Abstract
This work aimed at evaluating the potential of using natural deep eutectic systems (NADES) as cryoprotectant agents (CPAs). Several combinations between natural primary metabolites that have been identified in animals that live in extreme cold climates were prepared. All systems showed very little cytoxicity towards L929 cells at concentrations high as 1–2 M. Moreover, this cell line was highly tolerant to 10% (w/v) of NADES when compared to Me2SO. To test NADES as CPAs, two cell lines were used, L929 and HacaT cells. After freeze/thawing cycle, it was possible to observe that for L929 cells, NADES presented similar behaviour to Me2SO. For Hacat cell line a significant improvement on post-thawing recovery was observed. Moreover, the results presented herein showed that NADES do not need to be removed from the freezing media after thawing the cells, which is a great advantage of these materials. Additionally, we have shown that NADES can act as CPA when cells are frozen at −20 °C. In overall, the results demonstrate the high potential of NADES to be used in cryobiology as alternative CPAs.
Keywords: Natural deep eutectic systems, Cryobiology, Natural metabolites, Freeze-thawing, Biomaterials, Cryopreservation
1. Introduction
The preservation of biological material in a stable state is fundamental for biological/medical sciences. However, the cryopreservation of mammalian cells with high post-thawing cell survival remains a major challenge. Many studies have been reported for the cryopreservation of mammalian cells [1,2,14,17,26,28] but cellular function can be highly affected by environmental changes during the freezing process [28], such as cryopreservation solutions, biomaterials, freezing methods, and freezing/preservation temperatures.
Cryoprotection usually involves the treatment of cells with a cryoprotectant agent (CPA), which will cause osmotic dehydration of cells, thus preventing the formation of intracellular ice crystals that would cause the bursting of cells. The use of a CPA can also play an important role during the thawing step because they can also avoid the recrystallization process. If the heating process is too slow, small ice crystals will recrystalize into bigger ones which will cause cell death [15].
The use of dimethyl sulfoxide (Me2SO) as CPA remains the standard protocol used in cryopreservation of mammalian cells in common laboratories. However, it is known that it is toxic for cells, because it penetrates the cell membrane immediately after contact, causing cellular damage by altering mitochondrial membrane potential and increasing reactive oxygen species, and must be removed as soon as the cells are thawed [24]. Me2SO remains a solvent of choice in biomedical research, despite the physiological interference and cytotoxicity. However, less toxic alternatives would allow to preserve more cell and tissue types for biomedical research.
Deep eutectic systems (DES) have gained attention in the last years due to their sustainable properties such as high biodegradability, cost-effectiveness, and low toxicity [7,22]. These systems can be formed by natural primary metabolites, such as sugars, amino acids, organic acids, or choline derivative, and therefore called natural deep eutectic systems (NADES). The components are mixed at a particular molar ratio, presenting a high melting point depression, compared to its individual constituents, therefore becoming liquid at room temperature or near room temperature [22]. The small additions of water can tailor their intrinsic viscosity, as well as, decrease their toxicity [10].
Some components of NADES, such as trehalose, glucose, sorbitol, and proline, are known to be produced by animals and plants that live in extreme cold environments and are involved in many metabolic pathways during winter [8,13,16]. For the past few years, many reports have been published using these metabolites in combination with Me2SO to improve post-thawing cell viability [12,28,33,34].
Many authors reported the mixture of trehalose with Me2SO to cryopreserve different cells lines, such as stem cells, primary hepatocytes, and HepG2 cells [[5], [17], [18], [21], [27], [28], [32]]. In all cases the mixture showed better results than Me2SO or trehalose alone. On the other hand, betaine showed, by DSC, the ability to supresses water crystallization. When used alone in GLC-82, HeLa and MCF-10 cells, frozen in the presence of betaine, the post-thawing viability was significantly higher [34]. Additionally, a combination of proline and trehalose has been reported for the cryopreservation of red blood cells (RBCs) [11]. In clinical practice, glycerol is the standard CPA for RBCs, but its toxicity and complex deglycerolization limit its application. In this study this mixture resulted in the lowest post-thawing haemolysis and promoted normal morphology, as well as normal enzymatic activity, of RBCs.
The combination of these metabolites into a eutectic system seems to be highly promising. In fact, Castro et al. [6] have shown the potential of a NADES formed by glycerol and trehalose as CPA. Moreover, in that study, the authors have shown that, likewise other CPAs, NADES can also alter the thermal behaviour of water, suppressing crystallization, causing the vitrification of water or at least reducing the temperature of crystallization and the morphology of the crystal, therefore, presenting a high potential to be used as CPA [9].
Therefore, herein we present our latest results regarding the application of NADES for the cryopreservation of mammalian cells. These are based on different combinations of natural compounds that have been reported for their cryoprotective properties, such as sorbitol, glycerol, trehalose, glucose, sucrose, proline, and betaine [11,18,25,29,31]. Furthermore, we aimed to understand the possibility of using these systems without having to remove them during the thawing step. If successful, these systems could be applied in other applications such as the cryopreservation of stem cells and bone marrow, and red blood cells for transplants.
2. Experimental section
2.1. Materials and methods
Trehalose dihydrate (Hayashibara Co., Okayama, Japan), glycerol (≥99.5%, Sigma-Aldrich), Glucose monohydrate (Cmd Chemicals, Funchal, Portugal), Betaine (>97%, TCI), D-(+)-Xylose (≥99%, Sigma-Aldrich), D-Sorbitol (≥98%, Sigma-Aldrich), Sucrose (Cmd Chemicals, Funchal, Portugal), dl-Proline (99%, Aldrich).
2.2. Preparation of NADES
NADES were prepared by gently mixing the corresponding components (see Table 1, Results) and heating the mixture at 40–60 °C, with a constant stirring, until a clear liquid was obtained [19].
Table 1.
Preparation of NADES.
| NADES | Components |
Molar Ratio | Water Content (%)* | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| TreGluW | Trehalose | Glucose | Water | 1:2:13 | 26.9 ± 0.3 | |
| TreGluSorW | Trehalose | Glucose | Sorbitol | Water | 1:2:1:13 | 17.2 ± 0.6 |
| GluProGlyW | Glucose | Proline | Glycerol | Water | 3:5:3:20 | 21.5 ± 1.3 |
| GlyGluSorW | Glycerol | Glucose | Sorbitol | Water | 1:1:1:3 | 11.9 ± 0.6 |
| BetGlySucW | Betaine | Glycerol | Sucrose | Water | 2:3:1:5 | 10.3 ± 0.4 |
| BetTreW | Betaine | Trehalose | water | 4:1:10 | 22.0 ± 0.3 | |
| GlySucSorW | Glycerol | Sucrose | Sorbitol | Water | 2:1:2:10 | 16.3 ± 0.5 |
| BetGlyTreW | Betaine | Glycerol | Trehalose | Water | 2:3:1:5 | 12.6 ± 0.3 |
| BetSucProW | Betaine | Sucrose | Proline | Water | 5:2:2:21 | 20.2 ± 0.5 |
| GlyGlu | Glycerol | Glucose | 4:1 | 3.70 ± 0.2 | ||
| GlyTreSorW | Glycerol | Trehalose | Sorbitol | Water | 2:1:2:10 | 20.4 ± 0.8 |
| FruGluTreW | Fructose | Glucose | Trehalose | Water | 1:1:1:11 | 27.1 ± 0.8 |
| ProGlySorW | Proline | Glycerol | Trehalose | Water | 1:1:1:3 | 13.0 ± 0.5 |
| BetXylW | Betaine | Xylose | Water | 2:1:3 | 24.4 ± 0.2 | |
| TreGluGlyW | Trehalose | Glucose | Glycerol | Water | 1:2:2:3 | 13.1 ± 0.2 |
| GlyFruSorW | Glycerol | Fructose | Sorbitol | Water | 1:1:1:3 | 29.5 ± 0.5 |
| TreFruW | Trehalose | Fructose | Water | 1:2:13 | 32.6 ± 0.9 | |
| FruGluSucW | Fructose | Glucose | Sucrose | Water | 1:1:1:11 | 24.5 ± 0.5 |
2.3. Polarized Optical Microscopy (POM)
Optical characterization was carried out at room temperature using a transmission mode of an Olympus BX-53-F2 (Olympus Iberia, Barcelona, Spain). A droplet of NADES was placed on a microscope glass slide and then observed. The images were obtained with an equipped camera (Olympus SC50) and software Olympus stream start 2.3.
2.4. Nuclear magnetic resonance (NMR)
The NADES were slightly diluted in dimethyl sulfoxide-d6 (Me2SO-d6, 99.9 atom%, Sigma). NMR experiments were recorded on a Bruker Advance III 400 spectrometer. Chemical shifts (δ) are expressed in ppm and are reported with the solvent reference (Me2SO-d6, δ 2.50 ppm). MestreNova 9.0 software was used for spectral processing and analysis.
2.5. Viscosity measurements
The viscosity studies on NADES were carried out using a MCR102 Modular Compact Rheometer (Anton Parr) fitted with a parallel plate geometry with 50 mm of diameter (PP50, Anton Parr) and 1 mm of gap. All the measurements were performed under controlled stress conditions and at a constant shear rate of 10 s−1. All samples were equilibrated at 25 °C for 5 min, before performing the temperature scan from 40 °C to −5 °C at 1.6 °C/min.
2.6. Differential scanning calorimetry (DSC)
DSC experiments were carried out in a DSC Q2000 from TA Instruments Inc. (TZero technology) operating in heat Flow T4P mode. The measurements were carried out under anhydrous nitrogen at a flow rate of 50 mL min-1. All samples were encapsulated in an aluminium pan with a hermetic lid and at least 2 cooling runs between −90 °C and 40 °C were performed at a rate of 10 °C/min.
Solutions of 10 wt% of Me2SO and the system GlyGlu were prepared and analysed by DSC. Also, thermograms of pure milliQ water, GlyGlu and Me2SO were obtained for comparison. Values of Tm and Tc were obtained from peak maximum.
2.7. Cell culture
Biological studies were conducted in L929 (DSMZ - German Collection of Microorganisms and cell culture GmbH) and HacaT cell lines (DKFZ, Heidelberg). L929 cells were grown in Eagle's Minimum Essential Medium (MEM, with 1.5 g/L sodium bicarbonate, non-essential amino acids, l-glutamine and sodium pyruvate, Corning), supplemented with 10% fetal bovine serum (FBS, Corning) and 1% penicillin-streptomycin (Corning). HaCaT cells were grown in Dulbelco's Modified Eagle Medium (DMEM with 4,5 g/L glucose, l-glutamine and sodium pyruvate, Corning) supplemented with 10% fetal bovine serum (FBS, Corning) and 1% penicillin-streptomycin (Corning). Both cell lines were cultured in a humidified incubator at 37 °C, with 5% CO2.
2.8. Cell viability assay
Cells were incubated for a predetermined period of time in a 96-well plate at a density of 1.0–1.5 × 104 cells/well. Each NADES was added at predetermined concentrations and incubated at 37 °C and 5% CO2. Control cells were incubated only with complete media. Cell monolayers were washed with PBS and cell viability was evaluated using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega), which is based on tetrazolium active component ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS). The amount of formazan product was measured in a microplate reader (VICTOR Nivo ™, PerkinElmer, USA) at 490 nm, as absorbance is directly proportional to the number of viable cells in culture. Cell viability was expressed as percentage of cells exposed to extracts vs control cells.
2.9. Freezing cells
After trypsinization, confluent cells were harvested and collected by centrifugation (200 g, 10 min). Then 1.5 × 106 cells/mL were resuspended in ice cold freezing media (MEM + FBS, 70:30 for L929 cells and DMEM + FBS, 90:10, for HacaT cells) containing 10% (w/v) of NADES or 10% (v/v) Me2SO. The cryovials were immediately placed in a freezing container (Corning® CoolCell™ LX Cell Freezing Container) and directly transferred to −80 °C freezer or to −20 °C for a period of 2 weeks prior to the post-thawing assays. When cells are frozen with the individual components of the eutectic system GlyGlu (4:1), described in section 3.5, the freezing media contained each component at the same molar ratio as in the mixture (4 mol for glycerol and 1 mol for glucose).
2.10. Thawing cells
The cryovials were rapidly warmed up in a 37 °C water bath for 1 min, then transferred to a falcon tube containing warmed complete media and centrifuged (200 g, 10 min). In order to test the effect of the presence of the CPA in post-thawing, after discarding the supernatant solution, the cells were resuspended in complete media containing 1% of the corresponding CPA (NADES or Me2SO) and plated in 96-well plates at 1.5 × 104 cells/well. The centrifugation and resuspension in 1% of CPA steps were only necessary to count cells and guarantee that the same number of cells were plated among all wells. Cell viability, proliferation and morphology was evaluated after 24, 48 and 72 h, and compared with those frozen using Me2SO. After 24 h of the thawing, culture media was replaced by fresh complete media. Cells were sub-cultured up to 3 passages.
2.11. DNA quantification
The Quant-IT PicoGreen dsDNA Assay Kit (Invitrogen Life Technologies, Scotland) was used for DNA quantification according to the instructions from the manufacturer. Briefly, 24 h after thawing the cells, osmotic and thermal shock was used to extract the DNA. Triplicates were collected for each sample. The absorbance was read in a microplate reader (VICTOR Nivo ™, PerkinElmer, USA), using 485 and 528 nm as excitations and emission wavelengths, respectively. The amount of DNA was calculated from a standard curve, which was constructed by measuring the absorbance of several DNA solutions with concentrations ranging from 0 to 1000 ng/mL.
2.12. Cell morphology
After 24 h, 48 h, and/or 72 h of thawing, the cells were fixed with 10% (v/v) formalin solution (neutral buffered, Sigma-Aldrich) and stained with 4,6-diamidino-2-phenyindole (DAPI, Corning) and phalloidin–tetramethylrhodamine B isothiocyanate (phalloidin, Sigma-Aldrich) to visualize F-actin filaments and cell nuclei, respectively. Briefly, DAPI (2 μg/mL) and phalloidin (2.5 μg/mL) were added to each well and incubated for 30 min at room temperature and protected from light. After washing the cells with PBS, the morphologic changes were visualized in the dark using an inverted microscope (Axio Vert A1, Zeiss, Oberkochen, Germany).
2.13. Live/dead assay
Live/Dead assay was performed after thawing the cells to evaluate the post-thawing rate survival, using calcein AM/propidium iodide (PI) staining. At predetermined timepoints, cell monolayers were incubated for 20 min at 37 °C in the presence of calcein AM (0.25 μM, Corning) and then at room temperature for 5 min with PI (20 μg/mL, EMD Millipore Corp., Burlington, MA, USA). Then, cells were washed with PBS and analysed in an inverted microscope (Axio Vert A1, Zeiss, Oberkochen, Germany).
2.14. Statistical analysis
Statistical analysis was performed using the software GraphPad Prism 7.00. For the initial screening, two-way ANOVA and Dunnet's comparison test were performed to compare statistical differences between NADES and Me2SO. Later, Tukey's comparison test was used to compare either the best systems and Me2SO, and between the best systems. Statistical differences are represented by asterisk.
3. Results
3.1. Preparation of NADES
In this work, several NADES were prepared by gently mixing the corresponding components (Table 1) and heating the mixture until a stable and clear liquid was obtained. The successful formation of NADES was confirmed by Polarized Optical Microscopy (POM) showing the absence of crystals (Figure S1). Additionally, 1D and 2D NMR studies were conducted to confirm the spatial interaction between the different components of these systems through hydrogen bonding. Clear displacement of OH group signals from the components and water is visible in proton data, while spatial interaction is visible between the OH group from NADES components and water in the 2D NOESY spectra, demonstrating that these groups are affected by the formation of hydrogen bonds.
In the case of the system GlyGlu, which has no water as component of the system, the interaction between the OH groups of glycerol and glucose is evident. Moreover, in the systems with high amount of water (>20%), such as the system TreGluW, the interaction between water molecules and the OH groups from the components is still present in NOESY spectra (Figure S2), corroborating the theory suggested by Dai et al. [10] that NADES structure is retained even when water is present up to 50% (v/v).
The NMR studies presented herein were performed with very concentrated solution of NADES in highly dried Me2SO, so that there are no interferences from the water commonly present in this deuterated solvent. However, when the systems have components that are insoluble in Me2SO (e.g., betaine) a very small amount of water was added to facilitate the analysis.
3.2. Viscosity measurements
High viscosities can restrict the diffusion processes of frozen concentrated solutions affecting cells response during the freezing process; therefore, viscosity plays an important role in cryoprotection. Most NADES are highly viscous at room temperature, though their viscosity is highly dependent on their components’ nature, water content, and temperature. On the other hand, the gold standard CPA, Me2SO, presents very low viscosity. However, pure Me2SO is known to crystalize at ca. 18 °C.
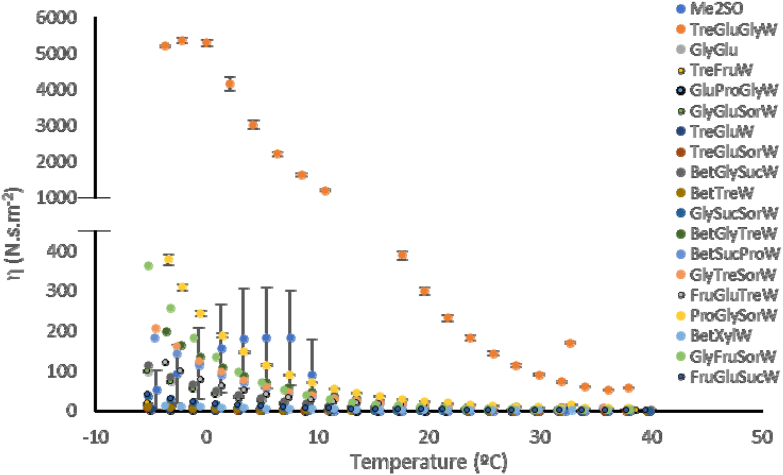
Thus, viscosity of all systems was measured and compared with Me2SO in a temperature range from −5 °C to 40 °C. In Fig. 1, differences between NADES and Me2SO viscosity can be observed. The viscosity data of the remaining systems is displayed in Figure S3.
Fig. 1.
Viscosities of NADES and Me2SO as a function of temperature, measured using a rheometer.
Most NADES are more viscous than Me2SO specially at temperature as low as −5 °C, which in some cases can be beneficial when used as CPA, though others that are too viscous compromising the desired effect. Among all NADES, TreGluGlyW showed the highest values of viscosity with values higher than 5000 N s m−2 at −5 °C, though it is not the system with the lowest amount of water. Other example is the system GlyGlu which is the system with the lowest water content (ca. 3%) although is not the more viscous one. Also, the system TreFruW has the highest water content (ca. 30%), however it is not least viscous systems. Considering these results, we can say that the viscosity depends on the water content but it is not directly proportional, and the type of components are in fact relevant for this property. Hence, it is important to understand the relevance of NADES viscosity in terms of cryoprotective properties.
3.3. Thermal behaviour of NADES
One of the major problems in cryopreservation is the risk of cell disruption by the presence of water crystals, hence the ability to avoid the formation of such crystals will lead to the design of interest components to be used as CPAs. To study the influence of the NADES in water thermal behaviour, we have prepared a solution of 10% (w/v) of Me2SO and of the system GlyGlu, and analysed them by DSC (Figure S3). The thermogram of pure GlyGlu showed only glass transition events. In Table 2 are presented the values of the melting temperatures (Tm) and crystallization temperatures (Tc) obtained from the thermograms.
Table 2.
Melting temperatures for pure water, and for mixtures of water with 10 wt% of NADES and Me2SO. Melting enthalpy values are also presented. All these values refer to the ones obtained in the second heating scan of each DSC experiment.
| Solution | Tm (°C) | ΔHm (J.g−1) | Tc (°C) |
|---|---|---|---|
| 10% GlyGlu + 90% H2O | −1.46 | 182.7 | −23.55 |
| 10% Me2SO + 90% H2O | −3.35 | 306.8 | −26.42 |
| Pure milliQ water | 5.40 | 312.9 | −9.85 °C |
From the results, we can say that 10% of NADES has a very similar influence in the thermal behaviour of water than 10% of Me2SO, with very similar melting and crystallization temperatures. Both 10% of Me2SO and 10% of GlyGlu are able to decrease the melting temperature of water by ca. 6 °C and decrease the crystallization temperature by ca. 20 °C.
3.4. Cytotoxicity evaluation
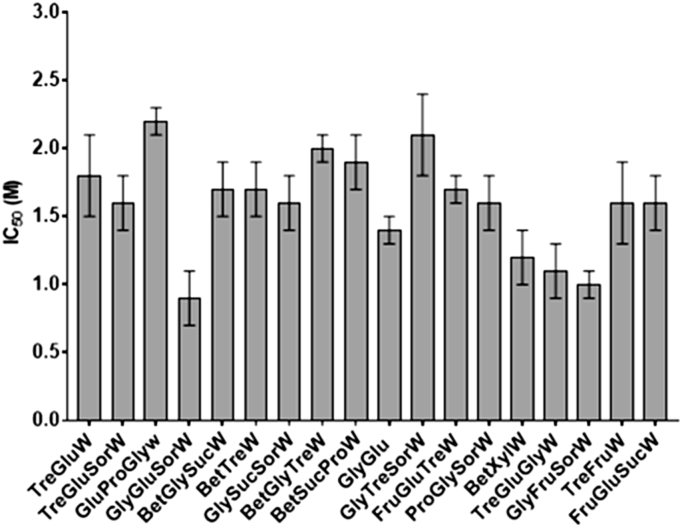
An initial screening of cytotoxicity was performed in L929 cells, commonly used for cytocompability studies, and the IC50 of all systems was determined (Fig. 2) by culturing these cells in the presence of different concentration of NADES. After a 24 h incubation period, MTS assay was used to measure cell viability. Then, the IC50 value was taken from an IC50 curve (log [NADES] vs %viability).
Fig. 2.
Inhibitory concentration (IC50) of NADES after L929 cells have been exposed for 24 h with different concentrations of the systems. IC50 was determined following MTS assay.
From the results, it is possible to see that L929 cells can tolerate high concentrations without losing their viability.
Moreover, we understand that the concentration and type of each component on the system will most likely influence the cytotoxicity. For example, BetGlySucW and BetGlyTreW only differ in one component, the first system contains sucrose while the latter contains trehalose, but the ratio between the components is the same (2:1:3:5). It is visible that sucrose confers a slightly more cytotoxic effect to the system (IC50 = 1.7 M) than trehalose (IC50 = 2.0 M).
In general, the amount of CPA in the slow-freezing protocol is <10%. Therefore, and considering the results presented in Fig. 2, only the systems with an IC50 lower than the concentration corresponding to 10% (w/v) were selected for further studies (GlyGluSorW, BetXylW, GlyFruSorW, TreFruW, FryGluSucW) (Table S1).
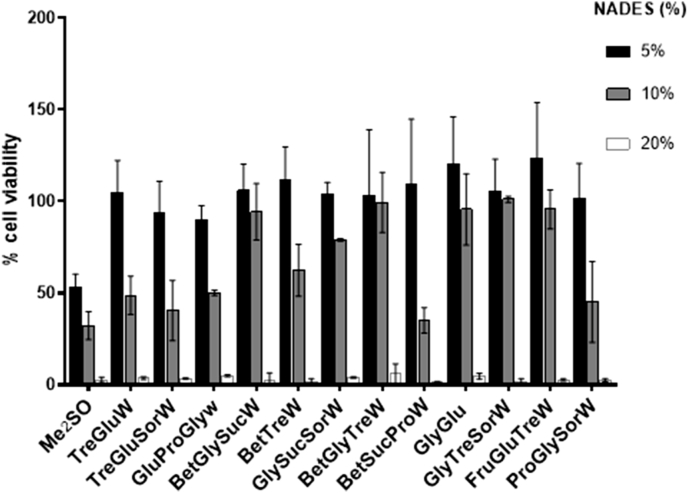
In order to anticipate the effect of these systems during the cryopreservation process and to understand the concentration limit that the cells can tolerate without significant death, we have incubated L929 cells in the presence of different amounts (5%, 10% and 20%) of each NADES for 24 h. Then, using the MTS assay the viability of cells was determined (Fig. 3).
Fig. 3.
Cell viability determined, following the MTS assay, after 24 h of incubation with different percentages of NADES.
As expected, high cell viability was obtained when cells were incubated with 5% of NADES. All systems showed viabilities two times higher than Me2SO. Moreover, L929 cells were quite tolerant to 10% of NADES, and again all systems resulted in higher cell viabilities than Me2SO. Also, at 10% the systems BetGlySucW, BetGlyTreW, GlyGlu, GlyTreSorW, and FruGluTreW presented no cytotoxicity However, 20% of all systems, including Me2SO, severely affected cell viability.
3.5. Cell recovery from freezing-thawing process
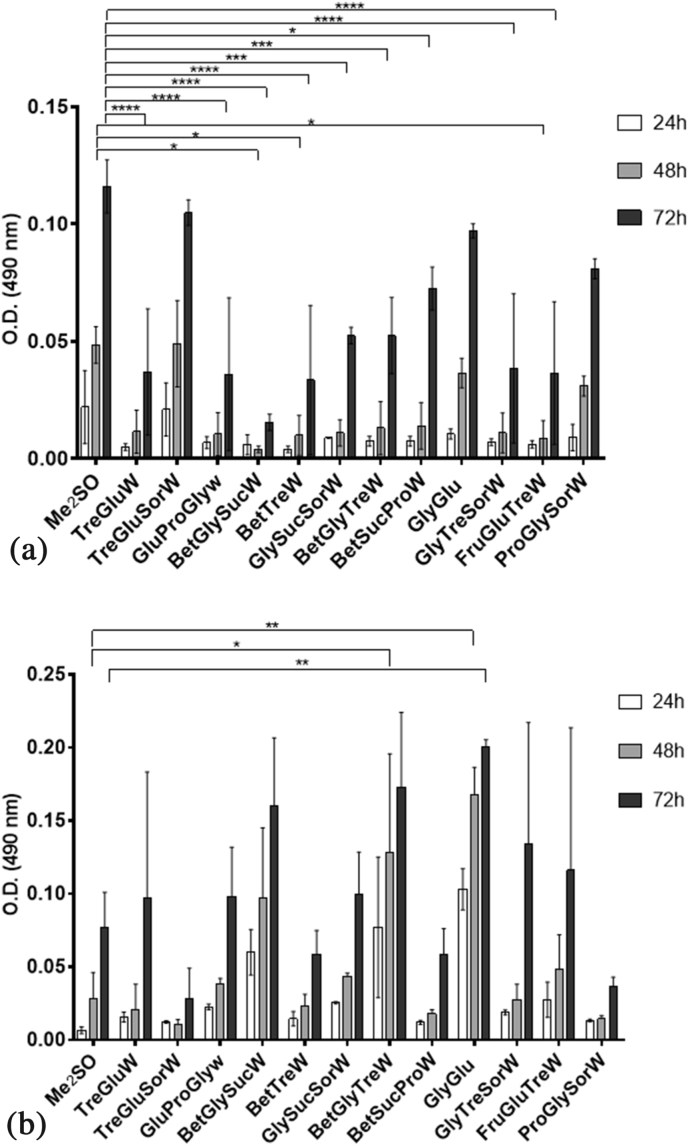
After studying the cytotoxic effect of each NADES in L929 cells, they were used as CPAs to cryopreserve both L929 and HacaT cells in freezing media composed by 90% of culture media and 10% of a CPA, either a NADES (w/v) or Me2SO (v/v)). L929 cells are very easy to culture and maintain after thawing, but HacaT cells are a more sensitive cell line, slightly more difficult to culture after thawing. Therefore, they were also chosen for this study to evaluate their resilience when frozen with NADES. The potential of each NADES as CPA was screened. The vials from each freezing media were thawed and the viability after 24 h, 48 h, and 72 h was evaluated (Fig. 4A and B).
Fig. 4.
Initial screening of the post-thawing viability, using the MTS assay, after cryopreservation with 10% (v/v) Me2SO or 10% (w/v) NADES. A. in L929 cells. Data represent means ± SD (n = 2). Statistically significant differences were determined by Dunnett's multiple comparisons test and are represented in asterisks: *p < 0.05; ***p < 0.005 and ****p = 0.0001, two-way ANOVA. The absence of asterisks means that there are no significant differences when compared with values of Me2SO at the corresponding time points. B. in HacaT cells. Statistically significant differences were determined by Dunnett's multiple comparisons test and are represented in asterisks: *p < 0.05, and **p < 0.005, two-way ANOVA. The absence of asterisks means that there are no significant differences when compared with values of Me2SO at the corresponding time points.
The results showed that for L929 cells three systems (TreGluSorW, GlyGlu and ProGlySorW) presented results very similar to Me2SO. On the other hand, for HacaT cells it was interesting to see that most NADES worked significantly better than Me2SO, especially the system GlyGlu. With the previous results in mind, we have selected the three best systems for each cell line to further study in terms of post-thawing morphology and proliferation. The results were then compared with the standard CPA, Me2SO. Therefore, for L929 cells we have selected the systems TreGluSorW, GlyGlu, and ProGlySorW and for HacaT cells we have selected BetGlySucW, BetGlyTreW, and GlyGlu.
Three different vials of both cell lines containing each formulation as CPA were rapidly thawed and cultured in the presence of 1% of each formulation to mimic the non-removal of the CPA. After 24 h and 72 h, viability, morphology, and proliferation were evaluated (Fig. 5, Fig. 6).
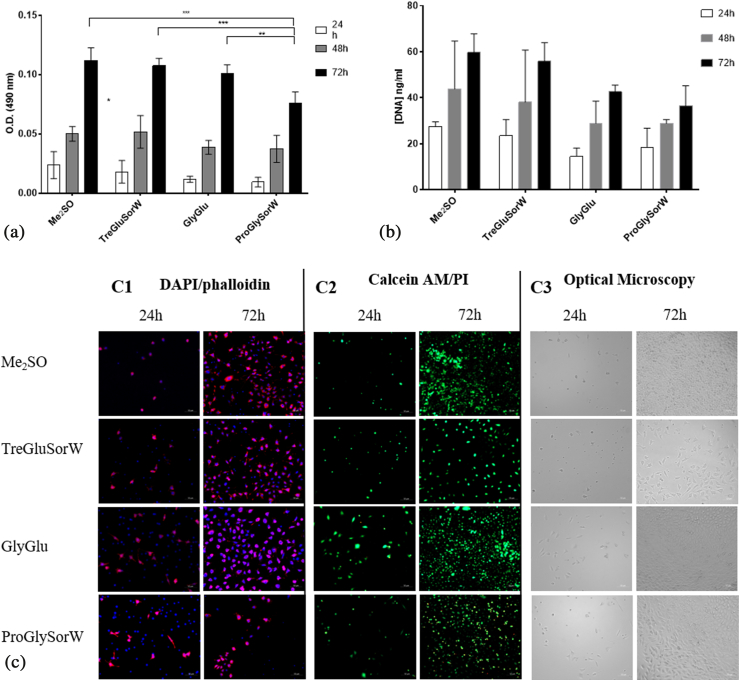
Fig. 5.
Assessment of the post-thawing recovery of L929 cells. MTS assay (A) and DNA Assay (B) after 24 h, 48 h, and 72 h of culture. Data represent means ± SD (n = 3). Statistically significant differences were determined by Tukey's multiple comparisons test and are represented in asterisks, *p < 0.05 **p < 0.02; ***p < 0.001 and ****p = 0.0001, two-way ANOVA. The absence of asterisks means that there are no significant differences between NADES and between NADES and Me2SO at the corresponding time points. Post-thawing DAPI/Phalloidin assay (cells nuclei were stained blue by DAPI and F-actin filaments in red by phalloidin) (C1), Live/Dead fluorescence assay (living cells were stained green by calcein AM and dead cells red by PI) (C2) and Optical microscopy (C3) of L929 cells at 24 h and 72 h.
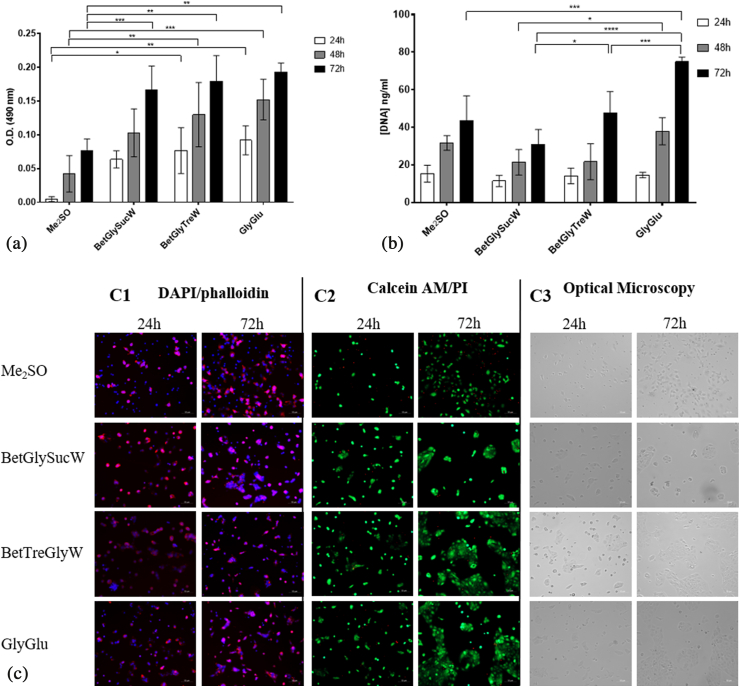
Fig. 6.
Assessment of the post-thawing recovery of HacaT cells. MTS assay (A) and DNA Assay (B) after 24 h, 48 h, and 72 h of culture. Data represent means ± SD (n = 3). Statistically significant differences were determined by Tukey's multiple comparisons test and are represented in asterisks: *p < 0.05 **p < 0.02; ***p < 0.001 and ****p = 0.0001, two-way ANOVA. The absence of asterisks means that there are no significant differences between NADES and between NADES and Me2SO at the corresponding time points. Post-thawing DAPI/Phalloidin assay (cells nuclei were stained blue by DAPI and F-actin filaments in red by phalloidin) (C1), Live/Dead fluorescence assay (living cells were stained green by calcein AM and dead cells red by PI) (C2) and Optical microscopy (C3), of HacaT cells at 24 h and 72 h.
The results indicate that for L929 cells a similar behaviour is observed when cells were cryopreserved with NADES and Me2SO, with the exception of the system ProGlySorW, which had a lower performance (Fig. 5A). In terms of DNA content (Fig. 5B), the same trend is observed, with ProGlySorW results showing a slightly lower content when compared with other NADES and Me2SO. These results are corroborated by the live/dead assay (Fig. 5C), where it is visible that the system GlyGlu is the best NADES, with very similar results when compared with Me2SO. It is relevant to mention that the system TreGluSorW is composed only by non-penetrating cryoprotectants, and yet has a similar behaviour as of Me2SO. However, the cryoprotective mechanism used by sugar molecules is still unclear.
Regarding the HacaT cells it is possible to see that NADES worked very well as CPA in this cell line, with high post-thawing viabilities. The system GlyGlu afforded a post-thawing viability two-fold higher than Me2SO, therefore being the most promising system (Fig. 6A). Significant differences can be seen in DNA content between cells cryopreserved with NADES and cells cryopreserved with Me2SO (Fig. 5A). For the system GlyGlu about two-fold increase on DNA content is observed when compared with Me2SO.
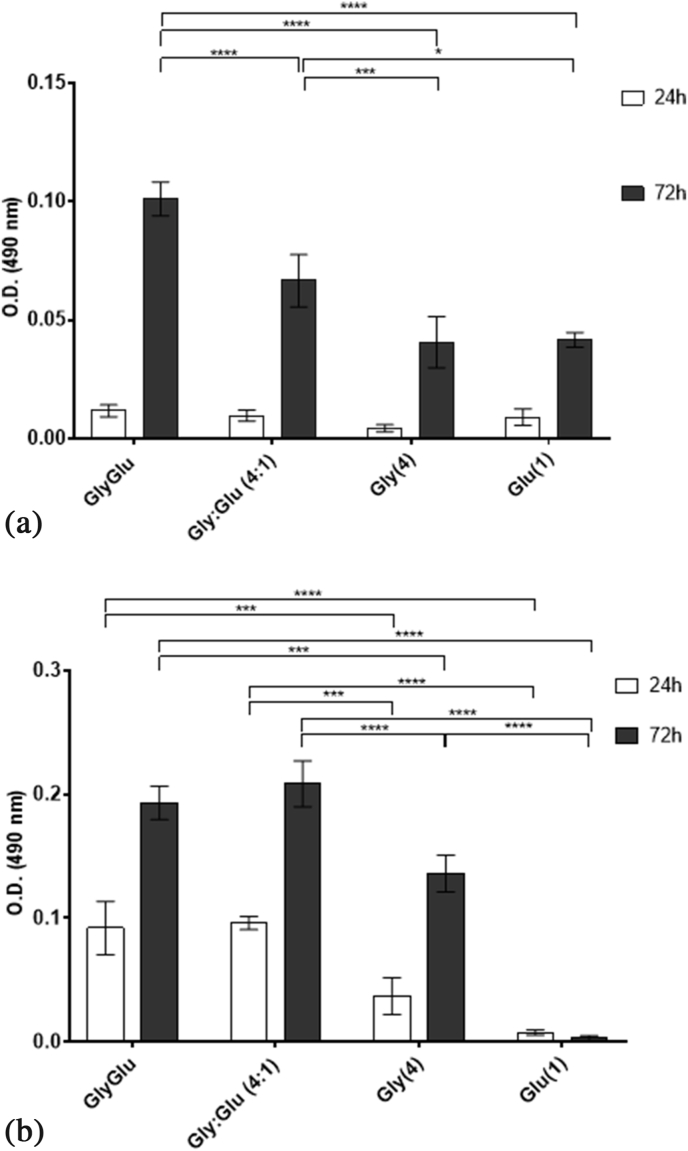
After 72 h, cells presented a normal and stretched morphology (Fig. 6-C1) and the live/dead assay confirmed the viabilities obtained by the MTS assay (Fig. 6-C2). At this point another question remained unanswered, whether the cryoprotective effect of a eutectic system results in the sum of each individual cryoprotective effect of all components of that system. Therefore, we have selected the system GlyGlu and both cell lines were frozen with glucose or glycerol in the same proportion as in the NADES. Later, the post-thawing viabilities were determined to answer the question (Fig. 7). As mentioned earlier when water is added to a NADES at a certain ratio, usually above 50% (w/v) the eutectic system becomes a eutectic mixture of those components. In this study, NADES are diluted to specific concentration when they are added to the cell cultures. Therefore, we needed to understand if the effect seen in the cells resulted from the eutectic system itself or if the effect resulted from the synergy between the compounds in solution. For example, in a different study our group have shown that a eutectic system had a higher anti-proliferative effect than the physical mixtures of the components of that system, even when diluted [22]. Hence, we have prepared the physical mixtures (e.g., a mixture of each compound in the same molar ratio than in the eutectic system) and tested it as CPA in both cell lines, for comparison. The pure components were also tested alone in the same concentration as they are in the NADES.
Fig. 7.
Comparison of the post-thawing viability, using the MTS assay, between NADES and the corresponding physical mixture and each individual component. A. in L929 cells, and B. in HacaT cells, after 24 h and 72 h of culture. Data represent means ± SD (n = 3). Statistically significant differences were determined by Tukey's multiple comparisons test and are represented in asterisks: ***p < 0.001 and ****p < 0.0001, two-way ANOVA.
In both cells lines there is a higher post-thawing viability when the NADES were used as CPA. Nevertheless, the results suggested that in the case if L929 cells the cryoprotective effect resulted in the synergistic effect of both components. However, in the case of HacaT that did not occur and the eutectic system had a better performance in terms of cryoprotection.
After thawing the cells, the same study was conducted for different passages (P1 and P2). The results showed that after the first passage, cells presented high values of viability and an improvement of proliferation is observed, suggesting that NADES did not affect cellular activity (Figure S4 and S5).
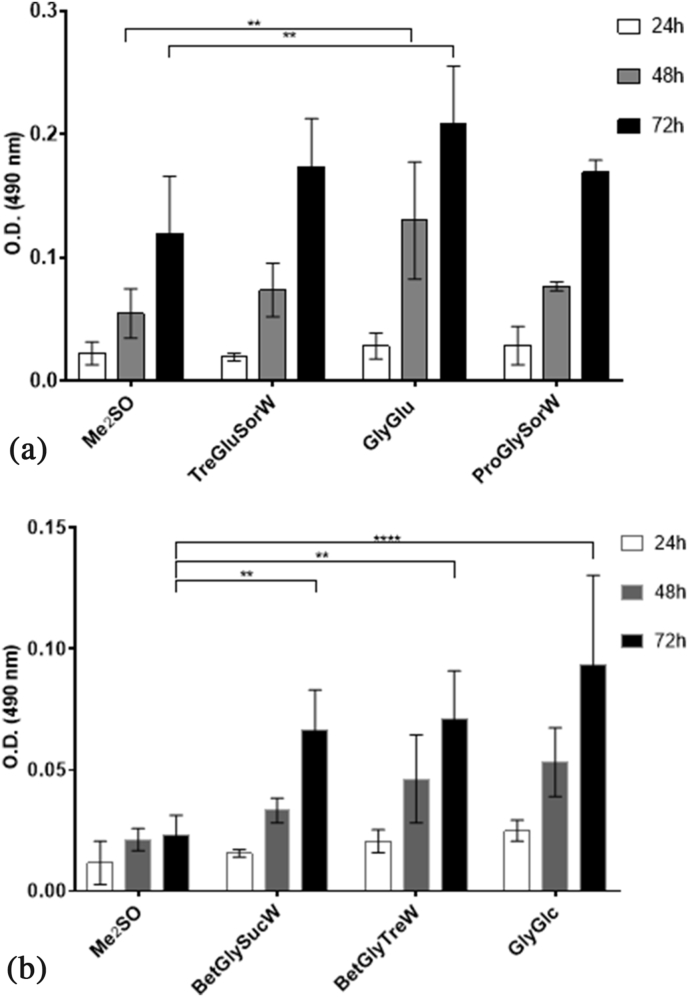
In general, cryopreservation is carried out at −80 °C or −196 °C (liquid nitrogen). However, it seemed interesting to assess the possibility of using these systems to preserve cells in a regular freezer at −20 °C, therefore cells were also frozen at −20 °C using either Me2SO or the best systems for each cell line (Fig. 8). Although experiments were carried out at different time intervals, the results presented in this report are for an exact freezing time of 2 weeks. All experiments were performed in carefully controlled conditions, including freezing time, and the results an average of 3 independent experiments (each one with triplicates).
Fig. 8.
Post-thawing viability of A. L929 cells and B. HacaT cells, frozen at −20 °C, using the MTS assay. Statistically significant differences were determined by Tukey's multiple comparisons test and are represented in asterisks: *p < 0.05; **p < 0.01 and ****p < 0.0001, two-way ANOVA.
Remarkably, the results have shown that in both cells lines, NADES were able to cryoprotect cells at −20 °C. In L929 cells, significant differences can be found between post-thawing viabilities when GlyGlu was used, after 48 h and 72 h (Fig. 8A). On the other hand, for HacaT cells all systems showed significant differences between Me2SO and NADES at the same periods of time.
4. Discussion
The goal of this work was to find alternative CPAs to overcome the toxicity problems associated to Me2SO, the gold standard CPA used. Moreover, if possible, find a system that does not have to be removed from the freezing media during the thawing step. Therefore, several NADES were designed and prepared (Table 1), composed by natural metabolites, commonly found in animals that live in extreme cold environments, such as trehalose, glucose, proline and sorbitol [15]. To prepare these systems was necessary to take in consideration the ratio between hydrogen bond donors (HBD) and hydrogen bond acceptors (HBA). Moreover, for most systems water was necessary to be added to form a eutectic mixture. The exception was the system GlyGlu. The confirmation of the successful formation of these systems was accomplished by POM and NMR. All NADES showed no crystals in POM images (Figure S1) and the displacement of OH group signals in 1H NMR proton spectra confirmed the establishment of hydrogen bonds. Through the analysis of 2D 1H–1H NOESY spectra, we observed the spatial heteromolecular interaction between water molecules and the OH groups present in the components of the NADES, supporting the establishment of hydrogen bonds between them. For the system that does not have water as a component, GlyGlu, interactions between the OH groups from glycerol and glucose were detected (Figure S2).
Most NADES are highly viscous at room temperature, mostly due to the hydrogen bond network between the components. However, the viscosity can interfere with the ability of certain compounds/mixture to be used as CPA [19] and most systems presented herein showed higher viscosities than Me2SO specially at −5 °C (Fig. 1). Interestingly, cell viability was not significantly compromised by the highly viscous systems, showing that these cells are robust enough to tolerate such viscosities, suggesting that the nature/concentration of the components present in the NADES plays the major role when it comes to their cytotoxicity.
In terms of cytotoxicity, L929 cells were able to tolerate very high concentrations of NADES, mostly due to the fact that they are metabolites present in living organisms. Furthermore, a small amount (5 wt%) of all NADES promoted cell growth due to the fact that they are mainly composed by sugars. Additionally, some NADES (TreGluW, TreGluSorW, GluProGlyW and BetSucProW) caused a decrease in cell viability when used at 10 wt%, while the others were able to maintain high cell viability after 24 h of incubation.
One of the major challenges regarding the use of Me2SO is that it needs to be removed immediately after thawing, generally by centrifugation that causes some cell death [3,4,28]. It is relevant to say that the post-thawing studies were conducted in the presence of 1% of NADES or Me2SO, to show that NADES does not need to be removed during the thawing step. Moreover, the post-thawing viabilities were obtained after centrifugation and plating, which also cause some cell death.
In this study two different cell lines were used, L929 and HacaT cells, to understand the potential of using the systems in different cell lines. In the first cell line, both NADES and Me2SO showed a very similar behaviour in terms of post-thawing viability, without affecting the morphology of cells.
Surprisingly, the post-thawing viability of HacaT cells frozen with NADES resulted in higher viability and proliferation rates than those frozen with Me2SO (Fig. 6). This cell line is very sensitive and Me2SO is even more toxic for them. Also, we believe that this cell line has a higher proliferation rate in an environment rich in sugars since they are usually in high glucose culture medium, and the eutectic systems presented in this study are all composed by sugar derivatives. Therefore, cells can recover faster and better when frozen with 10% of NADES. When the individual components of the system GlyGlu were tested to understand the effect of each compound in the freezing process (Fig. 7), both cell lines presented a higher post-thawing recovery for the DES in comparison with the individual components, suggesting that it is the synergetic effect of both components that results in the cryoprotective properties of NADES. Moreover, in L929 cells there is a slightly difference between the effect on post-thawing viability of NADES and the one conferred by the physical mixture. That may be explained by the fact that although the NADES is too diluted, there might be some interaction between glucose and glycerol that will confer a slightly stronger cryoprotective effect. On the other hand, in HacaT cells the effect was very similar between cells frozen with NADES or physical mixture, probably due to the fact that while glycerol protects the cells during the freezing process, glucose (from NADES or the mixture) helps the cells to recover after the thawing. The glucose alone will cause cell death during the freezing process.
Interestingly, NADES were also able to confer a much higher cryoprotection effect than Me2SO when both cell lines were frozen at −20 °C (Fig. 8). Although Me2SO can decrease the water crystallization point at the same proportion as GlyGlu (Figure S3) its toxicity seems to prevail at −20 °C, while in the case of NADES the ability to prevent ice crystallization overcomes the toxicity factor. The results suggest that there must be a balance between the ability to suppress water ice crystals formation and low toxicity in order to find the most suitable CPA. However, long-term studies are needed to understand if these eutectic mixtures can be used as CPA for long-term storage at this temperature.
In summary, in this work we have shown that NADES are in fact an emerging class of CPA. They have shown very low toxicity towards two different cell lines and a major advantage when compared with Me2SO, the fact that they do not need to remove from the freezing media after the cells are thawed and they can be added directly to the culture flask after dilution with complete media.
5. Conclusions
In this work we have prepared several eutectic mixtures with high potential as CPAs. These presented low cytotoxicity towards two cell lines, showing the versatility of such systems. Also, we have shown their ability to cryoprotect these cells at different temperatures. The major advantage of these systems is the fact that they are composed by biocompatible compounds and therefore they do not need to be removed after thawing the cells. This fact would be of great importance if they could be used in stem cells, bone marrow or organs for transplants, hence avoiding the toxic effects of Me2SO.
Scientists are focused on finding new approaches to promote normal, predictable and timely return to function of the cells after cryopreservation, and we do believe that NADES can contribute for those findings may be the answer to many problems found in cryobiology.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme, under grant agreement No ERC-2016-CoG 725034. This work was also supported by the Associate Laboratory for Green Chemistry – LAQV, financed by national funds from FCT/MCTES (UID/QUI/50006/2019) and by FCT/MCTES through the project CryoDES (PTDC/EQU-EQU/29851/2017), the PhD grant SFRH/BD/148510/2019 and the research contract IF/01146/2015.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cryobiol.2021.05.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bailey T.L., Wang M., Solocinski J., Nathan B.P., Chakraborty N., Menze M.A. Protective effects of osmolytes in cryopreserving adherent neuroblastoma (Neuro-2a) cells. Cryobiology. 2015;71(3):472–480. doi: 10.1016/j.cryobiol.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Baust J.M., Buskirk R.V.A.N., Baust J.G. Cell viability improves following inhibition of cryopreservation-induced apoptosis. Vitro Cell Dev. Biol. 2000;36(4):262–270. doi: 10.1290/1071-2690(2000)036<0262:cvifio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Baust J.G., Gao D., Baust J.M. Cryopreservation, Organogenesis. 2009;5(3):90–96. doi: 10.4161/org.5.3.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best B.P. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res. 2015;18:422–436. doi: 10.1089/rej.2014.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan S.S., Gross S.A., Acker J.P., Toner M., Carpenter J.F., Pyatt D.W. Cryopreservation of stem cells using trehalose: evaluation of the method using a human hematopoietic cell line. Stem Cell. Dev. 2004;13(3):295–305. doi: 10.1089/154732804323099226. [DOI] [PubMed] [Google Scholar]
- 6.Castro V.I.B., Craveiro R., Silva J.M., Reis R.L., Paiva A., Duarte A.R.C. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology. 2018;83:15–26. doi: 10.1016/j.cryobiol.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Chemat F., Vian M.A., Ravi H.K., Khadhraoui B., Hilali S., Serino P., Tixier A.F. Review of alternative solvents for green extraction of food and natural products: panorama, principles, applications and prospects. Molecules. 2019;24:3007. doi: 10.3390/molecules24163007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y.H., van Spronsen J., Dai Y., Verberne M., Hollmann F., Arends I.W.C.E., Verpoorte R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011;156(4):1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craveiro R., Aroso I., Flammia V., Carvalho T., Viciosa M.T., Dionísio M., Barreiros S., Reis R.L., Duarte A.R.C., Paiva A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016;215:534–540. 2016. [Google Scholar]
- 10.Dai Y., Witkamp G.-J., Verpoorte R., Choi Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14–19. doi: 10.1016/j.foodchem.2015.03.123. 2015. [DOI] [PubMed] [Google Scholar]
- 11.Dou M., Lu C., Sun Z., Rao W. Natural cryoprotectants combinations of L-proline and trehalose for red blood cells cryopreservation. Cryobiology. 2019;91:23–29. doi: 10.1016/j.cryobiol.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Eroglu A. Cryopreservation of Mammalian Oocytes by Using Sugars: intra and extracellular raffinose with small amounts of dimethylsulfoxide yields high cryosurvival, fertilization, and development rates. Cryobiology. 2010;60(3S):S54–S59. doi: 10.1016/j.cryobiol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertrudes A., Craveiro R., Eltayari Z., Reis R.L., Paiva A., Duarte A.R.C. How do animals survive extreme temperature amplitudes? The role of natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017;5:9542–9553. [Google Scholar]
- 14.Hara J., Tottori J., Anders M., Dadhwal S., Asuri P., Mobed-Miremadi M. Trehalose effectiveness as a cryoprotectant in 2D and 3D cell cultures of human embryonic kidney cells. Artif. Cells, Nanomed., Biotechnol. 2017;45(3):609–616. doi: 10.3109/21691401.2016.1167698. [DOI] [PubMed] [Google Scholar]
- 15.Jan H., Hong-Mei H., Oliver H., Ingrid V.R., Philippe I.H.B., Markus G. Direct measurement of water states in cryopreserved cells reveals tolerance toward ice crystallization. Biophys. J. 2016;110(4):840–849. doi: 10.1016/j.bpj.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesus A.R., Meneses L., Duarte A.R.C., Paiva A. Natural deep eutectic systems—a new era of cryopreservation. Adv. Bot. Res. 2021;97 ISSN 0065-2296. [Google Scholar]
- 17.Katenz E., Vondran F.W.R., Schwartlander R., Pless G., Gong X., Cheng X., Neuhaus P., Sauer I.M. Cryopreservation of primary human hepatocytes: the benefit of trehalose as an additional cryoprotective agent. Liver Transplant. 2007;13(1):38–45. doi: 10.1002/lt.20921. [DOI] [PubMed] [Google Scholar]
- 18.Martinetti D., Colarossi C., Buccheri S., Denti G., Memeo L., Vicari L. Effect of trehalose on cryopreservation of pure peripheral blood stem cells. Biomedical Reports. 2017;6(3):314–318. doi: 10.3892/br.2017.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneses L., Santos F., Gameiro A.R., Paiva A., Duarte A.R.C. Preparation of binary and ternary deep eutectic systems. JoVE. 2019;152 doi: 10.3791/60326. [DOI] [PubMed] [Google Scholar]
- 21.Ntai A., La Spada A., De Blasio P., Biunno I. Trehalose to cryopreserve human pluripotent stem cells. Stem Cell Res. 2018;31:102–112. doi: 10.1016/j.scr.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Paiva A., Craveiro R., Aroso I., Martins M., Reis R.L., Duarte A.R.C. Natural deep eutectic solvents – solvents for the 21st century. ACS Sustain. Chem. Eng. 2014;2(5):1063–1071. [Google Scholar]
- 24.Dludlaa Phiwayinkosi V., Jacka Babalwa, Viraragavana Amsha, Pheiffera Carmen, Johnsona Rabia, Louwa Johan, Christo J., Muller F. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicology Reports. 2018;5:1014–1020. doi: 10.1016/j.toxrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pojprasath T., Lohachit C., Techakumphu M., Stout T., Tharasanit T. Improved cryopreservability of stallion sperm usinga sorbitol-based freezing extender. Theriogenology. 2011;75:1742–1749. doi: 10.1016/j.theriogenology.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Sharp D.M.C., Picken A., Morris T.J., Hewitt C.J., Coopman K., Slater N.K.H. Amphipathic polymer-mediated uptake of trehalose for dimethyl sulfoxide-free human cell cryopreservation. Cryobiology. 2013;67(3):305–311. doi: 10.1016/j.cryobiol.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokich B., Osgood Q., Grimm D., Moorthy S., Chakraborty N., Menze M.A. Cryopreservation of hepatocyte (HepG2) cell monolayers: impact of trehalose. Cryobiology. 2014;69(2):281–290. doi: 10.1016/j.cryobiol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Sun H., Glasmachek B., Hofmann N.m. Compatible solutes improves cryopreservation of human endothelial cells. Cryoletters. 2012;33(6):485–493. [PubMed] [Google Scholar]
- 29.Villaverde A.I.S.B., Fioratti E.G., Penitenti M., Ikoma M.R.V., Tsunemi M.H., Papa F.O., Lopes M.D. Cryoprotective effect of different glycerol concentrations on domestic catspermatozoa. Theriogenology. 2013;80:730–737. doi: 10.1016/j.theriogenology.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Vysotskaya O.N., Popov A.S., Butenko R.G. The advantage of glucose over sucrose in cryopreservation of strawberry meristems. Russ. J. Plant Physiol. 1999;46(2):255–257. [Google Scholar]
- 32.Wu C.F., Tsung H.C., Zhang W.J., Wang Y., Lu J.H., Tang Z.Y., Kuang Y., Jin W., Cui L., Liu W., Cao Y.L. Improved cryopreservation of human embryonic stem cells with trehalose. Reprod. Biomed. Online. 2005;11(6):733–773. doi: 10.1016/s1472-6483(10)61692-6. [DOI] [PubMed] [Google Scholar]
- 33.Yang J., Cai N., Zhai H., Zhang J., Zhu Y., Zhang L. Natural zwitterionic betaine enables cells to survive ultrarapid cryopreservation. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M., Oldenhof H., Sieme H., Wolkers W.F. Freezing-induced uptake of trehalose into mammalian cells facilitates ccryopreservation. Biochim. Biophys. Acta. 2016;1858:1400–1409. doi: 10.1016/j.bbamem.2016.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.