Abstract
To describe the clinical features and the risk factors for nontuberculous mycobacteria (NTM) and Talaromyces marneffei (TM) co-infections in HIV-negative patients. A multicenter retrospective study in 13 hospitals, and a systematic literature review were performed of original articles published in English related to TM/NTM co-infections. HIV-negative patients with TM and NTM co-infections comprised Group 1; TM-only infection Group 2; NTM-only infection Group 3; and healthy volunteers Group 4. Univariate logistic analysis was used to estimate the potential risk factors of TM/NTM co-infections. A total of 22 cases of TM and NTM co-infections were enrolled. Of these, 17 patients (77.3%) had a missed diagnosis of one of the TM or NTM pathogens. The anti-IFN-γ autoantibodies (AIGAs) titer, white blood cell (WBC), neutrophil counts (N), erythrocyte sedimentation rate (ESR), C reactive protein (CRP), globulin, and immunoglobulin G (IgG) levels of Group 1 were higher than those of the other groups, whereas the levels of CD4+T cells was lower than those of other groups. There was a significant negative correlation between the AIGA titers and the number of CD4+T cells (P < 0.05). Factors including the ratio of the actual values to the cut-off values of AIGAs, WBC, N, HGB, CD4+T cells, IgG, IgM, IgA, serum globulin, ESR, and CRP were taken as potential risk factors for TM and NTM co-infection. Most patients with TM and NTM co-infection had a missed diagnosis of one of the TM or NTM pathogens. The levels of AIGAs, WBC, N, ESR, and CRP in TM and NTM co-infections were remarkably higher than in mono-infection. High-titer AIGAs may be a potential risk factor and susceptibility factor for co-infection of TM and NTM in HIV-negative hosts.
Subject terms: Immunology, Microbiology, Bacteria, Clinical microbiology, Fungi, Infectious-disease diagnostics
Talaromyces marneffei (TM) and nontuberculous mycobacteria (NTM) are opportunistic intracellular pathogens, with a strong association toward acquired immunodeficiency syndrome (AIDS) and other immunocompromised conditions1–4. Recently, an increasing number of TM and NTM mono-infection have been reported in HIV-negative patients5–7, especially in adults producing anti-IFN-γ autoantibodies (AIGAs)8–12. Refractory and relapsing TM and NTM infections often occur due to the high rates of misdiagnosis and inappropriate therapy leading to poor prognosis13,14. Thus, timely diagnosis of TM and NTM co-infections and differential diagnosis between mono- and co-infections are key to improve prognosis.
However, systematic clinical cohort studies of TM and NTM co-infections in HIV-negative hosts are lacking. Here, we report 22 HIV-negative adult patients, who suffered from co-infections by TM and NTM due to AIGAs. This study aimed to describe the clinical features and address the risk factors for NTM and TM co-infections in HIV-negative individuals.
Results
Patient demographics
A total of 22 HIV-negative patients with disseminated TM and NTM co-infection were enrolled in our study Group 1, including 14 patients from the multicenter retrospective cohort and 8 patients from the literature review cohort13–19.
In Group 1, simultaneous diagnosis with TM and NTM co-infections (Group 1A) was only made in 5 patients. The majority of patients with TM/NTM co-infections (17 patients, 77.3%) was firstly diagnosed with only one of the pathogens (group 1B), including 8 cases of initially missed diagnosis of TM, and 9 cases of initially missed diagnosis of NTM. TB was the most common presumed diagnosis in groups 1, 2 and 3. TB was the most common misdiagnosis in Groups 1, 2, and 3. Baseline patient characteristics are presented in Table 1. Sex, age, and underlying disease distribution were not significantly different between the three groups.
Table 1.
Baseline demographics and clinical characteristics of the 106 participants.
| Variable | Group 1 (n = 22) | Group 2 (n = 22) | Group 3 (n = 22) | Group 4 (n = 40) | P-value |
|---|---|---|---|---|---|
| Age (year) | 52 (42, 57) | 61 (46, 66) | 60 (50, 62) | 49 (33, 57) | 0.180 |
| Sex, female n (%) | 9 (40.9) | 8 (36.4) | 11 (50.0%) | 22 (55.0) | 0.480 |
| BMI (kg/m2) | 19.5 (18.2, 20.4) | 19.5 (17.4, 22.6) | 19.5 (17.0, 21.6) | – | 0.915 |
| Underlying disease* | 9 (40.9) | 9 (40.9) | 7 (31.8) | – | 0.798 |
| AIGAs positive** | 20 (100.0)a, b, c | 12 (54.5)d | 8 (36.4) | 0 (0) | 0.000 |
| AIGAs titers (ng/mL)g | 58,931.1 (32,343.8, 81,530.2)a, b, c | 16,070.4 (3496.1, 24,673.5)d, e | 12,302.2 (2523.1, 9068.4)f | 1497.4 (1192.3, 3177.7) | 0.000 |
| WBC × 109cells/Lg | 21.9 (18.1, 23.9)b | 20.8 (13.8, 30.3)d | 7.0 (5.4, 8.4) | ND | 0.000 |
| N × 109 cells/Lg | 18.5 (13.6, 19.9)b | 16.3 (11.8, 25.1)d | 4.5 (3.5, 6.6) | ND | 0.000 |
| L × 109 cells/Lg | 1.3 (0.8, 1.7) | 1.1 (0.62, 2.1) | 1.2 (0.9, 1.4) | ND | 0.769 |
| HGB g/Lg | 84.0 (60.4, 88.8)b | 71 (63.0, 97.6)d | 120 (110.9, 134.8) | ND | 0.000 |
| ESR mm/hg | 106.0 (90.0, 119.0)b | 95.5 (59.6, 113.25)d | 26.0 (8.0, 49.0) | ND | 0.000 |
| CRP mg/Lg | 166.9 (136.9, 200.0)b | 133.6 (92.5, 192.0)d | 10 (8.9, 13.9) | ND | 0.000 |
| CD4+T cell cells/μLg | 173 (105, 396)a, b | 676 (519, 1088) | 674 (547, 839) | ND | 0.001 |
| CD8+T cell cells/μLg | 378 (231, 709.5) | 470 (311,852) | 378 (231, 709) | ND | 0.651 |
| CD3+T cell cells/μLg | 549 (268, 806)a, b | 1246 (806,1796.7) | 1053 (725, 1602.5) | ND | 0.013 |
| IgG g/Lg | 29.5 (18.8, 39.1)a, b | 22.5 (12.3, 28.0)d | 14.3 (10.1, 18.2) | ND | 0.003 |
| IgA g/Lg | 2.3 (2.1, 4.3) | 2.7 (2.3, 3.5) | 2.4 (1.7, 4.0) | ND | 0.931 |
| IgM g/Lg | 2.0 (1.2, 2.9)a, b | 1.1 (0.6, 1.8) | 0.71 (0.7,1.6) | ND | 0.004 |
| Globulin g/Lg | 45.9 (36.8, 53.55)a, b | 33.7 (21.7. 58.9)d | 28.5 (24.2, 37.9) | ND | 0.011 |
Bold values indicate significant difference between groups or in univariate logistic regression analysis.
aIndicates statistical significance between Groups 1 and 2.
bIndicates statistical significance between Groups 1 and 3.
cIndicates statistical significance between Groups 1 and 4.
dIndicates statistical significance between Groups 2 and 3.
eIndicates statistical significance between Groups 2 and 4.
fIndicates statistical significance between Groups 3 and 4.
gA total number of 14 patients in Group 1 had AIGAs titer, WBC, N, L, HGB, ESR, CRP, globulin, immunoglobulins (IgG, IgA, IgM), and lymphocytes subpopulations (CD4+T cell, CD8+T cell, CD3+T cell) data.
Data are expressed as median ± interquartile range. Kruskal–Wallis H test was used to determine statistical significance among the 3 or 4 groups, followed by a 2 by 2 comparison across groups through a Fisher’s exact test. P < 0.05 indicates statistical significance.
Group 1 = patients with TM and NTM co-infections; Group 2 = patients with TM mono-infection; Group 3 = patients with NTM mono-infection; Group 4 = healthy control volunteers.
*Indicates the nature of the underlying disease in three groups. Group 1: 5 cases with Sweet’s syndrome, 1 case with malignant tumor, 1 case with cystic fibrosis, 1 case with Behcet’s syndrome, and 1 case with diabetes; Group 2: 1 case with thalassemia, 1 case with Sjogren's syndrome, 1 case with ankylosing spondylitis, 1 case with major trauma or surgery, 1 case with hyperthyroidism, 2 cases with glucocorticoids and or immunosuppressive agents, 1 case with hypertension, and 1 case of diabetes. Group 3: 3 cases with major trauma or surgery, 3 cases with hypertension, and 1 case with diabetes.
**Serums from 14 participants in Group 1, all patients in Groups 2 and 3, and 40 health volunteers were tested for anti-IFN-γ autoantibodies. Six of eight patients in the literature review cohort in Group 1 were defined as AIGA-positive, while the last 2 patients were not assessed. Thus, a total of 20 patients were tested for AIGAs in Group 1.
BMI body mass index, AIGAs anti-IFN-γ auto-antibodies, ND no data, WBC white blood cell, N neutrophil counts, L lymphocyte counts, HGB haemoglobin, ESR erythrocyte sedimentation rate, CRP C-reactive protein, Ig immunoglobulin. Normal range: IgG, 8–18 g/L; IgA, 2.01–2.69 g/L; IgM, 0.84–1.32 g/L; CD4+T cell, 410–1590 cells/μL; CD8+T cell, 190–1140 cells/μL; CD3+T cell, 690–2540 cells/μL.
Laboratory findings and clinical features
Laboratory findings are shown in Table 1 and Fig. 1. Routine bloodwork including, erythrocyte sedimentation rate (ESR), C reactive protein (CRP) lymphocyte phenotyping, and serum immunoglobulin G (IgG)] were performed for 14 patients from the retrospective study, and were not available in patients from the literature review cohort. White blood cell (WBC), neutrophil counts (N), ESR, and CRP in Groups 1 and 2 were significantly higher than in Group 3 (P < 0.001). Hemoglobin (HGB) in Groups 1 and 2 were lower than in Group 3 (P < 0.05). Globulin, IgG, and IgM levels of Group 1 were higher than those of the other groups. CD4+ T and CD3+ T lymphocyte counts in Group 1 were lower than normal reference values and that in Groups 2 and 3, respectively (P < 0.05) (Table 1, Fig. 1A–C).
Figure 1.
Comparison of biochemical indexes between groups. (A) White blood cell (WBC), neutrophil (N) counts, erythrocyte sedimentation rate (ESR), and C reactive protein (CRP) were significantly increased in Groups 1 and 2 compared with Group 3 (P < 0.001). (B) Globulin, IgG, and IgM in Group 1 were higher than normal reference values and higher than Groups 2 and 3 (P < 0.01). (C) CD4+T and CD3+T-lymphocyte counts in Group 1 were lower than normal reference values and lower than Groups 2, 3, and 4 (P < 0.01). (D, E) After combined treatment, all parameters in patients were improved.
Significant differences in clinical manifestations were found (P = 0.002) through Chi-square statistical tests for all the clinical manifestations between Groups 1, 2, and 3 in the three groups (Table 2). The most common clinical features in Group 1 were lymphadenopathy, fever, and cutaneous lesions, followed by cough, weight loss, and ostealgia. Fever, lymphadenopathy, and ostealgia were more common in Groups 1 and 2. However, weight loss and cough were more common in Group 3. Chest high resolution computed tomography (HRCT) was also conducted in the three groups (Table 2), showing significant differences in the prevalence of mediastinal lymphadenopathy, fibrous cords, pleural effusion and/or pleural thickening, bronchiectasis, and cavitary lesions. Mediastinal lymphadenopathy was more common in Groups 1 and 2, whereas fibrous cord, cavitary lesions, and bronchiectasis were more common in Group 3.
Table 2.
Symptoms and imaging findings of Chest HRCT in three groups.
| Variable | Group 1 (n = 22) | Group 2 (n = 22) | Group 3 (n = 22) | P-value |
|---|---|---|---|---|
| Symptoms, n (%) | 0.002 | |||
| Fever | 19 (86.4)b | 21 (95.5)c | 7 (31.8) | 0.000 |
| Lymphadenopathy | 20 (90.9)b | 20(86.4)c | 7 (31.8) | 0.000 |
| Cutaneous lesions | 19 (86.4)a, b | 10 (45.5)c | 2 (9.1) | 0.000 |
| Ostealgia | 12 (54.5)b | 11 (50)c | 1 (4.5) | 0.000 |
| Weight loss | 13 (59.1)a | 8 (36.4)c | 16 (72.7) | 0.047 |
| Cough and sputum production | 14 (63.6)b | 15 (68.2)c | 22 (100) | 0.007 |
| Hepatosplenomegaly | 4 (18.2) | 6 (27.2) | 2 (9.1) | 0.438 |
| Shiver | 4 (18.2) | 3 (13.6) | 2 (9.1) | 0.715 |
| Pectoralgia | 8 (36.4) | 2 (9.1) | 7 (31.8) | 0.086 |
| Shortness of breath | 5 (22.7) | 5 (22.7) | 9 (40.9) | 0.307 |
| Abdominal pain | 3 (13.6) | 3 (13.6) | 1 (4.5) | 0.483 |
| Imaging features of Chest HRCT, n (%)* | 0.023 | |||
| Number of patients assessed | 20 | 22 | 22 | – |
| Pulmonary consolidation | 17 (85.0) | 21 (95.5) | 19 (86.4) | 0.189 |
| Mediastinal lymphadenopathy | 11 (55.0)b | 10 (45.5)c | 1 (4.5) | 0.002 |
| Fibrous cords | 8 (40.0)b | 9 (40.9)c | 16 (72.7) | 0.032 |
| Pleural effusion/pleural thickening | 12 (60)b | 18 (81.8)c | 7 (31.8) | 0.003 |
| Nodular lesions | 9 (45.0) | 9 (40.9) | 4 (18.2) | 0.182 |
| Tracheal inflated sign | 3 (15.0) | 5 (22.7) | 1 (4.5) | 0.383 |
| Ground glass opacities | 3 (15.0) | 1 (4.5) | 2 (9.1) | 0.603 |
| Pericardial effusion | 2 (10.0) | 7 (31.8) | 2 (9.1) | 1.000 |
| Bronchiectasis | 1 (5.0)b | 1 (4.5)c | 7 (31.8) | 0.009 |
| Cavitary lesions | 1 (5.0)b | 3 (13.6)c | 9 (40.9) | 0.003 |
Bold values indicate significant difference between groups or in univariate logistic regression analysis.
aIndicates statistical significance between Groups 1 and 2.
bIndicates statistical significance between Groups 1 and 3.
cIndicates statistical significance between Groups 2 and 3.
Data are presented as n (%). Fisher’s exact test and Kruskal–Wallis H test were used to calculate P-values. P < 0.05.
Group 1 = patients with TM and NTM co-infections, Group 2 = patients with TM infections only, Group 3 = patients with NTM infections only. HRCT high resolution computed tomography.
*Two patients from the systematic literature review did not undergo HRCT. Thus, a total of 20 patients received HRCT.
These indexes were also compared between Groups 1A and 1B, which showed no significant differences (Supplementary Table 1). However, the CD4+T lymphocytes and CD3+T lymphocytes in Group 1A were lower than in Group 1B. In addition, patients in Group 1A receiving combined treatment (anti-fungal with anti-NTM treatment) showed a significant decrease in the inflammatory indexes (WBC, N, ESR, and CRP). By contrast, in Group 1B, the inflammatory indexes did not decrease, but rather increased following a single regimen therapy (anti-fungal or anti-NTM treatment). However, upon identifying the second pathogen and providing combined treatment, these inflammatory indexes, symptoms, and signs in patients improved (Fig. 1D, E, Supplementary Table 2).
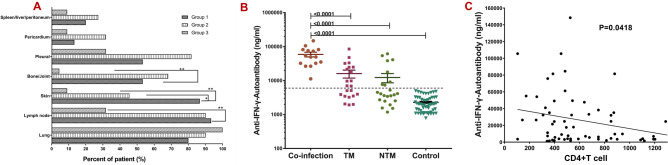
Comparing the involved sites of the three groups (Supplementary Table 3, Fig. 2A), lymph nodes, skin, and bone/joints were the most commonly infected sites in Groups 1 and 2. Lung involvement was more common in Groups 2 and 3, with pleural as the most commonly involved site in Group 2.
Figure 2.
Comparison of sites involved among three groups. (A) Lymph nodes were most commonly involved (90.1%), followed by the skin (86.4%) and bone/joint (54.5%) in Groups 1 and 2. (B) Comparing the anti-IFN-γ autoantibodies titer between groups, titers in Groups 1, 2, and 3 were remarkably higher than the healthy volunteer group, with patients in Group 1 showing the highest anti-IFN-γ autoantibodies titer. (C) The correlation between AIGA titers and the number of CD4+T cells.
Microbiology and pathology in patients with concomitant or sequential infections by TM and NTM
TM was most commonly isolated from respiratory specimens (14 cases), including bronchoalveolar lavage fluid (BALF) (7 cases), sputum (5 cases), and lung tissue (2 cases), followed by blood (5 cases), purulent secretion (4 cases), and lymph nodes (3 cases) in Group 1. By contrast, NTM was most commonly isolated from lymph nodes (7 cases), sputum (4 cases), and blood (4 cases) in Group 1.
Granulomatous lesions (12 cases), followed by non-specific inflammation (11 cases) and suppurative lesions (7 cases), were the most common histological findings in 27 pathological specimens from Group 1. Further, positive PAS staining (40.7%) of tissues and secretions were more frequent than acid-fast (AFB) staining (14.8%) in Group 1.
The distribution of rapid- and slow-growing nontuberculous mycobacterial species was similar in Group 1 (Supplementary Table 4). In these patients, the most commonly isolated species was Mycobacterium abscessus (4/11, 36.4%), followed by Mycobacterium chelonae and Mycobacterium kansasii (3/11, 27.6%). In addition to TM and NTM, other common co-infecting pathogens in Group 1 were Staphylococcus aureus, Aspergillus, Salmonella, and Burkholderia. Moreover, one patient was infected by up to six pathogens during the course of disease.
Increased AIGA levels in TM and NTM co-infection
Serums obtained from 14 participants in Group 1, all patients in Groups 2 and 3 (n = 22 in each group), and 40 health volunteers were tested for AIGAs. Furthermore, six of the eight patients in the literature review cohort in Group 1 were defined as AIGA-positive, the other 2 patients were not assessed. The positivity rate of AIGAs was significantly different across groups, specifically 100% (20/20), 81.8%, and 63.6% in Groups 1, 2, and 3, respectively (P = 0.000). When comparing AIGA titers between the groups, Groups 1, 2, and 3 were remarkably higher than the healthy volunteer group, with Group 1 showing the highest AIGA titer (Table 1, Fig. 2B). Meanwhile, there was a significant negative correlation between AIGA titers and the number of CD4+T cells (P < 0.05, Fig. 2C).
Univariate analysis logistic regression analyses for risk factors of TM and NTM co-infections
We analyzed risk factors for developing TM/NTM coinfections in group 1 compared to groups 2 and 3. We found that factors including the ratio of the actual values to the cut-off values of AIGAs, WBC, N, HGB, CD4+T cells, IgG, IgM, IgA, serum globulin, ESR, and CRP were taken as potential risk factors for TM and NTM co-infection (Table 3).
Table 3.
Results of univariate analysis for risk factors of TM and NTM co-infection (n = 66).
| Variable | Univariate analysis | ||
|---|---|---|---|
| P | HR | 95%CI | |
| Age (year) | 0.103 | 0.961 | 0.917–1.008 |
| BMI (kg/m2) | 0.948 | 1.006 | 0.834–1.214 |
| Relative nAIGA titer | 0.000 | 1.840 | 1.331–2.544 |
| WBC × 109cells/L | 0.018 | 1.081 | 1.013–1.152 |
| L × 109cells/L | 0.760 | 0.998 | 0.986–1.011 |
| N × 109cells/L | 0.025 | 1.083 | 1.010–1.161 |
| HGB g/L | 0.037 | 0.976 | 0.953–0.998 |
| CD4+T cell cells/μL | 0.001 | 0.998 | 0.996–1.000 |
| CD8+T cell cells/μL | 0.823 | 1.000 | 0.999–1.002 |
| CD3+T cell cells/μL | 0.316 | 1.000 | 0.999–1.000 |
| IgG g/L | 0.008 | 1.107 | 1.1027–1.193 |
| IgA g/L | 0.856 | 1.1051 | 0.616–1.793 |
| IgM g/L | 0.007 | 3.892 | 1.459–10.382 |
| Globulin g/L | 0.020 | 1.058 | 1.009–1.109 |
| ESR mm/h | 0.003 | 1.033 | 1.012–1.056 |
| CRP mg/L | 0.002 | 1.019 | 1.007–1.031 |
Bold values indicate significant difference between groups or in univariate logistic regression analysis.
Relative nAIGA titer indicates the ratio of the actual value to the cut-off value of nAIGA. BMI body mass index, nAIGAs neutralizing anti-IFN-γ auto-antibodies, ND no data, WBC white blood cell, N neutrophil counts, L lymphocyte counts, HGB haemoglobin, ESR erythrocyte sedimentation rate, CRP C-reactive protein, Ig immunoglobulin.
Treatment and outcome
The prognosis and outcomes of patients in Group 1 was worse than that of patients in Groups 2 and 3, especially in cases of persistent and/or relapsed infections (P < 0.001) (Table 4).
Table 4.
Comparison of the outcomes between three groups in 66 HIV-negative participants.
| Variable | Group 1 (n = 22) | Group 2 (n = 22) | Group 3 (n = 22) | P-value |
|---|---|---|---|---|
| Prognosis and outcomes | 0.043 | |||
| Cured | 7 (31.8) | 14 (63.6) | 14 (63.4) | |
| Persistent or relapse infection* | 13 (59.1) | 2 (9.1) | 5 (22.7) | |
| Death | 1 (4.5) | 6 (27.3) | 2 (9.1) | |
| Lost | 1 (4.5) | 0 | 0 | |
Data are expressed as number and percentage (%). Fisher’s exact test and Kruskal–Wallis H test were used to determine statistical significance among the groups. P < 0.05 was taken as significant.
Group 1 = patients with TM and NTM co-infections, Group 2 = patients with TM infection only, and Group 3 = patients with NTM infection only.
*Persistent or Relapse infection: In Group 1, the infection condition of patients may have been only TM persistent infection, only TM recurrent infection, only NTM persistent infection, only NTM recurrent infection, both TM persistent and recurrent infection, both NTM persistent and recurrent infection, both TM persistent infection and NTM recurrent infection, or both NTM persistent infection and TM recurrent infection. Detailed prognostic information for Group 1 can be found in Table 3 which described the treatment and patient outcomes in Group 1. In Group 2, there was one case with persistent infection and one case with relapse infection. In Group 3, there was one case with persistent infection and four cases with relapse infections.
Treatment outcomes are presented in Table 3 among 22 patients: 19 received anti-NTM medical treatment and 22 received anti-fungal treatment. Furthermore, 1 case was lost to follow-up, 1 died from multiple organ failure, 7 were effectively cured of both TM and NTM), 9 relapsed, and 6 had persistent infection. Of the 13 patients with positive AIGA, only 1 patient (P17) received AIGA treatment. Upon receiving combined methylprednisolone and rituximab treatment, the AIGA titer of P17 decreased from more than 1: 10,000 to 1: 5000 after 2 courses of therapy. The total treatment time, including anti-fungal and anti-NTM, was 40 months (6–114 months) (Table 5).
Table 5.
Treatment and patient outcomes in Group 1.
| Patient | AIGAs | TM therapy | NTM therapy | AIGAs treatment | Duration | Outcome* |
|---|---|---|---|---|---|---|
| P1 | Positive | VCZ + AMB 2w. Secondary prophylaxis VCZ 12 m | RFP + EMB + MXFX + CLR 6 m, then relapse after 1 m of withdrawal, changed to Biapenem + LVFX for 5 m | None | 12 m | TM effective; NTM relapse |
| P2 | Positive | Intravenous VCZ for 2w then oral VCZ 5 m | EMB + INH + RFP | None | 15 m | TM relapse; NTM persistent infection |
| P3 | Positive | Intravenous VCZ for 2w then oral VCZ for 6 m | LVFX + EMB | None | 12 m | TM relapse; NTM persistent infection |
| P4 | Positive | AMB for 2w, then oral VCZ 12 m | CLR + MXFX + RZA + SMZ for 7 m | None | 20 m | TM and NTM effective |
| P5 | Positive | Oral ICZ for 24 m | MXFX + EMB for 36 m | None | 36 m | TM and NTM effective |
| P6 | Positive | AMB for 2w, secondary prophylaxis oral ICZ for 4 m | CLR + MXFX for 6 m then relapse, change to MXFX + IMP for 6 m | None | 12 m | TM and NTM relapse |
| P7 | Positive | Oral ICZ for 12 m | INH + RFP + EMB + PZA | None | 36 m | TM effective; NTM persistent infection |
| P8 | Positive | AMB for 2w, then oral ICZ | CLR + AMK 7 m | None | 43 m | TM and NTM effective |
| P9 | Positive | Intravenous VCZ for 3 days then oral VCZ | None | None | 3 days | Death |
| P10 | Positive | Oral ICZ | None | None | 60 m | TM and NTM persistent infection |
| P11 | Positive | AMB for 2w, then oral ICZ | None | None | 6 m | Lost to follow-up |
| P12 | Positive | AMB for 2w, then oral ICZ for 18 m. VCZ for 60 m for relapse | CLR + MXFX | None | 78 m | TM and NTM both relapse |
| P13 | Positive | AMB for 2w, then oral VCZ | CLR + CXT + MXFX for 12 m; then AMK + IMP + AZM for 6 m for relapse | None | 18 m | TM effective; NTM relapse |
| P14 | Positive | ICZ for 12w | IMP + CLR for 36 m | None | 40 m | TM and NTM Effective |
| P1513 | NA | Micafungin | AZM + RFP + EMB | None | – | TM and NTM effective |
| P1614 | Positive | AMB | RIF + EMB + CLR + CIP then relapse, changed to CIP + INH + RIF + CLR | None | 60 m | TM and NTM persistent infection |
| P1715 | NA | AMB for 5 m, then oral ICZ for 25 m | RFP + EMB + CLR for 19 m | None | 41 m | TM and NTM effective |
| P1816 | Positive | LAMB for 2w, then oral ICZ for 6 m | IMP + AMK for 1 m, then AMK + CLR + CIP for 3 m then relapse, changed to CLR + EMB for 1 m | Rituximab plus methylprednisolone | 55 m | TM Effective; NTM relapse |
| P1917 | Positive | AMB + ICZ for 2w, then oral ICZ | LXFX | None | – | TM and NTM effective |
| P1018 | Positive | ICZ for 10 m | INH + RFP + PZA + EMB + MXFX for 24 m then relapse, and changed to INH + RFP + PZA + EMB + CLR + SMZ 6 m | None | 69 m | TM effective, NTM relapse |
| P2119 | Positive | ICZ | IMP for 6 m then relapse, then changed to MEM + AMK + TGC | None | 78 m | TM effective; NTM persistent infection |
| P2219 | Positive | AMB for 2w, then oral ICZ for 10w | INH + EMB + CLR + AMK + OFLX for 22 m, then changed to EMB + CLR + AMK + OFLX | None | 114 m | TM effective; NTM relapse |
NA anti-IFN-γ autoantibodies not detected, AMB amphotericin B, LAMB amphotericin B liposome, VCZ voriconazole, ICZ itraconazole, EMB ethambutol, RFP rifampin, CIP ciprofloxacin, INH isoniazid, PZA pyrazinamide, OFLX ofloxacin, CXT cefoxitin, IMP imipenem, AMK amikacin, CLR clarithromycin, LXFX levofloxacin, MXFX moxifloxacin, SMZ sulfamethoxazole, MEM meropenem, TGC tigecycline, AIGAs anti–interferon-γ autoantibodies.
*For 14 patients the outcome assessment was performed at their last outpatient follow-up. For 8 patients that were part of the systematic literature review, their outcome assessment was extracted from the literature. For the following patients, the duration between the time the treatment was stopped, and the outcome assessment was respectively: 12 months for P15; 19 months for P16; 10 months for P18; 12 months for P19; and 6 months for P22. For the following patients, the outcome assessment time was performed when they were discharged: P17, P21, and P21.
Discussion
To our knowledge, this is the first report showing the differences between TM and NTM co-infection and their respective mono-infections. Some clinical differences were noticed across groups. The severity of inflammation (WBC, N, ESR, CRP), inflammatory anemia, and prevalence of involved sites in TM and NTM co-infection were more evident than in TM or NTM mono-infection, especially when compared. Noteworthy, when patients received single active antifungal or single anti-NTM treatment, some symptoms improved while others worsened. Inflammatory markers (WBC, N, CRP, ESR) did not significantly decline or increase, but did not maintain normal levels, indicating the presence of double or multiple infections, especially in patients with high-titer AIGAs.
Univariate analysis for risk factors of TM and NTM co-infection found that high level of AIGAs, WBC, N, HGB, IgG, IgM, IgA, serum globulin, ESR, and CRP and low level of CD4+T cells were taken as potential risk factors for TM and NTM co-infection. Most importantly, the titer of AIGAs was significantly positively correlated with the number of sites involved, which suggested that the titer of AIGAs was associated with disseminated infection. Thus, high-titer AIGAs may represent a potential risk factor and susceptibility factor for co-infection of TM and NTM in HIV-negative hosts. Monitoring the AIGA titer is the most important step in screening for co-infections or disseminated infections.
IFN-γ is produced principally by T lymphocytes and natural killer cells after stimulation with microbial products and interleukin (IL)-1220. Patients with positive AIGAs often suffer from recurrent infections, especially due to NTM8,9,11. Because IFN-γ is an activator of macrophage differentiation and a pro-inflammatory activator of innate immunity, the blockade effects of the AIGAs on IFN-γ present in the serum of patients with NTM are hypothesized to regulate the antimicrobial function of macrophages20. Recently, a study showed that AIGAs can neutralize IFN-γ, affect the activation of the IFN-γ receptor (IFN-γR), and downregulate the production of its downstream factors, such as TNF-α and IL-12, and inhibit IFN γ-STAT-1 phosphorylation11. IFN-γ is also an essential activator of CD4+T cell differentiation into Th1 cells21. In the present study, the AIGA titers and positive rates of patients with co-infection were significantly higher than those of other groups, while their CD4+T and CD3+T cell levels were significantly lower than those of other groups. Meanwhile, there was a significant negative correlation between AIGA titers and the number of CD4+T cells. Thus, the neutralizing and blockade effects of the AIGAs may be related to the low level of CD4+T cells, which may be the reason for patients susceptible to opportunistic pathogens, especially intracellular pathogens.
TM and NTM showed very similar clinical manifestations such as fever, anemia, weight loss, cough, expectoration, and skin lesions. They both can involve skin lesions, respiratory system, and bone, leading to local or disseminated infections. High recurrence and/or persistent infection rates (59.1%) was found in TM and NTM co-infected patients, primarily due to misdiagnosis and/or missed diagnoses as each other or TB. In HIV-negative individuals with TM and NTM co-infection, only one pathogen (TM or NTM) was discovered in the early stages of disease in most patients (77.3%). Moreover, inflammatory markers in TM and NTM co-infection were higher than in NTM mono-infection, though no significant difference was found between simultaneous and successive TM and NTM. These suggest that most patients found to have sequential TM and NTM infections were in fact infected with both TM and NTM simultaneously; however, one pathogen was missed at diagnosis, resulting in poor prognosis.
Furthermore, TM histopathology often manifests as granuloma, but caseous granuloma is rare, which was characteristic of positive TM cultures in this study. Second, it is more difficult to make a differential diagnosis of NTM from TB because of its similar histopathology and acid-fast staining. Thus, even if it is positive for acid-fast staining, metagenomic next-generation sequencing and culture of mycobacteria is essential to detect NTM, especially when anti-tuberculosis treatment is not effective. Third, TM and NTM co-infection has a higher inflammatory index and dissemination than NTM infection, which may be related to AIGAs and TM. Fourth, when a single treatment (anti-fungal or anti-tuberculosis branch) is not effective for a patient, potential co-infection with other pathogens should be considered, especially in patients with positive AIGAs.
Conclusion
High-titer AIGAs represent an independent risk factor for TM and NTM co-infection in HIV-negative hosts. AIGA may be a major susceptibility factor for intracellular pathogens such as TM and NTM. Further, poor prognosis of TM and NTM co-infection may be due to misdiagnosis and/or missed diagnoses. Therefore, AIGA screenings in patients with unexplained recurrent or multiple microbial infections may serve as an indicator of acquired immunodeficiency.
Limitations
There are important limitations to our study. First, the number of participants and reports was small, reflecting that AIGA disease and co-infection of TM and NTM is still rarely recognized. Second, it is unclear when the AIGA is positively detected or activated by infection. Despite these limitations, this is the first comprehensive description of TM and NTM co-infection in AIGA-associated immunodeficiency syndrome.
Methods
Study design and patients
Guangxi, China cohort
For this multicenter, observational, retrospective cohort study, we screened for TM and NTM co-infection (Group 1) in HIV-negative patients from 13 hospitals between January 1st, 2012, and January 1st, 2020. Group 2 comprised patients with TM mono-infections and Group 3 of NTM mono-infections. All patients were HIV-negative. The healthy controls were recruited after completing a multicenter retrospective study and a systematic literature review. Healthy control volunteers (Group 4) were enrolled to match the gender, age, and HIV-negative condition of Group 1. Demographic and clinical data were recorded on standardized forms.
The 13 participating centers included: (1) The Eighth Affiliated Hospital of Sun Yat-Sen University; (2) The First Affiliated Hospital of Guangxi Medical University; (3) The Affiliated Tumor Hospital of Guangxi Medical University; (4) The Second Affiliated Hospital of Guangxi Medical University; (5) The Hospital of Guangxi Zhuang Autonomous Region; (6) Nan Xishan Hospital of Guangxi Zhuang Autonomous Region; (7) Nanning Second People's Hospital; (8) Nanning Forth People's Hospital; (9) Nanning Eighth People's Hospital; (10) Yiyang Central Hospital; (11) Liuzhou First People's Hospital; (12) Guigang First People's Hospital; and (13) Guilin First People's Hospital.
This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2018.KY-E-094). The clinical trial was registered on www.clinicaltrials.gov (NCT03819348). Written informed consent was provided by all healthy participants in this study. All methods were performed in accordance with the relevant guidelines.
Systematic literature review cohort
For a systematic review of articles related to TM/NTM co-infection, original articles published in English from Jan 2004 to July 2019 were reviewed using the following electronic databases: PubMed, Web of Science, Embase, and BIOSIS. Screening of relevant studies was based on combinations of keywords, such as “non-tuberculosis”, “non-tuberculous”, “non tuberculosis”, “nontuberculous”, “nontuberculous mycobacterium”, “nontuberculosis mycobacteria”, “NTM”, “MOTT”, “atypical mycobacterium”, “penicilliosis”, “Penicillium marneffei”, “Talaromycosis”, “Talaromyces marneffei”, “T. marneffei”, and “P. marneffei”. Inclusion criteria for the systematic literature review consisted of the following: (1) TM and NTM diagnosis based on exact pathogen, isolated NTM, and TM from clinical specimens; (2) articles clearly stating the HIV infection status; and (3) only HIV-uninfected subjects with TM and NTM co-infection were included. Informed consent was waived for patients in the literature review due to the nature of the study.
The data presented in this study result from a merge of these 2 cohorts (Guangxi cohort and literature review cohort). Clinical outcomes definitions: (1) Cured (no recurrence of TM and/or NTM infection for at least six months after discontinuation of antifungal/anti-NTM therapy); (2) persistent or relapsed infection (persistent infection: no improvement of clinical symptoms after antifungal/anti-NTM treatment, relapsed infection: improvement of clinical symptoms, negative pathogen detection after antifungal/anti-NTM effective treatment, followed by the reappearance of pathogen-associated infectious signs and/or positive pathogen testing); and (3) death. A disseminated disease was defined as an infection in at least two noncontiguous and sterile sites.
Diagnostic criteria for NTM and TM
Each patient fulfilled the diagnostic criteria of each disease. NTM was diagnosed following the 2007 American Thoracic Society (ATS)/Infectious Disease Society of America guidelines22,23. TM infection was diagnosed as follows: (1) positive cultures for TM, characterized by dimorphic fungi that grew either as a mold at 25 °C or as yeast at 37 °C; (2) characteristic morphology of the yeast form of TM, confirmed by cytology and histopathology from tissues and secretions using Periodic Acid-Schiff (PAS) staining or Wright’s stain, including a transverse septum23; or (3) TM and/or NTM isolated by metagenomic next-generation sequencing from clinical specimens.
Anti-IFN- γ autoantibody assay
Serum samples obtained under sterile conditions before the patient received antimicrobial therapy treatment and during the active stage of the infection. Serum samples were retrieved from a serum bank and stored at − 80 °C. AIGAs were detected in all participants. All serum samples were tested at the first thaw. The detection of AIGAs was performed using an enzyme-linked immunosorbent assay kit (Cloud-Clone Corp. Wuhan, China) whose detection range is 12–200 ng/ml. According to the manufacturer’s protocols: the serum samples from patients were 1:1500 diluted, and serum samples from a healthy control were 1:600 diluted by phosphate-buffered saline (PBS). The normal range for the anti–IFN-γ–autoantibody concentration was defined by the 99th percentile for the healthy controls and was estimated using the log-normal distribution. Outlying concentrations were classified as positive for anti–IFN-γ autoantibodies1,6.
IFN-γ, IL-4, IL-6, IL-8, TNF-α assay
Serum samples obtained under sterile conditions before the patient received antimicrobial therapy treatment and during the active stage of the infection. Serum samples were retrieved from a serum bank and stored at − 80 °C. IFN-γ, IL-4, IL-6, IL-8, TNF-α were detected in all participants. All serum samples were tested at the first thaw. The detection of IFN-γ, IL-4, IL-6, IL-8, TNF-α was performed using a human enzyme-linked immunosorbent assay kit (Cloud-Clone Corp. Wuhan, China) according to the manufacturer’s instructions.
Statistical analysis
Continuous variables were expressed as median ± interquartile range. Differences between groups were compared using Kruskal–Wallis H or Mann–Whitney U tests. Dunn-Bonferroni test was used for post-hoc comparisons. Chi-square or Fisher’s exact tests were used to compare categorical variables. Spearman’s correlation coefficient was used for ranked data to measure the dependence of two nonparametric variables. Univariate logistic analysis was used to estimate risk factors of co-infection. We used SPSS (version 25.0), and GraphPad Prism (version 7) for statistical analysis and graph illustrations, and a two-sided P-value of 0.05 or less was considered significant.
Ethical approval
This study was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2018.KY-E-094). The clinical trial was registered on www.clinicaltrials.gov (NCT03819348). Written informed consent was provided by all participants in the prospective cohort study.
Consent to participate
All study participants provided informed consent, and the study design was approved by the appropriate ethics review board.
Consent for publication
Written informed consent for publication was obtained from all participants.
Supplementary Information
Acknowledgements
The authors thank Meng Li, Professor of Microbiology, Department of Microbiology laboratory, the First Affiliated Hospital of Guangxi Medical University.
Author contributions
Y.Q., J.H., and Y.L. made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and drafting of the manuscript. J.Z. and Y.Q. made substantial contributions to the conception and design of the study; acquisition, analysis, and interpretation of the data; and critical revision of the manuscript for important intellectual content. W.Z. conceived of the study, participated in its design, and helped to draft the manuscript. J.Z. gave final approval of the version to be published. M.P., J.C., H.Z., X.S., and D.Q. participated in analysis and interpretation of the data and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China ((No. NSFC81760010 and 82060364)) and the Science and Technology Department of Guangxi Zhuang Autonomous Foundation of Guangxi Key Research and Development Program (No. GuikeAB20238025).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
We used SPSS (version 25.0), and GraphPad Prism (version 7) for statistical analysis and graph illustrating, and P-value < 0.05 was considered significant.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ye Qiu, Jie Huang and Yu Li.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95686-0.
References
- 1.Le T, Kinh NV, Cuc NTK, Tung NLN, Lam NT, Thuy PTT, Cuong DD, Phuc PTH, Vinh VH, Hanh DTH, et al. A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. N. Engl. J. Med. 2017;376(24):2329–2340. doi: 10.1056/NEJMoa1613306. [DOI] [PubMed] [Google Scholar]
- 2.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017;17(11):e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 3.Binder AM, Adjemian J, Olivier KN, Prevots DR. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2013;188(7):807–812. doi: 10.1164/rccm.201307-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, Luo J, Huang H. The prevalence of non-tuberculous mycobacterial infections in mainland China: Systematic review and meta-analysis. J. Infect. 2016;73(6):558–567. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg. Microbes. Infect. 2016;5:e19. doi: 10.1038/emi.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu Y, Feng X, Zeng W, et al. Immunodeficiency disease spectrum in HIV-negative individuals with talaromycosis. J. Clin. Immunol. 2021;41(1):221–223. doi: 10.1007/s10875-020-00869-5. [DOI] [PubMed] [Google Scholar]
- 7.Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019;54(1):1900250. doi: 10.1183/13993003.00250-2019. [DOI] [PubMed] [Google Scholar]
- 8.Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, Lin PC, Chen HJ, Chou CH, Feng JY, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121(8):1357–1366. doi: 10.1182/blood-2012-08-452482. [DOI] [PubMed] [Google Scholar]
- 9.Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, Yeh CF, Fung CP, Sun HY, Huang CT, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Med. (Baltimore) 2016;95(25):e3927. doi: 10.1097/MD.0000000000003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng W, Qiu Y, Tang S, Zhang J, Pan M, Zhong X. Characterization of anti-interferon-gamma antibodies in HIV-negative patients infected with disseminated Talaromyces marneffei and cryptococcosis. Open Forum Infect. Dis. 2019;6(10):ofz208. doi: 10.1093/ofid/ofz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J, Ning XQ, Ding JY, et al. Anti-IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J. Exp. Med. 2020;217(12):e20190502. doi: 10.1084/jem.20190502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theresa A, Sagel SD, Sontag MK, et al. The clinical course of a Mexican female with cystic fibrosis and the novel genotype S531P/S531P. J. Cyst. Fibros. 2008;7(5):454–456. doi: 10.1016/j.jcf.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Lee WI, Huang JL, Wu TS, et al. Patients with inhibitory and neutralizing auto-antibodies to interferon- resemble the sporadic adult-onset phenotype of Mendelian Susceptibility to Mycobacterial Disease (MSMD) lacking Bacille Calmette-Guerin (BCG)-induced diseases. Immunobiology. 2013;218(5):762–771. doi: 10.1016/j.imbio.2012.08.281. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Huang X, Zhang X, et al. Coinfection of disseminated Talaromyces marneffei and Mycobacteria kansasii in a patient with papillary thyroid cancer. A case report. Med. (Baltimore). 2017;96(52):e9072. doi: 10.1097/MD.0000000000009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruetpongpun N, Khawcharoenporn T, Damronglerd P, et al. Disseminated Talaromyces marneffei and Mycobacterium abscessus in a patient with anti-interferon-γ autoantibodies. Open Forum Infect. Dis. 2016;3(2):ofw093. doi: 10.1093/ofid/ofw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Liu D, He X, et al. Sweet’s syndrome associated with Talaromyces marneffei and Mycobacterium abscessus infection due to anti-interferon-gamma autoantibodies. Indian J. Dermatol. 2018;63(5):428–430. doi: 10.4103/ijd.IJD_362_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampitak T, Suwanpimolkul G, Browne S, et al. Anti-interferon-c autoantibody and opportunistic infections: Case series and review of the literature. Infection. 2011;39(1):65–71. doi: 10.1007/s15010-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 19.Tang BS, Chan JF, Chen M, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin. Vaccine Immunol. 2010;17(7):1132–1138. doi: 10.1128/CVI.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krisnawati DI, Liu YC, Lee YJ, et al. Blockade effects of anti-interferon- (IFN-) γ autoantibodies on IFN-γ-regulated antimicrobial immunity. J. Immunol. Res. 2019;2019(30):1629258. doi: 10.1155/2019/1629258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X, Nakayamada S, Kubo S, et al. Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis. 2018;77(9):1354–1361. doi: 10.1136/annrheumdis-2017-212652. [DOI] [PubMed] [Google Scholar]
- 22.Nseir S, Grailles G, Soury-Lavergne A, et al. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin. Microbiol. Infect. 2010;16(7):902–908. doi: 10.1111/j.1469-0691.2009.03027.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoenigl M, Strenger V, Buzina W, et al. European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J. Antimicrob. Chemother. 2012;67(8):2029–2033. doi: 10.1093/jac/dks155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
We used SPSS (version 25.0), and GraphPad Prism (version 7) for statistical analysis and graph illustrating, and P-value < 0.05 was considered significant.