Abstract
Cutaneous mast cells (MCs) express Mas-related G protein-coupled receptor-X2 (MRGPRX2; mouse ortholog MrgprB2), which is activated by an ever-increasing number of cationic ligands. Antimicrobial host defense peptides (HDPs) generated by keratinocytes contribute to host defense likely by two mechanisms; one involving direct killing of microbes and the other via MC activation through MRGPRX2. However, its inappropriate activation may cause pseudoallergy and likely contribute to the pathogenesis of rosacea, atopic dermatitis, allergic contact dermatitis, urticaria and mastocytosis. Gain- and loss-of-function missense single nucleotide polymorphisms in MRGPRX2 have been identified. The ability of certain ligands to serve as balanced or G protein-biased agonists have been defined. Small molecule HDP mimetics have been developed that display both direct antimicrobial activity and activate MCs via MRGPRX2. In addition, antibodies and reagents have been generated that modulate MRGPRX2 expression and signaling. In this article, we provide a comprehensive update on MrgprB2 and MRGPRX2 biology. We propose that harnessing MRGPRX2’s host defense function by small molecule HDP mimetics may provide a novel approach for the treatment of antibiotic-resistant cutaneous infections. By contrast, MRGPRX2-specific antibodies and inhibitors could be utilized for the modulation of allergic and inflammatory diseases that are mediated via this receptor.
Keywords: G protein–coupled receptor, host defense peptides, MRGPRX2, MrgprB2, mast cells, neurogenic inflammation, missense mutation
INTRODUCTION
Mast cells (MCs) are granulated tissue-resident cells of hematopoietic lineage that are best known for their roles in allergic reactions, but they also play an important role in host defense and wound healing.1–3 In human, most MCs found in the skin contain tryptase, chymase, carboxypeptidase and cathepsin, and are known as MCTC. By contrast, majority of MCs that are found in the lung and gut express only tryptase, and are known as MCT.4 In rodents, connective tissue MCs (CTMCs) resemble MCTC and are found in the nasopharynx, skin, peritoneal cavity, and muscularis propria, whereas mucosal MCs (MMCs) resemble MCT and populate the mucosa of the gastrointestinal tract. While both subtypes express a high affinity IgE receptor (FcεRI), MCTC and CTMCs undergo degranulation via an IgE/FcεRI-independent pathway by a diverse group of basic molecules including neuropeptides, antimicrobial host defense peptides (HDPs), FDA-approved drugs and the cationic polymer compound 48/80 (C48/80). Until recently, these secretagogues were thought to activate CTMCs and MCTC via direct activation of G proteins through a receptor-independent mechanism.5, 6
MRGPRX2 is an acronym for Mas-related G protein-coupled receptor X2, which is one of nine members of the human MRGPR family.7 In 2006, Tatemoto et al.,8 provided the first demonstration that MRGPRX2 is expressed in MCs. Using transfected cell lines, it was demonstrated that basic secretagogues that induce degranulation in MCTC selectively activate this receptor. Recent transcriptome analysis revealed that while most MRGPR family members are expressed in peripheral neurons, MRGPRX2 is found predominantly in skin MCs.9–11 Human basophils and eosinophils have recently been shown to express MRGPRX2.12–14 However, functional consequence of this expression is not clear as basophils from healthy individuals do not respond to opiates, vancomycin and fluoroquinolones, which activate MCs via MRGPRX2.15–19
A major complication in studying MRGPRX2 function in vivo is that the gene cluster containing the nine human MRGPRX members is dramatically expanded in mice, consisting of 22 potential Mrg coding genes. In 2015, McNeil et al.,20 made the seminal observation that murine CTMCs express the transcript of a single family member, MrgprB2, and demonstrated that it is the mouse ortholog of human MRGPRX2.20 It is noteworthy that there is only ~53% overall sequence similarity between MrgprB2 and MRGPRX2.21 This difference is reflected in the concentrations of ligands that are required to activate these receptors.20, 22 Despite these differences, MrgprB2 promotes host defense against bacterial infection, but also contributes to neurogenic inflammation, pain, atopic dermatitis (AD), allergic contact dermatitis (ACD), non-histaminergic itch and pseudoallergy in mice.20, 23–27
Most of the studies performed in the last 5 years with MrgprB2 and MRGPRX2 focused on cutaneous health and disease. In this article, we first review the potential roles of MrgprB2 and MRGPRX2 on cutaneous host defense and then describe how their inappropriate activation may contribute to the pathogenesis of rosacea, AD, ACD, non-histaminergic itch, pseudoallergy and mastocytosis. Clinical implications of naturally occurring missense MRGPRX2 mutations in health and disease are also discussed. Furthermore, we propose that harnessing MRGPRX2’s host defense properties could provide a novel therapeutic approach for the treatment of antibiotic-resistant cutaneous infections. By contrast, inhibiting its inappropriate activation may lead to the development of new treatment options for a number of allergic and inflammatory disorders.
MrgprB2 AND MRGPRX2 IN MC-MEDIATED HOST DEFENSE AGAINST BACTERIAL INFECTION
Cells related to MCs (test cells) have been recognized in the invertebrate urochordate, which appeared approximately 500 million years ago.28 These cells contain heparin and histamine and undergo degranulation in response to C48/80, a potent basic secretagogue for mouse CTMCs and human MCTC via MrgprB2 and MRGPRX2, respectively.29, 30 It has been proposed that test cells represent ancient effector cells of the innate immunity in primitive chordates and raises the interesting possibility that MrgprB2 and MRGPRX2 contribute to host defense in mice and humans, respectively. Interestingly, MRGPRX2 gene has undergone positive selection in human evolution supporting its possible beneficial role.31
Quorum-sensing molecules (QSMs) including competence-stimulating peptides (CSPs) are secreted by bacteria to signal population density. Pundir et al.,25 made the remarkable observation that CSP-1 causes degranulation, TNF-α secretion, reactive oxygen species (ROS) and prostaglandin D2 (PGD2) generation in mouse PMCs via MrgprB2, resulting in the inhibition of bacterial growth and prevention of biofilm formation. Nasopharynx, peritoneum and skin are enriched with MrgprB2-expressing CTMCs and are the major portal of entry for bacteria.25 There is now evidence to show that MrgprB2 confers protective immunity against bacterial infection at all three sites. In a pneumococcal nasopharyngeal colonization model, MrgprB2MUT mice exhibit impaired bacterial clearance when compared to wild-type (WT) mice. In a peritoneal infection model, MrgprB2MUT mice display higher bacterial load after infection with vancomycin-resistant Enterococcus faecium (E. faecium) than WT mice. Similarly, in a dermal infection model, subcutaneous infection with Streptococcus pyogenes (S. pyogenes) or Pseudomonas aeruginosa (P. aeruginosa) results in the development of footpad edema that is significantly larger and display a prolonged progression in MrgprB2MUT mice when compared with WT mice. In these infection models, WT mice display increased MC density, higher TNF-α level and elevated neutrophil count when compared to MrgprB2MUT mice. In addition, pharmacological activation of MrgprB2 with either C48/80 or CSP-1 eliminates bacteria and reduces disease score. These findings suggest that activation of CTMCs via MrgprB2 provides an important mechanism for host defense by sensing bacteria-associated QSMs and secreting antibacterial mediators.25
In addition to mouse PMCs, CSP-1 also causes degranulation, ROS, PGD2 and TNF-α production in a human MC line Laboratory of allergic diseases 2 (LAD2) that endogenously expresses MRGPRX2, and these responses are substantially reduced in MRGPRX2-silenced cells.25 Co-culture of LAD2 cells with clinical isolates of Streptococcus pneumoniae (S. pneumoniae), which produce CSP-1, results in the suppression of bacterial growth but this suppression is attenuated in MRGPRX2-silenced cells. It is well known that biofilms allow bacteria to subvert immune responses and establish chronic infections.32 Interestingly, LAD2 cells reduce biofilm viability, and this response is decreased in MRGPRX2-silenced cells.25 These findings suggest that activation of MRGPRX2 by QSMs plays an important role for antibacterial defense in humans through MC degranulation, ROS production and subsequent recruitment of neutrophils (Fig. 1A).
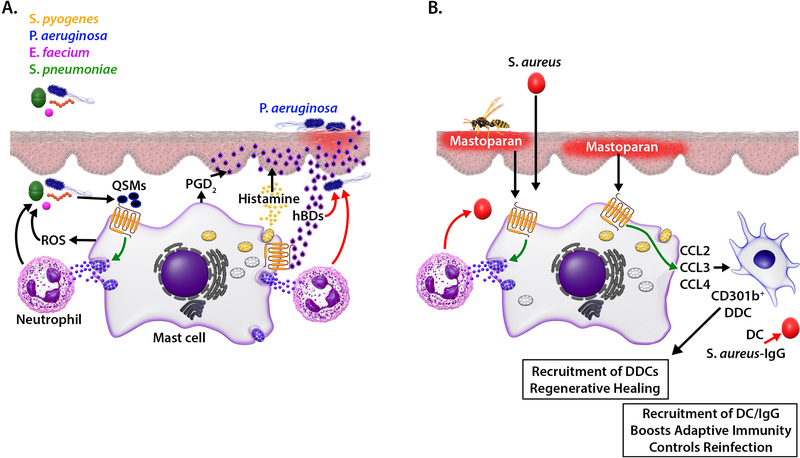
FIG 1.
Contribution of MRGPRX2 in host defense to bacterial infection and cutaneous wound healing. (A) QSMs released from bacteria activate MCs via MRGPRX2, resulting in degranulation, reactive oxygen species (ROS) generation and recruitment of neutrophils which collectively participate in bacterial killing (red arrows). In addition, skin infection with pathogen such as P. aeruginosa induces human β defensin (hBD) from keratinocytes, which kill bacteria directly and activate MCs via MRGPRX2. MC mediators (histamine, PGD2) cause additional hBD secretion from keratinocytes, which causes further MC activation and recruitment of neutrophils, resulting in resolution of infection. (B) Based on studies with mice, we propose that topical application of mastoparan activates cutaneous MCs via MRGPRX2 to control S. aureus skin infection. Activated MCs release chemokines and recruit CD301b+ dermal dendritic cells to mediate regenerative healing. Mastoparan treatment also elicits S. aureus–specific IgG to induce antibacterial adaptive immunity to control reinfection.
The emergence of antibiotic-resistant infections poses a tremendous public health concern globally and requires urgent need to develop novel therapy for their treatment.33, 34 P. aeruginosa, a Gram-negative bacterium, is a common cause of nosocomial skin wound infection and presents a rising therapeutic challenge due to its ability to form biofilms and increase antibiotic resistance.35, 36 Zimmerman et al.,3 recently showed that in a topical P. aeruginosa infection model in mice, MCs contribute to both bacterial clearance and wound healing. The authors found that MCs infected with P. aeruginosa in vitro are unable to decrease bacterial load unless they are co-cultured with keratinocytes. Interestingly, P. aeruginosa infection of MC/keratinocyte co-culture results in the secretion of MC-derived mediators, which cause the release of mouse β-defensin (mBD)-14 (Defb14, ortholog of human β-defensin (hBD)-3) from keratinocytes.3 Based on these findings, it has been proposed that in P. aeruginosa skin infection, MC-derived mediators promote the induction and secretion of HDPs, which control infection through their direct antimicrobial activities.3
Zhang et al.,37 showed that Defb3 and Defb14 activate MrgprB2 to induce degranulation in mouse PMCs. Furthermore, activation of MRGPRX2 in human MCs results in histamine and PGD2 release, which further promotes hBD production from keratinocytes.38–42 Moreover, infection of human keratinocytes with P. aeruginosa results in the induction of hBD2.43 It now appears that most of the HDPs that display direct antimicrobial activity also activate MCs via MRGPRX2.41, 42, 44 Thus, it is likely that during microbial infection, both QSMs produced from bacteria and HDPs generated from infected keratinocytes activate MRGPRX2 to generate mediators that promote the recruitment of neutrophils, resulting in the clearance of microbial infection (Fig. 1A).
The amphipathic peptide mastoparan found in Hymenoptera venom activates murine and human MCs via MrgprB2 and MRGPRX2, respectively, and displays direct antimicrobial activity.20, 45 In a mouse model of Staphylococcus aureus (S. aureus)-induced skin infection, topical application of mastoparan promotes neutrophil recruitment and clears infection,45 thus functioning similar to endogenously derived HDPs such as hBDs. In addition to degranulation, activation of LAD2 cells by mastoparan results in the generation of chemokines; CCL2, CCL3 and CCL4, which contribute to the mobilization of CD301b+ dermal dendritic cells (DDCs) and antigen presenting dendritic cells (DCs). DDCs play critical role in promoting re-epithelialization of sterile wounds.46 Additionally, topical application of mastoparan to S. aureus-infected wound results in significantly higher level of S. aureus–specific total IgG when compared to vehicle treated control. This increased IgG is associated with reduced lesion size following reinfection in a mouse model of S. aureus-induced skin infection.45 Thus, it is likely that activation of MRGPRX2 by mastoparan not only accelerates bacterial clearance and promotes regenerative healing, but also enhances adaptive immunity, which provides protection against reinfection (Fig. 1B).
ROSACEA
MCs are found beneath the epithelia in close proximity to nerve endings and blood vessels, and likely serve as a functional homeostatic regulatory unit.47 Rosacea is a chronic inflammatory skin condition that is provoked by several triggers such as an imbalance in commensal skin microbiota, ultraviolet (UV) radiation and is exacerbated by psychosocial stress.48 Although the pathogenesis of rosacea is not fully determined, emerging evidence suggests that it results from dysregulated host defense, excessive production of the HDP LL-37, aberrant activation of cutaneous MCs and abnormal neurovascular signaling (Fig. 2).49, 50 Patients with rosacea display increased baseline expression of LL-37, which likely results from the activation of Toll-like receptors (TLRs) on keratinocytes following microbial infection.51 The number and activity of MCs are increased in the skin of rosacea patients when compared to control subjects.52, 53 Although no animal models are available that fully reproduce rosacea in humans, “rosacea-like” features are observed following intradermal administration of LL-37 in WT mice but not in MC-deficient Wsh/Wsh mice.53 Neutrophils that are recruited following MC activation also serve as an important source of LL-37.54 Thus, the cross-talk between activated MCs, keratinocytes and neutrophils, that amplify the generation of LL-37, provides a vicious cycle for the continuous activation of MCs to sustain chronic cutaneous inflammation in rosacea (Fig. 2).
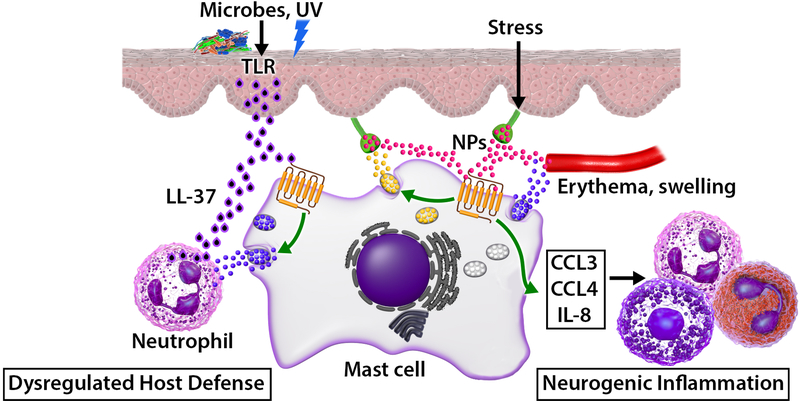
FIG 2.
Upregulation of LL-37, induction of SP and aberrant activation of cutaneous MCs via MRGPRX2 contributes to rosacea. Microbial assault induces excessive release of LL-37 from keratinocytes which activates MCs via MRGPRX2. Activated MCs release mediators to promote neutrophil recruitment, which generate additional LL-37 to cause chronic inflammation and rosacea pathogenesis via further activation of MCs via MRGPRX2. Moreover, stress induces the release of SP from the sensory neurons, which also activates MCs by MRGPRX2, resulting in the release of chemokines and cytokines. Recruitment of inflammatory cells leads to neurogenic inflammation and exacerbation of rosacea.
Neurogenic inflammation is a physiological process in the skin by which mediators are released directly from the cutaneous nerves to initiate an inflammatory reaction, resulting in erythema, swelling, and pain. Classic experiments performed in the late 19th and early 20th century showed that electrically stimulated dorsal roots induce vasodilation, resulting in neurogenic inflammation. It was thought that this response resulted from the release of the neuropeptides (NPs) SP and calcitonin gene-related peptide (CGRP) from nociceptors, which directly act on vascular endothelial cells to cause vasodilation and increased capillary permeability.55 As discussed below, it is now realized that NPs released from sensory neurons not only act on the vasculature but also activate cutaneous MCs via MRGPRX2 to induce degranulation and chemokine/cytokine generation, which may contribute to rosacea exacerbation and the pathogenesis of AD.56
The expression of NPs; SP, pituitary adenylate cyclase-activating polypeptide (PACAP), vasoactive intestinal peptide (VIP), are increased in rosacea, which activate MCs via MRGPRX2.8, 49, 57, 58 In turn, histamine, tryptase and other MC-derived mediators can lead to the release of NPs from sensory nerve endings, which creates a bidirectional loop between the MCs and sensory nerves. Moreover, SP causes degranulation in human skin MCs via MRGPRX257 and induces chemokines, IL-8, CCL2, CCL3 and CCL4 in LAD2 cells.27 Thus, it is likely that activation of MCs by LL-37 through MRGPRX2 initiates the pathogenesis of rosacea. Furthermore, release of chemokines by NPs via MRGPRX2 promotes neurogenic inflammation and rosacea exacerbation leading to itching, flushing, erythema, and/or burning sensations (Fig. 2).48, 59
ATOPIC DERMATITIS
Atopic dermatitis (AD) or atopic eczema is a chronic inflammatory skin disease clinically characterized as intense pruritus and T-helper-2 (TH2)-associated hypersensitivity to wide spectrum of allergens including those derived from house dust mites (HDMs).60 The skin of AD patients display altered barrier function and are colonized with exotoxin-producing bacteria such as staphylococcal enterotoxin B (SEB)-secreting S. aureus.61 Additionally, loss-of-function mutation in filaggrin, a component of skin barrier function, has been shown to be risk factor for AD.62 Cutaneous nerve fibres of patients with AD display greater expression of SP and protease activated receptor 2 (PAR2) when compared to healthy skin.63, 64 Furthermore, AD skin shows increased MC numbers that are found within or in close proximity to epidermis and interact with SP containing nerve bundles displaying signs of degranulation.65–67 Nattkemper et al.,64 used RNA sequencing to analyze the complete transcriptome of skin samples of AD patients and healthy controls. They found that genes encoding SP (Tac1) and its receptor MRGPRX2 are upregulated in AD when compared to healthy controls. Thymic stromal lymphopoietin (TSLP) is a cytokine that is released by epithelial cells and its aberrant activation is a hallmark of atopic diseases, including AD.68 Babina et al.,69 recently showed that TSLP serves as a novel priming factor for SP-induced degranulation in human skin MCs. These findings suggest that MC-nociceptor interaction contributes to the pathogenesis of AD.
The exact mechanism via which MC-nociceptor interaction contributes to the pathogenesis of AD is not known. However, using a well-established clinically relevant mouse model of AD, Serhan et al.,24 showed that repeated epicutaneous exposure to HDM strain Dermatophagoides farinae (D. farinae) and SEB results in the development of moderate to severe AD like skin lesions characterized by a dysregulation of skin barrier architecture and an exacerbated pathogenic type 2 immune response. Although a variety of immune cells can generate SP,70–72 its expression in mouse skin is restricted to a subpopulation of transient receptor potential vanilloid type 1 (TRPV1+) sensory neurons.24 Furthermore, mice deficient in Tac1 or WT mice treated with resiniferatoxin (RTX), which selectively ablates TRPV1+ nociceptors, are protected from the development of AD- like pathology. Application of D. farinae + SEB causes the release of SP from cultured dorsal root ganglia (DRG) neurons but not from DRG neurons treated with RTX. In addition, cysteine but not serine protease activity of D. farinae is required for inducing Ca2+ mobilization in TRPV1+ DRG neurons ex vivo. Furthermore, unlike the response in WT mice, D. farinae + SEB does not induce AD-like features in MC-deficient or MrgprB2MUT mice.24 Interestingly, most of activated sensory neurons in the skin are found either in direct contact or in the close proximity with dermal MCs. These findings suggest that cysteine-protease-dependent release of SP from TRPV1+ nociceptors and the subsequent activation of MCs via MrgprB2 contribute to the early stages in the development of AD in mice.
Given that the expression of Tac1 and MRGPRX2 genes are upregulated in the skin of AD patients when compared to control skin,64 it is likely that studies reported in mice have translational relevance to the human disease (Fig. 3A). More than 90% of AD patients are colonized with S. aureus in the lesional skin, whereas most healthy individuals do not harbor this pathogen. Furthermore, S. aureus δ-toxin induces degranulation in human MCs via MRGPRX2.73 S. aureus also induces the expression and release of hBD2 from human keratinocytes.74 Harder et al.,75 showed that expression and secretion of these HDPs (hBD2, hBD3) are increased in the skin of AD patients when compared to healthy skin. Furthermore, MCs are abundantly localized close to and within the epidermis in AD.76 These HDPs cause MC chemotaxis, degranulation, PGD2 and IL-31 production via the activation of MRGPRX2.41, 42, 77, 78 These findings suggest that in addition to SP and S. aureus δ-toxin, increased expression and secretion of HDPs can contribute to the pathogenesis of AD.79 Thus, targeting MRGPRX2 and its signaling may provide an attractive therapeutic approach for the treatment of AD (Fig. 3A).
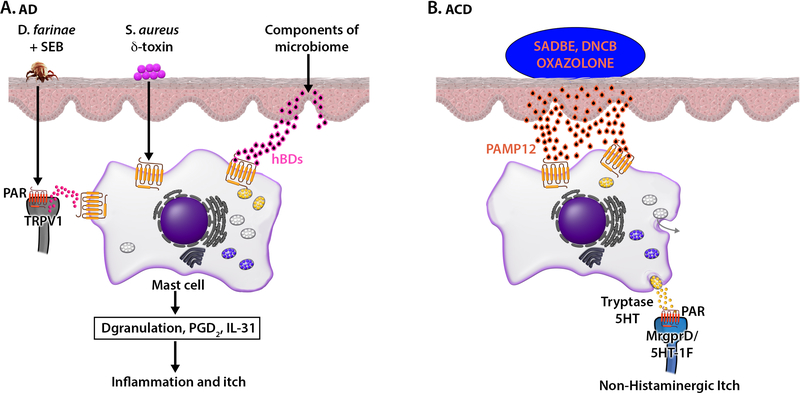
FIG 3.
MRGPRX2 contributes to AD, ACD and non-histaminergic itch. (A) Dermatophagoides farinae (D. farinae) + staphylococcal enterotoxin B (SEB) activate TRPV1+Tac1+ sensory neurons to release SP, which activates cutaneous MCs via MRGPRX2. S. aureus secreted δ-toxin and hBDs released from keratinocytes activate human MCs via MRGPRX2. Activated MCs release mediators and cytokines resulting in itch sensations associated with AD. (B) Based on studies performed with mice, we propose that haptens such as SADBE, DNCB and oxazolone cause the release of PAMP12 from keratinocytes, which activate cutaneous MCs via MRGPRX2 to release tryptase and 5HT. Tryptase cleaves proteinase-activated receptors (PARs) present on MrgprD and 5-HT-1F expressing itch sensory neurons to induce itch sensation.
ALLERGIC CONTACT DERMATITIS AND NON-HISTAMINERGIC ITCH
Allergic contact dermatitis (ACD) is an intensely pruritic skin disease; caused by repeated exposure to haptens that penetrate the skin barrier to induce a type IV hypersensitivity reaction and does not respond to antihistamine treatment.80, 81 Itch in ACD is thought to be mediated by nociceptors that have their cellular bodies in DRG and projections in the skin where they are found in close proximity to MCs. Compared to a healthy control skin, lesional skin from ACD patients display increased number of MCs as well as the MRGPRX2 ligand pro-adrenomedullin peptide 12 (PAMP12).26 However, unlike IgE-mediated itch, the response in ACD is not modulated by anti-histamines.80, 81 This could reflect differences in the activation programs of human MCs via MRGPRX2 and FcεRI that diverge temporally and spatially for granule-derived mediator release.82 It is noteworthy that subcutaneous injection of PAMP12 or application of the haptens, squaric acetyl dibutyl acid (SADBE), oxalozone, or dinitrochlorobenzene (DNCB) induces itch behaviour in WT mice but this response is significantly reduced in MrgprB2MUT mice.26 While both histamine (H1 and H4) and serotonin (5HT2 and 5HT7) receptor antagonists inhibit IgE-mediated histaminergic itch, they have no significant effect on PAMP12-induced response. Moreover, both scratching bouts and immune cells recruitment, two pathological traits classically associated with ACD, are significantly reduced in MrgprB2MUT mice. It is noteworthy that activation of human MCs via MRGPRX2 and murine MCs via MrgprB2 elicit similar spatial and temporal MC degranulation pattern indicating that in vitro studies with mouse PMCs and in vivo studies with MrgprB2MUT mice could provide insight on the mechanism of non-histaminergic itch in ACD.26
At concentrations of antigen and PAMP12 that similarly activate mouse PMCs, as measured by β-hexosaminidase release, there is a remarkable difference in the release of pruritogens. Unlike IgE, PAMP12 is a weak stimulant for histamine and serotonin release but strongly induces the release of tryptase β2.26 It now appears that this differential release of MC mediators results in the excitation of different sensory neuron subpopulations. Thus, majority of the neurons that respond to antigen/IgE also respond to histamine. By contrast, neurons that respond to PAMP12 also respond to the pruritogenic ligands chloroquine (MrgprA3 agonist), serotonin (5-HT-1F receptor agonist) and β-alanine (MrgprD agonist). It is therefore possible that PAMP12-mediated activation of MCs via MrgprB2 results in the release of tryptase, which activates PARs on sensory neurons to induce itch. Xing et al.,83 recently showed that histamine H1 and H2 receptors are enriched in MrgprA3+ neurons but the possibility that these histamine receptors contribute to MrgprB2-mediated itch has not been determined. The demonstration that ACD skin displays increased numbers of MCs as well as the MRGPRX2 ligand PAMP12 suggests that mouse models for ACD likely reflect the situation in humans and provides an explanation why anti-histamines are ineffective for its treatment (Fig. 3B).26 However, one report showed that intradermal injection of PAMP12 in human subjects provokes transient itch and erythema and that these responses are blocked by an H1 receptor antagonist.84 Future studies with larger human subject populations is needed to resolve this discrepancy.
Compared to human skin MCs, colon MCs express MRGPRX2 at a low level.85 However, a recent report indicated that PAMP12-MRGPRX2 interaction contributes to the pathogenesis of ulcerative colitis (UC). In the colon, MCs are found mostly in the mucosa and connective tissue, generally clustered at epithelial surfaces.86 It has been shown that adrenomedullin (ADM, proteolytic precursor of PAMP12) is upregulated in activated fibroblasts and secretory epithelial cells in inflamed UC when compared to uninflamed tissue. Furthermore, exposure of lamina propria cells obtained from inflamed UC biopsies to an MRGPRX2 agonist results in the release of MC protease carboxypeptidase-3 (CPA-3). This response is absent in cells obtained from control biopsies. Based on these findings, it has been proposed that ADM generated from inflamed epithelial cells and activated fibroblasts in UC is proteolyzed by CPA-3 secreted from MCs to generate PAMP12, resulting in continuous MC activation through MRGPRX2. Thus, it is possible that MRGPRX2 contributes to both ACD and UC via the activation MCs through the same ligand, PAMP12.26, 86
PSEUDOALLERGY
Allergic reactions that occur during perioperative procedures and anaesthesia in response to neuromuscular blocking drugs (NMBDs; such as atracurium, mivacurium, tubocurarine, rocuronium cisatracurium), opiates, antibiotics, iodinated contrast media, plasma expanders and dyes may be severe and life-threatening.87–89 These reactions represent a diagnostic challenge for allergists as many of these drugs are administered simultaneously and reactions are often mediated independently of IgE.90, 91 The mechanism of their actions, however, remained unknown until the seminal observation by McNeil et al.,20 that many of these drugs activate human MCTC and murine CTMCs via MRGPRX2 and MrgprB2, respectively. Moreover, in murine models of anaphylaxis, fluoroquinolones and the NMBD cisatracurium cause increased vascular permeability but this response is substantially attenuated in MC-deficient and MrgprB2MUT mice.20, 92, 93 As these drugs also induce degranulation in human MCs via MRGPRX2, it has been proposed that activation of this receptor is responsible for non-IgE-mediated drug-induced anaphylactoid reactions.20, 94, 95
McNeil et al.,20 showed that rocuronium induces Ca2+ mobilization in transfected HEK293 cells expressing MrgprB2 and MRGPRX2 with EC50 values of 22.2 μg/mL and 263 μg/mL, respectively. Consistently, rocuronium at a concentration of 20 μg/mL induces significant degranulation in WT mouse PMCs but this response is abolished in PMCs from MrgprB2−/− mice.22 However, the possibility that rocuronium activates human MCs via MRGPRX2 has been a subject of controversy. For example, initial studies with rocuronium at a concentration of up to 2 mg/mL failed to demonstrate degranulation in LAD2 cells and transfected RBL-2H3 cells.94, 96, 97 Although rocuronium (1640 μM; ~1 mg/ml) induces transient Ca2+ mobilization in human CD34+-derived MCs, it does not provide sufficient signal for degranulation.98 However, a more recent study with transfected RBL-2H3 cells and LAD2 cells showed that rocuronium causes significant degranulation at 500 μg/mL and reaching a maximal at 2 mg/mL. Interestingly, human skin MCs also respond to rocuronium (2 mg/mL) for robust degranulation, as measured by β-hexosaminidase release and cell surface expression of lysosomal-associated membrane protein 1 (LAMP-1).22 The reason for the discrepancy is not clear but could reflect differences in the batches of rocuronium used or different assays used to measure MC activation.22
Spoerl et al.,99 reported 3 cases of rocuronium-induced hypersensitivity; these patients had no increase in serum IgE and basophil activation test (BAT) was negative. Sugammadex is a γ-cyclodextrin that encapsulates aminosteroid NMBDs such as rocuronium, preventing its interaction with the nicotinic receptor, and thereby reversing neuromuscular blockade.99, 100 Sugammadex inhibits both rocuronium-induced MRGPRX2-mediated signaling in MCs in vitro and irritative skin reactions in vivo.96, 99 Based on some of these findings, it has been proposed that MRGPRX2 plays an important role in drug-induced pseudoallergy and that this reaction should be re-classified as type A adverse reaction.99, 101 Furthermore, Navinés-Ferrer et al.,94 showed that sera obtained from patients who had experienced anaphylactoid reactions to NMBDs induce degranulation in LAD2 cells via MRGPRX2.
Suzuki et al., 97 recently reported a case of perioperative anaphylactic reaction to rocuronium. In this patient intradermal skin testing with undiluted rocuronium (10 mg/mL) resulted in a positive reaction. However, total IgE and specific IgE to rocuronium were negative. Sequence analysis of genomic DNA of this patient revealed three amino acid mutations (M196I, L226P and L237P) in MRGPRX2.97 These mutations are located in MRGPRX2’s 5th and 6th transmembrane (TM) domains in close proximity to its ligand binding pocket.102–104 It was proposed that these mutations enhance the affinity of MRGPRX2 for rocuronium, thus providing genetic evidence for the role of MRGPRX2 on rocuronium-induced hypersensitivity. However, Chompunud Na Ayudhya et al.,22 showed that while rocuronium induces degranulation in RBL-2H3 cells expressing WT MRGPRX2, cells expressing the mutated receptors (M196I, L226P and L237P) either respond normally to rocuronium or display loss of-function phenotype. These findings suggest that, at least in this patient, MRGPRX2 does not contribute to rocuronium hypersensitivity. Furthermore, based on the results of triple testing (skin testing, quantification of specific IgE and BAT) in a clinical study conducted with 140 patients suspected of perioperative hypersensitivity to rocuronium, it was concluded that in most patients, the hypersensitivity reaction mainly resulted from IgE-mediated MC activation.15, 105
It is possible that MRGPRX2 contributes to drug-induced pseudoallergy in patients with other cutaneous disorders in which MRGPRX2 expression is upregulated. Expression of MRGPRX2 is increased in cutaneous MCs of patients with chronic spontaneous urticaria (CSU) when compared to control subjects.57 Furthermore, icatibant and atracurium induce significantly greater wheal reaction in CSU patients when compared to control subjects.19 Thus, relative contribution of FcεRI and MRGPRX2 on pseudoallergy is likely to be complex and additional studies are required before adopting a paradigm shift regarding IgE/FcεRI versus MRGPRX2 for drug-induced hypersensitivity reactions.101
MASTOCYTOSIS
Mastocytosis is characterized by a pathologic increase of MCs in tissues and is often associated with mutations in KIT, the receptor for stem cell factor.106 Given that skin MCs express MRGPRX2, it is possible that symptoms associated with cutaneous mastocytosis involve the activation of this receptor through HDPs and NPs generated from epithelium and nociceptors. However, this possibility has yet to be tested.
Systemic mastocytosis is associated with proliferation and accumulation of MCs in different tissues with approximately 50% risk of developing anaphylaxis.106, 107 Giavina-Bianchi et al.,108 recently reported a case of a patient with systemic mastocytosis who developed 5 episodes of anaphylaxis in response to insect venom and ciprofloxacin used for a urinary tract infection treatment. Serum specific IgE levels to bee, wasp, fire ant and allergen components were negative.108 Skin testing to Hymenoptera venom and ciprofloxacin was negative. Given that mastoparan found in Hymenoptera venom and ciprofloxacin activate human MCs via MRGPRX2, it was proposed that activation of this receptor contributed to the patient’s life-threatening reaction. However, absence of positive skin reaction to Hymenoptera venom and ciprofloxacin is surprising.109 It is noteworthy that this particular patient was diagnosed with systemic mastocytosis with increased numbers of MCs in the duodenal and gastric mucosa and the presence of spindle-shaped MCs in the bone marrow without cutaneous involvement.108 This may explain why intradermal test concentrations of ciprofloxacin and Hymenoptera extracts used did not induce cutaneous MC activation.108, 110
AGONIST-MRGPRX2 INTERACTION AND LOSS-OF-FUNCTION SINGLE NUCLEOTIDE POLYMORPHISMS (SNPs)
MRGPRX2 is a member of class A GPCRs, which shares a common structure of seven transmembrane (TM) α-helices.111 Ligand binding on the extracellular region of GPCRs leads to conformational rearrangements of the intracellular region to initiate downstream signaling cascades.112 In the GPCR database (GPCRdb) nomenclature for class A GPCRs, the first number denotes the helix (1–7) and the second number indicates the residue position relative to the most conserved position, which is assigned the number 50. Thus, 4×55 denotes a residue in TM4, which is at 5 positions after the most conserved residue (4×50) and 4×46 denotes a residue in TM4 at 4 positions before the most conserved residue.
A unique feature of MRGPRX2 is that it is activated by a diverse range of cationic ligands, resulting in a multitude of biological responses. Using molecular modeling and docking studies, Lansu et al.,102 identified negatively charged Glu164 (4×60) and Asp184 (5×38) in MRGPRX2 making ionic contacts with cationic opioid ligands. Accordingly, mutations E164Q and D184N that retain the steric property of the WT residues but remove the negative charges resulted in loss of receptor activation by dextromethorphan, morphine, and related opioid ligands. Reddy et al.,103 also predicted that SP-binding pocket in MRGPRX2 consists of a number of structurally conserved hydrophilic residues along with a buried glutamic acid residue Glu164. Accordingly, replacement of Glu164 with a positively charged Arg (E164R) results in loss of MRGPRX2 activation by SP. However, this mutant responds normally to the HDP, LL-37. Based on these findings, it has been proposed that different ligands interact with different amino acid residues on MRGPRX2’s predicted ligand-binding pocket to induce MC degranulation and biological responses.103
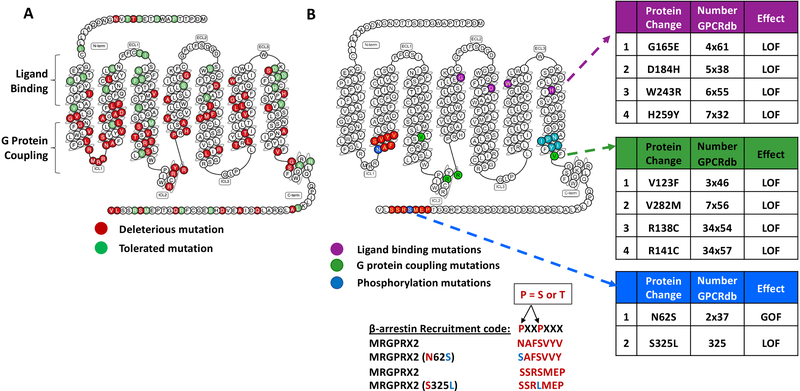
Single nucleotide polymorphisms (SNPs) are one of the most common genetic variants found among individuals. Missense SNPs in GPCRs may affect their structure leading to variation in their expression and functional properties. This may contribute to individual differences in responses to receptor activation.113, 114 Analysis of GPCRdb reveals the presence of 107 missense SNPs in MRGPRX2 with 72 mutations predicted to be deleterious (Fig. 4A). Alkanfari et al.,104 found that transfection of cDNA encoding naturally occurring missense variants in MRGPRX2’s potential ligand binding region, G165E (4×61, rs141744602) and D184H (5×38, rs372988289) in a MC line (RBL2H3) results in loss-of-function phenotype for degranulation in response to NPs (SP and hemokinin-1), HDP (hBD3), and a cationic drug (bradykinin B2 receptor antagonist, icatibant). Two additional SNPs found within the receptor’s 6th and 7th TM domains, W243R (6×55, rs150365137) and H259Y (7×32, rs140862085) also render the receptor unresponsive to all the ligands tested (Fig. 4B). Although cells expressing W243R and H259Y variants did not survive the stable transfection procedure, they expressed normally in transiently transfected cells. It is therefore possible that replacement of the bulky Trp with a positively charged Arg in variant W243R and His with Tyr in variant H259Y influences both the receptor stability and its ability to interact with diverse ligands.
FIG 4.
MRGPRX2 single nucleotide polymorphisms (SNPs). (A) Snake diagram of secondary structure of MRGPRX2 with each circle representing amino acid residue with one letter code. Solid background in red denotes deleterious and green denotes tolerated SNPs in MRGPRX2. (B) SNPs for ligand binding (purple), G protein coupling (green) and β-arrestin binding (blue) are shown. Conserved sequence on MRGPRX2 for β-arrestin binding (β-arrestin recruitment code) and mutations identified within this code are also shown.
MRGPRX2-G PROTEIN COUPLING AND LOSS-OF-FUNCTION SNPs
Despite diversity of ligands, most class A GPCRs including MRGPRX2 interact with a relatively small family of G proteins in order to transduce signals for cell activation, suggesting the presence of conserved activation patterns among the different receptors. Venkatakrishnan et al.,115 analysed the pattern of contact between structurally equivalent residues from the crystal structures of 27 class A GPCRs. From this analysis, it became clear that in the inactive state of class A GPCRs, the residue at position 6×37 is in contact with a conserved hydrophobic residue at position 3×46. Upon receptor activation, this interaction is rearranged so that residue at 3×46 breaks contact from residue 6×37 and forms a new contact with a tyrosine residue at 7×53 within the highly conserved NPXXY motif of TM7. This rearrangement results in the activation of G proteins.
The residues at positions 3×46, 6×37 and 7×53 in MRGPRX2 are Val, Ile, and Tyr, respectively. These residues are either large hydrophobic or aromatic, which likely facilitate contact formation during the receptor conformational rearrangement.115 Using Ala substitution mutations and transfection studies in RBL-2H3 cells, Chompunud Na Ayudhya et al.,116 showed that V123A (3×46) had no effect of Ca2+ mobilization but partially inhibited SP-induced degranulation whereas I225A (6×37) and Y279A (7×53) are essential for both SP-induced Ca2+ mobilization and degranulation. These findings suggest that MRGPRX2 utilizes a “barcode” similar to those found in other class A GPCRs for transducing signal from ligand binding to G protein activation.
Chompunud Na Ayudhya et al.,116 then searched for SNPs within or close to these conserved residues in MRGPRX2 and found three missense variants; V123F (3×46), T224A (6×36) and V282M (7×56). Using site directed mutagenesis and transfection approaches, the authors reported that SNPs V123F (3×46) and V282M (7×56) display loss-of-function phenotype for SP-induced Ca2+ mobilization and degranulation (Fig. 4B). In addition to the conformational changes in TM helices, recent crystallography and spectroscopy studies have shown that intracellular loops of GPCRs also interact with G proteins.117, 118 Not surprisingly, two additional SNPs in MRGPRX2’s second intracellular loop (ICL2), R138C (34×54) and R141C (34×57) were also identified to display loss-of-function phenotype for SP-induced Ca2+ mobilization and degranulation. Thus, it appears that combination of residues in MRGPRX2’s TM and ICL2 domains transmit signal following MRGPRX2 activation by SP for Ca2+ mobilization and degranulation (Fig. 4B). It is noteworthy that the nature of the G protein to which MRGPRX2 couples to depends on the cell type on which the receptor is expressed.20, 116 There is a conserved sequence of NPXXY motif, termed as G protein coupling code present in 7th TM helix (Fig. 4B). It also remains to be determined whether other MRGPRX2 ligands utilize the same residues on the receptor to couple to the same or different G proteins in transfected HEK293, RBL-2H3 and cells that endogenously expressing the receptor such as LAD2 cells, human skin MCs and human CD34+ cell-derived MCs.
MRGPRX2 DESENSITIZATION; GAIN AND LOSS OF-FUNCTION SNPs
For most GPCRs, agonist-induced receptor phosphorylation by G protein receptor kinases (GRKs) and the subsequent recruitment of the adapter proteins β-arrestin leads to their desensitization.119 MRGPRX2 possesses a “β-arrestin binding code” in its carboxyl terminus containing Ser325 (Fig. 4B).120 Despite this, MRGPRX2 is resistant to phosphorylation and desensitization in response to LL-37.41 However, Chompunud Na Ayudhya et al.,116 found the naturally occurring variant (S325L, rs779903448) responds to SP for enhanced Ca2+ mobilization and degranulation when compared to the WT receptor. It is therefore possible that differences in the susceptibility of MRGPRX2 to undergo desensitization reflects the nature of the agonist used for receptor activation.41, 116
Chen et al.,86 recently found that a missense N62S mutation (rs10833049) in MRGPRX2’s first intracellular domain, which results in the creation of a “β-arrestin binding code”, serves as a protective genetic variant for UC. The authors showed that activation of MRGPRX2 by a synthetic high affinity agonist ZINC3573 and the endogenous ligand PAMP12 results in β-arrestin recruitment, which is associated with receptor desensitization. However, ZINC3573 and PAMP12-induced β-arrestin recruitment and receptor desensitization are significantly enhanced in cells expressing the MRGPRX2 N62S variant when compared to the WT receptor. Given that PAMP12 precursor is upregulated in the colon of patients with UC, it has been proposed that individuals harboring N62S mutation would be protected from developing this disease because of loss-of-function phenotype for MC activation due to enhanced receptor phosphorylation, β-arrestin recruitment and desensitization (Fig. 4B). This finding has important implication for ACD, which may be mediated via PAMP12-MRGPRX2 interaction.26 Thus, individuals harboring this mutation may also be protected from developing ACD. Of note, LL-37, which does not promote MRGPRX2 desensitization, only weakly activates β-arrestin and this response is not regulated by the MRGPRX2 N62S mutation.41, 86 These findings raise the interesting possibility that unlike ACD and UC, individuals harboring this mutation may not be protected from developing rosacea, which is thought to be mediated via MRGPRX2 activation by LL-37. Future studies will needed to determine impact of MRGPRX2 polymorphisms on a variety of cutaneous and other MC-mediated disorders.
BALANCED AND G PROTEIN-BIASED SIGNALING BY MRGPRX2: ROLE OF β-ARRESTIN-1
In addition to G proteins, most GPCR agonists activate an additional signaling pathway that involves the recruitment of β-arrestins.121 This pathway was initially characterized for its role in GPCR desensitization and internalization122 but subsequent studies showed that it also serves an important role in G protein-independent downstream signaling for cell migration, growth, and differentiation.86, 123 GPCR agonists that preferentially activate either G proteins or β-arrestins are known as G protein-biased and β-arrestin-biased, respectively. However, agonists that activate both pathways are known as balanced agonists. Thus, while an angiogenic HDP AG-30/5C, icatibant, codeine and C48/80 induced Ca2+ mobilization and degranulation through MRGPRX2 via G proteindependent pathway, there is a remarkable difference in their ability to cause β-arrestin recruitment.124, 125 Thus, while C48/80 and codeine induce β-arrestin recruitment and cause substantial MRGPRX2 internalization, AG-30/5C does not.124, 125 MCs express both β-arrestin1 and β-arrestin2.126 However, β-arrestin1, but not β-arrestin2 contributes to MRGPRX2 internalization.125 Chen et al.,86 recently showed that cells expressing the gain of function MRGPRX2 phosphorylation variant N62S responds to PAMP12 for enhanced extracellular signal-regulated kinase (ERK) phosphorylation when compared to the WT receptor. Given that ERK phosphorylation is required MRGPRX2-mediated chemokine generation,127 it raises the interesting possibility that agonists that serve as balanced ligands could provide a greater signal for chemokine generation than G protein biased agonists. This possibility remains to be determined.
β-ARRESTIN2 IS A NOVEL REGULATOR FOR CIPROFLOXACIN-INDUCED PSEUDOALLERGY AND IgE-MEDIATED ANAPHYLAXIS
Emerging evidence suggests that while β-arrestin-1 contributes to MRGPRX2 desensitization, internalization and down signaling for ERK phosphorylation by balanced agonists, β-arrestin2 regulates MC degranulation via the modification of signaling pathways downstream of Ca2+ mobilization.86, 125, 128 For example, ciprofloxacin does not cause MRGPRX2-mediated β-arrestin recruitment as determined by transcriptional activation following arrestin translocation (Tango) assay.102 Despite this, ciprofloxacin-induced degranulation in mouse PMCs in vitro and vascular permeability in vivo are enhanced in mice with MC-specific deletion of β-arrestin2.128 Furthermore, absence of β-arrestin2 in mouse bone marrow-derived MCs (BMMCs) has no effect on early F cε RI signaling such as antigen-induced Syk phosphorylation or Ca2+ mobilization but results in enhanced degranulation. Consistent with the in vitro findings, MC-specific deletion of β-arrestin2 enhanced IgE-mediated passive cutaneous anaphylaxis. This interesting finding indicates that β-arrestin2 negatively regulates both MrgprB2 and FcεRI-mediated MC degranulation to attenuate drug-induced pseudoallergy and IgE-mediated anaphylaxis, suggesting that this adapter protein could be targeted for the modulation of a diverse array of MC-mediated disorders.
PRECLINICAL ANIMAL MODELS TO STUDY MRGPRX2 FUNCTION IN VIVO
Although MrgprB2 is regarded as the murine ortholog of human MRGPRX2, there is only ~53% sequence homology between the two receptors, resulting in important differences in their interaction with agonists and antagonists. Thus, while ZINC3573 activates MRGPRX2, it is inactive against MrgprB2.129 By contrast, rocuronium at a concentration of 20 μg/ml causes significant degranulation in murine PMCs but requires ~100-fold higher concentration to induce similar level of response in human skin MCs.22 The reason for this difference is not clear. However, C48/80, a polymer with a strong positive charge and multiple bulky hydrophobic moieties, is one of the most potent MRGPRX2 agonists known with a potency of ~130-fold higher than for MrgprB2. Although rocuronium has one quaternary amine and another amine that is mostly positively charged at pH 7.4, its hydrophobic steroidal backbone lacks the aromatic ring present in higher affinity MRGPRX2 agonists such as ZINC3573 and C48/80.7, 20, 102 Thus, in addition to positive charges, the hydrophobic moieties may be required for MRGPRX2 activation but may interfere with MrgprB2 activation. This may explain why rocuronium has higher potency for MrgprB2 when compared to MRGPRX2.
With the exception of rocuronium, most agonists tested have lower affinity for MrgprB2 than MRGPRX2.20 Despite SP’s lower affinity for MrgprB2, it utilizes this receptor to promote neurogenic inflammation and AD in mice.20, 24, 27 Because of the close anatomical co-localization of MCs and TRPV1+ nociceptors in the mouse dermis, it has been proposed that local concentration of SP may reach the high activation threshold to activate MrgprB2 in vivo.24 Even if this occurs, it is highly unlikely that potential MRGPRX2 antagonists at therapeutic concentrations will block MrgprB2 response in vivo. Indeed, Ogasawara et al.,130 recently identified two small molecule MRGPRX2 antagonists that specifically block MRGPRX2-mediated activation of human MCs but have no effect on MrgprB2-mediated responses. Thus, from a therapeutic standpoint, mice expressing MrgprB2 are unlikely to serve as an appropriate in vivo model to screen novel MRGPRX2 antagonists.
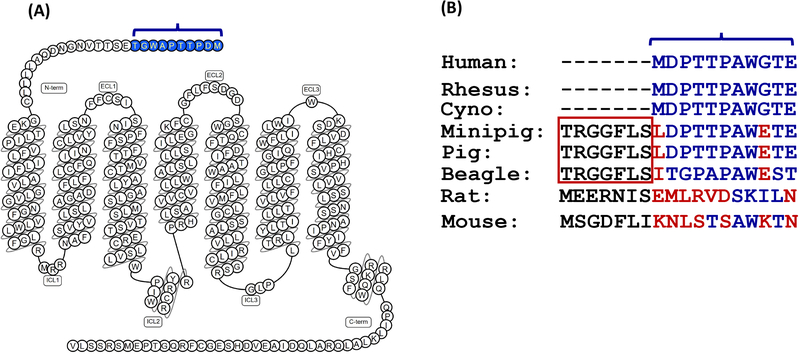
Dogs, minipigs and non-human primates are generally used for preclinical drug safety studies. Histamine-like symptoms or anaphylaxis are sometimes observed in these animal studies, indicating the involvement of MRGPRs. Predicted open-reading frames for canine and porcine MRGPRX2 orthologs display distinctive elongated N-terminal domains, which are not present in primates (human, Rhesus, Cynomolgus) or rodents (Fig. 5A–B).131, 132 Interestingly, truncation of the excess N-terminal domain results in comparable Ca2+ mobilization response to human MRGPRX2 to the archetypal ligand, C48/80. Although MRGPRX2 is sometimes referred to as “primate-exclusive”, it is not possible to predict candidate MRGPRX2 of non-human primates using available genomic sequences.131 However, human MRGPRX2 is the only ortholog that requires Gα16 for optimal C48/80-induced Ca2+ mobilization in transfected HEK293 cells, indicating the unique properties from related species.131 This species-disparity restricts our ability to reliably survey the toxicity of newly developed drugs and to screen for novel MRGPRX2 antagonists in vivo.
FIG 5.
Sequence disparity in the N-terminus of Mas-related GPCRs in different species. (A) Snake diagram of secondary structure of MRGPRX2 with solid blue representing first 10 N-terminal amino acids. (B) Comparison of N-terminal sequence of human MRGPRX2 with the corresponding rhesus, cynomolgus (Cyno), minipig, pig, beagle, rat and mouse receptors.
To overcome the translational challenges discussed above, Suzuki et al.,133 recently showed that an antagonistic DNA aptamer that targets MRGPRX2, inhibits anaphylactic shock to SP in MC-deficient rats engrafted with RBL-2H3 cells stably expressing MRGPRX2. Similar MC knock-in procedure can be developed in MC-deficient mice by engrafting MrgprB2−/− BMMCs transfected with MRGPRX2 to study the function of this human receptor function in vivo.134 The recently reported humanized mice that develop MRGPRX2-expressing human MCs with a partially functioning human immune system may provide an appropriate model to study the function of this receptor in vivo.129
HDPs AND SMALL MOLECULE HDP MIMETICS (smHDPMs) AS POTENTIAL THERAPEUTICS FOR THE TREATMENT OF ANTIBIOTIC-RESISTANT MICROBIAL SKIN INFECTIONS
Antibiotics have been used for the treatment of microbial infections since the early 1900s but emergence of multidrug-resistant strains of microbes possess a tremendous public health concern globally.135, 136 A large number of HDPs are in clinical trials reflecting their therapeutic potentials.136 Their mechanisms of action include (a) direct antimicrobial activity via the cell membrane (b) indirect antimicrobial activity via immune modulation and (c) inhibition of intracellular functions.137 However, it now appears that most of the HDPs that display direct antimicrobial activity also activate MCs via MRGPRX2.138 Based on these findings, it has been proposed that antimicrobial activity of HDPs reflect their ability to harness MRGPRX2’s immunomodulatory activity.34, 45 From a therapeutic view point, HDPs have clear advantages over conventional antibiotics, which include slower emergence of resistance, broad-spectrum activity, and the ability to favorably modulate MRGPRX2-MC-mediated host immune response.44,136, 138,139
Most studies evaluating clinical potentials of HDPs for antimicrobial activity involve their topical application at the site of infection.136 At these sites, it is desirable for a therapeutic agent to display antimicrobial activity and to harness MC’s immunomodulatory and wound healing properties.2, 3 Arifuzzaman et al.,45 recently showed that multiple application of an MRGPRX2 and MrgprB2 agonist on infected mouse skin clears bacterial infection and promotes healing without any local or systemic side effects. The lack of systemic reactions likely reflects the small volume of MRGPRX2 agonist applied locally, which activates predominantly in cutaneous MCs, resulting in responses that are less intense and more transient than IgE-mediated MC activation.82 Furthermore, MrgprB2-mediated induction of adaptive immunity results in increased IgG and IgA but not IgE.140
Although HDPs have important therapeutic potential for topical administration, their metabolic instability and cellular cytotoxicity have limited their utility.136 To overcome these limitations, a series of small molecule HDP mimetics (smHDPMs) have been developed.141, 142 These compounds are relatively inexpensive to synthesize and have distinct advantages over HDPs in terms of stability, bioavailability, and low toxicity. Furthermore, smHDPMs exhibit potent antibacterial and antifungal activities and thus these compounds could be used as potential therapeutic agents for the treatment of drug-resistant microbial infections in general.143 In addition, smHDPMs, which are non-cytotoxic and display direct antimicrobial activity, also activate murine and human MCs via MrgprB2 and MRGPRX2, respectively.34 Thus, harnessing this novel feature of HDPs/smHDPMs to activate MC’s host defense properties in addition to their antimicrobial activities expands their clinical potential.
Despite the difference in sequences between MrgprB2 and MRGPRX2, smHDPMs tested thus far appear to activate human and murine MCs with similar efficacy.34 This important finding suggests that outcome of preclinical studies with smHDPMs in mouse model of skin infection is likely to be relevant in human. As discussed above, smHDPMs have tremendous advantages over HDPs mainly due to ease of synthesis and lack of toxicity. Moreover, several approaches including lipidation has been used to increase hydrophobicity and membrane activity.142 Protegrin-1 is a HDP that activates human MCs via MRGPRX244 and its lipidated peptidomimetic, murepavadin (also known as POL7080), is an antibiotic with high activity against a broad panel of clinical isolates including multidrug-resistant Pseudomonas bacteria. Compared to 13 antibiotics and their combination tested, murepavadin demonstrated highest activity against P. aeruginosa recovered from cystic fibrosis patients, raising possibility that inhalation route of administration can be utilized for such conditions.144 A topical formulation of the drug is also being developed for the treatment of antibiotic-resistant P. aeruginosa skin infection.145, 146 Thus, if murepavadin and other smHDPMs kill microbes directly and harness MC’s host defense properties via the activation of MRGPRX2, they could serve as novel “antibiotics” for the treatment of antibiotic-resistant skin infections, including diabetic foot ulcer infections.136, 137
INHIBITION OF MRGPRX2 AND ITS SIGNALING AS POTENTIAL THERAPEUTIC STRATEGIES FOR THE TREATMENT OF ALLERGIC AND INFLAMMATORY SKIN DISEASES
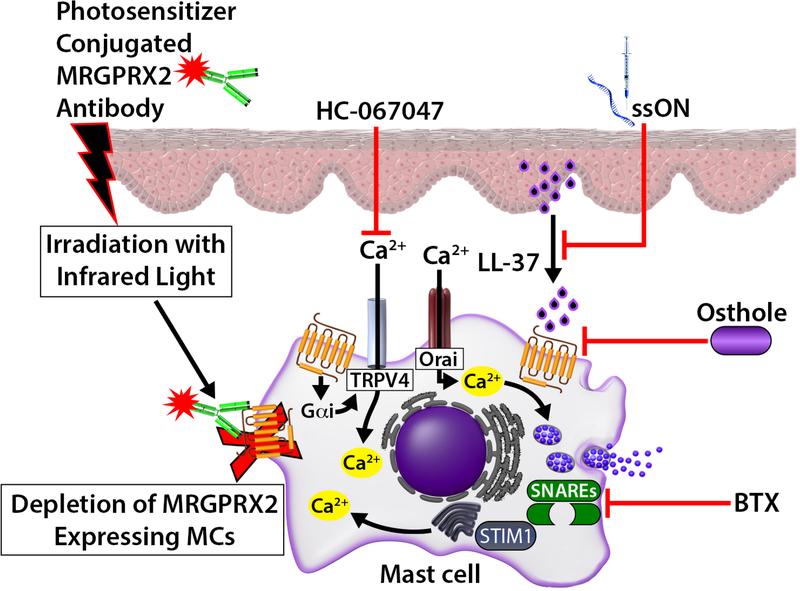
MRGPRX2 likely functions as a double-edged sword, contributing to host defense to microbial infection but its inappropriate activation leading to inflammatory skin diseases. Thus, inhibition of MRGPRX2 and its signaling could provide novel strategies for the modulation of cutaneous diseases such as rosacea, AD, ACD and chronic urticaria. MRGPRX2-based photoimmunotherapy to selectively deplete MCs in the skin has been developed.85 Injection of photosensitizer-conjugated MRGPRX2 antibody in the skin and subsequent irradiation with near infrared light could selectively deplete MRGPRX2 expressing skin MCs.85 Dondalska et al.,147 recently showed that an immunomodulatory single-stranded oligonucleotide (ssON) inhibited LL-37/MRGPRX2 signaling and MC degranulation without affecting IgE-mediated response. Additionally, ssON treatment ameliorates LL-37-induced inflammation in a mouse model of rosacea. Callahan et al.,127 showed that osthole, a natural plant coumarin attenuates SP and LL-37-induced Ca2+ mobilization, degranulation and chemokine/cytokine production in LAD2 cells and human primary skin MCs in vitro. It also inhibits LL-37-mediated rosacea-like features in vivo. Molecular docking analysis suggests that osthole does not compete with MRGPRX2 agonist, but rather regulates MRGPRX2 activation via allosteric modification. Furthermore, osthole reduces the expression of MRGPRX2 in human MCs indicating that it can be utilized for the treatment of MRGPRX2-mediated skin disorders (Fig. 6).
FIG 6.
Modulation of MRGPRX2 and its signaling in cutaneous MCs. Injection of photosensitizer conjugated with MRGPRX2 antibody can selectively destroy targeted skin MCs. Single-stranded oligonucleotide (ssON) inhibits LL-37-induced degranulation in human MCs and reduces LL-37-induced experimental rosacea in mice. Osthole, TRPV4 inhibitor HC-067047 and Botulinum toxin (BTX) inhibit different component of MRGPRX2 and its signaling in human MCs to block LL-37induced rosacea in mice.
Increased Ca2+ mobilization that occurs almost immediately after MRGPRX2 activation is essential for the release of inflammatory mediators.41 Occhiuto et al.,148 showed that store-operated calcium entry (SOCE) via the calcium sensor, stromal interaction molecule 1 (STIM1), regulates MC response to LL-37. Furthermore, a SOCE blocker prevents the development of LL-37-induced rosacea-like features in mice. Orai channels are activated upon the depletion of internal calcium stores and likely contribute to Ca2+ increased in response to MRGPRX2 actvation.42 By contrast, Mascarenhas et al.,149 showed that in LL-37-induced rosacea-like features in mice, there is a 672 substantial upregulation of the Ca2+-permeable transient receptor potential vanilloid-type 4 (TRPV4) ion channel, which colocalizes with murine MCs. TRPV4 expression is also upregulated in human CD34+ cell-derived primary MCs treated with LL-37 for 24h. In addition, treatment of primary human MCs with pertussis toxin (inhibitor of Gαi/Go family of G proteins) or CRISPR/Cas9-mediated knockdown of MRGPRX2 results in the inhibition of LL-37-induced TRPV4 expression and MC degranulation.149 Furthermore, pharmacological inhibition of TRPV4 by HC-067047 results in inhibition of MRGPRX2-mediated MC degranulation. Based on these findings, it has been proposed that TRPV4 expressed in cutaneous MCs contributes to the pathogenesis of rosacea.149,150 It is therefore possible that both SOCE/Orai and TRPV4 activation in response to MRGPRX2 contribute to rosacea and other MC-mediated inflammatory skin diseases (Fig. 6). As such, inhibitors of these Ca2+ entry pathways could be targeted for the modulation of MRGPRX2-mediated disorders.
Botulinum toxin (BTX) is a neurotoxin produced by the bacterium Clostridium botulinum that inhibits the release of acetylcholine at the neuromuscular junction level and has been used to treat a variety of muscular/neuromuscular conditions.151 Intradermal injection of BTX has been shown to improve rosacea symptoms via an unknown mechanism.151–153 The fusion between vesicles and the plasma membrane requires a specific set of proteins including soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), including synaptosomal associated protein-25 (SNAP-25) and vesicle-associated membrane protein (VAMP).154 Choi et al.,152 recently showed that BTX inhibits C48/80 (agonist for MRGPRX2 and MrgprB2)-induced degranulation in human and murine MCs and that this is associated with decreased cleavage of SNAP-25 and VAMP2. Furthermore, in a mouse model of LL-37-induced rosacea, mice pre-treated with BTX showed significantly less dermal MC degranulation and erythema compared to control. Although direct proof that MRGPRX2 expressed in MCs plays a major role in the pathogenesis of rosacea is lacking, there is now growing body of evidence that LL-37 and SP activate MCTC via this receptor.8, 41, 57, 138 Thus, a variety of approaches targeting MRGPRX2 and its signaling (Ca2+ mobilization, SNARE, VAMP) may provide novel approaches for the modulation of rosacea and other MRGPRX2-mediated inflammatory skin diseases (Fig. 6).
CONCLUDING REMARKS AND FUTURE DIRECTIONS
In our last review published in this journal in 2016, we postulated that MRGPRX2 contributes to host defense, neurogenic inflammation, pain itch and chronic inflammatory skin diseases.21 Since then, tremendous progress has been made and studies with WT and MrgprB2MUT mice led to the realization of many of these predictions.24–27, 45 Furthermore, a number of reviews and opinion articles have been published summarizing different aspects of MRGPRX2 and MrgprB2 biology.7, 155–161 However, this current perspective article provides a comprehensive analysis of MRGPRX2/MrgprB2 biology with particular emphasis on how it could be targeted for MC-mediated health and disease.
While MRGPRX2 was originally thought to be expressed predominantly in cutaneous MCs, recent studies indicate that it is also expressed in colon MCs and contributes to the pathogenesis of UC.86 Although human lung MCs express little to no MRGPRX2, its expression is upregulated in lung MCs of individuals who died from asthma when compared to individuals who died from other causes and has been proposed as a new biomarker for allergic asthma.57, 162–164 Furthermore, the NP hemokinin-1 released from bronchial cells and major basic protein (MBP) and eosinophil cationic protein (ECP) activate human MCs via MRGPRX2.57, 165 It now appears that human basophils and eosinophils express MRGPRX2 but whether or not they respond to agonists that activate the receptor in MCs is the subject of controversy.12, 13, 19 Nevertheless, it is clear that expression of MRGPRX2 is not limited to cutaneous MCs as previously thought. Thus, we anticipate that the next few years will herald a new era when basic research with MRGPRX2 and in vivo studies with MrgprB2 will be translated into disease processes and it will be expanded beyond its current cutaneous boundary into other systems such as the airways, synovium and the gut.72, 85, 114, 162 Furthermore, we anticipate that MRGPRX2 agonists and antagonists will be further developed for the modulation of MRGPRX2 in health and disease.
What is currently known:
Unlike FcεRI, which is expressed in all MCs, MRGPRX2 is found predominantly in human skin MCs with low levels in lung and gut MCs.
Bacterial quorum sensing peptides and HDPs activate MCs via MRGPRX2 and likely contribute to host defense and cutaneous wound healing.
In mice, MrgprB2 promotes host defense and contributes to pseudoallergy, neurogenic inflammation, pain, atopic dermatitis, non-histaminergic itch and allergic contact dermatitis.
Expression of MRGPRX2 is upregulated in skin MCs of patients with chronic spontaneous urticaria and lung MCs of individuals who died from asthma-related causes.
MC-specific mediators and a precursor of MRGPRX2 agonist (PAMP-12) are upregulated in ulcerative colitis.
Agonist binding sites, G protein coupling domains, phosphorylation sites and β-arrestin binding codes on MRGPRX2 have been partially identified.
Certain MRGPRX2 ligands act as balanced agonists whereas others serve as G protein-biased agonists.
Individuals harboring a missense MRGPRX2 variant (N62S) are protected from the development of ulcerative colitis due to enhanced β-arrestin recruitment and receptor desensitization.
What more do we need to know:
Why MRGPRX2 is predominantly expressed in skin MCs and what local and epigenetic factors regulate its expression?
Does MRGPRX2 contribute to mastocytosis and mast cell activation syndrome?
What factors regulate the expression of MRGPRX2 in basophils and eosinophils and do they contribute to the pathogenesis of asthma and asthma exacerbation?
Are the expression of MRGPRX2 agonists or their proteolytic precursors increased in inflammatory diseases of the skin or other organs?
Do individuals harboring missense MRGPRX2 mutations display resistance or susceptibility to bacterial infection and inflammatory diseases?
Can the current pipeline of smHDPMs, which are being developed for direct antibacterial activities, be utilized to harness MRGPRX2’s immunomodulatory properties for the treatment of antibiotic-resistant skin infections?
Can small molecule antagonists or antibodies targeting MRGPRX2 be utilized for the treatment of rosacea, pain, atopic dermatitis, allergic contact dermatitis, itch, ulcerative colitis and other diseases that depend on MCs?
Acknowledgments
Funding: This work was supported by National Institutes of Health grants R01-AI124182, R01-AI143185 and R01-AI149487 to Hydar Ali.
Abbreviations used
- ACD
Allergic contact dermatitis
- AD
Atopic dermatitis
- C48/80
Compound 48/80
- CSP
Competence-stimulating peptide
- DCs
Dendritic cells
- DDCs
Dermal dendritic cells
- DRG
Dorsal root ganglia
- GPCR
G protein-coupled receptor
- hBD
Human β-defensin
- HDM
House dust mite
- HDP
Host defense peptide
- LAD2
Laboratory of allergic diseases 2
- MCs
Mast cells
- MRGPRX2
Mas-related G protein–coupled receptor-X2
- MrgprB2
Mas-related G protein–coupled receptor-B2
- NPs
Neuropeptides
- NMBDs
Neuromuscular blocking drugs
- PAMP
Pro-adrenomedullin peptide
- PAR
Protease activated receptor
- PMCs
Peritoneal mast cells
- PGD2
Prostaglandin D2
- QSMs
Quorum-sensing molecules
- ROS
Reactive oxygen species
- SEB
Staphylococcal enterotoxin B
- SNPs
Single nucleotide polymorphisms
- SP
Substance P
- smHDPMs
Small molecule host defense peptide mimetics
- SOCE
Store-operated calcium entry
- TM
Transmembrane
- TRPV
Transient receptor potential vanilloid
- UC
Ulcerative colitis
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Galli SJ, Gaudenzio N, Tsai M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu Rev Immunol 2020; 38:49–77. [DOI] [PubMed] [Google Scholar]
- 2.Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB J 2006; 20:2366–8. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann C, Troeltzsch D, Gimenez-Rivera VA, Galli SJ, Metz M, Maurer M, et al. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc Natl Acad Sci U S A 2019; 116:20500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol 2005; 115:1162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science 1993; 262:1569–72. [DOI] [PubMed] [Google Scholar]
- 6.Aung G, Niyonsaba F, Ushio H, Kajiwara N, Saito H, Ikeda S, et al. Catestatin, a neuroendocrine antimicrobial peptide, induces human mast cell migration, degranulation and production of cytokines and chemokines. Immunology 2011; 132:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeil BD. Minireview: Mas-related G Protein-coupled receptor X2 Activation by Therapeutic Drugs. Neurosci Lett 2021:135746. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin Eindependent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 2006; 349:1322–8. [DOI] [PubMed] [Google Scholar]
- 9.Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018; 159:1325–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014; 123:e58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer DF, Barrett NA, Austen KF, Immunological Genome Project C. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol 2016; 17:878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy 2020; 75:2229–42. [DOI] [PubMed] [Google Scholar]
- 13.Wedi B, Gehring M, Kapp A. Reply to Sabato V et al. “Surface expression of MRGPRX2 expression on resting basophils: An area of controversy”. Allergy 2020; 75:2424–7. [DOI] [PubMed] [Google Scholar]
- 14.Sabato V, Elst J, Van Houdt M, Bridts C, Mertens C, Ebo DG. Surface expression of MRGPRX2 on resting basophils: An area of controversy. Allergy 2020; 75:2421–2. [DOI] [PubMed] [Google Scholar]
- 15.Van Gasse AL, Elst J, Bridts CH, Mertens C, Faber M, Hagendorens MM, et al. Rocuronium Hypersensitivity: Does Off-Target Occupation of the MRGPRX2 Receptor Play a Role? J Allergy Clin Immunol Pract 2019; 7:998–1003. [DOI] [PubMed] [Google Scholar]
- 16.Van Gasse AL, Sabato V, Uyttebroek AP, Elst J, Faber MA, Hagendorens MM, et al. Immediate moxifloxacin hypersensitivity: Is there more than currently meets the eye? Allergy 2017; 72:2039–43. [DOI] [PubMed] [Google Scholar]
- 17.Leysen J, De Witte L, Sabato V, Faber M, Hagendorens M, Bridts C, et al. IgE-mediated allergy to pholcodine and cross-reactivity to neuromuscular blocking agents: Lessons from flow cytometry. Cytometry B Clin Cytom 2013; 84:65–70. [DOI] [PubMed] [Google Scholar]
- 18.Elst J, Sabato V, Mertens C, Garvey LH, Ebo DG. Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br J Anaesth 2020; 125:e448–e50. [DOI] [PubMed] [Google Scholar]
- 19.Shtessel M, Limjunyawong N, Oliver ET, Chichester K, Gao L, Dong X, et al. MRGPRX2 Activation Causes Increased Skin Reactivity in Patients with Chronic Spontaneous Urticaria. J Invest Dermatol 2021; 141:678–81 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mastcell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015; 519:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol 2016; 138:700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Oskeritzian CA, Ali H. MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants. Cells 2021; 10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Y, Peng B, Che D, Zheng Y, Kong S, Liu R, et al. Imiquimod-related dermatitis is mainly mediated by mast cell degranulation via Mas-related G-protein coupled receptor B2. Int Immunopharmacol 2020; 81:106258. [DOI] [PubMed] [Google Scholar]
- 24.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol 2019; 20:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pundir P, Liu R, Vasavda C, Serhan N, Limjunyawong N, Yee R, et al. A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe 2019; 26:114–22 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019; 50:1163–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019; 101:412–20 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crivellato E, Travan L, Ribatti D. The phylogenetic profile of mast cells. Methods Mol Biol 2015; 1220:11–27. [DOI] [PubMed] [Google Scholar]
- 29.Cavalcante MC, Allodi S, Valente AP, Straus AH, Takahashi HK, Mourao PA, et al. Occurrence of heparin in the invertebrate styela plicata (Tunicata) is restricted to cell layers facing the outside environment. An ancient role in defense? J Biol Chem 2000; 275:36189–6. [DOI] [PubMed] [Google Scholar]
- 30.Cavalcante MC, de Andrade LR, Du Bocage Santos-Pinto C, Straus AH, Takahashi HK, Allodi S, et al. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata). J Struct Biol 2002; 137:31321. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Liu Y, Lin AA, Cavalli-Sforza LL, Zhao Z, Su B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene 2005; 352:30–5. [DOI] [PubMed] [Google Scholar]
- 32.Lewis K Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007; 5:48–56. [DOI] [PubMed] [Google Scholar]
- 33.Ardal C, Balasegaram M, Laxminarayan R, McAdams D, Outterson K, Rex JH, et al. Antibiotic development - economic, regulatory and societal challenges. Nat Rev Microbiol 2020; 18:267–74. [DOI] [PubMed] [Google Scholar]
- 34.Alkanfari I, Freeman KB, Roy S, Jahan T, Scott RW, Ali H. Small-Molecule Host-Defense Peptide Mimetic Antibacterial and Antifungal Agents Activate Human and Mouse Mast Cells via Mas-Related GPCRs. Cells 2019; 8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther 2015; 13:605–13. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, McNeil BD. β-defensins are proinflammatory pruritogens that activate Mrgprs. J Allergy Clin Immunol 2019; 143:1960–2 e5. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa T, Kanda N, Hau CS, Tada Y, Watanabe S. Histamine induces human β-defensin-3 production in human keratinocytes. J Dermatol Sci 2009; 56:121–7. [DOI] [PubMed] [Google Scholar]
- 39.Kanda N, Ishikawa T, Watanabe S. Prostaglandin D2 induces the production of human βdefensin-3 in human keratinocytes. Biochem Pharmacol 2010; 79:982–9. [DOI] [PubMed] [Google Scholar]
- 40.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/−2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol 2001; 31:1066–75. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem 2011; 286:44739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. β-Defensins activate human mast cells via Mas-related gene X2. J Immunol 2013; 191:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dossel J, Meyer-Hoffert U, Schroder JM, Gerstel U. Pseudomonas aeruginosa-derived rhamnolipids subvert the host innate immune response through manipulation of the human βdefensin-2 expression. Cell Microbiol 2012; 14:1364–75. [DOI] [PubMed] [Google Scholar]
- 44.Gupta K, Kotian A, Subramanian H, Daniell H, Ali H . Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 2015; 6:28573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arifuzzaman M, Mobley YR, Choi HW, Bist P, Salinas CA, Brown ZD, et al. MRGPRmediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci Adv 2019; 5:eaav0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang B, Suwanpradid J, Sanchez-Lagunes R, Choi HW, Hoang P, Wang D, et al. IL-27 Facilitates Skin Wound Healing through Induction of Epidermal Proliferation and Host Defense. J Invest Dermatol 2017; 137:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forsythe P Mast Cells in Neuroimmune Interactions. Trends Neurosci 2019; 42:43–55. [DOI] [PubMed] [Google Scholar]
- 48.Ahn CS, Huang WW. Rosacea Pathogenesis. Dermatol Clin 2018; 36:81–6. [DOI] [PubMed] [Google Scholar]
- 49.Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol 2018; 40:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Wang YJ, Hao D, Wen X, Du D, He G, et al. The Theranostics Role of Mast Cells in the Pathophysiology of Rosacea. Front Med (Lausanne) 2019; 6:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007; 13:975–80. [DOI] [PubMed] [Google Scholar]
- 52.Aroni K, Tsagroni E, Kavantzas N, Patsouris E, Ioannidis E. A study of the pathogenesis of rosacea: how angiogenesis and mast cells may participate in a complex multifactorial process. Arch Dermatol Res 2008; 300:125–31. [DOI] [PubMed] [Google Scholar]
- 53.Muto Y, Wang Z, Vanderberghe M, Two A, Gallo RL, Di Nardo A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol 2014; 134:2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sorensen OE, Clemmensen SN, Dahl SL, Ostergaard O, Heegaard NH, Glenthoj A, et al. Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest 2014; 124:4539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall JS, Waserman S. Mast cells and the nerves--potential interactions in the context of chronic disease. Clin Exp Allergy 1995; 25:102–10. [DOI] [PubMed] [Google Scholar]
- 56.Helfrich YR, Maier LE, Cui Y, Fisher GJ, Chubb H, Fligiel S, et al. Clinical, Histologic, and Molecular Analysis of Differences Between Erythematotelangiectatic Rosacea and Telangiectatic Photoaging. JAMA Dermatol 2015; 151:825–36. [DOI] [PubMed] [Google Scholar]
- 57.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 2014; 134:622–33 e9. [DOI] [PubMed] [Google Scholar]
- 58.Schwab VD, Sulk M, Seeliger S, Nowak P, Aubert J, Mess C, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011; 15:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc 2011; 15:33–9. [DOI] [PubMed] [Google Scholar]
- 60.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 61.Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol 2000; 105:814–9. [DOI] [PubMed] [Google Scholar]
- 62.Visser MJ, Verberk MM, Campbell LE, McLean WH, Calkoen F, Bakker JG, et al. Filaggrin loss-of-function mutations and atopic dermatitis as risk factors for hand eczema in apprentice nurses: part II of a prospective cohort study. Contact Dermatitis 2014; 70:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003; 23:6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, et al. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J Invest Dermatol 2018; 138:1311–7. [DOI] [PubMed] [Google Scholar]
- 65.Jarvikallio A, Harvima IT, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res 2003; 295:2–7. [DOI] [PubMed] [Google Scholar]
- 66.Sugiura H, Maeda T, Uehara M. Mast cell invasion of peripheral nerve in skin lesions of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1992; 176:74–6. [PubMed] [Google Scholar]
- 67.Toyoda M, Morohashi M. Morphological assessment of the effects of cyclosporin A on mast cell--nerve relationship in atopic dermatitis. Acta Derm Venereol 1998; 78:321–5. [DOI] [PubMed] [Google Scholar]
- 68.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol 2019; 80:1013–21. [DOI] [PubMed] [Google Scholar]
- 69.Babina M, Wang Z, Franke K, Zuberbier T. Thymic Stromal Lymphopoietin Promotes MRGPRX2-Triggered Degranulation of Skin Mast Cells in a STAT5-Dependent Manner with Further Support from JNK. Cells 2021; 10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bost KL, Breeding SA, Pascual DW. Modulation of the mRNAs encoding substance P and its receptor in rat macrophages by LPS. Reg Immunol 1992; 4:105–12. [PubMed] [Google Scholar]
- 71.Weinstock JV, Blum AM. Release of substance P by granuloma eosinophils in response to secretagogues in murine schistosomiasis mansoni. Cell Immunol 1990; 125:380–5. [DOI] [PubMed] [Google Scholar]
- 72.Okamura Y, Mishima S, Kashiwakura JI, Sasaki-Sakamoto T, Toyoshima S, Kuroda K, et al. The dual regulation of substance P-mediated inflammation via human synovial mast cells in rheumatoid arthritis. Allergol Int 2017; 66S:S9–S20. [DOI] [PubMed] [Google Scholar]
- 73.Azimi E, Reddy VB, Lerner EA. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch (Phila) 2017; 2:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ommori R, Ouji N, Mizuno F, Kita E, Ikada Y, Asada H. Selective induction of antimicrobial peptides from keratinocytes by staphylococcal bacteria. Microb Pathog 2013; 56:35–9. [DOI] [PubMed] [Google Scholar]
- 75.Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U, et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol 2010; 130:1355–64. [DOI] [PubMed] [Google Scholar]
- 76.Groneberg DA, Bester C, Grutzkau A, Serowka F, Fischer A, Henz BM, et al. Mast cells and vasculature in atopic dermatitis--potential stimulus of neoangiogenesis. Allergy 2005; 60:907. [DOI] [PubMed] [Google Scholar]
- 77.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol 2010; 184:3526–34. [DOI] [PubMed] [Google Scholar]
- 78.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human βdefensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol 2002; 14:421–6. [DOI] [PubMed] [Google Scholar]
- 79.Shelley JR, Davidson DJ, Dorin JR. The Dichotomous Responses Driven by β-Defensins. Front Immunol 2020; 11:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011; 34:973–84. [DOI] [PubMed] [Google Scholar]
- 81.Kostner L, Anzengruber F, Guillod C, Recher M, Schmid-Grendelmeier P, Navarini AA. Allergic Contact Dermatitis. Immunol Allergy Clin North Am 2017; 37:141–52. [DOI] [PubMed] [Google Scholar]
- 82.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest 2016; 126:3981–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xing Y, Chen J, Hilley H, Steele H, Yang J, Han L. Molecular Signature of Pruriceptive MrgprA3(+) Neurons. J Invest Dermatol 2020; 140:2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasbak P, Eskesen K, Lind H, Holst J, Edvinsson L. The vasorelaxant effect of adrenomedullin, proadrenomedullin N-terminal 20 peptide and amylin in human skin. Basic Clin Pharmacol Toxicol 2006; 99:162–7. [DOI] [PubMed] [Google Scholar]
- 85.Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald HR. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020; 52:404–16 e5. [DOI] [PubMed] [Google Scholar]
- 86.Chen E, Chuang LS, Giri M, Villaverde N, Hsu NY, Sabic K, et al. Inflamed Ulcerative Colitis Regions Associated With MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology 2021; S00165085(21)00024-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Florvaag E, Johansson SG, Oman H, Venemalm L, Degerbeck F, Dybendal T, et al. Prevalence of IgE antibodies to morphine. Relation to the high and low incidences of NMBA anaphylaxis in Norway and Sweden, respectively. Acta Anaesthesiol Scand 2005; 49:437–44. [DOI] [PubMed] [Google Scholar]
- 88.Johansson SG, Florvaag E, Oman H, Poulsen LK, Mertes PM, Harper NJ, et al. National pholcodine consumption and prevalence of IgE-sensitization: a multicentre study. Allergy 2010; 65:498–502. [DOI] [PubMed] [Google Scholar]
- 89.Rouzaire P, Nosbaum A, Mullet C, Diot N, Dubost R, Bienvenu F, et al. Immediate allergic hypersensitivity to quinolones associates with neuromuscular blocking agent sensitization. J Allergy Clin Immunol Pract 2013; 1:273–9 e1. [DOI] [PubMed] [Google Scholar]
- 90.Hausmann O, Schnyder B, Pichler WJ. Etiology and pathogenesis of adverse drug reactions. Chem Immunol Allergy 2012; 97:32–46. [DOI] [PubMed] [Google Scholar]
- 91.Berroa F, Lafuente A, Javaloyes G, Ferrer M, Moncada R, Goikoetxea MJ, et al. The usefulness of plasma histamine and different tryptase cut-off points in the diagnosis of peranaesthetic hypersensitivity reactions. Clin Exp Allergy 2014; 44:270–7. [DOI] [PubMed] [Google Scholar]
- 92.Che D, Wang J, Ding Y, Liu R, Cao J, Zhang Y, et al. Mivacurium induce mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell Immunol 2018; 332:121–8. [DOI] [PubMed] [Google Scholar]
- 93.Liu R, Hu S, Zhang Y, Che D, Cao J, Wang J, et al. Mast cell-mediated hypersensitivity to fluoroquinolone is MRGPRX2 dependent. Int Immunopharmacol 2019; 70:417–27. [DOI] [PubMed] [Google Scholar]
- 94.Navines-Ferrer A, Serrano-Candelas E, Lafuente A, Munoz-Cano R, Martin M, Gastaminza G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep 2018; 8:11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Che D, Rui L, Cao J, Wang J, Zhang Y, Ding Y, et al. Cisatracurium induces mast cell activation and pseudo-allergic reactions via MRGPRX2. Int Immunopharmacol 2018; 62:24450. [DOI] [PubMed] [Google Scholar]
- 96.Fernandopulle NA, Zhang SS, Soeding PF, Mackay GA. MRGPRX2 activation in mast cells by neuromuscular blocking agents and other agonists: Modulation by sugammadex. Clin Exp Allergy 2020. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki Y, Liu S, Kadoya F, Takasaki Y, Yorozuya T, Mogi M. Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br J Anaesth 2020; 125:e446–e8. [DOI] [PubMed] [Google Scholar]
- 98.Elst J, Sabato V, Faber MA, Bridts CH, Mertens C, Van Houdt M, et al. MRGPRX2 and Immediate Drug Hypersensitivity: Insights from Cultured Human Mast Cells. J Investig Allergol Clin Immunol 2020:0. [DOI] [PubMed] [Google Scholar]
- 99.Spoerl D, D’Incau S, Roux-Lombard P, Harr T, Czarnetzki C. Non-IgE-Dependent Hypersensitivity to Rocuronium Reversed by Sugammadex: Report of Three Cases and Hypothesis on the Underlying Mechanism. Int Arch Allergy Immunol 2016; 169:256–62. [DOI] [PubMed] [Google Scholar]
- 100.Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, et al. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl 2002; 41:266–70. [DOI] [PubMed] [Google Scholar]
- 101.Spoerl D, Nigolian H, Czarnetzki C, Harr T. Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”? Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017; 13:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy VB, Graham TA, Azimi E, Lerner EA. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J Allergy Clin Immunol 2017; 140:1726–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alkanfari I, Gupta K, Jahan T, Ali H. Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human β-Defensin-3, and Icatibant. J Immunol 2018; 201:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ebo DG, Van der Poorten ML, Elst J, Van Gasse AL, Mertens C, Bridts C, et al. Immunoglobulin E cross-linking or MRGPRX2 activation: clinical insights from rocuronium hypersensitivity. Br J Anaesth 2021;126:e27–e29. [DOI] [PubMed] [Google Scholar]
- 106.Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35–45. [DOI] [PubMed] [Google Scholar]
- 107.Valent P, Akin C, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, et al. Mast cell activation syndrome: Importance of consensus criteria and call for research. J Allergy Clin Immunol 2018; 142:1008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giavina-Bianchi P, Goncalves DG, Zanandrea A, Borges de Castro R, Garro LS, Kalil J, et al. Anaphylaxis to quinolones in mastocytosis: Hypothesis on the mechanism. J Allergy Clin Immunol Pract 2019; 7:2089–90. [DOI] [PubMed] [Google Scholar]
- 109.Kelso JM. MRGPRX2 signaling and skin test results. J Allergy Clin Immunol Pract 2020; 8:426. [DOI] [PubMed] [Google Scholar]
- 110.Giavina-Bianchi P, Goncalves DG, Borges de Castro R, Zanandrea A, Weiler CR. Reply. J Allergy Clin Immunol Pract 2020; 8:426–7. [DOI] [PubMed] [Google Scholar]
- 111.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol 2013; 53:531–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Venkatakrishnan AJ, Deupi X, Lebon G, Heydenreich FM, Flock T, Miljus T, et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 2016; 536:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, et al. Pharmacogenomics of GPCR Drug Targets. Cell 2018; 172:41–54 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol 2018; 9:3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature 2013; 494:185–94. [DOI] [PubMed] [Google Scholar]
- 116.Chompunud Na Ayudhya C, Roy S, Alkanfari I, Ganguly A, Ali H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int J Mol Sci 2019; 20:5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 2011; 477:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 2017; 546:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gurevich VV, Gurevich EV. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol 2019; 10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, et al. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell 2017; 170:457–69 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gurevich VV, Gurevich EV. Biased GPCR signaling: Possible mechanisms and inherent limitations. Pharmacol Ther 2020; 211:107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A 2017; 114:2562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wingler LM, Lefkowitz RJ. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends Cell Biol 2020; 30:736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roy S, Ganguly A, Haque M, Ali H. Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B2 Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells. J Immunol 2019; 202:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Babina M, Wang Z, Roy S, Guhl S, Franke K, Artuc M, et al. MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway. J Invest Dermatol 2020;S0022–202X(20)32150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vibhuti A, Gupta K, Subramanian H, Guo Q, Ali H. Distinct and shared roles of β-arrestin-1 and β-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One 2011; 6:e19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Callahan BN, Kammala AK, Syed M, Yang C, Occhiuto CJ, Nellutla R, et al. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front Immunol 2020; 11:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roy S, Gupta K, Ganguly A, Ali H. β-Arrestin2 expressed in mast cells regulates ciprofloxacin-induced pseudoallergy and IgE-mediated anaphylaxis. J Allergy Clin Immunol 2019; 144:603–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mencarelli A, Gunawan M, Yong KSM, Bist P, Tan WWS, Tan SY, et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J Leukoc Biol 2020; 107:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogasawara H, Furuno M, Edamura K, Noguchi M. Novel MRGPRX2 antagonists inhibit IgEindependent activation of human umbilical cord blood-derived mast cells. J Leukoc Biol 2019; 106:1069–77. [DOI] [PubMed] [Google Scholar]
- 131.Grimes J, Desai S, Charter NW, Lodge J, Moita Santos R, Isidro-Llobet A, et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol Res Perspect 2019; 7:e00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hamamura-Yasuno E, Iguchi T, Kumagai K, Tsuchiya Y, Mori K. Identification of the dog orthologue of human MAS-related G protein coupled receptor X2 (MRGPRX2) essential for drug-induced pseudo-allergic reactions. Sci Rep 2020; 10:16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suzuki Y, Liu S, Ogasawara T, Sawasaki T, Takasaki Y, Yorozuya T, et al. A novel MRGPRX2-targeting antagonistic DNA aptamer inhibits histamine release and prevents mast cell-mediated anaphylaxis. Eur J Pharmacol 2020; 878:173104. [DOI] [PubMed] [Google Scholar]
- 134.Gaudenzio N, Sibilano R, Starkl P, Tsai M, Galli SJ, Reber LL. Analyzing the Functions of Mast Cells In Vivo Using ‘Mast Cell Knock-in’ Mice. J Vis Exp 2015:e52753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 2013; 13:1057–98. [DOI] [PubMed] [Google Scholar]
- 136.Magana M, Pushpanathan M, Santos AL, Leanse L, Fernandez M, Ioannidis A, et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis 2020; 20:e216–e30. [DOI] [PubMed] [Google Scholar]
- 137.Browne K, Chakraborty S, Chen R, Willcox MD, Black DS, Walsh WR, et al. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta K, Idahosa C, Roy S, Lee D, Subramanian H, Dhingra A, et al. Differential Regulation of Mas-Related G Protein-Coupled Receptor X2-Mediated Mast Cell Degranulation by Antimicrobial Host Defense Peptides and Porphyromonas gingivalis Lipopolysaccharide. Infect Immun 2017; 85:e00246–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silva ON, Torres MDT, Cao J, Alves ESF, Rodrigues LV, Resende JM, et al. Repurposing a peptide toxin from wasp venom into antiinfectives with dual antimicrobial and immunomodulatory properties. Proc Natl Acad Sci U S A 2020; 117:26936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med 2008; 14:536–41. [DOI] [PubMed] [Google Scholar]
- 141.Choi S, Isaacs A, Clements D, Liu D, Kim H, Scott RW, et al. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc Natl Acad Sci U S A 2009; 106:6968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scott RW, Tew GN. Mimics of Host Defense Proteins; Strategies for Translation to Therapeutic Applications. Curr Top Med Chem 2017; 17:576–89. [DOI] [PubMed] [Google Scholar]
- 143.Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother 2007; 51:4125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ekkelenkamp MB, Canton R, Diez-Aguilar M, Tunney MM, Gilpin DF, Bernardini F, et al. Susceptibility of Pseudomonas aeruginosa Recovered from Cystic Fibrosis Patients to Murepavadin and 13 Comparator Antibiotics. Antimicrob Agents Chemother 2020; 64:e01541–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tummler B Emerging therapies against infections with Pseudomonas aeruginosa. F1000Res 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jabbour JF, Sharara SL, Kanj SS. Treatment of multidrug-resistant Gram-negative skin and soft tissue infections. Curr Opin Infect Dis 2020; 33:146–54. [DOI] [PubMed] [Google Scholar]
- 147.Dondalska A, Ronnberg E, Ma H, Palsson SA, Magnusdottir E, Gao T, et al. Amelioration of Compound 48/80-Mediated Itch and LL-37-Induced Inflammation by a Single-Stranded Oligonucleotide. Front Immunol 2020; 11:559589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Occhiuto CJ, Kammala AK, Yang C, Nellutla R, Garcia M, Gomez G, et al. Store-Operated Calcium Entry via STIM1 Contributes to MRGPRX2 Induced Mast Cell Functions. Front Immunol 2019; 10:3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mascarenhas NL, Wang Z, Chang YL, Di Nardo A. TRPV4 Mediates Mast Cell Activation in Cathelicidin-Induced Rosacea Inflammation. J Invest Dermatol 2017; 137:972–5. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y, Moore CD, Zhang JY, Hall RP 3rd, MacLeod AS, Liedtke W. TRPV4 Moves toward Center-Fold in Rosacea Pathogenesis. J Invest Dermatol 2017; 137:801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scala J, Vojvodic A, Vojvodic P, Vlaskovic-Jovicevic T, Peric-Hajzler Z, Matovic D, et al. Botulin Toxin Use in Rosacea and Facial Flushing Treatment. Open Access Maced J Med Sci 2019; 7:2985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi JE, Werbel T, Wang Z, Wu CC, Yaksh TL, Di Nardo A. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J Dermatol Sci 2019; 93:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dayan SH, Ashourian N, Cho K. A Pilot, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of IncobotulinumtoxinA Injections in the Treatment of Rosacea. J Drugs Dermatol 2017; 16:549–54. [PubMed] [Google Scholar]
- 154.Lorentz A, Baumann A, Vitte J, Blank U. The SNARE Machinery in Mast Cell Secretion. Front Immunol 2012; 3:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z, Babina M. MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis? Exp Dermatol 2020; 29:1104–1111. [DOI] [PubMed] [Google Scholar]
- 156.Chompunud Na Ayudhya C, Roy S, Thapaliya M, Ali H. Roles of a Mast Cell-Specific Receptor MRGPRX2 in Host Defense and Inflammation. J Dent Res 2020:22034520919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Corbiere A, Loste A, Gaudenzio N. MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases? Exp Dermatol 2021; 30:193–200. [DOI] [PubMed] [Google Scholar]
- 158.Meixiong J, Basso L, Dong X, Gaudenzio N. Nociceptor-Mast Cell Sensory Clusters as Regulators of Skin Homeostasis. Trends Neurosci 2020; 43:130–2. [DOI] [PubMed] [Google Scholar]
- 159.Kuhn H, Kolkhir P, Babina M, Dull M, Frischbutter S, Fok JS, et al. Mas-related G protein-coupled receptor X2 and its activators in dermatologic allergies. J Allergy Clin Immunol 2021; 147:456–469.. [DOI] [PubMed] [Google Scholar]
- 160.Wang F, Yang TB, Kim BS. The Return of the Mast Cell: New Roles in Neuroimmune Itch Biology. J Invest Dermatol 2020; 140:945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thapaliya M, Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Ali H. Mast Cell-Specific MRGPRX2: a Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr Allergy Asthma Rep 2021; 21:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manorak W, Idahosa C, Gupta K, Roy S, Panettieri R Jr., Ali H Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir Res 2018; 19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Varricchi G, Pecoraro A, Loffredo S, Poto R, Rivellese F, Genovese A, et al. Heterogeneity of Human Mast Cells With Respect to MRGPRX2 Receptor Expression and Function. Front Cell Neurosci 2019; 13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.An J, Lee JH, Won HK, Kang Y, Song WJ, Kwon HS, et al. Clinical significance of serum MRGPRX2 as a new biomarker in allergic asthma. Allergy 2019;75:959–962. [DOI] [PubMed] [Google Scholar]
- 165.Ogasawara H, Furuno M, Edamura K, Noguchi M. Peptides of major basic protein and eosinophil cationic protein activate human mast cells. Biochem Biophys Rep 2020; 21:100719. [DOI] [PMC free article] [PubMed] [Google Scholar]