Abstract
Dendriform pulmonary ossification (DPO) is a rare condition characterized by metaplastic bone formation in the lung parenchyma. It has been reported to be often associated with primary lung diseases, such as usual interstitial pneumonia (UIP) or chronic aspiration of gastric acid; however, its clinical features and pathophysiology remain unclear, especially in idiopathic cases. We herein report five DPO cases, including three with an idiopathic origin. In all cases of idiopathic DPO, the pathological and radiological examinations showed localized pulmonary lesions suggesting inflammation or hemorrhaging.
Keywords: dendriform pulmonary ossification, inflammation, hemorrhaging, fibrosis, usual interstitial pneumonia, surgical lung biopsy
Introduction
Diffuse pulmonary ossification is a rare condition characterized by metaplastic bone formation in the lung parenchyma (1). It is usually an asymptomatic disease and is invisible on chest radiography. Several recent cases of diffuse pulmonary ossification were diagnosed thanks to developments in radiography; however, the condition often remains underdiagnosed or misdiagnosed (2).
Diffuse pulmonary ossification has been recognized in nodular and dendriform types. The nodular type of ossification has been linked to passive congestion due to chronic heart failure, mitral stenosis, and hypertrophic subaortic stenosis. The dendriform type of ossification, also known as dendriform pulmonary ossification (DPO), is idiopathic or occurs in association with primary lung diseases (1-3). DPO has often been reported in association with usual interstitial pneumonia (UIP) or chronic aspiration of gastric acid (4). However, the clinical features and pathophysiology of DPO remain unclear, especially the idiopathic cases.
We herein report our experience with five DPO cases, including three cases with an idiopathic origin.
Materials and methods
A retrospective study was conducted to examine the clinical features and pathophysiology of DPO cases in our hospital. This study was approved by the institutional review board of our hospital (Approval number: 679). We used an opt-out method so that patients and families could refuse to participate in the study.
The diagnosis of DPO
In this study, cases showing both pathological and radiological findings of DPO were diagnosed with DPO. Pathological findings of DPO were defined as interstitial branching spicules of bone and marrow elements in pathological specimens (5). Radiological findings of DPO were defined as nodules 1-5 mm in diameter in the peripheral interstitium forming contiguous lines of nodules connecting lobules on computed tomography (CT) and multiple calcified dense lesions visible on CT with a mediastinal window (4,6).
Subjects
In this study, the pathology reports of 313 consecutive cases diagnosed by a surgical lung biopsy (SLB) for diffuse lung diseases (DLDs) between April 1, 2000, and March 31, 2018, at our hospital were reviewed retrospectively. Subsequently, in cases showing pathological findings of DPO in the specimens, the findings of CT were evaluated. Cases showing both pathological and radiological findings of DPO were diagnosed with DPO. In addition, 4,634 cases that involved surgical resection of a lung tumor were reviewed according to the same criteria. The clinical data (i.e., laboratory data, results of pulmonary function tests, and clinical records) and radiological and pathological features were analyzed in the DPO cases.
Results
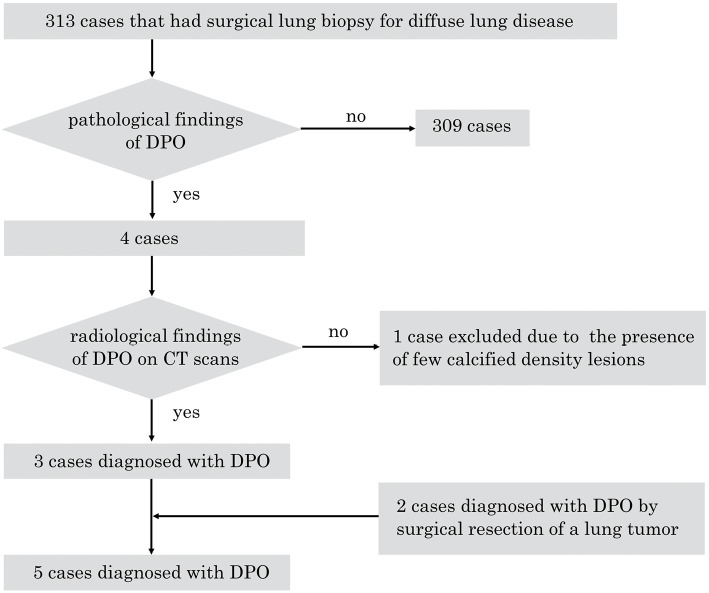
The case selection process is shown in Fig. 1. Of the 313 consecutive cases diagnosed by an SLB for DLDs in the abovementioned period at our hospital, 4 cases showed pulmonary ossification. All four cases showed pathological findings of DPO. Subsequently, in these four cases, the CT findings were evaluated.
Figure 1.
Flowchart of the study. DPO: dendriform pulmonary ossification
Three cases showed radiological findings of DPO, while one with fibrotic nonspecific interstitial pneumonia (NSIP) was excluded due to the presence of few calcified dense lesions on CT. Thus, 3 of 313 cases were ultimately diagnosed with DPO based on both pathological and radiological findings of DPO. In addition, of the 4,634 cases that involved surgical resection of a lung tumor, 2 cases were diagnosed with DPO. A total of five cases with DPO were thus identified.
The patient characteristics are shown in Table 1. The median age was 69 (range, 37-70) years old, and the 5 patients included 4 men and 1 woman. Four of the five cases had no smoking history. DPO has been reported as associated with recurrent acid aspiration and UIP (4). One case (case 4) showed demonstrable hiatal hernia, and another (case 5) showed gastroesophageal reflux disease (GERD) and UIP. Case 5 was diagnosed with idiopathic pulmonary fibrosis/UIP by multi-disciplinary discussion due to UIP patterns of SLB specimens, although the CT findings were not clear enough to suggest UIP.
Table 1.
Patient Characteristics.
| Case No. |
Age, years | Sex | Diagnostic opportunity | Smoking History | Past medical history | Main location of lesions* | Bone marrow elements** | Interstitial fibrosis** |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | F | Surgical resection of a lung tumor | Never | Sclerosing pneumocytoma, Diabetes mellitus, Uterine fibroids |
Both lower lobes | Present | Present |
| 2 | 69 | M | Surgical resection of a lung tumor | Ex-smoker | Lung adenocarcinoma, Coronary-pulmonary artery fistula, COPD, OMI, HT |
Both lower lobes | Present | Present |
| 3 | 37 | M | SLB for DLD | Never | Spinal disc herniation | Both lower lobes | Present | Present |
| 4 | 46 | M | SLB for DLD | Never | Demonstrable hiatal hernia | Both lower lobes | Present | Present |
| 5 | 70 | M | SLB for DLD | Never | IPF/UIP, GERD | Both lower lobes | Present | Present |
SLB: surgical lung biopsy, DLD: diffuse lung disease, COPD: chronic obstructive pulmonary disease, OMI: old myocardial infarction, HT: hypertension, IPF: idiopathic pulmonary fibrosis, UIP: usual interstitial pneumonia, GERD: gastroesophageal reflux disease
*: Radiological findings
**: Pathological findings
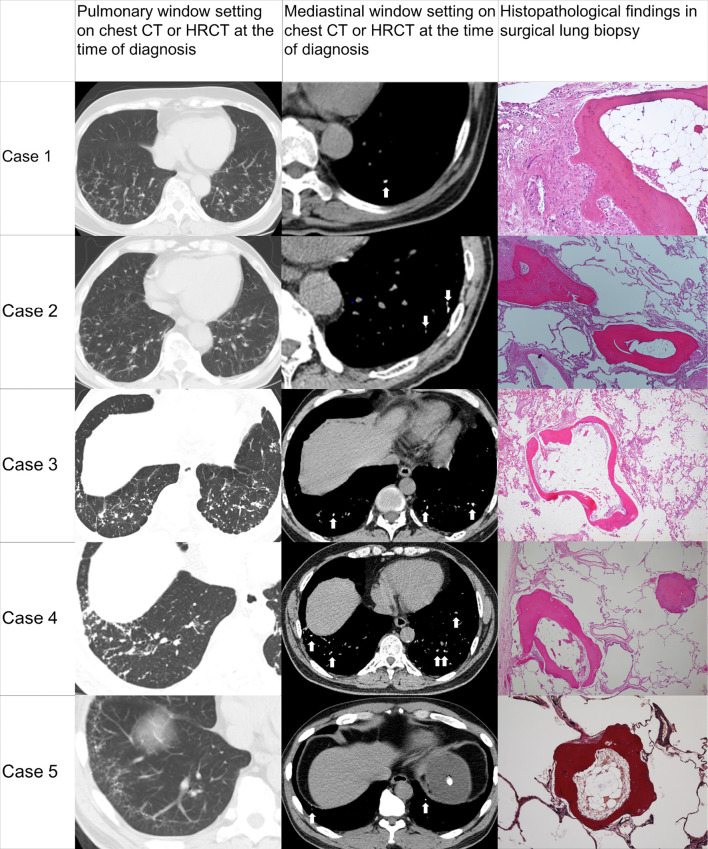
Chest CT or high-resolution CT (HRCT) images at the time of the diagnosis and histopathology of the SLB are shown in Fig. 2. Chest CT or HRCT demonstrated multiple tiny calcified dense lesions in the peripheral interstitium forming contiguous lines of nodules connecting lobules in all cases. The lesions of DPO tended to be located in both lower lobes in all cases. The laboratory findings are shown in Table 2. The surfactant protein-D (SP-D) levels were elevated in case 2. However, the elevation was not attributable to interstitial pneumonia according to pathological and radiological assessments. No other remarkable findings were observed on laboratory tests. The pulmonary function test results at the time of diagnosis are shown in Table 3. Although the values of %vital capacity, %total lung capacity (TLC), residual volume/TLC, and %diffusing capacity of the lungs for carbon monoxide were slightly low in cases 3 and 4, they were near the normal range. No other remarkable impairments were noted at baseline. No significant decline in respiratory function was observed during the follow-up period(Table 3). None of the DPO patients had died by the time of the study.
Figure 2.
CT or HRCT images at the time of the diagnosis and histopathological findings of the surgical lung biopsy. (Case 1) Hematoxylin and Eosin (H&E) staining, ×40. (Cases 2-4) H&E staining, ×20. (Case 5) Elastica van Gieson stain, ×20. Arrows indicate calcified dense lesions. HRCT: high-resolution computed tomography
Table 2.
Laboratory Data.
| Case No. |
WBC /µL |
Hb g/dL |
Plt ×104/µL |
AST U/L |
ALT U/L |
LDH U/L |
Cr mg/dL |
CRP mg/dL |
KL-6 U/mL |
SP-D ng/mL |
SP-A ng/mL |
Alb g/dL |
Ca mg/dL |
PT-INR | aPTT s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4,100 | 13.6 | 25.1 | 27 | 20 | 238 | 0.72 | 0.03 | 284 | N/A | N/A | 4.6 | 9.2 | 1.04 | 27.6 |
| 2 | 6,500 | 13.1 | 21.0 | 16 | 11 | 142 | 0.80 | 0.18 | 402 | 393.7 | 54.6 | 4.5 | 8.9 | 1.01 | 23.7 |
| 3 | 8,300 | 14.5 | 25.6 | 14 | 22 | 145 | 0.66 | 0 | 365 | <17 | 39.6 | 5.3 | 9.7 | 0.99 | 28.8 |
| 4 | 5,300 | 15.7 | 19.3 | 16 | 25 | 154 | 0.83 | 0.06 | 287 | 65.5 | N/A | 4.5 | 9.3 | 0.96 | 24.1 |
| 5 | 5,700 | 15.6 | 22.8 | 22 | 22 | 185 | 0.79 | 0.01 | 438 | 55.9 | 41.3 | 4.5 | 8.9 | 1.01 | 32.7 |
WBC: white blood cells, Hb: hemoglobin, Plt: platelets, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, Cr: creatinine, CRP: C-reactive protein, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, SP-A: surfactant protein-A, Alb: albumin, Ca: calcium, PTH: parathyroid hormone, PT-INR: prothrombin time-international normalized ratio, aPTT: activated partial thromboplastin time, N/A: not available
Table 3.
Pulmonary Function Test.
| Case No. |
VC* (L) |
%VC* (%) |
FEV1* (L) |
%FEV1* (%) |
FEV1%* (%) |
%TLC* (%) |
RV/TLC* (%) |
%DLco* (%) |
%DLco/VA* (%) |
ΔFVC** per year (%) |
ΔDLco** per year (%) |
Follow-up period (Y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.92 | 128.1 | 2.19 | 114.7 | 71.8 | 131.8 | 37.1 | 113.1 | 103.9 | -0.2 | 3.1 | 3.3 |
| 2 | 3.18 | 96.7 | 2.27 | 83.5 | 70.3 | 80.8 | 36.1 | 102.6 | 90.1 | N/A | N/A | N/A |
| 3 | 3.23 | 81.4 | 2.54 | 70.8 | 77.9 | 69.7 | 25.5 | 69.8 | 91.8 | 0.4 | -0.2 | 9.6 |
| 4 | 3.21 | 85.1 | 2.47 | 74.0 | 76.5 | 71.5 | 25.9 | 74.3 | 102.9 | -3.4 | 0.7 | 1.3 |
| 5 | 3.84 | 119.6 | 2.97 | 112.5 | 83.4 | 97.4 | 30.6 | 135.9 | 124.5 | -0.5 | -2.5 | 3.6 |
VC: vital capacity, FEV1: forced expiratory volume in one second, TLC: total lung capacity, RV: residual volume, DLco: diffusing capacity of the lungs for carbon monoxide, VA: alveolar ventilation, FVC: forced vital capacity, N/A: not available
*: Values at the time of diagnosis
**: Rate of change per year from the diagnosis to the last follow-up. Each parameter was calculated by the formula: ΔFVC per year: [(FVC in the latest pulmonary function test (L) – FVC at baseline (L))×100]/[(FVC at baseline (L))×(follow-up period (year))], ΔDLco per year: [(DLco in the latest pulmonary function test (ml/min/mmHg) – DLco at baseline (ml/min/mmHg))×100]/[(DLco at the baseline (ml/min/mmHg))×(follow-up period (year))].
Case Presentation
• Case 1
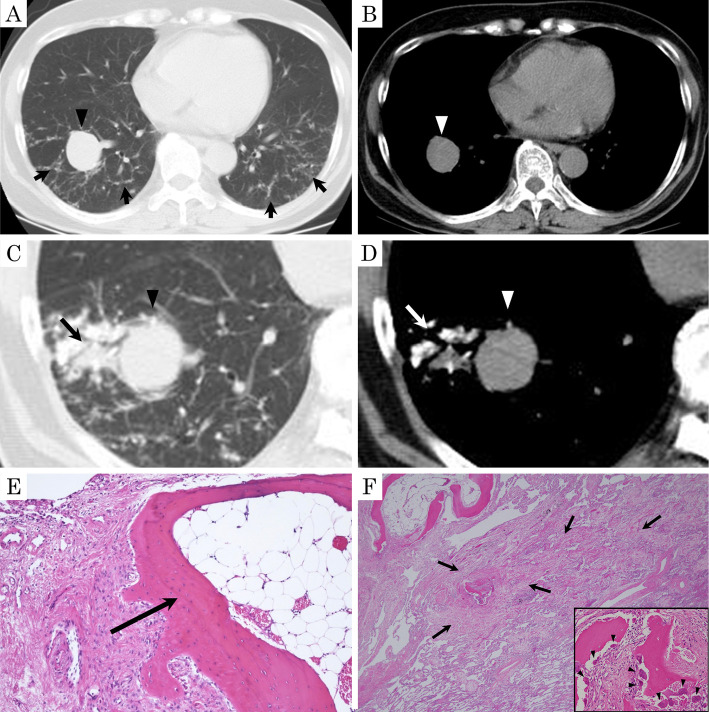
A 70-year-old woman visited our hospital due to pulmonary opacities. She had diabetes, which had been treated with oral antidiabetic agents and insulin. She had no remarkable family medical history, smoking history, or previous exposure to environmental allergens. An abnormality on a chest radiograph had been noted at a medical checkup when she was about 40 years of age; however, no close investigation had been conducted. A nodular shadow in the right lower lobe had been noted when she was 54 years of age. It had grown by the time she was 70 years of age, so she was referred to our hospital. There were no remarkable findings on laboratory or pulmonary function test results. Electrocardiogram and echocardiogram results showed no abnormalities. Chest CT at the first visit showed diffuse reticulonodular shadows in the bilateral lower lobes and a 3-cm nodule in the right lower lobe (Fig. 3A, B). About two months later, consolidation next to the nodular shadow in the right lower lobe, which included calcified densities, appeared on CT (Fig. 3C, D). At that time, the patient was asymptomatic.
Figure 3.
Chest CT images and histopathology of surgical lung biopsy specimens in Case 1. (A, B) Pulmonary window and mediastinal window settings of chest CT at the first visit showed diffuse reticulonodular shadows in both lower lobes (arrows) and a 3-cm nodular shadow in the right lower lobe (arrowhead). (C, D) Chest CT at 13 days before surgery showed consolidation (arrow) next to a 3-cm nodular shadow (arrowhead) in the right lower lobe, which included calcified densities. (E) The specimen obtained by a surgical lung biopsy revealed the presence of several irregularly shaped segments of interstitial bone, some of which contained fat marrow tissues (arrow) [Hematoxylin and Eosin (H&E) staining, ×40]. (F) Ossifications (inset) surrounded by osteoblasts (arrowheads) with intra-alveolar fibrosis and slight inflammatory cell infiltration (arrows), corresponding to consolidation in the right lower lobe (H&E staining, ×10), were observed.
Resection of the right lower lobe was performed for the diagnosis 13 days after CT. The nodule in the right lower lobe was diagnosed as a sclerosing pneumocytoma by a histological examination. In addition, the specimens showed multiple interstitial branching spicules of bone that were mainly located irregularly in the alveolar airspaces and focally within the walls. Some contained fat marrow tissues (Fig. 3E). These findings were compatible with DPO. Furthermore, fibrosis and slight intra-alveolar inflammatory cell infiltration, which included new bone formations surrounded by some osteoblasts, were identified, corresponding to the consolidation in the right lower lobe (Fig. 3F).
The patient was diagnosed with idiopathic DPO due to the lack of any underlying disease and received follow-up observation without treatment. DPO did not show any progression over the 40-month follow-up period.
• Case 2
A 69-year-old man visited our hospital for pulmonary opacities and cough. He had chronic obstructive pulmonary disease (COPD), old myocardial infarction, hypertension, and coronary-pulmonary artery fistula, which were treated with beta blocker, statin-based medicine, and aspirin. He did not have any remarkable family medical history. He was a former smoker and declared no previous exposure to environmental allergens. On follow-up CT performed at 69 years old, reticulonodular shadows were detected mainly in both lower lobes, and a nodular shadow in the right lower lobe was noted.
In order to diagnose and treat the nodular shadow, resection of the right lower lobe was performed. The nodule in the right lower lobe was diagnosed with squamous cell carcinoma. In addition, multiple interstitial branching spicules of bone including fat marrow tissues - findings compatible with DPO - were detected.
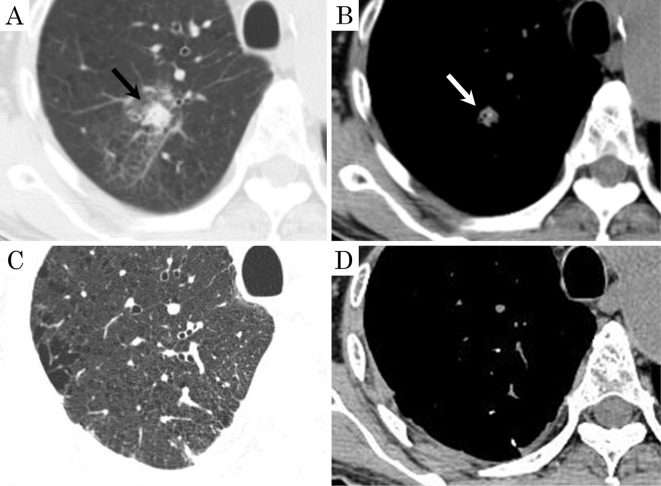
The patient was diagnosed with idiopathic DPO due to the lack of any underlying disease and received follow-up observation without treatment. During the follow-up period, consolidation including a calcified shadow suspected of being inflammation or hemorrhaging was observed in the right upper lobe on CT(Fig. 4A, B). After two months, CT showed that it had regressed spontaneously(Fig. 4C, D).
Figure 4.
Chest CT images in Case 2. (A, B) High-resolution CT showed consolidation including calcified density in the right upper lobe (arrow). (C, D) The consolidation had regressed spontaneously after two months.
• Case 3
A 37-year-old man visited our hospital for pulmonary opacities and cough. He did not have a remarkable medical history or smoking history. His brother and grandmother had had asthma, and his brother had died at a young age from unknown causes. He noted no previous exposure to environmental allergens. When he visited our hospital, CT showed reticulonodular shadows mainly in both lower lobes. To make a diagnosis, an SLB was performed. The pathological findings were compatible with DPO as in cases 1 and 2.
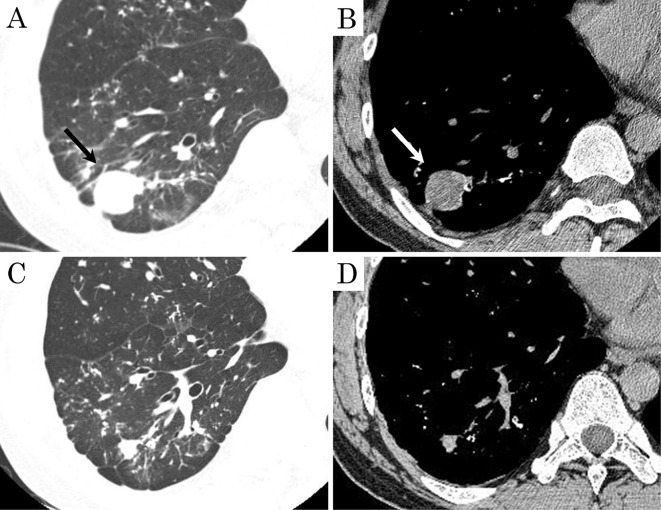
He was diagnosed with idiopathic DPO due to the lack of any underlying disease and received follow-up observation without treatment. During the follow-up period, a round 3-cm nodule was observed in the right lower lobe on CT (Fig. 5A, B). After about seven weeks, CT showed that the consolidation had regressed spontaneously (Fig. 5C, D). It was suspected of being a hematoma due to its well-circumscribed spherical shape, smooth surface, homogeneous density, and temporality.
Figure 5.
Chest CT images in Case 3. (A, B) High-resolution CT showed a round, 3-cm nodule in the right lower lobe (arrow). (C, D) The nodule had regressed spontaneously after seven weeks.
Discussion
The findings of our five DPO cases were nearly consistent with the previously reported common features of DPO patients, including slow progression, the lesion location, and co-existence of fibrosis; however, we further found that localized pulmonary lesions suspected of being inflammatory or hemorrhagic were observed in all three idiopathic DPO cases (cases 1-3).
In this retrospective study of 313 cases with DLDs that involved SLBs, 3 cases were definitely diagnosed with DPO, representing an average incidence of 0.96%.Diffuse pulmonary ossification has been reported to be present in 0.16% to 0.40% of autopsies (1,7). Limited to living patients with DLDs, the incidence of DPO may be rather high. In this retrospective study, pulmonary ossifications were located in both lower lobes in all five cases. Similarly, pulmonary ossifications were located in the lower lobes in 36 of 42 cases diagnosed with DPO in a previous study (8). In our case series, pulmonary function test results showed no remarkable impairment at baseline or significant decline during the follow-up period. None of the DPO patients met the criteria for progressive fibrosing interstitial lung disease (9) or had died at the time of study. DPO is typically considered to be indolent or slowly progressive, inducing a gradual decline in the pulmonary function (10-12). However, a DPO case that showed progressive restrictive ventilatory impairment was reported (13). Nevertheless, the risk factors for progression in DPO remain unclear.
DPO is frequently accompanied by interstitial fibrosis (5,7,14), and the association of these two entities has been suggested. As a factor causing ossification, transforming growth factor-beta, which is strongly implicated in idiopathic pulmonary fibrosis and other fibrotic pulmonary diseases, stimulates osteoblast and chondrocyte proliferation (3). In the present retrospective study, all five DPO cases showed interstitial fibrosis in lung tissues. Furthermore, on a histopathologic review of 313 cases with DLDs, ossification was identified in 1 (0.6%) of the 174 cases with UIP and 1 (0.9%) of the 112 cases with NSIP. Travis et al. reported that ossifications were identified during a pathologic examination of lung biopsy specimens in 12 of 56 (21%) cases of UIP and in 2 of 22 (9%) cases of fibrotic NSIP (15). Kim et al. reported that ossifications were identified during a pathologic examination of lung biopsy specimens in 5 of 75 (6.7%) cases of UIP and were not identified in any of 44 cases of NSIP (16). The frequency of ossifications in UIP was lower in this retrospective study than in the previous studies. Two reasons may explain this. First, false-negative biopsy results may have occurred due to sampling errors. Second, the difference in patient characteristics might have influenced the findings. Although it is difficult to compare the characteristics of the patients in our study with those in previous studies due to the lack of details, there is the possibility that the time interval between the onset of UIP and the SLB and the UIP severity might be involved.
In this retrospective study, case 4 showed demonstrable hiatal hernia, and case 5 showed GERD. In a previous report, 39 of 52 DPO cases without UIP (75%) showed obstructive sleep apnea, GERD, or a chronic neurologic disorder, and DPO was suggested to be associated with recurrent acid aspiration (4). Local acidity and hypoxemia can cause the differentiation of pulmonary fibroblasts, and possibly macrophages, into osteoblasts, so microscopic and chronic low-level acid aspiration can be expected to cause DPO (14). However, this previous report was based on a DPO diagnosis made using CT findings, and no radiologic definition for the entity of DPO has been established (17). The ossifications in DPO are not always visible on CT due to their microscopic size (7). They might thus be difficult to distinguish from calcifications on CT. Further studies with an accurate diagnosis of DPO made by pathological and radiological assessments are required to elucidate the pathogenesis of DPO.
DPO is reportedly often associated with primary lung diseases, such as UIP, or chronic aspiration of gastric acid (4). However, its clinical features and pathophysiology remain unclear. Three cases (cases 1-3) were considered to be idiopathic due to the lack of UIP, any risk factor for acid aspiration, or any other underlying disease. In case 1, in addition to ossifications compatible with DPO, fibrosis and slight inflammatory cell infiltration were observed in the lung tissues. Ossifications in the fibrosis and slight inflammatory cell infiltration were considered to be relatively recent because they co-existed with osteoblasts. This case suggested that fibrosis, which typically results from chronic inflammation, or inflammation itself might be related to new bone formation. Furthermore, cases 2 and 3 also showed temporary consolidation suspected of being caused by inflammation or hemorrhaging. Therefore, all three idiopathic DPO cases showed consolidation suspected of being caused by inflammation or hemorrhaging. This suggests that inflammation or hemorrhaging associated with fibrosis may contribute to bone formation in DPO. Inflammation itself is suggested to be involved in the pathogenesis of bone formation by promoting the transformation of cultured fibroblasts into osteoblasts (18,19). With respect to hemorrhaging, the organization of intra-alveolar hemorrhaging associated with chronic passive hyperemia and phagocytosis of intra-alveolar hemosiderin deposits are suggested to be involved in the pathogenesis of bone formation (20). Indeed, DPO was reported to occur secondarily to diffuse alveolar hemorrhaging (21,22), and ossifications in the lung parenchyma were reported to occur in vascular Ehlers-Danlos syndrome, which is caused by mutations in the type III collagen (COL3A1) gene and results in acute hemorrhaging and organizing pneumonia (23,24). However, localized consolidation in our cases could not explain the diffuse ossification seen in the whole lungs. In addition, the sample size in this retrospective study was quite small. The causality between ossifications in DPO and localized consolidation in our cases is thus unclear.
In conclusion, we described five DPO cases, including three cases of idiopathic origin. In all of the idiopathic cases, the pathological and radiological examinations showed localized pulmonary lesions suspected of having been caused by inflammation or hemorrhaging.
Author's disclosure of potential Conflicts of Interest (COI).
Toru Arai: Honoraria, Boehringer Ingelheim and Shionogi. Yoshikazu Inoue: Honoraria, Boehringer Ingelheim, Shionogi and Asahi Kasei.
Financial Support
Grant from Japanese Ministry of Health, Labour, and Welfare (Yoshikazu Inoue).
Acknowledgement
We thank all of the subjects who participated in the study.
References
- 1.Lara JF, Catroppo JF, Kim DU, da Costa D. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification: report of a 26-year autopsy experience. Arch Pathol Lab Med 129: 348-353, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Jamjoom L, Meziane M, Renapurkar RD. Dendriform pulmonary ossification: report of two cases. Indian J Radiol Imaging 23: 15-18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 165: 1654-1669, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Gruden JF, Green DB, Legasto AC, Jensen EA, Panse PM. Dendriform pulmonary ossification in the absence of usual interstitial pneumonia: CT features and possible association with recurrent acid aspiration. Am J Roentgenol 209: 1209-1215, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Ndimbie OK, Williams CR, Lee MW. Dendriform pulmonary ossification. Arch Pathol Lab Med 111: 1062-1064, 1987. [PubMed] [Google Scholar]
- 6.Kim DU, Guinee D, Mohammed TL. Case of the season: usual interstitial pneumonia with dendriform pulmonary ossification. Semin Roentgenol 50: 4-7, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology 38: 45-48, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Bussy S, Labarca G, Pires Y, Diaz JC, Caviedes I. Dendriform pulmonary ossification. Respir Care 60: e64-e67, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KR, Brown KK, Wells AU, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res 4: e000212, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peros-Golubicic T, Tekavec-Trkanjec J. Diffuse pulmonary ossification: an unusual interstitial lung disease. Curr Opin Pulm Med 14: 488-492, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Martinez JB, Ramos SG. Dendriform pulmonary ossification. Lancet 382: e22, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Ahari JE, Delaney M. Dendriform pulmonary ossification: a clinical diagnosis with 14 year follow-up. Chest 132: 701A, 2007. [Google Scholar]
- 13.Matsuo H, Handa T, Tsuchiya M, et al. Progressive restrictive ventilatory impairment in idiopathic diffuse pulmonary ossification. Intern Med 57: 1631-1636, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaderborg JM, Dunton RF. Rare clinical diagnosis of dendriform pulmonary ossification. Ann Thorac Surg 71: 2009-2011, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 24: 19-33, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Kim TS, Han J, Chung MP, Chung MJ, Choi YS. Disseminated dendriform pulmonary ossification associated with usual interstitial pneumonia: incidence and thin-section CT-pathologic correlation. Eur Radiol 15: 1581-1585, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Egashira R, Jacob J, Kokosi MA, et al. Diffuse pulmonary ossification in fibrosing interstitial lung diseases: prevalence and associations. Radiology 284: 255-263, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Popelka CG, Kleinerman J. Diffuse pulmonary ossification. Arch Intern Med 137: 523-525, 1977. [PubMed] [Google Scholar]
- 19.Reddi AH, Huggins CB. Cyclic electrochemical inactivation and restoration of competence of bone matrix to transform fibroblasts. Proc Nat Acad Sci U S A 71: 1648-1652, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green JD, Harle TS, Greenberg SD, Weg JG, Nevin H, Jenkins DE. Disseminated pulmonary ossification. A case report with demonstration of electron-microscopic features. Am Rev Respir Dis 101: 293-298, 1970. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi T, Fujimoto N, Miyamoto Y, et al. The rapid appearance and disappearance of dendriform pulmonary ossification after diffuse alveolar hemorrhage. Am J Respir Crit Care Med 193: 333-334, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Hsing AY, Meghoo C, Allan PF. Diffuse alveolar hemorrhage associated with dendriform pulmonary ossification. Chest 136: 35S, 2009. [Google Scholar]
- 23.Corrin B, Simpson CG, Fisher C. Fibrous pseudotumours and cyst formation in the lungs in Ehlers-Danlos syndrome. Histopathology 17: 478-479, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata Y, Watanabe A, Yamaguchi S, et al. Pleuropulmonary pathology of vascular Ehlers-Danlos syndrome: spontaneous laceration, haematoma and fibrous nodules. Histopathology 56: 944-950, 2010. [DOI] [PubMed] [Google Scholar]