Abstract
Fibrosis is a pathologic process characterized by the replacement of parenchymal tissue by large amounts of extracellular matrix, which may lead to organ dysfunction and even death. Fibroblasts are classically associated to fibrosis and tissue repair, and seldom to regeneration. However, accumulating evidence supports a pro-regenerative role of fibroblasts in different organs. While some organs rely on fibroblasts for maintaining stem cell niches, others depend on fibroblast activity, particularly on secreted molecules that promote cell adhesion, migration, and proliferation, to guide the regenerative process. Herein we provide an up-to-date overview of fibroblast-derived regenerative signaling across different organs and discuss how this capacity may become compromised with aging. We further introduce a new paradigm for regenerative therapies based on reverting adult fibroblasts to a fetal/neonatal-like phenotype.
Subject terms: Mechanisms of disease, Ageing, Diseases
Introduction
Tissue damage can have several causes, including mechanical forces, infections, toxins, ischemia, and autoimmune reactions1. In an ideal scenario, the response to injury is able to restore normal organ architecture and function, a process that is known as regeneration2. Independently of the organ, regeneration usually comprises three overlapping phases. Immediately after injury, apoptotic cells at the injury site signal toward macrophage and neutrophil recruitment for clearance of cell debris and avoid infection, thus initiating the inflammatory stage. From 2 to 10 days after injury, the proliferative stage begins and processes such as angiogenesis, extracellular matrix (ECM) deposition, and cell proliferation create new tissue and reduce the injured area2. Finally, the remodeling stage takes place, in which the tissue recovers the preceding organization and the underlying ECM is reorganized3. Dysregulation of any of these processes can trigger excessive ECM deposition, typically rich in collagen I, resulting in the formation of scar tissue—a process that is widely known as fibrosis. The latter is therefore associated to organ repair and negatively impacts organ function4.
Fibroblasts are cells of mesenchymal origin and are the main producers of ECM in homeostatic conditions and in response to injury5. These cells are found in virtually every tissue, but their molecular signature is not preserved between organs6. Fibroblasts are activated in the inflammatory stage of the wound healing in response to cytokines and growth factors such as transforming growth factor beta 1 (TGF-β1)7, interleukin 1 beta (IL-1β)8, interleukin 6 (IL-6) or platelet-derived growth factor (PDGF)9, and differentiate into myofibroblasts5. Less characterized cells like pericytes, mural cells typically associated with endothelial cells, are also capable of differentiating into myofibroblasts10. The latter display distinctive features such as increased cell size, high alpha smooth muscle actin (α-SMA) expression, and the presence of microfilaments that supports cell contraction. Importantly, myofibroblasts secrete great amounts of ECM and are therefore regarded as the culprits of fibrotic diseases and organ dysfunction after injury2. Current strategies to treat or reverse fibrosis focused on targeting myofibroblasts include inducing apoptosis or senescence, promoting dedifferentiation to fibroblasts and reprogramming into other cell types11. Recently, the use of chimeric antigen receptor (CAR)-T cells for targeting activated cardiac fibroblasts has shown great potential to reduce myocardial fibrosis supporting immunotherapeutic strategies as a new avenue to control fibrosis12. Yet, little anti-fibrotic therapies are effective and currently available in the clinical setting1.
Considering the well-described contribution of fibroblasts in the development of fibrosis, their role in other biological events is often neglected. However, highly regenerative organs like the intestine rely on fibroblast activity to maintain the stem cell niche13. In other organs like the liver or the lung, where regenerative mechanisms encompassing the proliferation of differentiated cells or progenitor cell differentiation are only activated after injury, fibroblasts are able to secrete growth factors and mitogens and produce structural components of ECM to restore normal tissue architecture14,15. Even in a non-regenerative organ like the heart, where fibroblast activity is mainly associated to fibrosis and organ dysfunction, neonatal fibroblasts and some ECM components have been shown to promote cardiomyocyte proliferation after injury16. Hence, one can hypothesize that fibroblasts in most organs are actively involved in repair as well as in regeneration. This review will focus on the characterization of fibroblast signatures in different organs and unveil potential targets for stimulating fibroblast-induced regeneration in wound healing and fibrotic diseases.
Organ-specific fibroblasts: surface markers and regenerative signals
Intestine
The intestine is an organ which self-renews its lining every few days, much owing to the presence of intestinal stem cells (ISC) in the crypts17. Fibroblasts, along with other cells, encompass a supporting niche for leucine-rich repeat-containing G-protein 5 (Lgr5)+ ISC18,19 (Fig. 1a) and were found to express GLI family zinc finger 1 (GLI-1), podoplanin, CD90, vimentin, and fibroblast-specific protein 1 (FSP-1)20–22. Some cells express α-SMA in physiological conditions, and are considered by some to be myofibroblasts23. Unsupervised clustering gene expression analysis also showed that human gastrointestinal fibroblasts segregate from fibroblasts isolated from other organs24. The major differences in gene expression concerned upregulation of some transcriptional regulators (e.g., Tcf21, Foxf1, Foxp2), signaling molecules (e.g., chemokine ligands, fibroblast growth factors), and ECM remodeling genes (e.g., Comp, Col3a1, Lama3).
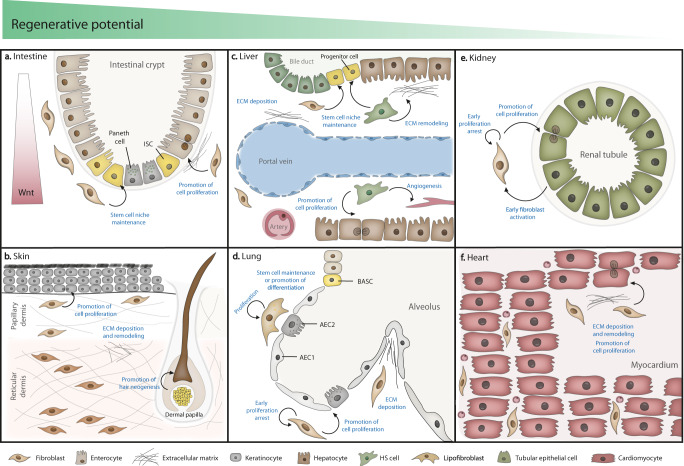
Fig. 1. The fibroblast as a mediator of regeneration in major organs.
a Intestinal fibroblasts are a component of the stem cell niche, secreting ECM, growth factors, and creating a Wnt gradient along the crypt promoting ISC proliferation/differentiation. b Dermal fibroblasts promote regeneration mainly by secreting specific ECM components, promoting wound healing. A specialized population of fibroblasts (dermal papilla) is also involved in hair neogenesis. c In the liver, portal fibroblasts and HS cells promote liver progenitor and hepatocyte proliferation after injury by multiple mechanisms. d Proliferative lung fibroblasts and lipofibroblasts promote regeneration by secreting structural ECM components and stimulating AEC2 cells with mitogens. e In the kidney, fibroblasts and epithelial cells communicate in a bidirectional fashion to coordinate tubular regeneration. f Specific ECM components putatively secreted by fibroblasts have been linked to cardiac regeneration in the neonatal heart.
The maintenance of the ISC niche by intestinal fibroblasts appears to be mediated by the establishment of a Wnt/BMP gradient, where Wnt signaling is the main driver of crypt proliferation. At the base of the crypt where ISC reside, fibroblasts secrete Wnts and BMP antagonists25,26. In a single-cell RNA-sequencing approach targeted for human intestinal CD90+ cells, the authors identified 11 clusters from which two were crypt niche cells27. Besides expressing the conventional fibroblast markers vimentin, collagen I, and collagen III, these cells also expressed noncanonical Wnt ligands, BMPs, and periostin. Gene ontology analysis showed enriched terms of “response to wound healing” and “regulation of epithelial cell proliferation.” In the same report, these cells were decreased in the biopsies of inflammatory bowel disease patients, who present exacerbated intestinal inflammation and decreased mucosal healing, which reveals a role of these cells in supporting epithelial renewal28. Furthermore, PDGFRα+ fibroblasts have been shown to secrete Wnt and R-spondin 3 in the pericryptal region29. R-spondin 3 acts as a Wnt enhancer and is vital to injury repair by inducing more differentiated Lgr4+ cells to regain Wnt production, and thus generate new crypts29,30. Gli1+ gp38+ colonic fibroblasts were also found to express Wnt2b and thus to be possible stem cell niche-supporting cells21. These cells were later found to be pericryptal CD90+ fibroblasts, capable of supporting endothelial cell proliferation and organoid growth in vitro. Subsequent differential gene expression analysis unveiled upregulated expression of stem cell niche factors, hepatocyte growth factor (HGF), and class 3 semaphorins (Sema III). Of note, inhibiting the binding of semaphorins to their receptors prevented CD90+ fibroblasts-mediated organoid growth. Altogether, these reports indicate that fibroblasts are key drivers of ISC maintenance and crypt proliferation through Wnt/BMP signaling. In line with this, age-associated decrease in regenerative ability has been correlated with a reduction in Wnt signaling, and exogenous Wnt administration rescued the aged ISC phenotype in an organoid model31,32.
Other reports suggest that these cells also directly promote epithelial cell proliferation by secreting HGF33 and expressing periostin34. Furthermore, in an injury setting, the inflammatory milieu sensed by tumor progression locus-2 (Tpl2) kinase-expressing subepithelial myofibroblasts triggers a compensatory epithelial proliferation via ERK, cycloxygenase 2 (Cox-2), and prostaglandin (PGE2)-activated signaling—a response, which is dysregulated in inflammatory bowel diseases35. In a similar fashion, the prostaglandin signaling pathway in Cox-2+ fibroblasts was found to positively influence stem cell antigen-1 (Sca-1)+ ISC expansion, and trigger epithelium regeneration or tumor formation, in case ISC underwent previous mutations36. Overall, intestinal fibroblasts directly stimulate cell proliferation and are critical for supporting the stem cell niche and epithelial renewal, a capacity that decreases with aging thus contributing toward a decline in the regenerative capacity of the organ in the elderly.
Skin
The skin is the largest organ in the body and a complex structure comprising the epidermis, dermis, hair follicles, and other appendages. In homeostasis, basal epidermal stem cells ensure the re-epithelization after normal skin shedding or after insults to the outermost layer of the skin, the epidermis37. Fibroblasts stimulate keratinocyte proliferation and differentiation in the epidermis mainly by the release of soluble factors such as IL-1, keratinocyte growth factor (KGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) as shown in studies using dermal equivalents with epidermal keratinocyte/fibroblast co-cultures38–40. Yet, the dermis, the thickest layer of the skin, is the most studied layer in wound healing37,41. Rognoni et al. postulate that an initial phase of active dermal fibroblast migration and proliferation followed by high ECM deposition phase, which negatively regulates fibroblast proliferation, is key to successful wound healing42. Dermal fibroblast progenitors expressing Twist-related protein 2 (Twist2/Dermo1), platelet-derived growth factor receptor α (PDGFRα) and Engrailed1 (En1) give rise to papillary fibroblasts and to reticular fibroblasts (as reviewed in ref. 43) which reside in the upper papillary dermis and the lower reticular dermis, respectively (Fig. 1b), and display a distinct signature. In the human skin, mutually exclusive CD90 and fibroblast activation protein (FAP) expression are enough to discriminate both populations44. Papillary and reticular fibroblasts influence the composition of the respective layer of the dermis. Well-organized fibrillary collagen bundles are abundant in the reticular dermis, while non-fibrillary collagens and proteoglycans like fibromodulin and decorin are more common in papillary dermis41,45. This suggests that these populations directly influence the ECM components of the dermis and may respond differently to injury. Jiang and Rinkevich reviewed the subsets of dermal fibroblasts implicated in fibrosis and concluded that no markers or spatial location within the dermis can discriminate fibrotic fibroblasts46. Conversely, other evidences point that papillary fibroblast favor scar-free wound healing by producing Wnt whereas reticular fibroblasts are fibrotic since readily synthetize collagenous ECM47,48. In fact, keratinocytes grown on papillary dermis-like ECM proliferate more than on reticular fibroblast-derived ECM49. In addition, reticular and papillary fibroblasts have been described to respond differently to aging50,51. In a comparative study, aged papillary fibroblasts display reduced capacity to proliferate and remodel ECM relatively to aged reticular fibroblasts51. The authors of this study suggest that the less-differentiated papillary fibroblasts may progressively disappear or differentiate into reticular fibroblasts with aging. These evidences collectively suggest that papillary fibroblasts are more pro-regenerative than reticular fibroblasts and that their stimulation can be key for regenerating skin without scarring52.
Aiming at understanding the mechanisms governing scarless skin regeneration, significant interest has been given to mammalian fetuses as they are able to regenerate their skin without scarring53–55. The fetal healing process was found to differ from the one of adults in various parameters such as inflammatory cell recruitment, TGF-β expression, and ECM secretion54,56. In fetal wound healing, ECM components like collagen III, hyaluronic acid, and matrix metalloproteinase (MMP) to inhibitor (TIMP) ratio are upregulated, which is thought to favor cell migration54. Mouse neonates can also regenerate their skin. The loss of regenerative potential within the dermis as mice age is correlated to a decrease in fibroblast proliferation, particularly reflected in the cellular density of the papillary layer, and a decrease in Wnt signaling57. A single-cell RNA-sequencing study comparing skin in developing (P2), regenerating (wounded P2), homeostatic (P21), scarring (wounded P21) conditions has unveiled that pro-regenerative fibroblasts are papillary fibroblasts expressing Lef158. The reactivation of Lef1 in adult animal dermis enhanced wound healing.
One of the few examples of adult human scar-free healing occurs in injuries of the oral mucosa. Here, the microenvironment is less inflammatory and fibroblast-secreted ECM is rich in ED-A form of fibronectin, chondroitin sulfate and has less elastin59. The authors postulated that chondroitin sulfate promotes faster wound closure. On the other hand, fibronectin ED-A, which is typically produced by fibroblasts, has been shown to promote a normal wound healing process likely by promoting epithelial cell migration60. An independent study showed that human oral mucosa fibroblasts are reportedly more prone to express glycoproteins and transcription factors that promote angiogenesis, cell migration and proliferation, and less prone to the expression of senescent markers than human dermal fibroblasts61.
Animal models with enhanced capacity to regenerate are important models to dissect regenerative mechanisms. Recently, a small mammal able to regenerate the skin and hair has been identified. Acomys, the spiny mouse, is a rodent whose skin thickness is very similar to Mus musculus but tears easily at the back or in the tail62. After injury, almost no α-SMA+ myofibroblasts are found at the lesion site62. The composition of the ECM is different in Acomys wounds and molecules like collagen triple helix repeat containing-1 (CTHRC-1), tenascin-C, fibronectin 1, laminin α1, and aggrecan are upregulated and correlated to a regeneration-inducing environment, although no mechanistic details have been attributed to these components individually63. However, CTHRC-1 has been described as a promoter of wound closure by recruiting anti-inflammatory macrophages64. Therefore, the underlying ECM may be responsible for regeneration in Acomys wounds. In fact, Brant et al. suggested that a low inflammatory environment, due to lesser induction of cytokines and chemokines, combined with the presence of fetal-like ECM may underlie scarless regeneration in this animal65. This evidence further supports fetal fibroblasts display a pro-regenerative phenotype.
Another challenge of skin regeneration is the restoration of hair follicles. Most adult mammals do not fully restore these structures after injury but the opposite has been demonstrated in full thickness wounds in mice and rabbits66,67. Hair follicle neogenesis requires coordination between stem cells that generate all components of the follicle and the dermal papilla niche, which comprises specialized fibroblasts derived from the papillary lineage68,69. Dermal papilla cells usually express CD133 and alkaline phosphatase70. In mice, Blimp1+ fibroblasts contribute to hair follicle formation through Wnt/β-catenin signaling activation in the dermal papilla71. Moreover, activation of Sonic hedgehog (Shh) in wound fibroblasts has been also correlated to dermal papilla formation and hair growth stimulation72. Hence, the stimulation of fibroblast-mediated Wnt or Shh signaling may pose as a promising strategy for hair follicle renewal.
In the skin, fibroblasts have been shown to influence dermal regeneration by secreting specific ECM components (reviewed elsewhere73) and, in the case of hair production, fibroblasts are also essential for regulating the stem cell niche. Of note, scarless free skin regeneration is restricted to fetal stages or to specific environments, such as the oral mucosa, in which fibroblast acquire a fetal-like phenotype. In adulthood, papillary fibroblasts have been shown to signal toward regeneration but further studies are required for unveiling involved mechanisms.
Liver
The two aforementioned organs, the skin and intestine, are epithelial organs with high cell turnover, in which cell renewal and tissue homeostasis are achieved through activation of a tissue-specific pool of stem/progenitor cells. Instead, the liver has low cell turnover but regenerates in a unique manner. Following an up to two-thirds mouse liver resection, the organ becomes fully recovered in ~2 weeks74. This efficient replacement is mainly at expenses of adult mature hepatocyte self-renewal, and to a lesser extent granted by the existence of progenitor cells74–76.
Portal fibroblasts and hepatic stellate cells (HS) or sinusoidal pericytes are the main populations of resident mesenchymal cells in the liver77 (Fig. 1c). At steady state, HS are in contact with hepatocytes and sinusoids, and portal fibroblasts are found surrounding the portal vein and bile ducts in the portal region. Despite some phenotypic heterogeneity, both are recognized by the expression of specific markers (reviewed in refs. 78,79). Single-cell RNA-sequencing approaches using mesenchyme-labelling PDGFRβ expression enabled the transcriptomic and spatial in-depth characterization of different subsets of fibroblasts and HS, both in healthy and injured livers80,81. Dobie et al. describe a population with upregulated ECM pathways (portal fibroblasts) and a population with upregulated vitamin A metabolism processes (HS). After centrilobular necrosis injury, a subset of lysophosphatidic acid receptor 1-positive (LPAR-1) HS is the main culprit for collagen secretion and fibrosis. Krenkel et al. find four subpopulations of myofibroblasts derived from HS in the injured liver, with the surface marker S100 calcium binding protein A6 (S100A6) being expressed in all of them. Indeed, HS appear to be the main source of myofibroblast-like cells following most injuries, having a central role in hepatic fibrosis and the underlying imbalance between ECM synthesis and degradation82. Portal myofibroblasts also play a part in liver fibrosis, with a particular role in biliary diseases83.
Interestingly, both HS and portal myofibroblasts can play a role in liver regeneration. What makes these cells pro-regenerative or pro-fibrotic is still not known, although some authors hypothesize that this response lies on yet to be described subpopulations15. Activated HS undergo changes such as the loss of vitamin A and upregulation of α-SMA, and become capable of releasing mitogens, like HGF, pleiotrophin, and epimorphin, to stimulate hepatocyte proliferation84. Activated HS also influence the recruitment of immune cells and angiogenesis by vascular endothelial growth factor (VEGF) and erythropoietin secretion85, and induce ECM remodeling via secretion of MMP-1, MMP-2, and angiopoietin15,86. HS promote the proliferation of progenitor cells through the secretion of IL-6, fibroblast growth factor 7 (FGF-7), lymphotoxin-beta, and Hh, as reviewed in ref. 87. HS have also been shown to become progenitor cells and support liver regeneration in a Hedgehog-regulated fashion after hepatectomy88. HS cells first differentiate into a myofibroblast phenotype to then repopulate the liver with newly formed hepatocytes. In parallel, portal fibroblasts also appear to promote survival of progenitor cells in the bile duct region via Hedgehog89 and by losing nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) expression90. Furthermore, portal fibroblasts in the regenerating rat liver express neural cell adhesion molecule (NCAM), an adhesion molecule that associates with collagens and provides binding sites to other cells, ensuring regrowth of portal tracts91.
Apart from contributing for both liver repair and regeneration after injury, HS have a role in reverting fibrosis. The concept of liver fibrosis reversal has been addressed in humans and animal models92 and has even been confirmed in cases of hepatitis after antiviral treatment93. Activated HS are either eliminated by apoptosis, become senescent, and later cleared by NK cells, or survive but become inactivated94–96. If HS become apoptotic, TIMP-1 production decreases and restitution of homeostatic MMP/TIMP ratio impedes buildup of new ECM and the existent collagenous matrix is degraded97–99. If HS become inactivated, fibrotic genes like Spp1, Col1a1, and Acta2 (α-SMA) are downregulated and activated HS acquire characteristics similar to those found in the quiescent counterparts95,100.
Changes in ECM after injury have also been shown to influence liver regeneration. For example, vitronectin and olfactomedin 4 (OLFM4) production in injured human livers promote cell migration and adhesion, while fibronectin and collagen I induce cell proliferation and differentiation of liver progenitors into hepatocytes101. MMP-2 and -9 are also key players in liver regeneration, as they affect ECM remodeling and the release of HGF and other growth factors102,103. Importantly, a decrease in ECM remodeling via MMP activity and subsequent increase in fibrosis is correlated with aging104.
In the liver, the same cells involved in fibrotic response can signal also for regeneration for which ECM composition and remodeling appears to be crucial. Yet, further studies are needed to pinpoint cellular subpopulations that may contribute mostly to regeneration instead of repair.
Lung
Alike the liver, the lung presents limited cell turnover in homeostasis. However, this organ has been proven to regenerate after injury (i.e., facultative regeneration) owing mostly to a pool of stem cells. The latter exit their quiescent state105, namely bronchioalveolar stem cells (BASC) that are able to originate both bronchiolar and alveolar epithelial cells106, and alveolar type II cells (AEC2) in alveoli107, where gas exchanges occur and the organ is most susceptible to external insults.
Surrounding alveoli are resident lung fibroblasts (Fig. 1d), which express PDGFRα, collagen I, CD146, vimentin, and desmin (reviewed in ref. 108). These cells contribute to the formation of an ECM that supports alveolar regeneration. For example, in vitro and in vivo studies show that elastin and collagen secreted by lung fibroblasts aid alveologenesis after birth by providing support to alveoli septation109,110. Fibroblast-secreted fibronectin is also important for endothelial cell adhesion, as shown in an in vitro study using fibroblast-derived matrices111.
Because PDGFRα was found to be important for alveolarization during development and realveolarization112, Endale et al. have recently traced PDGFRα+ fibroblasts spatial location throughout development113. At postnatal stages, these fibroblasts, which include subpopulations of myo and lipofibroblasts, co-localized with alveoli. In addition, in the human lung, a single-cell RNA-sequencing study unveiled the spatial distribution of at least eight stromal populations, and alveoli were found to be surrounded by myofibroblasts, alveolar fibroblasts, and lipofibroblasts114. Lipofibroblasts are best characterized by the expression of adipose differentiation-related protein (ADRP), peroxisome proliferator-activated receptor γ (PPARγ), parathyroid hormone 1 receptor (PTH1R)115, and transcription factor 21 (Tcf21)116. These cells are lipid-containing interstitial fibroblasts that are found in close contact with AEC2 cells and aid in surfactant production in alveoli117. Barkauskas et al. have proposed that AEC2 are adult stem cells, which are directly supported by PDGFRα+ lipofibroblasts in the formation of alveolospheres in vitro by stimulation of AEC2 proliferation and differentiation107. In fact, PDGFRα+ fibroblasts directly influence AEC2 regeneration after injury by proliferating and secreting IL-6, FGF-7, and BMP inhibitors118. A single-cell RNA-sequencing approach confirmed a role of alveolar lipofibroblasts in fibrosis119, although retinoic acid-metabolizing lipofibroblasts are also regarded as pro-regenerative cells after a seminal study demonstrated that retinoic acid administration induced regeneration in a rat emphysema model120,121. Retinoic acid, a bioactive metabolite of vitamin A, induces changes in gene expression by binding to specific transcription factors and has been previously linked to regeneration of urodele limbs, central nervous system, and lungs122.
Some fibroblast populations also signal directly for epithelial regeneration after pneumonectomy. For example, Fsp1+ fibroblasts are beneficial to regeneration if their proliferation is transient and contained to the first few days after injury when IL-6 and cell-cycle genes are upregulated123. PDGFRα+ fibroblasts increase in realveolarization after pneumonectomy112 and are the main source of KGF and HGF, which induce epithelial and endothelial proliferation after injury124. Noteworthy, the response to pneumonectomy seems to change with age. Compared to 3-month-old, 9-month-old murine fibroblasts have lower ability to proliferate in vitro and adopt a myofibroblastic phenotype125.
In sum, fibroblasts contribute to lung regeneration mainly by proliferating in early stages of injury, whilst secreting mitogens and structural ECM components. Yet, the influence of ECM composition in lung regeneration remains unclear as has been mainly addressed in vitro. This response changes with age, since the proliferative capacity of fibroblasts decreases in older animals, demonstrating the key role of this cell type orchestrating regeneration in this organ.
Kidney
The kidney is an organ with limited regenerative potential since quiescent tubular epithelial cells regain capacity to proliferate after acute injury but structural regeneration of the nephron—the functional unit of the kidney—is not achieved126,127.
Resident fibroblasts are sparsely dispersed between the tubules and peritubular capillaries (Fig. 1e). They express vimentin but not desmin128, FSP-1, cadherin-9129, and secrete erythropoietin to maintain homeostatic conditions in response to hypoxia130. Kidney-resident fibroblasts and pericytes express PDGFRβ and CD73, and derive from the same progenitor, suggesting that these populations are likely overlapping131, although the subject is still controversial132.
After acute kidney injury, kidney fibroblasts are able to promote tubular regeneration133–135. The latter depends on the bidirectional communication between tubular epithelial cells and fibroblasts133. The injured epithelium promotes early fibroblast activation by releasing Shh and TGF-β1-containing exosomes136,137. Then, activated fibroblasts promote tubular repair via Wnt/β-catenin signaling, retinoic acid production, and HGF secretion138–140. Apart from stimulating epithelial proliferation, HGF also inhibits TGF-β signaling in fibroblasts, preventing their further activation and fibrosis140. In fact, early fibroblast proliferation in acute kidney injury is required for regeneration137. In acute injuries, initial fibroblast/myofibroblast expansion regresses after regeneration, contrarily to what is observed in chronic injuries, in which regeneration is not present. This seems to relate with the downregulation of genes related to ECM organization, TGF-β, and MMP signaling in fibroblasts during the resolution phase of acute injuries whereas in chronic scenarios, these genes remain upregulated. In addition, expression of inflammation-associated molecules in fibroblasts from acute injuries seem to facilitate fibroblast clearing and ECM degradation141.
Evidently, the intercommunication between fibroblasts and epithelial cells of the kidney is crucial for tubule regeneration after acute injury. A control of early fibroblast proliferation and clearance is also necessary, alike what happens in the lung. In opposition to other organs, the ECM in the kidney has not been studied in the context of regeneration.
Heart
The heart does not regenerate after injury. Instead, lost cardiomyocytes are replaced by a fibrotic scar synthesized by fibroblasts that prevents ventricular wall rupture but contributes to functional decline142,143. Cardiac fibroblasts are uniformly dispersed throughout the interstitial space of the myocardium142,144 (Fig. 1f) and show a common embryonic lineage ancestry that can be traced to the endocardium and the proepicardial organ145,146. Multiple markers for cardiac fibroblasts have been reported (reviewed in refs. 147,148), but these are also found on perivascular cells and therefore two or more markers are normally combined to discriminate fibroblasts in the heart149,150.
In contrast to the adult mammalian heart, fetal and neonatal hearts regenerate following cardiac injury with minimal scarring151. In 2011, it was revealed for the first time that the neonatal heart of 1-day-old mice could regenerate after partial resection of the apex contrarily to as 7-day-old mice, which fail to regenerate their myocardium and develop significant fibrosis152. This regenerative response is therefore transient and relies on pre-existing cardiomyocyte proliferation153. Quaife-Ryan et al. compared all main cardiac populations (including CD90+ fibroblasts) in neonatal and adult hearts after myocardial infarction by RNA sequencing154, and found that adult fibroblasts are more responsive to injury than neonatal fibroblasts. Others show that neonatal fibroblasts display an intermediate phenotype between fetal and adult fibroblasts and contribute to the heart response to injury. Specifically, after neonatal cardiac injury, adult-like fibroblasts (PDGFRα+CD90+Sca-1+) exhibited increased expression of fibrotic-associated genes (Col1a1, Col3a1, Tgfb1, and Tgfb3), whereas fetal-like fibroblasts (PDGFRα +CD90+Sca-1−) had also increased expression of genes associated with improved cardiomyocyte proliferation (Fn1, Tbx20, Igf1, Igf2, Fstl1) and neovascularization (Vegfa)155. A recent single-cell RNA-sequencing approach characterizing non-myocyte populations in regenerative and reparative neonatal mice hearts has unveiled subsets of fibroblasts, which respond differently to injury. Contrarily to regenerative hearts, in which the subsets remain fairly constant after injury, in non-regenerative hearts, proliferating and activated fibroblasts become more prevalent156. These findings support that cardiac fibroblasts diversity may comprise cell subsets with pro-regenerative or pro-reparative phenotypes.
Cardiac fibroblasts impart on the response to injury through the production of ECM. In fact, ECM from regenerative hearts displays different components when compared those of reparative hearts157,158. Fibronectin, periostin, and agrin have all been correlated to a pro-regenerative setting16. In zebrafish, fibronectin is required for regeneration by promoting fibroblast migration and cardiomyocyte proliferation159,160. Embryonic mouse fibroblasts also express high levels of fibronectin, collagen, and heparin-binding EGF-like growth factor, which promote mitotic activation in cardiomyocytes during late development161. Increased deposition of agrin and periostin upon injury in P1 neonatal mice has been related to cardiac regeneration by activating the phosphoinositide 3-kinase and Yes-associated protein signalling pathway, respectively, and subsequent induction of cardiomyocyte re-entry in the cell cycle162–164. Even though tenascin-C has been reported as a pro-regenerative component in lower vertebrates165, in mice there are contradictory evidences regarding its role in the heart response to injury. Tenascin-C is highly expressed in the infarct zone, and different studies indicate that promotes neonatal regeneration by attenuating inflammation and promoting cardiomyocyte proliferation159,166. On the other hand, tenascin-C can be a predictor of fibrosis since high expression of tenascin-C in a fibrosis model is correlated to collagen deposition in later stages167. Although no evidence has been shown in mammals, hyaluronic acid is another component of the ECM that can promote heart regeneration in newts and zebrafish by promoting cell migration165.
Apart from ECM modulation, cardiac fibroblasts can signal for regeneration through the secretion of growth factors and cytokines, both in the adult168 and the neonatal156. Although the precise involvement of fibroblasts in neonatal regeneration is undefined, different studies have accessed the role of fibroblasts and fibroblasts-produced molecules in the adult response to injury. Furtado et al. have suggested that fibroblast expressing cardiogenic markers are associated to a regenerative response after injury, as the loss of Tbx20 expression hampered cardiac repair169. Fibroblasts express fibroblast growth factor 1 (FGF-1), which induces cardiomyocyte cell-cycle re-entry170,171. After myocardial infarction, an injection of FGF-1 in combination with p38 MAP kinase inhibition enhances the proliferation of both cardiomyocytes and endothelial cells, resulting in reduced scar and improved heart function170,172.
The discovery of the regenerative capacity of neonatal hearts has highlighted the importance of age in the response to injury. Since then, various studies have linked particular ECM components expressed in neonatal animals to regeneration, but the specific role of cardiac fibroblast in the cardiac regenerative response is poorly defined.
Fibrosis vs regeneration: the impact of aging
Most tissues exhibit a progressive decay in their regenerative capacity from the onset of development to the end of their lifespan. In fact, aging leads to a decline in tissue function, reducing the ability of tissues to repair after damage and maintain homeostasis. The recently reported Tabula Muris Senis study has provided insight on cell dynamics and profiles in aging mice across all organs173. In tissues like the intestine, aging is associated to a decay of adult stem cell regenerative potential. However, the aging phenotype of stem cells can be rescued when exposed to a “young” environment. For example, the aged phenotype of muscle stem, adipose mesenchymal stem, and hematopoietic stem cells have been rescued after exposure to the blood of a younger animal using a parabiosis model174. As noted before, the reactivation of Wnt in the intestine is capable of rejuvenating aged ISC32. In other organs, it is known that aging promotes the development of progressive fibrosis and ECM deposition linked to a low-grade inflammation (inflammaging), leading to a hindered regenerative capacity in the kidney175–177, the lung178,179, the liver180,181, and the heart182. Indeed, the ECM of the majority of the organs shows age-dependent biochemical and mechanical modifications104,182–185, which suggests a great influence of fibroblast activity and dynamics on the behavior of neighboring cells. Alongside, the secretome of fibroblasts is gradually modified from development to aging, imparting differently on organ function and response to injury. In different organs, fetal and adult fibroblasts have been associated with pro-regenerative and pro-fibrotic phenotypes, respectively. In the skin, fetal dermal fibroblasts favor scarless wound healing by expressing high levels of FGFs and TGF-β1, compared to adult fibroblasts186. In the heart, the transcriptome and epigenome of fetal and adult cardiac fibroblasts have been compared showing that besides being smaller and proliferating faster, fetal fibroblasts express high levels of IL-8 signaling, ephrin receptor signaling, and Notch signalling pathways, compared to adults187. Recently, single-cell RNA-sequencing analysis of mouse hearts at different ages has unveiled that the transition from neonatal to adult fibroblast state directly supports cardiomyocyte maturation188. The same report showed that, in in vitro co-culture studies, adult fibroblasts reduce immature cardiomyocyte proliferation and promote their electrophysiological maturation. The composition of fetal/neonatal and adult fibroblast-derived ECM is also different and young ECM has shown to be a preferable environment to maintain cardiac cells, compared to the adult counterpart. In addition, the composition and stiffness of the ECM has been show to directly influence the regenerative capacity of the neonatal heart189. Collectively, this evidence supports that, in two organs with such different regeneration potentials, younger fibroblasts provide pro-regenerative cues when compared to adult fibroblasts.
Aging is often related to cellular senescence190. Several reports on fibroblasts and aging indicate that fibroblast senescence is one of the underlying causes of poor regenerative capacity and of the formation of extensive fibrosis. Compared to younger counterparts, aged dermal fibroblasts show features of cell senescence, such as senescence-associated β-galactosidase, are less responsive to TGF-β activation and migrate less, ultimately leading to slower wound healing191. In idiopathic pulmonary fibrosis, which is more common in the elderly, lung fibroblasts express more α-SMA, collagen I, and secrete high levels of inflammatory cytokines192. A report of induced senescence in the lung shows that lung fibroblasts have decreased expression of collagen I, elastin, and fibronectin, which are important for alveologenesis193. In the heart, aging fibroblasts are pro-inflammatory and express Serpine1 and Serpine2, promoting age-associated endothelial dysfunction194. In the intestine, senescent fibroblasts secrete growth differentiation factor 15 (GDF15), a senescence-associated factor, which stimulates dysregulated epithelial cell proliferation, migration, and, ultimately, tumor formation195. On the other hand, other reports propose that cell senescence, occurring at least transiently, is key to limit fibrosis196–198. In the heart, senescent fibroblasts accumulate in the injury area after myocardial infarction and limit collagen production. Knocking down the senescence marker p53 resulted in reduced inflammation, which leads to the conclusion that cardiac fibroblast senescence is beneficial if limited in time199. In the liver, HS cell senescence and further clearing by immune cells is reported to limit fibrosis, with reduced secretion of ECM and increased ECM degradation94. Transient senescence after injury is beneficial by limiting fibrosis likely because it targets mostly myofibroblasts. This scenario contrasts with age-associated chronic senescence, in which homeostatic fibroblasts are the primary targets, and that ultimately leads to the loss of regenerative capacity, aberrant ECM deposition and organ fibrosis200.
In sum, the axis of repair-regeneration mediated by fibroblast activity in different organs seems to be profoundly affected by age (Fig. 2). The younger the organism, the better fibroblasts maintain homeostasis and respond to injury in a pro-regenerative fashion. Older fibroblasts appear to be heterogeneous and respond with varying healing rates to reprogramming and wounding201. Senescence, typically associated to aging, may be beneficial to regeneration, but only if limited in time. Furthermore, evidence that most regenerative ECM components are typically expressed by fetal/neonatal fibroblasts supports the view that young ECM is a pro-regenerative environment whereas the adult/aged ECM supports pro-reparative/fibrotic responses. In fact, most pro-regenerative ECM-related molecules mentioned above decrease their expression with aging (Table 1). Overall, whilst the beneficial effect of fibroblasts in regeneration is emerging in different organs, most studies fail to demonstrate causality. In this context, future studies addressing the impact of fibroblast abrogation (e.g., through genetic models, as performed in the heart202) on the regenerative response will be important to provide comprehensive understanding on the role of these cells in organ renewal.
Fig. 2. Correlation of the regenerative potential of organs with aging.
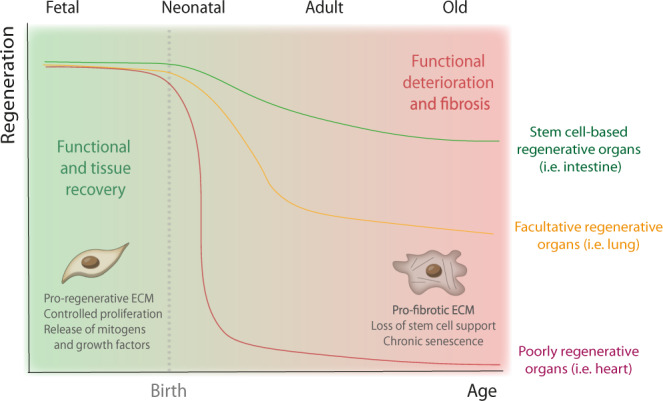
Whilst less regenerative organs, as the heart, decrease their regenerative capacity abruptly after birth, regenerative organs, such as the skin and intestine, experience a progressive decline in their renewal capacity with increasing age. Organs like the lung depend on specific signals to mount a regenerative response—facultative regeneration—however, this ability is also impaired in the elderly. The loss of regenerative capacity in most organs seem to associate with the transition from a “young” to an “old” fibroblast phenotype. Whilst young fibroblasts are pro-regenerative and promote healthy tissue recovery, aged fibroblasts fail to support the regenerative niche, ultimately contributing to fibrosis and loss of organ function.
Table 1.
ECM-associated molecules207 involved in the regeneration in in vivo studies of different organs and their transcriptional regulation with age.
| Organ | Function | Expression in aging | Model | Refs. | |
|---|---|---|---|---|---|
| Core matrisome | |||||
| Agrin | Heart | Promotes CM proliferation in regenerative hearts | ↓ | Mouse | 163 |
| Chondroitin sulfate/aggrecan | Skin | Enhances wound closure in oral mucosa and in Acomys | ↓ | Acomys, Human | 63,208 |
| Collagens | Liver | Collagen I: may induce progenitor cell differentiation | N/A | Mouse | 101 |
| Skin | Collagen III: favors cell migration in fetal wound healing | ↓ | Mouse, Rat | 209,210 | |
| CTHRC-1 | Skin | Promotes recruitment of M2 macrophages | N/A | Mouse | 64 |
| Elastin | Skin | Downregulated in oral mucosa | ↑ | Human | 208 |
| Lung | Present in alveolar septation | ↑ | Mouse | 211 | |
| Fibronectin | Skin | ED-A form: likely influences cell migration in oral mucosa | ↓ | Human, Rat | 208,212 |
| Liver | Induces cell proliferation and progenitor differentiation | ↓ | Mouse | 101,213 | |
| Lung | Promotes endothelial cell adhesion | ↓ | Rabbit | 111,214 | |
| Heart | Favors fibroblast migration and CM proliferation in zebrafish | ↓ | Rat, Zebrafish | 160,215 | |
| Hyaluronic acid | Skin | Promotes cell migration in scarless wound healing | ↓ | Human, Rabbit | 216,217 |
| Heart | Supports cell migration | N/A | Newt | 165 | |
| Laminins | Skin | Laminin α1: present in regenerative Acomys skin | N/A | Acomys | 63 |
| Olfactomedin | Liver | Promotes cell migration and adhesion | N/A | Mouse | 101 |
| Periostin | Intestine | Regulates epithelium proliferation | N/A | Human, Mouse | 34 |
| Heart | Promotes fibroblast migration in P1 regenerative hearts | ↓ | Mouse | 218 | |
| Tenascin-C | Skin | May help promote fibroblast and epithelial cell migration | ↓ | Human | 73,219 |
| Heart | Influences newt CM proliferation in vitro | ↓ | Mouse, Newt | 165,220 | |
| Vitronectin | Liver | Signals to increase in cell migration and proliferation | N/A | Rat | 101,221 |
| ECM affiliated proteins | |||||
| Semaphorin 3 | Intestine | Supports epithelial growth | ↓ | Mouse, Rat | 21,222 |
| ECM regulators | |||||
| MMPs | Skin | Increased MMP/TIMP ratio in fetal wound healing supports matrix degradation | ↓ | Rat | 223 |
| Liver | MMP-1, 2: promote ECM remodeling | = | N/A | 224 | |
| Secreted factors | |||||
| Angiopoietin | Liver | Promotes angiogenesis | N/A | Rat | 225 |
| Epimorphin | Liver | Stimulates hepatocyte proliferation | N/A | Mouse | 226 |
| Erythropoietin | Liver | Promotes angiogenesis | ↓ | Human | 227 |
| FGF-1 | Heart | Induces CM cell-cycle re-entry | N/A | Mouse | 171 |
| HGF | Intestine | Promotes cell proliferation | N/A | Mouse | 21 |
| Liver | Promotes cell proliferation | N/A | Mouse | 228 | |
| Kidney | Promotes cell proliferation and TGF-β antagonization | N/A | Mouse | 137 | |
| Pleiotrophin | Liver | Stimulates hepatocyte proliferation | N/A | Rat | 229 |
| Shh | Skin | Promotes hair follicle maintenance | ↓ | Mouse | 72,230 |
| VEGF | Liver | Promotes angiogenesis | ↓ | In vitro rat HS | 231,232 |
| Wnt | Intestine | Promotes stem cell niche maintenance | ↓ | Mouse | 26,29,32 |
| Skin | Promotes stem cell niche maintenance | ↓ | Mouse | 71,233 | |
N/A information not available.
Concluding remarks
Fibroblasts remain the main culprits of fibrotic diseases in most organs. The phenotypic heterogeneity of these cells is stressed by the observation that fibroblast markers in different organs mostly do not overlap. Yet, PDGFRα is present in resident fibroblasts from most organs. PDGFRα+ fibroblasts can differentiate into adipocytes and myofibroblasts in various organs, such as the lung and the heart113,203. All reported fibroblast markers also do not distinguish pro-fibrotic from pro-regenerative populations across organs and within the organ itself, although recent reports on synovial fibroblasts suggest the existence of discrete subpopulations of fibroblasts with opposite functions based on the expression of a single marker204,205. In the future, data collected from single-cell RNA-sequencing studies across organs will be of utmost importance to define the signature of homeostatic, pro-fibrotic, and pro-regenerative fibroblasts.
Apart from the well-known role of fibroblasts in tissue ECM maintenance in homeostasis and remodeling after injury, a new perspective is emerging on the pro-regenerative role of resident fibroblasts. In this context, they may act in a cell-intrinsic manner or by modulating the microenvironment of the stem cell niche, when existing, by: (i) producing matricellular components, namely growth factors or mitogens, (ii) activating signaling pathways (e.g., Wnt, Hedgehog, retinoic acid), (iii) proliferating in early and restricted time-period of injury, or (v) creating the scaffold that guides regeneration and immunomodulation.
Importantly, fibroblast-mediated regeneration seems to rely on the activation of embryonic/neonatal gene expression programs across different organs. Based on this evidence one can hypothesize that reverting the adult fibroblast/myofibroblast phenotype to a fetal or neonatal stage could be a promising therapeutic avenue that can be applied to different organs. Recently, a proof-of-principle study showed that in vitro rejuvenation of fibroblasts is achievable and reverted the fibroblast aging phenotype, which can be useful for regenerative medicine purposes206.
Acknowledgements
The authors would like to acknowledge Vasco Sampaio-Pinto and Perpétua Pinto-do-Ó for critical manuscript revision. This work was funded by the European Regional Development Fund (ERDF) through COMPETE 2020, Portugal 2020 and by FCT (Fundação para a Ciência e Tecnologia, ([POCI-01-0145-FEDER-030985 and POCI-01-0145-FEDER-031120] and); by FCT/Ministério da Ciência, Tecnologia e Inovação in the framework of individual funding [CEECINST/00091/2018] to D.S.N. and individual [SFRH/BD/144490/2019] to R.N.G.
Author contributions
R.N.G. designed, drafted, and revised the manuscript. F.M. designed, drafted, and revised the manuscript. D.S.N. designed, drafted, and revised the manuscript. All authors have approved the submission of this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/7/2023
A Correction to this paper has been published: 10.1038/s41536-023-00319-x
References
- 1.Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez AC, et al. Wound healing—a literature review. Bras. Dermatol. 2016;91:614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swonger JM, Liu JS, Ivey MJ, Tallquist MD. Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation. 2016;92:66–83. doi: 10.1016/j.diff.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv. Wound Care. 2013;2:215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, et al. The role of interleukin-1 in wound biology. Part II: in vivo and human translational studies. Anesth. Analg. 2010;111:1534–1542. doi: 10.1213/ANE.0b013e3181f691eb. [DOI] [PubMed] [Google Scholar]
- 9.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 10.Carlo SEDi, Peduto L. The perivascular origin of pathological fibroblasts. J. Clin. Invest. 2018;128:54–63. doi: 10.1172/JCI93558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun J-I, Lau LF. Resolution of organ fibrosis. J. Clin. Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aghajanian H, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balestrini JL, Niklason LE. Extracellular matrix as a driver for lung regeneration. Ann. Biomed. Eng. 2015;43:568–576. doi: 10.1007/s10439-014-1167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal MB. Hepatic stellate cells: fibrogenic, regenerative or both? Heterogeneity and context are key. Hepatol. Int. 2016;10:902–908. doi: 10.1007/s12072-016-9758-x. [DOI] [PubMed] [Google Scholar]
- 16.Hortells L, Johansen AKZ, Yutzey KE. Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J. Cardiovasc. Dev. Dis. 2019;6:29. doi: 10.3390/jcdd6030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 18.Meran L, Baulies A, Li VSW. Intestinal stem cell niche: the extracellular matrix and cellular components. Stem Cells Int. 2017;2017:7970385. doi: 10.1155/2017/7970385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastuła A, Marcinkiewicz J. Cellular Interactions in the intestinal stem cell niche. Arch. Immunol. Ther. Exp. (Warsz.). 2019;67:19–26. doi: 10.1007/s00005-018-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westendorp BF, et al. Indian Hedgehog suppresses a stromal cell-driven intestinal immune response. Cell. Mol. Gastroenterol. Hepatol. 2018;5:67–82.e1. doi: 10.1016/j.jcmgh.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpus ON, et al. Colonic CD90+ crypt fibroblasts secrete semaphorins to support epithelial growth. Cell Rep. 2019;26:3698–3708.e5. doi: 10.1016/j.celrep.2019.02.101. [DOI] [PubMed] [Google Scholar]
- 22.Kurashima Y, et al. Mucosal mesenchymal cells: secondary barrier and peripheral educator for the gut immune system. Front. Immunol. 2017;8:1787. doi: 10.3389/fimmu.2017.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi Y, et al. Gastrointestinal fibroblasts have specialized, diverse transcriptional phenotypes: a comprehensive gene expression analysis of human fibroblasts. PLoS ONE. 2015;10:e0129241–e0129241. doi: 10.1371/journal.pone.0129241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosinski C, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl Acad. Sci. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valenta T, et al. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15:911–918. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 27.Kinchen J, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho G, Cartwright JA, Thompson EJ, Bain CC, Rossi AG. Resolution of inflammation and gut repair in IBD: translational steps towards complete mucosal healing. Inflamm. Bowel Dis. 2020;26:1131–1143. doi: 10.1093/ibd/izaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greicius G, et al. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl Acad. Sci. 2018;115:E3173–E3181. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harnack C, et al. R-spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat. Commun. 2019;10:4368. doi: 10.1038/s41467-019-12349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pentinmikko N, et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571:398–402. doi: 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalapareddy K, et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017;18:2608–2621. doi: 10.1016/j.celrep.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Göke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am. J. Physiol. Liver Physiol. 1998;274:G809–G818. doi: 10.1152/ajpgi.1998.274.5.G809. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi Y, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J. Histochem. Cytochem. 2008;56:753–764. doi: 10.1369/jhc.2008.951061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roulis M, et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc. Natl Acad. Sci. 2014;111:E4658–E4667. doi: 10.1073/pnas.1415762111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, DuBois RN. Fibroblasts fuel intestinal tumorigenesis. Cell Res. 2020;30:635–636. doi: 10.1038/s41422-020-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015;5:a023267. doi: 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br. J. Dermatol. 2002;147:230–243. doi: 10.1046/j.1365-2133.2002.04871.x. [DOI] [PubMed] [Google Scholar]
- 39.Russo B, Brembilla NC, Chizzolini C. Interplay between keratinocytes and fibroblasts: a systematic review providing a new angle for understanding skin fibrotic disorders. Front. Immunol. 2020;11:648. doi: 10.3389/fimmu.2020.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Ghalbzouri A, Ponec M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004;12:359–367. doi: 10.1111/j.1067-1927.2004.012306.x. [DOI] [PubMed] [Google Scholar]
- 41.Stunova A, Vistejnova L. Dermal fibroblasts-a heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018;39:137–150. doi: 10.1016/j.cytogfr.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Rognoni E, et al. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018;14:e8174. doi: 10.15252/msb.20178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thulabandu V, Chen D, Atit RP. Dermal fibroblast in cutaneous development and healing. Wiley Interdisci. Rev. Dev. Biol. 2018;7:e307. doi: 10.1002/wdev.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korosec A, et al. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Invest. Dermatol. 2019;139:342–351. doi: 10.1016/j.jid.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 45.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 46.Jiang D, Rinkevich Y. Scars or regeneration?-Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 2020;21:617. doi: 10.3390/ijms21020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rippa AL, Kalabusheva EP, Vorotelyak EA. Regeneration of dermis: scarring and cells involved. Cells. 2019;8:607. doi: 10.3390/cells8060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philippeos C, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 2018;138:811–825. doi: 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janson D, Rietveld M, Mahé C, Saintigny G, El Ghalbzouri A. Differential effect of extracellular matrix derived from papillary and reticular fibroblasts on epidermal development in vitro. Eur. J. Dermatol. 2017;27:237–246. doi: 10.1684/ejd.2017.2984. [DOI] [PubMed] [Google Scholar]
- 50.Haydont V, Neiveyans V, Zucchi H, Fortunel NO, Asselineau D. Genome-wide profiling of adult human papillary and reticular fibroblasts identifies ACAN, Col XI α1, and PSG1 as general biomarkers of dermis ageing, and KANK4 as an exemplary effector of papillary fibroblast ageing, related to contractility. Mech. Ageing Dev. 2019;177:157–181. doi: 10.1016/j.mad.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS ONE. 2009;3:1–13. doi: 10.1371/journal.pone.0004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodley DT. Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol. Clin. 2017;35:95–100. doi: 10.1016/j.det.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch. A. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 54.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somasundaram K, Prathap K. Intra-uterine healing of skin wounds in rabbit foetuses. J. Pathol. 1970;100:81–86. doi: 10.1002/path.1711000202. [DOI] [PubMed] [Google Scholar]
- 56.Moore AL, et al. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip. Rev. Dev. Biol. 2018;7:e309. doi: 10.1002/wdev.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rognoni E, et al. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development. 2016;143:2522–2535. doi: 10.1242/dev.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phan QM, et al. Lef1 expression in fibroblasts maintains developmental potential in adult skin to regenerate wounds. Elife. 2020;9:e60066. doi: 10.7554/eLife.60066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glim JE, Everts V, Niessen FB, Ulrich MM, Beelen RHJ. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral. Biol. 2014;59:1048–1055. doi: 10.1016/j.archoralbio.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 60.Muro AF, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyoshi K, Horiguchi T, Tanimura A, Hagita H, Noma T. Gene signature of human oral mucosa fibroblasts: comparison with dermal fibroblasts and induced pluripotent stem cells. Biomed. Res. Int. 2015;2015:121575. doi: 10.1155/2015/121575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seifert AW, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maden M, Brant JO. Insights into the regeneration of skin from Acomys, the spiny mouse. Exp. Dermatol. 2019;28:436–441. doi: 10.1111/exd.13847. [DOI] [PubMed] [Google Scholar]
- 64.Qin S, et al. CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF-β and notch pathways. Biomed. Pharmacother. 2019;113:108594. doi: 10.1016/j.biopha.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 65.Brant JO, Lopez M-C, Baker HV, Barbazuk WB, Maden M. A comparative analysis of gene expression profiles during skin regeneration in Mus and Acomys. PLoS ONE. 2015;10:e0142931. doi: 10.1371/journal.pone.0142931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 67.Wier EM, Garza LA. Through the lens of hair follicle neogenesis, a new focus on mechanisms of skin regeneration after wounding. Semin. Cell Dev. Biol. 2020;100:122–129. doi: 10.1016/j.semcdb.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou L, et al. Activating β-catenin signaling in CD133-positive dermal papilla cells increases hair inductivity. FEBS J. 2016;283:2823–2835. doi: 10.1111/febs.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Telerman SB, et al. Dermal Blimp1 acts downstream of epidermal TGFβ and Wnt/β-catenin to regulate hair follicle formation and growth. J. Invest. Dermatol. 2017;137:2270–2281. doi: 10.1016/j.jid.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim CH, et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 2018;9:4903. doi: 10.1038/s41467-018-07142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75–76:12–26. doi: 10.1016/j.matbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Michalopoulos GK. Liver regeneration. J. Cell. Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kountouras J, Boura P, Lygidakis NJ. Liver regeneration after hepatectomy. Hepatogastroenterology. 2001;48:556–562. [PubMed] [Google Scholar]
- 76.Lu W-Y, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, et al. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front. Pharmacol. 2014;5:167. doi: 10.3389/fphar.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karin D, Koyama Y, Brenner D, Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation. 2016;92:84–92. doi: 10.1016/j.diff.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells RG. The portal fibroblast: not just a poor man’s stellate cell. Gastroenterology. 2014;147:41–47. doi: 10.1053/j.gastro.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dobie R, et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep. 2019;29:1832–1847.e8. doi: 10.1016/j.celrep.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krenkel O, Hundertmark J, Ritz TP, Weiskirchen R, Tacke F. Single cell RNA sequencing identifies subsets of hepatic stellate cells and myofibroblasts in liver fibrosis. Cells. 2019;8:503. doi: 10.3390/cells8050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res. 2017;352:420–426. doi: 10.1016/j.yexcr.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 83.Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim. Biophys. Acta - Mol. Basis Dis. 2013;1832:948–954. doi: 10.1016/j.bbadis.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 85.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 86.Yin C, Evason KJ, Asahina K, Stainier DYR. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin Y, Kong D, He K, Xia Q. Regeneration and activation of liver progenitor cells in liver cirrhosis. Genes Dis. 2020;8:623–628. doi: 10.1016/j.gendis.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swiderska-Syn M, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63:1333–1344. doi: 10.1136/gutjnl-2013-305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omenetti A, et al. Hedgehog-mediated mesenchymal–epithelial interactions modulate hepatic response to bile duct ligation. Lab. Investig. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 90.Lavoie G, Se J, Jhandier MN, Kruglov EA, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J. Biol. Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 91.Nakatani K, et al. Expression of NCAM in activated portal fibroblasts during regeneration of the rat liver after partial hepatectomy. Arch. Histol. Cytol. 2006;69:61–72. doi: 10.1679/aohc.69.61. [DOI] [PubMed] [Google Scholar]
- 92.Campana L, Iredale JP. Regression of liver fibrosis. Semin. Liver Dis. 2017;37:1–10. doi: 10.1055/s-0036-1597816. [DOI] [PubMed] [Google Scholar]
- 93.Marcellin P, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 94.Krizhanovsky V, et al. Senescence of activated stellate. Cells Limits Liver Fibros. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Troeger JS, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–83.e22. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kisseleva T, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. U. S. A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 2006;21:S84–S87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 98.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 99.Murphy FR, et al. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J. Biol. Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 100.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 101.Klaas M, et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 2016;6:27398. doi: 10.1038/srep27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olle EW, et al. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44:540–549. doi: 10.1002/hep.21314. [DOI] [PubMed] [Google Scholar]
- 103.Kim T-H, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 104.Delire B, et al. Aging enhances liver fibrotic response in mice through hampering extracellular matrix remodeling. Aging. 2016;9:98–113. doi: 10.18632/aging.101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salwig I, et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38:e102099. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts: busy multitaskers. Am. J. Pathol. 2016;186:2519–2531. doi: 10.1016/j.ajpath.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hogan BLM, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Branchfield K, et al. A three-dimensional study of alveologenesis in mouse lung. Dev. Biol. 2016;409:429–441. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soucy PA, Romer LH. Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 2009;28:273–283. doi: 10.1016/j.matbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Acciani T, Le Cras T, Lutzko C, Perl A-KT. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell Mol. Biol. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Endale M, et al. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev. Biol. 2017;425:161–175. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Travaglini KJ, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;587:619–625. doi: 10.1038/s41586-020-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Habiel DM, Hogaboam CM. Heterogeneity of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr. Pathobiol. Rep. 2017;5:101–110. doi: 10.1007/s40139-017-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park J, et al. The Tcf21 lineage constitutes the lung lipofibroblast population. Am. J. Physiol. Cell. Mol. Physiol. 2019;316:L872–L885. doi: 10.1152/ajplung.00254.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El Agha E, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261–273.e3. doi: 10.1016/j.stem.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zepp JA, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170:1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie T, et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018;22:3625–3640. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tahedl D, Wirkes A, Tschanz SA, Ochs M, Mühlfeld C. How common is the lipid body-containing interstitial cell in the mammalian lung? Am. J. Physiol. Cell. Mol. Physiol. 2014;307:L386–L394. doi: 10.1152/ajplung.00131.2014. [DOI] [PubMed] [Google Scholar]
- 121.Massaro GDC, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat. Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 122.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- 123.Paxson JA, et al. Global gene expression patterns in the post-pneumonectomy lung of adult mice. Respir. Res. 2009;10:92. doi: 10.1186/1465-9921-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plantier L, Boczkowski J, Crestani B. Defect of alveolar regeneration in pulmonary emphysema: role of lung fibroblasts. Int. J. Chron. Obstruct. Pulmon. Dis. 2007;2:463–469. [PMC free article] [PubMed] [Google Scholar]
- 125.Paxson JA, et al. Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS ONE. 2011;6:e23232. doi: 10.1371/journal.pone.0023232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J. Am. Soc. Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 127.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 128.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thedieck C, et al. Cadherin-9 is a novel cell surface marker for the heterogeneous pool of renal fibroblasts. PLoS ONE. 2007;2:e657. doi: 10.1371/journal.pone.0000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato Y, Yanagita M. Resident fibroblasts in the kidney: a major driver of fibrosis and inflammation. Inflamm. Regen. 2017;37:17. doi: 10.1186/s41232-017-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. 2012;27:183–193. doi: 10.1007/s00467-011-1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tan RJ, Zhou D, Liu Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis. 2016;2:136–144. doi: 10.1159/000446336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schiessl IM, et al. Renal interstitial platelet-derived growth factor receptor-β cells support proximal tubular regeneration. J. Am. Soc. Nephrol. 2018;29:1383–1396. doi: 10.1681/ASN.2017101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Castrop H. The role of renal interstitial cells in proximal tubular regeneration. Nephron. 2019;141:265–272. doi: 10.1159/000496278. [DOI] [PubMed] [Google Scholar]
- 136.Borges FT, et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou D, et al. Early activation of fibroblasts is required for kidney repair and regeneration after injury. FASEB J. 2019;33:12576–12587. doi: 10.1096/fj.201900651RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou D, et al. Fibroblast-specific β-catenin signaling dictates the outcome of AKI. J. Am. Soc. Nephrol. 2018;29:1257–1271. doi: 10.1681/ASN.2017080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakamura J, et al. Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int. 2019;95:526–539. doi: 10.1016/j.kint.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 140.Schievenbusch S, et al. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem. Biophys. Res. Commun. 2009;385:55–61. doi: 10.1016/j.bbrc.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 141.Higashi AY, Aronow BJ, Dressler GR. Expression profiling of fibroblasts in chronic and acute disease models reveals novel pathways in kidney fibrosis. J. Am. Soc. Nephrol. 2019;30:80–94. doi: 10.1681/ASN.2018060644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weber KT, et al. Remodeling and reparation of the cardiovascular system. J. Am. Coll. Cardiol. 1992;20:3–16. doi: 10.1016/0735-1097(92)90130-F. [DOI] [PubMed] [Google Scholar]
- 143.Fu X, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J. Cell. Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moore-Morris T, Cattaneo P, Puceat M, Evans SM. Origins of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ. J. 2016;80:2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Humeres C, Frangogiannis NG. Fibroblasts in the infarcted, remodeling, and failing heart. JACC Basic Transl. Sci. 2019;4:449–467. doi: 10.1016/j.jacbts.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tallquist MD. Cardiac fibroblast diversity. Annu. Rev. Physiol. 2020;82:63–78. doi: 10.1146/annurev-physiol-021119-034527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sampaio-Pinto, V., Silva, A. C., Pinto-do-Ó, P. & Nascimento, D. S. Cardiac regeneration and repair: from mechanisms to therapeutic strategies. in Concepts and Applications of Stem Cell Biology (eds. Rodrigues, G. & Roelen, B. A. J.) 187–211 (Springer International Publishing, 2020).
- 152.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Z, et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc. Natl Acad. Sci. 2019;116:18455–18465. doi: 10.1073/pnas.1905824116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quaife-Ryan GA, et al. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation. 2017;136:1123–1139. doi: 10.1161/CIRCULATIONAHA.117.028252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sampaio-Pinto V, et al. Neonatal apex resection triggers cardiomyocyte proliferation, neovascularization and functional recovery despite local fibrosis. Stem Cell Rep. 2018;10:860–874. doi: 10.1016/j.stemcr.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Z, et al. Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. Cell Rep. 2020;33:108472. doi: 10.1016/j.celrep.2020.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Silva AC, et al. Three-dimensional scaffolds of fetal decellularized hearts exhibit enhanced potential to support cardiac cells in comparison to the adult. Biomaterials. 2016;104:52–64. doi: 10.1016/j.biomaterials.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 158.Silva AC, Pereira C, Fonseca ACRG, Pinto-do-Ó P, Nascimento DS. Bearing my heart: the role of extracellular matrix on cardiac development, homeostasis, and injury response. Front. Cell Dev. Biol. 2021;8:1705. doi: 10.3389/fcell.2020.621644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Porrello ER, Olson EN. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014;13:556–570. doi: 10.1016/j.scr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 2013;382:427–435. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ieda M, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev. Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. U. S. A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bassat E, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Z, et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res. 2017;113:620–632. doi: 10.1093/cvr/cvx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 2013;382:457–469. doi: 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 167.Bhattacharyya S, et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016;7:11703. doi: 10.1038/ncomms11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Forte E, et al. Dynamic interstitial cell response during myocardial infarction predicts resilience to rupture in genetically diverse mice. Cell Rep. 2020;30:3149–3163.e6. doi: 10.1016/j.celrep.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Furtado MB, et al. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ. Res. 2014;114:1422–1434. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Engel FB, Hsieh PCH, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor induces mitosis, 2006. Proc. Natl Acad. Sci. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Palmen M, et al. Fibroblast growth factor-1 improves cardiac functional recovery and enhances cell survival after ischemia and reperfusion: a fibroblast growth factor receptor, protein kinase C, and tyrosine kinase-dependent mechanism. J. Am. Coll. Cardiol. 2004;44:1113–1123. doi: 10.1016/j.jacc.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 172.Engel FB, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Almanzar N, et al. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583:590–595. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 175.Gagliano N, et al. Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney. Cortex. J. Gerontol. Ser. A. 2000;55:B365–B372. doi: 10.1093/gerona/55.8.B365. [DOI] [PubMed] [Google Scholar]
- 176.Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am. J. Pathol. 1995;146:742–752. [PMC free article] [PubMed] [Google Scholar]
- 177.Valentijn FA, Falke LL, Nguyen TQ, Goldschmeding R. Cellular senescence in the aging and diseased kidney. J. Cell Commun. Signal. 2018;12:69–82. doi: 10.1007/s12079-017-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Calabresi C, et al. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp. Gerontol. 2007;42:1003–1011. doi: 10.1016/j.exger.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 179.Navarro S, Driscoll B. Regeneration of the aging lung: a mini-review. Gerontology. 2017;63:270–280. doi: 10.1159/000451081. [DOI] [PubMed] [Google Scholar]
- 180.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr. Opin. Gastroenterol. 2015;31:184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Timchenko NA. Aging and liver regeneration. Trends Endocrinol. Metab. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 182.Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. GeroScience. 2017;39:7–18. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Birch HL. Extracellular matrix and ageing. Subcell. Biochem. 2018;90:169–190. doi: 10.1007/978-981-13-2835-0_7. [DOI] [PubMed] [Google Scholar]
- 184.Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J. Cell Commun. Signal. 2018;12:35–43. doi: 10.1007/s12079-018-0459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Levi N, Papismadov N, Solomonov I, Sagi I, Krizhanovsky V. The ECM path of senescence in aging: components and modifiers. FEBS J. 2020;287:2636–2646. doi: 10.1111/febs.15282. [DOI] [PubMed] [Google Scholar]
- 186.Broker BJ, et al. Comparison of growth factor expression in fetal and adult fibroblasts: a preliminary report. Arch. Otolaryngol. Neck Surg. 1999;125:676–680. doi: 10.1001/archotol.125.6.676. [DOI] [PubMed] [Google Scholar]
- 187.Jonsson MKB, et al. A transcriptomic and epigenomic comparison of fetal and adult human cardiac fibroblasts reveals novel key transcription factors in adult cardiac fibroblasts. JACC Basic Transl. Sci. 2016;1:590–602. doi: 10.1016/j.jacbts.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Y, et al. Single-cell analysis of murine fibroblasts identifies neonatal to adult switching that regulates cardiomyocyte maturation. Nat. Commun. 2020;11:2585. doi: 10.1038/s41467-020-16204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Notari M, et al. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 2018;4:eaao5553. doi: 10.1126/sciadv.aao5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brun C, et al. Phenotypic and functional changes in dermal primary fibroblasts isolated from intrinsically aged human skin. Exp. Dermatol. 2016;25:113–119. doi: 10.1111/exd.12874. [DOI] [PubMed] [Google Scholar]
- 192.Álvarez D, et al. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. Cell. Mol. Physiol. 2017;313:L1164–L1173. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Woldhuis RR, et al. Link between increased cellular senescence and extracellular matrix changes in COPD. Am. J. Physiol. Cell. Mol. Physiol. 2020;319:L48–L60. doi: 10.1152/ajplung.00028.2020. [DOI] [PubMed] [Google Scholar]
- 194.Vidal R, et al. Transcriptional heterogeneity of fibroblasts is a hallmark of the aging heart. JCI Insight. 2019;4:e131092. doi: 10.1172/jci.insight.131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guo Y, et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell. 2019;18:e13013. doi: 10.1111/acel.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jun J-I, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging. 2010;2:627–631. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Meng X, Wang H, Song X, Clifton AC, Xiao J. The potential role of senescence in limiting fibrosis caused by aging. J. Cell. Physiol. 2020;235:4046–4059. doi: 10.1002/jcp.29313. [DOI] [PubMed] [Google Scholar]
- 198.Rhinn M, Ritschka B, Keyes WM. Cellular senescence in development, regeneration and disease. Development. 2019;146:dev151837. doi: 10.1242/dev.151837. [DOI] [PubMed] [Google Scholar]
- 199.Zhu F, et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE. 2013;8:e74535. doi: 10.1371/journal.pone.0074535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kumar A, Bano D, Ehninger D. Cellular senescence in vivo: from cells to tissues to pathologies. Mech. Ageing Dev. 2020;190:111308. doi: 10.1016/j.mad.2020.111308. [DOI] [PubMed] [Google Scholar]
- 201.Mahmoudi S, et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature. 2019;574:553–558. doi: 10.1038/s41586-019-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ivey MJ, Kuwabara JT, Riggsbee KL, Tallquist MD. Platelet-derived growth factor receptor-α is essential for cardiac fibroblast survival. Am. J. Physiol. Circ. Physiol. 2019;317:H330–H344. doi: 10.1152/ajpheart.00054.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Raffaella L, et al. Cardiac fibro-adipocyte progenitors express desmosome proteins and preferentially differentiate to adipocytes upon deletion of the desmoplakin gene. Circ. Res. 2016;119:41–54. doi: 10.1161/CIRCRESAHA.115.308136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wei K, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 2020;582:259–264. doi: 10.1038/s41586-020-2222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Croft AP, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Roy B, et al. Fibroblast rejuvenation by mechanical reprogramming and redifferentiation. Proc. Natl Acad. Sci. 2020;117:10131–10141. doi: 10.1073/pnas.1911497117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hynes RO, Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coolen NA, Schouten KCWM, Middelkoop E, Ulrich MMW. Comparison between human fetal and adult skin. Arch. Dermatol. Res. 2010;302:47–55. doi: 10.1007/s00403-009-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1988;187:493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- 210.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194:25–37. doi: 10.1159/000322399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Luo Y, et al. Spatial and temporal changes in extracellular elastin and laminin distribution during lung alveolar development. Sci. Rep. 2018;8:8334. doi: 10.1038/s41598-018-26673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J. Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Baloch Z, Klapper J, Buchanan L, Schwartz M, Amenta PS. Ontogenesis of the murine hepatic extracellular matrix: an immunohistochemical study. Differentiation. 1992;51:209–218. doi: 10.1111/j.1432-0436.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 214.Sinkin RA, Sanders RS, Horowitz S, Finkelstein JN, Lomonaco MB. Cell-specific expression of fibronectin in adult and developing rabbit lung. Pediatr. Res. 1995;37:189–195. doi: 10.1203/00006450-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 215.Farhadian F, et al. Fibronectin expression during physiological and pathological cardiac growth. J. Mol. Cell. Cardiol. 1995;27:981–990. doi: 10.1016/0022-2828(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 216.Longaker MT, et al. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann. Surg. 1989;210:667–672. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mast BA, et al. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11:63–68. doi: 10.1016/S0934-8832(11)80228-3. [DOI] [PubMed] [Google Scholar]
- 218.Snider P, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ. Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal. 2009;3:287–310. doi: 10.1007/s12079-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Imanaka-Yoshida K, Aoki H. Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front. Physiol. 2014;5:283. doi: 10.3389/fphys.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sano K, Asanuma-Date K, Arisaka F, Hattori S, Ogawa H. Changes in glycosylation of vitronectin modulate multimerization and collagen binding during liver regeneration. Glycobiology. 2007;17:784–794. doi: 10.1093/glycob/cwm031. [DOI] [PubMed] [Google Scholar]
- 222.Gonzales J, et al. Semaphorin 3A controls enteric neuron connectivity and is inversely associated with synapsin 1 expression in Hirschsprung disease. Sci. Rep. 2020;10:15119. doi: 10.1038/s41598-020-71865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dang CM, et al. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast. Reconstr. Surg. 2003;111:2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- 224.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sato T, et al. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J. Hepatol. 2001;34:690–698. doi: 10.1016/S0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 226.Watanabe S, et al. A novel hepatic stellate (Ito) cell-derived protein, epimorphin, plays a key role in the late stages of liver regeneration. Biochem. Biophys. Res. Commun. 1998;250:486–490. doi: 10.1006/bbrc.1998.9339. [DOI] [PubMed] [Google Scholar]
- 227.Dame C, et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood. 1998;92:3218–3225. doi: 10.1182/blood.V92.9.3218. [DOI] [PubMed] [Google Scholar]
- 228.Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology. 1992;16:1227–1235. [PubMed] [Google Scholar]
- 229.Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ. Cellular distribution of the new growth factor pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat. Embryol. 1992;186:387–406. doi: 10.1007/BF00185989. [DOI] [PubMed] [Google Scholar]
- 230.Dashti M, Peppelenbosch MP, Rezaee F. Hedgehog signalling as an antagonist of ageing and its associated diseases. BioEssays. 2012;34:849–856. doi: 10.1002/bies.201200049. [DOI] [PubMed] [Google Scholar]
- 231.Holmes DIR, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol. 2005;6:209. doi: 10.1186/gb-2005-6-2-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 233.Makrantonaki E, et al. Identification of biomarkers of human skin ageing in both genders. Wnt signalling—a label of skin ageing? PLoS ONE. 2012;7:e50393. doi: 10.1371/journal.pone.0050393. [DOI] [PMC free article] [PubMed] [Google Scholar]