intercept and B-values, for each model differ and are shown in SA-Table 2a-d.
Model Performance:
Performance and validation measures are shown in Table III for each complication composite. Model discrimination using AUC was stable across bootstrapping and external validation; yielding good reliability and predictive power. The bootstrapped models tested on the external cohort presented AUC that were: Overall 0.70 (95% CI: 0.68–0.72), Infectious 0.67 (95% CI: 0.66–0.69), Internal Organ 0.74 (95% CI: 0.69–0.79) and Hematologic 0.84 (95% CI: 0.82–0.87). Model accuracy, evaluated by the Brier Score, returned improvement across all complication composites when comparing the development cohort to the external cohort: Overall 0.05 to 0.04, Infectious 0.04 to 0.03, Internal Organ 0.006 to 0.003 and Hematologic 0.012 to 0.009. Internally, the HLT showed that the four models were calibrated well with all p-values above 0.05; Overall 0.21, Infectious 0.25, Internal Organ 0.14 and Hematologic 0.44. The four models retained good discrimination and accuracy on the external cohort but the calibration changed marginally. However, with good discriminative ability and accuracy, the models were recalibrated on the external cohort and the calibration improved substantially. Calibration plots and ROC curves on the cohorts were generated; displayed in SA-Figure 2-4.
Table III.
Performance and Calibration Measures
| Development Cohort | External Validation Cohort | |||
|---|---|---|---|---|
| Apparent Model | Internal Validation Bootstrapped Model | External Validated | Recalibrated model | |
| Overall Complication | ||||
| C-Statistic | 0.72 | 0.72 | 0.70 | 0.70 |
| Slope | 1.00 | 0.99 | 0.91 | 1.00 |
| Intercept | 0.00 | −0.02 | −0.33 | 0.00 |
| Brier Score | 0.05 | 0.05 | 0.04 | 0.04 |
| HLT | 0.21 | 0.21 | 0.02 | 0.24 |
|
| ||||
| Infectious Complication | ||||
| C-Statistic | 0.70 | 0.67 | 0.67 | 0.67 |
| Slope | 1.00 | 0.99 | 0.95 | 1.00 |
| Intercept | 0.00 | −0.04 | −0.19 | 0.00 |
| Brier Score | 0.04 | 0.04 | 0.03 | 0.03 |
| HLT | 0.25 | 0.25 | 0.51 | 0.60 |
|
| ||||
| Internal Organ Complication | ||||
| C-Statistic | 0.81 | 0.80 | 0.74 | 0.74 |
| Slope | 1.00 | 0.98 | 0.88 | 1.00 |
| Intercept | 0.00 | −0.08 | −0.67 | 0.00 |
| Brier Score | 0.006 | 0.006 | 0.004 | 0.003 |
| HLT | 0.14 | 0.14 | 0.84 | 0.97 |
|
| ||||
| Hematologic Complication | ||||
| C-Statistic | 0.84 | 0.84 | 0.84 | 0.84 |
| Slope | 1.00 | 0.98 | 0.96 | 1.00 |
| Intercept | 0.00 | −0.06 | −0.31 | 0.00 |
| Brier Score | 0.01 | 0.01 | 0.009 | 0.009 |
| HLT | 0.44 | 0.44 | 0.42 | 0.86 |
HLT: Hosmer Lemeshow test statistic; C-Statistic: concordance statistic or area under the curve
Risk Calculator:
All four models served as a foundation to construct the risk calculator which is accessible at www.breastcalc.org. The variability in predicting a complication solely depends on a patients risk profile. The online platform, shown in Figure I, represents the interactive website appearance and how patients can input their demographic information. We recommend following national guidelines for axillary surgery therefore we automatically input axillary management for patients diagnosed with IvBC (sentinel lymph node biopsy, at a minimum), whereas patients with DCIS do not undergo lymph node management; surgeon discretion to change input if needed. The majority of patients undergoing reconstructive surgeries are admitted overnight for monitoring purposes and are referred to as inpatient here but if surgeons practice outpatient reconstructive surgery that input is modifiable. Using the risk calculator, we illustrate two hypothetical patients with differing risk profiles presenting at a surgical consultation and their complication probabilities for each surgical intervention, Table IV. Patient 1 is a typical, “low risk,” surgical patient with few risk factors that would be concerning for complications post-operatively. Her risk profile suggests an overall 1.5% risk probability if a partial mastectomy was chosen but if reconstruction is desired, oncoplastic surgery offers the lowest risk probability at 6.0% when compared to other mastectomy reconstructive procedures. Patient 2 has a more significant past medical history and is interested in a reconstructive operation. Collectively, patient 2 has known complication risk factors including smoking, inpatient care and obesity, increasing the risk for infectious and hematologic complications (11, 13, 14, 21, 33). Her risk ranges between 3.3 – 21.8%, for the five surgical interventions. Her probability for a reconstructive surgery complication is three times higher compared to a partial mastectomy; lowest with Oncoplastic surgery at 9.4% or 10.9 % with Mastectomy with implant reconstruction; to minimize complications smoking cessation would be recommended (smokers have a 145% higher odds of complications, SA-Table2a).
Table IV.
Estimated Risk for a Complication in Two Hypothetical Patients
| Patient 1: 46 y/o white female with PMH of HTN, normal BMI, presenting with DCIS | Complication Composite | |||
|---|---|---|---|---|
| Infectious | Hematologic | Internal Organ | Overall | |
| Partial Mastectomy | 1.2 % | 0.1 % | 0.1 % | 1.5 % |
| Oncoplastic Surgery* | 3.5 % | 2.0 % | 0.4 % | 6.0 % |
| Mastectomy Alone | 3.1 % | 0.6 % | 0.2 % | 3.9 % |
| Mastectomy Implant Reconstruction* | 4.7 % | 1.6 % | 0.4 % | 6.6 % |
| Mastectomy with Muscular Flap Reconstruction* | 7.0 % | 9.6 % | 0.8 % | 15.9 % |
|
| ||||
| Patient 2: 66 y/o white female with PMH of obesity, COPD, smoking, presenting with IvBC | Complication Composite | |||
| Infectious | Hematologic | Internal Organ | Overall | |
| Partial Mastectomy | 2.7 % | 0.2 % | 0.4 % | 3.3 % |
| Oncoplastic Surgery* | 6.3 % | 1.9 % | 1.1 % | 9.4 % |
| Mastectomy Alone | 5.3 % | 0.5 % | 0.5 % | 6.3 % |
| Mastectomy Implant Reconstruction* | 8.2 % | 1.6 % | 1.1 % | 10.9 % |
| Mastectomy with Muscular Flap Reconstruction* | 13.6 % | 6.0 % | 2.2 % | 21.8 % |
Reconstructive procedures are treated as inpatient; PMH, past medical history; HTN, hypertension; BMI, body mass index; DCIS, ductal carcinoma in situ; IvBC, invasive breast cancer; COPD, chronic obstructive pulmonary disease
DISCUSSION
Modernization of medicine insinuates adapting to our patients. As breast surgeons it is imperative to illustrate evidence based medicine that informs patients about their unique risk for differing breast cancer surgery options. Since the Women’s Health and Cancer Rights Act (WHCRA), passed in 1998, reconstructive rates for mastectomy procedures and oncoplastic surgery have increased dramatically (4, 8, 11, 34, 35). With this rise in breast reconstruction and subsequent complexity of the operation, it has become increasingly important to address patient risk profiles during the pre-operative decision making process (36). Predictive models or risk calculators applied in clinical practice embody personalized medicine that are effective decision aids with conjunction to medical, surgical and anesthesia consultations (37–40). The growing influence of predictive models, or risk calculators, is most likely attributed by the ability to compute a risk probability unique to a patient. This surpasses the imprecision of stratifying patients only into “high risk” or “low risk” groups. The aim of the BCSRc was to provide a decision aid for patients and inform them of their individual complication risk for each surgical intervention. To our knowledge, our study is the first to assess short-term post-operative complications and predict a risk probability for five surgical categories a patient can choose from when diagnosed with IvBC or DCIS.
Women presenting for a surgical consultation are offered a wide variety of surgical interventions depending on oncologic requirements. The majority of patients acknowledge surgery is not risk-free, however few are aware of their unique risk profiles and able to discern how their risk factors may influence outcomes. With the BCSRc a patient’s risk for surgery-related complications can now be easily assessed. The two hypothetical scenarios, listed in Table IV, have two patients with very different pre-operative risk factors. Here the calculator illustrates how these differing patient profiles can influence complications and on occasion a oncologic procedure without reconstruction may be superior in dealing with the urgent issue at hand by minimizing complications. After the initial surgery, during the remission follow-up period, a timely breast reconstruction can be discussed with preoperative medical optimization.
The BCSRc is a model for all breast cancer patients to use that incorporates comorbidities, patient information and surgical factors that previous predictive models do not include. Complications post-operatively are multifactorial, they depend on the surgical approach, extent of surgery and patient characteristics. Most surgeons acknowledge the impact of pre-operative functional status, CHF, COPD, DM, HTN or dyspnea may have on patients but are unable to quantify this risk. Interestingly, elective surgery for patients with COPD or CHF has been associated with higher internal organ complications and two-fold increase in readmission status (41, 42). Pre-emptive screening and medical optimization following ACC/AHA guidelines is common practice but here we can start by using these risk factors for an appropriate risk-benefit discussion (43). Complications directly relate to readmission for inpatient care and by informing patients of the surgery associated with the lowest risk of complication, we can indirectly decrease the risk of nosocomial complications in the future(44). Identifying and quantifying these comorbidities preoperatively may allow for better stratification of patient risk and better matching of patients with different operative procedures in order to lower post-operative morbidity (45).
Study Strengths and Limitations
Previous breast surgical calculators such as the Breast reconstruction assessment score are limited in their reconstructive scope because they only focus on post-mastectomy reconstruction (26). The advantage of the BCSRc calculator is that it aids both the oncologic and reconstructive surgeon in most of the breast surgical options for breast cancer patients since it also includes breast conservation options (partial mastectomy alone, oncoplastic surgery). In addition, our models performance measures (i.e. HLT, AUC, brier score) on external validation have superior accuracy and precision compared to previous predictive models (26, 46). In using a nationwide cohort of patients for model development and validation this model may also be more broadly generalizable. Integrating patient comorbidities individually, instead of using a scoring system such as the ASA score, is more precise and improves the accuracy of the model; scoring systems predictability is controversial and can under or overestimate probabilities for complications(32).
Our model also incorporates the American Society for Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines that recommend axillary surgery for all patients diagnosed with IvBC whereas axillary surgery with a diagnosis of DCIS should only be considered in mastectomy procedures.(47) If a patient requires an axillary lymph node dissection than with a surgeon’s guidance the input needs to be changed appropriately (sentinel lymph node biopsy to axillary lymph node biopsy). Nevertheless, axillary surgeries increase operative time and can be underestimated especially if an intraoperative obstacle requires attention.
Oncologic factors (i.e. cancer stage, radiotherapy, hormonal receptor status, hereditary genetic factors) unfortunately were not included in the NSQIP dataset, thereby precluding us from determining how their role may influence complications for each surgical intervention (31). However, by following NCCN guidelines patients can choose either, breast conservation or mastectomy interventions as both groups offer oncologic safety and the surgery type is a patient right to choose. Interpretation of the NSQIP database categorizing by CPT coding may vary (31, 35). We used a coding protocol similar to one used at our institution and recent guidelines setting a consensus CPT classification system (48). Complications such as, graft or flap failure and hematoma without transfusion were not included in our analysis nor included in the NSQIP database, thus surgeon discretion is required for complications associated with ischemia or flap failure (i.e. mastectomy with muscular flap reconstruction) and conservative management with hematoma formation. Lastly, long term complications were not recorded in the NSQIP database, thereby potentially hindering our complication results from the final long term outcomes (over 1 month). Overall complication did not include return to the operating room as a complication as this was viewed as a treatment for a complication. In a future studies we will further study the need for operative management when associated with complications for each surgical intervention.
Our study focused on acute post-operative complications limited to the NSQIP 30 day prospective data collection thus complications that occur after this would not be included nor provide patients with information regarding delays in adjuvant therapy such a chemoradiotherapy. It is important to note that one of the most frequent types of breast reconstruction involving pre-pectorally placed implants has little long-term data given its relative newness while acknowledging that short-term complication rates do not differ significantly from the more traditional, sub-pectoral/dual plane technique.(49) Nevertheless, long-term complications in pre-pectoral implant placement may include differences in capsular contracture rates, and future studies investigating this will likely follow over time. Further prospective studies or extending the use of these models with a database that includes long-term complications, graft complications or oncologic factors, such as chemotherapy and radiation therapy, would be crucial and a prospect for future research assessing complications.
CONCLUSION
The BCSRc is the first published risk calculator generalizable for all female breast cancer surgical patients and can calculate individualized, complication risk probabilities for five surgical interventions. Evidence based medicine drives meaningful medical advancements, but often fails to deliver the value to the general public due to the lack of translatability. In parallel, population-based risk estimates often lack reliability for patients with diverse risk profiles. Here we presented a modern, patient-centered decision aid to improve health concerns. The breast cancer surgical risk calculator incorporates our model in scalable, informative, decision making platform enabling physicians and patients to use personal information to determine a patient’s complication risk estimates. Identifying patients into “low-risk” or “high-risk” is imprecise. Using the BCSRc physicians can provide, accurate, objective information to patients. Breast surgeons can now better inform breast cancer patients, lower postoperative complications and offer appropriate guidance.
Supplementary Material
Synopsis:
An online pre-operative surgical risk calculator that calculates a post-operative complication probability for five interventions from individualized patient risk factors. The risk calculator serves as a tool to support patient-centered decision making and reduce the risk of post-operative complications.
Acknowledgment Section
Role of the Funder/Sponsor: The ACS NSQIP were solely the source of data used for this study, herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. This project does not represent the views or plans of the ACS or the ACS NSQIP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest and Disclosure of Funding:
Brian Czerniecki - Intellectual property on dendritic cell cancer vaccine.
The described study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, and Award Number TL1TR002546 and the American Cancer Society grant # CRP-17-112-06-COUN.
LIST OF ABBREVIATIONS
- ACC/AHA
American College of Cardiology/American Heart Association
- ASA class
American Society of Anesthesiologists classification
- ASCO
American Society for Clinical Oncology
- AUC
Area under the curve
- BCSRc
Breast cancer surgical risk calculator
- BMI
Body mass index
- CHF
Chronic heart failure
- COPD
Chronic obstructive pulmonary disease
- CPT
Current Procedural Terminology
- DCIS
Ductal carcinoma in situ
- DM
Diabetes mellitus
- HTN
Hypertension
- ICD-9
International Classification of Diseases Ninth Revision
- ICD-10
International Classification of Diseases Tenth Revision
- IvBC
Invasive breast cancer
- NCCN
National Comprehensive Cancer Network
- NSQIP
National Surgical Quality Improvement Program
- PUF
Participant user files
- U.S.
United States
Footnotes
Conflicts of Interest Disclosures: All authors report no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.The L GLOBOCAN 2018: counting the toll of cancer. Lancet. 2018;392(10152):985. [DOI] [PubMed] [Google Scholar]
- 2.Henley SJ, Thomas CC, Lewis DR, Ward EM, Islami F, Wu M, et al. Annual report to the nation on the status of cancer, part II: Progress toward Healthy People 2020 objectives for 4 common cancers. Cancer. 2020;126(10):2250–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan A, Sharma MM, Kumar K. Evaluation of Surgical Outcomes of Oncoplasty Breast Surgery in Locally Advanced Breast Cancer and Comparison with Conventional Breast Conservation Surgery. Indian J Surg Oncol. 2016;7(4):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. [DOI] [PubMed] [Google Scholar]
- 5.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg. 2017;265(3):581–9. [DOI] [PubMed] [Google Scholar]
- 6.Albornoz CR, Matros E, Lee CN, Hudis CA, Pusic AL, Elkin E, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg. 2015;135(6):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter SA, Lyons GR, Kuerer HM, Bassett RL Jr., Oates S, Thompson A, et al. Operative and Oncologic Outcomes in 9861 Patients with Operable Breast Cancer: Single-Institution Analysis of Breast Conservation with Oncoplastic Reconstruction. Ann Surg Oncol. 2016;23(10):3190–8. [DOI] [PubMed] [Google Scholar]
- 8.Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. [DOI] [PubMed] [Google Scholar]
- 9.Jeevan R, Mennie JC, Mohanna PN, O’Donoghue JM, Rainsbury RM, Cromwell DA. National trends and regional variation in immediate breast reconstruction rates. Br J Surg. 2016;103(9):1147–56. [DOI] [PubMed] [Google Scholar]
- 10.Cil TD, Cordeiro E. Complications of Oncoplastic Breast Surgery Involving Soft Tissue Transfer Versus Breast-Conserving Surgery: An Analysis of the NSQIP Database. Ann Surg Oncol. 2016;23(10):3266–71. [DOI] [PubMed] [Google Scholar]
- 11.Carlson GW. Trends in autologous breast reconstruction. Semin Plast Surg. 2004;18(2):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anker CJ, Hymas RV, Ahluwalia R, Kokeny KE, Avizonis V, Boucher KM, et al. The Effect of Radiation on Complication Rates and Patient Satisfaction in Breast Reconstruction using Temporary Tissue Expanders and Permanent Implants. Breast J. 2015;21(3):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer (Dove Med Press). 2017;9:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg. 2007;245(5):665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losken A, Styblo TM, Carlson GW, Jones GE, Amerson BJ. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg. 2007;59(3):235–42. [DOI] [PubMed] [Google Scholar]
- 16.Tenofsky PL, Dowell P, Topalovski T, Helmer SD. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg. 2014;207(3):398–402; discussion [DOI] [PubMed] [Google Scholar]
- 17.Bolliger M, Kroehnert JA, Molineus F, Kandioler D, Schindl M, Riss P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur Surg. 2018;50(6):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazaure HS, Roman SA, Sosa JA. Association of postdischarge complications with reoperation and mortality in general surgery. Arch Surg. 2012;147(11):1000–7. [DOI] [PubMed] [Google Scholar]
- 19.Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394(10211):1807–15. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Gass J, Burke MB, Kopkash K, El-Tamer MB, Holmes DR, et al. Results from the American Society of Breast Surgeons Oncoplastic Surgery Committee 2017 Survey: Current Practice and Future Directions. Ann Surg Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Cordeiro E, Jackson TD, Elnahas A, Cil T. Higher rate of breast surgery complications in patients with metastatic breast cancer: an analysis of the NSQIP database. Ann Surg Oncol. 2014;21(10):3167–72. [DOI] [PubMed] [Google Scholar]
- 22.Evans TA, Duquette S, Soleimani T, Christensen LF, Munshi I, Cohen A, et al. Trends in Surgical Treatment of Breast Cancer in the Veterans Affairs System. JAMA Surg. 2017;152(3):305–6. [DOI] [PubMed] [Google Scholar]
- 23.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losken A, Hart AM, Broecker JS, Styblo TM, Carlson GW. Oncoplastic Breast Reduction Technique and Outcomes: An Evolution over 20 Years. Plast Reconstr Surg. 2017;139(4):824e–33e. [DOI] [PubMed] [Google Scholar]
- 25.Rezai M, Knispel S, Kellersmann S, Lax H, Kimmig R, Kern P. Systematization of Oncoplastic Surgery: Selection of Surgical Techniques and Patient-Reported Outcome in a Cohort of 1,035 Patients. Ann Surg Oncol. 2015;22(11):3730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Mlodinow AS, Khavanin N, Hume KM, Simmons CJ, Weiss MJ, et al. Individualized Risk of Surgical Complications: An Application of the Breast Reconstruction Risk Assessment Score. Plast Reconstr Surg Glob Open. 2015;3(5):e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen MA, Nickel KB, Margenthaler JA, Fox IK, Ball KE, Mines D, et al. Development of a Risk Prediction Model to Individualize Risk Factors for Surgical Site Infection After Mastectomy. Ann Surg Oncol. 2016;23(8):2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorarinsson A, Frojd V, Kolby L, Liden M, Elander A, Mark H. Patient determinants as independent risk factors for postoperative complications of breast reconstruction. Gland Surg. 2017;6(4):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto WK, Munhoz AM, Okada A, Montag E, Arruda EG, Fonseca A, et al. Influence of advanced age on postoperative outcomes and total loss following breast reconstruction: a critical assessment of 560 cases. Rev Col Bras Cir. 2018;45(2):e1616. [DOI] [PubMed] [Google Scholar]
- 30.Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Todd C. Pretreatment health measures and complications after surgical management of elderly women with breast cancer. Br J Surg. 2015;102(6):653–67. [DOI] [PubMed] [Google Scholar]
- 31.Jonczyk MM, Jean J, Graham R, Chatterjee A. Trending Towards Safer Breast Cancer Surgeries? Examining Acute Complication Rates from A 13-Year NSQIP Analysis. Cancers (Basel). 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steyerberg EW. Clinical prediction models : a practical approach to development, validation, and updating. New York: Springer; 2009. [Google Scholar]
- 33.Bennett KG, Qi J, Kim HM, Hamill JB, Pusic AL, Wilkins EG. Comparison of 2-Year Complication Rates Among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg. 2018;153(10):901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labor USdo 2018;Pages. Accessed at United States department of Labor at https://www.dol.gov/general/topic/health-plans/womens on 11/1/20182018.
- 35.Jonczyk MM, Jean J, Graham R, Chatterjee A. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancer Facts & Figures 2020. In: Society AC, ed. Atlanta: American Cancer Society; 2020. [Google Scholar]
- 37.Basta MN, Bauder AR, Kovach SJ, Fischer JP. Assessing the predictive accuracy of the American College of Surgeons National Surgical Quality Improvement Project Surgical Risk Calculator in open ventral hernia repair. Am J Surg. 2016;212(2):272–81. [DOI] [PubMed] [Google Scholar]
- 38.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 39.Wilcox T, Smilowitz NR, Xia Y, Berger JS. Cardiovascular Risk Scores to Predict Perioperative Stroke in Noncardiac Surgery. Stroke. 2019;50(8):2002–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopecky KE, Urbach D, Schwarze ML. Risk Calculators and Decision Aids Are Not Enough for Shared Decision Making. JAMA Surgery. 2019;154(1):3–4. [DOI] [PubMed] [Google Scholar]
- 41.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–95. [DOI] [PubMed] [Google Scholar]
- 42.Hammill BG, Curtis LH, Bennett-Guerrero E, O’Connor CM, Jollis JG, Schulman KA, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108(4):559–67. [DOI] [PubMed] [Google Scholar]
- 43.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Developed in collaboration with the American College of Surgeons, American Society of Anesthesiologists, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Vascular Medicine Endorsed by the Society of Hospital Medicine. J Nucl Cardiol. 2015;22(1):162–215. [DOI] [PubMed] [Google Scholar]
- 44.Glance LG, Kellermann AL, Osler TM, Li Y, Mukamel DB, Lustik SJ, et al. Hospital Readmission After Noncardiac Surgery: The Role of Major Complications. JAMA Surgery. 2014;149(5):439–45. [DOI] [PubMed] [Google Scholar]
- 45.Smit-Fun V, Buhre WF. The patient with chronic heart failure undergoing surgery. Curr Opin Anaesthesiol. 2016;29(3):391–6. [DOI] [PubMed] [Google Scholar]
- 46.Blough JT, Vu MM, Qiu CS, Mlodinow AS, Khavanin N, Fine NA, et al. Beyond 30 Days: A Risk Calculator for Longer Term Outcomes of Prosthetic Breast Reconstruction. Plast Reconstr Surg Glob Open. 2018;6(12):e2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyfer BJ, Jonczyk M, Jean J, Graham RA, Chen L, Chatterjee A. Analysis of Surgical Trends for Axillary Lymph Node Management in Patients with Ductal Carcinoma In Situ Using the NSQIP Database: Are We Following National Guidelines? Annals of Surgical Oncology. 2020. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee A, Gass J, Patel K, Holmes D, Kopkash K, Peiris L, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(11):3436–44. [DOI] [PubMed] [Google Scholar]
- 49.Manrique OJ, Banuelos J, Abu-Ghname A, Nguyen M-D, Tran NV, Martinez-Jorge J, et al. Surgical outcomes of prepectoral versus subpectoral implant-based breast reconstruction in young women. Plastic and Reconstructive Surgery Global Open. 2019;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.