Abstract
Dietary fish oil supplementation provides n-3 long-chained polyunsaturated fatty acids for supporting fish growth and metabolism and enriching fillet with eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; c22:6n-3). Two experiments were performed as a 3 × 2 factorial arrangement of dietary treatments for 16 wk to determine effects and mechanisms of replacing 0%, 50%, and 100% fish oil with DHA-rich microalgae in combination with synthetic vs. microalgal source of astaxanthin in plant protein meal (PM)- or fishmeal (FM)- based diets for juvenile rainbow trout (Oncorhynchus mykiss). Fish (22 ± 0.26 g) were stocked at 17/tank and 3 tanks/diet. The 100% fish oil replacement impaired (P < 0.0001) growth performance, dietary protein and energy utilization, body indices, and tissue accumulation of DHA and EPA in both diet series. The impairments were associated (P < 0.05) with upregulation of hepatic gene expression related to growth (ghr1and igf1) and biosynthesis of DHA and EPA (fads6 and evol5) that was more dramatic in the FM than PM diet-fed fish, and more pronounced on tissue EPA than DHA concentrations. The source of astaxanthin exerted interaction effects with the fish oil replacement on several measures including muscle total cholesterol concentrations. In conclusion, replacing fish oil by the DHA-rich microalgae produced more negative metabolic responses than the substitution of synthetic astaxanthin by the microalgal source in juvenile rainbow trout fed 2 types of practical diets.
Keywords: Astaxanthin, fish oil, microalgae, n-3 polyunsaturated fatty acid, rainbow trout, sustainability
Introduction
Aquaculture production is expanding 3% to 4% annually (OECD-FAO, 2019). While the expansion has enhanced the global per capita fish consumption to over 20 kg a year (FAO, 2016), it leads to continuously increasing expense of fish oil as a highly demanded ingredient of aquafeeds (Olsen and Hasan, 2012). This is because fish oil is rich in omega-3 long-chained polyunsaturated fatty acids (n-3 LC-PUFA) including eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; c22:6n-3; Turchini et al., 2009; Perez-Velazquez et al., 2019, Fukada et al., 2020). These n-3 LC-PUFA in fish oil are essential nutrients for many fish species. They not only promote fish growth and nutrient metabolism but also are accumulated in the fillet to offer cardioprotective health benefits to consumers (Burr et al., 1989; Kris-Etherton et al., 2002; Raatz et al., 2013). In contrast, conventional plant oils contain no EPA or DHA. Their use in aquaculture feed translates into low fillet n-3 PUFA enrichments and compromised health values of the product (Caballero et al. 2002; Betiku et al., 2016; Cleveland et al., 2018). Likewise, fishmeal (FM) becomes increasingly expensive and plant-based protein is considered as a common replacement. Because protein sources in diets affect nutrient requirements of fish (García-Ortega et al., 2016), it is crucial to find appropriate fish oil substitutions under different dietary protein bases, without compromising fish growth performance and the fillet value for sustaining the world aquaculture production.
Microalgae have been explored as a DHA source in comparison with that of genetically modified plant oils (Amaro et al., 2011). Algal-based products can partially replace dietary fish oil or be used in combination with plant oils, without negatively affecting growth of Pacific white shrimp, Atlantic salmon, rainbow trout, and gilthead sea bream (Ju et al., 2012; Kiron, 2012; Vizcaíno et al., 2016; Santigosa et al., 2020). Those products also enhance the accumulation of DHA in the fillet and improve its ultimate health value (Peterson et al., 2019; Fukada et al., 2020). Although these studies illustrate the potential for microalgae as a replacement for fish oil-derived DHA, it remains largely unclear how its efficacy varies with the main source of dietary protein (plant meal (PM) vs. FM). Meanwhile, little is known as how supplemental microalgal DHA affects biochemical and molecular mechanisms regulating fatty acid biosynthesis and lipid metabolism in the liver and white muscle of fish.
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is a red-orange carotenoid. Whereas this compound is commonly supplemented in salmonid diets primarily for the pigmentation of the muscle tissue (Martínez et al., 2019), it also exhibits antioxidant properties and may prevent the oxidation of unsaturated fatty acids including DHA (Torrissen et al., 1989; Kobayashi et al., 1997). Effects of dietary astaxanthin on growth or reproductive performance were shown to be mixed: none in rainbow trout (Rehulka 2000; Yesilayer and Erdem 2011) but positive in the blood parrot fish (Li et al., 2018), golden pompano (Xie et al., 2017), and red swamp crayfish (Cheng and Wu, 2019). Although both synthetic and natural sources of astaxanthin are used as dietary supplements (Pan and Chien, 2009), the relative efficacy of synthetic and microalgal astaxanthin in diets for the rainbow trout and their interactions with the microalgal DHA replacement of fish oil have not been tested (Martínez et al., 2019). Because the aquafeed industry is interested in replacing fish oil and synthetic astaxanthin (SA) with microalgal sources at the same time, it is necessary to find out the relative potency or efficacy of these replacements and their collective or interactive impacts on fish growth performance and health status. Linking the growth performance and nutrient metabolism status to the related gene expression of fish in response to these replacements may help explore new strategies to overcome resultant adverse effects from the replacements.
Therefore, we performed 2 parallel experiments with 3 × 2 factorial arrangement of dietary treatments in juvenile rainbow trout (Oncorhynchus mykiss). The 2 experiments were run on 2 practical types of basal diets: FM-based protein source or PM/soybean meal-based protein source. There were 3 levels (0%, 50%, and 100%) of fish oil replacements by DHA-rich microalgae, and 2 sources of astaxanthin (synthetic: SA vs. algal-based: AA). Our objectives were to determine effects and mechanisms of these dietary treatments on growth performance, nutrient retention, lipid and fatty acid profiles, and gene expression-related to growth and biosynthesis of n-3 LC-PUFA.
Material and Methods
Experimental design and diets
Two feeding trials were conducted for 16 weeks as a 3 × 2 factorial arrangement of dietary treatments: 3 levels of fish oil substitutions (0%, 50%, and 100% of DHA basis) with increasing levels of DHA-rich Aurantiochytrium microalgal meal (Heliae Development, LLC, Gilbert, AZ) (Tolba et al., 2019) and 2 sources of astaxanthin as a synthetic form (SA; 4-ascorbyl polyphosphate Rovomix Stay-C 35; Carophyll pink, DSM Nutritional Products Ltd., Basel, Switzerland) or a microalgal form (AA; Haematococcus pluvialis, Heliae, Gilbert, AZ; Magnuson et al., 2018; Sun et al., 2018) at 80 mg astaxanthin/kg. The factorial arrangement of dietary treatments was replicated in two diet series: (1) the PM diet series that contained only plant-based protein sources and (2) the FM diet series that contained both FM and plant-based protein sources. Diet formulations and proximate analyses are presented in Supplementary Table 1 (PM diet series) and Table 2 (FM diet series). The analyzed fatty acid profiles of various ingredients and diets are presented in Supplemental Table 3 (fish oil, DHA-rich microalgae, and soy oil), Supplementary Table 4 (PM diet series), and Supplemental Table 5 (FM diet series). The treatments were randomly assigned to 3 replicate tanks each, making tank the experimental unit. Experimental diets were formulated to contain 44% crude protein, 18% crude lipid, available lysine, methionine, and threonine at 3.8%, 1.30%, and 2.1%, and total phosphorus at 1.0%, respectively. The basal diets (0% substitution of fish oil) in both series were formulated to meet or exceed all nutrient requirements of rainbow trout (NRC, 2011). Diets were prepared by cooking extrusion (DNDL-44, Buhler AG, Uzwil, Switzerland) as described by Cogliati et al (2019).
Table 2.
Growth performance and body indices of juvenile rainbow trout fed FM-based diets containing microalgae to replace fish oil and synthetic astaxanthin
| Diets1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fish oil replacement2 | 0% | 50% | 100% | Fish oil | ASTA | P-values11 | ||||
| Astaxanthin source | SA | AA | SA | AA | SA | AA | RMSE | Level | Source | Interaction |
| Growth performance | ||||||||||
| Average fish Wt (g) | 386a | 367a | 353a | 380a | 169b* | 147b | 15 | <0.0001 | 0.47 | 0.025 (100SA > 100AA) |
| Percent increase3 (%) | 1680a | 1610a | 1530a | 1660a | 678b* | 591b | 67 | <0.0001 | 0.90 | 0.027 (100SA > 100AA) |
| BW/day4 (%) | 0.80b | 0.77b | 0.83b | 0.78b | 1.24a | 1.43a* | 0.07 | <0.0001 | 0.36 | 0.030 (100SA < 100AA) |
| FCR5 | 0.92b | 0.88b | 0.96b | 0.89b | 1.52a | 1.77a* | 0.09 | <0.0001 | 0.32 | 0.024 (100SA < 100AA) |
| Body indices | ||||||||||
| Survival (%) | 93 | 100 | 98 | 100 | 97 | 98 | 3.7 | 0.52 | 0.08 | 0.43 |
| VSI6 (%) | 10.7b | 9.6b | 11.7a | 13.1a | 12.2a | 12.2a | 1.03 | 0.004 | 0.80 | 0.14 |
| FR7 (%) | 59.3 | 59.6 | 59.5 | 60.8 | 57.0 | 56.4 | 2.38 | 0.06 | 0.77 | 0.82 |
| HSI8 (%) | 1.1b | 1.1b | 1.3a | 1.3a | 1.2b | 1.2b | 0.12 | 0.04 | 0.70 | 0.96 |
| PRE9 (g) | 40.6a | 45.6a | 40.9a | 45.6a | 22.3b* | 17.9b | 3.12 | <0.0001 | 0.26 | 0.04 (100SA > 100AA) |
| ERE10 (kcal/g) | 55.0a | 53.6a | 53.6a | 61.1a | 29.9b | 27.1b | 4.24 | <0.0001 | 0.59 | 0.11 |
1SA, synthetic astaxanthin; AA, microalgal astaxanthin; ASTA, astaxanthin.
2Percent of fish oil replaced by mciroalgae on a crude lipid basis of DHA.
3Percent increase (%) = (final weight – initial weight) × 100/initial weight.
4BW/day (%) = (tank feed consumption total/average tank weight)/study duration × 100.
5FCR, feed conversion ratio = g feed consumed/g weight gained.
6VSI= gut weight/body weight × 100.
7FR= fillet wt × 2/body weight × 100.
8HSI= liver weight/body weight × 100.
9PRE, protein retention efficiency = g protein gain × 100/g protein fed.
10ERE, energy retention efficiency = kcal energy gain × 100/ kcal energy fed.
11Probability associated with the F-statistic. A significant (P < 0.05) main effect without significant interaction allowed data to be pooled. Significant main effects are denoted under level and source.
a,b,cMeans without sharing a common letter are different (P < 0.05). Direct comparisons of means across the 2 treatments should be made conditionally if there was an interaction.
*SA vs. AA, P < 0.05.
Fish culture, sampling, and index calculations
Experimental fish were raised in accordance with guidelines approved by the Cornell University Animal Care and Use Committee and the U.S. Fish and Wildlife Service. Rainbow trout from a single lot were obtained from a commercial producer (Troutlodge, Inc., Sumner, WA) and cultured at the Bozeman Fish Technology Center (Bozeman, MT). Fish were stocked at 17 fish/tank (initial body weight = 22 ± 0.26 g). Lighting was maintained on a 13:11 hour diurnal cycle. Tanks (200 L) were configured in a partial recirculating system (14 °C) with biofiltration, solids removal and UV treatment of the water. Fish were fed to apparent satiation twice a day, 6 d a week for 16 wk, and feed intake was determined by weighing buckets before and after feeding.
Ten fish from the initial population were killed for determination of initial whole-body proximate composition. During the growth trial, fish were weighed every 4 wk for the determination of feed conversion ratio (FCR), feed intake, and weight gain. At the end of the study, 4 fish from each tank were randomly selected for whole body composition, and 3 additional fish were dissected for determinations of hepatosomatic index (HSI), visceral somatic index (VSI), and fillet ratio (FR).
Proximate analyses of diets, ingredients, and whole body
Dry matter was analyzed according to standard methods (Abu and Hamza, 1995). Crude protein was determined by the Dumas method (Abu and Hamza, 1995) on a Leco TruSpec N instrument (LECO Corporation, St. Joseph, MI). Total energy was determined by isoperibol bomb calorimetry (Parr 6300, Parr Instrument Company Inc., Moline, IL). Lipid was determined by petroleum ether extraction using an Ankom XT10 (Ankom Technologies, Macedon, NY).
Profiles of fatty acids and lipids in tissues and(or) diets
Lipids were extracted from diets, muscle (fish fillets), liver, and(or) heart tissues according to Folch et al (1957) as performed in previous studies (Magnuson et al., 2018; Sun et al., 2018; Liu et al., 2019). After 200 mg of diets, 1 g of muscle, and 500 mg of liver and heart each were weighed, the tissue was cut into small pieces, frozen with liquid nitrogen, and powdered with tertiary butylhydroquinone (100 mg/500 mg tissue) to prevent lipid oxidation in a Waring blender for 2 min. As described by Christie (2010), the lipid was methylated using methanolic sulfuric acid (4%) in anhydrous methanol at 90 °C for 60 min. A gas chromatograph (Agilent 6890N, Agilent Technologies, Santa Clara, CA) fitted with the internal standard of tridecanoic acid and external standard of methyl ester (Sigma-Aldrich Co., St. Louis MO) were used to determine peaks and concentrations of fatty acids. A fused-silica capillary column coated with CP-SIL 88 (100 m Å–0.25 mm inner diameter, 0.2 mm film thickness) was used to separate fatty-acid methyl esters (Varian Inc., Lake Forest, CA). The oven temperature was programmed at 140 °C and increased to 220 °C (4 °C/min). Concentrations of total cholesterol (TC), triglyceride (TG), and nonesterified fatty acid (NEFA) in the muscle and liver were determined using a Wako Chemicals kit (Richmond, VA), and concentrations of total phospholipid in the tissues were determined using a kit from Fujifilm Wako Diagnostics (Mountain View, CA).
Gene expression related to growth and biosynthesis of n-3 LC-PUFA in the liver
Hepatic gene expression of growth hormone receptor (ghr), insulin-like and growth factor (igf), fatty acid desaturases 2 and 6 (fads2, 6), elongases 2 and 5 (elovl 2, 5), and acetyl-CoA oxidase (acox) were determined using elongation factor (elf) as a reference gene. The mRNA abundances of these genes were quantitated using conventional protocols as previously described (Cleveland and Evenhuis, 2010; Cleveland et al., 2020). Briefly, RNA was isolated using Tri Reagent (Millipore-Sigma, St. Louis, MO) and cDNA was produced using random primers and M-MLV reverse transcriptase. The PCR utilized SYBR Green Master Mix (Applied Biosystems, Foster City, CA) with an ABI7900 Sequence Detection System (Applied Biosystems). Fold changes in gene expression were log2 transformed prior to regression analysis. Primer sequences are shown in Supplementary Table 6.
Statistical analysis
Data were analyzed using 2-way ANOVA (3 × 2 factorial arrangement of dietary treatments) with Software R Studio (version 1.1.463, R Foundation for Statistical Computing, Vienna, Austria). The tank was used as the experimental unit. Duncan’s multiple-range method was used to compare treatment means. Stepwise regressions were run using SPSS statistical software (Ver. 26.0 for Mac, SPSS, Inc., Chicago, IL). The significance level for differences was P < 0.05.
Results
Growth performance and body condition indices
The DHA-rich microalgae substitutions for fish oil in the PM diet series affected (P < 0.05 or P < 0.0001) all measures of growth performance and body indices except for survival or FR (Table 1). The 100% replacement of fish oil resulted in poorer (P < 0.05) growth, FCR, protein retention efficiency (PRE), and energy retention efficiency (ERE) than the 0% or 50% replacement. In addition, the 50% and 100% replacements also enhanced (P < 0.05) VSI and HSI relative to the 0% fish oil replacement. In contrast, the astaxanthin source did not exert significant main effects on any measures of growth performance or body indices. However, there were interactions (P < 0.05) between the 3 levels of fish oil substitutions and 2 sources of astaxanthin on each of the 4 measures of growth performance and PRE. At the 100% replacement level, SA supplemented diets produced superior (P < 0.05) responses in fish growth to those supplemented with AA.
Table 1.
Growth performance and body indices of juvenile rainbow trout fed plant PM-based diets containing microalgae to replace fish oil and SA
| Diets1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fish oil replacement2 | 0% | 50% | 100% | Fish oil | ASTA | P-values11 | ||||
| Astaxanthin source | SA | AA | SA | AA | SA | AA | RMSE | Level | Source | Interaction |
| Growth performance | ||||||||||
| Average fish Wt (g) | 386a | 367a | 353a | 380a | 169b* | 147b | 15 | <0.0001 | 0.47 | 0.025 (100SA > 100AA) |
| Percent increase3 (%) | 1680a | 1610a | 1530a | 1660a | 678b* | 591b | 67 | <0.0001 | 0.90 | 0.027 (100SA > 100AA) |
| BW/day4 (%) | 0.80b | 0.77b | 0.83b | 0.78b | 1.24a | 1.43a* | 0.07 | <0.0001 | 0.36 | 0.030 (100SA < 100AA) |
| FCR5 | 0.92b | 0.88b | 0.96b | 0.89b | 1.52a | 1.77a* | 0.09 | <0.0001 | 0.32 | 0.024 (100SA < 100AA) |
| Body indices | ||||||||||
| Survival (%) | 93 | 100 | 98 | 100 | 97 | 98 | 3.7 | 0.52 | 0.08 | 0.43 |
| VSI6 (%) | 10.7b | 9.6b | 11.7a | 13.1a | 12.2a | 12.2a | 1.03 | 0.004 | 0.80 | 0.14 |
| FR7 (%) | 59.3 | 59.6 | 59.5 | 60.8 | 57.0 | 56.4 | 2.38 | 0.06 | 0.77 | 0.82 |
| HSI8 (%) | 1.1b | 1.1b | 1.3a | 1.3a | 1.2b | 1.2b | 0.12 | 0.04 | 0.70 | 0.96 |
| PRE9 (g) | 40.6a | 45.6a | 40.9a | 45.6a | 22.3b* | 17.9b | 3.12 | <0.0001 | 0.26 | 0.04 (100SA > 100AA) |
| ERE10 (kcal/g) | 55.0a | 53.6a | 53.6a | 61.1a | 29.9b | 27.1b | 4.24 | <0.0001 | 0.59 | 0.11 |
1SA, synthetic astaxanthin; AA, microalgal astaxanthin, ASTA, astaxanthin.
2Percent of fish oil replaced by microalgae on a crude lipid basis of DHA.
3Percent increase (%) = (final weight – initial weight) × 100/initial weight.
4BW/Day (%) = (tank feed consumption total/average tank weight)/study duration × 100.
5FCR, feed conversion ratio = g feed consumed/g weight gained.
6VSI= gut weight/body weight × 100.
7FR= fillet weight × 2/body weight × 100.
8 HSI= liver weight/body weight × 100.
9PRE, protein retention efficiency = g protein gain × 100/g protein fed.
10ERE, energy retention efficiency = kcal energy gain × 100/kcal energy fed.
11Probability associated with the F-statistic. A significant (P < 0.05) main effect without significant interaction allowed data to be pooled. Significant main effects are denoted under level and source.
a, bMeans without sharing a common letter are different (P < 0.05). Direct comparisons of means across the 2 treatments should be made conditionally if there was an interaction.
*SA vs. AA, P < 0.05.
The fish oil substitutions in the FM diet series also affected (P < 0.05 or P < 0.0001) all measures of growth performance and body indices except for survival (Table 2). In fact, the negative effects of the replacements on final body weights, percentage increase of body weights, and FCR were evident at the level of 50% fish oil replacement and were further affected by the 100% replacement. Meanwhile, the 100% replacement group elevated (P < 0.05) relative feed intake, and decreased (P < 0.05) FR, HSI, PRE, and ERE when compared with the 0% and 50% replacement groups. In addition, VSI was elevated (P < 0.05) in the 50% and 100% replacement groups compared with the 0% replacement. There were main effects (P < 0.05) of the astaxanthin source and interactions (P < 0.05) between the 3 levels of fish oil replacement and the source of astaxanthin on final body weight and percent increase of body weight. At the level of 50% replacement, SA produced better (P < 0.05) responses of these 2 measures than AA.
The fish oil substitutions contributed to a reduction (P < 0.05) in total body moisture and(or) decreased (P < 0.05) total body protein concentrations of fish fed both PM and FM diets (Supplemental Table 7). The 50% replacement in the PM diets elevated (P < 0.05) total body energy content compared with 0% or 100% replacement. Significant interactive effects indicate that SA led to higher (P < 0.05) total body moisture but lower (P < 0.05) total body lipid content than AA in fish fed the PM diets with 50% replacement of fish oil.
Fatty acid and lipid profiles in the tissues
The fish oil substitutions in the PM (Table 3) and FM (Table 4) diet series produced dose-dependent decreases (P < 0.05) of the EPA concentrations in the muscle and liver. That led to undetectable levels of EPA in both tissues at the 100% replacement. Similar dose-dependent decreases (P < 0.05) in the muscle and(or) liver total n-3 fatty acid concentrations and(or) n-3/n-6 fatty acid ratios were also caused by the fish oil replacements. In comparison, only the 100% replacement caused ~30% to 45% decreases (P < 0.05) in the muscle and liver DHA concentrations. The replacements produced mixed or variable effects on total concentrations of n-6 fatty acids and(or) PUFA, and little effect on total concentrations of SFA (saturated fatty acid) or MUFA (monounsaturated fatty acid). Likewise, the source of astaxanthin showed neither main effects nor interactions with the fish oil replacements on any of the fatty acid profiles in the 2 tissues with only 1 exception (muscle MUFA in the FM diet series).
Table 3.
Profiles of selected fatty acids, lipids, and phospholipid in muscle (fillet) and liver tissues of juvenile rainbow trout fed plant PM-based diets containing microalgae to replace fish oil and synthetic astaxanthin
| Diets1 | P-values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fish oil replacement2 | 0% | 50% | 100% | |||||||
| Astaxanthin source | SA | AA | SA | AA | SA | AA | RMSE | Fish oil level | ASTA source | Interaction |
| Muscle | ||||||||||
| Fatty acid (mg/g tissue) | ||||||||||
| 18:3n-3 (ALA) | 0.11 | 0.12 | 0.16 | 0.24 | 0.13 | 0.15 | 0.02 | 0.33 | 0.15 | 0.95 |
| 20:5n-3 (EPA) | 0.24a | 0.23a | 0.14b | 0.17b | 0.00c | 0.00c | 0.04 | <0.001 | 0.24 | 0.60 |
| 22:6n-3 (DHA) | 0.49a | 0.49a | 0.48a | 0.47a | 0.28b | 0.27b | 0.04 | <0.001 | 0.79 | 0.93 |
| n-3 | 0.84a | 0.83a | 0.79a | 0.87a | 0.42b | 0.43b | 0.09 | <0.001 | 0.60 | 0.99 |
| n-6 | 0.86b | 0.94b | 1.45ab | 1.75a | 1.43ab | 1.67a | 0.15 | 0.008 | 0.24 | 0.69 |
| n-3/n-6 | 0.96c | 0.89c | 0.54b | 0.50b | 0.29a | 0.26a | 0.12 | <0.001 | 0.15 | 0.60 |
| SFA | 1.36 | 1.42 | 1.62 | 1.59 | 1.18 | 1.35 | 0.07 | 0.46 | 0.63 | 0.74 |
| MUFA | 1.53 | 1.63 | 1.72 | 1.70 | 1.17 | 1.40 | 0.08 | 0.14 | 0.51 | 0.76 |
| PUFA | 1.69 | 1.78 | 2.30 | 2.66 | 1.93 | 2.17 | 0.05 | 0.28 | 0.33 | 0.78 |
| TG (mg/g protein) | 280a | 150b | 146b | 132b | 123b | 173b | 23.5 | 0.06 | 0.26 | 0.01 |
| TC (mg/g protein) | 11.8a | 10.2ab | 10.1ab | 9.21b | 9.22b | 9.08b | 0.43 | 0.07 | 0.27 | 0.45 |
| NEFA (mg/g protein) | 285a | 187b | 192b | 215ab | 217ab | 160b | 17.4 | 0.08 | 0.05 | 0.42 |
| Phospholipid (mg/mg tissue) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.001 | 0.44 | 0.32 | 0.95 |
| Liver | ||||||||||
| Fatty acid (mg/g tissue) | ||||||||||
| 18:3n-3 (ALA) | 0.04 | 0.00 | 0.02 | 0.06 | 0.08 | 0.04 | 0.01 | 0.14 | 0.51 | 0.89 |
| 20:5n-3 (EPA) | 0.44a | 0.48a | 0.20b | 0.35b | 0.01c | 0.00c | 0.08 | <0.001 | 0.27 | 0.38 |
| 22:6n-3 (DHA) | 2.67ab | 3.16a | 2.40ab | 2.48ab | 2.01b | 1.86b | 0.20 | <0.001 | 0.38 | 0.35 |
| n-3 | 3.14ab | 3.64a | 2.61ab | 3.20ab | 2.13b | 1.90b | 0.27 | 0.003 | 0.38 | 0.35 |
| n-6 | 1.07b | 1.17b | 1.44ab | 1.71ab | 2.30a | 2.30a | 0.22 | <0.001 | 0.51 | 0.83 |
| n-3/n-6 | 3.00c | 3.10c | 1.83b | 1.87b | 0.95a | 0.83a | 0.40 | <0.001 | 0.91 | 0.29 |
| SFA | 2.57 | 3.00 | 2.71 | 3.00 | 3.23 | 3.07 | 0.10 | 0.31 | 0.52 | 0.41 |
| MUFA | 2.39 | 2.68 | 2.31 | 2.31 | 2.38 | 2.19 | 0.07 | 0.43 | 0.90 | 0.44 |
| PUFA | 4.34 | 4.99 | 4.32 | 5.19 | 4.96 | 4.50 | 0.15 | 0.91 | 0.47 | 0.36 |
| TG (mg/g protein) | 21.9b | 32.1ab | 22.2b | 39.8a | 30.3ab | 29.3ab | 2.74 | 0.43 | <0.01 | 0.12 |
| TC (mg/g protein) | 24.3 | 27.7 | 24.7 | 21.3 | 23.9 | 23.5 | 0.84 | 0.15 | 0.93 | 0.23 |
| NEFA (mg/g protein) | 848 | 807 | 738 | 740 | 793 | 874 | 22.5 | 0.89 | 0.71 | 0.21 |
| Phospholipid (mg/mg tissue) | 0.017ab | 0.020a | 0.014b | 0.013b | 0.023a | 0.014b | 0.002 | 0.97 | 0.42 | 0.06 |
1SA, synthetic astaxanthin; AA, microalgal astaxanthin; ASTA, astaxanthin.
2Percent of fish oil replaced by microalgae a crude lipid basis of DHA.
a,b,cMeans in the same row without a common letter differ (P < 0.05). Direct comparisons of means across the 2 treatments should be made conditionally if there was an interaction.
Table 4.
Profiles of selected fatty acids, lipids, and phospholipid in muscle (fillet) and liver tissues of juvenile rainbow trout fed FM-based diets containing microalgae to replace fish oil and synthetic astaxanthin
| Diets1 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fish oil replacement2 | 0% | 50% | 100% | |||||||
| Astaxanthin source | SA | AA | SA | AA | SA | AA | RMSE | Fish oil level | ASTA source | Interaction |
| Muscle | ||||||||||
| Fatty acid (mg/g tissue) | ||||||||||
| 18:3n-3 (ALA) | 0.07c | 0.11b | 0.13b | 0.12b | 0.17a | 0.15a | 0.01 | <0.001 | 0.98 | 0.16 |
| 20:5n-3 (EPA) | 0.19c | 0.25c | 0.12b | 0.10b | 0.00a | 0.01a | 0.04 | <0.001 | 0.16 | 0.06 |
| 22:6n-3 (DHA) | 0.45ab | 0.54a | 0.47ab | 0.42ab | 0.31b | 0.38b | 0.03 | 0.002 | 0.26 | 0.77 |
| n-3 | 0.71ab | 0.90a | 0.72ab | 0.64ab | 0.48b | 0.54b | 0.06 | <0.001 | 0.25 | 0.25 |
| n-6 | 0.64c | 0.85c | 1.06b | 0.95b | 1.43a | 1.30a | 0.12 | <0.001 | 0.89 | 0.08 |
| n-3/n-6 | 1.10c | 1.06c | 0.69b | 0.68b | 0.34a | 0.42a | 0.13 | <0.001 | 0.88 | 0.22 |
| SFA | 1.30 | 1.64 | 1.46 | 1.37 | 1.54 | 1.40 | 0.05 | 0.99 | 0.68 | 0.06 |
| MUFA | 1.34 | 1.84 | 1.47 | 1.34 | 1.53 | 1.27 | 0.08 | 0.22 | 0.78 | 0.03 |
| PUFA | 1.35b | 1.76ab | 1.79ab | 1.60ab | 1.97a | 1.90a | 0.09 | 0.01 | 0.65 | 0.09 |
| TG (mg/g protein) | 77.0ab | 76.9ab | 151a | 49.7b | 62.0 | 51.4b | 15.4 | 0.41 | 0.08 | 0.83 |
| TC (mg/g protein) | 7.03ab | 8.28ab | 9.15a | 4.90b | 11.0a | 7.16ab | 0.84 | 0.18 | 0.01 | 0.02 |
| NEFA (mg/g protein) | 176a | 143a | 344b | 128a | 146a | 132a | 33.9 | 0.67 | 0.04 | 0.84 |
| Phospholipid (mg/mg tissue) | 0.019a | 0.015ab | 0.016ab | 0.012b | 0.015ab | 0.019a | 0.001 | 0.89 | 0.36 | 0.04 |
| Liver | ||||||||||
| Fatty acid (mg/g tissue) | ||||||||||
| 18:3n-3 (ALA) | 0.00 | 0.00 | 0.37 | 0.01 | 0.13 | 0.11 | 0.06 | 0.36 | 0.25 | 0.93 |
| 20:5n-3 (EPA) | 0.30a | 0.47a | 0.28a | 0.13b | 0.00b | 0.05b | 0.07 | <0.001 | 0.61 | 0.31 |
| 22:6n-3 (DHA) | 2.14b | 3.30a | 3.32a | 2.64ab | 2.08b | 2.08b | 0.23 | 0.07 | 0.48 | 0.06 |
| n-3 | 2.44b | 3.77a | 3.97a | 2.78ab | 2.21b | 2.24b | 0.32 | 0.07 | 0.88 | 0.17 |
| n-6 | 0.78b | 1.05b | 1.82a | 1.49ab | 1.90a | 2.31a | 0.23 | <0.001 | 0.53 | 0.75 |
| n-3/n-6 | 3.13a | 3.58a | 2.24ab | 1.87ab | 1.20b | 1.02b | 0.42 | <0.001 | 0.84 | 0.09 |
| SFA | 2.20 | 3.26 | 2.91 | 3.35 | 3.11 | 3.28 | 0.19 | 0.27 | 0.15 | 0.51 |
| MUFA | 2.17 | 3.12 | 3.41 | 3.03 | 2.54 | 2.32 | 0.20 | 0.60 | 0.70 | 0.16 |
| PUFA | 3.50 | 4.94 | 6.32 | 4.51 | 4.29 | 4.53 | 0.38 | 0.64 | 0.93 | 0.50 |
| TG (mg/g protein) | 26.7a | 16.2b | 23.6a | 17.8b | 23.0a | 24.0a | 1.64 | 0.12 | <0.001 | <0.001 |
| TC (mg/g protein) | 25.8a | 26.6a | 25.9a | 22.8b | 21.1b | 22.4b | 0.93 | 0.001 | 0.72 | 0.8 |
| NEFA (mg/g protein) | 743a | 543b | 632ab | 572b | 681ab | 719a | 32.7 | 0.14 | 0.03 | 0.01 |
| Phospholipid (mg/mg tissue) | 0.015ab | 0.022a | 0.015ab | 0.016ab | 0.013b | 0.017ab | 0.001 | 0.02 | 0.08 | 0.87 |
1SA, synthetic astaxanthin; AA, microalgal astaxanthin; ASTA, astaxanthin.
2Percent of fish oil replaced by microalgae on a crude lipid basis of DHA.
a,b,cMeans in the same row without a common letter differ (P < 0.05). Direct comparisons of means across the 2 treatments should be made conditionally if there was an interaction.
The 100% fish oil replacement in the FM diet series decreased (P < 0.05) liver TC and phospholipid concentrations compared with the 0% and(or) 50% replacements (Table 4). Compared with SA, AA in both diet series decreased (P < 0.05) the muscle TC and NEFA concentrations at 2 or all 3 levels of fish oil replacements than SA (Tables 3 and 4). However, AA in the PM diet series exerted tissue-specific and the fish oil replacement level-dependent effects (up, down, or no change) on the muscle and liver total TG concentrations. Furthermore, AA in the FM diet series produced lower (P < 0.05) concentrations of TG and NEFA in the liver compared with SA at the 0% and 50% fish oil replacements (Table 4). In the PM diet series, all heart fatty acid profiles were affected (P < 0.05) by the fish oil substitution level, but not at all by the astaxanthin source (Supplemental Table 8). In the FM diet series, more heart fatty acid profiles were affected (P < 0.001 to 0.07) by the astaxanthin source than the fish oil replacement (Supplemental Table 9).
Responses of liver gene expression in the liver
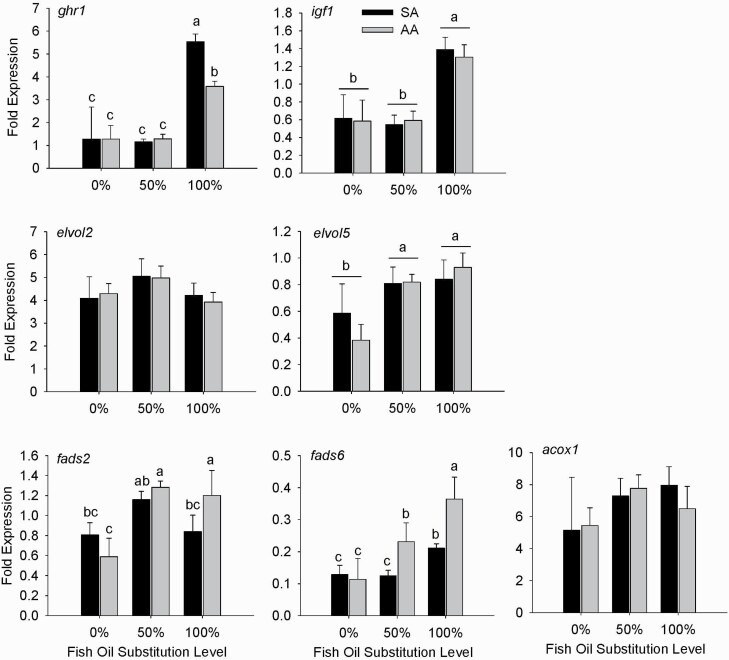
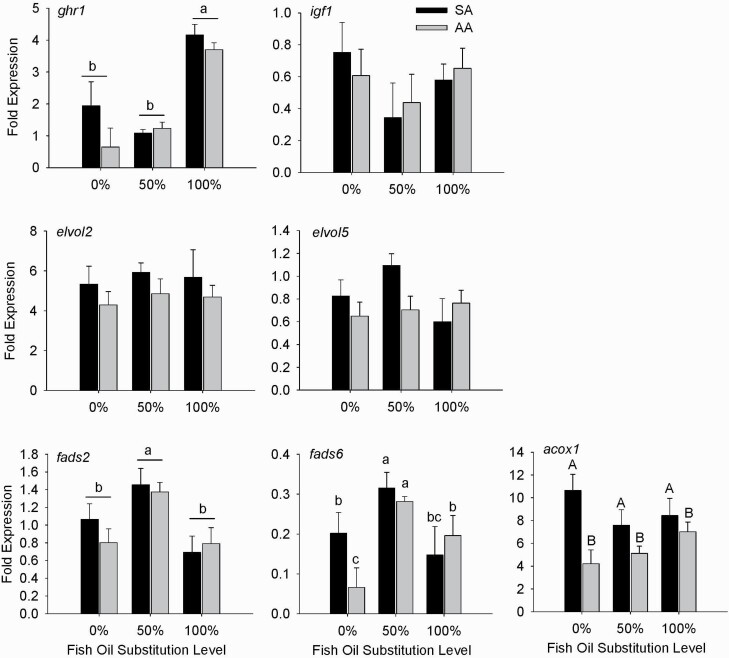
In both, the PM (Figure 1) and FM (Figure 2) diet series, the 100% substitution of fish oil elevated expression of ghr1 (P < 0.001) in the liver by ~2-fold compared with the 0% and 50% replacement levels. However, AA attenuated (P < 0.05) the upregulation of hepatic ghr1 mRNA by the 100% replacement in the PM diet series. Only in the PM diet series, the 100% fish oil replacement enhanced (P < 0.05) the hepatic igf1 expression compared with the 0% and 50% replacements.
Figure 1.
Effects of fish oil substitutions (0%, 50%, and 100%) by DHA-rich microalgae and sources of astaxanthin (SA vs. AA) on hepatic gene expression in fish fed the PM diet series. Bars indicate means ± SEM. Lowercase letters indicate differences (P < 0.05) between means. Uppercase letters indicate differences (P < 0.05) due to astaxanthin source (SA vs. AA). Abbreviations: ghr, growth hormone receptor; igf, insulin-like growth factor; fads, fatty acid desaturase; elovl, elongation of very long fatty acids protein; acox, acetyl-CoA oxidase; elf, elongation factor.
Figure 2.
Effects of fish oil substitutions (0%, 50%, and 100%) by DHA-rich microalgae and sources of astaxanthin (SA vs. AA) on hepatic gene expression in fish fed the FM diet series. Bars indicate means ± SEM. Lowercase letters indicate differences (P < 0.05) between means. Uppercase letters indicate differences (P < 0.05) due to astaxanthin source (SA vs. AA). Abbreviations: ghr, growth hormone receptor; igf, insulin-like growth factor; fads, fatty acid desaturase; elovl, elongation of very long fatty acids protein; acox, acetyl-CoA oxidase; elf, elongation factor.
While hepatic expressions of the fatty acid elongase genes (elovl2, elovl5) were similar among the FM series diets, the 50% and 100% replacements of fish oil in the PM diet series enhanced the expression of elovl5 (P < 0.001) over the 0% replacement. Within each diet series, expression of at least one desaturase gene (fads2 and(or) fads6) was affected by both the fish oil replacement level and the astaxanthin source. In the FM diet series, hepatic mRNA abundances of fads2 and fads6 were elevated (P < 0.05) by the 50% fish oil replacement compared with the other 2 replacement levels. Additionally, at the 0% replacement level, AA decreased (P < 0.05) the fads6 expression compared with SA. In the PM diet series, there was an interaction between fish oil replacement level and the astaxanthin type on the expressions of both fads2 and fads6. Compared with SA, AA upregulated (P < 0.05) hepatic fads2 expression at only the 100% fish oil replacement, but the fads6 expression at both the 50% and 100% fish oil replacements. Finally, hepatic acox1 expression was not altered by the fish oil replacement level in either diet series. However, in the FM diet series, AA decreased (P < 0.001) the acox1 expression compared with SA.
Stepwise regression analysis
When the fatty acid profiles in the diets, muscle, liver, and heart were used as the independent variables (Xs) for stepwise regression analyses, the remaining (significant) Xs for the growth performance as dependent variables (Y) of fish fed the PM diets were concentrations of diet n-6 fatty acids, muscle n-3 fatty acids, and heart PUFA (Supplemental Table 10). In comparison, concentrations of heart ALA were the only remaining X for the responses of growth performance of fish fed the FM diets (Supplemental Table 11).
When the fatty acid profiles in the diets and tissues and the gene expression in the liver were used as Xs for the stepwise regressions of muscle DHA and EPA concentrations (Y) in the PM diet series, each of the regressions came out 5 remaining Xs including three entries of various fatty acids (ALA, EPA, DHA, MUFA, and PUFA) in the heart, DHA or MUFA concentrations in the liver, n-3 fatty acid concentrations in the muscle, and n-6 fatty acid concentrations in the diets (Supplemental Table 10). In the FM diet series, the remaining Xs for the muscle DHA concentrations was liver EPA and heart MUFA concentrations, and for the muscle EPA concentrations (Y) were the diet EPA and DHA, muscle DHA and ALA, and liver PUFA concentrations (Supplemental Table 11).
When only the liver gene expression profiles were used as Xs for the stepwise regression, ghr1 and(or) fads6 remained as the 2 genes related to liver DHA and EPA concentrations (y), while igf1 was the remaining X for the liver NEFA concentrations in the fish fed the PM diets (Supplemental Table 10). In contrast, elvol5 and ghr1 were the only remaining gene for liver EPA and TC concentrations, respectively, while no gene expression was correlated with liver DHA concentration in fish fed the FM diets (Supplemental Table 11).
Discussion
Our study clearly demonstrates that the 100%, but not the 50%, substitution of fish oil by the DHA-rich microalgae produced consistent impairments in growth performance, dietary nutrient utilization (PRE and ERE), and body indices (VSI and HSI) of rainbow trout. Previous studies in salmonids showed similar effects of the substitution on growth (Sprague et al., 2015; Gong et al., 2019; Peterson et al., 2019; Santigosa et al., 2020; Sarker et al., 2020), nutrient digestibility and utilization (Gong et al, 2019; Peterson et al., 2019; Atalah et al., 2007), and body indices (Perez-Velazquez, 2018; Jiang et al., 2019). Comparatively, our findings add several new insights into the field.
Firstly, we have revealed that only the 100% replacement caused consistent negative responses in various measures in fish fed the PM diet series, whereas the 50% replacement was sufficient to produce the negative effects on several measures in fish fed the FM diet series. Seemingly, fish fed the FM diet series became more susceptible to the fish oil replacement than fish fed the PM diet. Although decreased palatability of microalgal diets was reported (Sarker et al., 2020), feed intakes were greater in the 100% replacement diets than the lower replacement diets in the present study. Secondly, the stepwise regression analyses indicated that the remaining (significant) independent variables for the growth performance of fish fed the PM diets included concentrations of diet n-6 fatty acids, muscle n-3 fatty acids, and heart PUFA, instead of just heart ALA concentrations for that of fish fed the FM diets. Thirdly, SA appeared to perform better than AA for growth performance and FCR of fish fed the 100% fish oil replacement in PM diets and of 50% fish oil replacement in FM diets. A possible explanation could be that high levels of algal meal (as both sources of DHA and astaxanthin) in the diets might lower growth performance and prevent astaxanthin absorption when algae oils and astaxanthin occurred in an encysted form (Choubert and Henrich, 1993). In addition, our study did not show consistent or any main effect of the astaxanthin source on growth performance of fish fed either diet series. Our findings are consistent with previous results that dietary astaxanthin in grow-out diets did not provide a growth benefit to rainbow trout (Rehulka, 2000; Choubert et al., 2006; Yesilayer and Erdem, 2011).
It is novel to illustrate the up-regulation of hepatic expression of ghr1 in response to the consistent suppression of growth performance of fish fed both PM and FM diet series by the 100% replacement of fish oil. In addition, hepatic igf1 expression was enhanced by the replacement in the fish fed the PM diets. Because the growth hormone (GH)/insulin-like growth factor (IGF) axis is part of the endocrine system generally recognized for its positive effects on growth (Wood et al. 2005), upregulations of their gene expression might represent a feedback response to the depressed growth. However, upregulation of GH and GH receptor is commonly reported during feed deprivation (Gabillard et al., 2006, Cleveland et al., 2009; reviewed in Bergan-Roller and Sheridan, 2018) which likely stimulates GH-induced lipolysis (Bergan et al., 2015). Although fish were not deprived of feed in the current study, growth rates were dramatically reduced with 100% fish oil replacement, despite exhibiting significantly higher feed intake, suggesting a nutritional insufficiency and subsequent GH response akin to the feed deprived condition. Increased ghr1 expression might be directly linked to the fatty acid profiles of the diet; in goldfish DHA decreased while EPA increased ghr1 expression in the hepatopancreas (Bertucci et al. 2017). Therefore, low levels of dietary DHA in the 100% replacement group might be attenuating DHA-inhibition of ghr1 expression. Increased liver sensitivity to GH signaling might also contribute to higher igf1 expression in the PM series since GH induction of hepatic IGF is a well-established regulatory mechanism (Wood et al., 2005). Plasma IGF concentrations also progressively increase with elevated feed intake in rainbow trout (Cleveland and Burr, 2011), so higher levels of consumption in the 100% replacement diet might also contribute to elevated igf1 expression. However, upregulations in igf1 were correlated with reduced growth performance, suggesting a disruption in IGF signaling capacity.
Another interesting finding from the present study is that replacing fish oil by the DHA-rich microalgae in both PM and FM diet series progressively reduced the EPA concentrations to undetectable in the liver and muscle, whereas their DHA concentrations were more resistant and exhibited reductions only at the 100% replacement level. Apparently, the fatty acid profiles of the 50% replacement diets (PM and FM series) were sufficient to maintain tissue DHA levels and the lack of EPA in the DHA-rich microalgae aggravated the depletion of tissue EPA by the 100% fish oil replacement. While our result supports the notion that tissue fatty acid content reflects dietary composition (Sargent et al., 2003), it raises questions requiring new conceptual explanations. The first question is that the ~40% reduction in dietary DHA concentrations between the 0% and 50% fish oil replacement diets did not translate into any reduction in liver or muscle DHA accumulation. Similarly, there was only an ~40% reduction in muscle DHA from the 100% replacement diet, despite the ~70% reduction in dietary DHA. These observations suggest a compensatory endogenous DHA synthesis and/or enhanced DHA retention during reduced DHA intake. The study done by Colombo et al (2018) indicated that the DHA concentration stored in the muscle tissue were 1.23 times higher than the DHA concentration in diet, which likely was due to de novo synthesis. The subsequent question is why the increased expression of fads genes in the 50% replacement in the FM diet series failed to sustain through the 100% replacement level, when the increased expression of elvol5, fads2, and fads6 during replacement of fish oil indeed supported an increased capacity for DHA synthesis. Previous studies in salmonids and other fish species have shown upregulation of n-3 and n-6 LC-PUFA biosynthetic genes such as fads2 and fads6 during consumption of vegetable oil-based diets with low DHA levels (Zheng et al. 2004; Panserat et al. 2009; Katan et al. 2019). This response likely occurs from reduction of an inhibitory effect of dietary DHA on expression of these genes, rather than a stimulatory effect of low C18-PUFA (Gregory et al. 2016). In line with this view, our stepwise regression analysis found out that the liver DHA concentrations in the PM diet series and EPA in the FM diet series were negatively correlated with the hepatic expression of fads6 and evol5, respectively. However, it is fascinating, but unclear to us, that the hepatic ghr1 and igf1 expression remained as the only or one of the 2 significant independent variables of genes related to liver DHA, EPA, NEFA, and (or) TC concentrations.
Our study also depicted different stepwise regressions of the muscle DHA and EPA concentrations with dietary and tissue fatty acid profiles. In the PM diet series, the 2 n-3 LC-PUFA in the muscle were strongly correlated with 3 different types of fatty acids in the heart. In the FM diet series, the muscle EPA concentrations were correlated with 3 more independent variables including dietary EPA and DHA concentrations than those for the muscle DHA concentrations. Presumably, these 2 LC-PUFA in the muscle had different regulations of biosynthesis and accumulation. Meanwhile, our study shows no major effect of the astaxanthin source (SA vs. AA) on the fatty acid concentrations in the liver or muscle, which is consistent with previous studies (Pan and Chien, 2009; Xie et al., 2020). However, there was an effect of the astaxanthin source on liver TG, NEFA, and acox1 expression in the FM diet series, particularly in the 0% and 50% fish oil replacement groups. These findings are consistent with that an inhibition of acox1 in mice fed high-fat diets reduced liver and serum TG and had beneficial effects on biomarkers of oxidative stress (Zeng et al. 2017). The TG and NEFA responses in mice were also be induced by astaxanthin (Jia et al. 2016; Bhuvaneswari et al. 2010), suggesting that AA might be more effective in regulating these mechanisms in rainbow trout than SA.
Seemingly, data from PM and FM diet series could have been combined and analyzed as 3 × 2 × 2 factorial arrangement of dietary treatments (3 levels of fish oil substitution, 2 sources of astaxanthin, and 2 types of major proteins). That would have allowed us to make direct comparisons between the PM and FM diets statistically or to reveal other informative patterns from the data. However, we have eventually chosen to analyze the PM and FM diet data separately. This is because our most important objective was to determine impacts and mechanisms of substitutions of fish oil-DHA and SA by microalgal sources. Thus, the interaction effects of these 2 substitutions were a focal point and new to the field. However, a 3-way interaction might complicate or deviate the analysis away from that focus. Meanwhile, the PM and FM diets contained a number of different ingredients and their nutrient compositions were not fully identical, although we tried our best to formulate these 2 types of diets with similar crude protein and lipid concentrations and balanced the diets for lysine, methionine, threonine, and phosphorus. Therefore, potential confounding effects arisen from these actual and presumed differences should not be neglected or overlooked, when our dietary treatments were incorporated at relatively low concentrations. Furthermore, differences between PM- and FM-based diets have been documented previously. A 3-way data analysis in the present study would have generated complicated tables and figures that might be overwhelming or distractive from the main findings.
It is worth noting that the analyzed proximate composition of the experimental diets exhibited some variation from the formulated levels of 44% protein and 18% fat, particularly in the PM series in which crude protein levels ranged from 41.1% to 45.8% while crude lipid ranged from 18.4% to 21.2%. Algal-based ingredients can be resilient to traditional analysis methods (Laurens et al. 2012). Therefore, we speculate that the variation was largely the artifact of digestion or extraction of the plant- and/or algal-derived ingredients during compositional analysis, instead of actual differences in macronutrient values between diets. Supporting this concept is that growth performance was a function of the fish oil substitution, but did not correlate with differences in analyzed concentrations of crude protein or other nutrients. For example, the 2 PM diets that resulted in the numerically fastest growth rates exhibited the lowest (0%/SA) and highest (50%/AA) percent lipid and energy content. Likewise, both PM and FM diets with the 50% and 100% substitutions of fish oil had lower concentrations of EPA, DHA, and(or) total n-3 LC-PUFA than the diets with 0% fish oil replacement. Those lower concentrations than the anticipated values might be due to the lack of ALA or EPA in the microalgae supplement and(or) incomplete extraction/recovery of these fatty acids including DHA from the cooked and extruded fish diets.
In summary, results from our study support that replacing up to 50% of fish oil from a control diet by a DHA-rich microalgal meal in the both PM and FM diets might still sustain growth performance, body indices, and tissue LC-PUFA concentrations of juvenile rainbow trout. However, a full replacement of fish oil by the microalgae produced negative effects on all those measures in fish fed either diet series. The impairments were associated with upregulations of hepatic ghr1, igf1, fads6, and evol5 expression, and seemed to be more severe in the FM than PM diet-fed fish and more pronounced on the tissue EPA than DHA concentrations. The 2 sources of astaxanthin: synthetic and microalgal, exerted only a few interactions with the fish oil replacement levels on several measures. Future research should elucidate the underlying mechanisms for the negative effects of the 100% fish oil replacement and to explore strategies to alleviate these negative effects on rainbow trout feeding.
Supplementary Material
Acknowledgments
This research was funded by DOE MAGIC grant DE-EE0007091, USDA grant 2019-69012-29905, and Cornell University Hatch grants NYC-127302. Mention of trade names is solely for accuracy and does represent endorsement by the federal government. The federal government is an equal opportunity provider and employer.
Glossary
Abbreviations
- DHA
docosahexaenoic acid (c22:6n-3)
- EPA
eicosapentaenoic acid (c20:5n-3)
- FCR
food conversion ratio
- FR
fillet ratio
- HPLC
high-performance liquid chromatography
- HSI
hepatosomatic index
- MUFA
monounsaturated fatty acid
- n-3 LC-PUFA
omega-3 long-chained polyunsaturated fatty acids
- NEFA
non-esterified fatty acid
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- TC
total cholesterol
- TG
triglyceride
- VSI
visceral somatic index
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abu, T., and Hamza M.. . 1995. Factors affecting protein extractability of defatted karkade (hibiscus sabdariffa)seed flour. King Saud University. Agric. Sci. 7:179–186. [Google Scholar]
- Amaro, H. M., Guedes A. C., and Malcata F. X.. 2011. Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy. 88(10):3402–3410. doi: 10.1016/j.apenergy.2010.12.014 [DOI] [Google Scholar]
- Atalah, E., Hernández-Cruz C. M., Izquierdo M. S., Rosenlund G., Caballero M. J., Valencia A., and Robaina L.. 2007. Two microalgal Crypthecodiniumcohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata). Aquaculture. 270: 178–185. doi: 10.1016/j.aquaculture.2007.04.009 [DOI] [Google Scholar]
- Bergan, H. E., Kittilson J. D., and Sheridan M. A.. . 2015. Nutritional state modulates growth hormone-stimulated lipolysis. Gen. Comp. Endocrinol. 217-218:1–9. doi: 10.1016/j.ygcen.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Bergan-Roller, H. E., and Sheridan M. A.. . 2018. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen. Comp. Endocrinol. 258:119–133. doi: 10.1016/j.ygcen.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Bertucci, J. I., Blanco A. M., Canosa L. F., and Unniappan S.. . 2017. Direct actions of macronutrient components on goldfish hepatopancreas in vitro to modulate the expression of ghr-I, ghr-II, igf-I and igf-II mRNAs. Gen. Comp. Endocrinol. 250:1–8. doi: 10.1016/j.ygcen.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Betiku, O. C., Barrows F. T., Ross C., and Sealey W. M.. 2016. The effect of total replacement of fish oil with DHA-Gold((R)) and plant oils on growth and fillet quality of rainbow trout (Oncorhynchus mykiss) fed a plant-based diet. Aquac. Nutr. 22: 158–169. doi: 10.1111/anu.12234 [DOI] [Google Scholar]
- Bhuvaneswari, S., Arunkumar E., Viswanathan P., and Anuradha C. V.. 2010. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 45: 1406–1414. doi: 10.1016/j.procbio.2010.05.016 [DOI] [Google Scholar]
- Burr, M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., and Deadman N. M.. . 1989. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Caballero, M. J., Obach A., Rosenlund G., Montero D., Gisvold M., and Izquierdo M. S.. 2002. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture. 214:253–271. doi: 10.1016/s0044-8486(01)00852-3 [DOI] [Google Scholar]
- Cheng, Y., and Wu S.. 2019. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkii. Aquaculture. 512:734341. doi: 10.1016/j.aquaculture.2019.734341 [DOI] [PubMed] [Google Scholar]
- Choubert, G., and Heinrich. O.. 1993. Carotenoid pigments of the green alga Haematococcus pluvialis: assay on rainbow trout, Oncorhynchus mykiss, pigmentation in comparison with synthetic astaxanthin and canthaxanthin. Aquaculture. 112:217–226. doi: 10.1016/0044-8486(93)90447-7 [DOI] [Google Scholar]
- Choubert, G., Mendes-Pinto M. M., and Morais R.. . 2006. Pigmenting efficacy of astaxanthin fed to rainbow trout Oncorhynchus mykiss: effect of dietary astaxanthin and lipid sources. Aquaculture. 257:429–436. doi: 10.1016/j.aquaculture.2006.02.055 [DOI] [Google Scholar]
- Christie, W. W., and Han. X.. 2010. Lipid analysis: isolation, separation, identification and lipidomic analysis. 4th ed. Bridgwater: Oily Press Lipid Library Series; p. 145–158. [Google Scholar]
- Cleveland, B. M., and Burr G. S.. 2011. Proteolytic response to feeding level in rainbow trout (Oncorhynchus mykiss). Aquaculture. 319(1–2):194–204. doi:10.1016/j.aquaculture.2011.06.043 [Google Scholar]
- Cleveland, B. M., and Evenhuis J. P.. . 2010. Molecular characterization of atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in rainbow trout (Oncorhynchus mykiss): expression across tissues in response to feed deprivation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 157:248–257. doi: 10.1016/j.cbpb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Cleveland, B. M., Gao G., and Leeds T. D.. . 2020. Transcriptomic response to selective breeding for fast growth in rainbow trout (Oncorhynchus mykiss). Mar. Biotechnol. (NY). 22:539–550. doi: 10.1007/s10126-020-09974-3. [DOI] [PubMed] [Google Scholar]
- Cleveland, B. M., Raatz S., Hanson B. K., Wickramaratne A., and Picklo M. J.. . 2018. Deposition and mobilization of lipids varies across the rainbow trout fillet during feed deprivation and transition from plant to fish oil-based diets. Aquaculture. 491:39–49. doi: 10.1016/j.aquaculture.2018.03.012 [DOI] [Google Scholar]
- Cleveland, B. M., Weber G. M., Blemings K. P., and Silverstein J. T.. . 2009. Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 297:R1332–R1342. doi: 10.1152/ajpregu.00272.2009. [DOI] [PubMed] [Google Scholar]
- Cogliati, K. M., Unrein J. R., Sealey W. M., Barrows F. T., Hakanson O., Chitwood R., and Schreck C. B.. . 2019. Low-lipid diets fed at reduced ration: Effects on growth, body composition, and survival of juvenile Chinook Salmon. J. Fish Wildlife Manag. 10(2):500–508. doi: 10.3996/062018-JFWM-059 [DOI] [Google Scholar]
- Colombo, S. M., Parrish C. C., and Wijekoon M. P. A.. . 2018. Optimizing long chain-polyunsaturated fatty acid synthesis in salmonids by balancing dietary inputs. PLos One 13:e0205347. doi: 10.1371/journal.pone.0205347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . 2016. Global per capita fish consumption rises above 20 kilograms a year.http://www.fao.org/news/story/en/item/421871/icode/ Accessed September 23, 2020.
- Folch, J., Lees M., and Sloane Stanley G. H.. . 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Fukada, H., Kitagima R., Shinagawa J., Morino H., and Masumoto T.. 2020. Effects of complete replacement of fish oil with plant oil mixtures and algal meal on growth performance and fatty acid composition in juvenile yellowtail Seriola quinqueradiata. Fish. Sci. 86(1):107–118. doi: 10.1007/s12562-019-01361-9 [DOI] [Google Scholar]
- Gabillard, J. C., Kamangar B. B., and Montserrat N.. . 2006. Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 191:15–24. doi: 10.1677/joe.1.06869. [DOI] [PubMed] [Google Scholar]
- García-Ortega, A., Kissinger K. R., and Trushenski J. T.. 2016. Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture. 452:1–8. doi:10.1016/j.aquaculture.2015.10.020 [Google Scholar]
- Gong, Y. Y., Bandara T., Huntley M., Johnson Z. I., Dias J., Dahle D., Sorensen M., and Kiron V.. . 2019. Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture. 501:455–464. doi:10.1016/j.aquaculture.2018.11.049 [Google Scholar]
- Gregory, M. K., Collins R. O., Tocher D. R., James M. J., and Turchini G. M.. . 2016. Nutritional regulation of long-chain PUFA biosynthetic genes in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 115:1721–1729. doi: 10.1017/S0007114516000830. [DOI] [PubMed] [Google Scholar]
- Jia, Y., Wu C., Kim J., Kim B., and Lee S. J.. . 2016. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 28:9–18. doi: 10.1016/j.jnutbio.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang, M., Zhao H. H., Zai S. W., Shepherd B., Wen H., and Deng D. F.. . 2019. A defatted microalgal meal (Haematococcus pluvialis) as a partial protein source to replace fishmeal for feeding juvenile yellow perch Perca flavescens. J. Appl. Phycol. 31(2):1197–1205. doi: 10.1007/s10811-018-1610-3 [DOI] [Google Scholar]
- Ju, Z. Y., Deng D. F., and Dominy W.. . 2012. A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture. 354: 50–55. doi: 10.1016/j.aquaculture.2012.04.028 [DOI] [Google Scholar]
- Katan, T., Caballero-Solares A., Taylor R. G., Rise M. L., and Parrish C. C.. . 2019. Effect of plant-based diets with varying ratios of ω6 to ω3 fatty acids on growth performance, tissue composition, fatty acid biosynthesis and lipid-related gene expression in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. D Genomics Proteomics 30:290–304. doi: 10.1016/j.cbd.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Kiron, V., Phromkunthong W., Huntley M., Archibald I., and De Scheemaker G.. . 2012. Marine microalgal from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 18(5): 521–531. doi: 10.1111/j.1365-2095.2011.00923.x [DOI] [Google Scholar]
- Kobayashi, M., Kakizono T., Nishio N., Nagai S., Kurimura Y., and Tsuji Y.. . 1997. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 48(3):351–356. doi: 10.1007/s002530051061 [DOI] [Google Scholar]
- Kris-Etherton, P. M., Harris W. S., and Appel L. J.; American Heart Association. Nutrition Committee . 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Laurens, L. M., Dempster T. A., Jones H. D., Wolfrum E. J., Van Wychen S., McAllister J. S., Rencenberger M., Parchert K. J., and Gloe L. M.. . 2012. Algal biomass constituent analysis: method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem. 84:1879–1887. doi: 10.1021/ac202668c. [DOI] [PubMed] [Google Scholar]
- Li, F., Huang S., Lu X., Wang J., Lin M., An Y., Wu S., and Cai M.. . 2018. Effects of dietary supplementation with algal astaxanthin on growth, pigmentation, and antioxidant capacity of the blood parrot (Cichlasoma citrinellum & Cichlasoma synspilum). J. Oceanol. Limnol. 36: 1851–1859. doi: 10.1007/s00343-019-7172-7 [DOI] [Google Scholar]
- Liu, G., Magnuson A. D., Sun T., Tolba S. A., Starkey C., Whelan R., and Lei X. G.. . 2019. Supplemental methionine exerted chemical form-dependent effects on antioxidant status, inflammation-related gene expression, and fatty acid profiles of broiler chicks raised at high ambient temperature1. J. Anim. Sci. 97:4883–4894. doi: 10.1093/jas/skz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson, A. D., Sun T., Yin R., Liu G., Tolba S., Shinde S., and Lei X. G.. . 2018. Supplemental microalgal astaxanthin produced coordinated changes in intrinsic antioxidant systems of layer hens exposed to heat stress. Algal Res. 33: 84–90. doi: 10.1016/j.algal.2018.04.031 [DOI] [Google Scholar]
- Martínez, J. M., Gojkovic Z., Ferro L., Maza M., Álvarez I., Raso J., and Funk C.. . 2019. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 289:121694. doi: 10.1016/j.biortech.2019.121694. [DOI] [PubMed] [Google Scholar]
- NRC . 2011. Nutrient requirements of fish. Washington, DC: National Research Council, The National Academies Press. [Google Scholar]
- OECD-FAO . 2019. OECD-FAO agricultural outlook 2019–2029.http://www.agri-outlook.org/commodities/Fish.pdf Accessed September 23, 2020.
- Olsen, R. L., and Hasan M. R.. . 2012. A limited supply of fishmeal: impact on future increases in global aquaculture production. Trends Food Sci. Technol. 27(2):120–128. doi: 10.1016/j.jpgs.2012.06.003 [DOI] [Google Scholar]
- Pan, C. H., and Chien Y. H.. . 2009. Effects of dietary supplementation of alga Haematococcus pluvialis (Flotow), synthetic astaxanthin and β-carotene on survival, growth, and pigment distribution of red devil, Cichlasoma citrinellum (Günther). Aquac. Res. 40(8):871–879. doi: 10.1111/j.1365-2109.2008.02153.x [DOI] [Google Scholar]
- Panserat, S., Hortopan G. A., Plagnes-Juan E., Kolditz C., Lansard M., Skiba-Cassy S., and Corraze G.. . 2009. Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver. Aquaculture. 294(1–2):123–131. doi: 10.1016/j.aquaculture.2009.05.013 [DOI] [Google Scholar]
- Perez-Velazquez, M., Gatlin D. M. III, González-Félix M. L., and García-Ortega A.. . 2018. Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture. 487: 41–50. doi: 10.1016/j.aquaculture.2018.01.001 [DOI] [Google Scholar]
- Perez-Velazquez, M., Gatlin D. M. III, González-Félix M. L., García-Ortega A., de Cruz C. R., Juárez-Gómez M. L., and Chen K.. . 2019. Effect of fishmeal and fish oil replacement by algal meals on biological performance and fatty acid profile of hybrid striped bass (Morone crhysops♀× M. saxatilis♂). Aquaculture. 507:83–90. doi: 10.1016/j.aquaculture.2019.04.011 [DOI] [Google Scholar]
- Peterson, B. C., Burr G. S., Barrows F. T., Block S., Bowzer J., and Buentello A.. . 2019. Growth performance of Atlantic salmon smolts fed diets containing heterotrophic algal biomass as replacement of fish oil. N. Am. J. Aquac. 81(4):364–371. doi: 10.1002/naaq.10104 [DOI] [Google Scholar]
- Raatz, S. K., Rosenberger T. A., Johnson L. K., Wolters W. W., Burr G. S., and M. J.Picklo, Sr. 2013. Dose-dependent consumption of farmed Atlantic salmon (Salmo salar) increases plasma phospholipid n-3 fatty acids differentially. J. Acad. Nutr. Diet. 113:282–287. doi: 10.1016/j.jand.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehulka, J. 2000. Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture. 190:27–47. doi: 10.1016/S0044-8486(00)00383-5 [DOI] [Google Scholar]
- Santigosa, E., Constant D., Prudence D., Wahli T., and Verlhac-Trichet V.. . 2020. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 51: 649–665. doi: 10.1111/jwas.12699 [DOI] [Google Scholar]
- Sargent, J. R., Tocher D. R., and Bell. J. G.. 2003. The lipids. In: Fish Nutrition. San Diego: Academic Press, pp. 182–246. doi: 10.1016/j.aquaculture.2015.10.020 [DOI] [Google Scholar]
- Sarker, P. K., Kapuscinski A. R., Vandenberg G. W., Proulx E., and Sitek A. J.. . 2020. Towards sustainable and ocean-friendly aquafeeds: evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elementa Sci. Anthropocene. 8:5. doi: 10.1525/elementa.404 [DOI] [Google Scholar]
- Sprague, M., Walton J., Campbell P. J., Strachan F., Dick J. R., and Bell J. G.. . 2015. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 185:413–421. doi: 10.1016/j.foodchem.2015.03.150. [DOI] [PubMed] [Google Scholar]
- Sun, T., Yin R., Magnuson A. D., Tolba S. A., Liu G., and Lei X. G.. . 2018. Dose-dependent enrichments and improved redox status in tissues of broiler chicks under heat stress by dietary supplemental microalgal astaxanthin. J. Agric. Food Chem. 66:5521–5530. doi: 10.1021/acs.jafc.8b00860. [DOI] [PubMed] [Google Scholar]
- Tolba, S. A., Sun T., Magnuson A. D., Liu G. C., Abdel-Razik W. M., El-Gamal M. F., and Lei X. G.. . 2019. Supplemental docosahexaenoic-acid-enriched microalgae affected fatty acid and metabolic profiles and related gene expression in several tissues of broiler chicks. J. Agric. Food Chem. 67:6497–6507. doi: 10.1021/acs.jafc.9b00629. [DOI] [PubMed] [Google Scholar]
- Torrissen, O. J., Hardy R. W., and Shearer K. D.. . 1989. Pigmentation of salmonids-carotenoid deposition and metabolism. Rev. Aquatic Sci. 1:209–225. doi: 10.1016/0044-8486(89)90478-X [DOI] [Google Scholar]
- Turchini, G. M., Torstensen B. E., and Ng W. K.. . 2009. Fish oil replacement in finfish nutrition. Rev. Aquac. 1(1):10–57. doi: 10.1111/j.1753-5131.2008.01001.x [DOI] [Google Scholar]
- Vizcaíno, J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., . et al. 2016. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44(D1):D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A. W., Duan C., and Bern H. A.. . 2005. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 243:215–285. doi: 10.1016/S0074-7696(05)43004-1. [DOI] [PubMed] [Google Scholar]
- Xie, J. J., Chen X., Liu Y. J., Tian L. X., Xie S. W., and Niu J.. . 2017. Effects of dietary astaxanthin on growth performance, hepatic antioxidative activity, hsp70, and hif-1α gene expression of juvenile golden pompano (Trachinotus ovatus). Isr J Aquacult-Bamid. 69:112–125. http://hdl.handle.net/10524/57050. [Google Scholar]
- Xie, J., Fang H., He X., Liao S., Liu Y., Tian L., and Niu J.. 2020. Study on mechanism of synthetic astaxanthin and Haematococcus pluvialis improving the growth performance and antioxidant capacity under acute hypoxia stress of golden pompano (Trachinotus ovatus) and enhancing anti-inflammatory by activating Nrf2-ARE pathway to antagonize the NF-κB pathway. Aquaculture. 518:734657. doi.org/10.1016/j.aquaculture.2019.734657 [Google Scholar]
- Yesilayer, N., and Erdem M.. 2011. Effects of oleoresin paprika (Capsicum annum) and synthetic carotenoids (Canthaxantin and Astaxanthin) on pigmentation levels and growth in rainbow trout Oncorhynchus mykiss W. J. Anim. Vet. Adv. 10(14): 1875–1882. doi: 10.3923/javaa.2011.1875.1882 [DOI] [Google Scholar]
- Zeng, J., Deng S., Wang Y., Li P., Tang L., and Pang Y.. . 2017. Specific inhibition of Acyl-CoA Oxidase-1 by an acetylenic acid improves hepatic lipid and reactive oxygen species (ROS) metabolism in rats fed a high fat diet. J. Biol. Chem. 292:3800–3809. doi: 10.1074/jbc.M116.763532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. Z., Tocher D. R., Dickson C. A., Bell J. G., and Teale A. J.. 2004. Effects of diets containing vegetable oil on expression of genes involved in highly unsaturated fatty acid biosynthesis in liver of Atlantic salmon (Salmo salar). Aquaculture. 236: 467–483. doi: 10.1016/j.aquaculture.2004.02.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.