Abstract
Rationale:
Atopic dermatitis(AD) causes sleep disturbance, yet the epidemiology is not known.
Objective:
Estimate the US prevalence of sleep disturbance and its impact on psychological and neurocognitive function.
Methods:
Cross-sectional survey of 180 parent-child dyads with AD, using stratified sampling based on disease severity(POEM, Patient Oriented Eczema Measure,mild(n=30)/moderate(n=75)/severe (n=75)), age, and race (White/Black or African American/Other). Symptoms of sleep and psychologic health were assessed using PROMIS(Patient Reported Outcome Measurement Information System). To estimate prevalence of sleep disturbance, we calculated weights using post-stratification adjustment making marginal frequencies of AD severity, race, and age similar to marginal frequencies in the 2007 National Survey of Children’s Health(NSCH). Unweighted regression models examined associations with sleep disturbance.
Results:
In children 5–17 years with AD, we estimated sleep disturbance occurs in 66.9% [95% CI: 53.3–80.5%; 3,116,305 children]. The odds of severe sleep disturbance(worse than 95% of US children) were highest in moderate/severe vs mild AD (2.03[1.00–4.10], P=0.0495/8.68[1.82–41.49], P=0.0068). Predictors of parent-proxy reported sleep disturbance were itch intensity(adjusted β [95% CI]:1.33[0.62,2.04]) and low income(<$50k:6.64[2.05,11.23], $50to<100k:4.75[0.35,9.14]). Controlling for disease severity, itch intensity, and significant socio-demographics- parent-proxy reported sleep disturbance was associated with increased severity of sleep-related impairment, depression, fatigue, and anxiety, in addition to worse inattention and impulsivity. In fully adjusted models, children who self-reported sleep disturbance(T-score ≥60) had increased odds of sleep-related impairment(1.20[1.11–1.29]), depression(1.13[1.03, 1.24]), fatigue(1.28[1.06–1.54]), and anxiety(1.16[1.02–1.31]).
Conclusions:
Sleep disturbance is a common symptom of AD affecting ~3 million US children and associated with neuropsychiatric impairment, including depression, anxiety, and inattention. Clinicians should screen for these symptoms in school-aged children, particularly with moderate-to-severe AD.
Keywords: atopic dermatitis, pediatric, quality of life, sleep, PROMIS, attention, mental health, depression, anxiety, neurocognitive function
Introduction
Atopic dermatitis (AD), a disorder characterized by itchy skin, affects 10–20% of US children.(1) Sleep disturbance is consistently reported in 60% of children with AD and its occurrence is even higher during times of disease flare.(2, 3) Although poor sleep is one of the most distressing aspects to patients and their families,(4, 5) the number of US children with AD suffering from sleep disturbance has not been quantified.
Assessment of sleep can be performed by objective measures, such as activity monitors (actigraphy) or clinical sleep studies (polysomnography), or by patient or parent-proxy report. Patient or parent-proxy sleep assessment is considered a meaningful outcome to capture the lived experiences of sleep.(6) Our group has demonstrated the reliability and validity of the PROMIS (Patient Reported Outcome Measurement Information System) Sleep measures in school-aged children (5–17y) with AD.(7) These measures are freely available and are the most psychometrically robust measure of patient- and parent-proxy report of sleep, allowing accurate quantification of the burden of sleep disturbance in children with AD. They can be used for epidemiologic assessment and clinically to compare to the general US population of children.
In addition to the need to quantify the national burden of sleep disturbance in AD, there is a gap in knowledge about patients at highest risk. Several studies have found disease severity as a key risk factor of sleep disturbance.(7–9) From the general sleep literature, the risk of poor sleep is higher in Black vs. White children,(10) but the risk by race in AD has not been assessed. AD and sleep disturbance are also associated with inattention and psychological symptoms (i.e. depression and anxiety).(11) In data analyzed from over 350,000 children, AD was associated with increased odds of ADD/ADHD (1.14 [95% CI, 1.03–1.26]). Moreover, in children with severe AD and fewer than 4 nights of adequate sleep per week, the odds of ADD/ADHD were up to 34.90 [15.01–82.24].(12) In a national study of adults with AD, patients with the highest level of sleep disturbance had the highest odds of anxiety and depression.(13) In children, the relationship between AD, sleep disturbance, inattention, and psychological symptoms (such as depression and anxiety) has been poorly addressed. Our overall objective was to estimate the prevalence of sleep disturbance and its impact on psychological and neurocognitive function in a sample of US children with AD. We hypothesized that children with more severe disease would experience more sleep disturbance and poorer psychological/neurocognitive function.
Methods
Selection of study cohort
We conducted a cross-sectional survey study of 180 children (5–17 years) with AD between May-July 2019 across the United States, out of 1545 screened (see Figure E1). Stratified sampling by disease severity occurred by Patient Oriented Eczema Measure (POEM)(14) (mild (0–7), n=30; moderate (8–16), n=75; and severe (17–28), n=75), and within disease severity strata: age (5–8; 9–12;13–17 years), region of the US (Midwest, NE, SE, NW, SW) and race (White/Black or African-American/other or multiracial). Other inclusion criteria were: ages 5–17 years, parent report of AD diagnosis by a healthcare provider and scratching ≥1 night in the past week. Exclusion criteria were defined by parent-report to questions about their child: sleep disturbance ≥2 nights per week due to asthma or hay fever, medication-induced itching, liver or kidney disease leading to chronic itch, active sleep apnea, restless leg syndrome, insomnia, narcolepsy, sleep disordered breathing, or urticaria. Screening questions were administered to the parent proxy, who must have slept in the same home as the child for ≥6 of the last 7 nights.
Survey participants were recruited by the National Eczema Association and Op4G. Informed consent was obtained electronically. The Institutional Review Boards at Lurie Children’s Hospital and Northwestern University approved the study protocol and design.
Statistical Analysis of the unweighted sample.
Summary statistics were tabulated to describe the study cohort (Table E1). Frequency and prevalence by POEM disease severity were generated for socio-demographic characteristics, clinical description, sleep habits, and questionnaire responses. Rao-Scott Chi-Square tests and t-tests were used to examine associations for categorical and continuous variables, respectively.
Applying weighted frequencies from National Survey of Children’s Health (NSCH) to our study sample to estimate US prevalence of sleep disturbance and sleep-related impairment.
Weighted frequencies to more accurately estimate the US prevalence of sleep disturbance and sleep related impairment in children with AD were constructed using post-stratification factor adjustment for AD severity, race, and age based on the 2007 National Survey of Children’s Health (NSCH) (the most recent study which queried about atopic dermatitis). Population estimates of AD from NSCH were generated similar to a previous study.(15) Briefly, from the NSCH, we identified a cohort of children 5–17 years who had been diagnosed by a healthcare provider with eczema or skin allergy in the past 12 months and had seen a healthcare professional for medical care at least once in the past 12 months. Weights were constructed to make the marginal frequencies of AD severity, race, and age similar to those in the 2007 NSCH. Thus, estimates based on the weighted data better represent the estimated 4.7 million children (ages 5–17 years) with AD in the United States than estimates based on the raw stratified sample.
Regression modeling on the unweighted sample to evaluate the association between disease severity, sleep disturbance, itch, psychologic symptoms and neurocognitive function
A-priori we planned to conduct unweighted logistic regression models to evaluate whether there were statistically significant differences in sleep disturbance or sleep-related impairment across disease severity groups. Crude (i.e., unadjusted) and adjusted odds ratios (OR) with 95% confidence interval (CI) are reported. Survey-weighted procedures in SAS version 9.4 (SAS Institute, Cary, NC) with Taylor series linearization were used to generate national estimates of sleep disturbance and impairment in AD.
Variables included in the regression models
AD disease severity was evaluated using parent-proxy report of the Patient Oriented Eczema Measure (POEM). The full 7-question score was used for patient disease severity characterization as described above. To isolate the effect of sleep and itch in regression models, we calculated a composite-adjusted POEM measure that removed the sleep and itch items. Itch severity was assessed using a Numerical Rating Scale (NRS) of 0–10 (10 is worst) response to the question “In the past 7 days, how bad was your child’s itch on average?”.
Questions were administered to parent proxy for all participants and self-reported for participants aged ≥ 8 years old. Demographic questions were completed by the parent to minimize burden on the child. The primary outcome in our study was Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance measure.(16) The PROMIS Sleep Disturbance measure focuses on perceptions of sleep quality, sleep depth, and restoration associated with sleep, as well as difficulties and concerns with getting to sleep or staying asleep. A secondary outcome, the PROMIS Sleep-related Impairment measure, focuses on perceptions of alertness, sleepiness, and tiredness during usual waking hours, and the perceived functional impairments during wakefulness associated with sleep problems or impaired alertness. All PROMIS measures were scored using EAP-scoring, which makes use of each individual’s pattern of item responses, and the IRT scores were converted to T-scores using a linear transformation (T=10*X+50). The presence and severity of sleep disturbance and sleep related impairment was determined based on previously established strata: for parent proxy (≥60–65, severe ≥66) and for patient-report (≥60–64, severe ≥65).(17)
The PROMIS Pediatric/Parent Proxy Profile 25 was used to assess depression, anxiety, fatigue, and peer relationships. Neurocognitive function was assessed using the MacArthur Health Behavioral Questionnaire (MacArthur HBQ) Inattention and Impulsivity subscales, with response options of 0=never/not true, 1= sometimes or somewhat true or 2=often or very true. A subscale score is computed by averaging the 6-inattention items and 9-impulsivity items.(18, 19)
Median and interquartile range (IQR) of PROMIS scores for sleep disturbance were estimated. Multivariable linear regression models were constructed to examine socio-demographic (sex, age, race, parental income, parental education attainment) and clinical (adjusted POEM, NRS of itch) associations of sleep disturbance. Model 1 examined the association of sleep disturbance as measured by PROMIS (dependent variable) and the adjusted POEM measure without sleep and itch items (independent variable). Model 2 added Numeric Rating Scale (NRS) of itch. Model 3 examined adjusted POEM, NRS of itch, and all socio-demographic characteristics that were significant in bivariable models. We separately constructed models for parent- and child-reported sleep disturbance. Crude and adjusted regression coefficients (unstandardized β) with 95% CI were estimated.
Regression models were also constructed to determine association of sleep disturbance with psychologic symptoms and impaired neurocognitive functioning. We examined individual domains of PROMIS (sleep-related impairment, depression, fatigue, peer relationships, and anxiety), as well as MacArthur HBQ measures of inattention and impulsivity. Models were constructed with similar stepwise variable addition of adjusted POEM, NRS of itch, and socio-demographic characteristics significant in bivariable models for each outcome.
All data processing and analyses were conducted in SPSS v26 and SAS v9.4. A two-sided P<0.05 was selected a priori to denote statistical significance. This study was conducted in accordance with all Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Results
Patient characteristics
A total of 180 children and adolescents with AD and parent dyads were surveyed. POEM disease severity was associated with higher parent educational attainment, parent- and child-reported sleep disturbance and sleep-related impairment, PROMIS profile measures (depression, anxiety, physical function, and pain interference), inattention, impulsivity, itch, and CDLQI QOL burden, and inversely associated with body mass index (Table 1). In particular, increased AD severity was associated with medium effect sized, increased problems across sleep disturbance domains: difficulty falling asleep, problem with sleep, trouble sleeping, time to fall asleep, worry about being able to fall asleep, waking and trouble falling back asleep, tossing and turning, and snoring (Cramer’s V ≥0.25, P≤.0001 for all)(20) (Table E2).
Table 1.
Patient Characteristics
| Variable | Disease severity by Patient Oriented Eczema Measure (POEM) | |||
|---|---|---|---|---|
|
| ||||
| Mild (n=30) | Moderate (n=75) | Severe (n=75) | P-value | |
| Male, n (%) | 18 (60.0) | 40 (53.3) | 42 (56.0) | 0.82 |
| Age, mean (SD) | 11.1 (3.7) | 10.6 (3.6) | 11.0 (3.6) | 0.78 |
| Race, n (%) | 1.00 | |||
| Black | 10 (33.3) | 24 (32.0) | 24 (32.0) | |
| White | 11 (36.7) | 27 (36.0) | 27 (36.0) | |
| Other | 9 (30.0) | 24 (32.0) | 24 (32.0) | |
| Hispanic/Latino, n (%) | 2 (6.7) | 11 (14.7) | 10 (13.3) | 0.58 |
|
| ||||
| Parent POEM, mean (SD) | 5.1 (1.9) | 11.6 (2.3) | 21.0 (3.2) | <0.01 |
| Child POEM, mean (SD) (n=133) | 4.2 (2.6) | 11.5 (5.1) | 18.5 (7.0) | <0.01 |
|
| ||||
| Region, n (%) | 0.77 | |||
| Northeast | 4 (13.3) | 11 (14.7) | 13 (17.3) | |
| South | 12 (40.0) | 32 (42.7) | 26 (34.7) | |
| Midwest | 6 (20.0) | 14 (18.7) | 10 (13.3) | |
| West | 8 (26.7) | 18 (24.0) | 26 (34.7) | |
| Rural/Urban, n (%) | 0.34 | |||
| Urban | 13 (43.3) | 32 (42.7) | 27 (36.0) | |
| Suburban | 13 (43.3) | 34 (45.3) | 44 (58.7) | |
| Rural | 4 (13.3) | 9 (12.0) | 4 (5.3) | |
|
| ||||
| Asthma, n (%) | 7 (23.3) | 13 (17.3) | 11 (14.7) | 0.57 |
| Allergic Rhinitis, n (%) | 12 (40.0) | 25 (33.3) | 15 (20.0) | 0.07 |
| Food Allergy, n (%) | 7 (23.3) | 21 (28.0) | 28 (37.3) | 0.28 |
| ADHD, n (%) | 2 (6.7) | 16 (21.3) | 12 (16.0) | 0.19 |
|
| ||||
| Parental Education, n (%) | <0.01 | |||
| Associate’s Degree or below | 22 (73.3) | 38 (50.7) | 21 (28.0) | |
| Bachelor’s Degree | 6 (20.0) | 21 (28.0) | 23 (30.7) | |
| Master’s Degree or higher | 2 (6.7) | 15 (20.0) | 31 (41.3) | |
| Household Income, n (%) | 0.97 | |||
| Under $50,000 | 13 (43.3) | 37 (49.3) | 34 (45.3) | |
| $50,000 to <$100,000 | 12 (40.0) | 25 (33.3) | 29 (38.7) | |
| >$100,000 | 3 (10.0) | 10 (13.3) | 8 (10.7) | |
| Parental Age, mean (SD) | 41.4 (5.8) | 39.6 (6.5) | 41.2 (6.1) | 0.20 |
|
| ||||
| BMI, mean (SD) | 21.0 (5.8) | 18.5 (4.4) | 18.8 (4.0) | 0.03 |
|
| ||||
| Parent-Proxy Reported Sleep Timing/Habits | ||||
| Bed time, weekdays, mean hh:mm (SD) | 21:14 (1:00) | 21:30 (0:59) | 21:29 (1:09) | 0.45 |
| Bed time, weekends, mean hh:mm (SD) | 22:23 (1:30) | 22:32 (1:39) | 22:19 (1:40) | 0.69 |
| Wake time, weekdays, mean hh:mm (SD) | 07:05 (1:26) | 07:24 (1:16) | 07:15 (1:40) | 0.60 |
| Wake time, weekends, mean hh:mm (SD) | 8:51 (1:48) | 8:44 (1:40) | 8:50 (1:46) | 0.93 |
| Sleep Onset Latency, weekdays, n (%) | 0.32 | |||
| ≤30 minutes | 20 (66.7) | 43 (57.3) | 38 (50.7) | |
| >30 minutes | 10 (33.3) | 32 (42.7) | 37 (49.3) | |
| Sleep Onset Latency, weekends, n (%) | 0.63 | |||
| ≤30 minutes | 21 (70.0) | 47 (62.7) | 45 (60.0) | |
| >30 minutes | 9 (30.0) | 28 (37.3) | 30 (40.0) | |
| Naps on weekdays, n (%) | 9 (30.0) | 20 (26.7) | 27 (36.0) | 0.46 |
| Naps on weekends, n (%) | 5 (16.7) | 16 (21.3) | 16 (21.3) | 0.85 |
|
| ||||
| Parent-Proxy Reported Sleep, mean (SD) | ||||
| Sleep Disturbance | 55.7 (7.5) | 59.8 (10.8) | 67.1 (9.5) | <0.01 |
| Sleep-Related Impairment | 52.6 (11.8) | 57.4 (7.8) | 62.1 (12.6) | 0.03 |
|
| ||||
| Child Reported Sleep, mean (SD) (n=133) | ||||
| Sleep Disturbance | 60.5 (7.8) | 65.0 (7.8) | 70.3 (7.2) | <0.01 |
| Sleep-Related Impairment | 56.9 (9.9) | 63.9 (8.8) | 67.7 (10.8) | <0.01 |
|
| ||||
| Parent-Proxy Reported PROMIS Profile, mean (SD) | ||||
| Depression/Sadness | 49.8 (9.3) | 55.4 (10.8) | 62.3 (13.1) | <0.01 |
| Peer Relationships | 40.9 (11.1) | 43.0 (9.7) | 43.2 (9.1) | 0.51 |
| Anxiety | 48.9 (10.9) | 55.5 (11.1) | 62.0 (14.1) | <0.01 |
| Physical Function/Mobility | 50.3 (8.3) | 45.4 (9.8) | 40.1 (9.9) | <0.01 |
| Pain Interference | 52.6 (9.0) | 57.4 (7.7) | 60.7 (7.9) | <0.01 |
|
| ||||
| Child Reported PROMIS Profile, mean (SD) (n=133) | ||||
| Depression/Sadness | 49.3 (9.4) | 54.4 (10.7) | 57.7 (11.4) | 0.01 |
| Peer Relationships | 44.4 (10.7) | 46.5 (10.9) | 43.4 (10.1) | 0.27 |
| Anxiety | 46.8 (10.3) | 52.1 (11.7) | 56.1 (12.5) | 0.01 |
| Physical Function/Mobility | 51.2 (9.1) | 47.7 (10.3) | 41.6 (9.9) | <0.01 |
| Pain Interference | 46.9 (9.7) | 54.0 (9.6) | 59.3 (8.4) | <0.01 |
|
| ||||
| CDLQI, mean (SD) | 6.2 (6.5) | 11.7 (7.1) | 16.8 (6.6) | <0.01 |
|
| ||||
| Parent-Reported PIQ-C, mean (SD) | 43.4 (7.6) | 50.5 (7.2) | 57.3 (7.1) | <0.01 |
| Child-Reported PIQ-C, mean (SD) (n=133) | 40.7 (8.6) | 48.5 (9.1) | 53.9 (7.9) | <0.01 |
|
| ||||
| Inattention, MacArthur Score, mean (SD) | 0.6 (0.6) | 0.9 (0.6) | 1.1 (0.5) | <0.01 |
|
| ||||
| Impulsivity, MacArthur Score, mean (SD) | 0.5 (0.6) | 0.8 (0.6) | 1.0 (0.6) | <0.01 |
|
| ||||
| Parent-Reported Itch NRS, mean (SD) | 3.8 (2.2) | 5.4 (1.9) | 7.6 (1.3) | <0.01 |
| Child-Reported Itch NRS, mean (SD) (n=133) | 3.4 (2.5) | 5.2 (2.4) | 7.1 (2.2) | <0.01 |
POEM=Patient Oriented Eczema Measure; ADHD=Attention Deficit Hyperactive Disorder; BMI=Body Mass Index; PROMIS= Patient-Reported Outcomes Measurement Information System; CDLQI=Children’s Dermatology Life Quality Index; PIQ-C=PROMIS Pediatric Itch Questionnaire-Child; NRS=Numeric Rating Scale; hh:mm= hours: minutes
Prevalence of sleep disturbance and sleep-related impairment in the US population of children using weighted estimates
Across disease severity groups, a weighted 66.9% [95% CI: 53.3–80.5%] and 61.2% [46.8–75.5%] of children and adolescents with AD had parent-proxy reported sleep disturbance and sleep-related impairment (PROMIS T-score ≥60), respectively. Thus, an estimated 3,116,305 children ages 5–17 years with AD had sleep disturbance, and 2,849,265 had sleep-related impairment (Table 2). In bivariable logistic regression models, increasing AD disease severity was associated with increased prevalence of sleep disturbance, PROMIS T-score ≥60–65 (crude OR [95% CI], moderate AD: 2.71 [1.20–6.13] P=0.02 and severe AD: 7.30 [0.99–53.88], P=0.05) and severe sleep disturbance, PROMIS T-score ≥66 (moderate AD: 2.03 [1.00–4.10], P=0.05 and severe AD: 8.68 [1.82–41.49], P=0.007) (Figure 1). Black/African-American and White children with AD had similar levels of sleep disturbance in models adjusted for disease severity (adjusted OR [95% CI]: 1.51 [0.65–3.50], P=0.33).
Table 2.
Estimated national prevalence of sleep disturbance and sleep-related impairment, by AD severity and race.
| No sleep disturbance | Sleep disturbance | Severe sleep disturbance | No sleep-related impairment | Sleep-related impairment | Severe sleep-related impairment | |
|---|---|---|---|---|---|---|
| POEM severity of AD | ||||||
| Mild | 1,246,572 (40.3%) | 636,118 (21.0%) | 1,143,962 (37.8%) | 1,456,317 (48.1%) | 860,844 (28.4%) | 709,490 (23.4%) |
| Moderate | 262,342 (20.6%) | 309,683 (24.3%) | 704,275 (55.2%) | 312,222 (24.5%) | 267,908 (21.0%) | 696,170 (54.5%) |
| Severe | 30,920 (8.8%) | 25,359 (7.2%) | 296,909 (84.1%) | 3,8335 (10.9%) | 28,747 (8.1%) | 286,106 (81.0%) |
| Race | ||||||
| White | 970,961 (34.3%) | 336,321 (11.9%) | 1,520,678 (53.8%) | 1,234,209 (43.6%) | 54,1172 (19.1%) | 1,052,580 (37.2%) |
| Black | 289,252 (23.7%) | 512,547 (42.0%) | 419,385 (34.3%) | 25,8677 (21.2%) | 50,4947 (41.3%) | 457,560 (37.5%) |
| Other | 279,621 (46.1%) | 122,292 (20.1%) | 205,083 (33.8%) | 313,989 (51.7%) | 111,380 (18.3%) | 181,626 (29.9%) |
| Total * | 1,539,834 (33.1%) | 971,160 (20.9%) | 2,145,145 (46.1%) | 1,806,874 (38.8%) | 1,157,499 (24.9%) | 1,691,766 (36.3%) |
Reflects total number and percentage of US population with atopic dermatitis that experience sleep disturbance or sleep-related impairment respectively.
Figure 1:
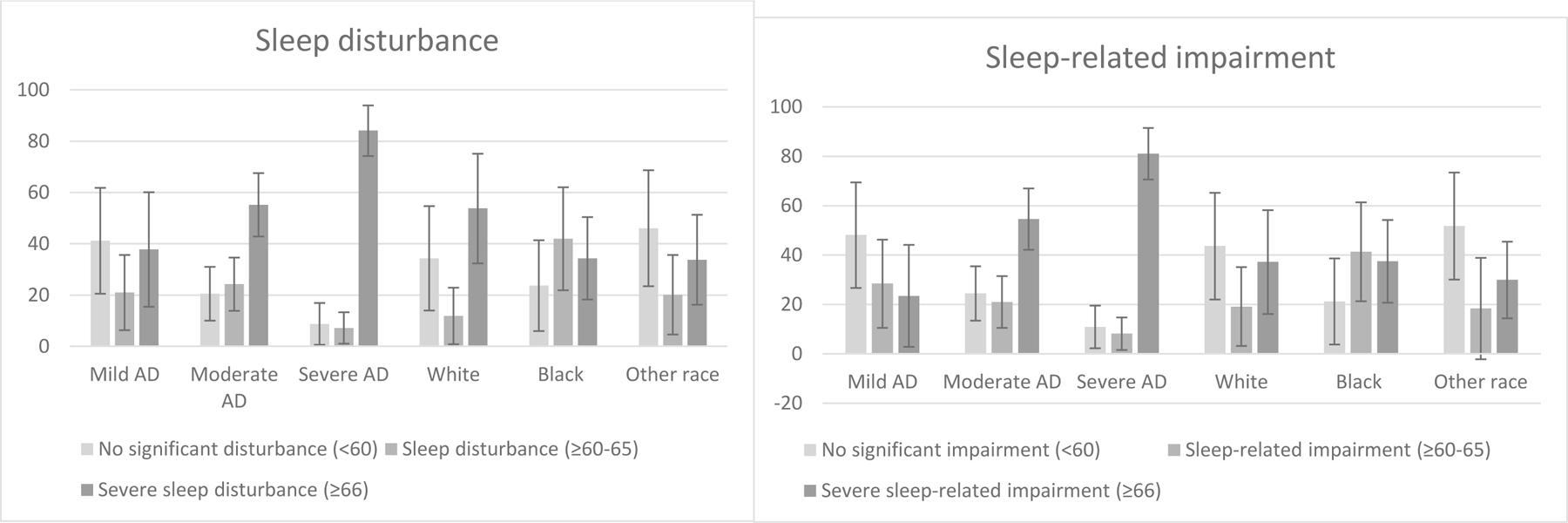
Weighted proportion of children with sleep disturbance and sleep-related impairment, by atopic dermatitis severity and race. Error bars reflect 95% confidence interval.
AD severity was similarly associated with stepwise increases in both any sleep-related impairment, PROMIS T-score ≥60–65 (moderate AD: 2.86 [132–6.23], P=0.008, severe AD: 7.62 [1.23–47.12], P=0.03), and severe sleep-related impairment, PROMIS T-score ≥66 (moderate AD: 3.92 [1.88–8.19], P=0.0003, severe AD: 13.93 [3.16–61.34], P=0.0005). Black vs. White race was associated with increased sleep-related impairment after controlling for disease severity (2.68 [1.14–6.31], P=0.02).
Predictors of sleep disturbance in the unweighted sample
In multivariable models using stepwise variable addition, parent-proxy reported sleep disturbance T-score was associated with increased itch intensity (adjusted β [95% CI]: 1.33 [0.62, 2.04]), income (<$50k: 6.64 [2.05, 11.23], $50 to <100k: 4.75 [0.35, 9.14]), and parent with a Master’s degree (4.67 [1.80, 7.54]) (Table 3A). Sleep disturbance T-score reported by children was associated with adjusted POEM (0.46 [0.08, 0.83]) and increased itch intensity (1.37 [0.58, 2.16) (Table 3B).
Table 3:
Stepwise linear regression model evaluating the associations between atopic dermatitis disease severity, itch and significant sociodemographic factors and the outcome of sleep disturbance
| Table 3A. Parent-proxy reported sleep disturbance (n=180) | Table 3B. Child self-reported sleep disturbance (n=133) | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Multivariable model |
Multivariable model |
||
| Adjusted β [95% CI] | P-value | Adjusted β [95% CI] | P-value | |
| Model 1 | ||||
| POEM without itch/sleep | 0.77 [0.53, 1.01] | <.0001 | 0.95 [0.66, 1.24] | <.0001 |
| Model 2 | ||||
| POEM without itch/sleep | 0.38 [0.11, 0.66] | 0.007 | 0.47 [0.12, 0.83] | 0.009 |
| Numeric Rating Scale Itch | 1.32 [0.61, 2.03] | 0.0003 | 1.37 [0.57, 2.16] | 0.0009 |
| Model 3 | ||||
| POEM without itch/sleep | 0.24 [−0.07, 0.54] | 0.13 | 0.46 [0.08, 0.83] | 0.02 |
| Numeric Rating Scale Itch | 1.33 [0.62, 2.04] | 0.0003 | 1.37 [0.58, 2.16] | 0.0008 |
| Parental income | ||||
| Under $50,000 | 6.64 [2.05, 11.23] | 0.005 | ||
| $50,000 to <$100,000 | 4.75 [0.35, 9.14] | 0.03 | ||
| >$100,000 | 1.00 [ref] | - | ||
| Parental Education | ||||
| ≤Associate’s Degree | 1.00 [ref] | - | 1.00 [ref] | - |
| Bachelor’s Degree | 1.58 [−0.85, 4.02] | 0.20 | −0.12 [−3.42, 3.18] | 0.94 |
| ≥Master’s Degree | 4.67 [1.80, 7.54] | 0.002 | 1.04 [−2.07, 4.16] | 0.51 |
Bold-face indicates statistical significance. Model 3 controls for sociodemographic factors significantly associated with sleep disturbance in bivariable models.
Psychologic symptoms and Neurocognitive impairment in children with sleep disturbance in the unweighted sample
Children with sleep disturbance had significant decrements in psychologic and neurocognitive functioning across multiple domains. In fully-adjusted models controlling for adjusted POEM disease severity, itch intensity, and all significant socio-demographics from our stepwise models, parent-proxy reported sleep disturbance was associated with increased severity of sleep-related impairment, depression, fatigue, and anxiety, in addition to worse inattention and impulsivity (Table 4). Moreover, in similarly adjusted models, children with parent-proxy reported sleep disturbance (T-score ≥ 60) had increased odds of sleep-related impairment (1.46 [1.24–1.72]), depression (1.19 [1.08, 1.31]), fatigue (1.28 [1.14–1.45]), and anxiety (1.29 [1.13–1.46]). Child self-reported sleep disturbance was similarly associated with sleep-related impairment, depression, fatigue, and anxiety, and those with a T-score ≥ 60 had increased odds of sleep-related impairment (1.20 [1.11–1.29]), depression (1.13 [1.03, 1.24]), fatigue (1.28 [1.06–1.54]), and anxiety (1.16 [1.02–1.31]).
Table 4.
Association of sleep disturbance* with psychologic symptoms and neurocognitive function.
| Crude β [95% CI] | P-value | POEM adjust Adj β [95% CI]** |
P-value | POEM+NRS itch Adj β [95% CI]** |
P-value | Fully-adjusted Adj β [95% CI]*** |
P-value | |
|---|---|---|---|---|---|---|---|---|
| Parent proxy response 5–17 years (n=180) | ||||||||
| PROMIS pediatric profile | ||||||||
| Sleep-related impairment | 1.08 [0.96, 1.19] | <.0001 | 1.00 [0.85, 1.14] | <.0001 | 1.00 [0.84, 1.15] | <.0001 | 1.01 [0.86, 1.17] | <.0001 |
| Depression | 0.89 [0.71, 1.08] | <.0001 | 0.74 [0.53, 0.95] | <.0001 | 0.72 [0.51, 0.93] | <.0001 | 0.67 [0.46, 0.88] | <.0001 |
| Fatigue | 0.96 [0.79, 1.12] | <.0001 | 0.88 [0.69, 1.07] | <.0001 | 0.87 [0.69, 1.06] | <.0001 | 0.76 [0.56, 0.97] | <.0001 |
| Peer relationships | −0.06 [−0.28, 0.15] | 0.55 | −0.08 [−0.36, 0.20] | 0.57 | −0.08 [−0.38, 0.22] | 0.61 | −0.10 [−0.43, 0.23] | 0.54 |
| Anxiety | 0.93 [0.73, 1.14] | <.0001 | 0.79 [0.55, 1.03] | <.0001 | 0.81 [0.58, 1.05] | <.0001 | 0.79 [0.57, 1.01] | <.0001 |
| MacArthur HBQ ADHD symptoms | ||||||||
| Inattention | 0.03 [0.02, 0.05] | <.0001 | 0.03 [0.02, 0.04] | <.0001 | 0.03 [0.02, 0.04] | <.0001 | 0.03 [0.02, 0.04] | <.0001 |
| Impulsivity | 0.03 [0.02, 0.04] | <.0001 | 0.02 [0.01, 0.04] | <.0001 | 0.03 [0.02, 0.04] | <.0001 | 0.02 [0.01, 0.04] | <.0001 |
|
| ||||||||
| Child self-report 8–17 years (n=133) | ||||||||
| PROMIS pediatric profile | ||||||||
| Sleep-related impairment | 0.93 [0.82, 1.05] | <.0001 | 0.81 [0.66, 0.95] | <.0001 | 0.79 [0.62, 0.96] | <.0001 | 0.87 [0.71, 1.03] | <.0001 |
| Depression | 0.67 [0.48, 0.86] | <.0001 | 0.60 [0.39, 0.81] | <.0001 | 0.66 [0.47, 0.84] | <.0001 | 0.66 [0.46, 0.86] | <.0001 |
| Fatigue | 0.83 [0.71, 0.96] | <.0001 | 0.69 [0.52, 0.86] | <.0001 | 0.67 [0.49, 0.86] | <.0001 | 0.69 [0.51, 0.87] | <.0001 |
| Peer relationships | −0.13 [−0.37, 0.10] | 0.27 | −0.11 [−0.41, 0.20] | 0.48 | −0.13 [−0.45, 0.20] | 0.44 | −0.13 [−0.48, 0.22] | 0.46 |
| Anxiety | 0.69 [0.51, 0.87] | <.0001 | 0.61 [0.40, 0.82] | <.0001 | 0.68 [0.49, 0.87] | <.0001 | 0.69 [0.49, 0.88] | <.0001 |
PROMIS T-score parent-proxy sleep disturbance or child self-report sleep disturbance, respectively
Adjusted for POEM composite severity score without sleep or itch items
Adjusted for POEM composite without sleep or itch items, itch NRS, and significant socio-demographics from Table 2
Discussion
This study found significant parent-proxy reported sleep disturbance occurs in a weighted 66.9% [95% CI: 53.3–80.5%] of US children with AD, ~3 million school aged children. More than half of these children had severe sleep disturbance, meaning their sleep is worse than 95% of children in the general population.(17) We quantified the sleep disturbance burden by disease severity groups to identify the high percentage affected, severe (91.3%) vs. moderate (79.5%) vs. mild (58.8%) patients. We previously identified that children with moderate/severe disease have ~50 minutes less sleep per night than age/sex/racially matched controls.(9) As such, it might not be surprising that most children with AD have significant sleep disturbance. Yet, it is profound and devastating to think about the impact this might have on psychologic and neurocognitive function.
Although this does not imply causality, we noted that depression, as well as anxiety, assessed by parent-proxy report on PROMIS measures, were significantly associated with poor sleep (β=0.67 [0.46, 0.88], p<0.01 and 0.79 [0.57, 1.01], p<0.01, respectively), even when controlling for disease severity, itch and sociodemographic variables. Parent and child responses were fairly concordant in the present study. This suggests that poor sleep is associated with mental health comorbidities in AD, an association also found in adult AD cohorts.(13, 21) Although peer relationships can worsen with AD,(22) we did not find it associated with sleep disturbance. Inattention and impulsivity were also significantly associated in fully adjusted models with sleep disturbance, the small unstandardized β reflecting the narrow range of numbers used for these scores (β=0.03 [0.02, 0.04], p<0.01 and 0.02 [0.01, 0.04], p<0.01, respectively). The most commonly noted symptoms of inattention in our cohort were: losing things, jumping from one activity to another, not seeming to listen, and difficulty following directions or instructions (Table E3).
Although we did not study sleep longitudinally, one previous cohort study from the United Kingdom among almost 5000 children with AD affirms that sleep disturbance in AD is experienced throughout childhood in children with active AD(23) as well as in their parents.(24) This suggests that psychologic and neurocognitive effects are ongoing.(22, 25) Although causation cannot be proven, longitudinal studies investigating the impact of childhood sleep disturbance in general populations have suggested an increased risk of anxiety, inattention and impulsivity.(26, 27) In fact, more long-term childhood sleep deficits are associated with more deficits in both cortical gray matter and white matter, including in the prefrontal cortex.(28) Specific brain activation pathways are shared in studies associating sleep disturbance and ADHD,(29) and in AD brain imaging studies, the prefrontal cortex is also an area of interest.(30) Systemic inflammation, such as elevated IL-6 levels in sleep disturbance, might also be part of the association between AD, neurocognitive and psychologic impairment.(31) Further work in this area is needed.
Stepwise linear regression modeling in this study also provides putative evidence of associations driving sleep disturbance in this age group. Itch was associated with sleep disturbance in fully adjusted models, in both parent-proxy and child report. Interestingly, in the model using parent-proxy reported sleep disturbance, lower income and having a Master’s degree each were associated with a greater likelihood of experiencing sleep disturbance. In child self-report, there was no significant contribution of sociodemographic variables. In this study, race was not significantly associated with an increased risk of sleep disturbance in fully adjusted models. This also points out that parent versus child-report, while fairly concordant, still captures different perspectives about the child’s sleep. Children can self-report starting at ~5 years old using PROMIS assessments, but might under-report symptoms.(7) If possible, clinicians should capture data from both parent/children, and rely more heavily on self-report in older children, while pursuing flagged values from either source. Clinical algorithms are acceptable which include either parent or child report.(7) Figure 2 is our purported algorithm for clinical use to screen and monitor sleep disturbance in school aged children with AD.
Figure 2.
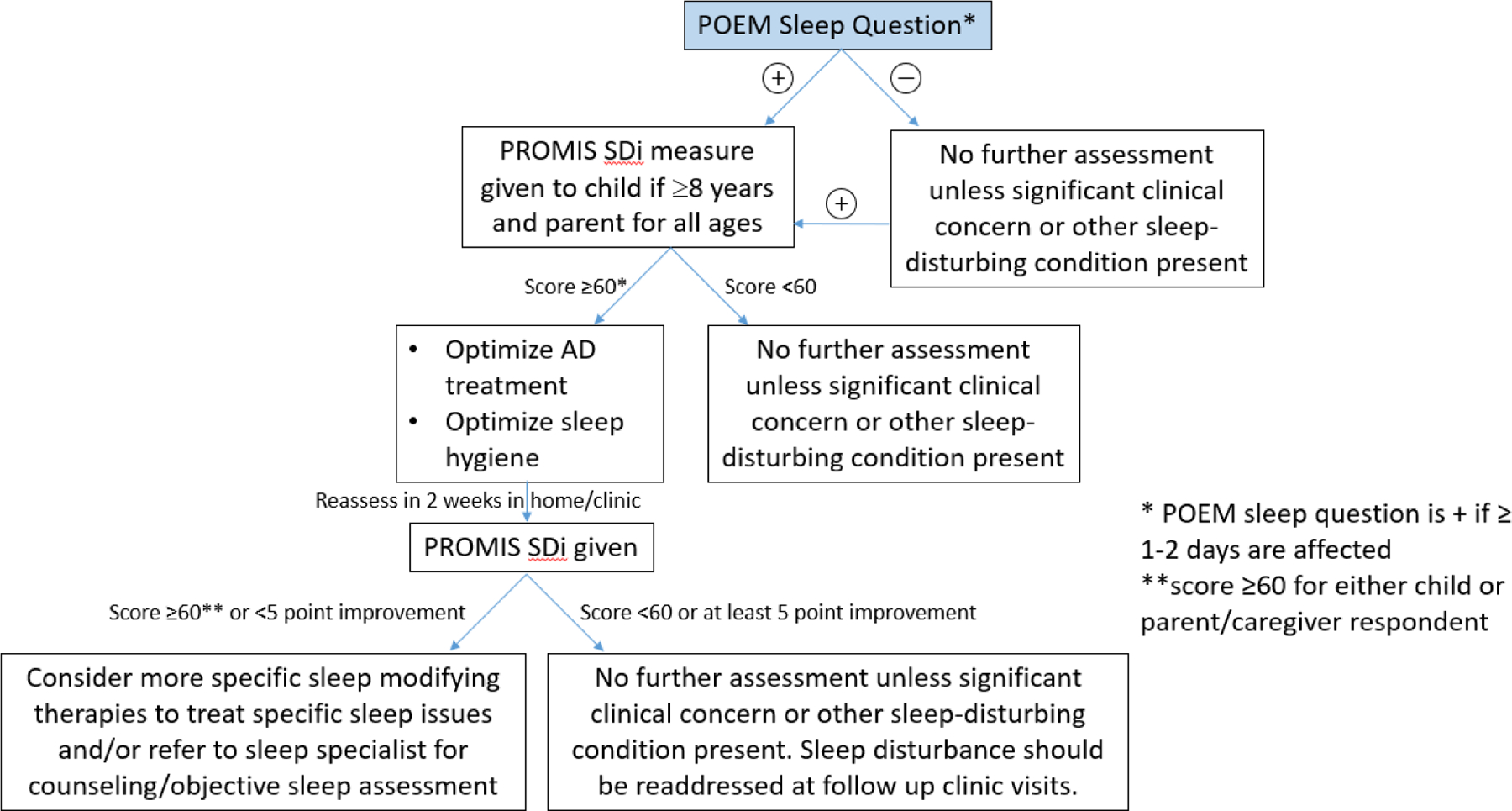
Clinical screening algorithm for sleep disturbance in school aged children with AD.
Reprinted with permission from Fishbein et al. Journal of the American Academy of Dermatology, 2020.
SDi= Sleep Distrubance, PROMIS= Patient Reported Outcome Measurement Information System
Limitations
This study has some limitations. First, disease severity and assessments were all based on parent-proxy or patient report; objective data was not available. Indeed, we have previously demonstrated that objective sleep disturbance (actigraphy) and PROMIS sleep assessment are distinct domains.(7) Our study is limited to cross-sectional data, so the longitudinal impact of AD on sleep was indeterminable. Additionally, our population-based estimates have inherent limitations as they were not sampled directly, and limited based on extrapolation of older data from 2007 NSCH. This data source did not include PROMIS questions about sleep and was chosen only for an estimate of population-based AD prevalence in the US. It was not used to generate a comparator group. Our cohort excluded patients with poorly controlled asthma, poorly controlled allergic rhinitis and other sleep disturbing conditions, which might be underestimating the prevalence and severity of sleep disturbance in AD. Given that the study questionnaire focused on AD and quality of life, respondents were not be blinded to the intent of the study. Although we utilized a stratified sampling approach to ensure adequate distribution of samples across all strata and allow for estimation of the sleep disturbance within each strata, it is a limitation that our estimates are based off a relatively small sample. Finally, minority populations frequently have worse AD severity, we used a targeted stratification plan wherein race was evenly represented across disease severity group. Although this is a strength of the study, it does unfortunately limit the ability to assess race-severity associations in these data.
Conclusions
As suggested in practice guidelines(32, 33) and from our findings, all children with AD should be screened for sleep disturbance, particularly those with worse itch and moderate/severe disease severity. One large cross-sectional study in adults gives hope that controlled disease can improve sleep, depression and anxiety.(34) Although some degree of impairment is still present compared to control patients, significant improvement is possible. In addition to sleep disturbance, psychologic and neurocognitive effects of AD are important to screen for in the clinic setting. Those with moderate or severe disease are at greatest risk of sleep disturbance, and have the largest treatment burden. Parents, patients and providers should align on a personalized medication and treatment regimen to avoid these severe consequences of AD.
The large prevalence of AD and the high frequency of sleep disturbance make this topic an important, yet long understudied problem.(2, 35) A publication from the sleep medicine literature further underscores the recognition from other fields that AD has the potential to induce devastating neurocognitive effects from prolonged sleep disturbance.(3) Our ongoing work focuses on uncovering the mechanism of sleep disturbance in AD to develop more targeted treatment approaches. In the meantime, we refer clinicians to our previously published algorithm to screen, assess and treat sleep disturbance in children with AD.(7)
Supplementary Material
Figure E1. Flowchart of participants.
*For demographic characteristics such as age, sex, race where that particular strata was already filled, participants who were over quota were not invited to complete the remainder of survey
Table E1. Unweighted prevalence of sleep disturbance and sleep-related impairment, by AD severity and race, in the study cohort (n=180).
Table E2. Domains of sleep disturbance by AD severity.
*Effect size calculated by Cramer’s V given multiple variable levels.
** Rao-Scott χ2 test comparing almost clear/mild vs moderate vs severe/very severe AD.
Bold text indicates statistical significance.
Table E3. Concordance of inattention and impulsivity items, by AD disease severity.
*Rao-Scott χ2 test comparing almost clear/mild vs moderate vs severe/very severe AD.
** Rao-Scott χ2 test comparing almost clear/mild/moderate vs severe/very severe AD.
Bold text indicates statistical significance.
What is already known about this topic?
Sleep disturbance is common in atopic dermatitis(AD). Patient Reported Outcome Measurement Information System(PROMIS) sleep disturbance is a meaningful outcome to capture the lived experiences of sleep, which our group has validated in pediatric AD.
What does this article add to our knowledge?
~3 million US children experience AD-induced sleep disturbance. This equates to 67% of all children with AD, and 91% with severe disease. Sleep disturbance in AD is associated with neuropsychiatric impairment- depression, anxiety, and inattention.
How does this study impact current management guidelines?
Clinicians should screen for sleep disturbance and neuropsychiatric symptoms in school-aged children, particularly with moderate-to-severe AD.
Funding Support:
The Agency for Healthcare Research and Quality (grant number K12HS023011 to AF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality
Abbreviations used:
- AD
atopic dermatitis
- PROMIS
Patient Reported Outcome Measurement Information System
- POEM
Patient Oriented Eczema Measure
- NSCH
National Survey of Children’s Health
- OR
odds ratios
- CI
confidence interval
- NRS
Numerical Rating Scale
- MacArthur HBQ
MacArthur Health Behavioral Questionnaire
- IQR
interquartile range
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- SDi
Sleep Disturbance
- ADHD
Attention Deficit Hyperactive Disorder
- BMI
Body Mass Index
- PIQ-C
PROMIS Pediatric Itch Questionnaire-Child
Footnotes
Administrative technical or material support: None
Study supervision: None
Financial disclosures: None relevant to this manuscript
Design and conduct of the study? No
Collection, management, analysis and interpretation of data? No
Preparation, review, or approval of the manuscript? No
Decision to submit the manuscript for publication? No
Conflicts of interest: None
References
- 1.Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. The journal of allergy and clinical immunology In practice. 2020;8(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishbein AB, Vitaterna O, Haugh IM, Bavishi AA, Zee PC, Turek FW, et al. Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. The Journal of allergy and clinical immunology. 2015;136(5):1170–7. [DOI] [PubMed] [Google Scholar]
- 3.Camfferman D, Kennedy JD, Gold M, Martin AJ, Lushington K. Eczema and sleep and its relationship to daytime functioning in children. Sleep medicine reviews. 2010;14(6):359–69. [DOI] [PubMed] [Google Scholar]
- 4.Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114(3):607–11. [DOI] [PubMed] [Google Scholar]
- 5. http://www.morethanskindeep-eczema.org/uploads/1/2/5/3/125377765/mtsd_report_-_digital_file_1.pdf.
- 6.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbein AB, Lor J, Penedo FJ, Forrest CB, Griffith JW, Paller AS. Patient-Reported Outcomes for Measuring Sleep Disturbance in Pediatric Atopic Dermatitis: cross sectional study of PROMIS Pediatric Sleep Measures and Actigraphy. Journal of the American Academy of Dermatology. 2020. [DOI] [PMC free article] [PubMed]
- 8.Treister AD, Stefek H, Grimaldi D, Rupani N, Zee P, Yob J, et al. Sleep and Limb Movement Characteristics of Children With Atopic Dermatitis Coincidentally Undergoing Clinical Polysomnography. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2019;15(8):1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishbein AB, Mueller K, Kruse L, Boor P, Sheldon S, Zee P, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: A case-control study. Journal of the American Academy of Dermatology. 2018;78(2):336–41. [DOI] [PubMed] [Google Scholar]
- 10.Rubens SL, Patrick KE, Williamson AA, Moore M, Mindell JA. Individual and socio-demographic factors related to presenting problem and diagnostic impressions at a pediatric sleep clinic. Sleep medicine. 2016;25:67–72. [DOI] [PubMed] [Google Scholar]
- 11.L LK, Cices A, Fishbein AB, Paller AS. Neurocognitive function in moderate-severe pediatric atopic dermatitis: A case-control study. Pediatric dermatology. 2019;36(1):110–4. [DOI] [PubMed] [Google Scholar]
- 12.Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. The British journal of dermatology. 2016;175(5):920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. The British journal of dermatology. 2019;181(3):554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. The British journal of dermatology. 2013;169(6):1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis : contact, atopic, occupational, drug. 2014;25(3):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest CB, Meltzer LJ, Marcus CL, de la Motte A, Kratchman A, Buysse DJ, et al. Development and validation of the PROMIS Pediatric Sleep Disturbance and Sleep-Related Impairment item banks. Sleep. 2018;41(6). [DOI] [PubMed] [Google Scholar]
- 17.Carle AC, Bevans KB, Tucker CA, Forrest CB. Using nationally representative percentiles to interpret PROMIS pediatric measures. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2020. [DOI] [PMC free article] [PubMed]
- 18.Lemery-Chalfant K, Schreiber JE, Schmidt NL, VANH CA, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing, and attention problems in young children: validation of the MacArthur HBQ. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1315–23. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong JM, Goldstein LH, & The MacArthur Working Group on, and OAMftMH, Research BQHMF, Network on Psychopathology and Development (David J. Kupfer C, Pittsburgh. Uo.
- 20.Cohen JSaftbsneLEA.
- 21.Li JC, Fishbein A, Singam V, Patel KR, Zee PC, Attarian H, et al. Sleep Disturbance and Sleep-Related Impairment in Adults With Atopic Dermatitis: A Cross-sectional Study. Dermatitis : contact, atopic, occupational, drug. 2018;29(5):270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan J, Takeshita J, Shin DB, Gelfand JM. Mental health impairment among children with atopic dermatitis: A United States population-based cross-sectional study of the 2013–2017 National Health Interview Survey. Journal of the American Academy of Dermatology. 2020;82(6):1368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of Atopic Dermatitis With Sleep Quality in Children. JAMA pediatrics. 2019;173(5):e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Assessment of Sleep Disturbances and Exhaustion in Mothers of Children With Atopic Dermatitis. JAMA dermatology. 2019;155(5):556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien LM, Gozal D. Neurocognitive dysfunction and sleep in children: from human to rodent. Pediatric clinics of North America. 2004;51(1):187–202. [DOI] [PubMed] [Google Scholar]
- 26.Cook F, Conway LJ, Giallo R, Gartland D, Sciberras E, Brown S. Infant sleep and child mental health: a longitudinal investigation. Archives of disease in childhood. 2020;105(7):655–60. [DOI] [PubMed] [Google Scholar]
- 27.Huhdanpää H, Morales-Muñoz I, Aronen ET, Pölkki P, Saarenpää-Heikkilä O, Paunio T, et al. Sleep Difficulties in Infancy Are Associated with Symptoms of Inattention and Hyperactivity at the Age of 5 Years: A Longitudinal Study. Journal of developmental and behavioral pediatrics : JDBP. 2019;40(6):432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocevska D, Muetzel RL, Luik AI, Luijk MP, Jaddoe VW, Verhulst FC, et al. The Developmental Course of Sleep Disturbances Across Childhood Relates to Brain Morphology at Age 7: The Generation R Study. Sleep. 2017;40(1). [DOI] [PubMed] [Google Scholar]
- 29.Shen C, Luo Q, Chamberlain SR, Morgan S, Romero-Garcia R, Du J, et al. What Is the Link Between Attention-Deficit/Hyperactivity Disorder and Sleep Disturbance? A Multimodal Examination of Longitudinal Relationships and Brain Structure Using Large-Scale Population-Based Cohorts. Biological psychiatry. 2020;88(6):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. The British journal of dermatology. 2009;161(5):1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soundararajan V, Lor J, Fishbein AB. Sleep Apnea and Skin. Current sleep medicine reports. 2020;6(3):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. The Journal of allergy and clinical immunology. 2013;131(2):295–9.e1–27. [DOI] [PubMed] [Google Scholar]
- 33.Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. Journal of the American Academy of Dermatology. 2014;70(2):338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckert L, Gupta S, Gadkari A, Mahajan P, Gelfand JM. Burden of illness in adults with atopic dermatitis: Analysis of National Health and Wellness Survey data from France, Germany, Italy, Spain, and the United Kingdom. Journal of the American Academy of Dermatology. 2019;81(1):187–95. [DOI] [PubMed] [Google Scholar]
- 35.Bender BG, Leung DY. Sleep disorders in patients with asthma, atopic dermatitis, and allergic rhinitis. The Journal of allergy and clinical immunology. 2005;116(6):1200–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Flowchart of participants.
*For demographic characteristics such as age, sex, race where that particular strata was already filled, participants who were over quota were not invited to complete the remainder of survey
Table E1. Unweighted prevalence of sleep disturbance and sleep-related impairment, by AD severity and race, in the study cohort (n=180).
Table E2. Domains of sleep disturbance by AD severity.
*Effect size calculated by Cramer’s V given multiple variable levels.
** Rao-Scott χ2 test comparing almost clear/mild vs moderate vs severe/very severe AD.
Bold text indicates statistical significance.
Table E3. Concordance of inattention and impulsivity items, by AD disease severity.
*Rao-Scott χ2 test comparing almost clear/mild vs moderate vs severe/very severe AD.
** Rao-Scott χ2 test comparing almost clear/mild/moderate vs severe/very severe AD.
Bold text indicates statistical significance.


