Abstract
A 60-year-old Japanese woman was admitted to our hospital with a fever and shortness of breath occurring immediately after using hairspray. Chest high-resolution computed tomography (HRCT) showed ground-glass opacities (GGOs) predominantly distributed around the bronchovascular bundles, and a pathological evaluation by a transbronchial lung cryobiopsy (TBLC) revealed fibrotic non-specific interstitial pneumonia (f-NSIP). Her symptoms disappeared without the use of corticosteroids, and GGOs on HRCT improved markedly over time. This case suggests that a pathological evaluation by a TBLC for lung injury due to inhalation pathogen exposure may provide a more accurate diagnosis and a better understanding of the pathology from bronchial to interstitial lesions than transbronchial lung biopsy.
Keywords: hairspray, inhalation, interstitial pneumonia, acute lung injury, transbronchial cryobiopsy
Introduction
Lung injury due to inhalation pathogen exposure is represented by hypersensitivity pneumonitis, eosinophilic pneumonia, pneumoconiosis, and lipoid pneumonia, but it is also widely known that some of these conditions develop after the use of hairspray, waterproof spray, isocyanate-containing paints, and insecticides when aerosol particles are generated at the time of use. A transbronchial lung cryobiopsy (TBLC) has been used in recent years as a promising diagnostic approach to assessing diffuse parenchymal lung diseases (DPLDs), replacing surgical lung biopsies as a safer method (1). For hypersensitivity pneumonitis, recently reported guidelines recommended performing a TBLC at the time of the diagnosis, but the utility of a TBLC for evaluating lung injury caused by hairspray has not yet been clarified (2).
We herein report the utility of a TBLC in assessing the clinical and pathological aspects of a case of lung injury due to hairspray inhalation.
Case Report
A 60-year-old Japanese woman with no significant medical history was admitted to our hospital for a chief complaint of fever and shortness of breath. She was a current smoker with a 30 pack-year history and had no allergies. She noticed a fever immediately after using hairspray to dye her hair in her home bathroom for several minutes on day X-11 and took cefcapene pivoxil hydrochloride hydrate for four days from day X-5. However, the fever continued, and her shortness of breath gradually worsened. She had only ever dyed her hair using hair-coloring agent at a beauty salon, and this was the first time she had used the hairspray.
Chest auscultation revealed fine crackles throughout her chest. Laboratory examinations revealed extremely high serum levels of C-reactive protein (7.7 mg/dL), but the levels of KL-6 (368 U/mL) and SP-D (139 mg/dL) were within the normal range. An arterial blood gas analysis showed a decreased partial pressure of oxygen (50.1 Torr) with an elevated alveolar-arterial oxygen gradient (62.3 Torr). Pulmonary function tests showed a forced vital capacity (FVC) of 2.69 L (% predicted, 105.1%), a forced expiratory volume in 1 second (FEV1) of 2.14 L, FEV1/FVC ratio of 79.5%, diffusing capacity of carbon monoxide (DLCO) of 12.8 mL/min/Torr (% predicted, 61.8%), and a % predicted DLCO/alveolar volume ratio of 77.9%.
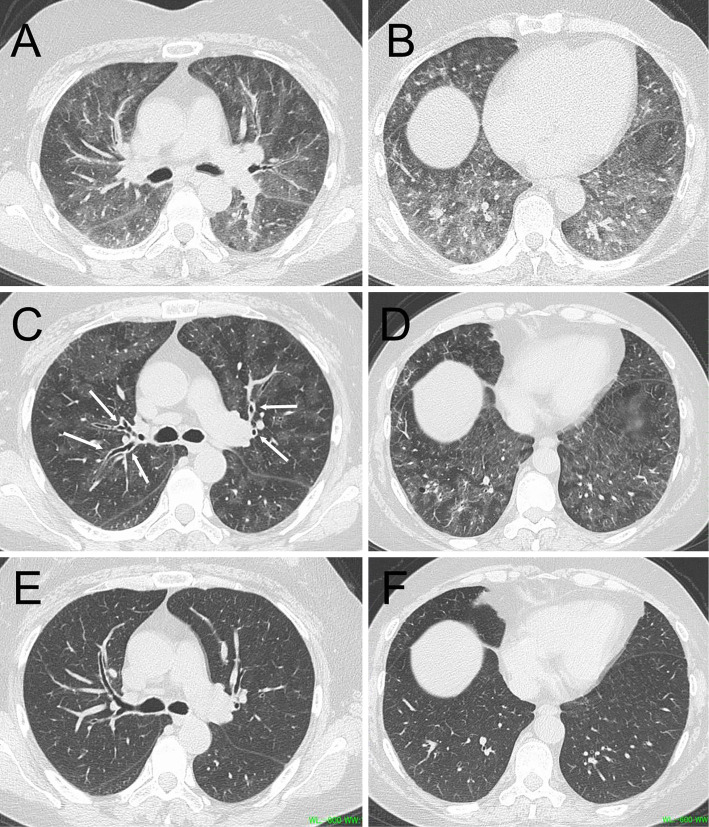
Chest high-resolution computed tomography (HRCT) showed ground-glass opacities (GGOs) predominantly distributed around the bronchovascular bundles along with peribronchial cuffing (Fig. 1C, D). Concurrently performed expiratory HRCT showed an extensive mosaic attenuation pattern (Fig. 1A, B).
Figure 1.
Chest high-resolution computed tomography (HRCT) images. Expiratory CT on admission showed an extensive mosaic attenuation pattern in both the upper (A) and lower lobes (B). Inspiratory CT on admission revealed numerous ground-glass opacities predominantly distributed around the bronchovascular bundles in both the upper (C) and lower lobes (D), along with peribronchial cuffing (arrows). Ground-glass opacities had disappeared on day 51 (E, F).
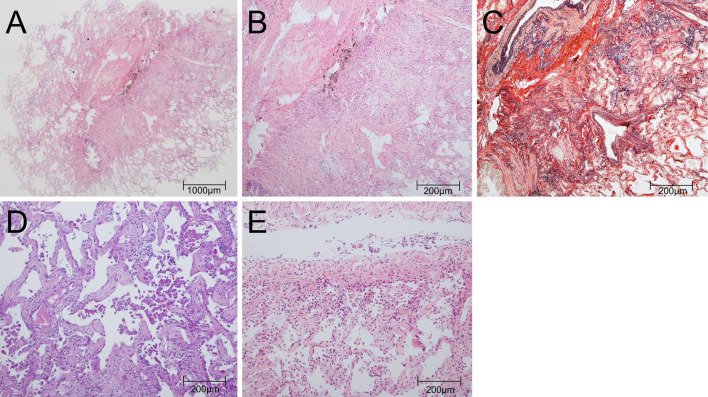
The bronchoalveolar lavage fluid (BALF) obtained from the right B4 had a recovery rate of 66.0%, a transparent appearance, lymphocyte CD4/CD8 ratio of 0.45, and contained 60.0% macrophages, 33.4% lymphocytes, 1.8% neutrophils, and 4.6% eosinophils. Microbiological tests for bacteria, fungi, acid-fast bacilli, and Pneumocystis jirovecii polymerase chain reaction in BALF were negative. A TBLC was performed at the right B8a and B8b using a flexible fiberoptic bronchoscope (1T-260; Olympus, Tokyo, Japan), an endotracheal tube (SACETT Suction Above Cuff Endotracheal Tube 8.0 mm; Smiths Medical International, Minneapolis, USA), a FogartyⓇ catheter (E-080-4F; Edwards Lifesciences, Irvine, USA), and the ERBE 1.9-mm cryoprobe (ERBECRYO 2 system; Erbe Elektromedizin, GmbH, Tubingen, Germany). The two specimens collected at the right B8a and B8b were 24 mm2 and 35 mm2 in size, respectively, and both specimens contained a wide range of alveoli with membranous bronchioles spanning two acini. The pathology of these specimens showed diffuse uniform thickening of the alveolar septum accompanied by deposition of collagen and elastic fibers, which is suggestive of fibrotic non-specific interstitial pneumonia (f-NSIP). Moderate intraluminal organization and mild infiltration of lymphocytes and eosinophils were also observed (Fig. 2A-C), and in some alveolar cavities, a cluster of eosinophilic macrophages was confirmed (Fig. 2D). The bronchial membrane collected in the right B9a using the same cryoprobe showed mild infiltration of lymphocytes and eosinophils (Fig. 2E).
Figure 2.
Histological images. (A) The lesion was characterized by diffuse thickening of the alveolar septum and moderate intraluminal organization (right B8a) [Hematoxylin and Eosin (H&E) staining, ×4]. (B) Mild infiltration of lymphocytes and eosinophils was observed (right B8a) (H&E staining, ×20). (C) In the alveolar septum, collagen deposition and the growth of elastic fibers were observed (right B8a) (Elastica van Gieson stain, ×20). (D) In some alveolar cavities, a cluster of eosinophilic macrophages was observed, but no apparent granulomas were seen (right B8a) (periodic acid-Schiff stain, ×20). (E) Mild infiltration of lymphocytes and plasma cells into the bronchial walls was also observed (right B9a) (H&E staining, ×20).
The pathological findings suggested f-NSIP due to inhaled pathogen exposure. No findings of granulomatous lesions, solitary multinucleated giant cells, peribronchiolar fibrosis, or hyperplasia of lymphoid follicles were observed. No significant increase in paired serum antibody titers of influenza virus, Mycoplasma pneumoniae, or Chlamydia pneumoniae was found, and an environmental provocation test conducted to rule out hypersensitivity pneumonitis was also negative.
Based on the onset of the disease after the start of hairspray use and the above findings, we diagnosed her with hairspray inhalation-induced interstitial pneumonitis. An additional interview with the patient revealed that the hairspray contained lanolin. The chief complaints of a fever and dyspnea gradually improved after hospitalization without the use of corticosteroids. There was no apparent recurrence after the patient was discharged, and the GGOs had disappeared on HRCT performed on day 51 (Fig. 1E, F).
Discussion
We herein report the first case, to our knowledge, of hairspray-induced interstitial pneumonitis that was histologically evaluated by a TBLC and that confirmed the findings of an f-NSIP pattern. The findings obtained from this case showed that the pathological evaluation by a TBLC for hairspray-induced pneumonitis may provide useful information to clinicians in terms of a differential diagnosis, and the clinical course may be self-limiting, even in the presence of an f-NSIP pattern.
Hairspray-induced interstitial pneumonitis was first reported by Bergmann et al. in 1958 (3), and various additional reports were made until the 1980s. However, there have been few reports in recent years, and it is considered to be a very rare disease (4,5). This is speculated to be due to the fact that polyvinylpyrrolidone and shellac, among the six etiological agents that have been linked to the pathogenesis (polyvinylpyrrolidone, polyvinyl acetate, shellac, dimethyl hydantoin formaldehyde resin, modified acid resins, and lanolin) are not recently being used in hairsprays (6,7). We experienced a case of developing acute lung injury in a patient using a hairspray containing lanolin, which is a potential pathogen pointed out by Draize et al. (6). Exposure to high levels of pathogens due to prolonged use of the hairspray in the closed environment of the bathroom may have been one of the factors that led to the onset of the disease. We believe that hairspray-induced interstitial pneumonitis should be considered as a differential diagnosis in acute-onset DPLD, even today.
The pathology of the TBLC specimens obtained in this case showed some suggestive findings. The specimens revealed diffuse, relatively homogeneous alveolar septal thickening and a preserved alveolar structure. Collagen deposition was also prominent and accompanied by mild lymphocytic infiltration. These findings were considered typical of an f-NSIP pattern. The pathological term “NSIP pattern” has been used historically in the context of chronic interstitial pneumonia, such as idiopathic interstitial pneumonias and connective tissue disease-associated interstitial lung diseases (CTD-ILD) (8). However, even in acute or subacute interstitial pneumonia, the NSIP pattern is often found along with diffuse alveolar damage and organizing pneumonia (9-11). When an NSIP pattern is observed in the clinical presentation of the acute course of the disease, CTD-ILD or drug-induced lung injury should be considered in the differential diagnosis (12), and we believe that lung injury due to inhalation pathogen should also be considered in addition to these conditions. In our search of the literature, we did not find any reports of NSIP in hairspray inhalation-induced pneumonitis. Although there have been several reported cases of surgical lung biopsies being performed up to the 1990s, surgical lung biopsies for acute lung injury can be challenging (5,13). In a recent transbronchial lung biopsy (TBLB) study of lung injury due to inhalation pathogen exposure, including hairspray, the presence of alveolar septal thickening and alveolitis was noted, but no case was diagnosed with an NSIP pattern (4,14,15). We speculate that the TBLB may not have led to the diagnosis of the overall tissue changes because of the inadequacy of the sample size obtained. As described above, the non-invasive evaluation of histopathological patterns in larger specimens than those obtained with a TBLB may be a major advantage of a TBLC over a surgical lung biopsy or TBLB.
In the present case, aggregation of eosinophilic macrophages and mild eosinophil infiltration were observed. Given the previous reports of phagocytes containing periodic acid-Schiff stain-positive granules and vacuolated macrophages in cases of hairspray-induced pneumonitis, the presence of eosinophilic or foamy macrophages is a finding that indicates some exposure to inhalant pathogens (4,16). The presence of mild inflammatory changes in the mucosa of bronchioles may also be a probable explanation for the findings of air trapping on HRCT in this case and may lead to a smoother understanding of the pathogenesis of the disease.
Of note, despite the pathology of f-NSIP, we confirmed that it improved spontaneously without the use of corticosteroids. We believe that hairspray inhalation-induced pneumonitis should be considered in the differential diagnosis of f-NSIP that shows a self-limiting course. Inhalation of hairspray in healthy subjects is only associated with brief, reversible small airway inflammation, and the risk of it leading to severe respiratory disturbance is low, as evidenced by the fact that the frequency of interstitial pneumonitis is also low in hairdressers who are continuously exposed to hairspray (4,17). It is therefore unlikely that corticosteroids will be required in this disease, which is presumed to be less pathogenic than isocyanate-induced or waterproofing spray-induced lung injury, in which patients can present with acute respiratory distress syndrome or diffuse alveolar hemorrhaging that leaves them with chronic respiratory dysfunction (15,18). However, it has also been pointed out that in the presence of a concomitant allergic predisposition, such as bronchial asthma or allergic rhinitis, or chronic bronchitis, the biological response to inhalation may be enhanced (17). There was no apparent comorbidity in our patient, but she was a smoker and had an elevated serum IgE level. Therefore, the possibility of airway lesions due to smoking or an allergic predisposition cannot be completely ruled out, and clinical symptoms may thus have been more likely to be overt or prolonged than had she not been a smoker. In patients with these factors, careful follow-up including chest imaging may be advisable.
In conclusion, a TBLC has the potential to contribute to a more accurate diagnosis and better aid in the inference of the etiology of hairspray-induced pneumonitis than transbronchial lung biopsy because of its ability to assess pathology from the bronchioles to the interstinum. In the case of spontaneous improvement of NSIP, it is necessary to consider hairspray inhalation-induced interstitial pneumonitis as one of the differential diagnoses. We need to collect more cases and examine the relationship between the clinical picture and the pathological findings in this disease.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 95: 188-200, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 202: e36-e69, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann M, Flance IJ, Blumenthal HT. Thesaurosis following inhalation of hair spray; a clinical and experimental study. N Engl J Med 258: 471-476, 1958. [DOI] [PubMed] [Google Scholar]
- 4.Nagata N, Kawajiri T, Hayashi T, Nakanishi K, Nikaido Y, Kido M. Interstitial pneumonitis and fibrosis associated with the inhalation of hair spray. Respiration 64: 310-312, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med 126: 1064-1070, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Draize J, Nelson AA, Newburger SH, Kelley EA. Inhalation toxicity studies of six types of aerosol hair sprays. Proc Sci Sect Toilet Goods Assoc 31: 28-32, 1959. [Google Scholar]
- 7.Kawajiri T, Nagata N, Morimoto Y, et al. Pathology and mechanism of lung toxicity following inhalation of hair spray in rats. Inhal Toxicol 16: 147-153, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188: 733-748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda T, Fujisawa T, Enomoto N, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J 28: 1005-1012, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 132: 214-220, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Kondoh Y, Taniguchi H, Kataoka K, et al. Prognostic factors in rapidly progressive interstitial pneumonia. Respirology 15: 257-264, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi H, Kondoh Y. Acute and subacute idiopathic interstitial pneumonias. Respirology 21: 810-820, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Wright JL, Cockcroft DW. Lung disease due to abuse of hairspray. Arch Pathol Lab Med 105: 363-366, 1981. [PubMed] [Google Scholar]
- 14.Hayashi H, Ishii T, Ishida T, et al. Drug-induced pulmonary damage due to inhalation of a waterproof spray. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 46: 35-38, 2008. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 15.Tabata H, Mochizuki Y, Nakahara Y, Kobashi Y, Kawamura T, Sasaki S. Hypersensitivity pneumonitis caused by isocyanate exposure during recreational painting. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 47: 1002-1007, 2009. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 16.Bergmann M, Flance IJ, Cruz PT, Klam N, Aronson PR, Joshi RA. Thesaurosis due to inhalation of hair spray. Report of twelve new cases, including three autopsies. N Engl J Med 266: 750-755, 1962. [DOI] [PubMed] [Google Scholar]
- 17.Schlueter DP, Soto RJ, Baretta ED, Herrmann AA, Ostrander LE, Stewart RD. Airway response to hair spray in normal subjects and subjects with hyperreactive airways. Chest 75: 544-548, 1979. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Murata K, Sasaki A, Okamoto S, Wada A, Takamori M. A case of spontaneously remitting diffuse alveolar hemorrhage following inhalation of waterproofing spray and cigarette smoke. J Jpn Soc Respir Endosc 37: 234-239, 2015. [Google Scholar]