Abstract
An 87-year-old man with hepatocellular carcinoma (HCC) presented with right-sided chest pain. Computed tomography revealed right bloody pleural effusion and an extravasation from an arterially enhanced mass in the right seventh posterior intercostal space. These findings indicated hemothorax from a rupture of HCC metastasis to the chest wall. Angiography of the intercostal arteries confirmed a hypervascular tumor, and transcatheter arterial embolization resulted in hemostasis. He was discharged with palliative care and remains alive after 9 months. Although hemothorax represents an unusual, life-threatening complication of HCC, our case suggests that transcatheter treatment can achieve hemostasis and a favorable survival even in elderly patients.
Keywords: hepatocellular carcinoma, chest wall metastasis, spontaneous rupture, hemothorax, transcatheter arterial embolization
Introduction
Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancer (1), which was estimated to have caused 841,100 incident cases and 781,600 mortalities worldwide in 2018 (2). A unique feature of HCC is the propensity to demonstrate a spontaneous rupture, which leads to hemoperitoneum (3,4). The incidence of ruptured HCC is especially high in Asian countries (up to 26%) where the tumor is prevalent (3,4). Although a rupture of HCC can cause severe conditions as a result of hemodynamic instability, a multimodal approach for the hemorrhage as well as the tumor has contributed to improving the management of such cases (3,4). In addition, the aggressive behavior of HCC is demonstrated by frequent metastasis to other organs, such as the lung, lymph node, and bone (5-9). Metastatic HCC generally shows arterial enhancement on contrast-enhanced computed tomography (CT), similar to the primary tumor (5,10). However, given the high prevalence and common extrahepatic spread of HCC, a rupture of the distant metastasis is considerably rare compared to that of the primary lesion. In particular, hemothorax, which results from a hemorrhage into the thoracic cavity, is an unusual presentation of HCC and it associated with a poor clinical outcome (11).
We herein describe a case of hemothorax secondary to spontaneous rupture of HCC metastasis to the chest wall, which was treated with transcatheter arterial embolization (TAE). Despite the advanced age of the patient, successful hemostasis and a relatively favorable survival were achieved.
Case Report
An 87-year-old man was admitted complaining of sudden-onset and persistent chest pain on the right side for 1 day. The patient developed HCC in segment 6 of the liver and received percutaneous radiofrequency ablation 4 years prior. He experienced local recurrence at the ablated site 2 years prior to this presentation, which was treated with transcatheter arterial chemoembolization (TACE). While the residual tumor was not identified, hepatitis C virus was eradicated with sofosbuvir and ledipasvir therapy 1 year previously. Another course of TACE was performed for a 1.5-cm intrahepatic recurrence abutting the gallbladder 2 months ago. However, his serum des-γ-carboxy prothrombin (DCP) level increased from the 214 mAU/mL pretreatment to 251 mAU/mL posttreatment, whereas the serum α-fetoprotein level remained persistently normal.
On admission, his vital signs were a body temperature of 36.0°C, blood pressure of 102/69 mmHg, pulse rate of 96/min, and respiratory rate of 16/min with percutaneous oxygen saturation of 97% on room air. A physical examination detected decreased breath sounds in the right chest. Laboratory results indicated a decreased hemoglobin level from 12.9 g/dL (1 month ago) to 10.3 g/dL. No significant change was observed on electrocardiography.
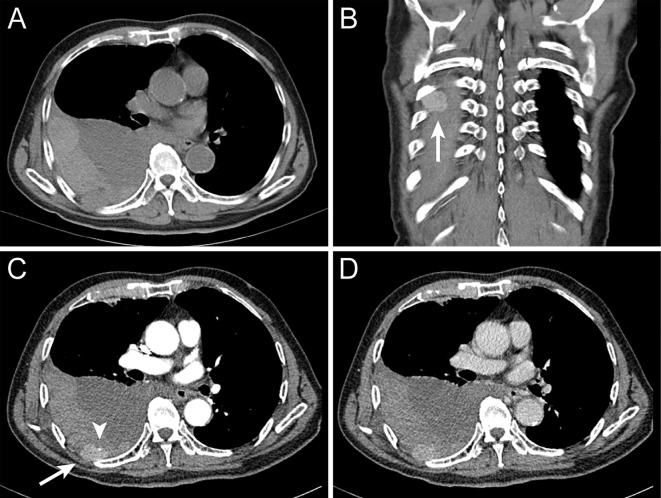
Chest radiography in the supine position revealed diffuse infiltration in the entire right lung (Fig. 1). Non-enhanced CT revealed massive right pleural effusion, the dorsal side of which showed a slightly high attenuation thus suggesting a hemorrhage (Fig. 2A). On dynamic CT, an arterially enhancing mass in the right seventh posterior intercostal space protruded into the thoracic cavity (Fig. 2B-D). A pinpoint extravasation was noted on the anterior surface of the lesion (Fig. 2C). There was no evidence suggesting an intrahepatic recurrence of HCC. These findings indicated hemothorax caused by a spontaneous rupture of HCC metastasis to the chest wall.
Figure 1.
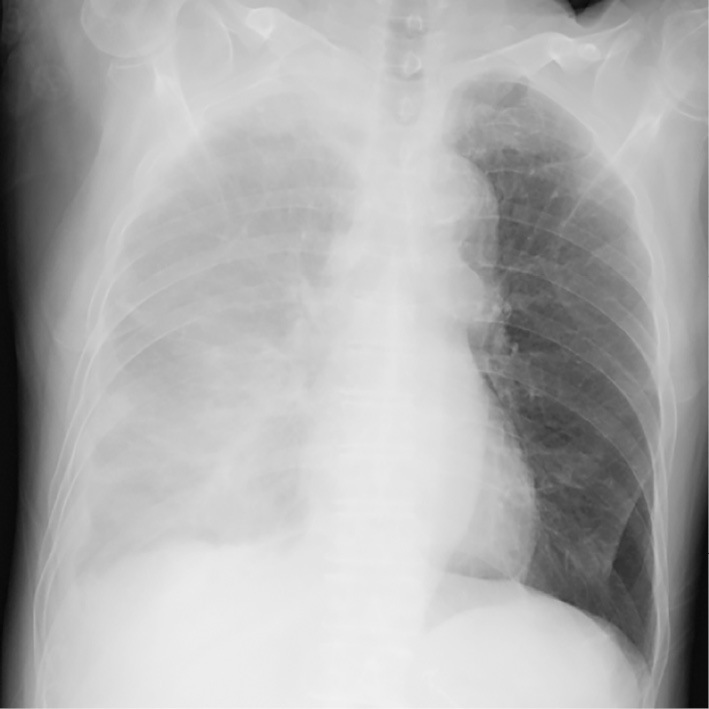
Supine chest radiography on admission showing diffuse infiltration in the entire right lung.
Figure 2.
Thoracic computed tomography (CT) on admission. Non-enhanced CT revealed massive right pleural effusion, the dorsal side of which showed slightly high attenuation suggesting hemorrhage (A). Arterial phase (B, C) and late phase (D) of contrast-enhanced CT. An arterially enhancing mass in the right seventh posterior intercostal space was protruding into the thoracic cavity (B, C) (arrow), compatible with metastasis from hepatocellular carcinoma. A pinpoint extravasation was noted on the anterior surface of the lesion (C) (arrowhead), indicating a spontaneous rupture of metastatic hepatocellular carcinoma.
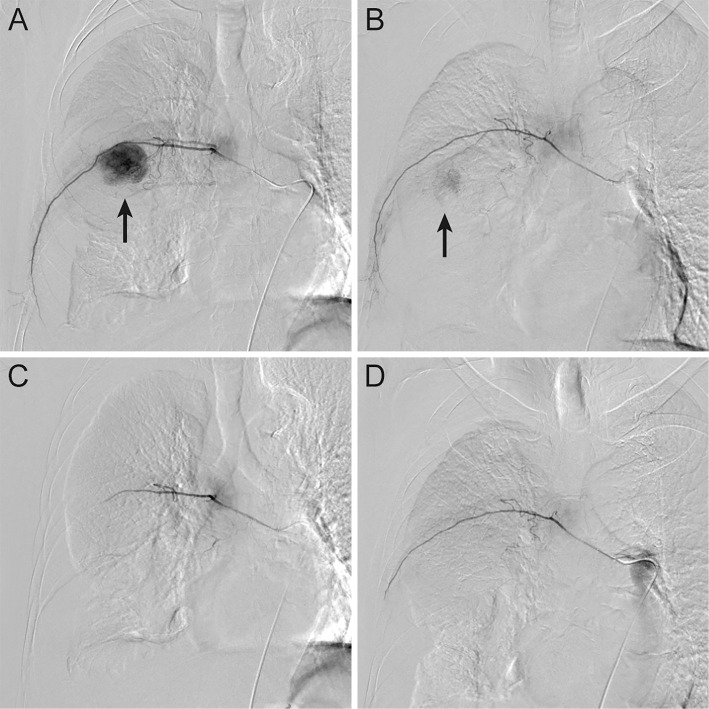
The patient refused chest drainage for the confirmation and management of hemothorax, but agreed to undergo transcatheter hemostasis for the chest wall lesion. Emergent angiography demonstrated a hypervascular tumor supplied predominantly by the right seventh intercostal artery (Fig. 3A) and partially by the right sixth intercostal artery (Fig. 3B), which was consistent with metastatic HCC. Although extravasation could not be identified on angiography, we performed selective embolization of these vessels using a gelatin sponge and microcoils based on the findings of contrast-enhanced CT. Post-embolization angiography of each artery confirmed complete disappearance of the tumor staining (Fig. 3C, D).
Figure 3.
Emergent angiography of the intercostal arteries. A hypervascular tumor was predominantly supplied by the right seventh intercostal artery (A) and partially by the right sixth intercostal artery (B) (arrow), which was consistent with metastatic hepatocellular carcinoma. After selective embolization of the feeding arteries with a gelatin sponge and microcoils, post-embolization angiography confirmed the complete disappearance of the tumor staining (C, D).
Following the treatment, his symptoms, such as fever, dyspnea, and chest pain, resolved conservatively. The hemoglobin and serum albumin levels decreased to the lowest levels of 7.9 g/dL and 2.7 g/dL, respectively, but blood transfusion or albumin supplementation was unnecessary. Because other liver function tests remained stable, these laboratory abnormalities were presumably caused by blood loss associated with hemothorax, not by hepatic decompensation. Although the thoracic metastasis was the only detected lesion of HCC, we withheld additional anticancer treatment because of his inadequate performance status and advanced age. The patient had been free of symptoms requiring management at the time of discharge, and we asked his primary care physician for future palliative care. While further radiological assessments were not performed, he visited our hospital with complete recovery of anemia and hypoalbuminemia 9 months after the onset of the hemothorax.
Discussion
This report highlights hemothorax as a complication of thoracic HCC metastasis in an elderly patient. To the best of our knowledge, no further case was reported after Yen et al. reviewed 21 cases with hemothorax from HCC referred to in the English literature (11). Notably, our patient was older than any of the previous cases. The most frequent symptoms of these cases were dyspnea followed by shock and chest pain (11). The HCC lesions responsible for hemothorax were metastases to the thoracic region, including the lung, mediastinum, pleura, rib, and chest wall, as well as primary tumors with either direct invasion or rupture into the thoracic cavity (11). Although bloody pleural effusion was not proved by thoracentesis in our patient, the extravasation on contrast-enhanced CT is useful for demonstrating a rupture of metastatic HCC, as also noted in previous reports (11-13). Whereas our patient recovered from hemothorax without chest tube insertion, the majority of previous cases received chest drainage probably because of massive hemothorax (14-24). However, it is notable that initial conservative management only with chest drainage failed in some cases, as illustrated by repeated thoracentesis (18-20) or continuous bloody drainage from the chest tube (21). These findings suggest the palliative role of chest drainage and the importance of hemostatic procedures in the treatment for hemothorax resulting from ruptured HCC.
Although a rapidly deteriorating general condition can preclude therapeutic intervention (16,25,26), transcatheter procedures have been the most common treatment for HCC with hemothorax and they were applied in 10 cases (Table) (11,13,15,18,20,21,23,24,27). The feeding arteries for ruptured thoracic HCC metastasis were identified as the intercostal artery (11,13), superficial cervical artery (15), inferior phrenic artery (21), and bronchial artery (24), whereas the inferior phrenic artery (18) as well as hepatic arteries (23,27) supplied the primary tumors responsible for hemothorax. Transcatheter treatment in this situation was found to be effective in achieving hemostasis in all cases except one (11,13,15,18,20,21,23,24,27). In our patient, we used a gelatin sponge and microcoils as embolic agents based on the CT findings indicating active bleeding from the chest wall metastasis of HCC, although extravasation was not evident on angiography. In contrast, all five previous cases with thoracic HCC metastasis who were successfully treated with TAE or TACE had extravasation on angiography (11,13,15,21,24). In four reports describing embolic agents, hemostasis was achieved by a gelatin sponge alone (15) or in combination with lipiodol (11) or microcoils (13), or polyvinyl alcohol particles with lipiodol (24). Due to the short-term survival (11,15,24) or subsequent treatments (13,21) as well as varying selection of embolic agents in these cases, long-term therapeutic effect of each agent is difficult to evaluate. In particular, it is unclear whether the treatment can be simplified with a gelatin sponge alone or if microcoils are necessary to achieve hemostasis in cases lacking extravasation on angiography.
Table.
Reported Cases with Hemothorax Secondary to Hepatocellular Carcinoma Treated with Transcatheter Procedures.
| No. | Reference | Age | Sex | Symptom | Responsible lesion | Feeding artery | Transcatheter treatment | Additional treatment | Follow-up | Outcome | Cause of death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Metastatic | |||||||||||
| 1 | 15 | 53 | M | Chest pain, dyspnea, shock | - | Rib | Superficial cervical artery | TAE | - | 3 months | Dead | Cancer death |
| 2 | 27 | 65 | M | Cough, fatigue | Direct invasion | - | Right and middle hepatic artery | TAI | - | 40 days | Dead | Rupture of esophageal varix |
| 3 | 18 | 64 | M | Dyspnea | Direct invasion | - | Inferior phrenic artery | TAE | - | 3 months | Dead | Cancer death |
| 4 | 20 | 59 | M | Dyspnea | Direct invasion | - | ND | TAE (ineffective) | Thoracoscopic RFA | 4 months | Dead | Cancer death |
| 5 | 21 | 68 | M | Chest pain, shock | - | Mediastinum (lymph node) | Inferior phrenic artery | TAE | Surgery, chemotherapy | 12 months | Alive | - |
| 6 | 23 | 56 | W | Epigastric pain, shock | Rupture | - | Right and left hepatic artery | TAE | Hepatectomy, RFA | 2 years | Alive | - |
| 7 | 13 | 71 | M | Shock | - | Chest wall | Intercostal artery | TAE | Radiotherapy | 15 months | Alive | - |
| 8 | 24 | 60 | M | Chest pain, dyspnea | - | Mediastinum (lymph node) | Bronchial artery | TACE | - | 4 months | Dead | ND |
| 9 | 11 | 60 | W | Dyspnea | - | Mediastinum | Intercostal artery | TAE | - | 20 days | Dead | Sepsis, MOF |
| 10 | Our case | 87 | M | Chest pain | - | Chest wall | Intercostal artery | TAE | - | 9 months | Alive | - |
M: man, W: woman, ND: not described, TAE: transcatheter arterial embolization, TAI: transcatheter arterial infusion, TACE: transcatheter arterial chemoembolization, RFA: radiofrequency ablation, MOF: multiorgan failure
Overall, HCC complicated with hemothorax has an unfavorable prognosis. Death within a few months was frequently observed in previous cases primarily due to shock, organ dysfunction (particularly respiratory and liver failure), and cancer progression (11). This was also true of the cases achieving transcatheter hemostasis for hemothorax (11,15,18,20,24,27), whereas 3 cases who survived for longer than 1 year received additional treatment for HCC (13,21,23). Based on these findings, it is remarkable that our patient survived at least 9 months after undergoing TAE alone, although no further anticancer therapy was applied partly because of his highly advanced age. Thus, our case suggests that TAE for hemothorax from ruptured HCC metastasis can lead to hemostasis and favorable survival outcome even in the elderly.
One possible explanation for the relatively long-term survival in our patient was the low primary tumor burden. It has been shown that the intrahepatic tumor status predicts the prognosis of patients with extrahepatic metastasis of HCC (8,9). However, metastatic HCC is generally associated with severe intrahepatic tumor condition (5-7). This finding is consistent with many previous reports describing that hemothorax from metastatic HCC occurred in cases with large and multiple primary lesions as well as repeated history of treatment for recurrence (11,16,21,22,24,26). In contrast, the intrahepatic lesion of our patient was well controlled despite sporadic locoregional therapies. In patients with localized intrahepatic HCC treated by transarterial therapies, extrahepatic metastasis is not the major form of disease progression (28). For this reason, we hardly anticipated the development of extrahepatic metastasis in our patient, although an increased serum DCP level after the latest course of TACE can be attributable to the chest wall lesion. Therefore, our case shows that thoracic metastasis and resulting hemothorax can occur even in a case with a low intrahepatic tumor burden. At present, however, it is unclear which population with HCC is most at risk of this unusual event, and thus may benefit from careful monitoring for extrahepatic metastasis.
In summary, we herein described a case of hemothorax resulting from spontaneously ruptured HCC metastasis to the chest wall. Although this life-threatening complication of HCC can occur in cases with a well controlled primary tumor status, TAE is an effective treatment that can lead to hemostasis and a favorable survival even in the elderly.
The patient provided written informed consent for the publication of this article and accompanying images.
Author's disclosure of potential Conflicts of Interest (COI).
Norifumi Kawada: Honoraria, Gilead Sciences; Research funding, Gilead Sciences.
References
- 1.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16: 589-604, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144: 1941-1953, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H, Mamada Y, Taniai N, Uchida E. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res 46: 13-21, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Moris D, Chakedis J, Sun SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol 117: 341-353, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology 216: 698-703, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 20: 1781-1787, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int 28: 1256-1263, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117: 4475-4483, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Jung SM, Jang JW, You CR, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol 27: 684-689, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Becker AK, Tso DK, Harris AC, Malfair D, Chang SD. Extrahepatic metastases of hepatocellular carcinoma: a spectrum of imaging findings. Can Assoc Radiol J 65: 60-66, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Yen CW, Hsu LS, Chen CW, Lin WH. Hepatocellular carcinoma with thoracic metastases presenting as hemothorax: a case report and literature review. Medicine (Baltimore) 97: e10945, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CK, Wu KC, Wu RH, Lui YH. Spontaneous hemothorax caused by metastasis of a rib tumour. CMAJ 178: 679, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagao E, Hirakawa M, Soeda H, Tsuruta S, Sakai H, Honda H. Transcatheter arterial embolization for chest wall metastasis of hepatocellular carcinoma. World J Radiol 5: 45-48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hino K, Ohumi T, Kojoh K, et al. A case of hepatocellular carcinoma associated with hemothorax from spontaneous rupture of the mediastinal metastasis. Acta Hepatol Jpn 28: 1383-1388, 1987. (in Japanese). [Google Scholar]
- 15.Kohno T, Yamamoto M, Iizuka H, Miura K, Yoshioka M, Sugahara K. Three cases of recurrent hepatocellular carcinoma with bone metastasis appearing with particular symptoms. Jpn J Gastroenterol Surg 23: 1887-1891, 1990. (in Japanese). [Google Scholar]
- 16.Akimaru K, Miyairi K, Tanaka H, et al. A rupture of lung metastasis of hepatocellular carcinoma causing haemothorax. J Gastroenterol Hepatol 8: 613-615, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Takagi H, Shimoda R, Uehara M, et al. Hepatocellular carcinoma with pleural metastasis complicated by hemothorax. Am J Gastroenterol 91: 1865-1866, 1996. [PubMed] [Google Scholar]
- 18.Masumoto A, Motomura K, Uchimura K, Morotomi I, Morita K. Case report: haemothorax due to hepatocellular carcinoma rupture. J Gastroenterol Hepatol 12: 156-158, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Ogata H, Tsuji H, Hashiguchi M, Azuma K, Shimono J, Fujishima M. Hepatocellular carcinoma with metastasis to the rib complicated by hemothorax. An autopsy case. Fukuoka Igaku Zasshi 90: 342-346, 1999. [PubMed] [Google Scholar]
- 20.Ishikawa T, Kohno T, Teratani T, Omata M. Thoracoscopic radiofrequency ablation therapy for hepatocellular carcinoma above the diaphragm associated with intractable hemothorax. Endoscopy 34: 843, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Shiozawa K, Fushida S, Tani T, Ishii K, Shimizu K, Miwa K. A case of hemothorax secondary to rupture of mediastinal lymph node metastasis of hepatocellular carcinoma. J Jpn Surg Assoc 64: 1358-1361, 2003. (in Japanese). [Google Scholar]
- 22.Wei YF, Wang HC, Chang YC. Hemothorax due to metastatic hepatocellular carcinoma presenting with massive hemoptysis. J Formos Med Assoc 105: 346-348, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ono F, Hiraga M, Omura N, et al. Hemothorax caused by spontaneous rupture of hepatocellular carcinoma: a case report and review of the literature. World J Surg Oncol 10: 215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SY, Seo KW, Jegal Y, et al. Hemothorax caused by spontaneous rupture of a metastatic mediastinal lymph node in hepatocellular carcinoma: a case report. Korean J Intern Med 28: 622-625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekiya Y, Yamada H, Yoshitsugu M, Ihori M, Takemura T. An autopsy case of hepatocellular carcinoma associated with right hemothorax from spontaneous rupture of the rib metastasis. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 89: 1310-1313, 1992. (in Japanese). [PubMed] [Google Scholar]
- 26.Sohara N, Takagi H, Yamada T, Abe T, Mori M. Hepatocellular carcinoma complicated by hemothorax. J Gastroenterol 35: 240-244, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kanou S, Yamada T, Hiramatsu N, et al. A case of hepatocellular carcinoma with direct invasion into the pleural cavity presenting as hemorrhage achieved hemostasis with intra-arterial injection of ethanol. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 93: 753-757, 1996. (in Japanese). [PubMed] [Google Scholar]
- 28.Senthilnathan S, Memon K, Lewandowski RJ, et al. Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology 55: 1432-1442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]