Figure 4.
INH binding to KatG
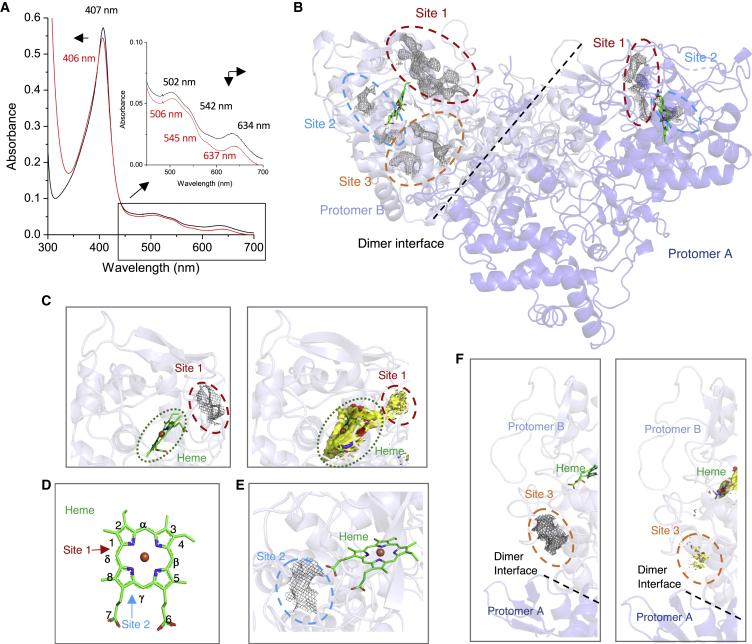
(A) UV-visible spectroscopy of WT KatG protein before and after the addition of INH. Black line shows WT resting state ferric KatG (6 μM) before the addition of INH and the red line after the addition of three INH equivalents. Inset shows the Q band region = and arrows indicate changes in wavelength and absorbance. All experiments were carried out at 20°C in 20 mM sodium phosphate, pH 7, 100 mM NaCl.
(B) Sites of INH binding to WT KatGINH. Protomer A is shown in purple and protomer B in light purple. Extra density identified is shown as a gray mesh. The three areas of extra density are circled and colored red for site 1, blue for site 2, and orange for site 3.
(C) Extra density for site 1 in protomer B near the heme corresponding to an identified hotspot (contour 14 cutoff, see the STAR methods). The hot spots are shown in yellow (hydrophobic), blue (hydrogen donor), and red (hydrogen acceptor), and the extra density for INH as a gray mesh. The heme is indicated by a green dashed circle and the binding site of INH as a red dashed circle.
(D) Heme b nomenclature with sites 1 and 2 shown with arrows and labeled.
(E) Extra density for site 2 in protomer B identified near the heme propionate groups. Site 2 is indicated with a blue dashed circle.
(F) Enlarged site 3 hotspot (cutoff contour 14), with the hotspot shown in yellow (hydrophobic), blue (hydrogen donor), and red (hydrogen acceptor), and extra density from INH binding shown as a gray mesh with site 3 indicated with an orange dashed circle.