Abstract
A pharmacist-driven methicillin-resistant Staphylococcus aureus (MRSA) nasal polymerase chain reaction (PCR)-based testing protocol with a 70% acceptance rate for vancomycin discontinuation within 24 hours of negative results significantly reduced unnecessary vancomycin use with an estimated cost avoidance of $40 per vancomycin course. We found high concordance (141 of 147, 96%) of culture-based versus PCR-based MRSA nasal screening.
Keywords: MRSA, pneumonia, rapid diagnostics, stewardship, vancomycin
Limiting unnecessary anti-methicillin-resistant Staphylococcus aureus (MRSA) therapy can reduce emergence of resistant Gram-positive isolates and minimize antimicrobial toxicity. Methicillin-resistant Staphylococcus aureus nasal screening is associated with a high negative predictive value (NPV) of 96.5% for MRSA pneumonia [1]. Clinical reports support the use of MRSA nasal screening as a powerful antimicrobial stewardship (AMS) tool to facilitate de-escalation of empiric antimicrobials in patients with pneumonia [2–6]. Clinicians may be reluctant to readily adopt this practice given concerns of discordance of MRSA nasal screens in patients with structural lung disease, lower screen performance in ventilator-associated pneumonia (VAP) (NPV 94.8%), or in critically ill patients with cultures pending when a negative nasal screen results [1]. One element determining test performance is the local MRSA prevalence, which, at our institution in 2018–2019, was 22% from all sources and 33% from respiratory cultures [7]. Our aims were to describe patterns of vancomycin use, concordance of polymerase chain reaction (PCR)-based versus culture-based nasal screening, and cost avoidance after implementation of a MRSA nasal PCR-based testing protocol.
METHODS
A nasal PCR protocol was designed to exclusively allow pharmacists to order testing if vancomycin was ordered for empiric pneumonia based on mandatory antibiotic indication fields (Supplementary Materials B). Pending and final test results were documented on vancomycin-per-pharmacy progress notes in the electronic medical record system (Supplementary Materials C). Team-based clinical and Infectious Disease pharmacists communicate the results with providers during clinical and AMS handshake rounds between 8:00 am and 3:00 pm; providers were ultimately responsible for vancomycin discontinuation.
Methicillin-resistant Staphylococcus aureus nasal PCR tests were performed at the Stanford Clinical Laboratory using GeneXpert MRSA (Cepheid, Sunnyvale, CA) 24 hours a day, 7 days a week, with a 4- to 6-hour turnaround time (TAT). This testing protocol was implemented independently of a preexisting process for state mandated MRSA nasal screening by culture (TAT 2 days) in selected patient populations such as intensive care unit admissions or 30-day hospital readmissions [8].
Our patients were ≥18 years old, hospitalized at a 613-bed tertiary care center, with a vancomycin order for suspected pneumonia between May 1, 2017 and August 31, 2017 in the Pre-PCR cohort and May 7, 2018 and December 31, 2019 in the post-PCR cohort. We excluded patients with extrapulmonary or undifferentiated sources of infection, cystic fibrosis, no vancomycin received, discharge or death within 24 hours of initiation, or any repeat vancomycin courses during the same hospitalization (Supplementary Materials A). Approval for this quality improvement project was waived by the Stanford University institutional review board.
The primary outcome was the proportion of patients in the Post-PCR group in which vancomycin was discontinued within 24 hours of negative PCR results. We compared Pre-PCR and Post-PCR groups in the secondary outcome analysis, which included time from negative MRSA PCR results to vancomycin discontinuation, number of vancomycin doses given and drug levels per patient, time of day of vancomycin discontinuation, concordance of MRSA nasal screening (PCR-based vs culture-based), hospital length of stay, 30- and 90-day mortality, nephrotoxicity (defined by a RIFLE category of injury or worse), and cost avoidance.
Cost avoidance was calculated based on the number of vancomycin doses, associated drug monitoring, and labor costs in patients before and after implementation of the PCR testing protocol. Vancomycin acquisition costs were estimated at $15 per 1-gram dose. Serum vancomycin levels were $37 at our institution. The cost for a MRSA PCR assay cassette was estimated at $36 [9]. We assumed (1) a maximum of 30 minutes for a pharmacist to initiate vancomycin or to manage each level and (2) a maximum of 30 minutes of phlebotomist time per vancomycin level drawn [10]. Hourly wages were obtained via the Bureau of Labor Statistics, US Department of Labor [11, 12].
Statistical Methods
Data were analyzed using SPSS 26.0 (IBM SPSS Statistics; IBM Corporation) software. Categorical variables were analyzed by χ 2 or Fisher’s exact test when appropriate. Continuous variables were analyzed by Student’s t test or Mann-Whitney U test, where appropriate. Categorical variables were represented by using frequencies and percentages, and continuous data are presented as the medians (interquartile ranges). Cost avoidance calculations were performed using Microsoft Excel (Microsoft Corp., Redmond, WA). P < .05 was considered statistically significant.
Patient Consent Information
Patient’s written consent was not required for this retrospective study. The design of the work was reviewed, and study approval was waived by the Stanford University institutional review board. The work conforms to standards currently applied in the country of origin. Data are available in Supplementary Material.
RESULTS
We included 610 of 813 screened patients in either the primary or secondary analysis (Table 1, Supplementary Materials A). Forty-one patients in the post-PCR group had a positive MRSA PCR. In the Post-PCR group, vancomycin duration was less than 24 hours in 317 of 453 (70%) of patients with a negative nasal PCR, with drug discontinuation before any drug levels being drawn in 263 (58%) of these patients. One patient had culture-confirmed MRSA pneumonia 4 days, and none had MRSA bacteremia after a negative PCR-based screen.
Table 1.
Baseline Characteristics
| Characteristics | Pre (n = 116) | Post (n = 494) | P Value |
|---|---|---|---|
| Age, median (IQR), years | 69 (59–78) | 66 (56–76) | .28 |
| Male, n (%) | 72 (62%) | 294 (60%) | .26 |
| Weight, median (IQR), kg | 67 (57–85) | 78 (64–92) | .35 |
| Body mass index, median (IQR), kg/m2 | 23.7 (22–28) | 28.5 (25–33) | .08 |
| Indication for Vancomycina, n (%) | .03 | ||
| CAP | 75 (65%) | 281 (57%) | |
| HAP | 24 (21%) | 161 (33%) | |
| VAP | 17 (15%) | 52 (11%) | |
| Culture-based MRSA nasal screening within 30 days of vancomycin initiation | 23 (20%) | 97 (20%) | 1.00 |
| Negative PCR-based MRSA nasal screening | 453 (92%) | ||
| Infectious Diseases consultation, n (%) | 18 (16%) | 109 (22%) | .12 |
| Treatment team, n (%) | .94 | ||
| BMT | 2 (2%) | 8 (2%) | |
| Hematology | 5 (4%) | 24 (5%) | |
| ICU | 56 (48%) | 218 (44%) | |
| Medicine | 32 (28%) | 145 (29%) | |
| Oncology | 6 (5%) | 37 (7%) | |
| Solid organ transplant | 10 (9%) | 35 (7%) | |
| Surgery | 5 (4%) | 27 (5%) | |
| Immunocompromised, n (%) | 44 (37%) | 191 (38%) | .92 |
| Bone marrow transplant | 2 (5%) | 16 (8%) | |
| Hematologic malignancy | 5 (11%) | 35 (18%) | |
| Solid organ transplant | 10 (23%) | 61 (32%) | |
| Lung transplant | 8 (18%) | 50 (26%) | |
| Solid tumor | 12 (27%) | 49 (26%) | |
| Otherb | 15 (16%) | 30 (34%) |
Abbreviations: BMT, bone marrow transplant; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction; VAP, ventilator-associated pneumonia.
aCAP and HAP were defined as onset of pneumonia within 48 hours of hospital admission and at least 48 hours after admission, respectively. VAP was defined as onset of pneumonia at least 48 hours after endotracheal intubation.
bReceived 20 mg or more of prednisone or equivalent daily for more than 14 days, biological agents in the preceding 30 days, or were infected with human immunodeficiency virus and had a CD4+ count <200.
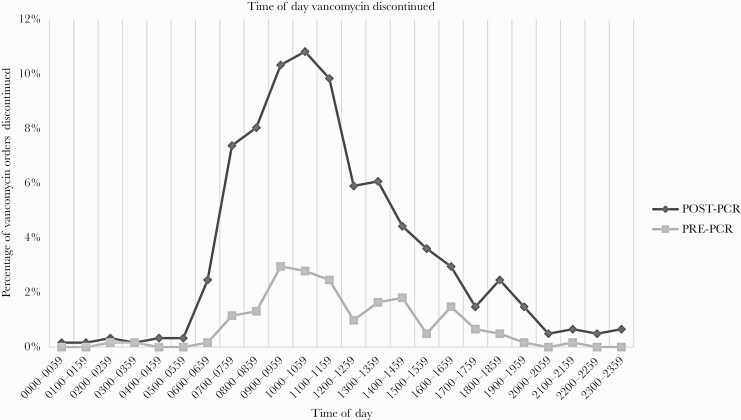
The median vancomycin duration was shorter in the Post-PCR group (1.29 days; 95% confidence interval [CI], 1.13–1.45) versus Pre-PCR group (1.98 days; 95% CI, 1.49–2.46) (P < .0005), a 34.8% reduction. Fewer vancomycin doses were administered per patient in the Post-PCR versus Pre-PCR group (2 [95% CI, 1–4] vs 3.5 [95% CI, 2–5] doses, P < .0001), a 43% (1.5 dose) decrease. In the Post-PCR group, 92% (453 of 494) of patients had a negative MRSA nasal PCR and a median time to vancomycin de-escalation of 15 hours (95% CI, 13–16). Vancomycin discontinuation was most frequent between 10:00 am and 12:00 pm (Figure 1). It is notable that vancomycin was discontinued between 7:00 pm and 7:00 am in 4.3% of patients in the pre-PCR group and 9.5% of the post-PCR group.
Figure 1.
Time of day of vancomycin discontinuation Pre-polymerase chain reaction (PCR) versus Post-PCR.
One hundred forty-seven patients (29.8%) underwent culture-based MRSA screening in addition to PCR-based screening in the post-PCR group. Of these 147 patients, 6 (4%) had discordant results (negative culture-based but positive PCR-based MRSA screen results) within 30 days (Supplementary Table S1).
Median aggregate costs per patient (composed of the costs associated with drug acquisition, serum vancomycin levels, the PCR assay cassette, and personnel time) were $175.66 in the pre-PCR group and $135.33 in the post-PCR group. Median cost avoidance was $40.33 per patient after PCR implementation. With approximately 700 PCR tests per year performed at our institution, this corresponds to a yearly total cost avoidance of $28 231.
Hospital length of stay (8.5 vs 9.8 days, P = .84), 30-day (22% vs 19.8%, P = .54) and 90-day mortality (24.1% vs 24.3%, P = 1.00), and nephrotoxicity (13.8% vs 8.7%, P = .10) were similar in Pre-PCR and Post-PCR groups and not included in cost analysis.
DISCUSSION
Our study revealed that although concerted efforts resulted in a high acceptance rate (70%) within 24 hours of negative PCR results, powerful prescribing social norms prevail and hinder earlier de-escalation. Communication of negative results were most impactful when occurring between 7:00 am and 4:00 pm at our institution, during multidisciplinary team rounds, day team coverage, and proactive pharmacist interventions (Figure 1). The low discontinuation rate between 7 pm and 7 am likely reflected deferred antibiotic decision-making by overnight “covering” providers, resulting in (1) a delayed median time to de-escalation of 15 hours after a negative MRSA PCR result and (2) a longer median overall vancomycin duration (31 hours) in our study compared to 27 hours previously reported [6]. Despite these delays, we report a higher discontinuation rate than previously reports (45.3%–55.2%) [2, 3].
These observations highlight potential next steps such as ongoing education, an electronic alert to daytime providers upon negative PCR results, and reassessment of laboratory MRSA PCR testing workflow (eg, batching of overnight tests).
We gathered data on 30-day concordance (96%) of 2 nasal screening methods to show that incidental culture-based MRSA screen results performed before diagnosis of pneumonia may be useful to withhold or discontinue anti-MRSA therapy, although limited by sample size. In addition, culture-based MRSA screening is less expensive, approximately $7 per test compared to $36 per PCR test [1, 9], and could decrease costs. The low 2% discordance between PCR-based and culture-based MRSA screening results within 5 days could have been due to de novo MRSA acquisition, limitation of testing characteristics of the nasal culture (87.6% sensitivity) [13], or poor nasal swab sampling technique. This timeframe is consistent with data supporting a 5- to 7-day repeat testing cutoff time [1, 14]. Community-acquired pneumonia guidelines recommend PCR-based but not culture-based screening in determining the need for anti-MRSA antibiotic initiation [2], but this warrants further study because culture-based screening demonstrated a 94.6% NPV for MRSA in respiratory cultures [15].
Delays in antibiotic decision-making by clinicians impacted and attenuated cost avoidance. Even though we included medication, monitoring, personnel, and PCR test costs in our estimate, we observed a modest cost avoidance of $40 compared to the $108 per patient estimate by Smith et al [3]. However, that study extrapolated cost savings based solely on medication and monitoring costs of a 72-hour anticipated vancomycin use period in those with a negative PCR result.
This was a retrospective, quality improvement study at a single teaching institution with limitations to its applicability to other settings. Our study did not account for patient benefits of limiting empiric vancomycin use. The Pre-PCR group study period was much shorter than the Post-PCR time period. However, upon further analysis, vancomycin duration in the Pre-PCR time period was still longer than a matched 4-month Post-PCR group (1.98 vs 1.13 days), but this did not impact our secondary outcome (number of vancomycin doses).
CONCLUSIONS
Our study identifies opportunities to improve the real-world application and financial impact of a pharmacist-driven MRSA nasal screening protocol. These results highlight the importance of aligning stewardship efforts with laboratory and antibiotic decision-making activities.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Stanford Health Care pharmacists, clinical microbiology laboratory scientists, Indre Budvytine, and Carol Nakamura.
Financial support. M. M. H. was funded by the National Institutes of Health (Grants NIH T32 AI 052073-11 A1 and T32 AI 007502-22).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Parente DM, Cunha CB, Mylonakis E, Timbrook TT. The clinical utility of methicillin-resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: a diagnostic meta-analysis with antimicrobial stewardship implications. Clin Infect Dis 2018; 67:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health Syst Pharm 2017; 74:1765–73. [DOI] [PubMed] [Google Scholar]
- 3.Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care 2017; 38:168–71. [DOI] [PubMed] [Google Scholar]
- 4.Boyce JM, Pop OF, Abreu-Lanfranco O, et al. A trial of discontinuation of empiric vancomycin therapy in patients with suspected methicillin-resistant Staphylococcus aureus health care-associated pneumonia. Antimicrob Agents Chemother 2013; 57:1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm 2018; 40:526–32. [DOI] [PubMed] [Google Scholar]
- 6.Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother 2017; 61:e02432–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niaz B, Nancy W. 2018. and 2019 Antibiograms. Stanford Clinical Microbiology–Antibiogram. Available at: https://med.stanford.edu/bugsanddrugs/clinical-microbiology.html. Accessed 29 December 2020.
- 8.BILL NUMBER: SB 1058. State of California Legislative Council. Available at: http://www.leginfo.ca.gov/pub/07-08/bill/sen/sb_1051-1100/sb_1058_bill_20080925_chaptered.html. Accessed 29 December 2020.
- 9.Henson G, Ghonim E, Swiatlo A, et al. Cost-benefit and effectiveness analysis of rapid testing for MRSA carriage in a hospital setting. Clin Lab Sci 2014; 27:13–20. [PubMed] [Google Scholar]
- 10.Lee BV, Fong G, Bolaris M, et al. Cost–benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect 2020; doi: S1198743X20307047. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Labor. Occupational Outlook Handbook. Pharmacists. Available at: https://www.bls.gov/ooh/healthcare/pharmacists.htm. Accessed 29 December 2020.
- 12.U.S. Department of Labor. Occupational Outlook Handbook. Phlebotomists. Available at: https://www.bls.gov/ooh/healthcare/phlebotomists.htm. Accessed 29 December 2020.
- 13.Luteijn JM, Hubben GA, Pechlivanoglou P, et al. Diagnostic accuracy of culture-based and PCR-based detection tests for methicillin-resistant Staphylococcus aureus: a meta-analysis. Clin Microbiol Infect 2011; 17:146–54. [DOI] [PubMed] [Google Scholar]
- 14.Ghasemzadeh-Moghaddam H, Neela V, van Wamel W, et al. Nasal carriers are more likely to acquire exogenous Staphylococcus aureus strains than non-carriers. Clin Microbiol Infect 2015; 21:998.e1–7. [DOI] [PubMed] [Google Scholar]
- 15.Mergenhagen KA, Starr KE, Wattengel BA, et al. Determining the utility of methicillin-resistant Staphylococcus aureus nares screening in antimicrobial stewardship. Clin Infect Dis 2020; 71:1142–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.