Abstract
The ‘Sepsis Six’ bundle was promoted as a deliverable tool outside of the critical care settings, but there is very little data available on the progress and change of sepsis care outside the critical care environment in the UK. Our aim was to compare the yearly prevalence, outcome and the Sepsis Six bundle compliance in patients at risk of mortality from sepsis in non-intensive care environments. Patients with a National Early Warning Score (NEWS) of 3 or above and suspected or proven infection were enrolled into four yearly 24-h point prevalence studies, carried out in fourteen hospitals across Wales from 2016 to 2019. We followed up patients to 30 days between 2016–2019 and to 90 days between 2017 and 2019. Out of the 26,947 patients screened 1651 fulfilled inclusion criteria and were recruited. The full ‘Sepsis Six’ care bundle was completed on 223 (14.0%) occasions, with no significant difference between the years. On 190 (11.5%) occasions none of the bundle elements were completed. There was no significant correlation between bundle element compliance, NEWS or year of study. One hundred and seventy (10.7%) patients were seen by critical care outreach; the ‘Sepsis Six’ bundle was completed significantly more often in this group (54/170, 32.0%) than for patients who were not reviewed by critical care outreach (168/1385, 11.6%; p < 0.0001). Overall survival to 30 days was 81.7% (1349/1651), with a mean survival time of 26.5 days (95% CI 26.1–26.9) with no difference between each year of study. 90-day survival for years 2017–2019 was 74.7% (949/1271), with no difference between the years. In multivariate regression we identified older age, heart failure, recent chemotherapy, higher frailty score and do not attempt cardiopulmonary resuscitation orders as significantly associated with increased 30-day mortality. Our data suggests that despite efforts to increase sepsis awareness within the NHS, there is poor compliance with the sepsis care bundles and no change in the high mortality over the study period. Further research is needed to determine which time-sensitive ward-based interventions can reduce mortality in patients with sepsis and how can these results be embedded to routine clinical practice.
Trial registration Defining Sepsis on the Wards ISRCTN 86502304 https://doi.org/10.1186/ISRCTN86502304 prospectively registered 09/05/2016.
Subject terms: Bacterial infection, Epidemiology, Fever, Antimicrobial therapy
Introduction
Sepsis is defined as dysregulated host response to infection with sequential organ failure. It is a complex disorder and is associated with high mortality1. Despite increased awareness, sepsis remains a major challenge and economic burden to healthcare globally2–5. To improve patient mortality, sepsis requires early recognition and urgent treatment6. Previously much attention was dedicated to the identification and treatment of patients at risk of poor outcomes within intensive care units (ICU)7,8. However, it is now known that the majority of patients with sepsis present in the emergency department (ED) and on general wards, with associated high mortality9–11.
Since the inception of the sepsis resuscitation bundle by the Surviving Sepsis Campaign (SSC) over a decade ago, completion rates have been reportedly low12–14. As the initial SSC bundle was heavily reliant on complex interventions, typically performed in a critical care environment, the ‘Sepsis Six’ bundle was promoted as a more deliverable tool outside of the critical care settings15. Although high-profile cases and systematic campaign from advocacy groups helped to increase awareness of the condition in the last decade, there is very little data available on the progress and change of sepsis care outside the critical care environment in the UK15,16. While the use of sepsis screening tools and the delivery of the ‘Sepsis Six’ bundle is now a key performance indicator in many institutions, external scrutiny of such initiatives is lacking17,18. The aim of our study was to examine the changes in care processes and outcomes over a four-year period, by utilising our yearly All Wales point-prevalence study on sepsis.
Methods
Study design and participants
We performed a secondary analysis on the patient populations recruited into four annual multi-centre 24-h point-prevalence studies conducted on the third Wednesday of October from 2016 to 2019. The study was conducted accordance with relevant guidelines and regulations including the Declaration of Helsinki. The Defining Sepsis on the Wards project was prospectively registered with an international trial registry (ISRCTN86502304).
Patients were recruited from each of the 14 acute hospitals across Wales, all of which had 24-h consultant cover in the ED and non-selective intake. Participating hospitals were identified through local collaborators via the Welsh Intensive Care Society Audit and Research Group. We screened all patients presenting to the ED and on the general wards. At the start of the study days at 08:00, data collectors systematically screened every patient on the acute in-patient wards within 4 h, then continued screening for any potential new participants until 07:59 the next morning. In each hospitals dedicated data collectors were stationed in the ED during the 24 h periods. We approached all patients with NEWS ≥ 3 in whom the treating clinical teams had a high degree of clinical suspicion of an infection (documented as such in the medical or nursing notes), and following the patients or their proxy, in cases of patients lacking capacity, gave written informed consent and were recruited to the study. Patients under 18 and those cared for in critical care or mental health units were excluded.
Local investigators were identified and were supported by three national coordinators. Key study information was provided through e-mails, face-to-face training and online video tutorials, which included the protocol, answers to key questions and description of the electronic case report form (eCRF). The details of the digital data collection platform developed for this study have been published previously19. Medical students working in pairs to ensure data validity and appropriate clinical knowledge, acted as data collectors, using tablets for electronic data collection and transfer. The tablets contained all supporting information needed for the study, including national formulary. Data collectors were supported by continuous online web-chat, which made the senior clinicians and the medical student national coordinators available throughout the study period. We referred patients to the clinical teams if the medical student data collectors felt they needed urgent medical attention due to their condition, in line with the requirements of the ethics approval. To facilitate linkage to national databases for the collection of follow-up data, we collected patient-identifiable data and entered it on to the secure data collection tool19. Further description of the methodology and performance of this platform is outlined in previous publications16,18–23.
We collected data from medical and nursing records on pre-admission patient characteristics, co-morbidities, physiological and laboratory values, Dalhousie clinical frailty score, and management actions such as the completion of the ‘Sepsis Six’ bundle and involvement of critical care outreach. In 2016, we conducted follow-up data collection for our primary outcome of all-cause mortality at 30 days from enrolment. In subsequent years (2017–2019) we conducted follow-up at 30 and 90 days.
Policy content: During the study period all of the participating hospitals were actively engaged in the Rapid Response to Acute Illness Learning Set (RRAILS) programme led by 1000 Lives Improvement. In 2013, all hospitals in Wales implemented the use of NEWS, with a score of six or above set to trigger the escalation of patients to senior decision makers or for consideration of referral to critical care outreach. RRAILS promoted the use of standardised sepsis screening tool across the hospital since 2008 (see Supplementary Figure 1). In 2018 the Welsh Government introduced a quality improvement performance indicator for the completion of ‘Sepsis Six’ in all acute hospitals based on the RRAILS tool.
Statistical analysis
Categorical variables are described as proportions and are compared using Chi square test. Continuous variables are described as median and interquartile range (IQR) and compared using Mann–Whitney U test. We plotted Kaplan–Meier survival curves and compared time-to-event data using log-rank testing. The starting point for the survival analysis was the data collection day. We estimated the respective hazard ratios (HRs) for the primary outcome within 30 days with a Cox proportional hazards model after adjustment for measured confounders. The model fit was assessed by the − 2 log likelihood statistics and Chi‐square test.
To increase sample size and to enable the inclusion of patients from all four study years, the primary analysis was performed on 30-day follow up results only. However, we also performed a subgroup analysis using the 90-day survival data using the results from the 2017 to 2019 studies. A two-tailed p-value < 0.05 was considered statistically significant. All statistical tests were calculated using SPSS 25.0 (SPSS Inc., Chicago, IL). Data visualisation was performed in R (Version 1.2.1335) with packages: ggplot2 (v3.3.3), dplyr (v1.0.5), UpSetR (v1.4.0), ComplexHeatmap (v2.7.8.1000) and sunburstR (v2.1.5), utilising repositories from Github (hms-dbmi/UpSetR, jokergoo/ComplexHeatmap and timelyportfolio/sunburstR)24,25.
Ethical approval and consent to participate
Ethical approval was granted by the South Wales Regional Ethics Committee (16/WA/0071, 15/04/2016) and patients or legal representatives gave written informed consent.
Results
Patient characteristics
Over the four annual 24-h point-prevalence study periods, we screened a total of 26,947 patients, of whom 1651 met inclusion criteria and were subsequently recruited (Fig. 1).
Figure 1.

Study flow diagram and eventual study sample. ED; emergency department.
Patient demographics and clinical characteristics for each year of study are shown in Table 1. The median age (IQR [range]) of participants was 73 years (60–82 [18–103]) and more females 852 (51.6%) than males 799 (48.4%) were recruited. The median (IQR) frailty score was 5 (3–6). Age, gender, and frailty of participants did not vary between years (Table 1).
Table 1.
Demographics, clinical characteristics and survival of patients in each year of study. Values are median (IQR [range]), number (proportion) or mean (95%CI).
| Year | ||||||
|---|---|---|---|---|---|---|
| 2016 (n = 380) | 2017 (n = 459) | 2018 (n = 413) | 2019 (n = 399) | All years (n = 1651) | P value | |
| Patient demographics | ||||||
| Age: median years | 74 (61–83 [18–100]) | 73 (62–84 [18–103]) | 73 (59–81 [19–99]) | 73 (60–81 [19–99]) | 73 (60–82 [18–103]) | 0.41 |
| Sex: male | 180 (47.4%) | 231 (50.3%) | 213 (51.6%) | 175 (43.9%) | 799 (48.4%) | 0.12 |
| Survival to 30 days | 380 (79.5%) | 372 (81.0%) | 343 (83.1%) | 332 (83.2%) | 1349 (81.7%) | 0.38 |
| Mean survival in 30-day follow-up (days) | 25.5 (24.5–26.4) | 26.6 (25.8–27.3) | 26.8 (26.0–27.6) | 26.9 (26.1–27.6–) | 26.5 (26.1–26.9) | 0.39 |
| Clinical characteristics | ||||||
| COPD | 112 (30.9%) | 118 (26.2%) | 117 (30.1%) | 135 (34.8%) | 482 (30.3%) | 0.06 |
| Diabetes | 75 (20.7%) | 98 (21.8%) | 89 (22.9%) | 71 (18.3%) | 333 (20.9%) | 0.44 |
| Drugs of abuse | 5 (1.4%) | 8 (1.8%) | 11 (2.8%) | 7 (1.8%) | 31 (1.9%) | 0.51 |
| Heart failure | 45 (12.4%) | 49 (10.9%) | 50 (12.9%) | 39 (10.1%) | 183 (11.5%) | 0.58 |
| Hypertension | 107 (29.5%) | 165 (36.7%) | 145 (37.3%) | 140 (36.1%) | 557 (35.0%) | 0.09 |
| Ischemic heart disease | 63 (17.4%) | 82 (18.2%) | 65 (16.7%) | 67 (17.3%) | 277 (17.4%) | 0.95 |
| Liver disease | 11 (3.0%) | 13 (2.9%) | 19 (4.9%) | 16 (4.1%) | 59 (3.7%) | 0.39 |
| Neuromuscular | 13 (3.6%) | 16 (3.6%) | 11 (2.8%) | 12 (3.1%) | 52 (3.3%) | 0.92 |
| Recent chemotherapy | 14 (3.9%) | 21 (4.7%) | 15 (3.9%) | 24 (6.2%) | 74 (4.7%) | 0.37 |
| Frailty score: median* | 5 (3–6) | 5 (3–6) | 4 (3–6) | 5 (3–6) | 5 (3–6) | 0.26 |
| DNA-CPR | 90 (24.1%) | 123 (27.5%) | 92 (24.5%) | 109 (27.9%) | 414 (26.1%) | 0.49 |
| NEWS ≥ 6 | 115 (30.3%) | 130 (28.3%) | 120 (29.1%) | 121 (30.3%) | 486 (29.4%) | 0.90 |
*Frailty score range was from 1 (“very fit”) to 9 (“terminally ill”) in all years.
Data was missing for frailty score for a total of 64 patients; 7 in 2016, 12 in 2017, 37 in 2018 and 8 in 2019.
COPD, Chronic Obstructive Pulmonary Disease, DNA-CPR, Do Not Attempt Cardiopulmonary Resuscitation order, NEWS, National Early Warning Score, IQR, interquartile range, 95%CI, 95% confidence interval.
Sepsis management
Overall, 289 (18.2%) patients were screened for sepsis using the ‘All Wales sepsis screening tool’. The ‘Sepsis Six’ bundle was completed on 223 (14.0%) occasions. There were no significant trends in completion rates of the screening tools between 2016 and 2019, nor in the proportion of patients seen by critical care outreach (Table 2).
Table 2.
Screening and management of patients in each year of study. Values are number (proportion).
| Year | ||||||
|---|---|---|---|---|---|---|
| 2016 (n = 373) | 2017 (n = 446) | 2018 (n = 380) | 2019 (n = 391) | All years (n = 1590) | P value | |
| Completed ‘Sepsis Six’ bundle | 44 (11.8%) | 63 (14.1%) | 58 (15.3%) | 58 (14.8%) | 223 (14.0%) | 0.53 |
| Completed All Wales screening tool | 59 (15.8%) | 100 (22.4%) | 62 (16.5%) | 68 (17.4%) | 289 (18.2%) | 0.06 |
| Number of patients seen by critical care outreach | 33 (8.8%) | 56 (12.6%) | 32 (8.6%) | 49 (12.5%) | 170 (10.7%) | 0.11 |
Data was missing for; Completed All Wales Screening tool for 4 patients in 2018; Data was also missing for number of patients seen by critical care outreach for 6 patients in 2018.
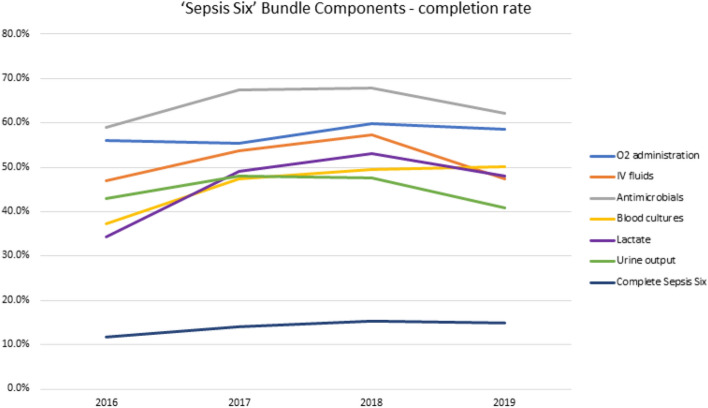
The completion of overall, as well as individual elements of the ‘Sepsis Six’ bundle over time is further presented in Fig. 2.
Figure 2.
‘Sepsis Six’ bundle completion rates during the study period. Data is presented for overall (dark blue line) and individual bundle elements: O2 administration (blue line), IV fluids (orange line), antimicrobials (grey line), blood cultures (yellow line), lactate (purple line), urine output measurement (green line).
When examined individual bundle elements, lactate measurement and obtaining blood cultures improved over time; however all elements were completed well below 70% of occasions (Fig. 2). We found no differences between organisations in completing ‘Sepsis Six’ bundles (as displayed in Supplementary Figure 2). Regardless of the number of bundle elements completed, we did not find any difference in the mortality across the years (Supplementary Figure 3). No discernible trends or patterns were identified when we examined the completion of individual and combined bundle elements (Fig. 3 and further interactive visualisation in Supplementary Figure 4 plus summary of most frequent combinations shown in Supplementary Figure 5) or when this was plotted against the patients’ NEWS across the study period (demonstrated in Supplementary Figure 6).
Figure 3.
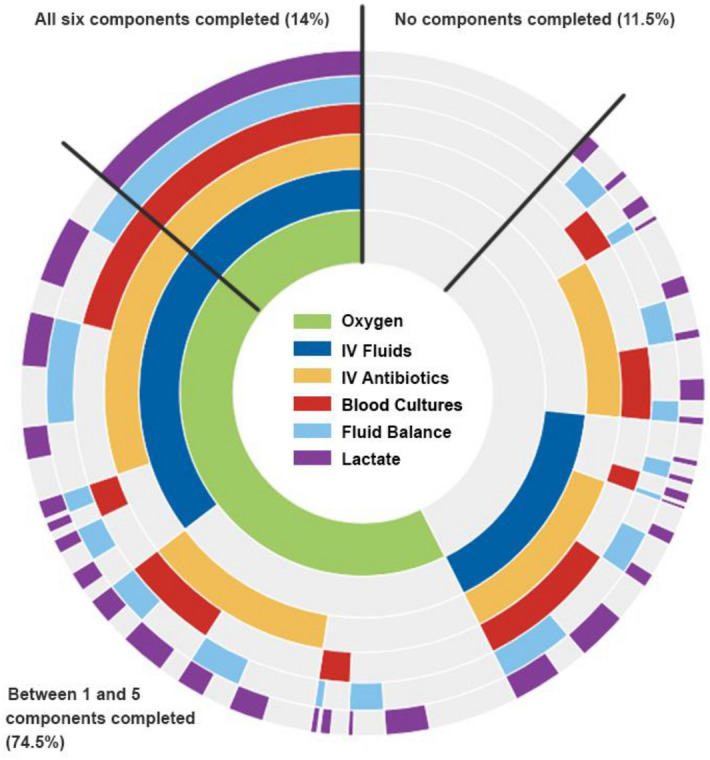
'Sepsis Six’ bundle element completion rates. A sunburst plot illustrating the frequency of completion of each component of the Sepsis Six bundle for the total events from 2016 to 2019 (n = 1588, with missing values removed). The coloured areas denote the Sepsis Six component has been completed, the grey areas denote where a component has not been completed. Working from the center, the frequency of each combination of Sepsis Six bundle components is illustrated. Plot created using R software (Version 1.2.1335), utilising packages ggplot (v 3.3.3) and sunburstR (v2.1.5)24,25. IV: intravenous.
Blood cultures were obtained from 632 (46.0%) patients, of which 89 (14.1%) were positive for growth. Sputum sampling had a substantially higher positivity rate (35.9%). Other microbiology samples were infrequently collected (Table 3).
Table 3.
Sepsis management—culture collection.
| Specimen | Collected (n = 1651) | Positive culture |
|---|---|---|
| Blood | 632 (46.0%) | 89 (14.1%) |
| Sputum | 170 (13.9%) | 61 (35.9%) |
| Urine | 455 (33.4%) | 86 (18.9%) |
| Wound | 112 (8.2%) | 54 (48.2%) |
| CSF | 8 (0.6%) | 0 (0%) |
CSF Cerebrospinal fluid.
Antimicrobials were administered to 743 (64.3%) patients. Piperacillin-tazobactam, followed by co-amoxiclav and clarithromycin were the commonly used antibiotics used over the four-year period and are illustrated in Supplementary Figure 7.
One hundred and seventy (10.7%) patients were seen by critical care outreach; the ‘Sepsis Six’ bundle was completed significantly more often in this group (54/170, 32.0%) than for patients who were not reviewed by critical care outreach (168/1385, 11.6%; p < 0.0001). However, when plotted as a patient pathway these effects became less pronounced (illustrated in the river-plot in Supplementary Figure 8).
In planned sensitivity analysis we found that the percentage number of patients with NEWS 6 or above (overall n = 486, 29.4%) did not change significantly over the study period (Table 1). In this group, more patients had a ceiling of care (such as ward level care only or not for intubation decision) and also DNA-CPR orders in place (19.7% vs 9.5%, p < 0.0001 and 37.8% vs 21.2%, p < 0.0001, respectively) compared to the less acutely unwell population. The completion of the ‘Sepsis Six’ bundle was significantly higher for patients with NEWS 6 or above (20.9% vs 11.1%, p < 0.0001) but unchanged over the study period, as was the completion rate for individual bundle elements (shown in Supplementary Figure 9).
Survival analysis
Overall, 1349 of 1651 patients (81.7%) survived to 30 days with a mean survival time of 26.5 days (95% CI 26.1–26.9). We found no difference in patient survival at 30 days between each year of study (Table 1 and Fig. 4).
Figure 4.
Survival difference of patients with sepsis presenting to emergency department or general wards in fourteen Welsh hospitals in the years; 2016 (blue line), 2017 (red line), 2018 (green line) and 2019 (orange line), p = 0.39.
We observed significantly higher mortality in patients with NEWS 6 or above (23.5% vs 16.1%, p < 0.0001). Overall 90-day survival for years 2017 – 2019 was 74.7% (949/1271). There was no difference in patient survival at 90 days between each year (see Kaplan–Meier curve in Supplementary Figure 10).
Risk factors of mortality
On multivariate regression analysis, we identified older age, heart failure, recent chemotherapy, higher frailty score and do not attempt cardiopulmonary resuscitation (DNA-CPR) orders as significantly associated with increased mortality in patients with sepsis (Table 4).
Table 4.
Multivariate Cox regression analysis of the risk factors for mortality in sepsis patients. Values are Hazards Ratio (95%CI).
| Variables | Hazards ratio (95% CI) | P value |
|---|---|---|
| Demographics | ||
| Age | 1.04 (1.031.05) | < 0.0001 |
| Male | 1.30 (0.96–1.74) | 0.09 |
| Co-morbidities | ||
| COPD | 0.95 (0.70–1.30) | 0.77 |
| Diabetes | 0.81 (0.55–1.18) | 0.26 |
| Drugs of abuse | 0.46 (0.06–3.37) | 0.45 |
| HF | 1.50 (1.03–2.20) | 0.04 |
| HTN | 1.08 (0.80–1.46) | 0.61 |
| IHD | 0.87 (0.60–1.27) | 0.48 |
| Liver disease | 1.07 (0.49–2.32) | 0.86 |
| Neuromuscular | 1.33 (0.61–2.89) | 0.47 |
| Recent chemotherapy | 3.12 (1.86–5.21) | < 0.0001 |
| Frailty score | 1.17 (1.05–1.30) | < 0.01 |
| DNA-CPR | 1.47 (1.03–2.09) | 0.03 |
| NEWS ≥ 6 | 0.84 (0.59–1.20) | 0.34 |
| Management | ||
| Complete sepsis six bundle | 0.67 (0.42–1.08) | 0.10 |
| All Wales screening tool | 0.86 (0.58–1.29) | 0.48 |
| Seen by critical care outreach | 1.13 (0.72–1.77) | 0.60 |
COPD chronic obstructive pulmonary disease, HF heart failure, HTN hypertension, IHD ischemic heart disease, DNA-CPR do not attempt cardiopulmonary resuscitation order. NEWS National Early Warning Score.
Discussion
We identified that sepsis management in Wales (according to sepsis screening tool application and ‘Sepsis Six’ bundle compliance) has not altered over the four-year study period and that mortality remain largely unchanged.
We found the demographic of the study population remained the same for each year, consisting of predominately frail and elderly patients with significant comorbidities. Approximately a third of the patients had a high NEWS and this group had higher likelihood of care limitations and DNA-CPR orders in place. Over the study period, there was no change in the short or medium-term mortality in the cohort, with approximately three out of four patients alive at 90 days. Our data opposes beliefs expressed that within the last decade the implementation of resuscitation bundles has led to better recognition of sepsis, in turn increasing the reported incidence of sepsis and reducing its apparent mortality6,26,27. Our observations are supported by recent analysis of studies identifying sepsis using direct clinical indicators of infection and organ dysfunction, suggesting that over the last decade the incidence and mortality of sepsis has in fact remained stable28,29.
Our findings that older age and higher frailty score are both associated with increased risk of mortality from sepsis, within an elderly population with high comorbidity burden, emphasise the threat of sepsis to patients throughout our hospitals3,30–32. The observations that heart failure and previous chemotherapy are associated with higher mortality from sepsis, are not new and are supported by results from large international cohorts11,33,34.
‘Sepsis Six’ bundle completion remained low with a mean of 14.0% over four years. The lack of improvement in completion of bundles probably underlines the significant problem of sepsis recognition outside of the ICU35. Our results support previously published UK and international data and highlight a significant concern in the real-world operationalising of response, which show significantly lower compliance in comparison to the sepsis performance measure (SEP-1) initiative or the resuscitation bundle promoted by the SCC13,36. Alarmingly, only one in five patients received the full bundle in a group with higher risk of deterioration, i.e. NEWS 6 or above, whilst only one out of ten patients received the full bundle in the lower acuity group, with no change over the four years. However, we found that patients who were reviewed by critical care outreach were more often treated with the full ‘Sepsis Six’ bundle and had antibiotics administered. This result is in line with previous experiences, where introduction of a dedicated team has improved compliance with the bundle37. Whilst our study did not find any association between critical care outreach involvement and mortality, it is possible that illness severity is a confounding factor here. It’s important to note that critical care outreach provision was variable across Wales during the study period38. Not every organisation had these services available and none of the hospitals had 24/7 critical care outreach on site. Furthermore, the existing critical care outreach services were nurse-led and delivered and at the time of the study, they did not have appropriate privileges for drug prescription and in some cases ordering tests either. Taking this into account, the associated three-fold increase in the ‘Sepsis Six’ bundle completion is remarkable.
Our results point towards system failure to respond to sepsis as a medical emergency and highlight the need for policy change in the Welsh NHS in response to sepsis. Despite the introduction of the quality improvement target for ‘Sepsis Six’ bundle completion in 2017/2018 by the Welsh Government, we have seen little change across the study years17. This quality improvement target was not accompanied by financial incentives or any additional funding. Importantly, there is no publicly available report about the baseline measured by this methodology and any potential improvement attained since 2017/2018 in the Welsh NHS organisations. The implementation of care bundles have been shown to have significant institutional barriers, which may not be overcome by traditional plan-do-study-act quality improvement cycles39,40. Importantly, neither the ‘Sepsis Six’ bundle, nor the SEP-1 bundle has been tested in a robust randomised controlled trial (RCT) and their perceived effectiveness has been derived from observational before and after studies with high risk of bias41. We believe, based on the individual bundle element compliance figures, that our data may show the presence of clinical equipoise for an RCT to test whether a bundle approach indeed improves outcomes compared to the current apparent standard care of administering supplemental oxygen and antibiotics to the majority of the patients with NEWS above 6. RECOVERY and REMAP-CAP have demonstrated the potential efficiency and effectiveness of adaptive platform trials42–44, and the recently funded Sepsis Trials in Critical Care (SEPTIC) platform (NIHR 17/136/02) illustrates such an approach in sepsis management. Adaptive platform trials create opportunities for ‘learning health care systems’ which promote efficient knowledge generation and transfer, use simple and purposeful data systems with transparent quality metrics, and integrate these into clinical, academic and commissioning structures45–47. Considering the significant evidence gap in the ward based sepsis care demonstrated in our study, we propose that a similar platform trial is necessary to delineate which timely, ward-based interventions can reduce mortality in patients with sepsis at the highest risk of adverse outcomes46,48.
There are certain limitations to our study. Firstly, the dataset was designed to enable a sufficiently comprehensive list of clinical and laboratory parameters while being small enough to maintain data reliability. Data collection was performed by medical students at different stages of training, introducing potential bias. To counter this, robust online and in-person training was cascaded, and we ensured that medical student hospital leads in subsequent years had participated as data collectors16,20,21. We also maintained the core clinical leadership of the group throughout the study. Secondly, we have only collected longer-term outcome data and cause of death on a subset of patients and our long-term follow-up data is yet to be linked with the Welsh Secure Anonymous Information Linkage (SAIL) databank31,49. The true human cost of sepsis in terms of re-hospitalisation and patient reported outcomes cannot be estimated from our results. Thirdly, although one of the largest in-depth sepsis studies in the UK, the sample-size is relatively small. However, we could not see any differences in sepsis incidence or outcomes based on geographical area, hospital status or size and we ensured that all acute hospitals in Wales participated in each year of the study16,20,21. Lastly, the point-prevalence design might have led to a systematic underestimate of compliance with ‘Sepsis Six’ completion; however, despite being mandated in the NHS Wales Delivery Framework in 201717, there is no publicly available data generated by Welsh Health Boards to provide a comparison on longer-term longitudinal changes of this quality improvement index. Moreover, engagement of participating hospitals with our point-prevalence study has remained high and our results have been consistent across the study period.
Conclusions
In summary, our data suggests that despite efforts to increase sepsis awareness within the NHS, there is poor compliance with the sepsis care bundles and there has been no change in outcomes over the study period. Our results highlight the ongoing need for clinical trials to determine which time-sensitive ward-based interventions are most likely to reduce mortality in patients with highest risk of death and which should be adopted by learning healthcare systems.
Supplementary Information
Acknowledgements
The Authors would like to acknowledge the help of the Critical Care Outreach teams of the participating hospitals. The full list of collaborators is provided under the Welsh Digital Data Collection Platform Collaborators.
Author contributions
Conceptualization, M.K., B.S., T.Sz.; formal analysis, M.K., T.Sz.; investigation, M.K., H.U., B.S., R.J.P., T.C., D.D., M.S., D.P., S.T., A.P., L.O., S.W., I.R., J.N.C.H., B.P., E.W., F.A., H.M.P.C., J.B., J.H., J.H., J.N., L.W., L.H.T., M.W., P.M., T.R., U.H.A., V.M., Z.A., Z.N., Z.X.Tan, T.Sz.; methodology, M.K., H.U., B.S., T.Sz.; project administration, M.K., H.U., B.S., T.Sz.; resources, T.Sz.; supervision, T.Sz.; visualization, M.K., L.J.P.T.; writing – original draft, M.K., H.U., T.Sz.; writing – review & editing, all authors. All authors read and approved the final manuscript.
Funding
This work was supported by the Fiona Elizabeth Agnew Trust and the Welsh Intensive Care Society, and they had no access to the data and no role in study design, conduct, analysis or drafting this report.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maja Kopczynska and Harry Unwin.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Tamas Szakmany, Email: SzakmanyT1@cardiff.ac.uk.
The Welsh Digital Data Collection Platform collaborators:
Maria Hobrok, Moriah Thomas, Annie Burden, Nadia Youssef, Katherine Carnegie, Helena Colling-Sylvester, Natasha Logier, Meshari Alsaeed, Hannah Williams, Arfa Ayob, Nor Farzana, Sweta Parida, David Lawson, Emily Evans, Laura Jane Davis, Billie Atkins, Llywela Wyn Davies, Lee Sanders-Crook, Steffan Treharne Seal, Alice Cains, Katy Crisp, Sarah Venning, Ella Sykes, Stephanie Narine, Georgia Parry, Emily Angela Dillon, Qi Zhuang Siah, Ting Yang, Tyler Jones, Parvathi Thara, Emma Wood, Georgina St Pier, Richard Betts, Kyriaki Mitsaki, Mari Tachweed Pierce, Sioned Davies, Yakeen Hafouda, Erin Ifan, Grace Lacey, Francesca Mitchell, John Lynch, Michal Mazur, Lezia D’Souza, Bethan Ponting, Terrance Lau, Ruairidh Kerrigan, Lucy Morgan, Roshan Vindla, Claudia Zeicu, Becky James, Amirah Amin Ariff, Wan Binti Wan Azzlan, Charlotte Collins, Elizabeth Wickens, Alisa Norbee, Aliya Zulkefli, Thomas Haddock, Megan Thomas, Matthew Lee, Miriam Cynan, Nik-Syakirah Nik Azis, Imogen Hay, Catherine Russell, Margriet Vreugdenhil, Mustafa Abdimalik, Joseph Davies, Peter Havalda, Angharad Evans, Kate Robertson, Grace Gitau, Mei-yin Gruber, Thomas Telford, Anas Qarout, Naomi Nandra, Hannah Garrard, James Cutler, Rhiannon Tammy Jones, Amy Prideaux, Timothy Spence, Sarah Hardie, Harriet Seymour, Matthew Warlow, Shanali Thanthilla, Thomas Downs, Nina Foley, Chad McKeown, Akshita Dandawate, Holleh Shayan-Arani, Ellie Taylor, Oliver Kyriakides, Rachel Price, Ffion Haf Mackey, Emily Haines, Samuel Chun, Nilarnti Vignarajah, Tessa Chamberlain, Dongying Zhao, Nayanatara Nadeesha T. Tantirige, Naomi Dennehey, Georgina Evans, John Watts, Ceri Battle, Ryan Jones, Selina Jones, Charlotte James, James O’Hanlon, Isabella Bridges, Bethany Hughes, Leo Polchar, Elise Bisson, Charlotte Mykura, Lara Money, Joshua McKenna, Sarah Kinsman, Demiana Hanna, Emily Baker, Harrison Sprague, Liam Sharma, Tom Pontin, Emma Shore, Tamara Hughes, Sam Nightingale, Philby Baby, Matthew Shield, Alice Cross, Jenna Boss, Olivia Ross, George Ashton, Kimaya Pandit, Daniel Davies, Cameron Garbutt, Charlotte Johnston, Marcus Cox, Chantal Roberts, Alessia Waller, Laura Heekin, Kathy Wang, Rhianna Church, Shrina Patel, Marianne Broderick, Hannah Whillis, Daniel Craig Hathaway, Emel Yildirim, Caitlin Atkins, Elin Walters, Carys Durie, Robert James Hamilton Sinnerton, Benjamin Tanner, Julimar Abreu, Kiran Bashir, Vincent Hamlyn, Amelia Tee, Zoe Ann Hinchcliffe, Rita Otto, Georgie Covell, Megan Stone, Katherine Godfray, Rhidian Caradine, Hannah Beetham, Adanna Nicole Anomneze-Collins, Jeanette Tan, Yasmina Abdelrazik, Azizah Khan, Nabihah Malik, Aidan Clack, Tyler Thomas, Adam George Mounce, Anoopama Ramjeeawon, Ndaba Mtunzi, Duncan Soppitt, Jack Wellington, Robert Buchanan Ross, Danielle Lis, Rebecca Parsonson, Jude Joseph-Gubral, Ajitha Arunthavarajah, Aaron Harris, Henry Atkinson, Jessica Webster, Tim Burnett, Josephine Raffan Gowar, Sam DeFriend, Jasmine Whitaker, Elizabeth Beasant, Luis Macchiavello, Danyal Usman, Abdullah Mahdi, Tiffany Ye Tze Shan, Nick Savill, Jennifer Gee, Lizzie Hodges, Ami Desai, Hannah Rossiter, Matthew Taylor, Kevin Pinto, Eleanor Hartley, Oscar Emanuel, Rhiannon Long, Megan Selby, Alexandra Urquhart, Matthew Ashman, Elizabeth Adcock, Amelia Dickinson, Rebecca Jordache, Rym Chafai El Alaoui, Sophie Stovold, Sam Vickery, Nia Jones, Alice O’Donnell, Monty Cuthbert, Osa Eghosa, Muhammad Karim, Lowri Williams, Louise Tucker, Tom Downs, Rebecca Walford, Annabelle Hook, Adam Mounce, Emily Eccles, Ross Edwards, Kirtika Ramesh, Charlie Hall, Maria Lazarou, Rhidian Jones, Katy McGillian, Hari Singh Bhachoo, Zoe Teh, Vithusha Inpahas, Ruchi Desai, Yusuf Cheema, Andrew Hughes, Olivia Cranage, Felicity Bee, Khalid Osman, Humza Khan, Jennifer Pitt, Charlotte Pickwick, Jorge Carter, Fiona Andrew, Naseera Seedat, Roshni Patel, Alicia Boam, Jessica Randall, Beth Bowyer, Josh Edwards, Natasha Jones, Emma Walker, Ailsa MacNaught, Swagath Balachandran, Abbie Shipley, Jennifer Louise Kent, Bethany Davies, Emma Withers, Krishna Parmar, Lucie Webber, Angelica Sharma, Amy Handley, Alexandra Gordon, Lucy Allen, Rebecca Paddock, Harriet Penney, Lopa Banerjee, Chloe Victoria Vanderpump, Kate Harding, John Burke, Orsolya Minik, Nia Jarrett, Ellie Rowe, Adanna Anomneze-Collins, Harry Griffiths, Sarah Pengelly, Ffion Bennett, Ahmed Bilal, Abdullah El-badawey, Bethan Ellis, Luke Cook, Harriet Elizabeth Valentine Maine, Kiri Armstrong, Hannah Beresford, Timia Raven-Gregg, Tom Liddell-Lowe, Caitlin Ong, Harriet Reed, Frederika Alice St John, Weronika Julia Kozuch, Irukshi Anuprabha Silva, Sin Ting Natalie Cheng, Umme-Laila Ali, Noreena Syed, Luke Murphy, Thomas Grother, Harry Smith, Rachel Watson, Omar Marei, Emma Kirby, Anna Gilfedder, Lydia Maw, Sarah O’Connor, Charlotte Maden, Helena Jones, Hazel Preston, Nur Amirah Binti Maliki, Mark Zimmerman, Jessica Webber, Llewelyn Jones, Rebecca Phillips, Lauren McCarthy, Emily Hubbard, Leo Duffy, Abigail Guerrier Sadler, Owen Richards, Charles King, Charlotte Killick, Yusuf Chema, Kavita Shergill, Yi Huen Lillian Lau, Hannah Mustafa Ali, Lucas Wilcock, Molly Timlin, Ayeesha Rela, Daniel Smith, Sarah Ireland, Jennifer Evans, Nayanatara Poobalan, Jessica Pearce, Thivya V. Vadiveloo, Zoe Black, Daniel Elis Samuel, Humaira Hussain, Rebecca Creamer, Maham Zafar, Ahmad Almazeedi, Hannah Brunnock, Mekha Jeyanthi, Poorya Moghbel, Katie Kwan, Isobel Sutherland, Frank Davis, Abigail Rogers, Clare Chantrill, Amal Robertson, Jonathan Foulkes, Rahana Khanam, Jomcy John, Sarah Hannah Meehan, Huria Metezai, Hannah Dawson, Navrhinaa Vadivale, Camilla Lee, Amrit Dhadda, Sian Cleaver, Genna Logue, Joy Inns, Isabel Jones, Robyn Howcroft, Carys Gilbert, Matthew Bradley, Louise Pike, Rachel Keeling, Charldré Banks, Eleanor Cochrane, James McFadyen, Matthew Mo, Emily Ireland, Esme Brittain, Ihssen Laid, Charlotte Green, Adriel Mcforrester, Tu Xuong Michelle Ly, Mariana Nalbanti, Raven Joseph, Jack Tagg, Ayako Niina, Tyler Joshua Jones, Natalie Hoyle, Patrick Benc, Ellen Davies, Meng-Chieh Wu, David Fellows, Eloise Baxendale, Karishma Khan, Andrew Forrester, Oliver Moore, Hse Juinn Lim, Aimee Owen, Faris Hussain, Nima-banu Allybocus, Maneha Sethi, Harry Waring, Adeel Khan, Claire Smith, Nicholas Doyle, Mohammad Yahya Amjad, Luke Galloway, Paul Morgan, Gemma Ellis, Robert Lundin, Haamed Al Hassan, Bethan Markall, Namratha Kaur, Emmanuel Onyango, Heather Beard, Elliot Field, Ellen Nelson-Rowe, Lizzie Adcock, Amelia Stoddart, Frederika St John, Mathoorika Sivananthan, Rhys Jones, Sung Yeon Kwak, Lily Farakish, Holly Rhys-Ellis, Kate Moss, Tessa David, Talea Roberts, Annie Quy, Aniket Paranjape, Felicity Bee, Nutchanun Poolworaluk, Mary Keast, Si Liang Yao, Dion Manning, Isobel Irwin, Emelia Boggon, Ibrahim Alkurd, Genevieve Lawerece, Jade Brown, Emily Murphy, Evie Lambert, Jeremy Guilford, Mariam Almulaifi, Sashiananthan Ganesananthan, Berenice Cunningham-Walker, Chloe Spooner, Akanksha Kiran, Nabeegh Nadeem, Vidhi Unadkat, Esme Sparey, David Li, Jessica Smith, India Corrin, Amit Kurani, Paul McNulty, Ceri Brown, Wojciech Groblewski, Szilvia Szoke, Amelia Redman, Esther McKeag, Anastasia Donnir, Gaautham Ravishangar, Emanuela Howard, Charlotte Salmon, Sara Tanatova, Jasmine Kew, Megan Eilis Clark, Ellen Hannay, Olesya Godsafe, Christina Houghton, Francesca Lavric, Rachel Mallinson, Chris Littler, Harsha Reddy, Andrew Campbell, Benedict Soo, Rachel Evans, Georgina Donowho, Alexandra Cawthra, Maddison Davies, Matthew Lawrence Ashman, Jamie Scriven, James Vautrey, Shannon Seet, Imogen Britton, Abigail Hodgson, Emma Twohey, Joseph Robbins, Vanessa Yeo Yung Ling, Kimiya Asjadi, Carven Chin Yee Shean, Zoe McCarroll, Oritseweyimi Amatotsero, Antonia Ashaye, Josephine Acheampong, Ayowade Adeleye, Saber Ahmed, Alexandra Chrysostomou, Eshen Ang, Niamh McSwiney, Yin Yin Lim, Zong Xuan Lee, Svetlana Kulikouskaya, Nur Zulkifili, Sheryl Lim, Lim Xin, Adiya Urazbayeva, Nur Haslina Ahmad Hanif, Yau Ke Ying, Alice Coleclough, Eilis Higgins, Naomi Spencer, Tze Gee Ng, Sam Booth, Stephanie Wai Yee Ng, Christian P. Subbe, Isabella Patterson, Wen Li Chia, Abdullah Mukit, Hei Yi Vivian Pak, Felicity Lock, Mariana Nalmpanti, Shôn Alun Thomas, Tanisha Burgher, Alfred Wei Zhen Yeo, Siwan Powell Jones, Charlie Miles, Millicent Perry, Holly Burton, Katharine Powell, Luthfun Nessa, Aalaa Fadlalla, Rhian Morgan, Elizabeth Hodges, Amelia Heal, Chloe Scott, Alice Tayler, Abduahad Taufik, James Cochrane, Sieh Yen Heng, Alex Cooper, Henrik Graf von der Pahlen, Isabella Talbot, Robin Gwyn Roberts, Jessica Sharma Smith, Aisling Sweeney, Cerian Roberts, Laura Bausor, Chania Lambirnudi, Daniah Thomas, Elen Wyn Puw, Ronan A. Lyons, and Judith E. Hall
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95648-6.
References
- 1.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—A WHO resolution. N. Engl. J. Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M, et al. Sepsis among medicare beneficiaries: 1. The burdens of sepsis, 2012–2018. Crit. Care Med. 2020;48:276–288. doi: 10.1097/CCM.0000000000004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M, et al. Sepsis among medicare beneficiaries: 3. The methods, models, and forecasts of sepsis, 2012–2018. Crit. Care Med. 2020;48:302–318. doi: 10.1097/CCM.0000000000004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mearelli F, Orso D, Fiotti N, Altamura N, Breglia A, De Nardo M, et al. Sepsis outside intensive care unit: The other side of the coin. Infection. 2014;43:1–11. doi: 10.1007/s15010-014-0673-6. [DOI] [PubMed] [Google Scholar]
- 10.Esteban A, Frutos-Vivar F, Ferguson ND, Peñuelas O, Lorente JA, Gordo F, et al. Sepsis incidence and outcome: Contrasting the intensive care unit with the hospital ward. Crit. Care Med. 2007;35:1284–1289. doi: 10.1097/01.CCM.0000260960.94300.DE. [DOI] [PubMed] [Google Scholar]
- 11.Buchman TG, Simpson SQ, Sciarretta KL, Finne KP, Sowers N, Collier M, et al. Sepsis Among medicare beneficiaries: 2. The trajectories of sepsis, 2012–2018. Crit. Care Med. 2020;48:289–301. doi: 10.1097/CCM.0000000000004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baghdadi JD, Brook RH, Uslan DZ, Needleman J, Bell DS, Cunningham WE, et al. Association of a care bundle for early sepsis management with mortality among patients with hospital-onset or community-onset sepsis. JAMA Intern. Med. 2020;180:707–716. doi: 10.1001/jamainternmed.2020.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes A, Phillips G, Beale R, Cecconi M, Chiche JD, De Backer D, et al. The surviving sepsis campaign bundles and outcome: Results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study) Intensive Care Med. 2015;41:1620–1628. doi: 10.1007/s00134-015-3906-y. [DOI] [PubMed] [Google Scholar]
- 15.Daniels R, Nutbeam T, McNamara G, Galvin C. The sepsis six and the severe sepsis resuscitation bundle: A prospective observational cohort study. Emerg. Med. J. 2011;28:507–512. doi: 10.1136/emj.2010.095067. [DOI] [PubMed] [Google Scholar]
- 16.Szakmany T, Pugh R, Kopczynska M, Lundin RM, Sharif B, Morgan P, et al. Defining sepsis on the wards: Results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia. 2018;73:195–204. doi: 10.1111/anae.14062. [DOI] [PubMed] [Google Scholar]
- 17.NHSWalesDeliveryFramework2017_18.pdf [Internet]. [cited 2021 Mar 7]. Available from: https://ruralhealthandcare.wales/wp-content/uploads/2017/07/NHSWalesDeliveryFramework2017_18.pdf.
- 18.Frankling C, Patel J, Sharif B, Melody T, Yeung J, Gao F, et al. A Snapshot of compliance with the sepsis six care bundle in two acute hospitals in the West Midlands, UK. Indian J. Crit. Care Med. 2019;23:310–315. doi: 10.5005/jp-journals-10071-23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif B, Lundin RM, Morgan P, Hall JE, Dhadda A, Mann C, et al. Developing a digital data collection platform to measure the prevalence of sepsis in Wales. J. Am. Med. Inform. Assoc. 2016;23:1185–1189. doi: 10.1093/jamia/ocv208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopczynska M, Sharif B, Cleaver S, Spencer N, Kurani A, Lee C, et al. Red-flag sepsis and SOFA identifies different patient population at risk of sepsis-related deaths on the general ward. Medicine (Baltimore) 2018;97:e13238. doi: 10.1097/MD.0000000000013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopczynska M, Sharif B, Unwin H, Lynch J, Forrester A, Zeicu C, et al. Real world patterns of antimicrobial use and microbiology investigations in patients with sepsis outside the critical care unit: Secondary analysis of three nation-wide point prevalence studies. J. Clin. Med. 2019;8:1337. doi: 10.3390/jcm8091337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong AV, Arora N, Olusanya O, Sharif B, Lundin RM, Dhadda A, et al. Insertion rates and complications of central lines in the UK population: A pilot study. J. Intensive Care Soc. 2018;19:19–25. doi: 10.1177/1751143717722914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopczynska M, Sharif B, Pugh R, Otahal I, Havalda P, Groblewski W, et al. Prevalence and outcomes of acute hypoxaemic respiratory failure in wales: The PANDORA-WALES study. J. Clin. Med. 2020;9:3521. doi: 10.3390/jcm9113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. R Foudnationf for Statistical Computing, Vienna, Austria. 2019; available at: https://www.R-project.org.
- 25.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4 (2016). https://ggplot2.tidyverse.org.
- 26.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: A prospective cohort study. Lancet Infect. Dis. 2012;12:919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 27.Miller RR, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am. J. Respir. Crit. Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee C, Murphy MV, Li L, Platt R, Klompas M. Centers for disease control and prevention epicenters program. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin. Infect. Dis. 2015;60:88–95. doi: 10.1093/cid/ciu750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in us acute care hospitals. JAMA Netw. Open. 2019;2:e187571. doi: 10.1001/jamanetworkopen.2018.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopczynska M, Sharif B, Cleaver S, Spencer N, Kurani A, Lee C, et al. Sepsis-related deaths in the at-risk population on the wards: Attributable fraction of mortality in a large point-prevalence study. BMC Res. Notes. 2018;11:720. doi: 10.1186/s13104-018-3819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szakmany T, Hollinghurst J, Pugh R, Akbari A, Griffiths R, Bailey R, et al. Frailty assessed by administrative tools and mortality in patients with pneumonia admitted to the hospital and ICU in Wales. Sci. Rep. 2021;11:3407. doi: 10.1038/s41598-021-92874-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006;34:15–21. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 34.Knoop ST, Skrede S, Langeland N, Flaatten HK. Epidemiology and impact on all-cause mortality of sepsis in Norwegian hospitals: A national retrospective study. PLoS ONE. 2017;12:e0187990–e188013. doi: 10.1371/journal.pone.0187990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yealy DM, Huang DT, Delaney A, Knight M, Randolph AG, Daniels R, et al. Recognizing and managing sepsis: What needs to be done? BMC Med. 2015;13:98. doi: 10.1186/s12916-015-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deis AS, Whiles BB, Brown AR, Satterwhite CL, Simpson SQ. Three-hour bundle compliance and outcomes in patients with undiagnosed severe sepsis. Chest. 2018;153:39–45. doi: 10.1016/j.chest.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke J, Wood S, Hermon A, Szakmany T. Improving outcome of sepsis on the ward: Introducing the “Sepsis Six” bundle. Nurs. Crit. Care. 2019;24:33–39. doi: 10.1111/nicc.12358. [DOI] [PubMed] [Google Scholar]
- 38.Task and FInish Group on Critical Care Final Report 2019 [cited 10 July 2021] available from https://gov.wales/task-and-finish-group-critical-care-final-report.
- 39.Green SA, Bell D, Mays N. Identification of factors that support successful implementation of care bundles in the acute medical setting: A qualitative study. BMC Health Serv. Res. 2017;17:120. doi: 10.1186/s12913-017-2070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DJ, Aitken LM. Use of a single parameter track and trigger chart and the perceived barriers and facilitators to escalation of a deteriorating ward patient: A mixed methods study. J. Clin. Nurs. 2016;25:175–185. doi: 10.1111/jocn.13104. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Strich JR, Applefeld WN, Sun J, Cui X, Natanson C, et al. Driving blind: Instituting SEP-1 without high quality outcomes data. J. Thorac. Dis. 2020 doi: 10.21037/jtd.2019.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angus DC, Alexander BM, Berry S, Buxton M, Lewis R, Paoloni M, et al. Adaptive platform trials: Definition, design, conduct and reporting considerations. Nat. Rev. Drug Discov. 2019;18:797–807. doi: 10.1038/s41573-019-0034-3. [DOI] [PubMed] [Google Scholar]
- 43.Huang DT, McVerry BJ, Horvat C, Adams PW, Berry S, Buxton M, et al. Implementation of the Randomized Embedded Multifactorial Adaptive Platform for COVID-19 (REMAP-COVID) trial in a US health system—Lessons learned and recommendations. Trials. 2021;22:100. doi: 10.1186/s13063-020-04997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson E. RECOVERY trial: the UK covid-19 study resetting expectations for clinical trials. BMJ. 2020;369:m1626. [DOI] [PubMed]
- 45.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat. Med. 1984;3:409–420. doi: 10.1002/sim.4780030421. [DOI] [PubMed] [Google Scholar]
- 47.The REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically Ill patients with covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morain SR, Kass NE, Grossmann C. What allows a health care system to become a learning health care system: Results from interviews with health system leaders. Learn. Health Syst. 2017;1:e10015. doi: 10.1002/lrh2.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyons RA, Jones KH, John G, Brooks CJ, Verplancke J-P, Ford DV, et al. The SAIL databank: Linking multiple health and social care datasets. BMC Med. Inform. Decis. Mak. 2009;9:3. doi: 10.1186/1472-6947-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.