Abstract
Background:
Treatment guidelines for pneumonia recommend beta-lactam antibiotic-based therapy. Although reported penicillin allergy is common, more than 90% of patients with reported penicillin allergy are not allergic.
Objective:
We evaluated the association of a documented penicillin and/or cephalosporin (P/C) allergy to antibiotic use for the treatment of inpatient pneumonia.
Methods:
This was a national cross-sectional study conducted among Vizient, Inc. network hospitals that voluntarily contributed data. Among hospitalized patients with pneumonia, we examined the relation of a documented P/C allergy in the electronic health record to prevalence of first-line beta-lactam antibiotic administration and alternative antibiotics using multivariable log-binomial regression with Generalized Estimating Equations.
Results:
Of 2,276 inpatients receiving antibiotics for pneumonia at 95 US hospitals, 450 (20%) had a documented P/C allergy. Compared to pneumonia patients without a documented P/C allergy, patients with a documented P/C allergy had reduced prevalence of first-line beta-lactam antibiotic use (adjusted prevalence ratios [aPR] 0.79 [95%CI 0.69, 0.89]). Patients with high-risk P/C reactions (n=91) had even lower prevalence of first-line beta-lactam antibiotic use (aPR 0.47 [95% CI 0.35, 0.64]). Alternative antibiotics associated with a higher use in pneumonia patients with a documented P/C allergy included carbapenems (aPR 1.61 [95%CI 1.22, 2.13]) and fluoroquinolones (aPR 1.52 [95%CI 1.21, 1.91]).
Conclusions:
Inpatients with documented P/C allergy and pneumonia were less likely to receive recommended beta-lactams and more likely to receive carbapenems and fluoroquinolones. Inpatient allergy assessment may improve optimal antibiotic therapy for the 20% of inpatients with pneumonia and a documented P/C allergy.
Keywords: Allergy, Pneumonia, EHR Data
INTRODUCTION
Approximately 10% of patients in the United States (US) report an allergy to penicillin antibiotics and 2% report an allergy to cephalosporin antibiotics.1,2 However, up to 95% of these patients may be able to tolerate penicillin and other beta-lactam antibiotics; the prevalence of true immunoglobulin (Ig) E-mediated penicillin allergies are estimated to be 0.01 to 1%.3,4 Even confirmed IgE-mediated allergies appear to resolve at a rate of approximately 10% per year.5 Patients with a documented penicillin allergy are often prescribed alternative agents that may lead to inferior treatment outcomes or increase the risk of harm, including prolonged hospital length of stays, higher readmission rates, and adverse effects.6,7 Treatment with fluoroquinolones, clindamycin, and later generation cephalosporins is associated with an increased risk of Clostridioides difficile colitis.7–9 Furthermore, the unnecessary use of broad-spectrum antibiotics contributes to multidrug-resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE).7,9
Pneumonia is a common infection affecting approximately 450 million people per year and is a major cause of death across all age groups.10 Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the US with 1.5 million admissions per year; CAP additionally accounts for 4.5 million outpatient and emergency room visits annually.11 Hospital-acquired pneumonia (HAP) accounts for approximately 22% of all hospital-acquired infections; ventilator-acquired pneumonia (VAP), a particularly morbid subset of HAP, results in 13% mortality.12,13
The Infectious Disease Society of America recommends beta-lactam antibiotics as first-line pneumonia treatment.14,15 Inpatient CAP treatment consists of a beta-lactam (e.g., ceftriaxone) plus macrolide or respiratory fluoroquinolone monotherapy.14 Treatment of HAP and VAP includes an anti-pseudomonal beta-lactam (i.e., piperacillin-tazobactam, ceftazidime, cefepime) plus a non-beta-lactam anti-pseudomonal agent, such as an aminoglycoside, polymyxin, or fluoroquinolone, in addition to vancomycin or linezolid depending on risk factors for MRSA or VRE.15 Across all pneumonia types and treatment regimens, beta-lactam antibiotics are generally considered more effective and less toxic than alternative antimicrobial agents.16
In this study, we assessed the association of documented penicillin and/or cephalosporin (P/C) allergies on inpatient antibiotic selection for the treatment of pneumonia in a large national sample of US hospitals.
METHODS
Study Design and Data Collection
This is a secondary analysis of inpatients with pneumonia at short-term acute care hospitals from a large national cross-sectional study of hospitalized patients within Vizient.17,18 Vizient, Inc is the largest member-driven, health care performance improvement company in the US; over 3,200 acute care hospitals and more than 95% of all US academic medical centers are Vizient members. 19 The Acute Care Hospital Groups within Vizient maintains an email listserv of 147 contacts at Vizient member hospitals that was used to identify hospital participants for this study. Vizient member hospitals completed intake questionnaires from September 18, 2018 through October 12, 2018.18 Full study participation required hospitals to submit deidentified clinical details for inpatients receiving any antibiotic on a single assessment day within the study period (October 16, 2018 through January 13, 2019). All patient data were entered by employees of the hospital with access to electronic health records (EHRs) and antibiotic utilization data as part of their professional duties (e.g., clinical pharmacist).
In this study, we included all inpatients on antibiotics with a diagnosis of pneumonia from short-term acute care hospitals. Pneumonia was diagnosed by chart review and entered into the data collection field “Infection(s) treated (if known) (select all that apply).” We grouped pneumonia patients into those treated for pneumonia alone, pneumonia with bacteremia, pneumonia with sepsis, and pneumonia with other infections.
The exposure of interest was a P/C allergy documented in the EHR regardless of reaction. Reactions were reviewed by a board certified allergist-immunologist (KGB) and categorized into risk categories: high-risk, medium-risk, and low-risk Table E1. High-risk reactions included reactions that were potentially severe IgE (e.g., anaphylaxis, shortness of breath and wheezing, rash and shortness of breath, angioedema) or other immunologically-mediated phenotypes (e.g., acute interstitial nephritis, Stevens Johnson syndrome), and adverse reactions that may be contraindications such as bone marrow suppression and colitis.
Table E1.
Documented penicillin and/or cephalosporin reactions considered high-risk in patients with pneumonia
|
High Risk Reactions
|
| Acute Interstitial Nephritis |
| Anaphylaxis |
| Rash, GI upset, and itching |
| Rash, itching, and swelling |
| Angioedema |
| Hypotension |
| Stevens Johnson syndrome/toxic epidermal necrolysis/Drug rash eosinophilia and systemic symptoms (DRESS) syndrome/Acute generalized exanthematous pustulosis (AGEP) |
| Swelling |
| Rash and skin peeling |
| Peripheral eosinophilia |
| Tongue got puffy |
| Neutropenia |
| Patient notes bone marrow suppression |
| Colitis |
| Bronchospasm and fever |
| Shortness of breath and wheezing |
| Rash and shortness of breath |
| Shortness of breath |
| Shortness of breath and other |
| Cough and shortness of breath |
|
Medium Risk Reactions |
| Rash and GI Upset |
| Rash |
| Hives and diarrhea |
| Rash, hives and fever |
| Rash and hives |
| Rash, hives and itching |
| Rash and itching |
| Rash and fever |
| Rash and vomiting |
| Mental status change |
| Hives |
| Hives and itching |
|
Low Risk Reactions |
| Diarrhea and itching |
| Diarrhea |
| Dizziness |
| Fever |
| Itching and flushing |
| GI Upset |
| Not documented |
| Myalgia |
| Patient denies having this allergy |
| Tolerated a penicillin class antibiotic |
| Itching |
| Nausea |
| Nausea and vomiting |
| Palpitations |
| Vomiting |
The primary outcome was use of beta-lactam antibiotics indicated for treatment of pneumonia in hospitalized patients: ceftriaxone, piperacillin-tazobactam, cefepime, or ceftazidime. The secondary outcomes considered antibiotic use by drug and drug class. Cephalosporin antibiotics were grouped by generation, except we considered ceftazidime-avibactam and ceftolozane-tazobactam as “novel cephalosporins” and ceftaroline was not grouped. All antibiotics were received or pending administration on the assessment day.
We determined hospital characteristics, including geographic location, setting, and number of staffed beds for participating sites from the Definitive Healthcare database. Geographic location was modified into US census regions: Midwest, Northeast, South, and West.20 Setting was designated as urban or rural. Definitive Healthcare bed data come from the Medicare Cost Report, as self-reported by hospitals.21
Patient characteristics considered as potentially confounding factors included age, sex, race, inpatient location (e.g., intensive care unit), hospital day number, medical comorbidities (i.e., renal disease, diabetes), resistant organism (i.e., MRSA and VRE captured through infection control “flags”) colonization or infection, and type of pneumonia (pneumonia only, pneumonia and bacteremia or sepsis, or pneumonia with another infection). Renal disease was defined as an elevated creatinine on admission or renal failure of any type on the patient’s diagnoses, problem list, or admission note. A patient was considered diabetic if diabetes was listed in diagnoses, problem list, or admission note; if the patient was on diabetes medications at the time of their admission; or if the patient had a documented glycosylated hemoglobin≥6.5%.
Statistical Analysis
Numbers and frequencies were reported for categorical variables. Continuous variables were reported as means with standard deviations or medians with interquartile ranges, as appropriate considering their distributions. We examined the relation of documented P/C allergy to prevalent antibiotic use outcomes in unadjusted analyses, presenting numbers, frequencies, and p-values from Chi squared tests.
For the primary outcome of first-line indicated beta-lactam antibiotics and other commonly used drug classes with significant findings from univariable analyses, we examined the relation of documented P/C allergy to prevalent antibiotic use using log-binomial models with Generalized Estimating Equations models (to take into consideration correlation among patients at the same hospital) to estimate adjusted prevalence ratio (PR) and its 95% confidence interval (CI).22 Informed by directed acyclic graphs, we considered patient demographics, hospital day, intensive care unit location, resistant organisms, and whether there was pneumonia alone, pneumonia with bacteremia or sepsis, or pneumonia with other infections as potentially confounding variables warranting inclusion in the final models a priori.
We performed two sensitivity analyses. In the first, we assessed only patients with high-risk beta-lactam allergies in the multivariable models. In the second, we used E-value to quantify the minimum strength of association that an unmeasured and residual confounder must have with both the treatment and outcome, while simultaneously considering the measured covariates, to negate the observed treatment–outcome association.23,24
All p-values were 2-sided with p<0.05 considered statistically significant.25 Statistical analyses were performed in SAS version 9.4 (Cary, NC, US).
Institutional Review Board
This study was reviewed by the Partners Human Research Committee and determined to be exempt/non-human subjects research (Protocol 2018P001722).
RESULTS
Participating Sites and Patients
From 129 hospitals who submitted complete data for 10,729 hospitalized patients, we identified 2,276 inpatients with pneumonia from 95 short-term acute care US hospitals (Figure 1). All US census geographic regions were represented. Most hospitals were in an urban setting (89%). There were 37 (39%) academic medical centers with mean staffed beds 390 (standard deviation 283). Inpatient allergy consultations were available at 36 (38%) and inpatient penicillin skin testing was available at 28 (29%) of the hospitals. Almost all hospitals (98%) had a formalized antibiotic stewardship program.
Figure 1. Participating sites and patients.
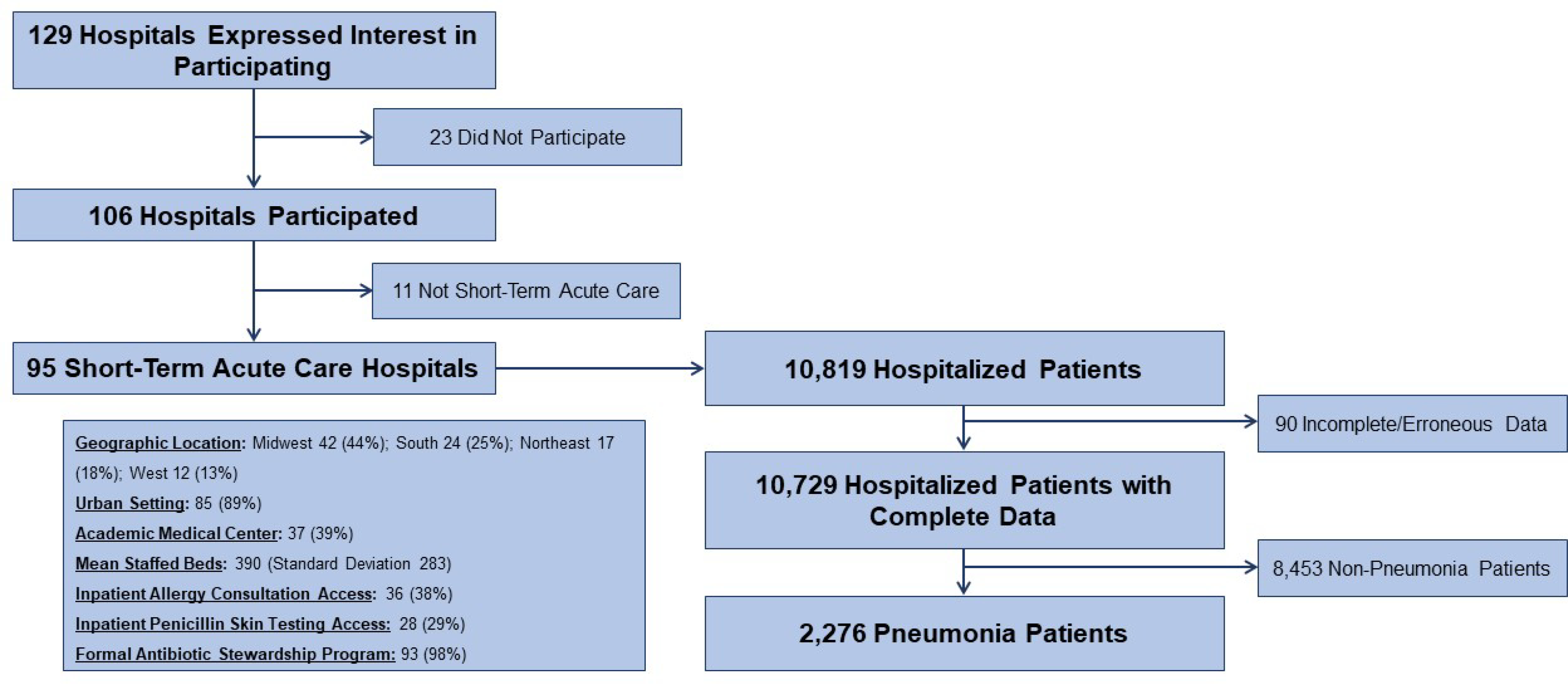
Of 129 hospitals who expressed interest in participating in this study, 95 (74%) acute care hospitals submitted their hospital characteristics and complete, cross-sectional patient data on 10,729 inpatients on antibiotics. This analysis considers the 2,276 (21%) treated for pneumonia.
Patient Characteristics
Of 2,276 patients receiving antibiotics for treatment of pneumonia, 450 (20%) had a documented P/C allergy and 1,826 (80%) did not have a documented P/C allergy (Table 1). The majority of patients had low or medium risk P/C allergies (n=359, 80%) but 91 patients (20%) had high risk P/C allergies (Figure 2). Hospital characteristics were comparable by allergy status. A similar proportion of patients with and without a documented allergy had access to an inpatient allergist (50% vs 48%) and inpatient penicillin skin testing (50% vs 48%). Patients with a documented allergy had similar age but were more frequently female (62% vs 44%) and white (74% vs 61%), compared with patients without a documented P/C allergy. Inpatient locations and hospital day were not meaningfully different by P/C allergy status. MRSA was more common in patients with documented P/C allergy (13% vs 11%). The number of specified infections in addition to pneumonia were similar P/C allergy status and most patients (78%) had pneumonia only.
Table 1.
Characteristics of pneumonia patients and hospitals by documented allergy status.
| Documented P/C Allergy (n = 450) | No P/C Allergy (n = 1,826) | |
|---|---|---|
| Hospital Characteristics | ||
|
| ||
| Geographic Location | ||
| Midwest | 231 (51) | 884 (48) |
| Northeast | 85 (19) | 346 (19) |
| South | 106 (24) | 459 (25) |
| West | 28 (6) | 137 (8) |
| Urban Setting | 420 (93) | 1,674 (92) |
| Bed Size | ||
| < 100 | 15 (3) | 94 (5) |
| 100–399 | 158 (35) | 690 (38) |
| ≥ 400 | 277 (62) | 1,042 (57) |
| Academic Medical Center | 243 (54) | 919 (50) |
| Number of Staffed Beds (Mean ± SD) | 527 ± 331 | 497 ± 327 |
| Inpatient Allergy Consultation Access | 225 (50) | 869 (48) |
| Inpatient Penicillin Skin Testing Access | 225 (50) | 869 (48) |
| Formal Antibiotic Stewardship Program | 448 (100) | 1,817 (100) |
| Allergist Part of Antibiotic Stewardship Program | 17 (4) | 83 (5) |
|
| ||
| Patient Characteristics | ||
|
| ||
| Age (Mean ± SD) | 63 ± 19 | 61 ± 21 |
| Female | 277 (62) | 795 (44) |
| Race | ||
| White | 334 (74) | 1,122 (61) |
| Black | 72 (16) | 419 (23) |
| Asian | 4 (1) | 32 (2) |
| Other | 40 (9) | 253 (14) |
| Inpatient Location | ||
| General Medical Floor | 215 (48) | 782 (43) |
| General Surgery Floor | 27 (6) | 109 (6) |
| Adult ICU | 89 (20) | 449 (25) |
| Cardiology/Telemetry | 53 (12) | 160 (9) |
| Oncology | 12 (3) | 79 (4) |
| Pediatric Floor | 11 (2) | 43 (2) |
| Pediatrics or Neonatal ICU | 1 (<1) | 36 (2) |
| Other | 32 (7) | 136 (7) |
| Hospital Day (Median, IQR) | 4 [2, 8] | 4 [2, 8] |
| Renal Disease | 97 (22) | 411 (23) |
| Diabetes | 160 (36) | 567 (31) |
| MRSA Colonization/Infection | 57 (13) | 193 (11) |
| VRE Colonization/Infection | 13 (3) | 46 (3) |
| Infection(s) Treated | ||
| Pneumonia only | 351 (78) | 1,422 (78) |
| Pneumonia and bacteremia | 9 (2) | 35 (2) |
| Pneumonia and sepsis | 28 (6) | 120 (7) |
| Pneumonia and other infections* | 59 (13) | 234 (13) |
Abbreviations: P/C, penicillin and/or cephalosporin; SD, standard deviation; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin resistant Enterococcus.
Other infections include: skin and soft tissue infections, surgical prophylaxis, urinary tract infection, intrabdominal infection, medical prophylaxis, Clostridioides difficile, neutropenic fever, endocarditis, and other
Figure 2.
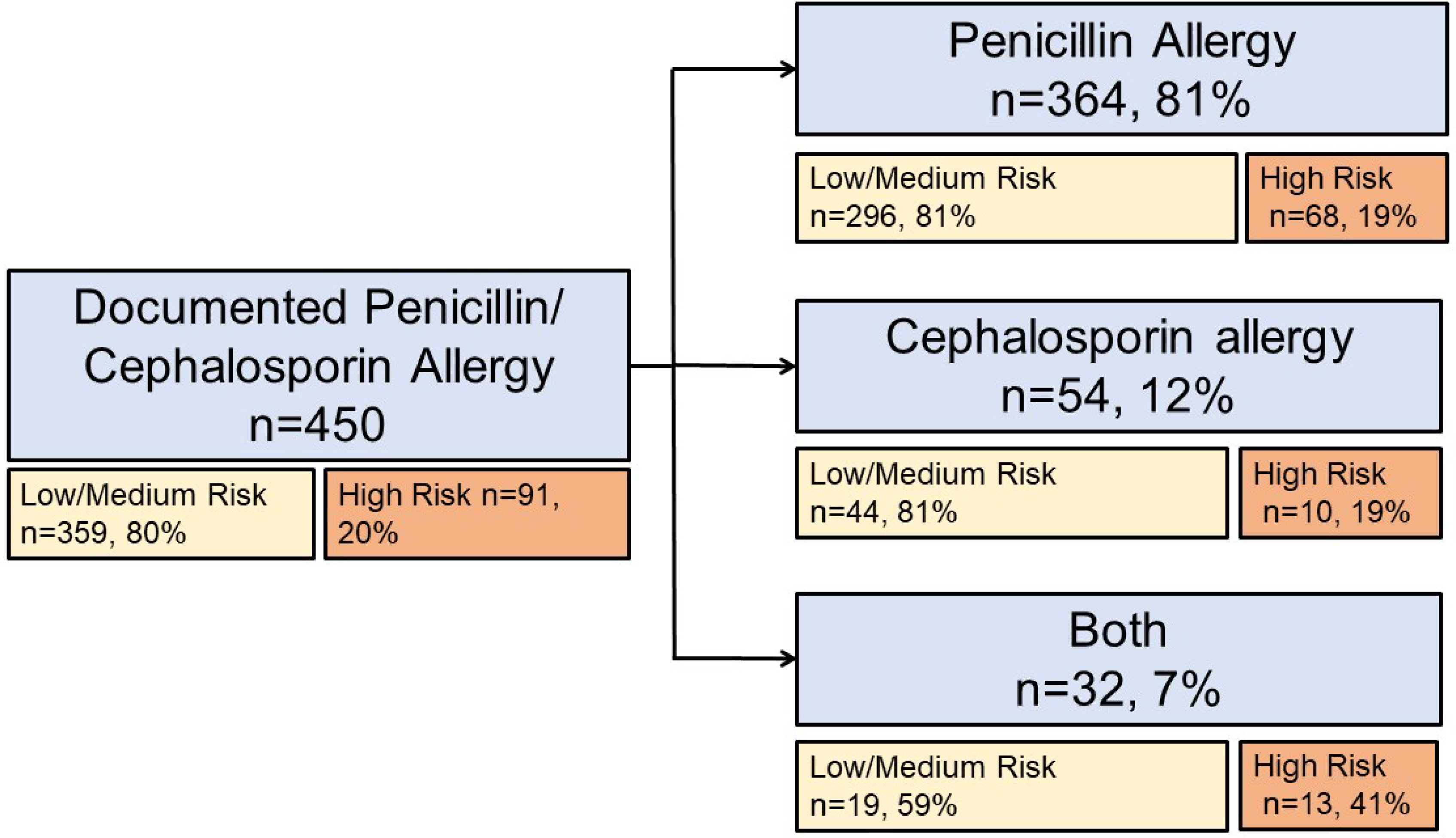
Penicillin and/or cephalosporin allergies documented in hospitalized inpatients with pneumonia (n=450). Risk categories were determined from coded and free text (i.e., “other”) reaction entries.
Antibiotic Use
First-line indicated beta-lactam antibiotics were less frequently used in patients with a documented P/C allergy (44% vs 57%, Table 2). Patients with a documented P/C allergy less frequently received penicillins (11% vs 33%), 3rd generation cephalosporins (18% vs 23%), and 4th generation cephalosporins (20% vs 13%). Many specific antibiotics were used more frequently in patients with a documented P/C allergy, including carbapenems (10% vs 6%), aztreonam (5% vs <1%), fluoroquinolones (23% vs 14%), clindamycin (4% vs 1%), and polymixins (2% vs <1%).
Table 2.
Antibiotic use among inpatients with pneumonia by documented beta-lactam allergy status
| All (n = 2,276) | Documented P/C (n = 450) | No Documented P/C Allergy (n = 1,826) | P-Value | |
|---|---|---|---|---|
| First-line Indicated Beta-Lactam Antibiotics * | 1241 (55) | 199 (44) | 1042 (57) | < 0.001 |
|
| ||||
| Beta-Lactam Antibiotics | ||||
|
| ||||
| Penicillins† | 650 (29) | 48 (11) | 602 (33) | <0.001 |
| Cephalosporins, 1st generation | 42 (2) | 8 (2) | 34 (2) | 0.91 |
| Cephalosporins, 2nd generation | 24 (1) | 8 (2) | 16 (1) | 0.09 |
| Cephalosporins, 3rd generation | 503 (22) | 79 (18) | 424 (23) | 0.01 |
| Cephalosporins, 4th generation | 327 (14) | 91 (20) | 236 (13) | <0.001 |
| Novel cephalosporins‡ | 14 (1) | 2 (<1) | 12 (1) | 0.61 |
| Ceftaroline | 6 (<1) | 2 (<1) | 4 (<1) | 0.40 |
| Carbapenems | 158 (7) | 44 (10) | 114 (6) | 0.01 |
| Aztreonam/Monobactams | 25 (1) | 24 (5) | 1 (<1) | <0.001 |
|
| ||||
| Non-Beta-Lactam Antibiotics | ||||
|
| ||||
| Macrolides | 550 (24) | 99 (22) | 451 (25) | 0.23 |
| Vancomycin | 526 (23) | 113 (25) | 413 (23) | 0.26 |
| Fluoroquinolones | 361 (16) | 102 (23) | 259 (14) | <0.001 |
| Doxycycline | 171 (8) | 46 (10) | 125 (7) | 0.01 |
| Metronidazole | 119 (5) | 33 (7) | 86 (5) | 0.03 |
| Sulfamethoxazole-trimethoprim | 112 (5) | 25 (6) | 87 (5) | 0.49 |
| Aminoglycosides | 67 (3) | 17 (4) | 50 (3) | 0.24 |
| Linezolid | 53 (2) | 17 (4) | 36 (2) | 0.02 |
| Clindamycin | 30 (1) | 17 (4) | 13 (1) | <0.001 |
| Polymixins¶ | 19 (1) | 10 (2) | 9 (<1) | <0.001 |
Abbreviations: P/C, penicillin and/or cephalosporin;
Piperacillin-tazobactam, ceftriaxone, cefepime, ceftazidime
Amoxicillin, amoxicillin-clavunate, ampicillin, ampicillin-sulbactam, nafcillin, oxacillin, penicillin G, and piperacillin-tazobactam
Ceftazidim-avibactam, ceftolozane-tazobactam
Colistimethate and polymyxin
In the adjusted multivariable regression model, patients with a documented P/C allergy had a reduced prevalence of first-line indicated beta-lactam antibiotic use (aPR 0.79 [95% CI 0.69, 0.89], Table 3, Table E2). Intensive care unit location (aPr 1.18 [95%CI 1.10, 1.25]) and pneumonia with bacteremia or sepsis (aPR 1.25 [95% CI 1.08, 1.45]) were associated with increased first-line beta lactam antibiotic use (Table E2).
Table 3.
Adjusted prevalence ratios for antibiotic use considering beta-lactam allergy compared to no beta-lactam allergy.
| All P/C Allergy (n=450) | High-Risk P/C Allergy (n=91) | |||
|---|---|---|---|---|
| Unadjusted Prevalence Ratio (95% CI) | Prevalence Ratio Adjusted (95% CI) | Unadjusted Prevalence Ratio (95% CI) | Prevalence Ratio Adjusted (95% CI) | |
| First-Line Indicated Beta-Lactams* | 0.77 (0.68, 0.88) | 0.79 (0.69, 0.89)† | 0.47 (0.35, 0.63) | 0.47 (0.35, 0.64)† |
| Carbapenems | 1.57 (1.18, 2.08) | 1.61 (1.22, 2.13)‡ | 2.15 (1.25, 3.70) | 2.22 (1.29, 3.82)‡ |
| Fluoroquinolones | 1.60 (1.28, 2.00) | 1.52 (1.21, 1.91)† | 1.59 (1.14, 2.22) | 1.56 (1.12, 2.17)† |
Abbreviations: P/C, penicillin and/or cephalosporin;
Piperacillin-tazobactam, ceftriaxone, cefepime, ceftazidime
Adjusted for age, white race, sex, hospital day, ICU location, resistant organism colonization/infection and type of pneumonia
Adjusted for, ICU location, and pneumonia type
Table E2.
Multivariable association between documented penicillin and/or cephalosporin allergy and prevalence of antibiotic treatment for pneumonia
| First-Line Beta-Lactam Treatment | ||
| PR [95% CI] | p-value | |
| Age | 1.00 (1.00, 1.00) | 0.05 |
| Female | 1.00 (0.94, 1.06) | 0.95 |
| White | 0.98 (0.90, 1.07) | 0.72 |
| Hospital day | 0.99 (0.99, 1.00) | < 0.001 |
| Intensive care unit location | 1.18 (1.10, 1.25) | < 0.001 |
| MRSA or VRE colonization/infection | 0.96 (0.86, 1.08) | 0.49 |
| Type of pneumonia | ||
| Pneumonia only | 1.00 (0.90, 1.12) | 0.99 |
| Pneumonia with bacteremia or sepsis | 1.25 (1.08, 1.45) | 0.002 |
| Pneumonia with other infections | ref | ref |
| Carbapenem Treatment | ||
| PR [95% CI] | p-value | |
| Intensive care unit location | 2.01 (1.52, 2.65) | <0.001 |
| MRSA or VRE colonization/infection | 2.11 (1.56, 2.85) | <0.001 |
| Fluoroquinolones Treatment | ||
| PR [95% CI] | p-value | |
| Age | 1.00 (1.00, 1.01) | 0.23 |
| Female | 1.00 (0.85, 1.19) | 0.97 |
| White | 1.20 (0.95, 1.53) | 0.13 |
| Hospital day | 1.00 (0.99, 1.01) | 0.53 |
| Intensive care unit location | 0.69 (0.55, 0.87) | 0.002 |
| MRSA or VRE colonization/infection | 0.74 (0.52, 1.06) | 010 |
| Type of pneumonia | ||
| Pneumonia only | 1.05 (0.78, 1.40) | 0.76 |
| Pneumonia with bacteremia or sepsis | 0.89 (0.57, 1.38) | 0.59 |
| Pneumonia with other infections | ref | ref |
Abbreviations: PR, prevalence ratio; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ref, reference
Considering only patients with high-risk P/C allergies, adjusted prevalence of first-line indicated beta-lactams was lower (aPR 0.47 [95%CI 0.35, 0.64]). To completely explain away the observed association (i.e., aPR=0.79), the association of potential residual confounder(s) with P/C documentation or use of first-line beta-lactam must be greater than 1.86.
In the adjusted multivariable regression model, patients with a documented P/C allergy had increased prevalence of carbapenem use (aPR 1.61 [1.22, 2.13], Table 3, Table E2). Considering only patients with high-risk P/C allergies, adjusted prevalence of carbapenem use was higher (aPR 2.22 [95%CI 1.29, 3.82]). To completely explain away the observed association (i.e., aPR=1.61), the association of potential residual confounder(s) with P/C allergies or use of carbapenem must be greater than 2.60.
In the adjusted multivariable regression model, patients with a documented P/C allergy had increased prevalence of fluoroquinolone use (aPR 1.52 [1.21, 1.91], Table 3, Table E2). Considering only patients with high-risk beta-lactam allergies, adjusted prevalence of fluoroquinolone use was the same (aPR 1.56 [95%CI 1.12, 2.17]). To completely explain away the observed association (i.e., aPR=1.52), the association of potential residual confounder(s) with P/C allergies or use of fluoroquinolone must be greater than 2.42.
Patients with a documented penicillin allergy had increase prevalence of 4th generation cephalosporin use (aPR 2.15 [95%CI 1.63, 2.83]) and tetracycline use (aPR 1.53 [95%CI 1.01, 2.30], Figure 3). Patients with a documented cephalosporin allergy had increase prevalence of vancomycin use (aPR 1.73 [95%CI 1.06, 2.81]). An increased prevalence of carbapenem use was observed in both patients with a penicillin (aPR 1.61 [95%CI 1.18, 2.21]) and cephalosporin allergy (aPR 2.37 [95%CI 1.28, 4.39]).
Figure 3:
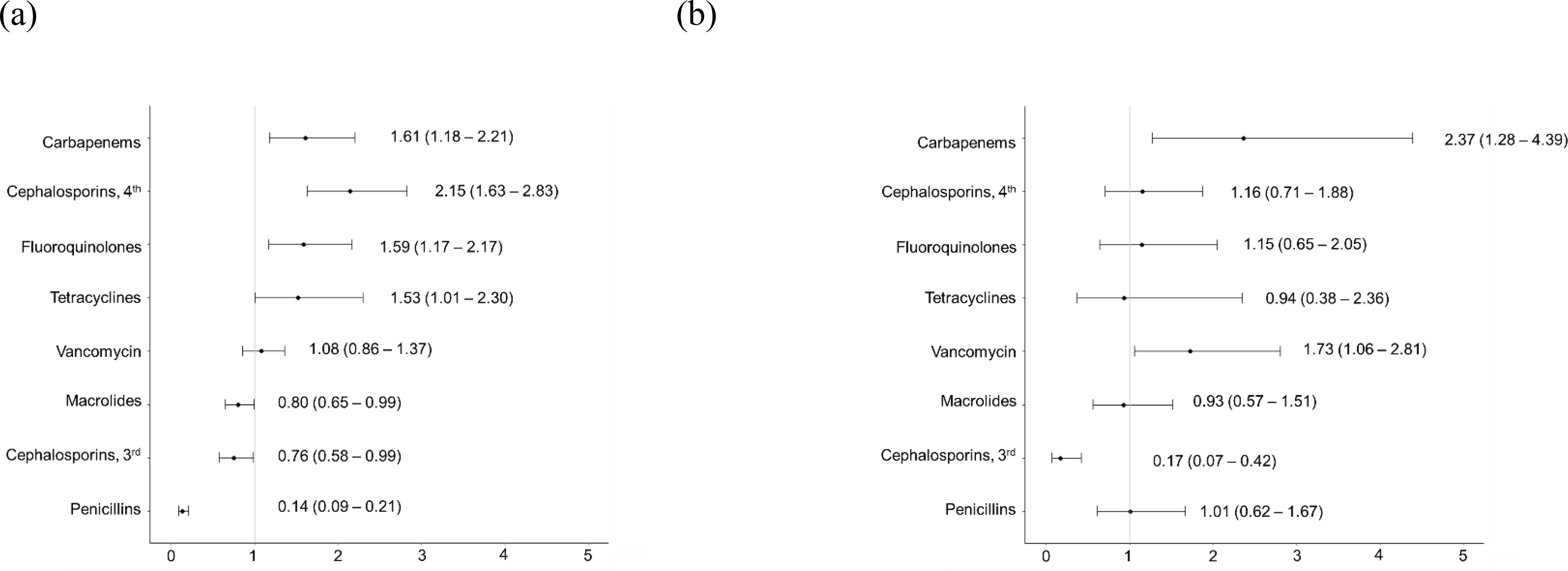
Multivariable assessment of the impact of a documented (a) penicillin allergy and (b) cephalosporin allergy on inpatient antibiotic use for US inpatients receiving antibiotics for pneumonia. The adjusted prevalence ratios and 95% confidence intervals are displayed. Adjusted prevalence ratios are adjusted for age, sex, hospital day, number of staffed beds, geographic location, and diabetes.
DISCUSSION
In this national cross-sectional study of 2,276 hospitalized patients who received antibiotics for the treatment of pneumonia from 95 US hospitals, the 20% who had an EHR-documented P/C allergy had a 21% lower risk of receiving a first-line indicated beta-lactam for pneumonia compared to those without a documented P/C allergy. A documented P/C allergy was also associated with an increased risk of pneumonia treatment with a fluoroquinolone antibiotic (53% increased use) and carbapenem antibiotic (61% increased use). Patients with higher risk P/C allergy histories had an even lower risk of receiving first-line beta-lactams (53% lower) and higher risk of receiving carbapenems (over 2-fold higher) for pneumonia treatment. Although uncommonly used for pneumonia, patients with a documented P/C allergy also had increased use of aztreonam, clindamycin, and polymixins in unadjusted analyses.
Beta-lactams serve as the backbone of antibiotic treatment of CAP, HAP, and VAP. However, documented beta-lactam allergies led to a 21% reduced selection of the guideline-recommended beta-lactams piperacillin-tazobactam, ceftriaxone, cefepime, and ceftazidime. Prevalence of use for these indicated beta-lactams was markedly lower (53% lower) for patients with documentation suggestive of higher risk P/C allergies, which further suggests that the allergy history is important to antibiotic choice. We additionally observed that a documented P/C allergy was associated with a 52% increased risk of fluoroquinolone treatment. Fluoroquinolones have important Food and Drug Administration “black box” warnings for tendon rupture, torsade de pointes, and abdominal aortic aneurysm/dissection.26 The increase in fluoroquinolone use may be due to avoidance of ceftriaxone in CAP treatment for patients with documented P/C allergies. However, use of ceftriaxone in patients with penicillin allergy is safe in most patients, even those with high-risk penicillin allergy histories. For patients with a cephalosporin allergy documented in the EHR, many other non-cross-reactive cephalosporins can be safely administered.1,2 For example, a patient with a nonanaphylactic allergy to cephalexin could be given ceftriaxone.1,2 US hospitals should identify best practice methods to safely increase guideline-recommended beta-lactam pneumonia treatments among inpatients with documented and unverified P/C allergies.
In our study, patients with a documented P/C allergy were treated with different beta-lactams and beta-lactam alternative antibiotics. There was a 61% increased use of carbapenems in adjusted analysis with unadjusted analyses additionally identifying increased frequency of use of fourth generation cephalosporins (cefepime) and aztreonam. These beta-lactam choices are more broad-spectrum than the recommended cephalosporins for CAP, such as ceftriaxone. The increased use of cefepime and carbapenems in patients with a documented beta-lactam allergy may reflect that provider cross-reactivity concerns are less with 4th generation cephalosporins and carbapenems. Some of the increased use of cefepime and carbapenems in patients with documented beta-lactam allergy may also be a result of the poor efficacy of aztreonam for Pseudomonas treatment.27 In our study, aztreonam was uncommonly used but almost exclusively reserved for patients with a documented beta-lactam allergy (5% vs <1%). While clindamycin was also uncommonly used overall, it was also more commonly used in patients with documented beta-lactam allergy (4% vs 1%); clindamycin is notably not guideline-recommended for the treatment of pneumonia.14 Selection of more broad-spectrum beta-lactams and beta-lactam alternatives may increase the risk of adverse effects leading to discontinuation of therapy, Clostridioides difficile colitis, and/or multidrug-resistant organisms.6
The World Health Organization,28 Centers for Disease Control and Prevention,29,30 President’s Council of Advisors on Science and Technology,31 and Presidential Advisory Council on Combating Antibiotic Resistant Bacteria32 have stressed the importance of antibiotic stewardship, both reducing inappropriate antibiotic use and prescribing the most targeted antibiotic.33,34 Antimicrobial stewardship recently adapted use of penicillin allergy assessments as a method of reducing inappropriate antibiotic use and prescribing targeted antibiotic therapy to improve patient safety and reduce antibiotic resistance.2,35 Tools to assess documented beta-lactam allergies in inpatient settings include institution-specific guidelines that promote penicillin skin testing and/or beta-lactam test doses or drug challenges (i.e., giving the indicated drug in a two-step observed setting).36 Implementation of penicillin allergy skin testing and drug challenges among inpatients have been associated with the safe increased use of beta-lactam antibiotics.9,36 In a systemic review of over 1,000 inpatients who received penicillin skin testing, 95% of patients were not allergic.37 However, since penicillin skin testing access is currently quite limited in the inpatient setting – in our study just 38% of hospitals caring for inpatients with pneumonia had access to inpatient allergy consultation and 29% had access to inpatient penicillin skin testing,18 hospital guidelines that use the reaction history to direct the use of different first-line beta-lactams in patients with documented beta-lactam allergies may be more feasible.38 Additional strategies include administration of amoxicillin directly to hospitalized patients with low-risk penicillin allergies with the goal of disproving and “delabeling” the penicillin allergy.39
While our study data came from a large sample of almost 100 diverse US hospitals, sites voluntarily participated in this study, and as such they were not sampled deliberately as to provide a representative national inpatient cohort. However, while this could impact our prevalence of documented P/C allergy estimate in pneumonia patients (20%), this would be less likely to impact our assessment of the relation of documented P/C allergy to antibiotic use for pneumonia patients. Given that all participating sites had active antibiotic stewardship programs, we consider that if a selection bias were present, it would bias our findings towards the null hypothesis and thus render our findings conservative. Cross-sectional data permitted us to assess antibiotics used or ordered on one day only, without consideration of antibiotic duration or cumulative utilization metrics such as days of therapy per 1000 patient days. We were also unable to determine whether inpatient allergy access or procedures impacted first-line beta-lactam treatment for pneumonia. While clinical data to validate the diagnosis of a bacterial pneumonia (e.g., chest x-ray, sputum culture) were not collected, the chart reviewed pneumonia diagnosis was determined by individuals who routinely perform auditing from health records for antibiotic stewardship practices. We did not have access to clinical data that guide pneumonia treatment, such as microbiology detail or type of pneumonia (CAP, HAP, VAP). Given that increased antimicrobial resistant organisms has been observed in patients with a documented penicillin allergy, it is possible there were differences in pneumonia organisms by P/C allergy. Although prior colonization or infection with resistant gram-negative rods (GNRs) can impact antibiotic treatment decisions, GNRs flags were not captured by our study. However, our multivariable adjustment notably included ICU location (as a proxy for pneumonia severity), pneumonia type, and MRSA/VRE colonization/infection in addition to age, sex, race, and hospital day. We also performed quantitative bias analyses using E-value to further assess unmeasured confounding and found that to completely explain away the weakest significant association we identified would require a potential unmeasured or residual confounder(s) greater than 1.86. This seems unlikely because we are not aware of any such potential confounder(s) and the magnitude would need to be greater than those we already included in our multivariable model.
In summary, we present the largest and most comprehensive US study to date demonstrating the prevalence and impact of documented P/C allergy on the treatment of inpatient pneumonia. The 20% of patients with a documented P/C allergy were treated less commonly with guideline-indicated beta-lactam antibiotics and more commonly with fluroquinolones and carbapenems. Methods to reduce or eliminate beta-lactam allergies documented in the EHR are needed to increase prescribing of more narrow-spectrum, guideline-recommended beta-lactam agents for the treatment of inpatient pneumonia.
Highlights Box:
What is already known about this topic?
Pneumonia affects over 450 million people per year, with treatment typically including beta-lactam antibiotics. Patients with unconfirmed penicillin or cephalosporin allergy may not receive beta-lactam antibiotics.
What does this article add to our knowledge?
We present the largest and most comprehensive US study to date demonstrating that pneumonia patients with a documented penicillin and/or cephalosporin allergy were treated less commonly with guideline-indicated beta-lactams.
How does this study impact current management guidelines?
Methods to reduce or eliminate unverified penicillin and/or cephalosporin allergies are needed to increase prescribing of guideline-recommended beta-lactams for the treatment of inpatient pneumonia.
Acknowledgements:
The authors thank Vizient, Inc. for facilitating this study through their hospital network. The authors thank Kristi Kuper, PharmD, BCPS, Christie Bertram, Pharm D, Arati Kurani, PharmD, BCPS, Jade Vitug, PharmD, Larry Huang,PharmD, Alyzeh Haider,MHA, and Sam Hohmann, PhD and for their project assistance.
Funding:
Dr. Blumenthal receives career development support from the National Institutes of Health (NIH) K01AI125631, the American Academy of Allergy Asthma and Immunology (AAAAI) Foundation, and the Massachusetts General Hospital (MGH) Claflin Distinguished Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AAAAI Foundation, nor the MGH. No funding for this study was received from Vizient, Inc.
Abbreviations:
- US
United States
- Ig
Immunoglobulin
- MRSA
Methicillin-resistant Staphylococcus aureus
- VRE
Vancomycin-resistant Enterococcus
- CAP
Community-acquired pneumonia
- HAP
Hospital-acquired pneumonia
- VAP
Ventilator-acquired pneumonia
- P/C
Penicillin and/or Cephalosporins
- HER
Electronic health record
- PR
Prevalence ratio
- CI
Confidence Interval
Footnotes
Conflicts of Interest
Dr. Blumenthal reports a beta-lactam allergy clinical decision support tool licensed to Persistent Systems. Megan Wimmer, Michael Postelnick, Christian Mancini, Xiaoqing Fu, Yuqing Zhang, Lucas Schulz, Tanaya Bhowmick, and Francesca Lee report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunology. Drug Allergy: An Updated Practice Parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA. 2019;321(2):188–99. [DOI] [PubMed] [Google Scholar]
- 3.Blanca M, Vega JM, Garcia JJ, Carmona MJ, Miranda A. Immediate Hypersensitivity Reactions to Penicillin and Related Antibiotics. Clin Exp Allergy. 1989;19(5):556–8. [DOI] [PubMed] [Google Scholar]
- 4.Park MA, Li JT. Diagnosis and Management of Penicillin Allergy. Mayo Clin Proc. 2005;80(3):405–10. [DOI] [PubMed] [Google Scholar]
- 5.Trubiano JA, Adkinson NF, Phillips EJ. Penicillin Allergy Is Not Necessarily Forever. JAMA. 2017;318(1):82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacFadden DR, LaDelfa A, Leen J, Gold WL, Daneman N, Weber E, et al. Impact of Reported Beta-Lactam Allergy on Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clin Infect Dis. 2016;63(7):904–10. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of Meticillin Resistant Staphylococcus aureus and Clostridium difficile in Patients with a Documented Penicillin Allergy: Population Based Matched Cohort Study. BMJ. 2018;361:k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepin J, Saheb N, Coulombe MA, et al. Emergence of Fluoroquinolones as the Predominant Risk Factor for Clostridium difficile-Associated Diarrhea: A Cohort Study During an Epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–60. [DOI] [PubMed] [Google Scholar]
- 9.Macy E, Contreras R. Health Care Use and Serious Infection Prevalence Associated with Penicillin “Allergy” In Hospitalized Patients: A Cohort Study J Allergy Clin Immunol. 2014;133(3):790–6. [DOI] [PubMed] [Google Scholar]
- 10.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral Pneumonia. Lancet. 2011;377(9773):1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 12.Magill SS, Edwards JR, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Survey of Health Care-Associated Infections. N Engl J Med. 2014;370(26):2542–3. [DOI] [PubMed] [Google Scholar]
- 13.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable Mortality of Ventilator-Associated Pneumonia: A Meta-Analysis of Individual Patient Data from Randomised Prevention Studies. Lancet Infect Dis. 2013;13(8):665–71. [DOI] [PubMed] [Google Scholar]
- 14.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picard M, Begin P, Bouchard H, et al. Treatment of Patients with a History of Penicillin Allergy in a Large Tertiary-Care Academic Hospital. J Allergy Clin Immunol Pract. 2013;1(3):252–7. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal KG, Kuper K, Schulz LT, Bhowmick T, Postelnick M, Lee F, et al. Association Between Penicillin Allergy Documentation and Antibiotic Use. JAMA Int Med. 2020;180(8)1120–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancini CM, Fu X, Zhang Y, et al. Penicillin Allergy Evaluation Access: A National Survey. Clin Infect Dis. 2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vizient. About Us. Vizient. Irving, TX, Published 2020, Available at https://www.vizientinc.com/about-us.Accessed January 30, 2020. [Google Scholar]
- 20.Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ Perception of Hospital Care in the United States. N Engl J Med. 2008;359(18):1921–31. [DOI] [PubMed] [Google Scholar]
- 21.Medicare Provider Cost Report Public Use Files. U.S. Centers for Medicare & Medicaid Services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Cost-Report.Accessed January 21, 2020,
- 22.Spiegelman D, Hertzmark E. Easy SAS Calculations for Risk or Prevalence Ratios and Differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA. 2019;321(6):602–3. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ. No Adjustments are Needed for Multiple Comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 26.Bayer HealthCare Pharmaceuticals Inc. CIPRO® (ciprofloxacin hydrochloride) tablet, for oral use and CIPRO® (ciprofloxacin hydrochloride), for oral suspension [package insert]. Silver Spring, MD: U.S. Food and Drug Administration, 1987. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s091,020780s048lbl.pdfAccessed October 23, 2020. [Google Scholar]
- 27.Hogan M, Bridgeman MB, Min GH, Dixit D, Bridgeman PJ, Narayanan N. Effectiveness of empiric aztreonam compared to other beta-lactams for treatment of Pseudomonas aeruginosa infections. Infect Drug Resist 2018;11:1975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. J Allergy Clin Immunol Pract. 2018;6(5):1649–54 e4. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Antibiotic Prescribing and Use: Appropriate Antibiotic Use. Atlanta, GA: U.S. Department of Health and Human Services, CDC. Available at https://www.cdc.gov/antibiotic-use/index.html.Accessed October 23, 2020. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Antibiotic Use in the United States: Progress and Opportunities - 2018 Update. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019. Available at https://www.cdc.gov/antibiotic-use/stewardship-report/pdf/stewardship-report-2018-508.pdf.Accessed October 23, 2020. [Google Scholar]
- 31.President’s Council of Advisors on Science and Technology. Report to the President on Combating Antibiotic Resistance. Washington DC: Executive Office of the President of the United States, 2014. Available at https://www.cdc.gov/drugresistance/pdf/report-to-the-president-on-combating-antibiotic-resistance.pdf.Accessed October 23, 2020, [Google Scholar]
- 32.Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria. Key Strategies to Enhance Infection Prevention and Antibiotic Stewardship: Report with Recommendations for Human and Animal Health. Washington DC: Executive Office of the President of the United States, 2018. Available at https://www.hhs.gov/sites/default/files/final-ips-report-10-03-2018.pdf). Accessed October 23, 2020. [Google Scholar]
- 33.Centers for Disease Control and Prevention. What CDC is Doing: Antibiotic Resistance Solutions Initiative. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2018. Available at https://www.cdc.gov/drugresistance/solutions-initiative/index.html.Accessed July 15, 2019. [Google Scholar]
- 34.Barlam TF, Cosgrove SE, Abbo LM, et al. Executive Summary: Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):1197–202. [DOI] [PubMed] [Google Scholar]
- 35.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62(10):e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfson AR, Huebner EM, Blumenthal KG. Acute Care Beta-Lactam Allergy Pathways: Approaches and Outcomes. Ann Allergy Asthma Immunol. 2019;123(1):16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical Outcomes Following Inpatient Penicillin Allergy Testing: A Systematic Review and Meta-Analysis. Allergy. 2017;72(9):1288–96. [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal KG, Li Y, Hsu JT, et al. Outcomes from an Inpatient Beta-Lactam Allergy Guideline Across a Large US Health System. Infect Control Hosp Epidemiol. 2019;40(5):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone CA Jr., Stollings JL, Lindsell CJ, et al. Risk-stratified Management to Remove Low-Risk Penicillin Allergy Labels in the ICU. Am J Respir Crit Care Med. 2020;201(12):1572–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


