Abstract
Background:
Our brain uses interoceptive signals from the body to shape how we perceive emotions in others; however, whether interoceptive signals can be manipulated to alter emotional perceptions is unknown. Alcohol has acute effects both on emotional processing and on the physiological substrates supporting interoception. In this registered report, we examine whether alcohol administration triggers physiological changes that alter interoceptive signals and manipulate emotional face processing. Such knowledge will broaden understanding of the mechanisms by which alcohol affects emotional face processing.
Methods:
Participants (n = 36) will be administered an alcohol or placebo beverage. Cardiovascular physiology will be recorded before and after administration. Participants will complete two behavioral tasks in which they view emotional faces presented in synchrony with different phases of the cardiac cycle (i.e., systole, diastole). This manipulation creates an index of how interoceptive signals amplify emotional face processing.
Hypotheses:
We hypothesize that, compared to placebo, alcohol administration will disrupt the cardiac amplification of emotional face processing. We further explore whether this disruption depends on the nature and magnitude of changes in cardiovascular physiology after alcohol administration.
Keywords: Interoception, emotional processing, alcohol administration, cardiac amplification
1. Introduction
A hallmark of alcohol intoxication is the disruption in emotional processing and facial expression detection (Miller et al., 2015) and such effects are thought to contribute to the development of addictive behaviors (Donadon & Osório, 2014; Lannoy et al., 2021). Alcohol may generate these effects through its pharmacological disruption of physiological signals and bodily states (Carbia et al., 2020). In this registered report, we examine whether acute alcohol administration affects emotional processing by disrupting underlying physiological mechanisms.
The ability of humans to detect emotions in others is paramount to our capacity to engage in social interactions. Facial expressions inform us of the emotional state of others and guide our behaviors. Evidence supports early theories that physiological signals are an essential component of how emotions are processed (Porges, 2001). Bodily responses to the environment play a crucial role in emotion (Lang, 1994), as stimuli are hypothesized to evoke a series of physiological responses, and the perception of these changes through sensory feedback mechanisms then generates emotional feelings (Friedman, 2010). It is in this context that the study of interoception, the perception of sensations from the body (Craig, 2002), creates a mechanistic framework for the role of physiological signals in emotion.
Interoceptive signals are relayed from the viscera through afferent pathways to different brain areas to influence cognition and emotion (Critchley & Harrison, 2013). These body-brain signals have been studied across different visceral domains (Garfinkel et al., 2016), but those emanating from the heart are particularly important for emotional processing (Critchley & Garfinkel, 2017). Directly relevant to the field of interoception are arterial baroreceptors, stretch sensitive mechanoreceptors that signal the timing and strength of each heartbeat to the brain. Baroreceptors remain silent during the upward deflection of the QRS complex in the electrocardiogram, corresponding to the diastolic phase, but become active around 300 ms after the R-wave, during the systolic phase to signal the contraction of the heart.
It is possible to experimentally utilize the structured temporal framework of cardiac activity to study the influence of interoceptive signals in emotional face processing by timing stimulus presentation to systole or diastole (Gray et al., 2010). Presenting stimuli at systole compared to diastole increases detection accuracy of fearful faces (Azevedo et al., 2018; Garfinkel et al., 2014). This provides foundational support for the dependence of objective attentional responses towards emotional faces on discrete cardiac interoceptive signals. Subjective measures of emotional face processing, such as intensity ratings, also show that cardiac signals shape the detection of fear (Garfinkel et al., 2014) and this has led to the assumption that cardiac interoceptive signals have a unique role in the processing of fear across cognitive domains (Garfinkel & Critchley, 2016). This phenomenon is known as cardiac amplification of emotional face processing, and offers an experimental perspective on the subtle interoceptive mechanisms that drive cognitive and emotional processing (Seth & Friston, 2016).
Our team recently published an emotional visual search task (Emo VST) to determine whether cardiac signals exclusively support fear processing (Leganes-Fonteneau et al., 2020). This novel task is derived from an attentional task that is sensitive to a variety of emotional faces (Calvo & Nummenmaa, 2008). In the Emo VST, participants detect an emotional face (happy, fear, sad, disgust) embedded within a visual array (Visual Search accuracy; VSA) and identify the emotion presented (Emotion Identification accuracy; EIA); the stimulus array is randomly synchronized with participants’ systole or diastole. Surprisingly, we found that detection accuracy of faces showing happy and disgust emotions was higher at systole than diastole, whereas detection accuracy for fearful faces was lower at systole. We also found that accuracy for identifying fear and sad emotions was lower at systole compared to diastole. The inhibition of fearful faces at systole can be interpreted as an attentional orientation towards surrounding faces as a possible source of imminent threat (Williams et al., 2005). An additional emotional intensity task revealed that both happy and fearful faces were subjectively rated as more intense at systole than diastole. These findings provide evidence that baroreceptors elicit undifferentiated signals of arousal that feed into cognitive operations and facilitate the processing of emotional faces (Cacioppo et al. 2000; Leganes-Fonteneau et al. 2020a).
As explained by Critchley and Garfinkel (2017), interoceptive signals in the cardiovascular axis can be decomposed in multiple dimensions. Higher-order levels of interoception, such as interoceptive sensitivity or meta-cognitive interoceptive awareness, are measurable from the conscious detection and subjective reporting of heart-beats. Intermediate-levels of interoception, such as cardiac amplification, can be captured as the modulation of emotional face processing by discrete baroreceptor firing. Finally, lower-order levels of interoception (e.g. baroreflex sensitivity, heart-rate evoked potentials) can be gauged directly from peripheral physiological signals.
The baroreflex is well-established as a 10-s body-brain feedback loop by which the cardiovascular system adapts heart-rate to cope with changes in arterial blood pressure (BP). Baroreflex sensitivity (BRS; the change in HR to 1mmHg change in BP) can be used to quantify heart-brain communication as vagally-mediated afferent responsiveness to cardiac output. Baroreflex activity, on the other hand, can be approximated from specific direct indices of heart rate that are derived from the ECG. More specifically, oscillations in heart rate around 0.1Hz offer an index of afferent cardiovascular activity because it directly arises from the baroreceptors (Cevese et al., 2001; Vaschillo et al., 2002) and corresponds to the 10-second periodicity of the baroreflex. This differs from the better known high frequency HRV signal (HF-HRV; 0.15–0.5 Hz) that is well established to represent parasympathetic cardiac activation and arousal inhibition via efferent vagal activation (Task Force, 1996). We propose that the amplitude of the HRV density spectrum at approximately 0.1Hz is uniquely suited as an index of interoceptive responsiveness to environmental stimuli (Leganes-Fonteneau et al. 2020b). Determining whether 0.1Hz HRV can act as a predictor of cardiac interoception may expand research into the physiological basis of interoception and bring about a more mechanistic characterization of individual differences. However, to the best of our knowledge, research linking BRS and 0.1Hz HRV to different levels of interoception is limited (Leganes-Fonteneau et al. In press). In this report, we will use measures of BRS and 0.1Hz HRV to understand the physiological basis of the cardiac amplification of emotional face processing. In doing so, we seek to characterize the direct physiological basis of intermediate-levels of interoception.
Prior studies have shown that 0.1Hz HRV can sensitively detect the effects of alcohol on the body (Vaschillo et al., 2008) and that alcohol dampens BRS (Abdel-Rahman et al., 1987). Thus, this report posits that alcohol administration could impact interoceptive signals, and through these changes, modify hedonic components and subjective feeling states (Paulus et al., 2009). This is supported by a relationship between 0.1Hz HRV and memory biases for alcohol cues after alcohol administration in persons with a family history of alcohol use disorder (Leganes-Fonteneau et al. 2020b). Interestingly, at a lower dose, alcohol did not generate a statistically significant and homogeneous disruption in 0.1Hz HRV at the level of the group mean; however, individual differences in the effect of alcohol on this HRV index correlated with alcohol attentional biases (Leganes-Fonteneau et al., 2021).
Beyond alcohol’s pharmacological influences on the body, its psychological effects are also well elucidated. One motive for alcohol consumption is to increase the desire to socialize (Sayette, 2017; Sayette et al., 2012) and to overcome a lack of confidence in social interactions (Smith et al., 1993), and this can be mediated by changes in emotional processing. Acute alcohol administration has an impact on face processing (Miller et al., 2015) by modulating the way we perceive emotions in others. Alcohol increases the perception of happy faces along with subjective ratings of empathy and positive mood (Dolder et al., 2017). A dose of 0.4g/kg of alcohol disrupted the processing of sad faces (Attwood et al., 2009; Kamboj et al., 2013) and some research further points towards a decrease in the perception of angry faces after drinking (Stevens et al., 2009) and a blunted processing of social signals of threat (Sripada et al., 2011), although results are mixed (Khouja et al., 2019). Finally, alcohol dampens disgust reactivity (Stafford et al., 2020), although whether this translates to emotional faces is not clear. None of these studies, however, address questions about the physiological mechanisms by which alcohol affects emotional processing. Such mechanistic studies are timely as the field of addiction continues to seek physiological and neural drivers of substance use behaviors (Eddie et al., 2020); interoceptive pathways may be one such driver underlying the changes in emotional face processing that are commonly seen among people with an alcohol use disorder (Donadon & Osório, 2014) as well as in non-clinical adolescent binge-drinking samples (Leganes-Fonteneau et al., 2019).
This report will replicate previous findings (Leganes-Fonteneau et al., 2020) and expand research into the pharmaco-physiological mechanisms of alcohol effects on emotional face processing in a placebo-controlled, two-session study. ECG and beat-to-beat BP will be recorded during a baseline task prior to and following beverage administration. Participants (n = 36) will then perform the Emo VST to obtain indices of VSA and EIA. The use of two independent indices allows to distinguish whether alcohol affects emotional detection (VSA) or identification (EIA), which has implications for intervention and treatment.
Pre-registered Hypotheses
We pre-register two hypotheses (see Design Table), each with two primary outcomes: Visual Search Accuracy (VSA) and Emotion Identification Accuracy (EIA). Initial manipulation checks will replicate the cardiac synchrony effects on VSA and EIA (Leganes-Fonteneau et al., 2020). We will also examine the interaction between beverage administration and emotional face processing.
Table 7. Design Table.
All hypotheses apply to two different DVs: VSA and EIA.
| Question | Hypothesis | Sampling plan (e.g. power analysis) | Analysis Plan | Interpretation given to different outcomes |
|---|---|---|---|---|
| Does alcohol generate an emotion-dependent effect on cardiac amplification? | H1: For some emotions, alcohol will increase the cardiac amplification and for other emotions alcohol will decrease the cardiac amplification. | We compute all power analyses to achieve a power = 0.90 assuming a small-medium effect size on the interaction between emotion and condition, cohen’s d = 0.3 For visual search accuracy we would need to recruit 35 participants. For emotion identification accuracy we would need to recruit 12 participants. Exclusions: Participants with a mean VSA <1/6 (chance level) in any session will be excluded. In addition, any technical errors in physiological data collection or incidents with participants not complying with the tasks or feeling sick because of alcohol administration will be registered, and their data excluded. In case of exclusions, more participants will be recruited to complete the sample stipulated by the power analysis, within the capabilities of the laboratory. Statistical and graphical inspection of outliers and influential datapoints will be performed. |
We will compute difference scores (systole minus diastole) for each emotion. A 2-way repeated measures anova will examine the interaction between emotion and beverage administration. Post-hoc analyses will compare difference scores for each emotion between alcohol and placebo. | If the result is significant, then alcohol effects on the cardiac amplification of emotional processing is emotion-dependent, and post-hoc analyses will allow determining for which emotions alcohol affects the cardiac amplification of their processing. If the result is non-significant, then there is a lack of evidence for alcohol generating its effects on emotional processing through interoceptive pathways. |
| Does alcohol affect the cardiac amplification of emotions? | H2: Alcohol will decrease the magnitude of change generated by systolic amplification. | We compute all power analyses to achieve a power = 0.90 assuming a small-medium effect size of alcohol on the magnitude of change score, cohen’s d = 0.3 For visual search accuracy we would need to recruit 18 participants. For emotion identification accuracy we would need to recruit 30 participants. |
The mean of the absolute difference scores (systole-diastole) for each emotion will be computed to obtain an index of magnitude of cardiac amplification score. A paired samples t-test will compare magnitude of cardiac amplification score during alcohol vs. Placebo. | If the result is significant and the magnitude of cardiac amplification score is higher for placebo than alcohol, then alcohol decreases the ability of cardiac signals to shape emotional processing. If the result is significant and the magnitude of cardiac amplification is higher for alcohol than placebo, then alcohol increases the ability of cardiac signals to shape emotional processing. No significant effects of beverage means lack of evidence for the effect of alcohol on the cardiac amplification of emotion. |
| Do individual differences in cardiac physiology (0.1Hz HRV and BRS) explain individual differences in cardiac amplification? | H3: 0.1Hz HRV and BRS will positively correlate with the absolute magnitude of cardiac amplification of emotional processing (i.e. Hypothesis 1), and the interaction between emotion and cardiac synchrony (i.e. Hypothesis 2). | This is an exploratory hypothesis. | We will measure 0.1Hz HRV and BRS at baseline (t0) in the placebo condition. We will run the correlation between 0.1Hz HRV and BRS and the absolute magnitude of cardiac amplification in emotional processing. We will use a series of linear-mixed models to examine the interaction of 0.1Hz HRV and BRS with emotion. A significant interaction would be followed up by simple slope analyses examining if difference scores (systole minus diastole) for different emotions correlate with physiological variables. |
If results are significant, then baroreceptor firing is the underlying mechanism for the cardiac amplification of emotional processing. If results are non-significant, then there is no evidence that 0.1Hz HRV and BRS, as measures of viscero-afferent baroreceptor firing, underlie individual differences in cardiac amplification. If results are found in the interaction with different emotions, then the role of afferent cardiovascular signals is emotion specific. |
| Does the alcohol effect on physiological indices correlate with its effect on cardiac amplification of emotion? | H4: Individual differences in physiological responses (0.1Hz HRV and BRS) to alcohol administration will correlate with the effect of alcohol on the cardiac amplification of emotion. | This is an exploratory hypothesis. | For the placebo and alcohol conditions, pre-to-post drink changes in 0.1Hz HRV and BRS will be computed (t1-t0). These difference scores will be introduced as a covariate in a series of linear-mixed models examining if changes in physiology predict the effect of alcohol on magnitude of cardiac amplification and on the interaction between condition and emotion. Significant interactions will be followed up by simple slope analyses. |
If results are significant and an interaction is found in the alcohol condition, then the effects of alcohol on the cardiovascular system are responsible for alcohol-induced changes in the cardiac amplification of emotional processing. If results are non-significant, then there is no evidence that changes 0.1Hz HRV and BRS after alcohol administration are responsible for a hypothetical effect of alcohol on the cardiac amplification of emotion. If results are found in the interaction with different emotions, then the effects of alcohol on the cardiovascular system are emotion-specific. |
Hypothesis 1: Alcohol will affect the cardiac amplification of faces differently depending on the emotion presented.
This hypothesis is based on prior evidence that alcohol administration has an emotion-dependent effect on face processing (Miller et al., 2015) and our initial evidence that the processing of different emotions can be improved or impaired as a function of cardiac synchrony (Leganes-Fonteneau et al., 2020). To test this, we will compute difference scores on VSA and EIA between systole and diastole independently for each emotional face. We will then examine whether there is an interaction between condition (alcohol vs placebo) and emotion (happy, sad, disgust, angry, fear) using a two-way ANOVA. A significant interaction will provide support for a selective disruption of the cardiac amplification of different emotional faces by alcohol administration. A non-significant interaction would imply a lack of evidence for a selective disruption of systolic amplification of emotional processing by alcohol. Significant post-hoc analyses will identify specific emotion-dependent alcohol effects on cardiac amplification, and we expect alcohol to increase the cardiac amplification of VSA and EIA for happy faces and to decrease it for all other emotions.
Hypothesis 2: Alcohol will dampen the ability of cardiac signals to influence emotional and cognitive processing.
Cardiac signals can amplify or dampen face processing depending on the emotion presented (Leganes-Fonteneau et al. 2020a). In addition, there is substantial evidence that alcohol dampens afferent cardiac signals (e.g., Koskinen et al. 1994; Vaschillo et al. 2008; Leganes-Fonteneau et al. 2020b). It is possible that alcohol mutes the ability of cardiac signals to guide cognitive and emotional processing, but the emotion-dependent directionality observed in the Emo VST could obscure a main effect of alcohol administration on cardiac amplification.
We will compute the absolute difference between systole and diastole for each emotional face type on VSA and EIA. We will then generate an overall mean difference per participant to obtain a magnitude of cardiac amplification score. In this way we will examine to which extent alcohol affects afferent cardiac signaling regardless of whether cardiac signals amplify or impair emotion-specific face processing. We note that alcohol administration may improve cardiac interoceptive accuracy (Leganes-Fonteneau et al. Unpublished Manuscript-b), thus the opposite effect might be observed.
We register two additional hypotheses to examine the physiological mechanisms underlying the effects observed in hypotheses 1 and 2, and the unique effect of alcohol administration on indices of cardiac interoceptive signaling. To the best of our knowledge, only our recent study (Leganes-Fonteneau et al. In Press) has examined related hypotheses; thus, we register these hypotheses as exploratory. We tentatively expect wide individual differences in cardiovascular adaptation to alcohol (Leganes-Fonteneau et al., 2021; Leganes-Fonteneau et al., 2020) and that the magnitude of these changes in afferent cardiovascular indices will correlate with the impact of alcohol on the link between cardiac signals and emotion.
Hypothesis 3: Individual differences in cardiovascular physiology will mediate the cardiac amplification of emotional faces independent of alcohol administration.
We will measure baseline physiology prior to beverage administration. To test the physiological basis of the cardiac amplification of different emotional faces, individual differences in 0.1Hz HRV and BRS at baseline will be correlated with the interaction between emotion and cardiac synchrony only in the placebo condition. Further, these physiological differences at baseline will be correlated with the magnitude of cardiac amplification score. Indices of HF-HRV will be also be included as a control covariate. No significant relationship could imply a lack of evidence for the involvement of baroreceptor activity in the systolic amplification of emotional face processing, despite both events co-occurring in time.
Hypothesis 4: The extent to which alcohol administration impacts physiological measures will parallel its effects on the cardiac amplification of emotional processing.
We will examine the physiological mechanisms by which alcohol administration disrupts the interaction between cardiac synchrony and emotion. We will compute pre-to-post beverage administration differences in cardiovascular physiology in the alcohol and placebo conditions. Independent models will introduce changes in 0.1Hz HRV, HF-HRV and BRS as covariates on the interaction between beverage, emotion and cardiac synchrony (Hypothesis 1) and between alcohol and magnitude of cardiac amplification score (Hypothesis 2). A lack of significant results, assuming alcohol does affect the systolic amplification of emotions, would imply that such effect occurs through another mechanism that is not related to baroreceptor functioning.
2. Methods
2.1. Ethics information
Ethical approval for this experiment will be obtained through the Rutgers University Arts and Sciences Institutional Review Board for the Protection of Human Subjects. Participants will read and sign an informed consent before the start of the experiment and will be compensated USD10/hour for their time with gift vouchers.
2.2. Pilot data
Previously published pilot data for the Emo VST task has shown that it is successful at identifying the effects of systolic amplification on emotional processing. For VSA, we found a significant interaction between emotion and cardiac synchrony, F(3,490) = 11.880, p<.001, Cohen’s f = .27, showing that VSA for happy and disgust faces was higher at systole than diastole, and that the opposite was true for fearful faces. For EIA, we also found a significant interaction between emotion and cardiac synchrony, F(3,490) = 6.090, p<.001, Cohen’s f = 0.16, and EIA was impaired at systole for fear and sad faces. Data for this report is available online: https://osf.io/s2ew9/?view_only=b0360195d2d3421a9623a3d9684492c9
For this experiment, we will add angry emotional faces to the existing fear, happy, sad and disgust ones because alcohol administration has been shown to acutely disrupt the processing of angry faces (Stevens et al., 2009). Power analyses use the existing data set, simulating the values for angry faces. We also simulate the effect of alcohol on magnitude of cardiac amplification score and on the interaction between emotion and cardiac amplification (see sampling plan section).
2.3. Participants:
A gender-balanced sample of 36 participants (see Sampling plan) will be recruited using fliers posted on social media or near Rutgers University, NJ, US. Eligibility criteria will be drinking more than three alcohol units per week in the past 3 months, being at least 21 years old and not reporting a history of learning disability, psychiatric disorders, treatment for substance use disorder, current AUD (assessed using AUDIT), or regular (weekly) illicit or prescription drug use. Pregnant women and participants with a Body Mass Index (BMI) 20% over- or under-weight will be excluded.
2.4. Emotional Visual Search task:
For this task, participants will sit ~60cm away from the screen and will be presented with a visual array consisting of 6 faces organized in a circular pattern around the center of the screen (stimulus array). For each trial, all faces will belong to the same model, but one of the faces, the target, will present a “full-blown” emotion (fear, sad, anger, disgust or happy), while the other faces will present neutral expressions, acting as distractors. Faces will be presented 135px away from the center of the screen. In total, participants will complete 250 trials (50 per emotion). Facial stimuli will be extracted from the Karolinska Directed Emotional Faces data base (Lundqvist et al., 1998);and will include 50 models (28 females). All faces will be cropped around the facial oval and presented in black and white.
Each trial will start with the presentation of a red fixation point that will remain on screen until the presentation of the stimulus array. The stimulus array will remain on screen for 100ms, followed by a black screen for 20ms. Next, participants will be presented with the visual search response array, in which 6 white ovals are presented at the location of the faces. Finally, participants will see the emotion identification response array, which will consist on 5 words in a row (Fear, Happy, Sad, Disgust, Anger). Both response arrays will remain on the screen until a response is detected. The mouse cursor will be reset to the center of the screen at the beginning of each trial. On 50% of the trials, the stimulus array will appear in synchrony with the participant’s R-wave (diastole), and on the remaining trials, a 300ms delay will be introduced between the R-wave and the presentation of visual array (systole). Systolic and diastolic trial presentation and emotion presentation will be fully randomized.
Participants’ task will be to select the oval corresponding to the location of the target emotional face as quickly and accurately as possible using the computer mouse (visual search accuracy, VSA) and to select which emotion they think was presented in the target stimulus using the mouse (emotion identification accuracy, EIA). Prior to the main task, participants will observe one example trial in which the stimulus array remains on screen for 500ms, and will complete a practice block in which 10 trials will be presented (2 per emotion), non-synchronized with participants’ cardiac phase, see Figure 1.
Figure 1 -. Emotional Visual Search task:
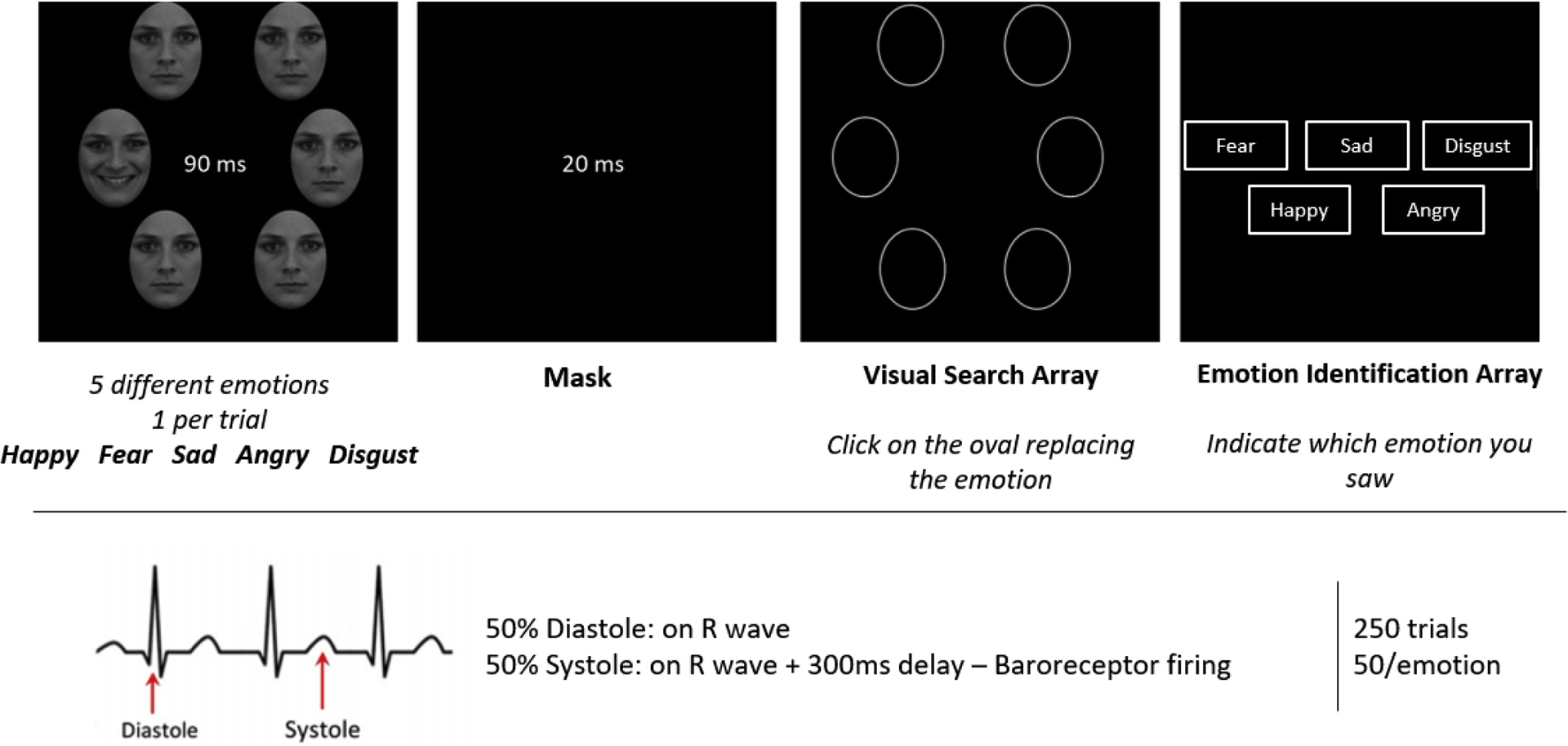
Participants will complete 250 trials (50 per emotion) in which a target emotional face will be presented surrounded by neutral distractors. Participants will indicate the location of the emotional target face (Visual Search Accuracy) and indicate which emotion they had observed (Emotion Identification Accuracy). On 50% of the trials, the faces appear synchronized with participants’ R-wave, and on 50% of the trials a 300ms delay will be introduced after the R-wave. In the Emotion Identification Array all emotion categories will appear in a single row (they are here represented stacked for graphical reasons).
The Matlab Psychtoolbox Code for the task will be made available on OSF.
2.5. ECG recordings and Cardiac synchronization:
Participants will be connected to a PowerLab 16/30 Acquisition System (ADInstruments, Colorado Springs, CO, US) to collect ECG data. Electrodes will be placed on their left ankle and on their left and right arm. In order to obtain BRS measures, a finger cuff will be placed to measure changes in beat-to-beat BP (Finometer MIDI - Finapres, Amsterdam, The Netherlands); all sampling rates will be 2000-Hz.
Participants’ physiological responses will be measured at baseline (t0) and 5 minutes after beverage administration (t1) using a Vanilla task (Jennings et al., 2007). In this task, participants are comfortably seated and presented with colored squares for 5 minutes. Each colored square remains on screen for 10 seconds and participants’ task is to silently count how many blue (t0) or red squares (t1) they see. Participants’ responses on the Vanilla task are irrelevant but used to avoid mind-wandering.
Stimulus synchronization with cardiac phase for the Emo VST will be performed in real time. Using Powerlab’s Labchart 8, a voltage trigger will be automatically sent through the output channel every time an R spike is detected in the ECG signal. This trigger will be detected through a National Instruments USB-6001 data acquisition device and integrated on Matlab. In this way, stimuli in diastolic trials will appear milliseconds after the occurrence of an R-spike, allowing stimuli to be paired with cardiac phase in a more accurate way than with heartbeat predictions (Leganes-Fonteneau et al. In press, Azevedo et al. 2017).
2.6. Alcohol administration procedure:
In a double-blind placebo-controlled experiment, participants will complete two sessions (within-subjects design) in which they will receive a drink containing alcohol or a placebo. Session order will be randomized and sex-balanced. The drink will consist of 0.4g/kg dose of alcohol (190-proof Everclear), mixed with sugar-free tonic water (Schweppes) and 5 drops of angostura bitters to make up a 200ml solution. In the placebo session, participants will receive 200ml of tonic water mixed with 5 drops of angostura. The 200ml solution will be divided in five 40ml-glasses presented on a tray. Alcohol will be sprinkled over the tray to provide olfactory cues and participants will be instructed to drink one glass at a time every 2 minutes at their own pace. We will measure BAC using Alco-Pro FST breathalyzer 10 minutes after the end of drink administration, and after the experimental task. The person preparing the beverages and administering the breathalyzer will be different than the person running the experiment.
2.7. Questionnaires:
Alcohol use behaviors will be measured using the 12-item alcohol use questionnaire (AUQ; Mehrabian & Russell 1978), out of which a binge drinking score also can be extracted by considering amount of drinks consumed in a 2-hour period as well as the number and percentage of times participants get intoxicated when drinking (Townshend & Duka, 2002). This questionnaire has been modified to include beverages (i.e. hard-seltzer) representative of the drinking patterns of student population in the US, and this modified version has already been used (Leganes-Fonteneau et al., 2021). The alcohol use disorder identification test (AUDIT) (Saunders et al., 1993) will measure problems related to alcohol consumption.
Participants will also complete a series of measures that will not be used in this report as they are not directly tied to the registered hypotheses. These measures can be useful to replicate and expand previous findings through secondary data analyses and to align this study with our larger research program. Before the first experimental session, participants will complete the Anticipated Effects of Alcohol Scale (Morean et al., 2012). In each session, before beverage administration and after each task, participants will complete measures of subjective light-headedness (Duka et al., 1998), Positive and Negative Affect Scale (Watson et al., 1988) and the Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993). Participants will also complete a baseline measure of cardiac interoceptive awareness using the heart rate discrimination task (Legrand & Allen, 2021). Participants will complete this interoceptive measure again after each Emo VST. At the end of the session, participants will indicate how many standard drinks they think they have consumed as basic placebo manipulation check. The inclusion of these additional measures will not affect or interact with the main hypotheses of this experiment and are unlikely to have any residual or carry-over effect on the other cardiac task. Any report on these secondary analyses will be fully transparent about the fact they were obtained in the context of this registered report.
2.8. Procedure:
Participants will complete an initial 5-min survey allowing us to determine eligibility criteria. Experimental sessions will be conducted in the laboratory, starting between 11:00 and 15:00. Participants will be instructed to have a light low-fat breakfast or lunch two hours before the beginning of the experiment.
Upon arrival to the laboratory, participants will sign consent forms and be weighed. Females will complete a pregnancy test to confirm negative status. Participants will complete questionnaires and the physiological equipment will be connected. The first session begins with the heart-beat discrimination task (unrelated to the registered hypotheses). Physiological recording begins during the first 5-minute Vanilla task (t0). After the beverage administration (10mins), participants will have a 5-min break during which they will remain seated and connected to the physiological equipment. The second Vanilla task will then start (t1), followed by a BAC measurement. The beat-to-beat BP equipment will then be disconnected and participants will complete the Emo VST and a BAC measurement (t2). An additional measure of cardiac interoception will be taken at the end of both sessions (unrelated to the registered hypotheses). Participants will remain in the laboratory until they reach a BAC < 3mg/dl. Those assigned to the placebo condition on the first session will be asked to remain in the laboratory for 20 minutes and the BAC will be measured twice to maintain the double blind for the second session, see Figure 2.
Figure 2 –
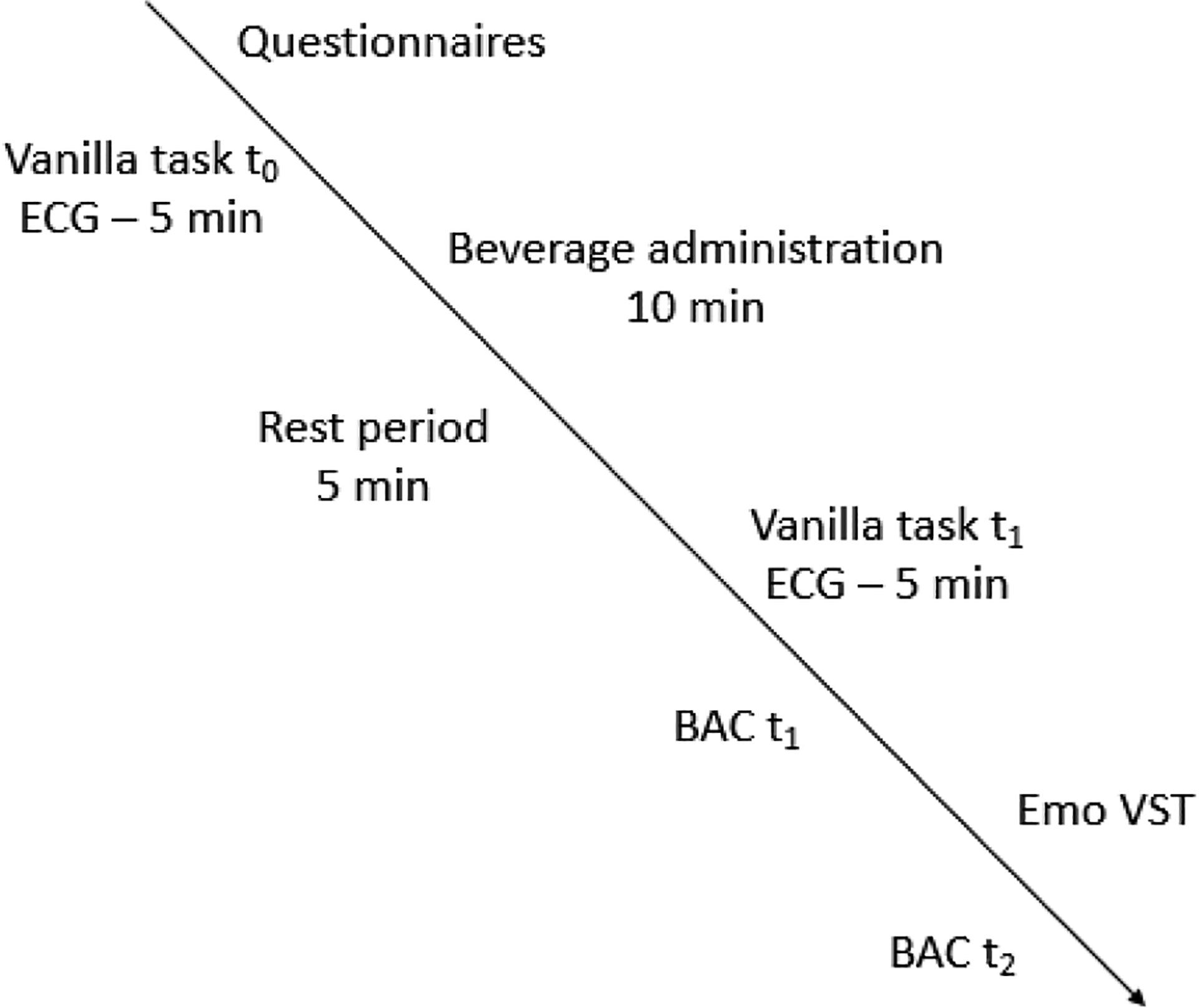
Experimental protocol
3. Data analysis
3.1. ECG data extraction:
Measures of BRS and HRV will be extracted using ANSLAB. Raw ECG and BP data will be inspected and post-processed to assure the quality of the recordings. 0.1Hz HRV will be computed as the peak power in the resonance frequency band (0.1Hz; 0.075–0.108 Hz). HF-HRV will be computed as the peak power in the high-frequency band (0.15–0.5 Hz). BRS will be calculated using a transfer function with systolic BP as the input and RRI as the output, computing the average value of TF(BP-RRI) in the low frequency range where BP-RRI coherence >0.5 (E. G. Vaschillo et al., 2012).
3.2. Proposed Analyses
Prior to the analyses, VSA and EIA (proportion of correct responses) data will be examined. Participants with a mean VSA <1/6 (chance level) in any session will be excluded. In addition, any technical errors in physiological data collection or incidents with participants not complying with the tasks or feeling sick because of alcohol administration will be registered, and their data excluded. In case of exclusions, more participants will be recruited to complete the sample stipulated by the power analysis, within the capabilities of the laboratory. Our laboratory has over a decade of experience performing similar studies and has only rarely encountered these problems.
VSA and EIA scores will be transformed using an arcsine square-root transformation. Physiological variables (0.1Hz HRV and BRS) were log10 transformed. PhysioΔ will be computed as the difference in physiological responses after drink administration (B1-B0) for 0.1Hz HRV and BRS. All analyses described below will be performed separately for VSA and EIA.
Manipulation checks:
A two-way repeated measures ANOVA with cardiac synchrony and emotion as within subjects’ factors will verify whether the Emo VST is susceptible to the effects of cardiac signals. A two-way repeated measures ANOVA with emotion and beverage as within subjects’ factors will verify the effects of alcohol administration on emotional processing.
Hypothesis 1:
In order to study whether alcohol will increase the cardiac amplification of some emotions and decrease it for other emotions, we will compute difference scores (systole-diastole) in accuracy for each emotion and introduce them in a two-way repeated measures ANOVA with beverage and emotion as within subjects’ effects. A significant two-way interaction will be followed up by paired-samples t-tests comparing difference scores for each emotion for alcohol and placebo.
Hypothesis 2:
In order to study the effects of alcohol administration on magnitude of cardiac amplification score, we will compute the absolute mean of the difference scores (systole-diastole) in accuracy for all emotions (1). A paired samples t-test will compare magnitude of cardiac amplification score after alcohol and placebo administration.
| (1) |
Hypothesis 3:
In order to study the cardiovascular mechanisms underlying individual differences in the cardiac amplification of emotional processing, 0.1Hz HRV, HF-HRV and BRS at baseline will be introduced as covariates of the scores obtained in the Emo VST in the placebo condition. For magnitude of cardiac amplification score, two Pearson’s correlation will examine this correlation. To study this effect independently for each emotion, three two-way linear-mixed models with emotion as a within subjects’ factor and 0.1Hz HRV, HF-HRV or BRS at baseline as continuous predictors will be performed. A significant interaction will be followed by a simple-slope analysis in which we will examine whether physiological responses correlate independently with the cardiac amplification of different emotions.
Hypothesis 4:
In order to study the physiological mechanisms by which alcohol administration disrupts the interaction between cardiac synchrony and emotion, we will compute the difference in physiological responding before and after beverage administration (PhysioΔ). For magnitude of cardiac amplification score we will conduct two 2-way linear mixed models with condition as within subjects’ factor and PhysioΔ after alcohol and placebo (0.1Hz HRV, HF-HRV or BRS separately) as a continuous predictor. A significant interaction will be followed up with a correlation between magnitude of cardiac amplification score and PhysioΔ separately for the alcohol and placebo conditions.
To study this effect independently for each emotion, we will conduct three three-way linear mixed models with condition and emotion as within subjects’ factors, and PhysioΔ after alcohol and placebo (0.1Hz HRV, HF-HRV or BRS separately) as a continuous predictor. A significant interaction will be followed up by 2-way models examining the interaction between PhysioΔ and emotion separately for alcohol and placebo, further followed up by simple slope analyses for each emotion.
Each model will be examined for influential data points through informal graphical techniques and formal statistical criteria. Q–Q plots of the raw residuals will be examined to check distributional assumptions (Garson, 2019; Littell et al., 2006; Tabachnick & Fidell, 2014; West et al., 2007). The plots of studentized and Pearson deleted residuals will be examined to identify influential data points. Cook’s D and Restricted Likelihood Distance (RLD) for each subject will be computed (Garson, 2019; West et al., 2007). For Cook’s D, a value > 1 is generally considered problematic (Fox, 2008), but the decision will be made in combination with a graphical examination of the distribution of D values. For RLD, in the absence of specific cut offs, extreme values that stand out from the rest of the distribution in a model will be considered to have an undue influence at the global level of all parameters (Garson, 2019). If any observations were determined to be unduly influential for the regression model, they will be removed from the analysis, and the regression will be rerun. Descriptive statistics and graphical representations of the outlier and assumption analyses will be provided as supplementary materials. F values will be used to determine significance of interactions and main effects.
A paired samples t-test will examine differences between alcohol and placebo sessions in the amount of standard drinks participants think they have consumed and descriptive statistics will be reported.
3.3. Sampling plan:
A power analysis determined the adequate sample size for the outcomes of VSA and EIA at a power level of 0.90 and α = .02. According to previous research, alcohol administration has a medium to large effect size on emotional processing (i.e. Felisberti & Terry 2015; Honan et al. 2018), but we conservatively consider small-medium effect sizes for the final sampling plan. The power analysis for Hypotheses 1 and 2 was conducted by using an “exemplary dataset” approach in which a dataset is constructed to specify the means, variances, and covariances of a set of hypothesized results (Littell et al., 2006). The hypothesized differences among independent variables are specified at particular levels of an effect size. These population parameters are then used by way of analytical approximation to compute the underlying non-centrality parameters associated with each statistical main effect and interaction of interest (Littell et al., 2006; Stroup, 2002). All power analyses were conducted using SAS PROC GLMPOWER. All power analyses were computed separately for VSA and EIA.
Hypothesis 1:
Previously published results (Leganes-Fonteneau et al. 2020a) were used as pilot data for happy, fear, sad, and disgust faces, assigning them to the placebo condition because they act as baseline control. All raw values of systole and diastole were arcsine square-root transformed as they were extracted on a percentile scale (0 – 1) and difference scores were subsequently computed (systole-diastole) as a measure of the effects of cardiac synchrony on each emotion (see Tables 1 and 2). As no pilot data were available for the angry faces, we simulated the plausible theoretical mean effects of cardiac synchrony on angry faces based on the visual inspection and extrapolation of the published data for the four emotions of happy, fear, sad, disgust. The SD value of cardiac synchrony on angry faces was derived by computing the mean of the SDs for the four observed emotional conditions in the pilot dataset. We specify, for the placebo condition, that the mean VSA for angry faces at systole would be 1.571, SD = 0.309, and for diastole would be 1.471, SD = 0.292, and that mean EIA for angry faces at systole would be 1.450, SD = 0.429, and for diastole would be 1.600, SD = 0.436.
Table 1 -.
Mean and SD of visual search accuracy for each emotion based on existing data.
| Diastole | Systole | Difference Score | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Disgust | 1.592 | 0.329 | 1.652 | 0.328 | 0.060 | 0.252 |
| Fear | 1.565 | 0.264 | 1.476 | 0.285 | −0.088 | 0.247 |
| Happy | 1.670 | 0.330 | 1.809 | 0.365 | 0.139 | 0.268 |
| Sad | 1.233 | 0.246 | 1.199 | 0.259 | −0.035 | 0.294 |
| Anger* | 1.471 | 0.292 | 1.571 | 0.309 | 0.100 | 0.265 |
We used previous results to interpolate means and SD for anger.
Table 2 -.
Mean and SD of emotion identification accuracy for each emotion based on existing data.
| Diastole | Systole | Difference Score | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Disgust | 1.492 | 0.369 | 1.552 | 0.394 | 0.060 | 0.396 |
| Fear | 1.668 | 0.460 | 1.451 | 0.360 | −0.217 | 0.502 |
| Happy | 2.222 | 0.371 | 2.177 | 0.361 | −0.045 | 0.455 |
| Sad | 1.573 | 0.543 | 1.342 | 0.602 | −0.231 | 0.702 |
| Anger* | 1.600 | 0.436 | 1.450 | 0.429 | −0.150 | 0.514 |
We used previous results to interpolate means and SD for anger.
Our main interest is the detection of a significant interaction between the two conditions (alcohol vs placebo) and the five emotions. Three exemplary datasets simulated the interaction between condition and emotion for small-medium (d = .35), medium (d = .5), and large (d = .8) effect sizes, based on Cohen’s (1988) d.
The interaction between condition and emotion at different levels of effect size was simulated. We computed the difference scores for each emotion in the alcohol condition with respect to their counterparts in the placebo condition according to the specified effect size (see equation 1). The equation for computing each hypothesized emotion in the alcohol condition (e.g. mean ) was derived from the equation for Cohen’s d (2):
| (2) |
in which and SDplacebo Happy are the observed mean and SD for the difference scores of happy faces in the placebo condition, and d varies based on which effect size is specified. Thus, a lower or upper value for the hypothesized effect of alcohol on each emotion can be obtained, which is then used to create an overall interaction pattern that corresponds to those effect sizes. We hypothesized that difference scores would decrease in the alcohol condition with respect disgust, fear, sad and anger faces in the placebo condition, and that they would increase in the alcohol condition for the happy faces. The SDs for each emotion in the placebo condition were also used to specify the SDs for their counterparts in the alcohol condition.
The power analysis for Hypothesis 1 required a 10 × 10 positive-definite correlation matrix among all the possible combinations of alcohol and emotion. Pilot data were available for four of the emotional faces in the placebo condition but not for anger (see Tables A1 and A2 in appendix), and therefore the correlations of anger with the other emotions in the placebo condition, all correlations in the alcohol condition, and the intercorrelations across placebo and alcohol, had to be simulated. This correlation matrix was first specified in the placebo condition. For that purpose, the observed values among the four placebo emotion categories in the pilot dataset were subjected to a principal factor analysis with an oblique promax rotation, in which all components with eigenvalues > 1 were retained. This yielded two factors with a correlation below .32, which is considered trivial as it explains only 10% of the variance (Tabachnick & Fidell, 2014), for both VSA and EIA. Thus, the factor analysis was repeated using an orthogonal varimax rotation, in which all components with eigenvalues > 1 were retained, again yielding a two-factor solution of loadings. The resulting loading tables were inspected to determine how the observed placebo-emotions clustered. Hypothesized loadings for angry faces were then specified based on scientific theorizing of how anger would relate to the clusters of observed emotions as defined by the extracted components. (see Tables 3 and 4). The matrix of loadings, A, for all emotions, including anger, was then turned back into a correlation matrix, R, among the five emotions in the placebo condition (see Tables 5 and 6) using the formula (3):
| (3) |
Table 3 -.
Factor loadings of the varimax rotated solution for difference scores in visual search accuracy.
| Factor 1 | Factor 2 | |
|---|---|---|
| Disgust | 0.161 | 0.700 |
| Fear | −0.129 | 0.729 |
| Happy | 0.743 | −0.100 |
| Sad | 0.748 | 0.136 |
| Anger* | 0.150 | 0.600 |
We used those results to interpolate loadings for Anger in each of the two factors.
Table 4 -.
Factor loadings of the varimax rotated solution for difference scores in emotion identification accuracy.
| Factor 1 | Factor 2 | |
|---|---|---|
| Disgust | 0.213 | 0.795 |
| Fear | 0.775 | 0.194 |
| Happy | 0.663 | −0.232 |
| Sad | 0.316 | −0.647 |
| Anger* | 0.600 | 0.150 |
We used those results to interpolate loadings for Anger in each of the two factors.
Table 5 -. Final correlation matrix among all repeated measures in the statistical model for Hypothesis 1 for visual search accuracy:
For the placebo condition (upper left triangular matrix) correlations are based on the loadings obtained in the factor analysis. For the alcohol condition (upper right triangular matrix), we assumed the same correlation matrix as in the placebo condition. For the intercorrelation between placebo and alcohol (lower left block), we assumed the same correlation matrix as in the placebo condition. For the correlation of each emotion between placebo and alcohol we assumed a stability coefficient of 0.7.
| PLACEBO | ALCOHOL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| disgust | fear | happy | sad | anger | disgust | fear | happy | sad | anger | |
| PLACEBO | ||||||||||
| Disgust | 1.000 | |||||||||
| Fear | 0.490 | 1.000 | ||||||||
| Happy | 0.049 | −0.169 | 1.000 | |||||||
| Sad | 0.216 | 0.003 | 0.542 | 1.000 | ||||||
| Anger | 0.444 | 0.418 | 0.051 | 0.194 | 1.000 | |||||
| ALCOHOL | ||||||||||
| Disgust | 0.700 | 0.490 | 0.049 | 0.216 | 0.444 | 1.000 | ||||
| Fear | 0.490 | 0.700 | −0.169 | 0.003 | 0.418 | 0.490 | 1.000 | |||
| Happy | 0.049 | −0.169 | 0.700 | 0.542 | 0.051 | 0.049 | −0.169 | 1.000 | ||
| Sad | 0.216 | 0.003 | 0.542 | 0.700 | 0.194 | 0.216 | 0.003 | 0.542 | 1.000 | |
| Anger | 0.444 | 0.418 | 0.051 | 0.194 | 0.700 | 0.444 | 0.418 | 0.051 | 0.194 | 1.000 |
Table 6 -. Final correlation matrix among all repeated measures in the statistical model for Hypothesis 1 for emotion identification accuracy:
For the placebo condition (upper left triangular matrix) correlations are based on the loadings obtained in the factor analysis. For the alcohol condition (upper right triangular matrix), we assumed the same correlation matrix as in the placebo condition. For the intercorrelation between placebo and alcohol (lower left block), we assumed the same correlation matrix as in the placebo condition. For the correlation of each emotion between placebo and alcohol we assumed a stability coefficient of 0.7.
| PLACEBO | ALCOHOL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| disgust | fear | happy | sad | anger | disgust | fear | happy | sad | anger | |
| PLACEBO | ||||||||||
| Disgust | 1.000 | |||||||||
| Fear | 0.319 | 1.000 | ||||||||
| Happy | −0.043 | 0.468 | 1.000 | |||||||
| Sad | −0.447 | 0.119 | 0.360 | 1.000 | ||||||
| Anger | 0.247 | 0.494 | 0.363 | 0.092 | 1.000 | |||||
| ALCOHOL | ||||||||||
| Disgust | 0.700 | 0.319 | −0.043 | −0.447 | 0.247 | 1.000 | ||||
| Fear | 0.319 | 0.700 | 0.468 | 0.119 | 0.494 | 0.319 | 1.000 | |||
| Happy | −0.043 | 0.468 | 0.700 | 0.360 | 0.363 | −0.043 | 0.468 | 1.000 | ||
| Sad | −0.447 | 0.119 | 0.360 | 0.700 | 0.092 | −0.447 | 0.119 | 0.360 | 1.000 | |
| Anger | 0.247 | 0.494 | 0.363 | 0.092 | 0.700 | 0.247 | 0.494 | 0.363 | 0.092 | 1.000 |
A principal factor analysis was then done on the computed correlation matrix to verify that it was positive definite and contained mathematically and scientifically plausible values.
For the alcohol condition, the intercorrelation matrix among the five emotions was specified to be equivalent to that of the placebo condition. This correlation matrix was also used to specify the intercorrelations among the 5 emotions across the placebo and alcohol conditions. The correlation for each emotion between its counterpart across the placebo and alcohol conditions (e.g., correlation for disgust between the placebo and alcohol conditions) was specified at .70 because we hypothesized that each emotion would have a moderately high degree of stability across drinking conditions within the same group of participants, without reaching a perfect correlation of 1 (see Tables 5 and 6). A principal factor analysis was then done on the final 10 × 10 correlation matrix to verify that it was positive definite and contained mathematically and scientifically plausible values.
The power analysis was then conducted to determine the sample size needed to detect small-medium, medium, and large condition × emotion interaction effects at a power level of .90. For VSA, we found that n = 36 would yield a power = 0.90 assuming a small-medium effect size, see Figure 3-A. For emotion identification accuracy, we found that n = 12 would yield a power = 0.90 assuming a small-medium effect size, see Figure 3-B.
Figure 3 -.
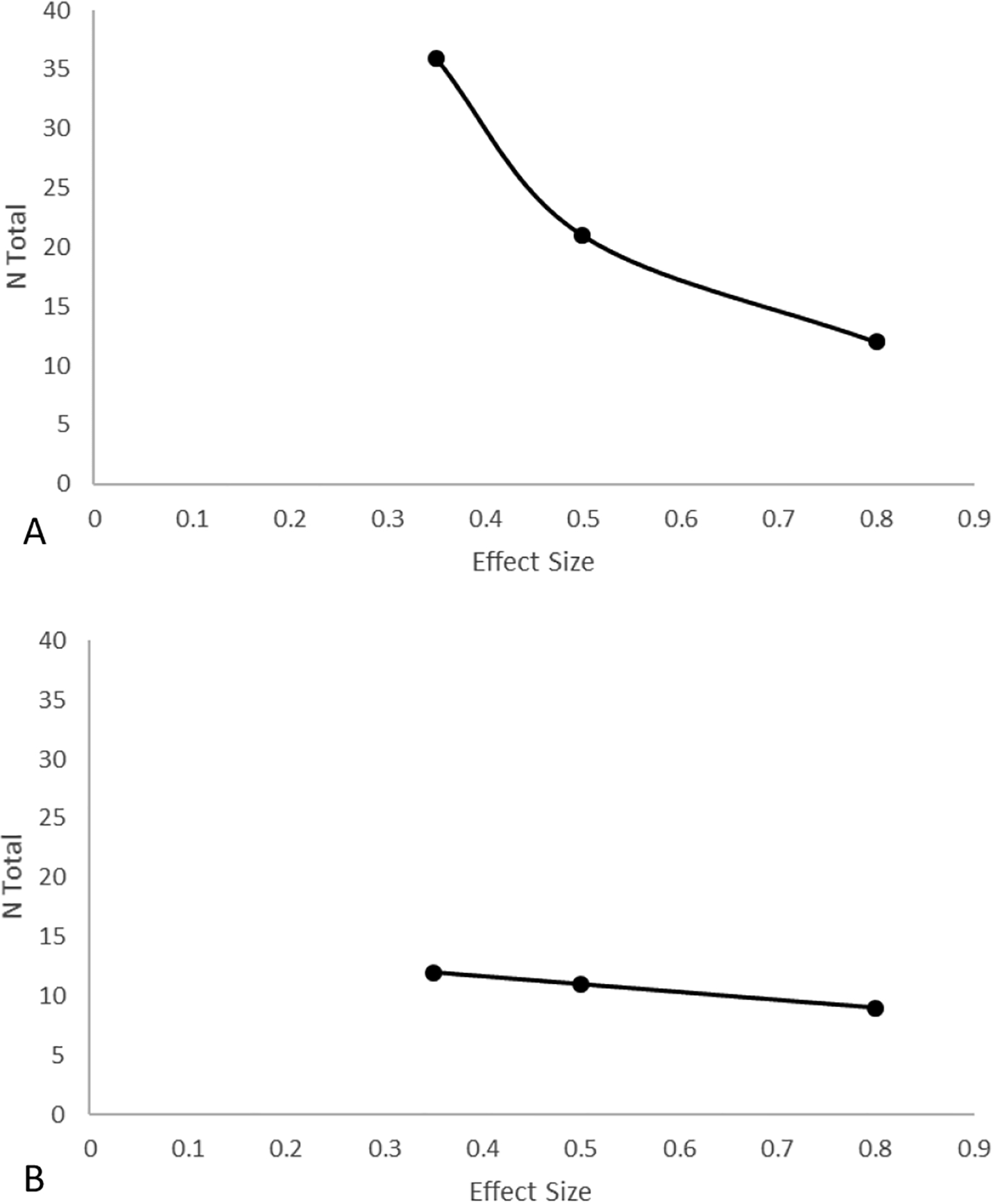
Power curve for Hypothesis 1 in visual search accuracy (A) and emotion identification accuracy (B) for a power = 0.90 and α = .02.
Hypothesis 2:
As a sequel to Hypothesis 1, we are interested in modelling MCA, irrespective of directionality of change. A power analysis determined the adequate sample size for the outcomes of VSA and EIA, separately.
To obtain an estimate of MCA for the placebo condition, we calculated the mean of the absolute difference scores (systole-diastole) for each of the observed emotions based on the existing data, VSA = 0.216, EIA = 0.394.
The variance of the averaged observed emotion values, , was computed using the equation for the variance of a composite distribution based on averaged multiple distributions (4):
| (4) |
in which, is the variance of each individual ith emotional category, Xi represents each individual ith emotional category, cov(Xi,Xj) is the covariance of each possible pairwise combination of the emotional categories, and n is the number of emotional categories. For VSA, SD = 0.0939, for EIA, SD = 0.2111.
For the alcohol condition, we specified a mean corresponding to each hypothesized level of effect size for each emotion as outlined above and then computed the average among the means. The correlation between the placebo and alcohol conditions again was specified as 0.70.
We obtained power estimates using PROC GLMPOWER, and for VSA, we found that n = 19 would yield a power = 0.90 assuming a small-medium effect size, see Figure 4-A. For EIA, we found that n = 31 would yield a power = 0.90 assuming a small-medium effect size, see Figure 4-B.
Figure 4 -.
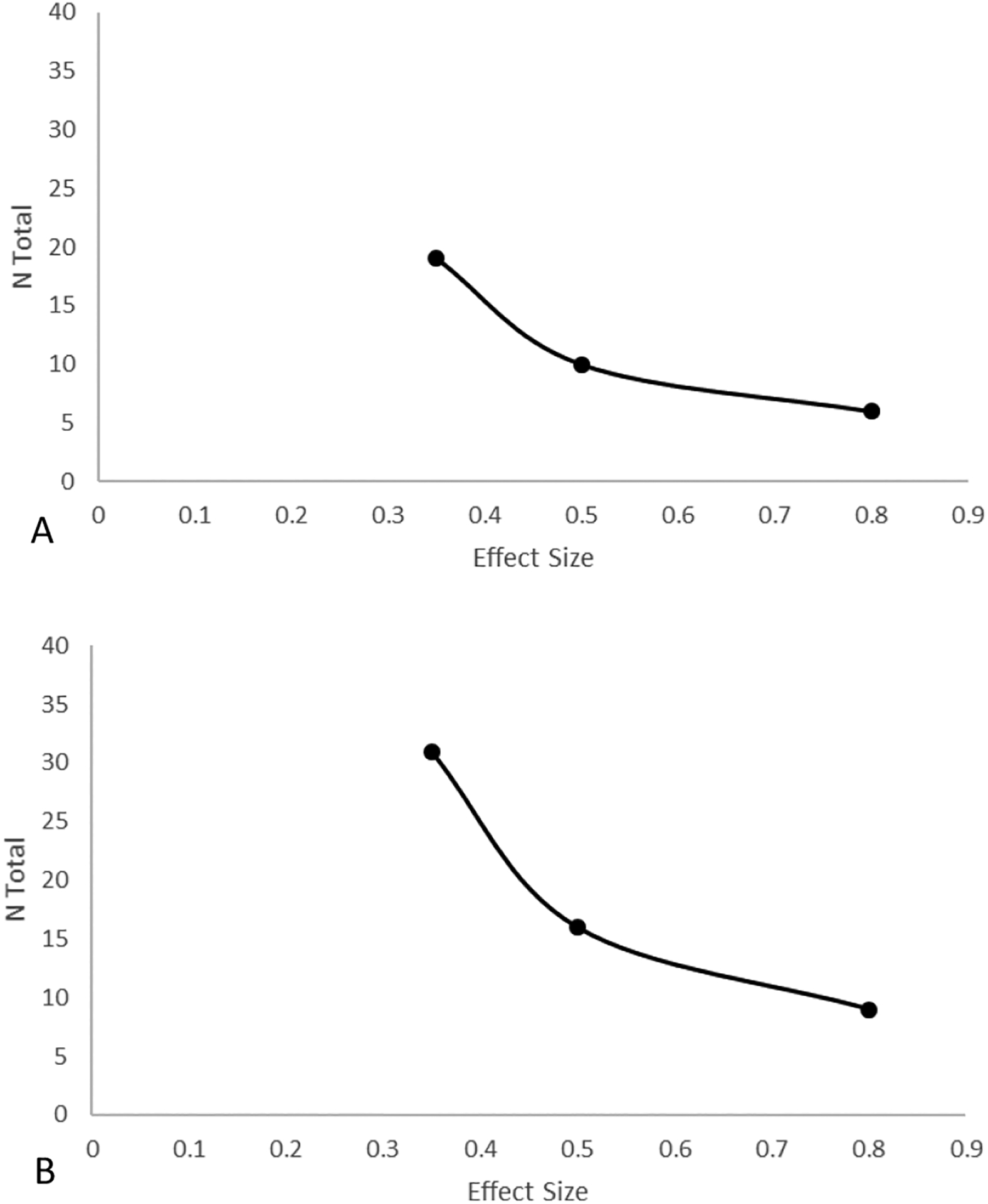
Power curve for Hypothesis 2 in visual search accuracy (A) and emotion identification accuracy (B) for a power = 0.90 and α = .02.
Supplementary Material
Disclosure:
We disclose no conflicts of interest.
This research was supported in part by grants R01AA023667 and K02AA025123 from the National Institute of Alcohol Abuse and Alcoholism.
MLF was responsible for conceptual and experimental design and manuscript writing. AP participated in data and power analysis. MEB and JFB participated in experimental design and manuscript writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
Previously published results used as pilot data are available on https://osf.io/s7auj/?view_only=7464b0cab0b141c89860c2baf342f648. All raw and compiled data collected during the experiment will be made available on OSF upon acceptance of the Stage 2 manuscript.
Code availability
The Matlab Psychtoolbox code for the experimental task, and the code used to generate the power analysis are available on https://osf.io/s7auj/?view_only=7464b0cab0b141c89860c2baf342f648. All codes will be shared publicly upon acceptance of the Stage 2 manuscript.
References:
- Abdel-Rahman ARA, Merrill RH, & Wooles WR (1987). Effect of acute ethanol administration on the baroreceptor reflex control of heart rate in normotensive human volunteers. Clinical Science, 72(1), 113–122. 10.1042/cs0720113 [DOI] [PubMed] [Google Scholar]
- Attwood AS, Ohlson C, Benton CP, Penton-Voak IS, & Munafò MR (2009). Effects of acute alcohol consumption on processing of perceptual cues of emotional expression. Journal of Psychopharmacology, 23(1), 23–30. 10.1177/0269881108089604 [DOI] [PubMed] [Google Scholar]
- Azevedo RT, Badoud D, & Tsakiris M (2018). Afferent cardiac signals modulate attentional engagement to low spatial frequency fearful faces. Cortex, 104, 232–240. 10.1016/j.cortex.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Azevedo RT, Garfinkel SN, Critchley HD, & Tsakiris M (2017). Cardiac afferent activity modulates the expression of racial stereotypes. Nature Communications, 8. 10.1038/ncomms13854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. In Handbook of emotions (2nd ed., pp. 173–191). [Google Scholar]
- Calvo MG, & Nummenmaa L (2008). Detection of emotional faces: Salient physical features guide effective visual search. Journal of Experimental Psychology: General, 137(3), 471–494. 10.1037/a0012771 [DOI] [PubMed] [Google Scholar]
- Carbia C, Lannoy S, Maurage P, López-Caneda E, O’Riordan KJ, Dinan TG, & Cryan JF (2020). A biological framework for emotional dysregulation in alcohol misuse: from gut to brain. In Molecular Psychiatry (pp. 1–21). Springer Nature. 10.1038/s41380-020-00970-6 [DOI] [PubMed] [Google Scholar]
- Cevese A, Gulli G, Polati E, Gottin L, & Grasso R (2001). Baroreflex and oscillation of heart period at 0.1 Hz studied by α-blockade and cross-spectral analysis in healthy humans. Journal of Physiology, 531(1), 235–244. 10.1111/j.1469-7793.2001.0235j.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Critchley H, & Garfinkel S (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7–14. 10.1016/J.COPSYC.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Dolder PC, Holze F, Liakoni E, Harder S, Schmid Y, & Liechti ME (2017). Alcohol acutely enhances decoding of positive emotions and emotional concern for positive stimuli and facilitates the viewing of sexual images. Psychopharmacology, 234(1), 41–51. 10.1007/s00213-016-4431-6 [DOI] [PubMed] [Google Scholar]
- Donadon MF, & Osório F. de L. (2014). Recognition of facial expressions by alcoholic patients: a systematic literature review. Neuropsychiatric Disease and Treatment, 10, 1655–1663. 10.2147/NDT.S65376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Stephens DN, Russell C, & Tasker R (1998). Discriminative stimulus properties of low doses of ethanol in humans. Psychopharmacology, 136(4), 379–389. 10.1007/s002130050581 [DOI] [PubMed] [Google Scholar]
- Eddie D, Bates ME, & Buckman JF (2020). Closing the brain–heart loop: Towards more holistic models of addiction and addiction recovery. Addiction Biology. 10.1111/adb.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felisberti F, & Terry P (2015). The effects of alcohol on the recognition of facial expressions and microexpressions of emotion: enhanced recognition of disgust and contempt. Human Psychopharmacology: Clinical and Experimental, 30(5), 384–392. 10.1002/hup.2488 [DOI] [PubMed] [Google Scholar]
- Friedman BH (2010). Feelings and the body: The Jamesian perspective on autonomic specificity of emotion. In Biological Psychology (Vol. 84, Issue 3, pp. 383–393). Elsevier. 10.1016/j.biopsycho.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, & Critchley HD (2016). Threat and the Body: How the Heart Supports Fear Processing. Trends in Cognitive Sciences, 20(1), 34–46. 10.1016/J.TICS.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, & Engels M (2016). Interoceptive dimensions across cardiac and respiratory axes. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), 20160014. 10.1098/rstb.2016.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, & Critchley HD (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(19), 6573–6582. 10.1523/JNEUROSCI.3507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson G (2019). Multilevel Modeling: Applications in STATA®, IBM® SPSS®, SAS®, R, & HLMTM. https://books.google.es/books?hl=en&lr=&id=LLKcDwAAQBAJ&oi=fnd&pg=PT15&dq=G.+David+Garson.+(2020).+Multilevel+modeling:+Applications+in+Stata&ots=iF63hlxw5i&sig=MP0xGbNIvHc9lMX1N3Sexn0urps
- Gray MA, Minati L, Paoletti G, & Critchley HD (2010). Baroreceptor activation attenuates attentional effects on pain-evoked potentials. Pain, 151(3), 853–861. 10.1016/j.pain.2010.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honan CA, Skromanis S, Johnson EG, & Palmer MA (2018). Alcohol intoxication impairs recognition of fear and sadness in others and metacognitive awareness of emotion recognition ability. Emotion, 18(6), 842–854. 10.1037/emo0000404 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (2007). Alternate Cardiovascular Baseline Assessment Techniques: Vanilla or Resting Baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Joye A, Bisby JA, Das RK, Platt B, & Curran HV (2013). Processing of facial affect in social drinkers: A dose-response study of alcohol using dynamic emotion expressions. Psychopharmacology, 227(1), 31–39. 10.1007/s00213-012-2940-5 [DOI] [PubMed] [Google Scholar]
- Khouja J, … A. A.-J. of, & 2019, undefined. (2019). Effects of acute alcohol consumption on emotion recognition in social alcohol drinkers. Journals.Sagepub.Com, 33(3), 326–334. 10.1177/0269881118822169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen P, Vlrolalnen J, & Kupari M (1994). Acute alcohol intake decreases short-term heart rate variability in healthy subjects. In Clinical Science (Vol. 87). [DOI] [PubMed] [Google Scholar]
- Lang PJ (1994). The Varieties of Emotional Experience: A Meditation on James-Lange Theory. Psychological Review, 101(2), 211–221. 10.1037/0033-295X.101.2.211 [DOI] [PubMed] [Google Scholar]
- Lannoy S, Duka T, Carbia C, Billieux J, Fontesse S, Dormal V, Gierski F, López-Caneda E, Sullivan EV, & Maurage P (2021). Emotional processes in binge drinking: A systematic review and perspective. Clinical Psychology Review, 101971. 10.1016/j.cpr.2021.101971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leganes-Fonteneau M, Buckman JF, Suzuki K, Pawlak A, & Bates ME (2020). More than meets the heart: systolic amplification of different emotional faces is task dependent. Cognition and Emotion. 10.1080/02699931.2020.1832050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leganes-Fonteneau M, Buckman J, Islam S, Pawlak A, Vaschillo B, Vaschillo E, & Marsha Bates M (n.d.). The Cardiovascular Mechanisms of Interoceptive Awareness: Effects of Resonance Breathing. International Journal of Psychophysiology, Stage 1 Re. [DOI] [PubMed] [Google Scholar]
- Leganes-Fonteneau Mateo, Bates ME, Vaschillo EG, & Buckman JF (2021). An interoceptive basis for alcohol priming effects. Psychopharmacology, 1–11. 10.1007/s00213-021-05796-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leganes-Fonteneau Mateo, Pi-Ruano M, & Tejero P (2019). Early Signs of Emotional Recognition Deficits in Adolescent High-Binge Drinkers. Substance Use and Misuse. 10.1080/10826084.2019.1662810 [DOI] [PubMed] [Google Scholar]
- Leganes-Fonteneau M, Buckman J, Pawlak A, Vaschillo B, Vaschillo E, & Bates M (2020). Interoceptive signaling in alcohol cognitive biases: Role of family history and alliesthetic components. Addiction Biology. 10.1111/adb.12952 [DOI] [PubMed] [Google Scholar]
- Legrand N, & Allen M (2021). The heart rate discrimination task: a psychophysical method to estimate the accuracy and precision of interoceptive beliefs. BioRxiv, 1, 2. 10.1101/2021.02.18.431871 [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, & Schabenberger O (2006). SAS for Mixed models. Analysis of Repeated Measured Data, 159–204. https://ci.nii.ac.jp/naid/10030959065/ [Google Scholar]
- Lundqvist D, Flykt A, & Ohman A (1998). The Karolinska directed emotional faces (KDEF). In CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet (pp. 91–630). Karolinska Institutet, Department of Clinical Neuroscience. 10.1017/S0048577299971664 [DOI] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, & Swift RM (1993). Development and Validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research, 17(1), 140–146. 10.1111/j.1530-0277.1993.tb00739.x [DOI] [PubMed] [Google Scholar]
- Mehrabian A, & Russell JA (1978). A questionnaire measure of habitual alcohol use. Psychological Reports, 43(3), 803–806. 10.2466/pr0.1978.43.3.803 [DOI] [PubMed] [Google Scholar]
- Miller MA, Bershad AK, & de Wit H (2015). Drug effects on responses to emotional facial expressions: recent findings. Behavioural Pharmacology, 26(6), 571–579. 10.1097/FBP.0000000000000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Treat TA (2012). The Anticipated Effects of Alcohol Scale: development and psychometric evaluation of a novel assessment tool for measuring alcohol expectancies. Psychological Assessment, 24(4), 1008–1023. 10.1037/a0028982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, & Schulteis G (2009). The role of interoception and alliesthesia in addiction. Pharmacology Biochemistry and Behavior, 94(1), 1–7. 10.1016/J.PBB.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De JR, Fuente ’ L, & Grant ’ M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Sayette MA (2017). The effects of alcohol on emotion in social drinkers. Behaviour Research and Therapy, 88, 76–89. 10.1016/J.BRAT.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, Kirchner TR, Levine JM, & Moreland RL (2012). Alcohol and Group Formation. Psychological Science, 23(8), 869–878. 10.1177/0956797611435134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, & Friston KJ (2016). Active interoceptive inference and the emotional brain. In Philosophical Transactions of the Royal Society B: Biological Sciences (Vol. 371, Issue 1708). Royal Society of London. 10.1098/rstb.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Abbey A, & Scott RO (1993). Reasons for drinking alcohol: Their relationship to psychosocial variables and alcohol consumption. Substance Use and Misuse, 28(9), 881–908. 10.3109/10826089309039662 [DOI] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, & Phan KL (2011). Effects of alcohol on brain responses to social signals of threat in humans. NeuroImage, 55(1), 371–380. 10.1016/j.neuroimage.2010.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford LD, Sekulla A, Morrison E, Fleischman DS, & Harvey AJ (2020). Alcohol and disgust: An intimate relationship. Drug and Alcohol Dependence, 206, 107780. 10.1016/j.drugalcdep.2019.107780 [DOI] [PubMed] [Google Scholar]
- Stevens S, Rist F, & Gerlach AL (2009). Influence of alcohol on the processing of emotional facial expressions in individuals with social phobia. British Journal of Clinical Psychology, 48(2), 125–140. 10.1348/014466508X368856 [DOI] [PubMed] [Google Scholar]
- Stroup WW (2002). Power analysis based on spatial effects mixed models: A tool for comparing design and analysis strategies in the presence of spatial variability. Journal of Agricultural, Biological, and Environmental Statistics, 7(4), 491–511. 10.1198/108571102780 [DOI] [Google Scholar]
- Tabachnick BG, & Fidell LS (2014). Using multivariate statistics new international edition. Pearson2012. [Google Scholar]
- Task Force O, & Of, the E. S. of C. and the N. A. S. (1996). Heart rate variability. Standards of measurement, physiologic interpretation, and clinical use. Circulation, 93(1043–1065). [PubMed] [Google Scholar]
- Townshend J, & Duka T (2002). Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol and Alcoholism. http://alcalc.oxfordjournals.org/content/37/2/187.short [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, & Ray S (2008). Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-Hz stimulation. Psychophysiology, 45(5), 847–858. 10.1111/j.1469-8986.2008.00673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, & Bates ME (2012). Measurement of vascular tone and stroke volume baroreflex gain. Psychophysiology, 49(2), 193–197. 10.1111/j.1469-8986.2011.01305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo E, Lehrer P, Rishe N, & Konstantinov M (2002). Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback, 27(1), 1–27. http://www.ncbi.nlm.nih.gov/pubmed/12001882 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, & Gatecki AT (2007). Linear mixed models: An overview. In A Practical Guide Using Statistical Software. [Google Scholar]
- Williams MA, Moss SA, Bradshaw JL, & Mattingley JB (2005). Look at me, I’m smiling: Visual search for threatening and nonthreatening facial expressions. Visual Cognition, 12(1), 29–50. 10.1080/13506280444000193 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


