Abstract
Small GTPases of the Ras superfamily, which include Ras-, Rho-, Rab-, Arf-, and Ran-family isoforms, are generally known to function as a nucleotide-dependent molecular switch in eukaryotic cells. In the GTP-loaded forms, they selectively recruit their cognate interacting proteins or protein complexes, termed “effectors,” to the cytoplasmic face of subcellular membrane compartments, thereby switching on the downstream effector functions, which are vital for fundamental cellular events, such as cell proliferation, cytoskeletal organization, and intracellular membrane trafficking. Nevertheless, in addition to acting as the classic nucleotide-dependent switches for the effectors, recent studies have uncovered that small GTPases themselves can be self-assembled specifically into homo-dimers or higher-order oligomers on membranes, and these assembly processes are likely responsible for their physiological functions. This Review focuses particularly on the self-assembly processes of Rab- and Arf-family isoforms during membrane tethering, the most critical step to ensure the fidelity of membrane trafficking. A summary of the current experimental evidence for self-assemblies of Rab and Arf small GTPases on lipid bilayers in chemically defined reconstitution system is provided
Keywords: Rab, Arf, Ras, Small GTPase, Membrane trafficking, Membrane tethering
Introduction
The Ras (Rat sarcoma) superfamily of small GTPases is composed of over 160 members in human cells, which are further classified into Ras-, Rho (Ras homologous)-, Rab (Ras-like protein in brain)-, Arf (ADP-ribosylation factor)-, and Ran (Ras-like nuclear protein)-subfamily protein isoforms (Bourne et al. 1990; Wennerberg et al. 2005; Rojas et al. 2012; Goitre et al. 2014). These Ras-superfamily small GTPases, for the most part, are small (around 20–35 kDa) monomeric GTP-binding and hydrolyzing proteins that cycle between the active GTP-loaded forms on the cytoplasmic surface of subcellular membrane compartments and the inactive GDP-loaded forms in the cytoplasm (Wennerberg et al. 2005; Goitre et al. 2014). In the GTP-loaded state, membrane-bound small GTPases specifically and physically interact with the cognate partner proteins or protein complexes, termed “effectors,” thereby selectively recruiting the effectors to their appropriate subcellular locations in eukaryotic endomembrane systems (Wennerberg et al. 2005; Goitre et al. 2014). A large number of the cognate effectors for Ras-superfamily small GTPases have been identified and found to be required for a variety of fundamental cellular processes, such as (i) cell proliferation, differentiation, and apoptosis (Ras-family GTPases and Ras effectors) (Karnoub and Weinberg 2008); (ii) cytoskeletal organization, cell adhesion, and cell migration (Rho-family GTPases and Rho effectors) (Heasman and Ridley 2008); and (iii) intracellular membrane trafficking in the secretory and endocytic transport pathways (Rab- and Arf-family GTPases and their effectors) (Stenmark 2009; Donaldson and Jackson 2011). Thus, in general, small GTPases are recognized as a molecular switch that allows the downstream effectors to function in those vital cellular events in a guanine nucleotide-dependent manner (Wennerberg et al. 2005; Goitre et al. 2014). Nevertheless, in addition to acting as the classic molecular switches via interacting with the cognate effectors, recent studies have demonstrated that several isoforms of the Ras superfamily, which include K-Ras/H-Ras (Muratcioglu et al. 2015; Spencer-Smith et al. 2017; Prakash et al. 2017; Zhou et al. 2018; Abankwa and Gorfe 2020; Lee et al. 2020; Van et al. 2021; Packer et al. 2021), endosomal Rabs (Lo et al. 2012; Tamura and Mima 2014; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019; Ueda et al. 2020), and Arf1/Arf6 (Beck et al. 2008; Beck et al. 2011; Diestelkoetter-Bachert et al. 2020; Fujibayashi and Mima 2021), can be specifically self-assembled into the homo-dimers, oligomers, or nanoclusters on membranes, thereby facilitating their physiological functions in eukaryotic cells. Here, this review particularly focuses on the self-assembly processes of Rab- and Arf-family small GTPases in intracellular membrane trafficking and thus summarizes the current experimental evidence for self-assemblies of Rab and Arf proteins on lipid bilayers in an in vitro reconstitution system (Figs. 1 and 2; Mima 2018; Segawa et al. 2019; Ueda et al. 2020; Fujibayashi and Mima 2021). The findings on self-assemblies of Rab and Arf small GTPases in trans between two distinct opposing membranes have expanded our understanding of the molecular basis of membrane tethering, which is a process of the initial physical contact between transport carriers (or vesicles) and their target subcellular compartments, conferring the compartmental specificity of intracellular membrane trafficking (Waters and Pfeffer 1999; Yu and Hughson 2010; Gillingham and Munro 2019).
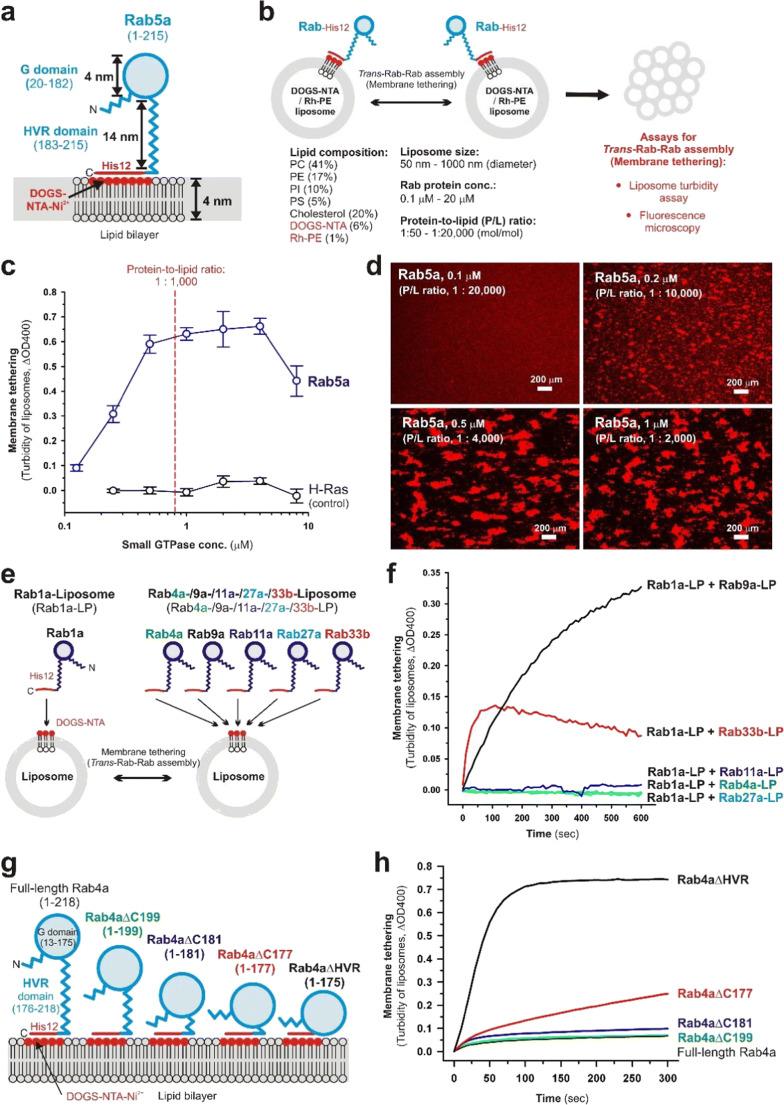
Fig. 1.
Self-assemblies of Rab small GTPases directly tether two distinct lipid bilayers. a Schematic representation of the membrane-anchored form of human Rab5a, which consists of 215 amino acid residues containing the globular Ras-superfamily GTPase domain (G domain; residues 20–182) and the flexible C-terminal hypervariable region domain (HVR domain; residues 183–215), in the in vitro chemically defined system reconstituted from purified recombinant Rab5a proteins with an artificial C-terminal polyhistidine tag (Rab5a-His12) and synthetic liposomes containing DOGS-NTA-Ni2+ lipids. b Schematic representation of reconstituted liposome tethering assays for investigating trans-Rab-Rab assemblies on lipid bilayers (liposome turbidity assay, fluorescence microscopy), showing the typical experimental conditions used. c Liposome turbidity assays for testing the intrinsic potency of human Rab5a and H-Ras (used as the negative control) to trigger the self-assemblies in trans, thereby physically tethering two distinct liposomal membranes. Purified Rab5a-His12 and H-Ras-His12 proteins were incubated with DOGS-NTA-bearing liposomes (400 nm in diameter) and then assayed for turbidity changes by measuring the optical density at 400 nm (ΔOD400; Mima 2018; Segawa et al. 2019). d Fluorescence microscopic imaging assays for Rab5a-mediated membrane tethering via trans-Rab-Rab self-assemblies. Rab5a-His12 proteins were incubated with the fluorescence-labeled liposomes (800 nm in diameter) bearing rhodamine-PE and DOGS-NTA-Ni2+ lipids and subjected to fluorescence microscopic observations of the rhodamine-labeled liposome clusters obtained (Mima 2018; Segawa et al. 2019). e Schematic representation of reconstituted membrane tethering assays for testing the heterotypic pairs of Rab-anchored liposomes, including Rab1a-liposomes (Rab1a-LP) plus Rab4a-LP, Rab9a-LP, Rab11a-LP, Rab27a-LP, and Rab33b-LP. f Heterotypic trans-assemblies of human Rab small GTPases drive reconstituted membrane tethering. Pre-incubated Rab1a-anchored liposomes (Rab1a-LP; 400 nm in diameter) were mixed with pre-incubated Rab4a-LP, Rab9a-LP, Rab11a-LP, Rab27a-LP, and Rab33b-LP (400 nm in diameter) and subsequently assayed for turbidity changes by measuring the optical density at 400 nm (ΔOD400; Segawa et al. 2019). g Schematic representation of the wild-type form and the C-terminal HVR-truncated and HVR-deleted mutant forms of human Rab4a, which include full-length Rab4a (residues 1–218), Rab4aΔC199 (residues 1–199), Rab4aΔC181 (residues 1–181), Rab4aΔC177 (residues 1–177), and Rab4aΔHVR (residues 1–175). h The flexible C-terminal HVR linker controls the intrinsic tethering potency of the globular Rab G domain. Purified full-length, HVR-truncated, and HVR-deleted Rab4a proteins with a polyhistidine tag (His12) at the C-terminus were incubated with DOGS-NTA-bearing liposomes (400 nm in diameter) and assayed for turbidity changes by measuring the optical density at 400 nm (ΔOD400; Ueda et al. 2020).
Fig. 2.
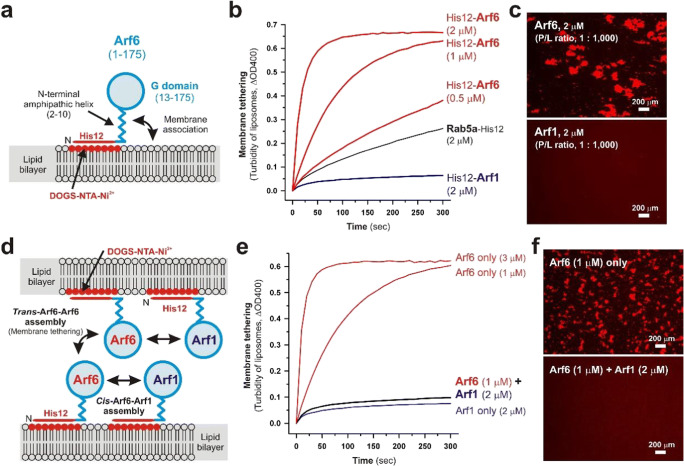
Self-assemblies of Arf small GTPases in trans and cis configurations on lipid bilayers. a Schematic representation of the membrane-anchored form of human Arf6, which consists of 175 residues containing the N-terminal amphipathic helix (residues 2–10) that can directly associate with lipid bilayers and the Ras-superfamily G domain (residues 13–175), in the chemically defined reconstitution system using purified recombinant Arf6 proteins with an N-terminal His12 tag (His12-Arf6) and synthetic liposomes bearing DOGS-NTA-Ni2+ lipids. b Human Arf6, but not Arf1, can act as a bona fide membrane tether, directly and physically tethering two distinct lipid bilayers. Purified His12-Arf6, His12-Arf1, and Rab5a-His12 (used as the positive control) were mixed with DOGS-NTA-bearing liposomes (200 nm in diameter; 1 mM lipids) and assayed for turbidity changes by measuring the optical density at 400 nm (ΔOD400; Fujibayashi and Mima 2021). c Fluorescence microscopic observations of liposome clusters induced by trans-Arf-Arf self-assemblies. His12-Arf6 (upper) and His12-Arf1 (lower) proteins were incubated with the fluorescence-labeled liposomes (200 nm in diameter; 2 mM lipids) baring rhodamine-PE and DOGS-NTA-Ni2+ lipids and then subjected to fluorescence microscopy (Fujibayashi and Mima 2021). d Schematic representation of trans-Arf6-Arf6 assemblies between two distinct opposing membranes, which drive membrane tethering, and heterotypic cis-Arf6-Arf1 assemblies on one membrane, which can prevent tethering-competent Arf6-Arf6 assemblies in trans. e Intrinsic tethering activity of Arf6 can be blocked by the presence of membrane-bound Arf1. Liposome turbidity assays were employed by incubating DOGS-NTA-bearing liposomes (200 nm in diameter; 1 mM lipids) with His12-Arf6 only, His12-Arf1 only, or both His12-Arf6 and His12-Arf1 (Fujibayashi and Mima 2021). f Fluorescence microscopy for testing the inhibitory effect of membrane-bound Arf1 on Arf6-mediated membrane tethering. Rhodamine-PE/DOGS-NTA-bearing liposomes (200 nm in diameter; 2 mM lipids) were incubated in the presence of Arf6 only (upper) or both Arf6 and Arf1 (lower) and then subjected to fluorescence microscopic observations of the liposome clusters formed (Fujibayashi and Mima 2021).
Self-assemblies of Rab-family small GTPases in trans between two distinct lipid bilayers
Rab-family small GTPases, constituting the largest branch of the Ras superfamily which includes over 60 Rab isoforms in human cells (Rojas et al. 2012), specifically localize to the cytoplasmic surface of their appropriate subcellular compartments in the GTP-loaded forms, interact with their cognate Rab effectors (e.g., myosin motor proteins, coiled-coil tethering proteins, and multisubunit tethering complexes) on the membrane surfaces and then cooperate with these effectors to achieve the sequential and fundamental steps of membrane trafficking: cytoskeletal transport, membrane tethering, membrane docking, and membrane fusion (Bonifacino and Glick 2004; Grosshans et al. 2006; Stenmark 2009; Hutagalung and Novick 2011). Along with the globular conserved Ras-superfamily GTPase domains (G domains; 160–170 residues; Fig. 1a) that bind guanine nucleotides and the effectors, Rab-family isoforms share the flexible C-terminal hypervariable region (HVR) domains (20–50 residues; Fig. 1a) that are post-translationally modified at the extreme C-terminal cysteine residues with isoprenyl lipid groups, thereby linking the globular Rab G domains to lipid bilayers through insertion of the lipid anchors (Hutagalung and Novick 2011). The membrane-bound forms of native Rab small GTPases were recapitulated in the chemically defined reconstitution systems using recombinant Rab proteins with a polyhistidine tag at the C-terminus (Rab-His12) and synthetic liposomal membranes bearing DOGS-NTA-Ni2+ (1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl) iminodiacetic acid]-succinyl}-Ni2+) lipids that specifically and stably capture the C-terminal His12 tails (Fig. 1a, b; Mima 2018). To further reconstitute the physiologically relevant state of membrane-anchored Rab proteins, liposomes were prepared with the lipid mixes containing five major lipid species, including PC (phosphatidylcholine), PE (phosphatidylethanolamine), PI (phosphatidylinositol), PS (phosphatidylserine), and cholesterol, using an extrusion method with 50- to 1000-nm pore-size filters (Fig. 1b; Mima 2018). This roughly mimicked the physiological lipid compositions (van Meer et al. 2008; Vance 2015; Yang et al. 2018) and sizes (Klumperman and Raposo 2014; Nguyen et al. 2017) of subcellular compartments in mammalian cells. Under these experimental conditions, the intrinsic capacities of Rab-family small GTPases to tether lipid bilayers via the self-assemblies in trans have been quantitatively evaluated by the two independent reconstituted tethering assays, turbidity assays, and fluorescence microscopic imaging assays (Fig. 1b; Tamura and Mima 2014; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019; Ueda et al. 2020).
By employing the reconstituted tethering assays for a number of representative human Rab-family isoforms functioning in the secretory and endocytic transport pathways (Rab1a, -2a, -3a, -4a, -5a, -6a, -7a, -9a, -11a, -14, -27a, and -33b), all the Rab isoforms, except for Rab27a, have been shown to exhibit their intrinsic tethering activities even in the absence of any other protein components associated with Rab-family GTPases, such as long coiled-coil tethering factors, multisubunit tethering complexes, and other types of Rab effectors (Tamura and Mima 2014; Inoshita and Mima 2017; Segawa et al. 2019). In particular, Rab5a, which is localized at early endosomes, clathrin-coated vesicles, and the plasma membrane, was found to be the most active, tethering-competent Rab isoform among those Rab-family isoforms tested (Segawa et al. 2019). When tested at a wide range of the Rab protein-to-lipid molar ratios from 1:100 to 1:6,400 (mol/mol) in turbidity assays (Fig. 1c) and also at the molar ratios from 1:2,000 to 1:20,000 in fluorescence microscopy (Fig. 1d), Rab5a was able to exhibit rapid and efficient tethering of liposomal membranes through the self-assemblies in trans at the ratios of 1:1,000–1:2,000 and further retain its significant tethering activities even at the ratios of 1:5,000–1:10,000 or below (Fig. 1c, d; Segawa et al. 2019). As demonstrated previously in comprehensive studies on the surface densities of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) family proteins in reconstituted SNARE-mediated membrane fusion (Ji et al. 2010; Zick et al. 2014; Hernandez et al. 2014), Rab protein densities on lipid bilayers (correlated with Rab-to-lipid molar ratios) are surely critical to reconstituting physiologically relevant Rab-mediated membrane tethering reactions. Although the densities of Rab-family proteins on membrane surfaces in vivo are largely unknown and likely to be variable among subcellular membrane compartments, the physiological Rab-to-lipid molar ratio was estimated from the average copy number of Rab proteins on synaptic vesicles from rat brain (total 25 Rab molecules per vesicle) reported in quantitative proteomic and lipidomic analyses of purified synaptic vesicles (Takamori et al. 2006). Using the copy number of synaptic Rabs (25 Rabs/vesicle; Takamori et al. 2006), the mean outer diameter of synaptic vesicles (42 nm; Takamori et al. 2006), the typical thickness of phospholipid bilayers (4 nm; Nagle and Tristram-Nagle 2000), and the average surface area of phospholipid headgroups (0.65 nm2; Nagle and Tristram-Nagle 2000), the Rab-to-lipid molar ratio of rat brain synaptic vesicles was calculated to be 1:560 (mol/mol; 25 Rab proteins and 14,100 lipids per vesicle; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019; Ueda et al. 2020). In addition, it should also be noted that, assuming that Rab proteins are typically a spherical 25-kDa molecule with a diameter of 4 nm (Erickson 2009), membrane-anchored Rab proteins can occupy only approximately 7% of the outer surface areas of liposomes in the reconstituted tethering assays, when tested at the physiological Rab-to-lipid molar ratio calculated above (Inoshita and Mima 2017; Mima 2018). Thus, experimental data of the reconstituted tethering reactions mediated by Rab5a and other Rab isoforms tested (except Rab27a) reflect that Rab-family small GTPases can exhibit their intrinsic capacities to efficiently and specifically tether two distinct lipid bilayers in the context of a physiologically relevant function (Fig. 1c, d; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019).
Comprehensive reconstitution experiments of Rab-mediated membrane tethering establish that several specific Rab-family isoforms in humans (Rab3a, Rab5a, Rab6a, Rab7a, etc.) can function as a highly potent membrane tether through their trans-assemblies in the “homotypic” arrangements (e.g., Rab5a-Rab5a, Rab6a-Rab6a, and Rab7a-Rab7a) at the physiologically relevant densities of Rab protein molecules on membrane surfaces, whereas some other Rab isoforms appeared to be significantly inefficient (Rab1a, Rab4a, Rab9a, Rab11a, Rab33b) or incompetent (e.g., Rab27a) in initiating “homotypic” Rab-mediated tethering under the same experimental conditions (Tamura and Mima 2014; Inoshita and Mima 2017; Segawa et al. 2019). Considering that intracellular membrane trafficking processes in eukaryotic endomembrane systems involve both “homotypic” and “heterotypic” membrane tethering and fusion events of subcellular compartments (Bonifacino and Glick 2004), it is conceivable that those less-active or inactive Rab-family isoforms in “homotypic” tethering may turn out to be an active membrane tether in a “heterotypic” fashion. Indeed, reconstituted tethering assays for testing the heterotypic combinations of Rab1a plus eight other Rab isoforms in humans revealed that Rab1a, which is primarily localized to the endoplasmic reticulum (ER) and Golgi apparatus compartments, cooperated exclusively with the three Golgi-resident Rab isoforms, Rab6a, Rab9a, and Rab33b, to synergistically promote heterotypic membrane tethering (Fig. 1e, f; Segawa et al. 2019). These heterotypic tethering reactions can be achieved by trans-assemblies of membrane-anchored Rab proteins (Fig. 1e, f; Segawa et al. 2019), as established in Rab-mediated tethering in a homotypic manner (Tamura and Mima 2014; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019). By assaying the combinations of Rab1a-anchored liposomes (Rab1a-LP) plus five distinct liposomes anchoring Rab4a, -9a, -11a, -27a, or -33b (Rab4a-LP, Rab9a-LP, Rab11a-LP, Rab27a-LP, or Rab33b-LP, respectively), Rab1a was shown to selectively recognize and associate in trans with the Golgi-resident Rab9a and -33b isoforms, but not Rab4a, -11a, and -27a isoforms, which are localized at endosomal or lysosomal compartments (Fig. 1e, f; Segawa et al. 2019). Experimental evidence from the chemically defined reconstitution system (Fig. 1e, f) supports the idea that “heterotypic” trans-assemblies of Rab small GTPases can directly drive membrane tethering and thus confer compartmental specificity of the tethering events in intracellular membrane trafficking.
Chemically defined reconstitution studies using synthetic Rab-anchored liposomes reveal that Rab-family small GTPases can directly and physically tether two distinct bilayers via their homotypic and heterotypic trans-assemblies in a physiological context, particularly in terms of the efficiency and specificity (Fig. 1a–f; Tamura and Mima 2014; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019). In spite of such research advances, however, the mechanistic basis of Rab small GTPase-mediated membrane tethering reactions still remains largely unknown. Among the structural features of Rab-family small GTPases (Fig. 1a), the non-conserved C-terminal HVR domains act as a relatively long flexible linker between the globular G domain (Fig. 1a; about 4 nm in diameter; Erickson 2009) and the membrane surface, which is calculated to be approximately 14 nm in length for the HVR domain of Rab5a (Fig. 1a), assuming the contour length of an amino acid of 0.4 nm/aa (Ainavarapu et al. 2007). While earlier cell biology-based studies reported the involvement of the C-terminal HVR linker domains in membrane targeting and intracellular localization of Rab-family small GTPases (Ali et al. 2004; Li et al. 2014), the critical roles of the HVR linkers in Rab-mediated membrane tethering have recently been uncovered by in vitro reconstitution experiments with the HVR-deleted and HVR-truncated mutant forms of endosomal Rab proteins in humans (Fig. 1g, h; Ueda et al. 2020). Strikingly, intrinsic tethering activities of the two endosomal Rab isoforms, Rab5a and Rab4a, were drastically enhanced by deletion of their flexible HVR linkers, yielding 5- to 50-fold higher tethering rates for the HVR-deleted Rab mutants than those for the full-length, wild-type Rab proteins (Ueda et al. 2020). Furthermore, by analyzing a series of sequentially HVR-truncated mutants of Rab4a (Fig. 1g, h), several N-terminal residues in the Rab4a HVR linker, which are located adjacent to the C-terminus of the G domain (Fig. 1g), were found to be critical to regulating the inherent tethering potency of the G domain on the membrane surface (Fig. 1g, h; Ueda et al. 2020). These findings indicate that the non-conserved flexible HVR linkers of Rab-family small GTPases define the intrinsic capacities of the globular G domains to drive membrane tethering via their self-assemblies in trans on lipid bilayers, through controlling the close attachment of the G-domains to membrane surfaces.
Trans- and cis-assemblies of Arf-family small GTPases on lipid bilayers
Small GTPases of the Arf family, as well as Rab small GTPases, function as essential protein components for intracellular membrane trafficking in eukaryotic cells, through cooperating with their cognate effectors, termed Arf effectors (D'Souza-Schorey and Chavrier 2006; Donaldson and Jackson 2011; Jackson and Bouvet 2014; Sztul et al. 2019). In particular, Arf-family small GTPases are generally known to play key roles in the formation of membrane-bound transport carriers (e.g., secretory and endocytic transport vesicles) at the donor membrane compartments by specifically interacting with the subunits of coat-protein complexes and cargo-adaptor complexes (Donaldson and Jackson 2011; Jackson and Bouvet 2014; Sztul et al. 2019). Arf1 associates with the COPI (coat protein complex I) subunits during vesicle formation in the retrograde Golgi-to-ER pathway (Spang et al. 1998; Bremser et al. 1999), and Arf6 is involved in the formation of clathrin-coated endocytic vesicles via binding to the AP-2 adaptor complex (Paleotti et al. 2005). It is noteworthy that prior studies on the self-assembly of Arf1 in a reconstituted system indicated that dimerization of Arf1 contributes to inducing membrane curvature and scission during the formation of coated vesicles (Beck et al. 2008; Beck et al. 2011; Diestelkoetter-Bachert et al. 2020). In addition to the pivotal roles in transport carrier formation, a number of Arf-family small GTPases, including Arl (Arf-like) GTPases, have been reported to physically associate with the non-coat Arf effectors, coiled-coil tethering proteins, and multisubunit tethering complexes, for example, golgin GMAP-210 for Arf1 (Drin et al. 2008), the exocyst complex for Arf6 (Prigent et al. 2003), Golgin-97 and Golgin-245 for Arl1 (Lu and Hong 2003), the GARP complex for Arl5 (Rosa-Ferreira et al. 2015), and the HOPS complex for Arl8 (Khatter et al. 2015). Although whether and how Arf small GTPases directly act upon the process of membrane tethering (perhaps together with the tethering factors described above) remain unclear, the recent reconstitution works on human Arf-family isoforms, Arf1 and Arf6, have revealed that Arf6 can function as a bona fide membrane tether, directly and physically linking two distinct lipid bilayers via the self-assembly in trans, whereas Arf1 retains little or no potency to initiate tethering in the absence of other tethering factors or protein components (Fig. 2a–c; Fujibayashi and Mima 2021). In the reconstitution experiments, human Arf1 and Arf6 were purified as the N-terminal His12-tagged forms (His12-Arfs; Fig. 2a–c) and specifically anchored to synthetic DOGS-NTA-bearing liposomes (Fig. 2a), mimicking the membrane-bound state of native Arf proteins that bind to membrane surfaces via a myristoyl lipid anchor at the N-terminus and an N-terminal amphipathic helix (Fig. 2a; Fujibayashi and Mima 2021). As previously established for reconstitution studies of Rab-mediated membrane tethering (Fig. 1; Tamura and Mima 2014; Inoshita and Mima 2017; Mima 2018; Segawa et al. 2019; Ueda et al. 2020), the intrinsic membrane tethering activities of purified His12-Arf proteins were investigated by liposome turbidity assays (Fig. 2b) and fluorescence microscopy (Fig. 2c), demonstrating the high potency of Arf6, but not Arf1, to drive rapid and efficient tethering of liposomal membranes (200 nm in diameter) at Arf-to-lipid molar ratios ranging from 1:2,000 to 1:500 (Fig. 2b, c; Fujibayashi and Mima 2021). Intriguingly, even though Arf1 was unable to trigger membrane tethering via the self-assembly in trans in the reconstituted tethering assays (Fig. 2b, c), the presence of membrane-bound Arf1 proteins on Arf6-anchored liposomes abolished the tethering potency of Arf6 almost completely (Fig. 2d–f). This indicates that heterotypic Arf1-Ar6 assemblies in a cis configuration can prevent homotypic Arf6-Arf6 self-assemblies in trans (Fig. 2d–f; Fujibayashi and Mima 2021). These experimental data in a reconstituted system suggest that self-assemblies of Arf-family small GTPases directly contribute to driving and regulating membrane tethering events in eukaryotic cells.
Conclusions and perspectives
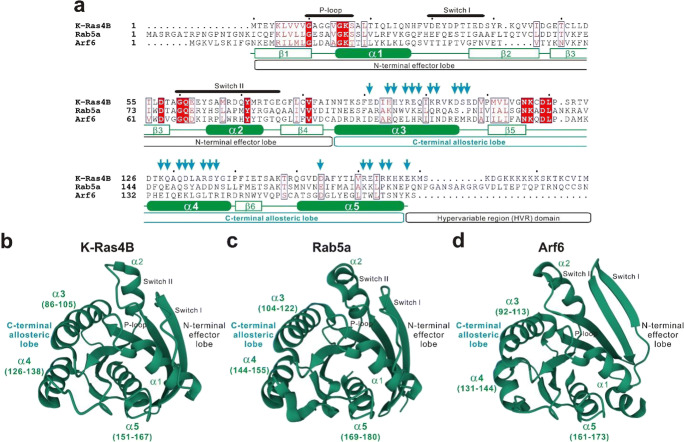
Recent biochemical studies by chemically defined reconstitution approaches using purified components have established that self-assemblies of Rab- and Arf-family small GTPases on lipid bilayers can directly mediate efficient and specific tethering of two distinct membranes in a physiological context: (i) A number of representative Rab-family small GTPases exhibit their inherent tethering functions at a physiological level of the surface densities of Rab proteins on membranes (Fig. 1a–d; Tamura and Mima 2014; Inoshita and Mima 2017; Segawa et al. 2019); (ii) both homotypic (e.g., Rab5a-Rab5a) and heterotypic (e.g., Rab1a-Rab9a) trans-assemblies of Rab small GTPases drive efficient and specific membrane tethering reactions (Fig. 1c–f; Segawa et al. 2019); (iii) the non-conserved flexible C-terminal HVR linkers of Rab small GTPases control the tethering-competent state of the globular G domains on membrane surfaces (Fig. 1g, h; Ueda et al. 2020); and (iv) in addition to Rab-family isoforms, the small GTPase Arf6 functions as a bona fide membrane tether through the self-assembly in trans (Fig. 2; Fujibayashi and Mima 2021). These findings provide novel insights into the mechanisms by which eukaryotic cells determine the directionality of intracellular membrane trafficking, since, in the current paradigm, the sequentially and structurally diverse classic tethering factors, such as long coiled-coil tethering proteins and multisubunit tethering complexes, are thought to primarily confer the spatiotemporal specificity of membrane trafficking by directly mediating membrane tethering in eukaryotic endomembrane systems (Bonifacino and Glick 2004; Yu and Hughson 2010; Kuhlee et al. 2015; Cheung and Pfeffer 2016; Spang 2016; Gillingham and Munro 2019). Future studies will need to be focused on the mechanistic details of membrane tethering mediated by Rab- and Arf-family small GTPases, particularly deciphering protein-protein interfaces in the globular G domains required for their trans-assemblies on lipid bilayers (Fig. 3). It should be noteworthy that recent advances in computational and experimental works on dimerization of oncogenic K-Ras4B have proposed two potential dimer interfaces in the Ras G domain (Zhou et al. 2018; Abankwa and Gorfe 2020; Van et al. 2021): the α3/α4 helical interface (Fig. 3; Muratcioglu et al. 2015; Prakash et al. 2017) and the α4/α5 helical interface (Fig. 3; Spencer-Smith et al. 2017; Prakash et al. 2017; Lee et al. 2020; Packer et al. 2021). Both of these two partially overlapping dimer interfaces are composed of abundant charged or hydrophilic amino acid residues and are located at the C-terminal allosteric lobe in the G domain, not within the N-terminal effector lobe which contains the p-loop, switch I, and switch II regions responsible for binding guanine nucleotides and the effectors (Fig. 3; Abankwa and Gorfe 2020). Although it remains unknown whether the two putative interfaces in the K-Ras4B dimer (Fig. 3; the α3/α4 and α4/α5 interfaces) are conserved through Rab- and Arf-family protein isoforms, comprehensive investigations on the α3-α4-α5 helical surfaces in the allosteric lobes of Rab and Arf small GTPases will advance our understanding of the molecular machinery of Rab- and Arf-mediated membrane tethering and, beyond that, the physiological significance of self-assemblies of Ras-superfamily small GTPases on lipid bilayers in general.
Fig. 3.
Putative protein-protein interfaces for self-assemblies of Ras, Rab, and Arf small GTPases. a Sequence alignment of human K-Ras4B (UniProtKB: P0116-2), Rab5a (UniProtKB: P20339), and Arf6 (UniProtKB: P62330). Amino acid sequences of these Ras-superfamily small GTPases were obtained from UniProtKB (https://www.uniprot.org), aligned using ClustalW (https://www.genome.jp/tools-bin/clustalw), and rendered by ESPript 3.0 (https://espript.ibcp.fr/ESPript/ESPript). Identical and similar amino acid residues in the sequence alignment are highlighted in red boxes and in red characters, respectively. The p-loop, switch I, and switch II regions; the N-terminal effector lobe; the C-terminal allosteric lobe; and the C-terminal hypervariable region (HVR) domain of K-Ras4B are indicated on the top or at the bottom of the sequence alignment. Secondary structures in the crystal structure of K-Ras4B (residues 1–167; PDB code, 3GFT), including five α-helices (α1–α5) and six β-strands (β1–β6), are also shown at the bottom of the alignment. Cyan arrows on the top of the alignment indicate putative key residues involved in the self-assembly of K-Ras4B, such as E91, H94, H95, R97, E98, Q99, K101, R102, D105, S106, E107, K128, Q129, Q131, D132, L133, R135, S136, Y137, D154, R161, E162, R164, K165, and E168 (Muratcioglu et al. 2015; Spencer-Smith et al. 2017; Prakash et al. 2017; Lee et al. 2020; Packer et al. 2021). b–d Crystal structures of human K-Ras4B (b PDB code, 3GFT), Rab5a (c PDB code, 1N6H), and Arf6 (d PDB code, 1E0S) proteins. The structures are labeled with the p-loop, switch I, and switch II regions; the N-terminal effector lobe; the C-terminal allosteric lobe; and five α-helices (α1–α5), where indicated.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abankwa D, Gorfe AA. Mechanisms of Ras membrane organization and signaling: Ras rocks again. Biomolecules. 2020;10:1522. doi: 10.3390/biom10111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainavarapu SR, Brujic J, Huang HH, Wiita AP, Lu H, Li L, Walther KA, Carrion-Vazquez M, Li H, Fernandez JM. Contour length and refolding rate of a small protein controlled by engineered disulfide bonds. Biophys J. 2007;92:225–233. doi: 10.1529/biophysj.106.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali BR, Wasmeier C, Lamoreux L, Strom M, Seabra MC. Multiple regions contribute to membrane targeting of Rab GTPases. J Cell Sci. 2004;117:6401–6412. doi: 10.1242/jcs.01542. [DOI] [PubMed] [Google Scholar]
- Beck R, Sun Z, Adolf F, Rutz C, Bassler J, Wild K, Sinning I, Hurt E, Brügger B, Béthune J, Wieland F. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci U S A. 2008;105:11731–11736. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, Prinz S, Diestelkötter-Bachert P, Röhling S, Adolf F, Hoehner K, Welsch S, Ronchi P, Brügger B, Briggs JA, Wieland F. Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J Cell Biol. 2011;194:765–777. doi: 10.1083/jcb.201011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Söllner TH, Rothman JE, Wieland FT. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/S0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Pfeffer SR. Transport vesicle tethering at the trans golgi network: coiled coil proteins in action. Front Cell Dev Biol. 2016;4:18. doi: 10.3389/fcell.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diestelkoetter-Bachert P, Beck R, Reckmann I, Hellwig A, Garcia-Saez A, Zelman-Hopf M, Hanke A, Nunes Alves A, Wade RC, Mayer MP, Wieland F. Structural characterization of an Arf dimer interface: molecular mechanism of Arf-dependent membrane scission. FEBS Lett. 2020;594:2240–2253. doi: 10.1002/1873-3468.13808. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Morello V, Casella JF, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–673. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujibayashi K, Mima J. The small GTPase Arf6 functions as a membrane tether in a chemically-defined reconstitution system. Front Cell Dev Biol. 2021;9:628910. doi: 10.3389/fcell.2021.628910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. Transport carrier tethering - how vesicles are captured by organelles. Curr Opin Cell Biol. 2019;59:140–146. doi: 10.1016/j.ceb.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Goitre L, Trapani E, Trabalzini L, Retta SF. The Ras superfamily of small GTPases: the unlocked secrets. Methods Mol Biol. 2014;1120:1–18. doi: 10.1007/978-1-62703-791-4_1. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hernandez JM, Kreutzberger AJ, Kiessling V, Tamm LK, Jahn R. Variable cooperativity in SNARE-mediated membrane fusion. Proc Natl Acad Sci U S A. 2014;111:12037–12042. doi: 10.1073/pnas.1407435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita M, Mima J. Human Rab small GTPase- and class V myosin-mediated membrane tethering in a chemically defined reconstitution system. J Biol Chem. 2017;292:18500–18517. doi: 10.1074/jbc.M117.811356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Bouvet S. Arfs at a glance. J Cell Sci. 2014;127:4103–4109. doi: 10.1242/jcs.144899. [DOI] [PubMed] [Google Scholar]
- Ji H, Coleman J, Yang R, Melia TJ, Rothman JE, Tareste D. Protein determinants of SNARE-mediated lipid mixing. Biophys J. 2010;99:553–560. doi: 10.1016/j.bpj.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatter D, Raina VB, Dwivedi D, Sindhwani A, Bahl S, Sharma M. The small GTPase Arl8b regulates assembly of the mammalian HOPS complex on lysosomes. J Cell Sci. 2015;128:1746–1761. doi: 10.1242/jcs.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb Perspect Biol. 2014;6:a016857. doi: 10.1101/cshperspect.a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlee A, Raunser S, Ungermann C. Functional homologies in vesicle tethering. FEBS Lett. 2015;589:2487–2497. doi: 10.1016/j.febslet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Lee KY, Fang Z, Enomoto M, Gasmi-Seabrook G, Zheng L, Koide S, Ikura M, Marshall CB. Two distinct structures of membrane-associated homodimers of GTP- and GDP-bound KRAS4B revealed by paramagnetic relaxation enhancement. Angew Chem Int Ed Eng. 2020;59:11037–11045. doi: 10.1002/anie.202001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yi L, Zhao L, Itzen A, Goody RS, Wu YW. The role of the hypervariable C-terminal domain in Rab GTPases membrane targeting. Proc Natl Acad Sci U S A. 2014;111:2572–2577. doi: 10.1073/pnas.1313655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SY, Brett CL, Plemel RL, Vignali M, Fields S, Gonen T, Merz AJ. Intrinsic tethering activity of endosomal Rab proteins. Nat Struct Mol Biol. 2012;19:40–47. doi: 10.1038/nsmb.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hong W. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol Biol Cell. 2003;14:3767–3781. doi: 10.1091/mbc.e03-01-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima J. Reconstitution of membrane tethering mediated by Rab-family small GTPases. Biophys Rev. 2018;10:543–549. doi: 10.1007/s12551-017-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratcioglu S, Chavan TS, Freed BC, Jang H, Khavrutskii L, Freed RN, Dyba MA, Stefanisko K, Tarasov SG, Gursoy A, Keskin O, Tarasova NI, Gaponenko V, Nussinov R. GTP-dependent K-Ras dimerization. Structure. 2015;23:1325–1335. doi: 10.1016/j.str.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle JF, Tristram-Nagle S. Structure of lipid bilayers. Biochim Biophys Acta. 2000;1469:159–195. doi: 10.1016/S0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Shteyn V, Melia TJ. Sensing membrane curvature in macroautophagy. J Mol Biol. 2017;429:457–472. doi: 10.1016/j.jmb.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MR, Parker JA, Chung JK, Li Z, Lee YK, Cookis T, Guterres H, Alvarez S, Hossain MA, Donnelly DP, Agar JN, Makowski L, Buck M, Groves JT, Mattos C. Raf promotes dimerization of the Ras G-domain with increased allosteric connections. Proc Natl Acad Sci U S A. 2021;118:e2015648118. doi: 10.1073/pnas.2015648118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleotti O, Macia E, Luton F, Klein S, Partisani M, Chardin P, Kirchhausen T, Franco M. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J Biol Chem. 2005;280:21661–21666. doi: 10.1074/jbc.M503099200. [DOI] [PubMed] [Google Scholar]
- Prakash P, Sayyed-Ahmad A, Cho KJ, Dolino DM, Chen W, Li H, Grant BJ, Hancock JF, Gorfe AA. Computational and biochemical characterization of two partially overlapping interfaces and multiple weak-affinity K-Ras dimers. Sci Rep. 2017;7:40109. doi: 10.1038/srep40109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rossé C, Camonis J, Chavrier P. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–1121. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Ferreira C, Christis C, Torres IL, Munro S. The small G protein Arl5 contributes to endosome-to-Golgi traffic by aiding the recruitment of the GARP complex to the Golgi. Biol Open. 2015;4:474–481. doi: 10.1242/bio.201410975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, Tamura N, Mima J. Homotypic and heterotypic trans-assembly of human Rab-family small GTPases in reconstituted membrane tethering. J Biol Chem. 2019;294:7722–7739. doi: 10.1074/jbc.RA119.007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. Membrane tethering complexes in the endosomal system. Front Cell Dev Biol. 2016;4:35. doi: 10.3389/fcell.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci U S A. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Smith R, Koide A, Zhou Y, Eguchi RR, Sha F, Gajwani P, Santana D, Gupta A, Jacobs M, Herrero-Garcia E, Cobbert J, Lavoie H, Smith M, Rajakulendran T, Dowdell E, Okur MN, Dementieva I, Sicheri F, Therrien M, Hancock JF, Ikura M, Koide S, O'Bryan JP. Inhibition of RAS function through targeting an allosteric regulatory site. Nat Chem Biol. 2017;13:62–68. doi: 10.1038/nchembio.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Sztul E, Chen PW, Casanova JE, Cherfils J, Dacks JB, Lambright DG, Lee FS, Randazzo PA, Santy LC, Schürmann A, Wilhelmi I, Yohe ME, Kahn RA. ARF GTPases and their GEFs and GAPs: concepts and challenges. Mol Biol Cell. 2019;30:1249–1271. doi: 10.1091/mbc.E18-12-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tamura N, Mima J. Membrane-anchored human Rab GTPases directly mediate membrane tethering in vitro. Biol Open. 2014;3:1108–1115. doi: 10.1242/bio.20149340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Tamura N, Mima J. Membrane tethering potency of Rab-family small GTPases is defined by the C-terminal hypervariable regions. Front Cell Dev Biol. 2020;8:577342. doi: 10.3389/fcell.2020.577342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van QN, Prakash P, Shrestha R, Balius TE, Turbyville TJ, Stephen AG. RAS nanoclusters: dynamic signaling platforms amenable to therapeutic intervention. Biomolecules. 2021;11:377. doi: 10.3390/biom11030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- Waters MG, Pfeffer SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/S0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee M, Fairn GD. Phospholipid subcellular localization and dynamics. J Biol Chem. 2018;293:6230–6240. doi: 10.1074/jbc.R117.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Prakash P, Gorfe AA, Hancock JF. Ras and the plasma membrane: a complicated relationship. Cold Spring Harb Perspect Med. 2018;8:a031831. doi: 10.1101/cshperspect.a031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M, Stroupe C, Orr A, Douville D, Wickner WT. Membranes linked by trans-SNARE complexes require lipids prone to non-bilayer structure for progression to fusion. eLife. 2014;3:e01879. doi: 10.7554/eLife.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]