Abstract
Objective
We aimed to examine the effects of isometric handgrip (IHG) training on home blood pressure (BP) levels in hypertensive Japanese patients undergoing treatment.
Methods
Fifty-three hypertensive patients (mean age, 61.7 years; 56.6% men) with a home systolic BP ≥135 mmHg and/or a home diastolic BP ≥85 mmHg were randomly assigned to either group A or B. As per the crossover design, group A performed 8 weeks of IHG training, followed by an equivalent training-free, control period, while the reverse protocol was performed by group B. The baseline characteristics were similar between both groups. The individualized daily IHG training comprised four sets of 2-min isometric contractions at 30% of the individual’s maximum voluntary contraction capacity, including 1 min of rest between sets, for ≥3 days/week. The outcome measure was morning and evening home BP readings taken over the last 2 weeks of the training and control periods.
Results
A combined data analysis for both groups showed that IHG training was significantly associated with the lowering of both systolic and diastolic BP in the morning (137.9±9.3 vs. 135.3±9.5 mmHg, p=0.007 and 83.0±9.5 vs. 81.2±9.3 mmHg, p<0.001, respectively) and evening (130.0±10.7 vs. 127.6±10.1 mmHg, p=0.003 and 75.8±10.4 vs. 73.8±9.2 mmHg, p<0.001, respectively), while no significant change was observed after the control period. A larger increase in the maximum grip strength due to IHG training was associated with greater BP reductions.
Conclusion
An 8-week period of IHG training significantly lowered both the morning and evening home BP in hypertensive Japanese patients undergoing treatment.
Keywords: home blood pressure, isometric handgrip training, hypertension, non-pharmacological treatment, grip strength
Introduction
Hypertension is a leading cause of cerebrovascular and cardiovascular diseases (1,2). Non-pharmacological treatments, such as a reduction of both salt and alcohol consumption, smoking cessation, and increase in physical activity, constitute basic preventive strategies that should be practiced by all hypertensive patients (3-5). Following a daily regimen of moderate aerobic exercise for ≥ 30 min is also recommended for hypertension treatment (6). A meta-analysis demonstrated that simple resistance training or isometric handgrip (IHG) training could significantly decrease blood pressure (BP) (7). IHG training is very practical because it does not require any special space or expensive equipment (8). This indoor exercise modality, unlike jogging or walking, is not limited by weather conditions. Additionally, the training is feasible for individuals who report difficulty in exercising either due to limited space, time constraints, or concurrent physical impairments, such as lower limb disability. Moreover, the Prospective Urban Rural Epidemiology (PURE) study (9) reported higher handgrip strength as an independent predictor for cardiovascular diseases, suggesting that IHG training might have favorable cardiovascular effects other than BP control.
The effects of IHG training on BP have been examined in different populations, including hypertensive (10-12) and normotensive (8,13) subjects, who demonstrated a significant decrease in BP following IHG training. However, the studies used BP values evaluated at a clinic as outcome measures. Moreover, most of the subjects were Caucasian, with only a few studies including Asians. The etiology of hypertension among Asians differs significantly from that in Caucasians owing to genetics (14,15) and lifestyles (16). To our knowledge, only one study has previously examined the effects of IHG training in untreated, hypertensive Japanese patients, and the study found that both brachial and central BP decreased significantly after 8 weeks of training (17). Although this result suggests that IHG training may lead to BP reduction in Asian subjects, more evidence of the beneficial effects of IHG training in this population is required. To the best of our knowledge, the effects of this exercise modality on home BP values have not yet been reported.
Using a randomized crossover study, we aimed to investigate our hypothesis that IHG training may significantly reduce home BP values in hypertensive Japanese patients. Home BP measurements are known to correlate with end organ damage in hypertensive patients and are considered better markers for predicting the risk of cardiovascular events than clinic BP values (6,18-20). We believe that if it is verified that IHG training reduces home BP measurements, the finding would validate the clinical and practical utility of this simple exercise modality.
Materials and Methods
Study design and patients
In this multicenter crossover study, we included outpatients who visited the clinics of Tohoku, Fukushima, and Aomori Rosai hospitals. The study was conducted in compliance with the Helsinki declaration for the study of human subjects and was registered in the UMIN Clinical Trial Registry (No. 000035866). The institutional review board of Tohoku Rosai Hospital approved the study protocol, and all study participants provided their written informed consent prior to study participation.
We recruited hypertensive patients who routinely monitored and recorded their BP at home. Patients with a mean systolic BP of ≥135 mmHg and/or those with a mean diastolic BP of ≥85 mmHg, measured in the morning over 5 days, met the study's eligibility criteria. The exclusion criteria were: 1) clinic BP measurement of ≥180/110 mmHg, 2) history of cerebrovascular or cardiovascular events within the previous 6 months, 3) concurrent chronic kidney disease of stage G3b or lower (21), 4) ongoing cancer treatment, and 5) ongoing hormone replacement therapy. Finally, 53 eligible patients (56.6% men, mean age = 61.7 years) were registered for the study.Height (cm) was measured manually using a height meter (BSM 370, InBody Japan, Tokyo, Japan). Abdominal circumference was measured at the level of the umbilicus at the end of expiration. Body weight and body mass index (BMI) were examined using a body composition analyzer (InBody 720, InBody Japan) (22).
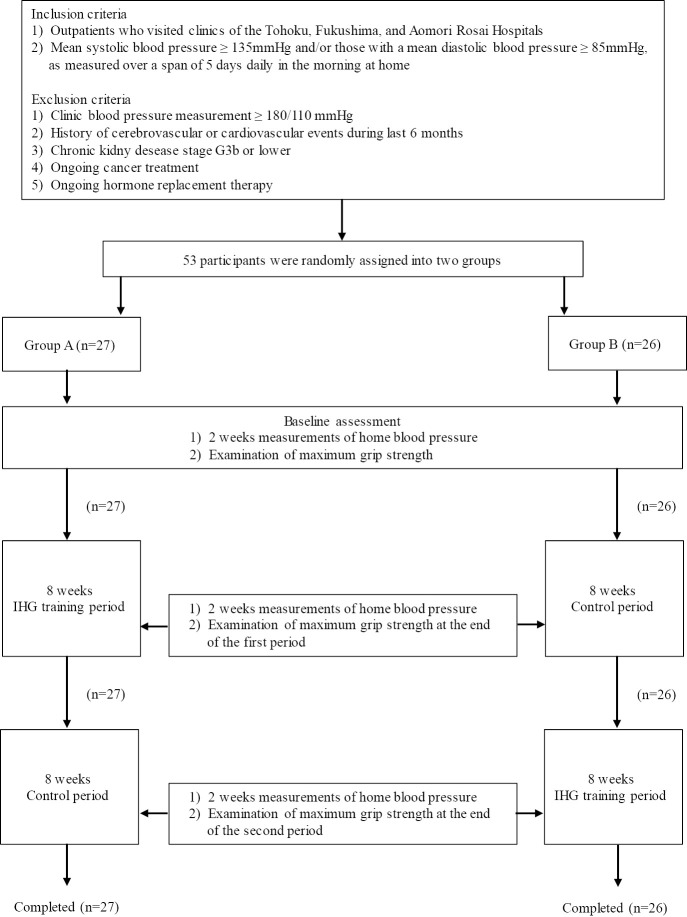
In this prospective crossover study conducted over 16 weeks, the patients were first randomized into either group A or B using the envelope method. Fig. 1 shows the outline of the study. After the baseline assessment, group A patients performed IHG training for an 8-week period, which was followed by a similar length of training-free, control period. Conversely, group B patients underwent the training regimen in the latter 8 weeks, with an initial exercise-free 8-week period.
Figure 1.
Flowchart of the study. All participants duly completed the study protocol. IHG training: isometric handgrip training
The IHG training protocol was determined according to previous reports (8,10-13). During the baseline assessment, the maximum grip strengths of both hands were determined for each participant. The subjects were asked to hold a handgrip spring dynamometer (GRIP-D, TAKEI, Niigata, Japan) in the right hand using a full grip. The subject compressed the handles of the dynamometer with the application of maximum effort for a few seconds. After adequate rest, the maximum grip of the left hand was examined in a similar fashion. Two measurements of grip strength were obtained for each hand, and the mean of the maximum grip strength values for each hand was regarded as the maximal grip strength. The examination of maximum grip strength was repeated at the end of the first and second 8-week periods (Fig. 1).
The subjects were guided to perform IHG training at home or at their workplace using a suitable device (DIGI-FLEX, FEI). We prepared four kinds of spring handgrip training devices with different levels of resistance (light, 4.54 kg; medium, 7.26 kg; heavy, 10.43 kg; very heavy, 14.06 kg). Each subject was given a device whose resistance level was close to 30% of his/her maximal isometric contraction. The subjects were asked to perform four sets of isometric handgrip contractions in the standing position using each hand for a 2-min duration, with 1 min of rest between sets. The isometric exercise was to be performed at least 3 days a week for 8 weeks. During the control period, subjects were instructed to continue their routine lifestyle. Antihypertensive medications were not changed during the entire study period unless marked BP fluctuations were observed.
Measurement of home BP
The patients were asked to measure their BP at home every morning and evening at baseline and during the last 2 weeks of the first and second 8-week periods (Fig. 1). Validated oscillometric monitors (HEM-7080IT Omron Healthcare, Kyoto, Japan) were supplied to all participants for self-monitoring of BP. The subjects measured their BP after resting for ≥2 min in a sitting position, within 1 hour of waking up in the morning (before breakfast and prior to taking antihypertensive medications) and before going to bed in the evening, as outlined in the Japanese guidelines for the management of hypertension (23). Two measurements were obtained at each time, and the mean BP was used as the representative value.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD) or as percentages. Group comparisons were performed using the t-test and Chi-square test for continuous and categorical variables, respectively. Inter-group differences were examined using the paired t-test. All statistical analyses were performed using the JMP (version 14.0 for Windows; SAS Institute, Cary, USA) software. A p value <0.05 was considered to be statistically significant.
Results
A total of 40, 9, and 4 participants were recruited from Tohoku, Fukushima, and Aomori Rosai hospitals, respectively. Of these 53 participants, 27 and 26 participants were randomly assigned to groups A and B, respectively. Participants in both groups duly completed the outlined study protocol (Fig. 1). Table 1 shows the baseline characteristics of all participants. The groups did not differ significantly in age, sex, BMI, maximum isometric strength, baseline home BP, heart rate, and prescribed antihypertensive medications. The frequencies of comorbidities such as diabetes, dyslipidemia, and hyperuricemia were also comparable between both study groups. None of the subjects required a change in antihypertensive medications throughout the study period.
Table 1.
Patient Characteristics.
| Variables | Group A (n=27) | Group B (n=26) | p value |
|---|---|---|---|
| Age, years | 62.3±11.7 | 61.2±13.3 | 0.748 |
| Sex (male), % | 51.9 | 61.5 | 0.477 |
| Height, cm | 163.5±10.2 | 163.2±10.9 | 0.911 |
| BMI, kg/m2 | 25.2±3.1 | 25.5±3.9 | 0.786 |
| Waist circumference, cm | 88.1±4.7 | 91.3±12.0 | 0.262 |
| Maximum grip strength, kg | 34.6±12.6 | 33.1±10.9 | 0.797 |
| Morning HSBP, mmHg | 136.9±8.2 | 141.0±11.1 | 0.129 |
| Morning HDBP, mmHg | 81.9±10.1 | 84.8±8.1 | 0.266 |
| Morning HHR, bpm | 66.8±9.5 | 67.2±8.1 | 0.860 |
| Evening HSBP, mmHg | 129.3±9.3 | 132.6±12.9 | 0.286 |
| Evening HDBP, mmHg | 75.4±10.7 | 77.0±9.5 | 0.574 |
| Evening HHR, bpm | 71.1±9.0 | 72.6±10.9 | 0.609 |
| Diabetes, % | 3.7 | 7.7 | 0.529 |
| Dyslipidemia, % | 37.0 | 26.9 | 0.430 |
| Hyperuricemia, % | 7.4 | 11.5 | 0.607 |
| Medication for hypertension, % | 74.1 | 76.9 | 0.810 |
| Calcium channel blocker, % | 51.9 | 61.5 | 0.477 |
| Angiotensin II receptor blocker, % | 48.2 | 65.4 | 0.206 |
| Diuretic, % | 25.9 | 23.1 | 0.810 |
| β blocker, % | 0.0 | 7.7 | 0.142 |
| Others, % | 14.8 | 15.4 | 0.954 |
| Medication for diabetes, % | 3.7 | 7.7 | 0.530 |
| Medication for dyslipidemia, % | 37.0 | 26.9 | 0.430 |
BMI: Body mass index, HSBP: home systolic blood pressure, HDBP: home diastolic blood pressure, HHR: home heart rate
Data are shown as mean±SD or percentage.
Table 2 shows values for morning and evening BP, heart rate, and maximum grip strength at baseline and at 8 and 16 weeks for both groups. The period of intervention was the first 8 weeks for group A and the latter 8 weeks for group B. In group A, both systolic and diastolic BP were significantly lowered in the morning and evening during the 8 weeks of intervention (p<0.05 and p<0.001, respectively). A similar tendency was observed in group B, although the fall in BP was not statistically significant. At 16 weeks, morning systolic BP (p<0.05) and both morning and evening diastolic BP (p<0.01 and p<0.05, respectively) were significantly lower than the baseline levels in group A. In group B, at 16 weeks, morning systolic BP (p<0.05) and evening systolic (p<0.01) and diastolic (p<0.05) BP readings were significantly lower than those at baseline. In addition, evening systolic and diastolic BP readings at 16 weeks were significantly lower than the values at 8 weeks (p<0.05 for both).
Table 2.
Group-wise Changes in Home Blood Pressure and Maximum Grip Strength between Baseline and 16 Weeks.
| Variables | Group A (n=27) | Group B (n=26) | ||||
|---|---|---|---|---|---|---|
| Baseline | 8 weeks | 16 weeks | Baseline | 8 weeks | 16 weeks | |
| Morning HSBP, mmHg | 136.9±8.2 | 134.0±8.1* | 133.0±7.3* | 141.0±11.1 | 138.9±10.4 | 136.7±10.8* |
| Morning HDBP, mmHg | 81.9±10.1 | 79.5±9.5** | 79.7±9.0** | 84.8±8.1 | 84.2±8.9 | 83.0±8.9 |
| Morning HHR, bpm | 66.8±9.5 | 68.2±9.6 | 68.2±8.7 | 67.2±8.1 | 68.5±8.0* | 69.2±10.2 |
| Evening HSBP, mmHg | 129.3±9.3 | 126.7±9.8* | 126.3±8.4 | 132.6±12.9 | 130.7±12.2 | 128.6±10.5**† |
| Evening HDBP, mmHg | 75.4±10.7 | 73.1±9.9** | 73.5±9.3* | 77.0±9.5 | 76.2±10.2 | 74.6±8.5*† |
| Evening HHR, bpm | 71.1±9.0 | 72.1±9.7 | 72.2±9.3 | 72.6±10.9 | 72.2±9.6 | 72.3±10.2 |
| Maximum grip strength, kg | 34.6±12.6 | 35.2±14.4 | 35.2±14.5 | 33.1±10.9 | 33.5±11.3 | 34.2±11.1* |
HSBP: home systolic blood pressure, HDBP: home diastolic blood pressure, HHR: home heart rate
Data are shown as mean±SD or percentage.
*p<0.05 vs. baseline,**p<0.01 vs. baseline,†p<0.05 vs. 8 weeks.
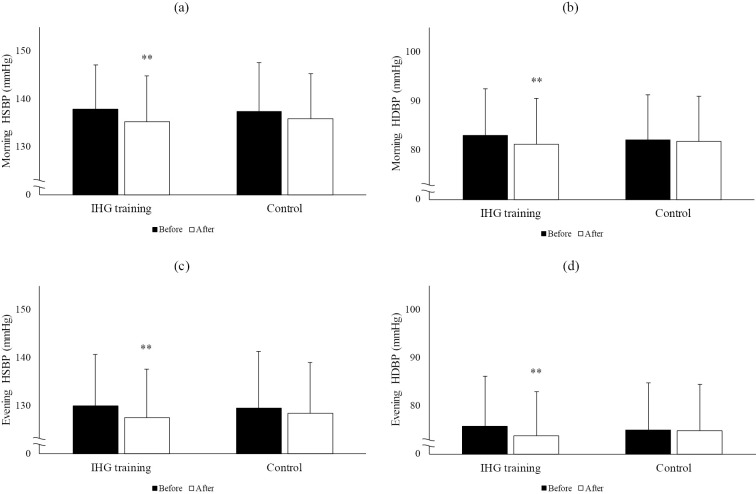
On a combined assessment of the data of both groups, IHG training was found to be significantly associated with a reduction of both the systolic and diastolic BP in the morning (systolic, p=0.007; diastolic, p<0.001) and evening (systolic, p=0.003; diastolic, p<0.001 for diastolic), while no significant changes in BP were observed during the training-free control period (Fig. 2). The heart rate remained unchanged in the training and control periods. The maximum grip strength increased significantly during the exercise intervention, while no change in maximum grip strength was observed during the control period (Fig. 3).
Figure 2.
Changes in the home blood pressure values before and after the control and IHG training periods. Each Figure shows (a) morning HSBP, (b) morning HDBP, (c) evening HSBP, (d) evening HDBP. IHG training: isometric handgrip training, HSBP: home systolic blood pressure, HDBP: home diastolic blood pressure. **p<0.01 vs. before.
Figure 3.
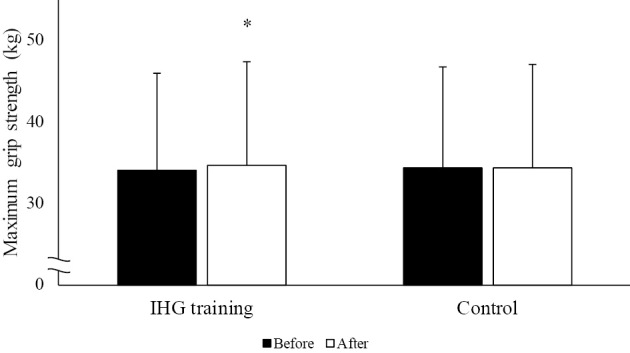
Changes in the maximum grip strength before and after the control and IHG training periods. IHG training: isometric handgrip training. *p<0.05 vs. before.
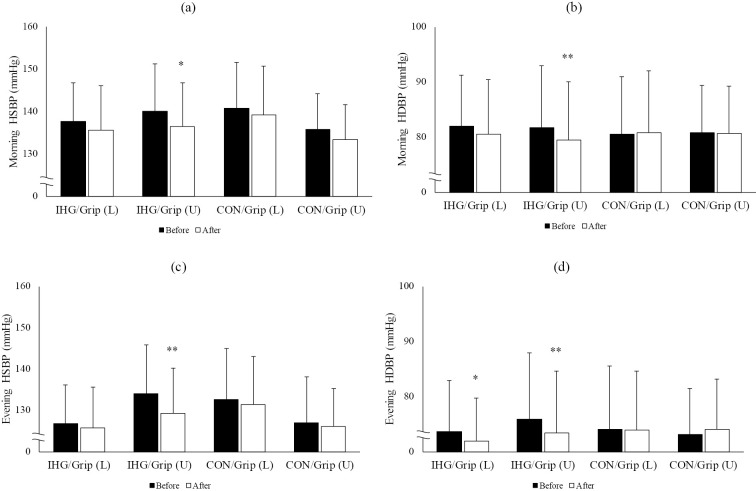
To further confirm the link between the changes in grip strength and blood pressure reduction, we compared the hypotensive effects of IHG training according to the changes in grip strength after the intervention (Fig. 4). The subjects were divided into two groups based on the changes in grip strength - an upper half and a lower half [IHG/Grip (U) and IHG/Grip (L), respectively]. The IHG/Grip (U) group showed a significant reduction in both morning and evening systolic and diastolic BPs compared to baseline, while the IHG/Grip (L) group showed a significant reduction in evening diastolic BP alone. We also performed a similar analysis using data collected just before and at the end of the control period. Again, the subjects were divided into two groups based on changes in grip strength - an upper [CON/Grip (U)] and a lower [CON/Grip (L)] half. There was no significant change in systolic or diastolic BP in the CON/Grip (U) and CON/Grip (L) groups, although the CON/Grip (U) group tended to show a greater BP reduction than the CON/Grip (L) group.
Figure 4.
Changes in the home blood pressure values according to the lower or upper half increase in maximum grip strength during the control or IHG training period. Grip (L) and grip (U) indicate lower and upper half increase in grip strength during a given period. (a), (b), (c), and (d) represent morning HSBP, morning HDBP, evening HSBP, and evening HDBP, respectively. CON: control period of group B, IHG: isometric handgrip training period, HSBP: home systolic blood pressure, HDBP: home diastolic blood pressure. *p<0.05 vs. before, **p<0.01 vs. before.
Discussion
We used a randomized crossover study design to examine the effects of IHG training on the resting BP in hypertensive Japanese patients undergoing treatment. The main outcome measure was the home BP level, which is superior to clinic-measured BP with respect to prognostic significance. We found that 8 weeks of IHG training significantly lowered both morning and evening BP. As shown in Table 2, the decreases in morning systolic and diastolic BP levels were 2.3 and 1.7 mmHg, respectively. Similarly, evening systolic and diastolic BP readings decreased by 2.1 and 1.8 mmHg, respectively. Our data are consistent with those of previous studies which applied similar exercise modalities and demonstrated an association between exercise and significantly decreased clinic BP readings in both Caucasian and Japanese hypertensive patients (10-12,17). The Ohasama study showed that an increase in the mean home systolic (10 mmHg) and diastolic (5 mmHg) BP measurements over 2 weeks was associated with a 31% and 20% increase in the risk of stroke and transient ischemic attacks, respectively (20). The real-world, large-scale prospective, observational home blood pressure measurement with Olmesartan Naïve patients to establish standard target blood pressure (HONEST) study involving >20,000 hypertensive Japanese patients receiving treatment demonstrated that the risk of cardiovascular events was lowest in patients with morning, home systolic BP readings of ≤124 mmHg, while the risk significantly increased when the controlled clinic systolic BP values were ≥145 mmHg (24). These results highlight the importance of lowering morning home systolic BP to 124 mm Hg, to minimize the risk of the occurrence of cardiovascular complications. In the present study, we used the mean home BP readings taken over 2 weeks as the outcome measure, and could therefore show the significant effects of IHG training in achieving better BP control.
The mechanisms of BP reduction due to IHG training are not well understood although favorable effects on vascular function may be involved. It has been shown that 4-8 weeks of IHG training significantly improved endothelial function, assessed using flow-mediated dilation, in hypertensive patients (25) and in those with chronic heart failure (26,27). A similar outcome was also observed in the elderly (28). Moreover, a similar exercise modality has been reported to significantly increase forearm blood flow in young and middle-aged populations (29), suggesting a mechanism of functional improvement in flow-mediated vasodilation.
As shown in Table 2, the BP reduction was slightly less in group B than in group A. This may have been due to the slightly lower pre-intervention BP levels (1.9-2.1 mmHg) in group B, which was possibly due to a possible placebo effect. Thus, the BP lowering effect of IHG training may have been underestimated in group B compared to group A. However, the BP values at 16 weeks were significantly lower than the values at baseline, even in group B patients (Table 2).
Moderate-intensity IHG exercise of up to 30% of the maximum voluntary muscle contraction capacity of the patient is reportedly safe because it is unlikely to increase cardiovascular risk by potentiating cardiac vagal activity, leading to an increased heart rate (10,30). In our study, the heart rate remained unchanged after IHG training; a finding which corroborated with the results of previous studies. Moreover, none of the subjects quit the study, which suggested that they found the application of the exercise modality feasible.
The hypotensive effects of IHG training were not substantially different from the placebo effects, as shown in group B. To further confirm the link between grip strength changes induced by IHG and blood pressure reduction, we compared the hypotensive effects between IHG/Grip (U) and IHG/Grip (L). Fig. 4 shows that the IHG/Grip (U) group showed significantly greater hypotensive effects than the IHG/Grip (L) group. Moreover, there was no clear relationship between the changes in the maximum grip strength and the changes in blood pressure in the control period. Thus, our data suggests that the increase in grip strength due to IHG training is associated with larger BP reductions.
A recent review has also shown that, compared to muscle mass, muscle strength is more involved in the improvement of vascular function and/or the risk reduction associated with resistance training (31). Fahs et al. showed that muscle strength is inversely associated with arterial stiffness assessed using pulse wave velocity (32). Moreover, consistent with the findings of the present study, it has been shown that low-level resistance training significantly increased muscle strength, but not muscle mass (31). Understanding the mechanisms underlying the relationship between muscle strength and blood pressure regulation requires further investigation.
This study is associated with several limitations. First, because IHG training was conducted at home, and not under medical supervision, it is unclear how accurately and diligently the subjects adhered to the prescribed training regimen. However, the subjects received regular follow-up calls to discuss the training protocol and to confirm and improve the quality of practice. In addition, questions or difficulties related to the training were adequately resolved during the calls. Second, we did not collect any information on lifestyle factors, such as the regular diet. Participation in this clinical study might have encouraged some subjects to favorably modify their lifestyle, which could have contributed towards a decrease in BP. Third, we did not include a washout period between the intervention and exercise-free control periods. Therefore, the effect of IHG training might have been underestimated in group B compared to group A because the intervention period preceded the control period in group A. Therefore, BP measurement taken just before the initiation of IHG training might have been comparatively lower in group B due to a placebo effect exerted by the preceding 8-week control period. However, in group B, the morning systolic BP readings and both evening systolic and diastolic BP levels at 16 weeks were significantly lower than the values at baseline, despite a statistically non-significant BP reduction during the 8-week interval. This finding supports the notions that IHG training can reduce BP and IHG training is superior to any possible placebo effect observed in group B.
In conclusion, this study showed that an 8-week IHG training period significantly lowered both morning and evening home BP levels in hypertensive Japanese patients receiving treatment. The IHG exercise modality can be widely applied in hypertensive patients because it is easy, safe, inexpensive, and does not have space specifications. Reduction in BP was associated with a significant increase in maximum grip strength. Mechanisms of how grip strength relates to blood pressure regulation needs further study.
The authors state that they have no Conflict of Interest (COI).
Financial support
This study was supported by a grant from the Japan Organization of Occupational Health and Safety (research grant on the preventive medicine model).
Acknowledgement
We would like to express our deepest gratitude to all subjects who participated in this study. We would also like to thank researchers from Fukushima and Aomori Rosai Hospitals for their cooperation in the successful completion of the study.
References
- 1.Noda H, Iso H, Saito I, et al. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res 32: 289-298, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda N, Saito E, Kondo N, et al. What has made the population of Japan healthy? Lancet 378: 1094-1105, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Lee LL, Watson MC, Mulvaney CA, Tsai CC, Lo SF. The effect of walking intervention on blood pressure control: a systematic review. Int J Nurs Stud 47: 1545-1561, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomized trials. Prev Med 44: 377-385, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Kelley GA, Kelly KS, Tran ZV. Walking resting blood pressure in adults: a meta-analysis. Prev Med 33: 120-127, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH2019). Hypertens Res 42: 1235-1481, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 58: 950-958, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Millar PJ, Bray SR, MacDonald MJ, McCartney N. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J Cardiopulm Rehabil Prev 28: 203-207, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet 18: 266-273, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AC, McCartner N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc 35: 251-256, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Badrov MB, Horton S, Millar PJ, McGowan CL. Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Psychophysiology 50: 407-414, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Farah BQ, Rodrigues SLC, Silva GO, et al. Supervised, but not home-based, isometric training improves brachial and central blood pressure in medicated hypertensive patients: a randomized controlled trial. Front Physiol 23: 961, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley RL, Dunn CL, Cox RH, Hueppchen NA, Scott MS. Isometric exercise training lowers resting blood pressure. Med Sci Sports Exerc 24: 749-754, 1992. [PubMed] [Google Scholar]
- 14.Kato N, Takeuchi F, Tabara Y, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation East Asians. Nat Genet 43: 531-538, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103-109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 30: 319-328, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto T, Hashimoto Y, Kobayashi R. Isometric handgrip training reduces blood pressure and wave reflections in East Asian, non-medicated, middle-aged and older adults: a randomized control trial. Aging Clin Exp Res 32: 1485-1491, 2020. [DOI] [PubMed] [Google Scholar]
- 18.Stergiou GS, Baibas NM, Kalogeropoulos PG. Cardiovascular risk prediction based on home blood pressure measurement: the Didima study. J Hypertens 25: 1590-1596, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Niiranen TJ, Asayama K, Thijs L, et al. Outcome-driven thresholds for home blood pressure measurement: international database of home blood pressure in relation to cardiovascular outcome. Hypertension 61: 27-34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkubo T, Asayama K, Kikuya M, et al. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens 22: 1099-1104, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713-735, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Bedogni G, Malavolti M, Severi S, et al. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr 56: 1143-1148, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Kario K, Shimada K, et al. The Japanese society of hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res 35: 777-795, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Saito I, Kushiro T, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy: primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension 64: 989-996, 2014. [DOI] [PubMed] [Google Scholar]
- 25.McGowan CL, Levy AS, Millar PJ, et al. Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291: H1797-1802, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 15: 210-214, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Katz SD, Yuen J, Bijou R, LeJemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol 82: 1488-1492, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Dobrosielski DA, Greenway FL, Welsh DA, Jazwinski SM, Welsch MA. Modification of vascular function after handgrip exercise training in 73- to 90-yr-old men. Med Sci Sports Exerc 41: 1429-1435, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. J Appl Physiol 62: 1063-1067, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 89: 327-334, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Figueroa A, Okamoto T, Jamie SJ, Fahs CA. Impact of high-and low-intensity resistance training on arterial stiffness and blood pressure in adults across the lifespan: a review. Pflugers Arch 471: 467-478, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Fahs CA, Heffernan KS, Ranadive S, Jae SY, Fernhall B. Muscular strength is inversely associated with aortic stiffness in young men. Med Sci Sports Exerc 42: 1619-1624, 2010. [DOI] [PubMed] [Google Scholar]