Abstract
Background
Cardiovascular disease (CVD), one of the most common comorbidities of coronavirus disease 2019 (COVID-19), has been suspected to be associated with adverse outcomes in COVID-19 patients, but their correlation remains controversial.
Method
This is a quantitative meta-analysis on the basis of adjusted effect estimates. PubMed, Web of Science, MedRxiv, Scopus, Elsevier ScienceDirect, Cochrane Library and EMBASE were searched comprehensively to obtain a complete data source up to January 7, 2021. Pooled effects (hazard ratio (HR), odds ratio (OR)) and the 95% confidence intervals (CIs) were estimated to evaluate the risk of the adverse outcomes in COVID-19 patients with CVD. Heterogeneity was assessed by Cochran’s Q-statistic, I2test, and meta-regression. In addition, we also provided the prediction interval, which was helpful for assessing whether the variation across studies was clinically significant. The robustness of the results was evaluated by sensitivity analysis. Publication bias was assessed by Begg’s test, Egger’s test, and trim-and-fill method.
Result
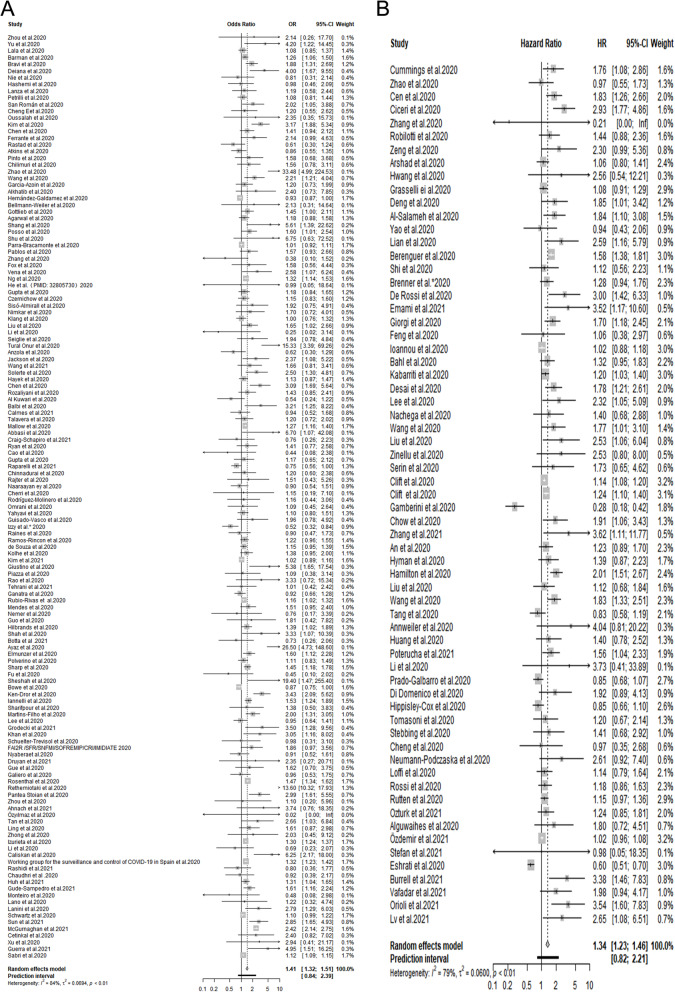
Our results revealed that COVID-19 patients with pre-existing CVD tended more to adverse outcomes on the basis of 203 eligible studies with 24,032,712 cases (pooled ORs = 1.41, 95% CIs: 1.32-1.51, prediction interval: 0.84-2.39; pooled HRs = 1.34, 95% CIs: 1.23-1.46, prediction interval: 0.82-2.21). Further subgroup analyses stratified by age, the proportion of males, study design, disease types, sample size, region and disease outcomes also showed that pre-existing CVD was significantly associated with adverse outcomes among COVID-19 patients.
Conclusion
Our findings demonstrated that pre-existing CVD was an independent risk factor associated with adverse outcomes among COVID-19 patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-11051-w.
Keywords: Coronavirus disease 2019, cardiovascular disease, adverse outcome, adjusted effect estimate
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global outbreak of coronavirus disease 2019 (COVID-19). Currently, the pandemic has affected more than 127,319,002 people in more than 200 countries and killed more than 2,785,838 people (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Previous studies have reported that several pre-existing medical conditions, such as hypertension, diabetes and so on, might accelerate disease progression of COVID-19 [1–3]. Cardiovascular disease (CVD), one of the most common comorbidities of COVID-19, has been observed to be associated with adverse outcomes among COVID-19 patients by Li et al. in a meta-analysis study [4]. Nevertheless, it is worth noting that the results of Li et al.’s study were based on the unadjusted effect estimates [4]. It is reported that age, sex, and co-existing diseases are known to affect the outcomes of COVID-19 patients [5–7], which may modulate the association between CVD and adverse outcomes in COVID-19 patients. Moreover, Zhou et al. observed that coronary heart disease (CHD), one of CVD, was strongly correlated with an increased risk of in-hospital mortality among COVID-19 patients in univariable analysis (odds ratio (OR) = 21.4, 95% confidence interval (CI): 4.64-98.76), but no significant correlation was observed in multivariable analysis (OR = 2.14, 95% CI: 0.26-17.79) [8]. The similar results were also observed by Robilotti et al. [9] and Louapre et al. [10]. Therefore, it is necessary to clarify whether pre-existing CVD was an independent risk factor associated with adverse outcomes in COVID-19 patients. In this study, we performed a quantitative meta-analysis on the basis of adjusted effect estimates.
Methods
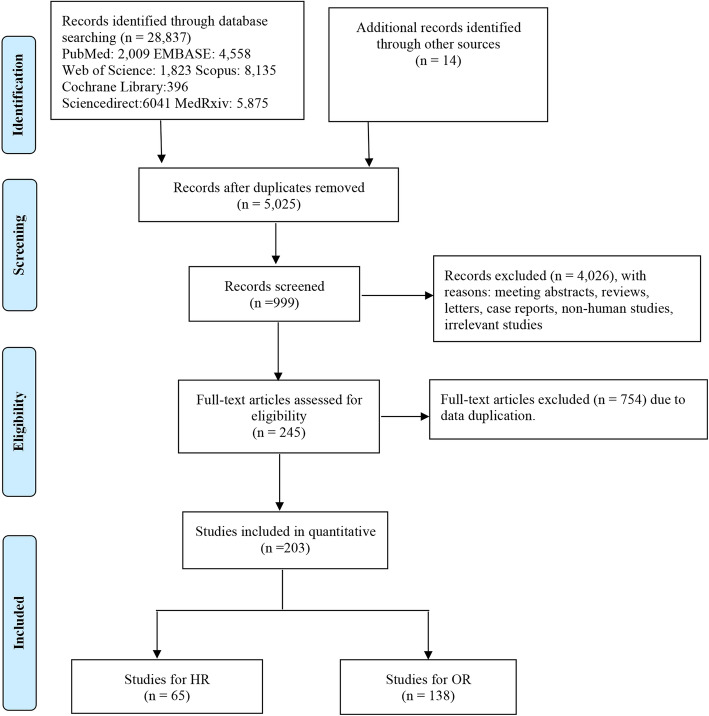
This is a quantitative meta-analysis on the basis of adjusted effect estimates. Admittedly, our study was not registered, but our meta-analysis was made in strict accordance with the process of systematic evaluation (Fig. 1). Moreover, our study is less likely to be biased by artificial bias because this study was carried out rigorously in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Online supplemental Table A1) [11].
Fig. 1.
Flow diagram of selection process
Literature search strategy
The databases of PubMed, Web of Science, MedRxiv, Scopus, Elsevier ScienceDirect, Cochrane Library and Embase were searched to obtain a complete data source up to January 7, 2021. The search strategies were as follows: (“COVID-19” OR “coronavirus disease 2019” OR “SARS-CoV-2” OR “2019-nCoV”) AND (“cardiovascular disease” OR “coronary heart disease” OR “cardiac disease” OR “heart disease” OR “heart failure” OR “coronary artery disease”) AND (“outcome” OR “severe” OR “critical” OR “severity” OR “fatality” OR “mortality” OR “death” OR “adverse outcome” OR “poor outcome” OR “clinical characteristics”). All the terms matched the MesH browser. Beyond that, the relevant references of preceding studies were also taken into account.
Eligibility criteria
The criteria for including studies were: (1) Subjects should be laboratory-confirmed COVID-19 patients; (2) Studies should report the correlation between CVD and COVID-19 patients and the data are available; (3) Studies should be published in English; (4) Studies should include the multivariate analysis. The studies with the largest sample size were selected for inclusion when studies were conducted in the same hospital and the overlapping period. There was no restriction for region of study. The exclusion criteria included case reports, review papers, comments, errata, repeated studies, studies only reporting the characteristics of COVID-19 patients with CVD, and studies without available full text.
Data extraction and quality assessment
Data were extracted independently by two investigators (J.X. and W.X.), including the following information: the first author, source of data, country, date of data collection, number of patients, mean/median age, the percent of males, study design, the percent of COVID-19 patients with CVD, adjusted effect estimates (hazard ratio (HR) or OR) and adjusted risk factors. When both OR and HR existed in the same article, it was preferred to include HR because cox regression took time into account. Two researchers negotiated to resolve it in case of any issues not covered by the criteria and Y.W. acted as arbiter. The quality of the included studies was evaluated by investigators according to the Newcastle-Ottawa Scale [12]. High-quality studies referred to studies with a score above 7.
Data synthesis
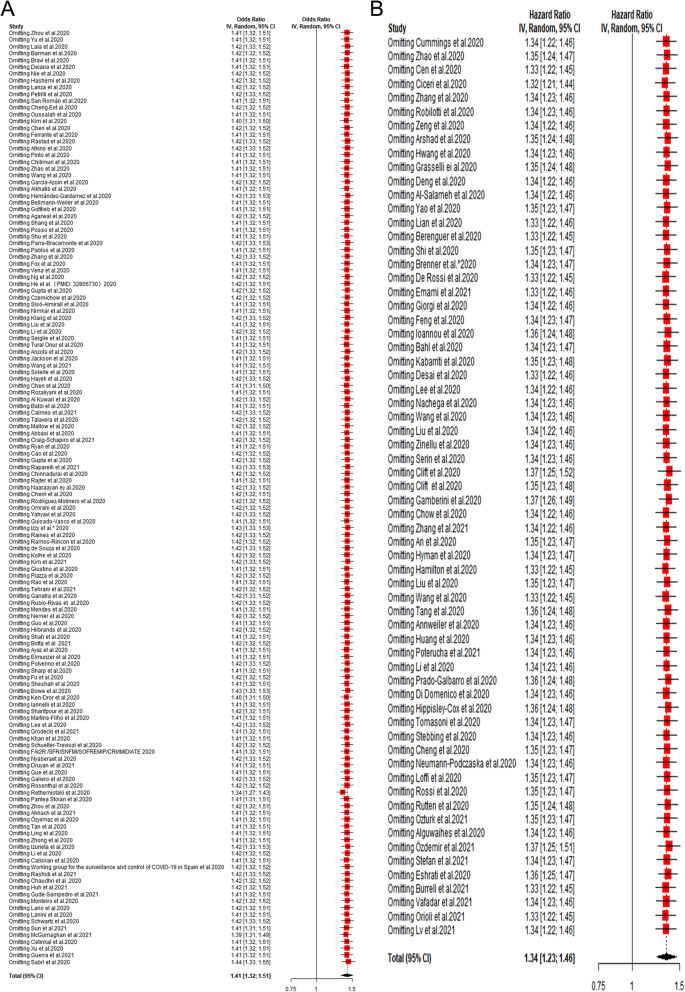
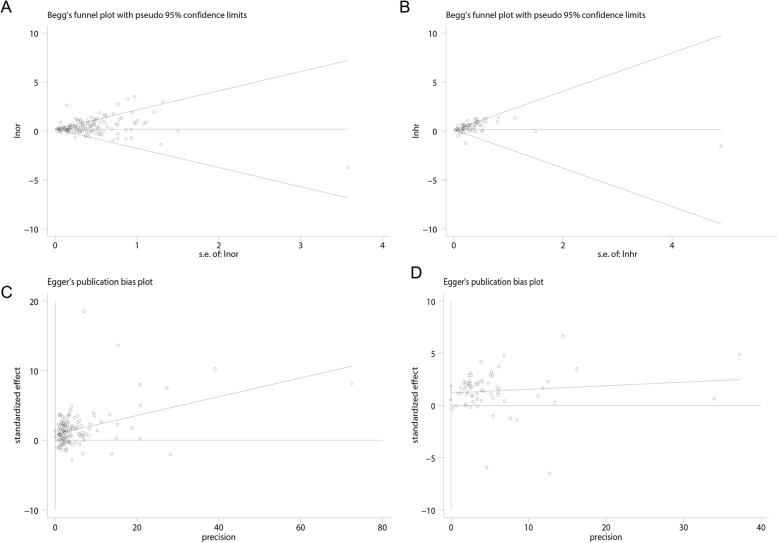
The major information such as study design and effect estimates were directly extracted from original articles. The research type of some articles was not clear and some articles provided both OR and HR. Besides, the calculation methods of HR and OR are different. The calculation of HR takes into account the concept of time, and OR is the approximate value of risk ratio. Therefore, pooled HR, OR and 95% confidence intervals (CIs) were separately calculated to address the risk of adverse outcomes in COVID-19 patients with a history of CVD. Heterogeneity was assessed by Cochran’s Q-statistic and I2 test, if no significant heterogeneity was observed (I2 ≤ 50%, P > 0.1), a fixed-effects model was adopted; otherwise, a random-effects model was applied [13]. In addition, we also provided the prediction interval, which was helpful for assessing whether the variation across studies was clinically significant [14, 15]. The robustness of the results was evaluated by sensitivity analysis which omitted one study at a time. Publication bias was assessed by Begg’s test [16], Egger’s test [17] and trim-and-fill method [18]. Subgroup analysis and meta-regression were conducted to determine the source of heterogeneity. Data analyses were conducted using Stata, version 12.0 (meta-program) and R, version 3.6.1 (netmeta package). A two-tailed P-value < 0.05 was regarded as significant.
Results
The flow chart of selection process is shown in Fig. 1. 5,025 records were retrieved after removing 23,826 duplicates, of which 245 studies were full-text assessed. Eventually, a total of 203 eligible studies with 24,032,712 patients were enrolled in our meta-analysis [2, 3, 8, 9, 19–210, 212–218]. 81 studies originated from Europe, 54 studies came from North America, 61 from Asia, 2 from Australia, and the remained 5 were not just from one country (Table 1). Among these studies, cardiac disease was mentioned in 63 studies, HF was involved in 35 studies, and CAD was involved in 35 studies (Table 2). Adjusted HR was reported in 65 studies and adjusted OR was reported in 138 studies (Table 2). The main characteristics of the selected studies are summarized in Table 1.
Table 1.
Main characteristics of the included studies
| Author (Year) | Country | Patients(n) | Mean/Median Age(years) | Male (%) | Study design | Kinds of diseases | CVD (%) | Adjusted effect estimate (95%CI) | Outcome | Confounders | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. (2020) [8] | China | 191 | 56·0 (46·0–67·0) | 119 (62) | Retrospective cohort study | Coronary heart disease | 18 (8) |
OR 2.14 (0.26-17.79) |
In-hospital death | Age, SOFA score | 7 |
| Yu et al. (2020) [19] | China | 333 | 50(35-63) | 172 (51.7) | Descriptive study | Heart disease | 24 (7.2) | OR 4.2 (1.2-14.2) | Severity | Age, sex, diabetes, HTN, respiratory disease | 8 |
| Cummings et al. (2020) [3] | USA | 257 | 62 (51–72) | 171 (67) | Prospective observational cohort study t | Chronic cardiac disease | 49 (19) | HR 1.76 (1.08-2.86) | In-hospital mortality | Age, gender, symptom duration before hospital, presentation, COPD or interstitial lung disease, diabetes, IL-6, D-dimer | 8 |
| Zhao et al. (2020) [20] | China | 1000 | 61 (46-70) | 466 (46.6) | Retrospective study | Coronary heart disease | 60 (6) | HR 0.972 (0.547-1.726) | Death | Age | 8 |
| Sabri et al. (2020) [21] | Iran | 60 | 54.1±15.5 | NR | Retrospective cohort study | Heart Disease | 10 (15.9) | OR 1.12 (1.08-1.14) | ICU admission | Pericardial effusion, blood oxygen saturation | 7 |
| Lala et al. (2020) [22] | USA | 2736 | 66.4 | 1630 (59.6) | NR | Coronary Artery Disease | 453 (16.6) |
OR 1.08 (0.85-1.37) |
Mortality | age, sex, BMI, race, ethnicity, history of CAD, history of AF, history of HF, history of HTN, history of CKD, history of DM, statin use, angiotensin converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) use, and CURB-65 score at hospital admission | 7 |
| Cen et al. (2020) [2] | China | 1007 | 61(49-68) | 493(49.0) | Multi-center observational study | Coronary artery disease | 65 (6.5) |
HR 1.828 (1.256-2.660) |
Disease progression was defined as progression to the severe or critical disease stage, or death | Age, sex, smoking history, HTN, diabetes, chronic obstructive lung disease, CAD, CRD, CVA, hepatitis B infection, anti-viral drug, aeration of anti-viral therapy | 7 |
| Ciceri et al. (2020) [23] | Italy | 410 | 65 (56-75) | 299 (72.9) | NR | Coronary artery disease | 51 (12.6) |
HR 2.93 (1.77-4.86) |
Death | Age, gender, cancer, radiographic assessment of lung edema score, WBC count, lymphocyte count, hemoglobin, platelets. | 7 |
| Barman et al. (2020) [24] | Turkey | 607 | 59.5±14.8 | 334 (55.02) | Multi-center retrospective study | Coronary artery disease | 116 (19.1) |
OR 1.26 (1.06-1.50) |
Mortality | Age, gender, HTN, diabetes, CAD, COPD, smoking, creatinine, uric acid, glucose | 7 |
| Bravi et al. (2020) [25] | Italy | 1603 | 58.0±20.9 | 758 (47.36) | Case-control, retrospective study | Major cardiovascular diseases | 258 (16.1) | OR 1.88 (1.32-2.70) | Severe or very severe/lethal | Age, gender, HTN, diabetes, cancer, COPD, renal disease | 7 |
| Deiana et al. (2020) [26] | Italy | 1223 | 80.4±10.6 | 499 (40.8) | Matched case-control study | CVD | 63 (64.9) | OR 4.0 (1.7-9.7) | Severity | Active tumors, diabetes, HIV, CLD, CRD, metabolic diseases, obesity, chronic neurological diseases, other pathologies | 7 |
| Zhang et al. (2020) [27] | China | 80 | 51.16±17.476 | 33 (41.25) | Retrospective cohort | Cardiac disease | 9 (11.25) |
HR 0.21 (0-22.09) |
Severity | Age, respiratory diseases, HTN, more than 2 kinds of diseases, WBC, neutrophil, LYM%, NEU%, NLR, FIB, CRP, TBIL, ALB, GFR, CK-MB, myoglobin, troponin | 7 |
| Nie et al. (2020) [28] | China | 671 | 43±15.09 | 377 (56.2) | NR | CVD | 70 (10.4) | OR 0.809 (0.306–2.142) | Severity | Age, gender, coexisting disorder (HTN, diabetes, respiratory diseases, diabetes, respiratory diseases), Animal/human transmission source contact, Contact with confirmed cases, Contact with confirmed cases, Contact with individuals who had been to Wuhan, Close to cluster outbreak, Visited hospital, Visited wet market, No contact, Days from illness onset to diagnosis, X-ray with pneumonia features, CT with pneumonia features, Blood routine test Leucocyte count, Lymphocyte count, Lymphocyte percentage, Neutrophil percentage | 7 |
|
Robilotti et al. (2020) [9] |
USA | 423 | 60.2 | 212 (50) | NR | Cardiac disorder | 84 (20) |
HR 1.44 (0.88-2.37) |
Severe respiratory illness, | Age, gender, race, BMI, smoking, asthma/COPD, cancer, major surgery, diabetes, HTN/CKI, Systemic chemotherapy, Chronic lymphopenia or corticosteroids, ICI | 8 |
|
Hashemi et al. (2020) [29] |
USA | 363 | 63.2±13.2 | 201 (55.37) | Multi-center retrospective study | Cardiac diseases | 39 (10.7) | OR 0.98 (0.46-2.09) | Death | CLD, age, obesity, gender, HTN, diabetes, hyperlipidemia, pulmonary disorders | 7 |
| Lanza et al. (2020) [30] | Italy | 222 | 66.4 (53.8–75.8) | 163 (73) | Observational retrospective study, | Heart disease | 27 (12.16) | OR 1.19 (0.58-2.44) | In-hospital death | Age, gender, smoke habit, CRP, Lung disease, cancer, diabetes, CKD, CURB-65a 1, CURB-65a 2, diabetes, BMI | 8 |
| Zeng et al. (2020) [31] | China | 461 | 45.00 (34.50-57.00) | 239 (51.84) | Multicenter retrospective study | CVD | 25 (5.42) |
HR 2.30 (0.99-5.38) |
Severity | Age, gender, HTN, diabetes, hematology, biochemistry, infection-related indices, coagulation function | 8 |
| Petrilli et al. (2020) [32] | USA | 5279 | 54 (38-66) | 2615 (49.5) | Prospective cohort study | Coronary artery disease | 704 (13.3) |
OR 1.08 (0.81-1.44) |
Mortality | Age, gender, BMI, race, COPD and asthma, diabetes, HTN, cirrhosis, CKD, CAD, immunosuppression, cancer, tobacco smoking | 8 |
| Arshad et al. (2020) [33] | USA | 2541 | 63.7±16.5 | 1298 (51.1) | Retrospective cohort study | Cardiovascular Comorbidity | 222 (8.7) | HR 1.062 (0.8-1.410) | Death | HCQ alone (vs. neither medication), azithromycin alone (vs. neither medication), HCQ+AZM (vs. neither medication), age, gender, ethic, BMI,lung comorbidity,CKI comorbidity,COPD, HTN,asthma, COPD,cancer,diabetes, percent O2 saturation < 95, admission to ICU, ventilator, given steroid, given tocilizumab | 7 |
| San Román et al. (2020) [34] | Spain | 522 | 68±15 | 294 (56) | NR | Heart disease | 68 (13.02) | OR 2.017 (1.050-3.876) | Severity | Age, SatO2 <90%, creatinine > 1.5 mg/dL, c-reactive protein> 10 mg/L | 7 |
| Cheng et al. (2020) [35] | China | 456 | 54.97±18.59 | 211 (46.27) | Retrospective cohort study | CVD | 52 (11.4) | OR 1.204 (0.554-2.619) | Any in-hospital disease progression | Age, gender, HTN, diabetes, CKD, neural system diseases, pulmonary disease, cancer, laboratory findings(leucocytes count, neutrophil count, lymphocyte count, NLR, platelet count, albumin, APTT, prothrombin time, INR, D-dimer, aspartate aminotransferase, creatinine, potassium, creatine kinase, lactate dehydrogenase, procalcitonin, C-reactive protein, erythrocyte sedimentation rate, IL-6 | 8 |
|
Oussalah et al. (2020) [36] |
France | 149 | 65 (54–77) | 91 (61.1) | Retrospective, longitudinal cohort study | CVD | 38 (25.5) |
OR 2.35 (0.35-15.68) |
Death | Age, COPD, gender, creatinine >10.1 mg/L, HTN | 8 |
| Kim et al. (2020)[37] | Korea | 9148 | 51* | 3556 (38.9) | Observational Study | Heart failure | 124 (1.4) |
OR 3.17 (1.88–5.34) |
Mortality | Gender, age, type of distiricts, high epidemic region and socio-economic status | 8 |
| Chen et al. (2020) [38] | China | 3309 | 62(49-69) | 1642 (49.6) | Retrospective | CVD | 242 (7.3) | OR 1.41 (0.94-2.13) | Death | Age, gender, HTN, diabetes, cerebrovascular disease, malignancy, CKI, COPD, days from onset to clinics (vs ≤5d), days from onset to admission (vs ≤12d) | 9 |
|
Ferrante et al. (2020) [39] |
Italy | 332 | 66.9 (55.4-75.5) | 237 (71.4) | Single-center cohort study | CAD | 49 (14.5) | OR 2.14 (0.99-4.63) | Death | Age, HTN, CVA, Cancer, eGFR, PaO/FiO2 ratio, PA diameter, baseline ACEI/ARB use | 7 |
| Rastad et al. (2020) [40] | Iran | 2597 | 54.8±16.9 | 1589 (53.7) | Retrospective cohort study | CVD | 314 (10.6) |
OR 0.61 (0.30, 1.24) |
In-hospital mortality | WBC, neutrophils, lymphocytes, serum concentrations, creatinine, LDH, AST, ALT, Hb, ESR, CRP, age | 8 |
| Hwang et al. (2020) [41] | South Korea | 103 | 67.62±15.32 | 52 (50) | Retrospective cohort study | CVD | 12 (12) | HR 2.556 (0.535–12.207)) | Mortality | Age, diabetes, CLD, Alzheimer’s dementia, stroke | 7 |
|
Grasselli ei al. (2020) [42] |
Italy | 3988 | 63 (56-69) | 3188 (79.9) | Retrospective, observational cohort study | Heart disease | 533 (13.4) |
HR 1.08 (0.91-1.29) |
Death | Age, gender, respiratory support, HTN, hypercholesterolemia, type 2 diabetes, Malignancy, COPD, ACE inhibitor therapy, ARB therapy, statin, diuretic, PEEP at admission, FiO2 at admission, PaO2/FiO2 at admission | 8 |
| Deng et al. (2020) [43] | China | 264 | 64.5 (53.3-74.0) | 130 (49.2) | Retrospective study | Coronary heart disease | 32 (12.1) | HR 1.855 (1.006-3.421) | Death | Age, gender, HTN, cTnI-ultra, CK-MB, MYO, NT-proBNP, Cr | 7 |
| AI-Salameh et al. (2020) [44] | France | 433 | 72±14.3 | 226 (52.1) | Observational cohort | CVD | 99 (31.2) |
HR 1.84 (1.1-3.08) |
Death | Age, diabetes, gender, abnormal LFTs | 7 |
| Atkins et al. (2020) [45] | UK | 507 | 74.3±4.5 | 311 (61.3) | NR | CHD | 108 (21.5) | OR 0.86 (0.55-1.36) | Death | Age, gender, race, education, atrial fibrillation, stroke, HTN, diabetes (type 2), CKD, depression, dementia, asthma, COPD, osteoporosis, osteoarthritis, delirium, pneumonia, falls/fragility fractures | 8 |
| Yao et al. (2020) [46] | USA | 242 | 66.1±18.3 | 104 (42.98) | Single-institution retrospective study | Heart Disease | 39 (13.6) | HR 0.94 (0.43-2.07) | Mortality | Zinc sulfate (yes vs no), age, gender, COPD, clinical severity, lopinavir/ritonavir, steroids, IL-6 receptor inhibitors, | 8 |
| Pinto et al. (2020) [47] | Italy | 1226 | 71.7±14.5 | 733 (59.8) | Observational cohort Study | CVD | NR (NR) | OR 1.58 (0.68–3.68) | Death | Age, sex, presence of metastatic disease, time since cancer diagnosis | 7 |
|
Chilimuri et al. (2020) [48] |
USA | 375 | 63.0 (52.0-72.0) | 236 (63) | Retrospective cohort study | CVD | 62 (17) | OR 1.56 (0.78-3.11) | Mortality | Age, gender, HTN, lymphocyte, creative protein, alanine aminotransferase, aspartate aminotransferase, creatine kinase | 8 |
| Lian et al. (2020) [49] | China | 232 | NR | 108 (46.5) | Retrospective study | Heart disease | 31 (13.36) | HR 2.587 (1.156-5.787) | Severity | Age, NLR, multiple mottling and ground-glass opacity | 8 |
| Zhao et al. (2020) [50] | USA | 641 | 58.9±17.5 | 358 (55.85) | Retrospective study | Heart failure | 20 (3.12) | OR 33.48 (4.99-224.45) | Mortality | LDH, procalcitonin, smoking history, SpO2, lymphocyte count, procalcitonin, LDH, COPD, SpO2, heart rate, age | 8 |
| Wang et al. (2020) [51] | USA | 1827 | 52.7±21.1 | 500 (32.6) | NR | CVD | 589 (32.2) | OR 2.21 (1.21-4.04) | Severity | Gender, race, marital status, Insurance type, smoking history, BMI, comorbidities (diabetes, COPD, CKD, CLD, HTN, allergic rhinitis), SABA, combination | 7 |
| Garcia-Azoin et al. (2020) [52] | Spain | 576 | 67.18±14.75 | 326 (56.6) | Retrospective cohort study | Cardiac disease | 154 (26.7) | OR 1.20 (0.730-1.999) | Mortality | mRS≥3, age, gender, HTN, diabetes, smoking, pulmonary disorders, cancer, chronic neurological disorders, immunosuppression | 7 |
|
Alkhatib et al. (2020) [53] |
USA | 158 | 57±15.1 | 61 (38.6) | Retrospective cross-sectional analysis | Heart Failure | 21 (13.3) | OR 2.4 (0.734-7.845) | Severity | Age, gender, diabetes, HTN, lung disease, CKD, BMI | 7 |
| Hernández-Galdamez et al. (2020) [54] | Mexico | 211003 | 45.7±16.3 | 115442 (54.71) | Cross-sectional study | CVD | 4949 (2.35) |
OR 0.93 (0.87-1.00) |
Death | At least one comorbidity/risk, CKD, immunosuppression, diabetes, COPD, HTN, asthma, obesity, smoking | 8 |
| Bellmann-Weiler et al. (2020) [55] | Australia | 259 | 66.8±14.3 | 157 (60.62) | Retrospective | CVD | 152 (58.62) | OR 2.127 (0.309–14.647) | Death | Age, CKD, COPD, eGFR, leukocytes, PCT, anemia, | 8 |
| Berenguer et al. (2020) [56] | Spain | 4035 | 70 (56 – 80) | 2433 (61) | Retrospective nationwide cohort study | Chronic heart disease | 932 (23.3) |
HR 1.58 (1.38-1.81) |
Death | Gender, age, HTN, diabetes, COPD, obesity, CKI stage 4, liver cirrhosis, chronic neurological disorder, cancer, dementia, headache, myalgia/arthralgia, anosmia, cough, sputum production, dyspnea, chest pain, vomiting/nausea, altered consciousness, low SaO2, WBC count, neutrophil-to-lymphocyte ratio, platelets, prolonged APTT, eGFR, ALT, CRP | 7 |
| Gottlieb et al. (2020) [57] | USA | 8673 | 41 (29 – 54) | 4045 (46.6) | Retrospective case-control study t | Congestive Heart Failure | 218 (14.7) |
OR 1.45 (1.00-2.12) |
Critical Illness | Age, gender, race, COPD, HTN, hyperlipidemia, diabetes, prior CVA, CKD, current ESRD, obstructive sleep apnea, bloodborne cancer, symptoms (anosmia, cough, headache, myalgias), labs(WBC, ALC,ANC/ALC, total Bilirubin, albumin, AST, ALT, LDH, lactate, D-Dimer, CRP, ferritin, troponin) | 8 |
|
Agarwal et al. (2020) [58] |
USA | 1126 | 67.9±13.7 | 630 (49.3) | Retrospective | CVD | 754 (59) |
OR 1.18 (0.88-1.57) |
Mortality | Treatment regimen (noninsulin only, insulin 1 noninsulin, insulin only), HTN, CKD, COPD | 7 |
| Shang et al. (2020) [59] | China | 2529 | 66 | 73 (64.6) | Retrospective | CHD | 28 (24.8) | OR 5.611 (1.392-22.623) | Death | Age, D-dimer, PCT, LYM, diabetes, CRP, BUN | 8 |
| Shi et al. (2020)[60] | Iran | 386 | 59.46±15.82 | 236 (61.1) | Prospective, single-center study | CVD | 97(25.1) | HR 1.121 (0.565-2.226) | Death | Age, diabetes, malignancy, CKD, CVA/TIA, previous ACEI/ARB use, ARDs, AKI | 7 |
| Posso et al. (2020) [62] | Spain | 834 | 60 | 400 (46.5) | Retrospective | Heart Failure | 37 (37.4) | OR 1.6 (1.01-2.55) | Death | Age, gender | 7 |
| Shu et al. (2020) [63] | China | 571 | 50.0 (38.0-59.0) | 278 (48.7) | Single-center, retrospective cohort study | Coronary heart disease | 12 (2.1) |
OR 6.75 (0.629-72.61) |
Severity | Smoke, HTN, diabetes, dyspnea, consolidation, interstitial abnormalities, lymphocyte counting | 8 |
| Parra-Bracamonte et al. (2020) [64] | Mexico | 142690 | 45 (34.0-57.0) | 79280 (56) | NR | Cardiopathy | 3521 (2.0) | OR 1.012 (0.92-1.112) | Mortality | Age, gender, smoking, hospitalized, pneumonia, comorbidity (HTN, obesity, diabetes, COPD, asthma, immunosuppressed, CKD, other complication) | 8 |
| Pablos et al. (2020) [65] | Spain | 456 | 65±17.9 | 182 (41) | Retrospective observational matched cohort study | Heart failure | 106 (23.2) | OR 1.57 (0.93-2.66) | Composite severe COVID-19 outcome | CTD, age, gender, obesity, diabetes, glucocorticoids (any dose), antivirals | 8 |
| Zhang et al. (2020) [66] | China | 461 | 51 (38-64) | 264 (57.3) | Multicenter study | Coronary heart disease | 25 (5.4) | OR 0.382 (0.096-1.526) | Critical illness | Age, gender, comorbidities (HTN, diabetes, CLD), types of previous surgery (gastrointestinal surgery, urogenital surgery, skeletal surgery, cardiovascular surgery, others), WBC, neutrophil, lymphocyte, LDH, hemoglobin, platelet, albumin, AST, ALT, DBIL, IBIL, TBIL, APTT, PT, D-dimer, creatinine, hs-CRP, procalcitonin, urea nitrogen, FBG, CT score) | 8 |
| Fox et al. (2020) [67] | USA | 389 | 66.2±14.2 | 208 (46.5) | Single-center retrospective analysis | CAD | 77 (19.79) | OR 1.579 (0.562–4.436) | In-hospital mortality | Age, BMI, gender, ethnic, Hispanic, others, COPD, asthma, CAD, HTN, atrial fibrillation, CKD | 7 |
| Vena et al. (2020) [68] | Italy | 317 | 71 (60-82) | 213 (67.2) | Retrospective study | CVD | 63 (19.9) |
OR 2.58 (1.07-6.25) |
All-cause in-hospital mortality | AKI, age, CRP, IL-6 | 7 |
| Ng et al. (2020) [69] | USA | 10482 | 66 | 6239 (59.5) | Retrospective study | Heart Failure | 920 (8.78) | OR 1.32 (1.14-1.53) | Death | Age, sex, race/ethnicity, BMI, diabetes mellitus, HTN, cancer, mechanical ventilation, use of vasoactive medication, hemoglobin, lymphocyte, blood urea nitrogen, albumin, C-reactive protein and ferritin | 8 |
| He et al. (2020) [70] | China | 288 | 48.5 (34.3-62) | 131(45.5) | Single-center, retrospective cohort study | CVD | 85 (29.5) | OR 0.986 (0.052-18.588) | Death | Age, CKD, exposure history in Wuhan >2 weeks, diarrhea, WBC count, lymphocyte count, creatinine, PCT, | 8 |
| Gupta et al. (2020) [71] | USA | 2626 | 63.99±16.49 | 1497(57.00) | Retrospective study | CAD | 516 (19.6) | OR 1.179 (0.844-1.647) | In-hospital mortality | Age, gender, CKD, exposure history in Wuhan >2 weeks, diarrhea, white blood cell count, lymphocyte count, creatinine | 6 |
| Czernichow et al. (2020) [72] | Europe | 5795 | 59.8±13.6 | 3791 (65.4) | Prospective cohort study | HF | 264 (4.55) | OR 1.15 (0.82-1.59) | Body mass index, age, diabetes, hypertension, dyslipidemia, sleep apnea, CKD, malignancies, history of smoking, gender | 8 | |
| Sisó-Almirall et al. (2020) [73] | Spain | 322 | 56.7±17.8 | 161 (50.0) | Multicenter, observational descriptive study | HF | 25(7.8) |
OR 1.92 [0.74–4.84] |
Death or ICU admission | Age, gender | 7 |
| Brenner et al*. (2020) [74] | Germany | 9548 | 62.1 | 4182 (43.8) | Ongoing statewide cohort study | CVD | 4186 (43.8) | HR 1.285 (0.936–1.763) | Mortality | Any cause, age, gender, cancer, respiratory disease, Season | 8 |
| De Rossi et al. (2020) [75] | Italy | 158 | 66.38±13.44 | 113 (71.52) | Retrospective cohort study | Heart disease | 33 (20.89) | HR 3.001 (1.422-6.332) | Mortality | GROUP, age, gender, diabetes, HTN, CRP at admission, time to hospitalization, Time to hospitalization | 7 |
| Nimkar et al. (2020) [76] | USA | 327 | 71 (59–82) | 182 (55.7) | Retrospective case series | Cardiac Disease | 98 (29.9) | OR 1.7 (0.7–3.9) | Mortality | AKI, ARDS, demographics (age, gender, race), HTN, diabetes mellitus, overweight (25 - 29.9), obese ( >= 30), underweight < 18.5 | 7 |
| Klang et al. (2020) [77] | USA | 1320 | 74.48±12.88 | 772 (58.48) | Multicenter observational retrospective study | CHD | 258 (19.55) | OR 1.00 (0.8–1.4) | Death | Age, CAD, HTN, diabetes, CKD, COPD, cancer, obesity, smoking | 7 |
| Emami et al. (2021) [78] | Iran | 1239 | 51.48±19.54 | 692 (55.9) | NR | CVD | 132 (10.7) | HR 3.52 (1.23–11.15) | Mortality | Age, diabetes, chronic liver disease, cancer, HIV, smoking, asthma, immunodeficiency disease | 5 |
| Liu et al. (2020) [79] | China | 2044 | 62.0 (51.0-70.0) | 1000 (48.92) | Mini-national multicenter, retrospective, cohort study | CHD | 199 (9.76) | OR 1.65 (1.02-2.66) | Critical disease (vs. moderate and severe disease) | Factors with effect modification, HTN, COPD, age, diabetes, tumor, CKD, cough | 6 |
| Giorgi et al. (2020) [61] | Italy | 2653 | 63.2 | 1328 (50.1) | Population-based prospective cohort | CHD | 168 (7.1) | HR 1.7 (1.2–2.5) | Death | Age, gender | 7 |
| Feng et al. (2020) [81] | China | 114 | 63.96±13.41 | 71 (62.3) | Single-center, prospective study | CVD | 31 (27.2) | HR 1.062 (0.380–2.970) | Poor outcome | Age, gender | 7 |
| Li et al. (2020) [82] | China | 199 | 67 (61-78) | 89 (44.7) | Retrospective study | CVD | NR (NR) | OR 0.250 (0.020-3.155) | Death | Age, CKD, HTN, Diabetes, d-dimer at admission, lymphocyte count at admission, fasting plasma glucose at admission, treatment with low molecular weight heparin, Antidiabetic drugs | 7 |
| Seiglie et al. (2020) [83] | USA | 450 | 63.32±17.13 | 259 (57.5) | Observational study | CHF | 52 (11.56) | OR 1.94 (0.78-4.85) | Death | Diabetes, BMI category (overweight, Obese), age, male, race/ethnicity (Hispanic, African American, other, unknown/missing), HTN, COPD/asthma, cancer (active), liver disease, renal disease | 7 |
| Tural Onur et al. (2020) [84] | Turkey | 301 | 57±18 | 206 (68.4) | Retrospectively | CVD | 19 (6.3) | OR 15.331 (3.394-69.272) | Death | Age, length of stay, lung cancer | 7 |
| Anzola et al. (2020) [85] | Italy | 431 | 65±16 | 263 (61) | Prospective study | CVD | 77 (18) | OR 0.618 (0.297-1.285) | Death | Age, lymphocyte count, creatinine, AST, CRP, diabetes, HTN, gender (male), | 7 |
| Ioannou et al. (2020) [86] | USA | 10131 | 61.6±15.9 | 9221 (91.0) | Longitudinal cohort study | CAD | 2203 (21.7) | HR 1.02 (0.88-1.18) | Death | Diabetes, cancer, HTN, congestive heart failure, cerebrovascular disease, dialysis, chronic kidney disease, cirrhosis, asthma, COPD, obstructive sleep apnea, obesity, hypoventilation, alcohol dependence, smoking, Charlson comorbidity body index score | 9 |
| Bahl et al. (2020) [87] | USA | 1461 | 62.0 (50.0–74.0) | 770 (52.7) | Multicentered cohort study | CVD | 163 (11.2) | HR 1.32 (0.95–1.83) | Mortality | Age, gender, race (Black/African American, White/Caucasian, other), diabetes mellitus, HTN, respiratory rate, blood oxygen saturation White blood cell count, hemoglobin, ALT, creatinine, d-dimer, procalcitonin, lactic acid | 6 |
|
Kabarriti et al. (2020) [88] |
USA | 5902 | 58 (44-71) | 2768 (46.9) | Cohort study | CVD | 1306 (22.1) | HR 1.20 (1.03-1.41) | Death | Age, gender, socioeconomic status (Lowest quartile, Second quartile, third quartile, highest quartile) | 8 |
| Jackson et al. (2020) [89] | USA | 51 | 60 (45–69) | 29 (56.9) | Retrospective observational cohort | CAD | 10 (19.6) | OR 2.37 (1.08–5.23) | Death | End-stage renal disease, neurologic disorders, | 6 |
| Desai et al. (2020) [90] | Italy | 575 | 64.8 (27-93) | 380 (66.09) | Single-center, retrospective, observational study | CVD | 155 (27.1) | HR 1.78 (1.21–2.61) | Death | Age, ACEi, therapy: LMWH | 8 |
| Wang et al. (2021) [91] | China | 663 | 58 (44-69) | 321 (48.4) | Retrospective | CVD | 164 (24.7) |
OR 1.66 (0.82-3.47) |
Poor therapeutic effect | Age, gender, respiratory diseases, urinary diseases, T2DM, severe and critical condition, Fever, Expectoration, dyspnea, chest tightness, muscle aches, dizziness, neutrophil count >6.3 × 10 per L, Lymphocyte count <1.1 × 10 per L, Hemoglobin <115 g/L, ALT >40 U/L, ALT >40 U/L, Cr >73 mmol/L, Cr >73 mmol/L, albumin <35 g/L, LDH >300 U/L, CRP >10 mg/L | 8 |
| Solerte et al. (2020) [92] | Italy | 169 | 69±1.0 | 115 (68) | Multicenter, case-control, retrospective, observational study | CVD | 53 (38) | OR 2.5 (1.30–4.81) | Mortality | Treatment with sitagliptin, age, gender, cancer, chronic kidney disease, use of hydroxychloroquine use of antiviral agents | 8 |
| Hayek et al. (2020) [93] | USA | 5019 | 60.42±14.86 | 3165 (63.06) | Multicenter cohort study | CAD | 676 (13.47) | OR 1.13 (0.87-1.47) | In-hospital cardiac arrest | Number of intensive care unit beds ( ≥100 (reference), 50-99, <50), age, gender, Black compared with non-Hispanic white, Hispanic compared with non-Hispanic white, body mass index per 5 kg/m2,current or former tobacco use, diabetes mellitus, HTN, coronary artery disease, congestive heart failure, kidney disease (chronic or end stage), COPD, active malignancy, mSOFA score per 2 units | 8 |
| Chen et al. (2020) [94] | China | 2828 | 60.0 (50.0-68.0) | 1442 (51.0) | single-center Retrospective cohort study | CHD | 181 (6.4) | OR 3.09 (1.69-5.64) | Adverse outcomes ( death, ARDS, respiratory failure and septic shock during hospitalization, mechanical ventilation, ICU admission, as well as clinical cure and discharges) | Age, COPD, AKI, Hs-CRP, neutrophil, lymphocyte, blood pressure | 5 |
| Lee et al. (2020) [95] | South Korea | 5061 | 45.44±17.92 | 2,229 (44%) | Retrospective cohort study | CVD | 49 (0.97) | HR 2.316 (1.053-5.094) | Mortality | Age, gender, cerebrovascular disease, HTN, diabetes, pulmonary disease, malignancy, CKD | 8 |
| Nachega et al. (2020) [96] | South Africa | 766 | 46 (34–58) | 500 (65.6) | Retrospective cohort study | Heart disease | 30 (3.9) | HR 1.40 (0.68–2.88) | Death | Age, gender, clinical stage at admission (mild or moderate, Severe or critical, HTN, diabetes, obesity, asthma/chronic obstructive pulmonary, chronic kidney disease, cancer, HIV, current tuberculosis, chloroquine/azithromycin–based, received oxygen | 8 |
| Rozaliyani et al. (2020) [97] | India | 4052 | 45.8±16.3 | 2169 (53.5) | Retrospective cohort study | Heart disease | 148 (6.9) | OR 1.43 (0.85-2.41) | Death | Age, gender, registered address (West Jakarta, Central Jakarta, South Jakarta, East Jakarta, North Jakarta, outside Jakarta, citizenship, foreigner), Symptoms (cough, fever, malaise, dyspnea, headache, nausea/emesis, Sore throat, cold/runny nose, myalgia, chills, abdominal pain, diarrhea, pneumonia), temperature, comorbidity (HTN, COPD, diabetes, renal disease, malignancy, immunological disorder, liver failure, Obesity) | 7 |
| Wang et al. (2020) [98] | China | 293 | 59.2 (42.8-73.1) | 138 (47.1) | Retrospective study | Coronary heart disease | 21 (7.2) | HR 1.771 (1.013-3.097) | Mortality | Age, gender, fever, cough, expectoration, dyspnea, catarrhal symptoms, neuromuscular symptoms, digestive symptoms, comorbidity, Hypertension, diabetes, cerebrovascular disease, COPD, chronic renal disease, chronic liver disease, malignancy, only one comorbidity, ≥2 comorbidities, complications, shock, acute cardiac injury, acute renal injury, acute liver injury, Only one complication, ≥2 complications | 8 |
| Liu et al. (2020) [99] | China | 77 | 63.6±3.6 | 48 (62) | Retrospective study | CVD | 15 (20) | HR 2.533 (1.108-6.306) | In-hospital death | HbA1C, age, gender, CRD | 8 |
| Al Kuwari et al. (2020) [100] | Qatar | 5685 | 35.8±12.0 | 5052 (88.9) | Case series | CVD | 250 (4.4) | OR 0.54 (0.24-1.22) | Severe or critical illness | Age, gender, Qatari nationality, HTN, diabetes mellitus, chronic lung disease, chronic kidney disease, cancer | 8 |
| Balbi et al. (2020) [101] | Italy | 340 | 68 (57–76) | 252 (74) | Retrospective observational study | CVD | 86 (25) | OR 3.21 (1.28–8.39) | Death | Age, SpO2, PaO2/FiO2 ratio, Brixia score | 6 |
| Calmes et al. (2021) [102] | Belgium | 493 | 58 ± 19 | 244 (49.49) | NR | Cardiopathy | 88 (18) | OR 0.94 (0.53-1.7) | Intensive care unit stay | Age, gender | 8 |
|
Talavera et al. (2020) [103] |
Spain | 576 | 67.18±14.75 | 325 (56.6) | Retrospective cohort study | Cardiological disorders | 154 (26.7) | OR 1.201 (0.716-2.016) | Mortality | Age, sex, hypertension, diabetes, smoking habit, cardiological disorders, pulmonary disorders, cancer, and chronic neurological disorders | 6 |
| Zinellu et al. (2020) [104] | Italy | 105 | 72.0 (59.5-80.0) | 70 (66.67) | Retrospective | CVD | 59 (56.19) | HR 2.53 (0.80-7.99) | In-hospital mortality | Age, gender, smoking status, intensity of care, respiratory disease, kidney disease, diabetes, cancer, De Ritis index ≥ 1.63 | 7 |
| Mallow et al. (2020) [105] | USA | 21676 | 64.9±17.2 | 11442 (52.8) | Retrospective cohort study | Severe heart disease | 12000 (55.4) | OR 1.27 (1.16-1.40) | Mortality | Age, gender, insurance (Medicaid as any payer), teaching status (nonteaching hospital vs teaching hospital), hospital bed Size, chronic lung disease, moderate to severe asthma, immunocompromised, obesity, diabetes, CKD with dialysis, liver disease, HTN, DNR, statin use in hospital | 8 |
| Abbasi et al. (2020) [106] | Iran | 262 | 58 (43–67) | 172 (65.6) | Retrospective cohort study | CAD | 78 (29.8) | OR 6.7 (1.08–42.2) | Mortality | Age, HTN, diabetes, chronic renal failure, hypoxia at admission, WBC, LYM count, LYM% less than 20%, Hb, Plt, AST, ALT, LDH, CRP, ESR, Cr, CT severity score | 6 |
| Craig-Schapiro et al. (2021) [107] | USA | 136 | 56.24±35.04 | 93 (68.38) | NR | CVD | 52 (38.23) | OR 0.76 (0.26-2.23) | Mortality | Waitlist status, age, gender, BMI, black, diabetes, pulmonary disease, history of stroke, smoking history, ACE / ARB use | 7 |
| Ryan et al. (2020) [108] | USA | 556 | 57±17 | 296 (53) | Retrospective case-control study | CVD | 71 (13) | OR 1.41 (0.77–2.58) | Composite of ICU Admission, Mechanical Ventilation, and Death | Age, immunocompromised status, dyspnea, vomiting, chronic kidney disease, COPD, diabetes mellitus, ACE inhibitor, gender, obesity, current or former smoker, obstructive sleep apnea, HTN, hyperlipidemia | 6 |
| Serin et al. (2020) [109] | Turkey | 2217 | 47.66±17.23 | 1175 (53) | NR | CAD | 165 (7.4) | HR 1.726 (0.645−4.618) | Mortality | COPD, chronic heart failure, HTN, diabetes mellitus, chronic renal failure, malignancy, without Involvement in CT, unilaterally, bilaterally, WBC, neutrophil, hemoglobin, C-Reactive Protein, D-Dimer, urea, aspartate aminotransferase, Lactate Dehydrogenase/Lymphocyte | 5 |
| Cao et al. (2020) [110] | China | 101 | 56.6±15.1 | 67 (66.3) | Retrospective, two-center study | CVD | 21 (20.8) | OR 0.439 (0.081–2.387) | Mortality | Age, respiratory rate, dyspnea, acute respiratory distress syndrome, diabetes, HTN, chronic pulmonary disease, bacterial infection | 7 |
| Gupta et al. (2020) [111] | USA | 3099 | 62 (51–71) | 2003 (64.6) | Multicenter cohort study | CAD | 390 (12.6) | OR 1.17 (0.65-2.13) | 28-day mortality | Age, gender, Non–white race, HTN, diabetes mellitus, BMI, chronic kidney disease, congestive heart failure, active malignancy, ≤3 days from hospital to ICU admission, lymphocyte count <1,000 mm3, PaO2:FiO2, altered mental status, ICU Day 1, secondary Infection, ICU Day 1, vasopressors, coagulation Component of SOFA Score, Liver component of SOFA Score, urine output (ml/day), initial RRT modality initial RRT modality, hospital size (no. pre-COVID ICU beds), regional density of COVID-19 (quartiles) | 7 |
| Raparelli et al. (2021) [112] | Italy | 3517 | 77.64±11.51 | 2346 (66.7) | Retrospective analysis | Congestive Heart Failure | 539 (15.7) | OR 0.75 (0.56-1.00) | Death | AGE, IHD, T2DM, dementia, COPD, CLD, CKD, AD, fever, SOB, cough, admission in ICU, AKI, acute cardiac injury, shock, antivirals, tocilizumab, length of stay | 6 |
| Chinnadurai et al. (2020) [113] | UK | 215 | 74 (60–82) | 133 (61.9) | Single-center observational study | CVD | 93 (43.3) | OR 1.20 (0.61–2.40) | Mortality | Age, care home resident, frailty, smoking, respiratory diseases | 6 |
| Rajter et al. (2020) [114] | USA | 280 | 59.6±15.9 | 153 (64.6) | NR | Cardiac Disease | 43 (15.4) | OR 1.51 (0.43-5.22) | Mortality | Treatment group (Ivermectin VS Control), age, gender, current or former smoker, Race (Black, Hispanic, Other, White), comorbidities (diabetes, pulmonary, HTBN, No comorbidities), BMI, severe presentation, Intubated at study entry, MAP < 70 mm Hg, corticosteroid treatment, peripheral white cell count, lymphocyte count | 7 |
| Naaraayan ey al. (2020) [115] | USA | 362 | 71 (59–82) | 200 (55.3) | Retrospective case series | Cardiac diseases | 119 (32.9) | OR 0.9 (0.5–1.4) | In-hospital mortality | age, sex, hypertension, diabetes, race, chronic obstructive pulmonary disease, renal disease and obesity | 6 |
| Cherri et al. (2020) [116] | Italy | 53 | 75 (68–83) | 32 (60.4) | Retrospective study | Cardiopathy | 20 (37.7) | OR 1.15 (0.187-7.13) | Mortality | Age, BMI, diabetes, active oncological disease | 7 |
| Rodríguez-Molinero et al. (2020) [117] | Spain | 418 | 65.4±16.6 | 238 (56.9) | Observational cohort study | Heart failure | 26 (6.22) | OR 1.16 (0.44–3.06) | Case fatality | Age, gender, diabetes mellitus, obesity, chronic kidney disease, HTN, atrial fibrillation, dementia, OSAS, Auto-immune disease | 6 |
| Clift et al. (2020) [118] | UK | 8256158 | 44.33±27.42 | 4111197 (49.8) | Cohort study | Heart failure | 96225 (1.17) | HR 1.14 (1.08–1.20) | Death | No learning disability, learning disability apart from down syndrome, down syndrome, males vs. females, Townsend material deprivation score (5-unit increase), White, Indian British, Pakistani British, Bangladeshi British, Other Asian British, Caribbean British, Black British, Chinese British, other ethnic group, not in care home or homeless, lives in residential or nursing home, Homeless according to GP records, No kidney failure, chronic kidney disease stage, chemotherapy grad, blood cancer, bone marrow or stem cell transplant in past 6 month, respiratory tract cancer, Radiotherapy in past 6 month, Solid organ transplant (excluding kidney and bone marrow), immunosuppressant drug, ≥4 scripts from GP in past 6 mo, Leukotriene or LABA, ≥4 scripts in past 6 month, Oral steroids, ≥4 scripts in past 6 month, Sickle celI disease or severe immunodeficiency, type 1 diabetes, type 2 diabetes, COPD, asthma, rare lung conditions (bronchiectasis, CF, or alveolitis), pulmonary hypertension or pulmonary fibrosis, coronary heart disease, stroke, atrial fibrillation, congestive heart failure, thromboembolism, peripheral vascular disease, congenital heart disease, dementia, Parkinson disease, Epilepsy MND, MS, myasthenia gravis, or Huntington disease, Cerebral palsy, severe mental illness, osteoporotic fracture (hip, spine, wrist, or humerus), rheumatoid arthritis or SLECirrhosis | 9 |
| Clift et al. (2020) [119] | UK | 6083102 | 48.21±18.57 | 3035409 (49.90) | Population based cohort study | Coronary heart disease | 215069 (3.54) | HR 1.24 (1.10-1.40) | Death | No learning disability, learning disability apart from down syndrome, down syndrome, males vs. females, Townsend material deprivation score (5-unit increase), White, Indian British, Pakistani British, Bangladeshi British, Other Asian British, Caribbean British, Black British, Chinese British, Other ethnic group, not in care home or homeless, lives in residential or nursing home, homeless according to GP records, No kidney failure, chronic kidney disease stage, chemotherapy grad, blood cancer, bone marrow or stem cell transplant in past 6 month, respiratory tract cancer, radiotherapy in past 6 month, solid organ transplant (excluding kidney and bone marrow), immunosuppressant drug, ≥4 scripts from GP in past 6 month, Leukotriene or LABA, ≥4 scripts in past 6 month, Oral steroids, ≥4 scripts in past 6 month, sickle celI disease or severe immunodeficiency, type 1 diabetes, type 2 diabetes, COPD, asthma, rare lung conditions (bronchiectasis, CF, or alveolitis), pulmonary hypertension or pulmonary fibrosis, coronary heart disease, stroke, atrial fibrillation, congestive heart failure, thromboembolism, peripheral vascular disease, congenital heart disease, dementia, Parkinson disease, epilepsy MND, MS, myasthenia gravis, or Huntington disease, cerebral palsy, severe mental illness, osteoporotic fracture (hip, spine, wrist, or humerus), rheumatoid arthritis or SLEcirrhosis | 9 |
| Gamberini et al. (2020) [120] | Italy | 2540 | 66 (59–72) | 300 (76.7) | Multicenter prospective observational study | Chronic ischemic heart disease | 35 (9) | HR 0.277 (0.181–0.423) | Mechanical ventilation | Age, SOFA score at ICU admission, renal replacement therapy during ICU stays, lowest PaO2/FiO2 within 5 days, CRS < 40 mL/cmH2O within 5 days, neurologic complications | 7 |
| Omrani et al. (2020) [121] | Qatar | 1409 | 39.82±14.2 | 1167 (82.8) | Retrospective cohort study | Coronary artery disease | 31 (2.4) | OR 1.090 (0.449–2.643) | Admission to ICU | Age, gender, diabetes mellitus, HTN, chronic liver disease, chronic kidney disease, BMI | 6 |
| Yahyavi et al. (2020) [122] | Iran | 2553 | 58.1±17.9 | 1498 (58.7) | Retrospective cohort study | CVD | 942 (36.9) | OR 1.1 (0.8-1.5) | Mortality | angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, chronic kidney disease, chronic pulmonary disease, diabetes mellitus, intensive care unit, diuretics, beta-blockers, and calcium channel blockers | 7 |
| Guisado-Vasco et al. (2020) [135] | Spain | 607 | 69±22.0 | 394 (65.02) | Retrospective, observational, longitudinal study | Chronic cardiac disease | 133 (22.62) | OR 1.956 (0.778-4.922) | In-hospital death | Age, gender, Chest X-ray score, hydroxychloroquine, tocilizumab, lopinavir/ritonavir, cyclosporine A, Glucocorticoids, Lymphocyte count at admission, Ferritin at admission, C-reactive protein at admission, lactate dehydrogenase (LDH) at admission, d-dimer at admission, Creatinine at admission, arterial hypertension, diabetes mellitus, chronic respiratory disease, PaO2/FiO2 | 7 |
| Izzy et al.* (2020) [124] | USA | 5190 | 52 (36–66) | 2378 (46) | NR | Coronary artery disease | 257 (5) | OR 0.52 (0.323–0.835) | ICU Admission | Age, gender, smoking status, last BMI, comorbidities (diabetes mellitus, hyperlipidemia, HTN, obstructive lung disease, interstitial lung disease, cerebrovascular disease, obstructive sleep apnea, CKD, transplantation, auto-immune diseases, malignancy), total comorbidities (0, 1–2, >2) | 8 |
| Chow et al. (2020) [125] | USA | 412 | 55 (41-66) | 244(52.9) | Retrospective, observational cohort study | CAD | 52 (12.62) | HR 1.91 (1.06-3.42) | In-hospital death | Age, gender, BMI, Ethnicity (African American, Asian, Hispanic/Latino), HTN, DM, renal disease, aspirin use | 6 |
| Raines et al. (2020) [126] | USA | 440 | 60.8±14.07 | 393 (89.32) | Retrospective | CVD | 364 (82.73) | OR 0.9 (0.47-1.73) | Mortality | Age, gender, race, BMI, immunodeficiency syndromes, pulmonary diseases, oncologic diseases, gastrointestinal diseases, renal diseases, hematologic diseases, endocrine diseases, neurologic problems, lifetime tobacco user | 7 |
| Ramos-Rincon et al. (2020) [123] | Spain | 2772 | 86.3 (83.2-89.6) | 1367 (49.4) | Nationwide, multicenter, retrospective, observational study | CVD | 855 (30.8) | OR 1.22 (0.96-1.54) | Mortality | Age, gender, degree of dependence (independent or mild, moderate, Severe), comorbidities ( Charlson comorbidity Index, non-atherosclerotic cardiovascular disease, atherosclerotic cardiovascular diseases, dementia, obesity, moderate-severe renal disease), symptoms (shortness of breath, anorexia, diarrhea), physical exam (Oxygen saturation < 90% (pulsi oximetry), temperature 37.8 ºC, HTN (systolic blood pressure<100 mmHg), tachycardia (>100 beats per minute), Tachypnoea (20 breaths per minute), confusion, pulmonary rales, qSOFA score 2 (high risk)), chest X-ray (normal, unilateral infiltrates, bilateral infiltrates), laboratory findings (leukocytes 10.0 x103/L, neutrophils 7.5 x103/L, Lymphocytes<0.800 x103/L, monocytes<0.500 x103/L, pH<7.40, PO2, PO2/FiO2 ratio < 200, glucose > 126 mg/dL, eGFR < 45ml/min/1.73m2, lactate dehydrogenase 500 U/L, AST,ALT, CRP, venous lactate, procalcitonin, interleukin-6, d-dimer, serum ferritin) | 6 |
| Zhang et al. (2021) [127] | China | 222 | 51.5 (34.0-65.3) | 90(40.54) | NR | Chronic cardiovascular disease | 44 (19.82) | HR 3.616 (1.111-11.776) | Mortality | Dyspnea, pharyngalgia, COPD, elevated myocardial enzymes, acute liver dysfunction, acute kidney injury | 6 |
| de Souza et al. (2020) [128] | Brazil | 9807 | 70.21±8.37 | 4662 (47.5) | Retrospective population-based study | CVD | 1192 (12.2) | OR 1.15 (0.95–1.39) | Mortality | Age, gender, initial symptoms reported (initial symptoms reported, fever, fatigue, headache, myalgia, odynophagia, dyspnea, diarrhea), comorbidities (diabetes, HTN, chronic lung disease, chronic kidney disease, obesity) | 8 |
| Kolhe et al. (2020) [129] | UK | 1161 | 72.1±16.0 | 657 (56.59) | Retrospective cohort study | Congestive cardiac failure | 207 (17.83) | OR 1.38 (0.95-1.99) | Mortality | Age, gender, ethnicity (White, Asian, Black, mixed, others, not stated), cerebrovascular disease, Dementia, chronic lung disease, connective tissue disorder, Diabetes with complication, paraplegia, chronic kidney disease, chronic liver disease, Cancer, treatment (ACEI or ARB use, ACEI or ARB use), AKI | 8 |
| Kim et al. (2021) [130] | USA | 10861 | 65 (54-77) | 6468(59.6) | NR | CAD | 1447 (13.3) | OR 1.02 (0.90-1.17) | Death | Age, gender, race/ethnicity, BMI, HTN, DM,CKD, end stage renal disease, cancer, asthma, COPD, smoking status, hospital type | 6 |
|
Giustino et al. (2020) [131] |
New York City & Milan | 305 | 63 (53–73) | 205 (67.2) | International, multicenter cohort study | Heart failure | 24 (7.9) | OR 5.38 (1.65-17.54) | In-Hospital Death | Age, Hispanic ethnicity, history of heart failure, cardiocirculatory shock, acute respiratory distress syndrome, acute kidney injury stage II or III, no cardiac injury (No cardiac injury vs cardiac injury with echocardiographic abnormalities) | 7 |
| An et al. (2020) [132] | Korea | 228 | 44.97±19.79 | 107 (46.9) | Cohort study | CVD | 70 (30.7) | HR 1.23 (0.89-1.70) | Mortality | Age, gender, income level, residence, household type, disability, symptom, infection route, underlying medical condition (none, HTN, diabetes mellitus, hyperlipidemia, cerebrovascular disease, cancer, chronic lung disease or asthma, chronic renal disease, mental illness, chronic liver disease) | 6 |
| Piazza et al. (2020) [133] | USA | 1114 | 50.6±18.3 | 511 (45.9) | Retrospective observational cohort analysis | CAD | 90 (8.1) | OR 1.09 (0.38–3.16) | Death | Major arterial or venous thromboembolic event (Age, gender, VTE prophylaxis, ARDS, d-dimer (decile)) | 7 |
| Rao et al. (2020) [134] | China | 240 | 48 (23–87) | 111 (46.250 | Retrospective cohort study | CVD | 43 (17.9) | OR 3.326 (0.721-15.336) | Severe pneumonia | Age | 7 |
| Tehrani et al. (2021) [136] | Sweden. | 255 | 66±17 | 150 (59) | Retrospective analysis | Chronic heart failure | 34 (13) | OR 1.01 (0.42-2.42) | Death | Age, HTN, chronic kidney disease, previous stroke | 8 |
| Hyman et al. (2020) [137] | USA | 755 | 63±13 | 483 (64.0) | Retrospective cohort study | Congestive heart failure or valve disorder | 30 (4.3) | HR 1.39 (0.87–2.23) | Mortality | Hospital site, baseline demography, preexisting comorbidities, laboratory findings at admission, maximum vital sign values | 7 |
| Hamilton et al. (2020) [138] | UK | 1032 | 71 (56–83) | 569 (55.1) | Retrospective review | Congestive Heart Failure | 129 (12.5) | HR 2.01 (1.51-2.67) | Mortality | AKI, cancer, other ethnicity, diabetes, gender, RAASi, race, dementia, myocardial infarction, age | 6 |
|
Liu et al. (2020) [139] |
China | 774 | 64 (54–73) | 452 (58.4) | Multicenter retrospective observational study | Chronic cardiac disease | 91 (11.8) | HR 1.12 (0.68–1.84) | Mortality | Time-varying exposure, age, gender, APACHE II score, COPD, diabetes, HTN, chronic kidney disease, chronic liver disease, stroke, malignancy, immunosuppression, fever at admission, systolic pressure at admission, leukocytes, hemoglobin, platelets, lymphocytes, d-dimer, total bilirubin, serum creatinine, procalcitonin, corticosteroids, corticosteroids, human immunoglobulin | 8 |
| Ganatra et al. (2020) [140] | USA | 2467 | 59 (18–101) | 1032 (42) | Retrospective study | CAD | 184 (7.0) | OR 0.92 (0.66–1.27) | Severe disease | Age, prior/current smoker, β-blockers, history of cancer, gender, diabetes mellitus, ACEi or ARB, HTN, COPD, CKD | 4 |
| Rubio-Rivas et al. (2020) [141] | Spain | 12066 | 68 (56–79) | 7052 (58.5) | Cohort study | Chronic heart failure | 809 (6.7) | OR 1.16 (1.02–1.32) | In-hospital mortality | Age, gender, BMI, clusters, comorbidity (Arterial hypertension, diabetes mellitus, hyperlipidemia, hyperlipidemia, chronic kidney disease, chronic hepatopathy, active cancer), Charlson’s index, heart rate upon admission, respiratory rate upon admission > 20 bpm, PaO2/FiO2 upon admission, lab test upon admission (CRP mg/L, LDH U/L), treatments during admission (Redeliver, tocilizumab, corticosteroids) | 9 |
| Mendes et al. (2020) [142] | Switzerland | 235 | 86.3±6.5 | 102 (43.4) | Retrospective monocentric cohort study | Heart failure | 66 (28.1) | OR 1.51 (0.95-2.40) | Mortality | Gender | 6 |
| Nemer et al. (2020) [143] | USA | 350 | 64±16 | 194 (55) | Prospective | Congestive heart failure | 42 (12) | OR 0.76 (0.17-3.39) | Primary composite outcome was defined as death, ICU transfer, or increased oxygen requirement. | Age, BMI, COPD, peripheral oxygen saturation on room air, CRP, lactate dehydrogenase level, abnormal troponin T level, abnormal d-dimer level, Abnormal chest x-ray findings | 8 |
| Guo et al. (2020) [144] | China | 350 | 43(32–56) | 173(49.4) | Retrospective, multicenter study | CVD | 15 (4.3) | OR 1.81 (0.42–7.84) | Severe COVID-19 | Age, gender, Wuhan exposure, family cluster case, smoking, comorbidity (HTN, diabetes, chronic kidney disease, chronic liver disease, cerebral infarction) | 6 |
| Hilbrands et al. (2020) [145] | Netherlands | 305 | 60±13 | 189(62) | Observational study | Heart failure | 64 (21) | OR 1.39 (1.02–1.89) | 28-day case-fatality | Age, gender | 5 |
| Wang et al. (2020) [146] | China | 7283 | 64 (53–71) | 3732 (51.2) | Retrospective observational study | CVD | 161 (2.2) | HR 1.83 (1.33-2.51) | Death | Age, gender, location (central area in Wuhan, Other areas), occupation (medical workers, retirees, others), diabetes, HTN, respiratory disease, number of symptoms at admission, date of onset (Dec 2019–9 Jan 2020, 10–22 Jan 2020, 23 Jan–1 Feb 2020, 2–25 Feb 2020) | 9 |
| Tang et al. (2020) [147] | USA | 752 | 73.9 (21.9-105.4) | 323 (43) | Cohort study | Coronary heart disease | 240 (31.91) | HR 0.83 (0.58-1.19) | Death | Age, gender, race, and facility | 8 |
| Annweiler et al. (2020) [173] | France | 77 | 88 (85−92) | 39 (50.6) | Retrospective quasi-experimental study | Cardiomyopathy | 42 (54.5) | HR 4.04 (0.81-20.30) | 14-day mortality | Age, gender, Iso resource groups score, severe undernutrition, history of cancer, history of HTN, glycated hemoglobin, number of acute health issue, use antibiotics, use of systemic corticosteroids, use treatments of respiratory disorder | 5 |
| Huang et al. (2020) [148] | China | 676 | 56.0 (39.0–68.0) | 314 (46.4) | Retrospective study | Heart Disease | 71 (10.5) | HR 1.40 (0.76–2.47) | Hospital mortality | Age, gender, HTN, Diabetes, cancer, d-dimer, CRP, PCT, LDH | 6 |
| Poterucha et al. (2021) [149] | USA | 887 | 64.1 | 513 (58) | Retrospective study | CAD | 104 (12.0) | HR 1.56 (1.04-2.33) | Mortality | AF/AFL, QRS abnormality, ST-T wave abnormality, Initial hs-cTnT ≥ 20 ng/L, age, gender, Hypertension, Diabetes, CKD, primary lung disease, Obesity, HFrEF, HFpEF, active cancer, history of cancer | 6 |
| Li et al. (2020) [150] | China | 100 | 62.0 (51.0–70.8) | 56 (56.0) | NR | CVD | 15 (15.0) | HR 3.73 (0.41–33.84) | Cardiac damage | Age, gender, Hypertension, diabetes, hyperlipidemia, white blood count, prothrombin time, d-dimer, creatinine interleukin-6, procalcitonin, hs-CRP | 6 |
| Prado-Galbarro et al. (2020) [151] | Mexico | 9487 | 31.37 (41.13-51.18) | 5050 (53.2) | Observational study | CVD | 171(1.8) | HR 0.85 (0.67-1.06) | Mortality | Age, gender, indigenous ethnicity, pneumonia, COPD, diseases associated with immunosuppression, additional comorbidity (Chronic diseases interaction, HTN, diabetes, obesity, chronic kidney disease, intensive care unit), region, density, mode of transport (driving, public transport, walking) | 8 |
| Shah et al. (2020) [152] | USA | 487 | 68.53±16.66 | 273 (56.06) | Retrospective review | Cardiomyopathy | 16 (3.28) | OR 3.33 (1.07-10.41) | Mortality | Age, gender, patient admitted from home, PMH HTN, PMH hyperlipidemia, PMH A. fib, , PMH CVA, PMH diabetes, PMH dementia, PMH active cancer, AKI, Dyspnea in ED noted as positive, initial CXR/CT findings | 7 |
| Botta et al. (2021) [153] | Netherlands | 553 | 67.0 (59.0–73.0) | 417 (75) | National, multicenter, observational cohort study | Heart failure | 25 (5.0) | OR 0.73 (0.26-2.08) | 28-day mortality | Ventilatory variables on day 0 (positive end-expiratory pressure, tidal volume, respiratory system compliance), PaO2/FiO2, laboratory tests on day 0* pH, Lactate, Creatinine), vital signs on day 0 (Heart rate, mean arterial pressure), organ support on day 0 (use of vasopressor, fluid balance), demographic characteristics (age, gender, BMI, HTN, diabetes, chronic kidney disease, COPD, use of angiotensin-converting enzyme inhibitor, use of angiotensin II receptor blocker) | 6 |
| Di Domenico et al. (2020) [154] | France | 310 | 64 (52–76) | 200 (64.5) | Single‑center retrospective study | Heart disease | 50 (16.2) |
HR 1.921 (0.893-4.135) |
Death | Age, diabetes, HTN, CKD, obesity, vascular disease, ever been a smoker | 7 |
|
Ayaz et al. (2020) [155] |
Pakistan | 66 | 50.6±19.1 | 40 (61) | Retrospective cohort study | Ischemic heart disease | 10 (15) |
OR 26.5 (4.7–147.8) |
Mortality | Age, diabetes, HTN, ICU admission, mechanical ventilation, bilateral infiltrates on chest radiography, neutrophil to lymphocyte ratio ≥3.3, INR ≥1.2 | 6 |
| Hippisley-Cox et al. (2020) [156] | UK | 8275949 | 48.47±18.41 | 4115973 (49.73) | Prospective cohort study | CVD | 433631 (5.24) |
HR 0.85 (0.66-1.10) |
Admission to ICU | ACE inhibitor, angiobrnsin enzyme blocker, gender, material deprivation, ethnicity, geographical region, smoking status, BMI, chronic renal disease, atrial fibrillation, type 1 diabetes, type 2 diabetes, hypertension, asthma, COPD, Beta-blockers, calcium channel blockers, other diabetes drugs, sulfonylureas, biguanides, anticoagulants, antiplatelets, statins, statins, potassium-sparing diuretics | 9 |
| Tomasoni et al. (2020) [157] | Italy | 692 | 66.5±13.3 | 415 (68.9) | Multicenter study | CAD | 148 (21.4) | HR 1.20 (0.67-2.14) | In-hospital mortality | Age, gender, smoker, HTN, hyper dyslipidemia, Diabetes, atrial fibrillation, COPD, CKD, Treatment before hospitalization (ACE-i/ARBs/ARNI, mineralocorticoids, Beta-blockers, direct oral anticoagulants, warfarin, Statins), baseline findings (heart rate, Oxygen saturation), laboratory measurements (PaO2/FiO2, red blood cell count, hemoglobin, hematocrit, lymphocytes count, platelets count, creatinine, eGFR (CKD-EPI), CRP on admission, procalcitonin, troponin, NT-proBNP, d-dimer, aspartate transaminase, albumin, international normalized Ratio) | 7 |
| Elmunzer et al. (2020) [158] | North American | 1846 | 59.9±16.4 | 1044 (56.6) | Large-scale retrospective cohort study | Congestive Heart Failure | 284 (15.4) |
OR 1.60 (1.12-2.28) |
Death | H2RA Use, PPI Use, age, gender, race, dementia, number of comorbidities, WBC at admission, platelets at admission, AST at admission, albumin at admission | 6 |
| Polverino et al. (2020) [159] | Italy | 3179 | 2171 (68.3) | Nationwide observational study | Coronary artery disease | 359 (11.3) | OR 1.11 (0.83-1.49) | Death | Age, gender, atrial fibrillation, blood cancer, COPD chronic renal failure, diabetes, HTN, obesity, organ cancer, stroke | 5 | |
| Sharp et al. (2020) [160] | USA | 21280 | 50 (34-66) | 9053 (42.5) | Retrospective cohort study | Congestive Heart Failure | NA (NA) |
OR 1.45 (1.18–1.77) |
Adverse outcomes (death, ARDS, respiratory failure and septic shock during hospitalization, mechanical ventilation, ICU admission, as well as clinical cure and discharges) | Age, gender, BMI, coagulopathy, diabetes, fluid and electrolyte disorders, other neurological disorders, weight Loss, heart rate, systolic BP, oxygen saturation, respiratory rate | 8 |
|
Stebbing et al. (2020) [161] |
Italy&Spain | 166 | 74.05±13.06 | 85 (51.2) | Observational studies | CVD | 48 (28.9) |
HR 1.41 (0.68-2.92) |
Death & admission to ICU | Age, gender, HTN, diabetes, chronic Obstructive Lung disease, cronic kidney disease, Solid cancer, Charlson Comorbidity Index, baseline PaO2/FiO2, lymphocyte count (/mcL), alanine aminotransferase, hydroxychloroquine, lopinavir/ritonavir, glucocorticoids, low molecular weight heparin, antibiotics | 6 |
| Fu et al. (2020) [162] | China | 355 | 43.5* | 193 (54.37) | Hospital-Based Retrospective Cohort Study | Heart disease | 20 (6.2) | OR 0.454 (0.102-2.010) | Myocardial injury | Age, gender, HTN, diabetes | 7 |
|
Sheshah et al. (2020) [163] |
Saudi Arabia | 300 | 49.7±13.2 | 259 (86.3) | Single-center, retrospective study | Coronary Artery Disease | 10 (3.3) | OR 19.4 (1.5-260) | Mortality | Age, gender, HTN, type 2 diabetes mellitus, chronic kidney disease, acute kidney injury, stroke, methylprednisolone, dexamethasone, hydroxychloroquine, azithromycin | 6 |
| Bowe et al. (2020) [164] | USA | 5216 | 70 (61–76) | 4908 (94) | Cohort study | CVD | 1588 (30.0) | OR 0.87 (0.76-1.01) | Severe AKI | Age, gender, race, Smoking status, HTN, diabetes mellitus type 2, ACEI/ARB, diuretics, anticoagulant, immunosuppressants, b-blocker, aspirin, eGFR category | 8 |
| Cheng et al. (2020) [165] | China | 220 | 59.5 (48.3-70.0) | 106 (48.2) | Retrospective, observational study | CAD | 22 (10.0) | HR 0.97 (0.35-2.68) | In-hospital death | Hypertension, history of cerebrovascular disease, History of diabetes mellitus, history of diabetes mellitus | 4 |
| Neumann-Podczaska et al. (2020) [166] | Poland | 50 | 74.8±9.4 | 35 (70.0) | Retrospective | Heart disease | 26 (52.0) | HR 2.61 (0.92–7.39) | 60-day mortality | Age, functional Capacity, Diabetes | 6 |
| Ken-Dror et al. (2020) [167] | UK | 429 | 70±18 | 242 (56.4) | Prospective cohort study | Chronic cardiac disease/congenital heart disease | 103 (31.3) | OR 3.43 (2.1-5.63) | Mortality | Self-reported feverishness 38°C, cough self-report, oxygen saturation, history of fever, cough, sore throat, chest pain, muscle aches myalgia, altered consciousness confusion, obesity as defined by clinical staff, diabetes with complications, dementia, malnutrition, current admission to ICU/IMC/HDU, non-invasive ventilation BIPAP/CPAP, invasive ventilation, high flow nasal canula oxygen therapy, clinical pneumonia, inotropes vasopressors, viral pneumonia, bacterial pneumonia, anemia | 7 |
| Iannelli et al. (2020) [168] | France | 8286 | 59.1±12.6 | 4296 (51.8) | Retrospective | Cardiac failure | 569 (6.9) | OR 1.53 (1.24–1.89) | Death | Age, gender, cancer, diabetes, bariatric surgery | 9 |
| Sharifpour et al. (2020) [169] | USA | 268 | 63±15 | 149 (55.6) | Cohort analysis | CAD | 36 (13.4) | OR 1.381 (0.498–3.826) | Mortality | Age, CRP Slope d1to7, CRP tests (count d1 to 7), CRRT, CRP Max d1to7, obesity (BMI> = 30kg/m2), intubation, SOFA score, HTN | 6 |
| Martins-Filho et al. (2020) [170] | Northeast Brazil | 1207 | 60 (46–73) | 724 (60) | Retrospective cohort study | Heart failure | 102 (8.45) |
OR 2.00 (1.31–3.04) |
Mortality | Infectious disease, kidney disease, age | 6 |
| Lee et al. (2020) [171] | Korea | 7339 | 47.1±19.0 | 2970 (40.1) | Nationwide Population-Based Retrospective Study | CVD | 455 (6.1) | OR 0.95 (0.64–1.40) | Death | Influenza, tuberculosis, COPD, pneumonia, asthma, DM, CKD, Chronic liver disease, HTN, malignancies, HIV infection, lopinavir/ritonavir, Hydroxychloroquine, ribavirin, type I interferon, Human immunoglobulin G, Oseltamivir, antibiotics, age, gender | 8 |
| Loffi et al. (2020) [172] | Italy | 1252 | 64.7±15.5 | 798 (63.74) | Retrospective, observational, single-center study | CAD | 124 (9.9) | HR 1.14 (0.79-1.63) | Death | Age, gender, LVEF<35%, CVA, atrial fibrillation, diabetes mellitus, hypertension, smoking, CKD | 5 |
| Grodecki et al. (2021) [175] | USA | 109 | 63.74±15.11 | 68 (62.39) | Prospective | Heart failure | 16 (14.68) | OR 3.5 (1.1-8.2) | Death | Age, gender, diabetes mellitus, hypertension, smoking history, chronic lung disease, history of coronary artery disease, epicardial adipose tissue volume (mL), epicardial adipose tissue attenuation, total pneumonia burden | 7 |
| Rossi et al. (2020) [80] | Italy | 590 | 76.2 (68.2–82.6) | 399 (67.6) | Retrospective observational study | CVD | 95 (16.1) | HR 1.180 (0.855–1.628) | Mortality | Age, gender, vital signs at admission (temperature, PaO2/FiO2, PaO2/FiO2<300), laboratory parameters (LDH, CRP, white blood cell count, lymphocyte’s rate), chronic diseases (hyperlipidemia, diabetes, atrial fibrillation, COPD, CKD, stroke, malignancy, 3 or more comorbidities), chronical drugs intake (ACEi, ARBs, CCBs, Alpha blockers, Diuretics, Beta blockers) | 6 |
| Khan et al. (2020) [177] | Saudi Arabia | 648 | 34±19 | 342 (52.8) | Retrospective cohort study | Cardiac diseases | 23 (3.5) | OR 3.05 (1.16-8.02) | ICU admission | Age, gender, smoker, comorbidities (one or more comorbidity, two or more comorbidity, diabetes mellitus, HTN, CRD, chronic kidney diseases, cancer/immunodeficiency), symptoms (fever, cough, sore throat, runny nose, headache, GI symptoms, myalgia), vital signs (temperature (≥38), heart rate ≥100, respiratory rate, respiratory rate, respiratory rate, DBP, oxygen saturation, oxygen saturation) | 7 |
| Rutten et al. (2020) [178] | Netherlands | 1538 | 84±8.7 | 554 (36.02) | Prospective cohort study | CVD | 53 (3.47) | HR 1.15 (0.97-1.35) | Mortality | Age, gender, comorbidity (Dementia, cerebrovascular disease, diabetes mellitus, chronic respiratory disease, reduced kidney function, Parkinson’s disease) | 6 |
| Schuelter-Trevisol et al. (2020) [179] | Brazil | 211 | 51.2* | 113 (53.6) | Cohort study | Chronic heart disease | 27 (12.9) | OR 0.98 (0.31-3.10) | Death | Age, gender, comorbidities (arterial hypertension, diabetes mellitus, obesity, neurologic/psychiatric diseases, chronic lung diseases, dyslipidemia, smoking habits, cancer, chronic kidney diseases, vascular diseases) | 6 |
|
FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE (2020) [174] |
France | 694 | 56.1±16.4 | 232 (33.4) | Observational, multicenter, French national cohort study | Coronary heart diseases | 68 (9.8) | OR 1.86 (0.97–3.56) | Severity | Age, gender | 8 |
| Nyaberaet al. (2020) [181] | USA | 290 | 77.6±8.3 | 150 (51.7) | Single-center retrospective cohort study | CAD | 80 (27.6) | OR 0.91 (0.52-1.62) | Mortality | BMI, age, COPD, asthma, DM, HTN, end-stage renal disease | 4 |
| Ozturk et al. (2021) [182] | Turkey | 1160 | 60.5 (47–71) | 627 (54.1) | Multicenter, retrospective, observational study | CVD | NR (NR) | HR 1.242 (0.850–1.815) | Death | Age, gender, diabetes mellitus, HTN, COPD, albumin, hemoglobin, lymphocyte count, platelet count, CRP increase, clinic presentation, COVID-19 diagnosis by RT-PCR, patient group, control group (HD group, RT group, CKD group) | 5 |
| Druyan et al. (2021) [183] | Israel | 181 | 62.71* | 107(59.1) | Single center study | Heart failure | 10 (5.52) | OR 2.35 (0.24-18.64) | Severe, critical or fatal COVID19 | Gender, AID, HTN, dyslipidemia, diabetes, malignancy, IHD, arrhythmia, obesity, pulmonary disease, smoking, CVA, renal failure, older age | 5 |
| Alguwaihes et al. (2020) [184] | Saudi Arabia | 439 | 55 (19–101) | 300 (68.3) | Single-center retrospective study | CVD | 44 (10.0) | HR 1.8 (0.7–4.4) | Death | Age, gender, comorbidities (obesity, HTN, diabetes mellitus, chronic kidney disease, congestive heart failure, stroke, smoking), medications (β-Blocker use, ACE inhibitor use, ARB Use), laboratory investigations (RBG, FPG, HbA1c>9.0%, bilateral lung infiltrates, neutrophil count>7.5, creatinine>90 μmol/l, ALT>65 U/l, 25(OH)D<12.5 nmol/l) | 7 |
|
Özdemir et al. (2021) [185] |
Turkey | 101 | 49.60±18 | 55 (54.4) | Retrospective study | Chronic heart failure | 10 (9.9) | HR 1.02 (0.98 – 1.10) | QTc prolongation | Baseline QTc, HCQ alone, HCQ + AZM | 6 |
| Gue et al. (2020) [186] | UK | 316 | 73.42±15.97 | 192 (61.1) | Single-center retrospective cohort | CAD | 48 (15.19) | OR 1.62 (0.76–4.07) | 30-day mortality | Age, gender, HTN, atrial fibrillation, oral anticoagulants, modified sepsis-induced coagulopathy score | 7 |
| Galiero et al. (2020) [187] | Italy | 618 | 65±15.2 | 379 (61.3) | Multicenter retrospective observational cohort study | Chronic Cardiac Disease | 166 (26.9) | OR 0.96 (0.53-1.76) | Mortality | Age, gender, Glasgow Coma Score/15, respiratory severity Scale, CKD, CLD, chronic respiratory disease, malignancies | 6 |
| Rosenthal et al. (2020) [188] | USA | 64781 | 56.1±19.9 | 31968 (49.3) | Retrospective cohort study | Myocardial infarction | 3717 (5.7) | OR 1.47 (1.34-1.62) | In-Hospital Mortality | Age, gender, race, payer type, admission point of origin, hospital region, hospital beds, hospital teaching status, hospital teaching status, Sepsis, acute kidney failure, hypokalemia, acidosis, acute liver damage, neurological disorder, baseline comorbidities (Cerebrovascular disease, COPD, dementia, diabetes, any malignant neoplasm, metastatic solid tumor, hemiplegia, AIDS, HTN, Hyperlipidemia) | 9 |
| Rethemiotaki et al. (2020) [189] | the World Health Organization dataset and Chi nese Center for Disease Control and Preventio | 44672 | 71* | 22981 (51.44) | NR | CVD | 92 (15.9) | OR 13.6 (10.3–17.9) | Death | Age, gender, occupation (service industry, farmer/laborer, health worker, retiree, other/none), province: (Hubei, Other), Wuhan-related exposure, comorbid condition (HTN, diabetes, chronic respiratory disease, cancer (any), none) | 8 |
| Pantea Stoian et al. (2020) [176] | China | 432 | NR | NR | Multiple-case, multiple-center | Heart failure | 30 (6.94) | OR 2.990 (1.612–5.546) | Death | Age, gender, HTN, obesity, diabetes type 2, dialysis, chronic kidney disease, COPD, supraventricular tachyarrhythmia, respiratory failure, Intercept | 7 |
| Zhou et al. (2020) [191] | China | 134 | 62.08±14.38* | 85 (63.4) | Retrospective | Coronary heart disease | 16 (11.94) | OR 1.098 (0.202–5.959) | Death | Gender, age, HTN, coronary heart disease, neutrophil, lymphocyte, ALT, IL-2, IL-6, TNF-α, D-dimer, and total CT score | 6 |
| Stefan et al. (2021) [192] | Romania | 37 | 64 (55–71) | 19 (51) | Retrospective, observational, single-center study | Coronary heart disease | 19 (51.0) | HR 0.98 (0.05–17.54) | In-hospital death | Age, hemodialysis vintage, obesity, current smoker, diabetes mellitus, Charlson comorbidity index, basal oxygen saturation, hemoglobin, lymphocytes, CRP, serum albumin, LDH, Lopinavir–ritonavir, Tocilizumab, hydroxychloroquine, glucocorticoids | 7 |
| Ahnach et al. (2021) [180] | Morocco | 101 | 50 (32–63) | 75 (51.72) | Retrospective study | CVD | 16 (11.03) | OR 3.74 (0.76–18.29 | Disease severity | Age, gender, HTN, diabetes, other disease, respiratory symptom, neutrophil, lymphocyte, eosinophil, CRP | 6 |
| Eshrati et al. (2020) [193] | Iran | 3188 | 55.05 ± 0.31 | 1925 (60.4) | Retrospective cohort study | CVD | 401 (12.6) | HR 0.60 (0.83-1.13) | death | Age, gender, immune disease, diabetes, liver disease, kidney disease, ,COPD, cancer, chronic nervous disease, type of treatment | 8 |
| Özyılmaz et al. (2020) [194] | Turkey | 105 | 45 (20–87) | 76 (72.3) | Single-center, retrospective, observational study | CAD | 14 (13.3) | OR 0.024 (0.000–1.207) | Mortality | Troponin I, C-Reactive protein, lymphocyte count, shortness of breath, HTN, hyperlipidemia, diabetes mellitus | 7 |
| Tan et al. (2020) [195] | China | 163 | 69.0 (62.0-78.0) | 109 (66.9) | Retrospective study | Chronic cardiac injury | 25 (15.3) | OR 2.660 (1.034-6.843) | Mortality | Age, gender, HTN, diabetes | 5 |
| Ling et al. (2020) [196] | UK | 444 | 74 (63-83) | 245 (55.2) | Cross-Sectional Multi-Centre Observational Study | Heart failure | 54 (12.2) | OR 1.61 (0.87–2.99) | Mortality | Age, gender, diabetes, non-Caucasian ethnicity, baseline serum 25(OH)D levels, vitamin D deficiency, treatment with cholecalciferol booster therapy, admission SpO2 < 96%, admission CRP > 73 mg/L, admission creatinine > 83 μmol/L, received CPAP, length of stay >11 days, diabetes (types 1 and 2 combined), admission glucose > 6·9 mmol/L, COPD, asthma, IHD, current or previous ACS, HTN, current or previous TIA or stroke, dementia, obesity, malignancy of solid organ, malignancy of skin, hematological malignancy, solid organ transplant, inflammatory arthritis, inflammatory bowel disease | 5 |
| Zhong et al. (2020) [197] | China | 126 | 66.3±10.6 | 56 (44.4) | Retrospective observational study | CVA | 21 (16.7) | OR 2.03 (0.45-9.08) | Death | Age, gender, ACEI/ARB, stains | 5 |
| Izurieta et al. (2020) [198] | USA | 12613 | 80.5* | 6496 (51.5) | Retrospective cohort study | Congestive Heart Failure | 3557 (28.2) | OR 1.30 (1.23, 1.36)) | Death | Age, gender, reason for entering medicare, ADI national rank, logged COVID-19 circulation rate by 100,000, logged population density by county, vaccination, presence of medical conditions (HTN, obesity, diabetes, hospitalized stroke/TIA, coronary revascularization, atrial fibrillation, hospitalized AMI, other cerebrovascular disease, COPD, asthma without COPD, interstitial lung disease, hypersensitivity pneumonitis, bronchiectasis, chronic liver disease, neurological/neurodevelopmental conditions), frailty conditions, immunocompromised status, estimated overall, interaction effects of age, dual-eligibility, and race, 80 years old vs. 65 years old, dual-eligible vs. non-dual-eligible, dual-eligible vs. non-dual-eligible, effects of being dual-eligible, by race, non-whites vs. whites, non-dual-eligible, non-whites vs. whites, dual-eligible | 8 |
| Burrell et al. (2021) [199] | Australia | 304 | 63.5 (53–72) | 140 (69%) | Prospective, observational cohort study | Chronic cardiac disease | 40 (20) | HR 3.38 (1.46–7.83) | Mortality | Age, gender, APACHE-II score on ICU day 1, comorbid conditions (comorbid conditions), | 5 |
| Li et al. (2020) [190] | China | 123 | 64.43±14.02 | 62 (50.41) | Retrospective study | CVD | 26 (21.14) | OR 0.686 (0.227–2.076) | Unfavorable clinical outcomes | Age, gender, diabetes, HTN, COPD, CT severity score, GGO volume, GGO volume percentage, consolidation volume, consolidation volume percentage | 4 |
| Caliskan et al. (2020) [200] | Turkey | 56 | 48±19.664 | NR | Retrospective observational study | CAD | 42 (7.4) | OR 6.252 (2.171-18.004) | Mortality | Former smoker, current smoker, age, COPD, diabetes, dementia, HTN, chronic renal failure, arrhythmia | 5 |
| Vafadar et al. (2021) [201] | Iran | 219 | 57.8±16.5 | 137 (62.6) | Retrospective cohort | Ischemic heart disease | 46 (22.37) | HR 1.98 (0.94–4.17) | Mortality | Respiratory rate, SpO2 ≤ 90%, WBC count, NLR, age | 6 |
| Working group for the surveillance and control of COVID-19 in Spain et al. (2020) [202] | Spain | 2612 | 83 (75–89) | 14680 (56.2) | NR | CVD | 11444 (59.9) | OR 1.32 (1.23-1.42) | Death | Gender, age, pneumonia, acute respiratory distress syndrome, acute renal failure, Diabetes, HTN, chronic lung disease, chronic renal disease, healthcare worker | 6 |
| Rashidi et al. (2021) [203] | Iran, Germany, USA | 1529 | 56 (32–80) | 832 (54.4) | Multi-center prospective study | Cardiac disease | 149 (9.7) | OR 0.80 (0.36–1.76) | Death | Age, gender, recent cancer, COPD, CKD, smoking, diabetes mellitus, HTN | 5 |
| Chaudhri et al. (2020) [204] | USA | 317 | 59.16±17.5 | 166 (52.37) | Single-center cohort study | Coronary artery disease | 27 (12) | OR 0.92 (0.39-2.17) | Key outcomes | Age, gender, history of ARB use,history of ACEI use, HTN, diabetes, CKD | 5 |
| Huh et al. (2021) [205] | South Korea | 219961 | 49.4 (18–116) | 104331 (47.4) | Retrospective case-control study | Chronic heart disease | 32457 (14.76) | OR 1.31 (1.04-1.65) | The requirement of any one of the following or death: supplementary oxygen, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation, and extracorporeal membrane oxygenation | Drugs commonly used for chronic conditions (angiotensin receptor blockers, angiotensin converting enzyme inhibitors, metformin, thiazolidinedione, Statins, NSAIDs), drugs with potential therapeutic effect, drugs with potential therapeutic effect, comorbidities (Charlson comorbidity index, mean (SD), Diabetes, HTN, chronic lung disease, asthma and allergic rhinitis, chronic liver disease, Chronic kidney disease, Malignancy, RA, SLE, GCA, and JIA, other connective tissue disease, chronic neurologic disease, Pancreatitis), healthcare utilization | 8 |
| Orioli et al. (2021) [206] | Belgium | 73 | 69±14 | 48 (66.67) | Retrospective study | CVD | 32 (43.8) | HR 3.54 (1.60-7.82) | In-hospital death | Diabetes, cognitive impairment, area of lung injury >50% | 6 |
| Gude-Sampedro et al. (2021) [207] | Spain | 10454 | 58.0±20.0 | 4172 (39.9) | Retrospective cohort study | Ischemic heart disease | OR 1.61 (1.20-2.33) | Death | Age, gender, lymphoma/leukemia, dementia, COPD, diabetes, chronic kidney disease | 9 | |
|
Monteiro et al. (2020) [208] |
USA | 112 | 61 (45–74) | 74 (66) | Retrospective, observational cohort study | CAD | 17 (15) | OR 0.48 (0.08–3.08) | Requiring mechanical ventilation | Age, gender, past medical history (obesity, diabetes, HTN, CKD), Tobacco exposure history | 4 |
| Lano et al. (2020) [209] | France | 122 | 73.5 (64.2–81.2) | 79 (65) | Observational cohort multicenter study | Congestive heart failure | 13 (11) | OR 1.222 (0.309–4.649) | Mortality | Age, atrial fibrillation, ARBs (current medication) | 8 |
| Lanini et al. (2020) [210] | Italy | 379 | 61.67±15.60 | 273 (72.03) | Longitudinal cohort study | CVD | 19 (5.01) | OR 2.79 (1.29-6.03) | Death | Age, gender, diabetes, neoplasm, obesity, chronic renal failure, COPD | 4 |
|
Schwartz et al. (2020) [212] |
Canada | 56606 | 31* | 29205 (51.59) | Cross-sectional study | CVD | 4465 (7.89) | OR 1.10 (0.99–1.22) | Death | Healthcare worker, age, comorbidities (asthma, COPD, renal conditions, diabetes, immune compromise or cancer, obesity, other medical conditions None), exposed to long-term care home, symptoms (fever and/or cough, other symptoms, missing, asymptomatic) | 9 |
| Sun et al. (2021) [213] | China | 3400 | 61 (50-68) | 1649 (48.5) | Retrospective cohort study | CVD | 343 (10.1) | OR 2.85 (1.65-4.94) | Death | Comorbid conditions (Neither HTN nor T2DM, Hypertension alone, T2DM alone, HTN and T2DM), age, gender, cerebrovascular disease, chronic kidney disease, chronic liver disease, chronic lung disease, endocrine/Immune system disease, tumor, ACEIs/ARBs treatment | 6 |
| McGurnaghan et al. (2021) [214] | Scotland | 319349 | 79.9 (71.4–85.7) | 180486 (56.5) | Cohort study | Any heart disease | 696 (64.3) | OR 2.425 (2.135–2.754) | Fatal or critical care unit-treated COVID-19 | Sociodemographic (age, gender, diabetes type, diabetes duration, care home resident, any hypoglycemia admission in past 5 years, deprivation index, ethnicity, comorbidities (any diabetic ketoacidosis admission in past 5 years, any hypoglycemia admission in past 5 years, ever admitted to hospital in past 5 years, asthma or chronic lower airway disease, neurological and dementia (excluding epilepsy),liver disease, immune disease or on immunosuppressants, any listed condition), other clinical measures (insulin pump use, flash glucose monitor use, HbA1c, BMI, systolic blood pressure, diastolic blood pressure, total cholesterol, Estimated glomerular filtration rate, albuminuria grade, retinopathy grading, tobacco smoking), drug exposures (any lipid lowering, any proton pump inhibitor, any non-steroidal anti-inflammatory drugs, any anticoagulants, Any antihypertensive, number of ATC level 3 drug classes (excluding for diabetes), number of diabetes drug classes prescribed) | 8 |
|
Cetinkal et al. (2020) [215] |
Turkey | 349 | 68.3±13.3 | 176 (50.43) | Retrospective single-center study | Heart failure | 38 (10.89) | OR 2.40 (0.82-7.01) | In-hospital mortality | Neutrophil to lymphocyte ratio, gender, age, diabetes mellitus, Use of RAAS blockers, chronic kidney disease, Smoking, COPD, d-dimer, LDH, procalcitonin, Ferritin | 6 |
| Xu et al. (2020) [216] | China | 61 | 63.62±10.78 | 33 (54.1) | Retrospective | Heart diseases | 7 (11.5) | OR 2.94 (0.42-21.78) | Severity | Age, gender, diabetes, HTN, hepatic dysfunction, mild-nonlung involvement | 4 |
| Lv et al. (2021) [217] | China | 409 | 50.47±12.43 | 188 (46) | Retrospective cohort Study | Heart disease | 51 (12.5) | HR 2.650 (1.079–6.510) | Death | Age, gender, fever, cough, sputum, tiredness, body aches, diarrhea, number of symptoms, HTN, diabetes, pulmonary disease, other comorbidities, CT ground-glass, opacity, CT bilateral pulmonary infiltration | 5 |
| Guerra et al. (2021) [218] | Spain | 447 | 55.0±22.5 | 190 (46.4) | Retrospective single center study | Coronary artery disease |
OR 4.95 (1.51-16.27) |
Mortality | Gender, HTN, COPD, cancer, diabetes, obesity, CLD, age | 6 |
*, studies included 2 two different cohort samples; HTN Hypertension, SOFA sequential organ failure assessment, ALT alanine aminotransferase, AST aspartate aminotransferase, ARDS acute respiratory distress syndrome, INR international normalized ratio, ICU intensive care unit, HF heart failure, IL-8 interleukin-8, AKI acute cardiac injury, CLD chronic lung diseases, CRD chronic renal disease, CKD chronic kidney disease, IL-6 interleukin-6, WBC white blood cell, NR not reported, HTN hypertension, HR hazard ratio, OR odds ratio, CI confidence interval, CHD, coronary heart disease, CVD cardiovascular disease, CAD coronary artery disease, CKD chronic kidney diseases, CLD chronic liver diseases, COPD chronic obstructive pulmonary disease, CRP C-reactive protein, hs-CRP high-sensitivity C-reactive protein, BMI body mass index, LYM% lymphocyte percentage, NEU% neutrophil percentage, NLR ratio of neutrophil to lymphocyte, FIB fibrinogen content, TBIL total bilirubin, ALB albumin, Cr creatinine, GFR glomerular filtration rate, CK-MB creatine kinase isoenzyme-MB, CT computerized tomography, PCT procalcitonin, GGO ground-glass opacity, ICI immune check point inhibitors, HCQ hydroxychloroquine, AZM azithromycin, APTT activated partial thromboplastin time, ACE angiotensin converting enzyme inhibitors, ARB angiotensin II receptor blockers, eGFR estimated glomerular filtration rate, PAD peripheral arterial disease, Hb hemoglobin, LDH lactate dehydrogenase, ESR erythrocyte sedimentation rate, MYO myoglobin, LFTs liver function tests, SABA short acting beta agonists, ESRD end-stage renal disease (on dialysis), ALC absolute lymphocyte count, ANC absolute neutrophil count, MV mechanical ventilation, APACHE II acute physiology and chronic health evaluation II, BUN blood urea nitrogen, CVA cerebrovascular accident, TIA transient ischemic attack, DBIL direct bilirubin, IBIL indirect bilirubin, PT prothrombin time, FBG fasting blood glucose.
Table 2.
The results of subgroup analysis
| Variables | Effects | NO. Of studies | Subgroup analysis | Prediction interval | |
|---|---|---|---|---|---|
| Pooled ES (95% CI) | I²,Tau², P value | ||||
| Sample size | |||||
| >=1000 | HR | 24 | 1.16 (1.03-1.32) | I² = 88%, τ² = 0.0697,P < 0.01 | 0.66-2.04 |
| OR | 53 | 1.41 (1.32-1.51) | I² = 84%, τ² = 0.0694,P < 0.01 | 0.84-2.39 | |
| <1000 | HR | 41 | 1.63 (1.41-1.88) | I² = 64%, τ² = 0.0957,P < 0.01 | 0.86-3.10 |
| OR | 83 | 1.57 (1.40-1.77) | I² = 57%, τ² = 0.0967, P < 0.01 | 0.84-2.95 | |
| Age | |||||
| >=60 | HR | 41 | 1.42 (1.25-1.61) | I² = 73%,τ² = 0.0914, P < 0.01 | 0.76-2.65 |
| OR | 78 | 1.49 (1.34-1.65) | I² = 86%, τ² = 0.1144,P < 0.01 | 0.75-2.95 | |
| <60 | HR | 23 | 1.18 (1.04-1.33) | I² = 81%, τ² = 0.0181,P < 0.01 | 0.77-1.80 |
| OR | 58 | 1.30 (1.19-1.42) | I² = 76%, τ² = 0.0379,P < 0.01 | 0.87-1.94 | |
| NR | HR | 1 | 2.59 (1.16-5.79) | - | - |
| OR | 2 | 1.75 (0.67-4.61) | I² = 88%, τ² = 0.4301,P < 0.01 | - | |
| Male (%) | |||||
| >=50 | HR | 44 | 1.41 (1.23-1.60) | I² = 83%, τ² = 0.1123,P < 0.01 | 0.71-2.80 |
| OR | 94 | 1.33 (1.23-1.44) | I² = 78%, τ² = 0.0558,P < 0.01 | 0.83-2.14 | |
| <50 | HR | 21 | 1.25 (1.13-1.38) | I² = 55%, τ² = 0.0179,P < 0.01 | 0.92-1.69 |
| OR | 36 | 1.42 (1.27-1.58) | I² = 56%, τ² = 0.0431,P < 0.01 | 0.92-2.20 | |
| NA | HR | 0 | - | - | - |
| OR | 8 | 2.25 (0.87-5.79) | I² = 98%, τ² = 1.6735,P < 0.01 | 0.08-65.97 | |
| Study design | |||||
| Retrospective/case series | HR | 38 | 1.50 (1.30-1.73) | I² = 81%, τ² = 0.1067,P < 0.01 | 0.76-2.96 |
| OR | 88 | 1.37 (1.28-1.47) | I² = 65%, τ² = 0.0269,P < 0.01 | 0.98-1.91 | |
| Prospective study | HR | 9 | 1.11 (0.74-1.67) | I² = 88%, τ² = 0.2724,P < 0.01 | 0.28-4.39 |
| OR | 7 | 1.31 (0.84-2.06) | I² = 77%, τ² = 0.2451,P < 0.01 | 0.32-5.34 | |
| Others | HR | 19 | 1.25 (1.12-1.39) | I² = 63%, τ² = 0.0214,P < 0.01 | 0.90-1.74 |
| OR | 43 | 1.45 (1.24-1.70) | I² = 93%, τ² = 0.1725,P < 0.01 | 0.62-3.42 | |
| Region | |||||
| Europe | HR | 27 | 1.31 (1.17-1.47) | I² = 83%,τ² = 0.0462, P < 0.01 | 0.83-2.08 |
| OR | 54 | 1.47 (1.33-1.64) | I² = 75%, τ² = 0.0725,P < 0.01 | 0.85-2.56 | |
| North America | HR | 12 | 1.16 (1.02-1.33) | I² = 52%,τ² = 0.0234, P = 0.02 | 0.80-1.69 |
| OR | 42 | 1.18 (1.08-1.29) | I² = 77%, τ² = 0.0333,P < 0.01 | 0.81-1.72 | |
| Asia | HR | 24 | 1.64 (1.24-2.16) | I² = 81%,τ² = 0.3015, P < 0.01 | 0.51-5.30 |
| OR | 37 | 1.55 (1.29-1.87) | I² = 68%, τ² = 0.1272,P < 0.01 | 0.73-3.29 | |
| Others | HR | 2 | 2.12 (0.89-5.01) | I² = 59%, τ² = 0.2289,P = 0.12 | - |
| OR | 5 | 3.54(0.86-14.60) | I² = 92%, τ² = 2.2249,P < 0.01 | 0.02-691.66 | |
| Disease | |||||
| CVD | HR | 27 | 1.36 (1.15-1.61) | I² = 79%, τ² = 0.1154,P < 0.01 | 0.66-2.80 |
| OR | 41 | 1.48 (1.24-1.76) | I² = 91%, τ² = 0.1984,P < 0.01 | 0.59-3.70 | |
| Cardiac disease | HR | 25 | 1.40 (1.17-1.69) | I² = 77%, τ² = 0.1141,P < 0.01 | 0.68-2.90 |
| OR | 38 | 1.43 (1.25-1.64) | I² = 84%, τ² = 0.0762,P < 0.01 | 0.80-2.55 | |
| HF | HR | 4 | 1.23 (1.05-1.44) | I² = 89%, τ² = 0.0173,P < 0.01 | 0.63-2.39 |
| OR | 31 | 1.46 (1.31-1.62) | I² = 59%, τ² = 0.0290,P < 0.01 | 1.01-2.10 | |
| CAD | HR | 9 | 1.48 (1.14-1.93) | I² = 70%, τ² = 0.0957,P < 0.01 | 0.67-3.29 |
| OR | 26 | 1.17 (1.02-1.35) | I² = 52%,τ² = 0.0416, P < 0.01 | 0.75-1.83 | |
| Others | HR | - | - | - | |
| OR | 2 | 1.63 (1.05-2.53) | I² = 33%, τ² = 0.0585,P = 0.22 | - | |
| Outcomes | |||||
| Mortality | HR | 55 | 1.39 (1.27-1.53) | I² = 76%, τ² = 0.0597,P < 0.01 | 0.85-2.30 |
| OR | 98 | 1.44 (1.32-1.56) | I² = 84%, τ² = 0.0840, P < 0.01 | 0.80-2.57 | |
| Severity | HR | 7 | 1.06 (0.70-1.60) | I² = 88%, τ² = 0.2418,P < 0.01 | 0.30-3.68 |
| OR | 25 | 1.22 (1.03-1.43) | I² = 66%, τ² = 0.0575,P < 0.01 | 0.72-2.06 | |
| Disease progression | HR | 3 | 1.65 (1.20-2.27) | I² = 0%, τ² = 0.000,P = 0.56 | 0.21-12.92 |
| OR | 15 | 1.63 (1.31-2.04) | I² = 68%, τ² = 0.0858,P < 0.01 | 0.84-2.39 | |
Note: ES, effect sizes; CI, confidence interval; OR, odds ratio; HR, hazards ratio.
Totally, our results revealed that COVID-19 patients who suffered from CVD tended more to adverse outcomes (pooled ORs = 1.41, 95% CIs: 1.32-1.51, prediction interval: 0.84-2.39; pooled HRs = 1.34, 95% CIs: 1.23-1.46, prediction interval: 0.82-2.21 Fig. 2). Subgroup analysis by sample size showed consistent results (pooled HRs = 1.16, 95% CIs: 1.03-1.32, prediction interval: 0.66-2.04; pooled ORs = 1.41, 95% CIs: 1.32-1.51, prediction interval: 0.84-2.39 for sample size >= 1000; pooled HRs = 1.63, 95% CIs: 1.41-1.88, prediction interval: 0.86-3.10; pooled ORs: 1.57, 95% CIs: 1.40-1.77, prediction interval: 0.84-2.95 for sample size < 1000; Table 2 and Fig. A1). The positive association between pre-existing CVD and adverse outcomes in COVID-19 patients was also observed in subgroup analysis by disease types (Table 2 and Fig. A2): cardiac disease (pooled HRs = 1.40, 95% CIs: 1.17-1.69, prediction interval: 0.68-2.90; pooled ORs = 1.43, 95% CIs: 1.25-1.64, prediction interval: 0.80-2.55), HF (pooled HRs = 1.23, 95% CIs: 1.05-1.44, prediction interval: 0.63-2.39; pooled ORs = 1.46, 95% CIs: 1.31-1.62, prediction interval: 1.01-2.10), and CAD (pooled HRs = 1.48, 95% CIs: 1.14-1.93, prediction interval: 0.67-3.29; pooled ORs = 1.17, 95% CIs:1.02-1.35, prediction interval: 0.75-1.83). In addition, subgroup analyses stratified by age, the proportion of males, region, disease outcomes and study design supported the above positive associations (Table 2 and Fig. A3-7). Sensitivity analysis indicated that our result was robust (Fig. 3A and B). There was no publication bias was detected by Begg’s test (OR: P = 0.233, HR: P = 0.054; Fig. 4A and B), while significant publication bias was found by Egger’s test (OR: P = 0.000, HR: P = 0.000; Fig. 4C and D). Therefore, the trim-and-fill method was adopted for further analysis. The results for HR showed that with the addition of 21 more studies, the results of the meta-analysis would be more robust but not reversed (pooled HRs = 1.11, 95% CIs: 1.01-1.14, fixed-effects model; pooled HRs = 1.16, 95% CIs: 1.06-1.26, random-effects model), and the OR results (pooled ORs: 1.18, 95% CIs: 1.16-1.20, fixed-effects model; pooled ORs: 1.21, 95% CIs: 1.12-1.30, random-effects model) showed that the results would be equally robust after adding 29 studies. However, there was high heterogeneity in our study. To find sources of heterogeneity, we conducted a meta-regression. However, adjustments for multivariate regression coefficients for sample size, age, proportion of males, study design, region, disease types, disease outcomes were not statistically significant (Table 3), suggesting that these were not sources of heterogeneity identified.
Fig. 2.
Forest plot of adjusted pooled effects for adverse outcomes associated with CVD in patients with COVID-19. A) Pooled OR; B) Pooled HR
Fig. 3.
Sensitivity analysis for pooled OR (A) and HR (B)
Fig. 4.
Publication bias for pooled OR (A and B) and HR (C and D)
Table 3.
The result of meta-regression
| Variables | HR | OR | ||||
|---|---|---|---|---|---|---|
| Tau² | t-value | P-value | Tau² | t-value | P-value | |
| Sample size | 0.0753 | -0.3248 | 0.0007 | 0.0931 | -0.1552 | 0.0449 |
| >=1000 | ||||||
| <1000 | ||||||
| Age | 0.0552 | - | 0.1123 | 0.0746 | - | 0.3495 |
| >=60 | 0.1404 | 0.1206 | 0.1006 | 0.1674 | ||
| <60 | ||||||
| NR | 0.7562 | 0.1143 | 0.1713 | 0.5027 | ||
| Male (%) | 0.0734 | 0.0351 | 0.7253 | 0.0997 | - | 0.0086 |
| >=50 | -0.0678 | 0.4355 | ||||
| <50 | ||||||
| NR | 0.4272 | 0.0119 | ||||
| Study design | 0.0774 | - | 0.0828 | 0.0796 | - | 0.8863 |
| Retrospective/case series | 0.1064 | 0.3152 | -0.0034 | 0.9647 | ||
| Prospective study | 0.1064 | 0.1628 | -0.0823 | 0.6301 | ||
| Others | ||||||
| Region | 0.0651 | - | 0.1800 | 0.0601 | - | <0.0001 |
| Europe | -0.1169 | 0.2910 | -0.0307 | 0.7439 | ||
| North America | -0.2287 | 0.0746 | -0.2362 | 0.0132 | ||
| Asia | ||||||
| Others | 0.3260 | 0.3447 | 1.3471 | <0.0001 | ||
| Disease | 0.0702 | - | 0.8655 | 0.1005 | - | 0.4005 |
| CVD | -0.1123 | 0.4286 | 0.1737 | 0.1365 | ||
| Cardiac disease | -0.0681 | 0.6418 | 0.1620 | 0.1741 | ||
| HF | -0.1221 | 0.5212 | 0.2230 | 0.0640 | ||
| CAD | ||||||
| Others | 0.82 | 0.413 | ||||
| Outcomes | 0.0694 | - | 0.0375 | 0.0810 | - | 0.1400 |
| Mortality | -0.0990 | 0.6880 | -0.1298 | 0.2733 | ||
| Severity | -0.4713 | 0.0915 | -0.2786 | 0.0528 | ||
| Disease progression | ||||||
Discussion
Many countries have been hit by the pandemic caused by SARS-CoV-2, numerous people lost their lives because of this. Meanwhile, health systems in every country were under so unprecedented strain that it was very important to find an effective marker to help implement bed grading management. What called for special attention was that earlier studies have shown COVID-19 patients with at least one underlying conditions, such as chronic kidney disease, HIV, diabetes and other comorbidities, have a poor disease course [2, 29, 211, 219, 220], which means that those patients with underlying diseases should be monitored more carefully in case of disease getting worse. Furthermore, it was reported that the risk of primary respiratory syndrome severity and adverse outcomes was increased in Middle East respiratory syndrome (MERS) patients with pre-existing CVD. The research by Li et al. [8] with unadjusted effect estimates showed that there was a positive association between CVD and adverse outcomes in patients with COVID-19, but the association might be confounded by other factors such as age, gender and comorbidities. Thus, we performed a quantitative meta-analysis on the basis of adjusted effect estimates to clarify whether pre-existing CVD was an independent risk factor associated with adverse outcomes in COVID-19 patients.
Our results based on adjusted effect estimates revealed that pre-existing CVD was significantly related to adverse outcomes in COVID-19 patients on the basis of 203 eligible studies with 24,032,712 cases. The significant association between pre-existing CVD and adverse outcomes in COVID-19 patients was still existent in further subgroup analyses stratified by the proportion of males, study design, disease types, sample size, region and disease outcomes, which suggests that our findings are relatively stable.
Similar to other meta-analyses, several limitations should be acknowledged in this present study. Firstly, data on drug and supportive treatments are not clear in the selected studies presently, thus, we could not evaluate the effects of treatments on the association between co-existing CVD and adverse outcomes in COVID-19 patients. Secondly, statistically significant results were more likely to be accepted and published than non-statistically significant results in similar studies, but in fact, the data of the meta-analysis mainly derived from the studies which have been published, which may lead to publication bias. Thirdly, the causal relationship of CVD and adverse outcomes in patients with COVID-19 cannot be confirmed on account of the inherent limitation of the observational study. Therefore, well-designed studies with larger sample sizes are needed for further verification.
Conclusions
In conclusion, our findings indicated that pre-existing CVD was an independent risk factor associated with adverse outcomes among COVID-19 patients. COVID-19 patients with a history of CVD might need more attention.
Supplementary Information
Additional file 1: Table A1. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Fig. A1. Subgroup analysis stratified by sample size. Fig. A2. Subgroup analysis stratified by type of disease. Fig. A3. Subgroup analysis stratified by age. Fig. A4. Subgroup analysis stratified by the proportion of male. Fig. A5. Subgroup analysis stratified by study design. Fig. A6. Subgroup analysis stratified by region. Fig. A7. Subgroup analysis stratified by outcome of disease.
Acknowledgements
We would like to thank Jian Wu, Yang Li and Hongjie Hou (All are from Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis.
Abbreviations
- CVD
Cardiovascular disease
- COVID-19
Coronavirus disease 2019
- CI
Confidence interval
- OR
Odds ratio
- HR
Hazard ratio
- CHD
Coronary heart disease
- CAD
Coronary artery disease
- HIV
Human immunodeficiency virus
- MesH
Medical Subject Headings
- HF
Heart failure
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
Authors’ contributions
H.Y. and Y.W. designed the study; J.X., W.X., X.L. and P.Z. searched literature and extracted the data; J.X., L.S. and Y.W. contributed to the statistical analyses and interpretation; J.X. drafted the manuscript. All the authors have read and approved the final manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81973105). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data relevant to the study are included in the article or uploaded as supplementary information.
Declarations
Ethics approval and consent to participate
Not required.
Consent for Publication
Not applicable
Competing interests
The authors declare not any potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liang X, Shi L, Wang Y, Xiao W, Duan G, Yang H, et al. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infect. 2020; 10.1016/j.jinf.2020.1006.1060. [DOI] [PMC free article] [PubMed]
- 2.Cen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 2020; 10.1016/j.cmi.2020.1005.1041. [DOI] [PMC free article] [PubMed]
- 3.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet (London, England) 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Guan B, Su T, Liu W, Chen M, Waleed KB, Guan X, Gary T, Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghesi A, Zigliani A, Masciullo R, Golemi S, Maculotti P, Farina D, et al. Radiographic severity index in COVID-19 pneumonia: relationship to age and sex in 783 Italian patients. Radiol Med. 2020;125(5):461–464. doi: 10.1007/s11547-020-01202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones J, Sullivan PS, Sanchez TH, Guest JL, Hall EW, Luisi N, Zlotorzynska M, Wilde G, Bradley H, Siegler AJ. Similarities and Differences in COVID-19 Awareness, Concern, and Symptoms by Race and Ethnicity in the United States: Cross-Sectional Survey. J Med Int Res. 2020;22(7):e20001. doi: 10.2196/20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafa NM. L AS: Characterisation of COVID-19 Pandemic in Paediatric Age Group: A Systematic Review and Meta-Analysis. J Clin Virol. 2020;128:104395. doi: 10.1016/j.jcv.2020.104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, Bogler Y, Caldararo M, Figueroa CJ, Glickman MS, Joanow A, Kaltsas A, Lee YJ, Lucca A, Mariano A, Morjaria S, Nawar T, Papanicolaou GA, Predmore J, Redelman-Sidi G, Schmidt E, Seo SK, Sepkowitz K, Shah MK, Wolchok JD, Hohl TM, Taur Y, Kamboj M. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, Deschamps R, Créange A, Wahab A, Pelletier J, Heinzlef O, Labauge P, Guilloton L, Ahle G, Goudot M, Bigaut K, Laplaud DA, Vukusic S, Lubetzki C, De Sèze J. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 14.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzer G, Carpenter J, Rücker G. Empirical evaluation suggests Copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol. 2010;63(3):282–288. doi: 10.1016/j.jclinepi.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Yu X, Sun X, Cui P, Pan H, Lin S, Han R, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis. 2020;67(4):1697–1707. doi: 10.1111/tbed.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Wang M, Zhang J, Gu J, Zhang P, Xu Y, et al. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. 2020;12(11):10070–10086. doi: 10.18632/aging.103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabri A, Davarpanah AH, Mahdavi A, Abrishami A, Khazaei M, Heydari S, et al. Novel coronavirus disease 2019: predicting prognosis with a computed tomography-based disease severity score and clinical laboratory data. Pol Arch Intern Med. 2020;130(7-8):629–634. doi: 10.20452/pamw.15422. [DOI] [PubMed] [Google Scholar]
- 22.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. JAm Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barman HA, Atici A, Sahin I, Alici G, Tekin EA, Baycan OF, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2020; Publish Ahead of Print. [DOI] [PMC free article] [PubMed]
- 25.Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, Martellucci CA, Parruti, Mantovani GL, Manzoli L, Shimosawa T. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLOS ONE. 2020;15(6):e0235248. doi: 10.1371/journal.pone.0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deiana G, Azara A, Dettori M, Delogu F, Vargiu G, Gessa G, Stroscio F, Tidore M, Steri G, Castiglia P. Deaths in SARS-Cov-2 positive patients in Italy: the influence of underlying health conditions on lethality. Int J Environ Res Public Health. 2020;17(12):4450. doi: 10.3390/ijerph17124450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Qin L, Li K, Wang Q, Zhao Y, Xu B, et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. doi: 10.3389/fcimb.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie Y, Li J, Huang X, Guo W, Zhang X, Ma Y, Wang H, Qi M, Tang X, Shen X, Dai X. Epidemiological and clinical characteristics of 671 COVID-19 patients in Henan Province, China. Int J Epidemiol. 2020;49(4):1085–1095. doi: 10.1093/ije/dyaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID‐19: a multicentre United States experience. Liver Int. 2020;40(10):2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanza E, Muglia R, Bolengo I, Santonocito GO, Lisi C, Angelotti G, Morandini P, Savevski V, Politi LS, Balzarini L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Euro Radiol. 2020;30(12):6770–6778. doi: 10.1007/s00330-020-07013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng Z, Ma Y, Zeng H, Huang P, Liu W, Jiang M, Xiang X, Deng D, Liao X, Chen P, Chen Y. Simple nomogram based on initial laboratory data for predicting the probability of ICU transfer of COVID‐19 patients: multicenter retrospective study. J Med Virol. 2021;93(1):434–440. doi: 10.1002/jmv.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell LF, Chernyak Y, Tobin K, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. IJID. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.San Roman JA, Uribarri A, Amat-Santos IJ, Aparisi A, Catala P, Gonzalez-Juanatey JR. The presence of heart disease worsens prognosis in patients with COVID-19. Rev Esp Cardiol. 2020;73(9):773–775. doi: 10.1016/j.recesp.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng B, Hu J, Zuo X, Chen J, Li X, Chen Y, Yang G, Shi X, Deng A. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26(10):1400–1405. doi: 10.1016/j.cmi.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oussalah A, Gleye S, Urmes IC, Laugel E, Callet J, Barbé F, et al. Long-term ACE inhibitor/ARB use is associated with severe renal dysfunction and acute kidney injury in patients with severe COVID-19: results from a referral center cohort in the Northeast of France. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 37.Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean national health insurance big data. J Korean Med Sci. 2020;35(26). [DOI] [PMC free article] [PubMed]
- 38.Chen J, Bai H, Liu J, Chen G, Liao Q, Yang J, Wu P, Wei J, Ma D, Chen G, Ai J, Li K. Distinct clinical characteristics and risk factors for mortality in female inpatients with coronavirus disease 2019 (COVID-19): a sex-stratified, large-scale cohort study in Wuhan, China. Clin Infect Dis. 2020;71(12):3188–3195. doi: 10.1093/cid/ciaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrante G, Fazzari F, Cozzi O, Maurina M, Bragato R, D’Orazio F, Torrisi C, Lanza E, Indolfi E, Donghi V, Mantovani R, Liccardo G, Voza A, Azzolini E, Balzarini L, Reimers B, Stefanini GG, Condorelli G, Monti L. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc Res. 2020;116(14):2239–2246. doi: 10.1093/cvr/cvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rastad H, Karim H, Ejtahed HS, Tajbakhsh R, Noorisepehr M, Babaei M, et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12:57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang JM, Kim JH, Park JS, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317–2324. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, Cabrini L, Carlesso E, Castelli G, Cattaneo S, Cereda D, Colombo S, Coluccello A, Crescini G, Molinari AF, Foti G, Fumagalli R, Iotti GA, Langer T, Latronico N, Lorini FL, Mojoli F, Natalini G, Pessina CM, Ranieri VM, Rech R, Scudeller L, Rosano AR, Storti E, Thompson BT, Tirani M, Villani PG, Pesenti A, Cecconi M, Agosteo E, Albano G, Albertin A, Alborghetti A, Aldegheri G, Antonini B, Barbara E, Bardelloni G, Basilico S, Belgiorno N, Bellani G, Beretta E, Berselli A, Bianciardi L, Bonanomi E, Bonazzi S, Borelli M, Bottino N, Bronzini N, Brusatori S, Cabrini L, Capra C, Carnevale L, Castelli G, Catena E, Cattaneo S, Cecconi M, Celotti S, Cerutti S, Chiumello D, Cirri S, Citerio G, Colombo S, Coluccello A, Coppini D, Corona A, Cortellazzi P, Costantini E, Covello DR, Crescini G, De Filippi G, Poli MD, Dughi P, Fieni F, Florio G, Molinari AF, Foti G, Fumagalli R, Galletti M, Gallioli GA, Gay H, Gemma M, Gnesin P, Grasselli G, Greco S, Greco M, Grosso P, Guatteri L, Guzzon D, Iotti GA, Keim R, Langer T, Latronico N, Lombardo A, Lorini FL, Mamprin F, Marino G, Marino F, Merli G, Micucci A, Militano CR, Mojoli F, Monti G, Muttini S, Nadalin S, Natalini G, Perazzo P, Perego GP, Perotti L, Pesenti A, Pessina CM, Petrucci N, Pezzi A, Piva S, Portella G, Protti A, Racagni M, Radrizzani D, Raimondi M, Ranucci M, Rech R, Riccio M, Rosano A, Ruggeri P, Sala G, Salvi L, Sebastiano P, Severgnini P, Sigurtà D, Stocchetti N, Storti E, Subert M, Tavola M, Todaro S, Torriglia F, Tubiolo D, Valsecchi R, Villani PG, Viola U, Vitale G, Zambon M, Zanella A, Zoia E. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Int Med. 2020;180(10):1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng P, Ke Z, Ying B, Qiao B, Yuan L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Int J Clin Chem. 2020;510:186–190. doi: 10.1016/j.cca.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al‐Salameh A, Lanoix AP, Bennis Y, Andrejak C, Brochot E, Deschasse G, et al. Characteristics and outcomes of ‐19 in hospitalized patients with and without diabetes. Diab/Metab Res Rev. 2021;37(3). [DOI] [PMC free article] [PubMed]
- 45.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, Melzer DA, Newman AB. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol Ser A. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, Jurado J, Penna ND, Celi LA. The minimal effect of zinc on the survival of hospitalized patients with COVID-19. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinto C, Berselli A, Mangone L, Damato A, Iachetta F, Foracchia M, Zanelli F, Gervasi E, Romagnani A, Prati G, Lui S, Venturelli F, Vicentini M, Besutti G, De Palma R, Rossi PG. SARSCoV-2 positive hospitalized cancer patients during the Italian Outbreak: the cohort study in Reggio Emilia. Biology. 2020;9(8):181. doi: 10.3390/biology9080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, et al. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York City. Western J Emerg Med. 2020;21(4):779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lian J, Jin C, Hao S, Zhang X, Yang M, Jin X, et al. High neutrophil-to-lymphocyte ratio associated with progression to critical illness in older patients with COVID-19: a multicenter retrospective study. Aging. 2020;12(14):13849–13859. doi: 10.18632/aging.103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PloS one. 2020;15(7):e0236618. doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146(4):808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Azorin D, Martinez-Pias E, Trigo J, Hernandez-Perez I, Valle-Penacoba G, Talavera B, et al. Neurological comorbidity is a predictor of death in Covid-19 disease: a cohort study on 576 patients. Front Neurol. 2020;11:781. doi: 10.3389/fneur.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alkhatib AL, Kreniske J, Zifodya JS, Fonseca V, Tahboub M, Khatib J, Denson JL, Lasky JA, Lefante JJ, Bojanowski CM. BMI is associated with coronavirus disease 2019 intensive care unit admission in African Americans. Obesity. 2020;28(10):1798–1801. doi: 10.1002/oby.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, Lima-Morales R, Hernández-Vicente IA, Lumbreras-Guzmán M, Méndez-Hernández P. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51(7):683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8):2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berenguer J, Ryan P, Rodríguez-Baño J, Jarrín I, Carratalà J, Pachón J, et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B, Jang T. Clinical course and factors associated with hospitalization and critical illness among COVID‐19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27(10):963–973. doi: 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43(10):2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang Y, Liu T, Wei Y, Li J, Shao L, Liu M, et al. Scoring systems for predicting mortality for severe patients with COVID-19. E Clin Med. 2020;24:100426. doi: 10.1016/j.eclinm.2020.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Xu L, Liang J, Zhao Q, He H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posso M, Comas M, Román M, Domingo L, Louro J, González C, Sala M, Anglès A, Cirera I, Cots F, Frías V-M, Gea J, Güerri-Fernández R, Masclans JR, Noguès X, Vázquez O, Villar-García J, Horcajada JP, Pascual J, Castells X. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a University Hospital in Spain. Arch Bronconeumol. 2020;56(11):756–758. doi: 10.1016/j.arbres.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Shu L, Wang X, Li M, Chen X, Ji N, Shi L, Wu M, Deng K, Wei J, Wang X, Yang C, Yan J, Feng G. Clinical characteristics of moderate COVID‐19 patients aggravation in Wuhan Stadium Cabin Hospital: A 571 cases of retrospective cohort study. J Med Virol. 2021;93(2):1133–1140. doi: 10.1002/jmv.26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52:93–98.e2. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pablos JL, Galindo M, Carmona L, Lledó A, Retuerto M, Blanco R, Gonzalez-Gay MA, Martinez-Lopez D, Castrejón I, Alvaro-Gracia JM, Fernández DF, Mera-Varela A, Manrique-Arija S, Vázquez NM, Fernandez-Nebro A. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B, Liu S, Lu Z, Dong Y, Zhang S. Previous cardiovascular surgery significantly increases the risk of developing critical illness in patients with COVID-19. J Infect. 2021;82(2):282–327. doi: 10.1016/j.jinf.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox T, Ruddiman K, Lo KB, Peterson E, DeJoy R, Salacup G, Pelayo J, Bhargav R, Gul F, Albano J, Azmaiparashvili Z, Anastasopoulou C, Patarroyo-Aponte G. The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol. 2021;58(1):33–38. doi: 10.1007/s00592-020-01592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vena A, Giacobbe DR, Di Biagio A, Mikulska M, Taramasso L, De Maria A, Ball L, Brunetti I, Loconte M, Patroniti NA, Robba C, Delfino E, Dentone C, Magnasco L, Nicolini L, Toscanini F, Bavastro M, Cerchiaro M, Barisione E, Giacomini M, Mora S, Baldi F, Balletto E, Berruti M, Briano F, Sepulcri C, Dettori S, Labate L, Mirabella M, Portunato F, Pincino R, Russo C, Tutino S, Pelosi P, Bassetti M, Alessandrini A, Camera M, Delfino E, De Maria A, Dentone C, Di Biagio A, Dodi F, Ferrazin A, Mazzarello G, Mikulska M, Nicolini L, Toscanini F, Giacobbe DR, Vena A, Taramasso L, Balletto E, Portunato F, Schenone E, Rosseti N, Baldi F, Berruti M, Briano F, Dettori S, Labate L, Magnasco L, Mirabella M, Pincino R, Russo C, Sarteschi G, Sepulcri C, Tutino S, Pontremoli R, Beccati V, Casciaro S, Casu M, Gavaudan F, Ghinatti M, Gualco E, Leoncini G, Pitto P, Salam K, Gratarola A, Bixio M, Amelia A, Balestra A, Ballarino P, Bardi N, Boccafogli R, Caserza F, Calzolari E, Castelli M, Cenni E, Cortese P, Cuttone G, Feltrin S, Giovinazzo S, Giuntini P, Natale L, Orsi D, Pastorino M, Perazzo T, Pescetelli F, Schenone F, Serra MG, Sottano M, Tallone R, Amelotti M, Majabò MJ, Merlini M, Perazzo F, Ahamd N, Barbera P, Bovio M, Campodonico P, Collidà A, Cutuli O, Lomeo A, Fezza F, Gentilucci N, Hussein N, Malvezzi E, Massobrio L, Motta G, Pastorino L, Pollicardo N, Sartini S, Vacca P, Virga V, Porto I, Bezante G, Bona RD, La Malfa G, Valbusa A, Ad VG, Barisione E, Bellotti M, Teresita A’, Blanco A, Grosso M, Piroddi MG, Moscatelli P, Ballarino P, Caiti M, Cenni E, Giuntini P, Magnani O, Sukkar S, Cogorno L, Gradaschi R, Guiddo E, Martino E, Pisciotta L, Cavagliere B, Cristina R, Francesca F, Garibotto G, Esposito P, Bellezza C, Harusha E, Rossi F, Arboscello E, Arzani L, De Mattei L, Spadaro M, Passalacqua G, Bagnasco D, Braido F, Riccio A, Tagliabue E, Gustavino C, Ferraiolo A, Monacelli F, Mahmoud M, Tagliafico L, Napolitano A, Fiorio M, Pizzonia M, Giannotti C, Nencioni A, Giuffrida S, Rosso N, Morando A, Papalia R, Passerini D, Tiberio G, Orengo G, Battaglini A, Ruffoni S, Caglieris S. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020. 10.1016/j.kint.2020.1007.1030. [DOI] [PMC free article] [PubMed]
- 69.He F, Luo Q, Lei M, Fan L, Shao X, Huang G, et al. Risk factors for severe cases of COVID-19: a retrospective cohort study. Aging. 2020;12(15):15730–15740. doi: 10.18632/aging.103803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta A, Madhavan MV, Poterucha TJ, DeFilippis EM, Hennessey JA, Redfors B, et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Res Sq [Preprint]. 2020:rs.3.rs-56210. [DOI] [PMC free article] [PubMed]
- 71.Czernichow S, Beeker N, Rives-Lange C, Guerot E, Diehl JL, Katsahian S, et al. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity. 2020;28(12):2282–2289. doi: 10.1002/oby.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sisó-Almirall A, Kostov B, Mas-Heredia M, Vilanova-Rotllan S, Sequeira-Aymar E, Sans-Corrales M, et al. Prognostic factors in Spanish COVID-19 patients: a case series from Barcelona. PloS one. 2020;15(8):e0237960. doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenner H, Holleczek B, Schöttker B. Vitamin D insufficiency and deficiency and mortality from respiratory diseases in a cohort of older adults: Potential for limiting the death toll during and beyond the COVID-19 pandemic? Nutrients. 2020;12(8):1–11. doi: 10.3390/nu12082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Rossi N, Scarpazza C, Filippini C, Cordioli C, Rasia S, Mancinelli CR, et al. Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. E Clin Med. 2020;25:100459. doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nimkar A, Naaraayan A, Hasan A, Pant S, Durdevic M, Suarez CN, et al. Incidence and risk factors for acute kidney injury and its effect on mortality in patients hospitalized from COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):687–695. doi: 10.1016/j.mayocpiqo.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klang E, Soffer S, Nadkarni G, Glicksberg B, Freeman R, Horowitz C, Reich DL, Levin MA. Sex differences in age and comorbidities for COVID-19 mortality in urban New York City. SN Compr Clin Med. 2020;2(9):1319–1322. doi: 10.1007/s42399-020-00430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emami A, Javanmardi F, Akbari A, Kojuri J, Bakhtiari H, Rezaei T, et al. Survival rate in hypertensive patients with COVID-19. Clin Exper Hypertens. 2021;43(1):77–80. doi: 10.1080/10641963.2020.1812624. [DOI] [PubMed] [Google Scholar]
- 78.Liu D, Cui P, Zeng S, Wang S, Feng X, Xu S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25:100471. doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi PG, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R, Forloni G. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLOS ONE. 2020;15(8):e0238281. doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng X, Li P, Ma L, Liang H, Lei J, Li W, et al. Clinical characteristics and short-term outcomes of severe patients with COVID-19 in Wuhan, China. Front Med. 2020;7:491. doi: 10.3389/fmed.2020.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G, Deng Q, Feng J, Li F, Xiong N, He Q. Clinical characteristics of diabetic patients with COVID-19. J Diab Res. 2020;2020:1652403. doi: 10.1155/2020/1652403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seiglie J, Platt J, Cromer SJ, Bunda B, Foulkes AS, Bassett IV, et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diab Care. 2020;43(12):2938–2944. doi: 10.2337/dc20-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tural Onur S, Altın S, Sokucu SN, Fikri B, Barça T, Bolat E, et al. Could ferritin level be an indicator of COVID-19 disease mortality? J Med Virol. 2021;93(3):1672–1677. doi: 10.1002/jmv.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anzola GP, Bartolaminelli C, Gregorini GA, Coazzoli C, Gatti F, Mora A, et al. Neither ACEIs nor ARBs are associated with respiratory distress or mortality in COVID-19 results of a prospective study on a hospital-based cohort. Intern Emerg Med. 2020;15(8):1477–1484. doi: 10.1007/s11739-020-02500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bahl A, Van Baalen MN, Ortiz L, Chen NW, Todd C, Milad M, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020;15(8):1485–1499. doi: 10.1007/s11739-020-02509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rafi K, Patrik Brodin N, Maron MI, Guha C, Kalnicki S, Garg MK, Racine AD. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an Urban Medical Center in New York. JAMA Network Open. 2020;3(9):e2019795. doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson BR, Gold JAW, Natarajan P, Rossow J, Fanfair RN, da Silva J, et al. Predictors at admission of mechanical ventilation and death in an observational cohort of adults hospitalized with coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 89.Desai A, Voza G, Paiardi S, Teofilo FI, Caltagirone G, Pons MR, Aloise M, Kogan M, Tommasini T, Savevski V, Stefanini G, Angelini C, Ciccarelli M, Badalamenti S, De Nalda AL, Aghemo A, Cecconi M, Boneschi FM, Voza A. The role of anti-hypertensive treatment, comorbidities and early introduction of LMWH in the setting of COVID-19: A retrospective, observational study in Northern Italy. Int J Cardiol. 2021;324:249–254. doi: 10.1016/j.ijcard.2020.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Liu Z, Li J, Zhang J, Tian S, Lu S, Qi M, Ma J, Qiu B, Weiguo Dong YX. Impacts of type 2 diabetes on disease severity, therapeutic effect, and mortality of patients with COVID-19. J Clin Endocrinol Metab. 2020;105(12):e4219–e4229. doi: 10.1210/clinem/dgaa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solerte SB, D'Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diab Care. 2020;43(12):2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayek SS, Brenner SK, Azam TU, Shadid HR, Anderson E, Berlin H, et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020;371:m3513. doi: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen R, Yang J, Gao X, Ding X, Yang Y, Shen Y, et al. Influence of blood pressure control and application of renin-angiotensin-aldosterone system inhibitors on the outcomes in COVID-19 patients with hypertension. J Clin Hypertens. 2020;22(11):1974–1983. doi: 10.1111/jch.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JH, Kim YC, Cho SH, Lee J, You SC, Song YG, et al. Effect of sex hormones on coronavirus disease 2019: an analysis of 5,061 laboratory-confirmed cases in South Korea. Menopause. 2020;27(12):1376–1381. doi: 10.1097/GME.0000000000001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nachega JB, Ishoso DK, Otokoye JO, Hermans MP, Machekano RN, Sam-Agudu NA, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103(6):2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rozaliyani A, Savitri AI, Setianingrum F, Shelly TN, Ratnasari V, Kuswindarti R, et al. Factors associated with death in COVID-19 patients in Jakarta, Indonesia: an epidemiological study. Acta Med Indones. 2020;52(3):246–254. [PubMed] [Google Scholar]
- 97.Wang Z, Ye D, Wang M, Zhao M, Li D, Ye J, et al. Clinical features of COVID-19 patients with different outcomes in Wuhan: a retrospective observational study. BioMed Res Int. 2020;2020:2138387. doi: 10.1155/2020/2138387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Wang W, Yang K, Li S, Yu X, Dong C, Zhang B. Glycemic control before admission is an important determinant of prognosis in patients with coronavirus disease 2019. J Diab Investig. 2021;12(6):1064–1073. doi: 10.1111/jdi.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al Kuwari HM, Abdul Rahim HF, Abu-Raddad LJ, Abou-Samra A-B, Al Kanaani Z, Al Khal A, Al Kuwari E, Al Marri S, Al Masalmani M, Al Romaihi HE, Al Thani MH, Coyle PV, Latif AN, Owen R, Bertollini R, Butt AA. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10(10):e040428. doi: 10.1136/bmjopen-2020-040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balbi M, Caroli A, Corsi A, Milanese G, Surace A, Di Marco F, Novelli L, Silva M, Lorini FL, Duca A, Cosentini R, Sverzellati N, Bonaffini PA, Sironi S. Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department. Eur Radiol. 2021;31(4):1999–2012. doi: 10.1007/s00330-020-07270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calmes D, Graff S, Maes N, Frix AN, Thys M, Bonhomme O, et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160–169. doi: 10.1016/j.jaip.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, Simón-Campo P, de Lera M, Chavarría-Miranda A, López-Sanz C, Gutiérrez-Sánchez M, Martínez-Velasco E, Pedraza M, Sierra Á, Gómez-Vicente B, Guerrero Á, Arenillas JF. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. 2020;419:117163. doi: 10.1016/j.jns.2020.117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zinellu A, Arru F, De Vito A, Sassu A, Valdes G, Scano V, et al. The De Ritis ratio as prognostic biomarker of in‐hospital mortality in COVID‐19 patients. Eur J Clin Investig. 2021;51(1). [DOI] [PMC free article] [PubMed]
- 104.Mallow PJ, Belk KW, Topmiller M, Hooker EA. Outcomes of hospitalized COVID-19 patients by risk factors: results from a United States hospital claims database. J Health Econ Outcomes Res. 2020;7(2):165–174. doi: 10.36469/jheor.2020.17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abbasi B, Akhavan R, Khameneh AG, Zandi B, Farrokh D, Rad MP, et al. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 106.Craig-Schapiro R, Salinas T, Lubetzky M, Abel BT, Sultan S, Lee JR, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21(4):1576–1585. doi: 10.1111/ajt.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ryan C, Minc A, Caceres J, Balsalobre A, Dixit A, Ng BKP, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 108.Serin I, Sari ND, Dogu MH, Acikel SD, Babur G, Ulusoy A, et al. A new parameter in COVID-19 pandemic: initial lactate dehydrogenase (LDH)/Lymphocyte ratio for diagnosis and mortality. J Infect Public Health. 2020;13(11):1664–1670. doi: 10.1016/j.jiph.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao Y, Han X, Gu J, Li Y, Liu J, Alwalid O, et al. Prognostic value of baseline clinical and HRCT findings in 101 patients with severe COVID-19 in Wuhan, China. Sci Rep. 2020;10(1):17543. doi: 10.1038/s41598-020-74497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. JASN. 2021;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raparelli V, Palmieri L, Canevelli M, Pricci F, Unim B, Lo Noce C, et al. Sex differences in clinical phenotype and transitions of care among individuals dying of COVID-19 in Italy. Biol Sex Differ. 2020;11(1):57. doi: 10.1186/s13293-020-00334-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chinnadurai R, Ogedengbe O, Agarwal P, Money-Coomes S, Abdurrahman AZ, Mohammed S, et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020;20(1):409. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021;159(1):85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naaraayan A, Nimkar A, Hasan A, Pant S, Durdevic M, Elenius H, et al. End-stage renal disease patients on chronic hemodialysis fare better with COVID-19: a retrospective cohort study from the New York Metropolitan Region. Cureus. 2020;12(9):e10373. doi: 10.7759/cureus.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cherri S, Lemmers DHL, Noventa S, Abu Hilal M, Zaniboni A. Outcome of oncological patients admitted with COVID-19: experience of a hospital center in northern Italy. Ther Adv Med Oncol. 2020;12:1758835920962370. doi: 10.1177/1758835920962370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodríguez-Molinero A, Gálvez-Barrón C, Miñarro A, Macho O, López GF, Robles MT, et al. Association between COVID-19 prognosis and disease presentation, comorbidities and chronic treatment of hospitalized patients. PloS one. 2020;15(10):e0239571. doi: 10.1371/journal.pone.0239571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clift AK, Coupland CAC, Keogh RH, Hemingway H, Hippisley-Cox J. COVID-19 mortality risk in down syndrome: results from a cohort study of 8 million adults. Ann Intern Med. 2021;174(4):572–576. doi: 10.7326/M20-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clift AK, Coupland CAC, Keogh RH, Diaz-Ordaz K, Williamson E, Harrison EM, Hayward A, Hemingway H, Horby P, Mehta N, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gamberini L, Tonetti T, Spadaro S, Zani G, Mazzoli CA, Capozzi C, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Omrani AS, Almaslamani MA, Daghfal J, Alattar RA, Elgara M, Shaar SH, et al. The first consecutive 5000 patients with Coronavirus Disease 2019 from Qatar; a nation-wide cohort study. BMC Infect Dis. 2020;20(1):777. doi: 10.1186/s12879-020-05511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yahyavi A, Hemmati N, Derakhshan P, Banivaheb B, Behnagh AK, Tofighi R, TehraniYazdi A, Kabir A. Angiotensin enzyme inhibitors and angiotensin receptor blockers as protective factors in COVID-19 mortality: a retrospective cohort study. Intern Emerg Med. 2021;16(4):883–893. doi: 10.1007/s11739-020-02523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guisado-Vasco P, Valderas-Ortega S, Carralón-González MM, Roda-Santacruz A, González-Cortijo L, Sotres-Fernández G, Martí-Ballesteros EM, Luque-Pinilla JM, Almagro-Casado E, La Coma-Lanuza FJ, Barrena-Puertas R, Malo-Benages EJ, Monforte-Gómez MJ, Diez-Munar R, Merino-Lanza E, Comeche-Casanova L, Remirez-de-Esparza-Otero M, Correyero-Plaza M, Recio-Rodríguez M, Rodríguez-López M, Sánchez-Manzano MD, Andreu-Vázquez C, Thuissard-Vasallo IJ, María-Tomé JME-S, Carnevali-Ruiz D. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort) EClinicalMedicine. 2020;28:100591. doi: 10.1016/j.eclinm.2020.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Izzy S, Tahir Z, Cote DJ, Al Jarrah A, Roberts MB, Turbett S, et al. Characteristics and outcomes of latinx patients with COVID-19 in comparison with other ethnic and racial groups. Open Forum Infect Dis. 2020;7(10):ofaa401. doi: 10.1093/ofid/ofaa401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, McCurdy MT, Tabatabai A, Kumar G, Park P, Benjenk I, Menaker J, Ahmed N, Glidewell E, Presutto E, Cain S, Haridasa N, Field W, Fowler JG, Trinh D, Johnson KN, Kaur A, Lee A, Sebastian K, Ulrich A, Peña S, Carpenter R, Sudhakar S, Uppal P, Fedeles BT, Sachs A, Dahbour L, Teeter W, Tanaka K, Galvagno SM, Herr DL, Scalea TM, Mazzeffi MA. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 125.Raines AM, Tock JL, McGrew SJ, Ennis CR, Derania J, Jardak CL, et al. Correlates of death among SARS-CoV-2 positive veterans: the contribution of lifetime tobacco use. Addict Behav. 2021;113:106692. doi: 10.1016/j.addbeh.2020.106692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramos-Rincon JM, Buonaiuto V, Ricci M, Martín-Carmona J, Paredes-Ruíz D, Calderón-Moreno M, et al. Clinical characteristics and risk factors for mortality in very old patients hospitalized with COVID-19 in Spain. J Gerontol Ser A Biol Sci Med Sci. 2021;76(3):e28–e37. doi: 10.1093/gerona/glaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L, Fan T, Yang S, Feng H, Hao B, Lu Z, et al. Comparison of clinical characteristics of COVID-19 between elderly patients and young patients: a study based on a 28-day follow-up. Aging. 2020;12(20):19898–19910. doi: 10.18632/aging.104077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Souza CD, de Arruda Magalhães AJ, Lima AJ, Nunes DN, de Fátima Machado Soares É, de Castro Silva L, et al. Clinical manifestations and factors associated with mortality from COVID-19 in older adults: retrospective population-based study with 9807 older Brazilian COVID-19 patients. Geriatr Gerontol Int. 2020;20(12):1177–1181. doi: 10.1111/ggi.14061. [DOI] [PubMed] [Google Scholar]
- 129.Kolhe NV, Fluck RJ, Selby NM, Taal MW, Remuzzi G. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLOS Med. 2020;17(10):e1003406. doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim TS, Roslin M, Wang JJ, Kane J, Hirsch JS, Kim EJ. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity. 2021;29(2):279–284. doi: 10.1002/oby.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.An C, Lim H, Kim DW, Chang JH, Choi YJ, Kim SW. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. 2020;10(1):18716. doi: 10.1038/s41598-020-75767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rao X, Wu C, Wang S, Tong S, Wang G, Wu G, et al. The importance of overweight in COVID-19: A retrospective analysis in a single center of Wuhan, China. Medicine. 2020;99(43):e22766. doi: 10.1097/MD.0000000000022766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tehrani S, Killander A, Åstrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. IJID. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hyman JB, Leibner ES, Tandon P, Egorova NN, Bassily-Marcus A, Kohli-Seth R, et al. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0254. doi: 10.1097/CCE.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hamilton P, Hanumapura P, Castelino L, Henney R, Parker K, Kumar M, et al. Characteristics and outcomes of hospitalised patients with acute kidney injury and COVID-19. PloS one. 2020;15(11):e0241544. doi: 10.1371/journal.pone.0241544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganatra S, Dani SS, Redd R, Rieger-Christ K, Patel R, Parikh R, et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020:1–10. [DOI] [PubMed]
- 140.Rubio-Rivas M, Corbella X, Mora-Luján JM, Loureiro-Amigo J, López Sampalo A, Yera Bergua C, et al. Predicting clinical outcome with phenotypic clusters in COVID-19 pneumonia: an analysis of 12,066 hospitalized patients from the Spanish registry SEMI-COVID-19. J Clin Med. 2020;9(11). [DOI] [PMC free article] [PubMed]
- 141.Mendes A, Serratrice C, Herrmann FR, Genton L, Périvier S, Scheffler M, Fassier T, Huber P, Jacques MC, Prendki V, et al. Predictors of in-hospital mortality in older patients with COVID-19: the COVIDAge study. J Am Med Dir Assoc. 2020;21(11):1546–1554.e1543. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nemer DM, Wilner BR, Burkle A, Aguilera J, Adewumi J, Gillombardo C, et al. Clinical characteristics and outcomes of non-ICU hospitalization for COVID-19 in a nonepicenter, centrally monitored healthcare system. J Hosp Med. 2021;16(1):7–14. doi: 10.12788/jhm.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo T, Shen Q, Zhou Z, Li J, Guo W, He W, et al. Combined interventions for severe novel coronavirus disease (COVID-19): experience from 350 patients. Infect Drug Resist. 2020;13:3907–3918. doi: 10.2147/IDR.S279255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang F, Cao J, Yu Y, Ding J, Eshak ES, Liu K, et al. Epidemiological characteristics of patients with severe COVID-19 infection in Wuhan, China: evidence from a retrospective observational study. Int J Epidemiol. 2021;49(6):1940–1950. doi: 10.1093/ije/dyaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang O, Bigelow BF, Sheikh F, Peters M, Zenilman JM, Bennett R, Katz MJ. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21(12):1767–1773.e1761. doi: 10.1016/j.jamda.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, Annweiler C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang Y, Lyu X, Li D, Wang L, Wang Y, Zou W, et al. A cohort study of 676 patients indicates D-dimer is a critical risk factor for the mortality of COVID-19. PloS one. 2020;15(11):e0242045. doi: 10.1371/journal.pone.0242045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Poterucha TJ, Elias P, Jain SS, Sayer G, Redfors B, Burkhoff D, et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc. 2021;10(1):e018476. doi: 10.1161/JAHA.120.018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, Zhang Y, Wang F, Liu B, Li H, Tang G, et al. Cardiac damage in patients with the severe type of coronavirus disease 2019 (COVID-19) BMC Cardiovasc Disord. 2020;20(1):479. doi: 10.1186/s12872-020-01758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Prado-Galbarro FJ, Sanchez-Piedra C, Gamiño-Arroyo AE, Cruz-Cruz C. Determinants of survival after severe acute respiratory syndrome coronavirus 2 infection in Mexican outpatients and hospitalised patients. Public Health. 2020;189:66–72. doi: 10.1016/j.puhe.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shah C, Grando DJ, Rainess RA, Ayad L, Gobran E, Benson P, Neblett MT, Nookala V. Factors associated with increased mortality in hospitalized COVID-19 patients. Ann Med Surg (2012) 2020;60:308–313. doi: 10.1016/j.amsu.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Di Domenico SL, Coen D, Bergamaschi M, Albertini V, Ghezzi L, Cazzaniga MM, et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: a single-center retrospective study. Intern Emerg Med. 2020:1–10. [DOI] [PMC free article] [PubMed]
- 155.Ayaz A, Arshad A, Malik H, Ali H, Hussain E, Jamil B. Risk factors for intensive care unit admission and mortality in hospitalized COVID-19 patients. Acute Crit Care. 2020;35(4):249–254. doi: 10.4266/acc.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ: Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020, 106(19):1503-1511. [DOI] [PMC free article] [PubMed]
- 157.Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020;22(12):2238–2247. doi: 10.1002/ejhf.2052. [DOI] [PubMed] [Google Scholar]
- 158.Elmunzer BJ, Wolf BJ, Scheiman JM, Tierney WM, Taylor JR. Association between preadmission acid suppressive medication exposure and severity of illness in patients hospitalized with COVID-19. Gastroenterology. 2021;160(4):1417–1422.e1414. doi: 10.1053/j.gastro.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Polverino F, Stern DA, Ruocco G, Balestro E, Bassetti M, Candelli M, et al. Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, italian observational study (ItaliCO). Front Cardiovasc Med. 2020;7. [DOI] [PMC free article] [PubMed]
- 160.Sharp AL, Huang BZ, Broder B, Smith M, Yuen G, Subject C, et al. Identifying patients with symptoms suspicious for COVID-19 at elevated risk of adverse events: The COVAS score. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed]
- 161.Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1). [DOI] [PMC free article] [PubMed]
- 162.Fu L, Li XY, Fei J, Xiang Y, Xiang HX, Li MD, et al. Myocardial injury at early stage and its association with the risk of death in COVID-19 patients: a hospital-based retrospective cohort study. Front Cardiovasc Med. 2020;7:590688. doi: 10.3389/fcvm.2020.590688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sheshah E, Sabico S, Albakr RM, Sultan AA, Alghamdi KS, Al Madani K, et al. Prevalence of diabetes, management and outcomes among Covid-19 adult patients admitted in a specialized tertiary hospital in Riyadh. Saudi Arabia. Diabetes Res Clin Pract. 2021;172:108538. doi: 10.1016/j.diabres.2020.108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng X, Cai G, Wen X, Gao L, Jiang D, Sun M, et al. Clinical characteristics and fatal outcomes of hypertension in patients with severe COVID-19. Aging. 2020;12(23):23436–23449. doi: 10.18632/aging.104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neumann-Podczaska A, Chojnicki M, Karbowski LM, Al-Saad SR, Hashmi AA, Chudek J, et al. Clinical characteristics and survival analysis in a small sample of older COVID-19 patients with defined 60-day outcome. Int J Environ Res Public Health. 2020;17(22). [DOI] [PMC free article] [PubMed]
- 167.Ken-Dror G, Wade C, Sharma S, Law J, Russo C, Sharma A, et al. COVID-19 outcomes in UK centre within highest health and wealth band: a prospective cohort study. BMJ Open. 2020;10(11):e042090. doi: 10.1136/bmjopen-2020-042090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iannelli A, Bouam S, Schneck AS, Frey S, Zarca K, Gugenheim J, et al. The impact of previous history of bariatric surgery on outcome of COVID-19. A nationwide medico-administrative French study. Obes Surg. 2020:1–9. [DOI] [PMC free article] [PubMed]
- 169.Sharifpour M, Rangaraju S, Liu M, Alabyad D, Nahab FB, Creel-Bulos CM, et al. C-Reactive protein as a prognostic indicator in hospitalized patients with COVID-19. PloS one. 2020;15(11):e0242400. doi: 10.1371/journal.pone.0242400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martins-Filho PR, Antunes de Souza Araújo A, Pereira LX, Quintans-Júnior LJ, de Souza Barboza W, Cavalcante TF, Feitosa de Souza M, de Oliveira Góes MA, Santos VS. Factors associated with mortality among hospitalized patients with COVID-19: a retrospective cohort study. Am J Trop Med Hyg. 2021;104(1):103–105. doi: 10.4269/ajtmh.20-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee SG, Park GU, Moon YR, Sung K. Clinical characteristics and risk factors for fatality and severity in patients with coronavirus disease in Korea: a nationwide population-based retrospective study using the Korean Health Insurance Review and Assessment Service (HIRA) database. Int J Environ Res Public Health. 2020;17(22). [DOI] [PMC free article] [PubMed]
- 172.Loffi M, Piccolo R, Regazzoni V, Di Tano G, Moschini L, Robba D, et al. Coronary artery disease in patients hospitalised with Coronavirus disease; 2019. (COVID-19) infection. Open Heart. 2020;7(2). [DOI] [PMC free article] [PubMed]
- 173.Grodecki K, Lin A, Razipour A, Cadet S, McElhinney PA, Chan C, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2021;115:154436. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khan A, Althunayyan S, Alsofayan Y, Alotaibi R, Mubarak A, Arafat M, Assiri A, Jokhdar H. Risk factors associated with worse outcomes in COVID-19: a retrospective study in Saudi Arabia. East Mediterr Health J. 2020;26(11):1371–1380. doi: 10.26719/emhj.20.130. [DOI] [PubMed] [Google Scholar]
- 175.Rutten JJS, van Loon AM, van Kooten J, van Buul LW, Joling KJ, Smalbrugge M, Hertogh C. Clinical suspicion of COVID-19 in nursing home residents: symptoms and mortality risk factors. J Am Med Dir Assoc. 2020;21(12):1791–1797.e1791. doi: 10.1016/j.jamda.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schuelter-Trevisol F, Raimundo LJ, Soccas HD, Antunes AF, Mohr RLD, Marcon CEM, et al. Assessment of patients with COVID-19 hospitalized in southern Santa Catarina. Rev Soc Bras Med Trop. 2020;53:1–5. doi: 10.1590/0037-8682-0579-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80(4):527-538. [DOI] [PMC free article] [PubMed]
- 178.Nyabera A, Lakhdar S, Li M, Trandafirescu T, Ouedraogo TS. The association between BMI and inpatient mortality outcomes in older adults with COVID-19. Cureus. 2020;12(10):e11183. doi: 10.7759/cureus.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ozturk S, Turgutalp K, Arici M, Odabas AR, Altiparmak MR, Aydin Z, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2021;35(12):2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Druyan A, Lidar M, Brodavka M, Levy I, Barzilai A, Pavlotsky F. The risk for severe COVID 19 in patients with autoimmune and/or inflammatory diseases: first wave lessons. Dermatol Ther. 2021;34(1):e14627. doi: 10.1111/dth.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alguwaihes AM, Al-Sofiani ME, Megdad M, Albader SS, Alsari MH, Alelayan A, et al. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Özdemir İH, Özlek B, Özen MB, Gündüz R, Çetin N, Bilge AR. Hydroxychloroquine/azithromycin treatment, QT interval and ventricular arrhythmias in hospitalised patients with COVID-19. Int J Clin Pract. 2021;75(2):e13896. doi: 10.1111/ijcp.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gue YX, Tennyson M, Gao J, Ren S, Kanji R, Gorog DA. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10(1):21379. doi: 10.1038/s41598-020-78505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Galiero R, Pafundi PC, Simeon V, Rinaldi L, Perrella A, Vetrano E, et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: findings from COVOCA study. PloS one. 2020;15(12):e0243700. doi: 10.1371/journal.pone.0243700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rethemiotaki I. A preliminary study of coronavirus disease 2019 in China: the impact of cardiovascular disease on death risk. Arch Med Sci Atherosclerotic Dis. 2020;5:e219–e223. doi: 10.5114/amsad.2020.98918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stoian AP, Pricop-Jeckstadt M, Pana A, Ileanu B-V, Schitea R, Geanta M, et al. Death by SARS-CoV 2: a Romanian COVID-19 multi-centre comorbidity study. Sci Rep. 2020;10(1). [DOI] [PMC free article] [PubMed]
- 188.Zhou S, Chen C, Hu Y, Lv W, Ai T, Xia L. Chest CT imaging features and severity scores as biomarkers for prognostic prediction in patients with COVID-19. Ann Transl Med. 2020;8(21):1449. doi: 10.21037/atm-20-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stefan G, Mehedinti AM, Andreiana I, Zugravu AD, Cinca S, Busuioc R, et al. Clinical features and outcome of maintenance hemodialysis patients with COVID-19 from a tertiary nephrology care center in Romania. Renal Fail. 2021;43(1):49–57. doi: 10.1080/0886022X.2020.1853571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ahnach M, Zbiri S, Nejjari S, Ousti F, Elkettani C. C-reactive protein as an early predictor of COVID-19 severity. J Med Biochem. 2020;39(4):500–507. doi: 10.5937/jomb0-27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eshrati B, Baradaran HR, Erfanpoor S, Mohazzab A, Moradi Y. Investigating the factors affecting the survival rate in patients with COVID-19: a retrospective cohort study. Med J Islamic Repub Iran. 2020;34:88. doi: 10.34171/mjiri.34.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Özyılmaz S, Ergün Alış E, Ermiş E, Allahverdiyev S, Uçar H. Assessment of the relationship between mortality and troponin I levels in hospitalized patients with the novel coronavirus (COVID-19). Medicina. 2020;56(12). [DOI] [PMC free article] [PubMed]
- 193.Tan X, Zhang S, Xu J, Zhou M, Huang Q, Duan L, et al. Comparison of clinical characteristics among younger and elderly deceased patients with COVID-19: a retrospective study. Aging. 2020;13(1):16–26. doi: 10.18632/aging.202139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong M-F, Jude EB. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12(12):3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhong Y, Zhao L, Wu G, Hu C, Wu C, Xu M, et al. Impact of renin-angiotensin system inhibitors use on mortality in severe COVID-19 patients with hypertension: a retrospective observational study. J Int Med Res. 2020;48(12):300060520979151. doi: 10.1177/0300060520979151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Izurieta HS, Graham DJ, Jiao Y, Hu M, Lu Y, Wu Y, Chillarige Y, Wernecke M, Menis M, Pratt D, Kelman J, Forshee R. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US medicare beneficiaries. J Infect Dis. 2021;223(6):945–956. doi: 10.1093/infdis/jiaa767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Burrell AJ, Pellegrini B, Salimi F, Begum H, Broadley T, Campbell LT, et al. Outcomes for patients with COVID-19 admitted to Australian intensive care units during the first four months of the pandemic. Med J Aust. 2021;214(1):23–30. doi: 10.5694/mja2.50883. [DOI] [PubMed] [Google Scholar]
- 198.Li Y, Shang K, Bian W, He L, Fan Y, Ren T, et al. Prediction of disease progression in patients with COVID-19 by artificial intelligence assisted lesion quantification. Sci Rep. 2020;10(1). [DOI] [PMC free article] [PubMed]
- 199.Caliskan T, Saylan B. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: a retrospective observational study. Rev Assoc Med Bras (1992) 2020;66(12):1679–1684. doi: 10.1590/1806-9282.66.12.1679. [DOI] [PubMed] [Google Scholar]
- 200.Moradi EV, Teimouri A, Rezaee R, Morovatdar N, Foroughian M, Layegh P, Kakhki BR, Koupaei SRA, Ghorani V. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. 2021;40:11–14. doi: 10.1016/j.ajem.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.The first wave of the COVID-19 pandemic in Spain: characterisation of cases and risk factors for severe outcomes, as at 27 April 2020. Eurosurveillance. 2020;25(50) [DOI] [PMC free article] [PubMed]
- 202.Rashidi F, Barco S, Kamangar F, Heresi GA, Emadi A, Kaymaz C, et al. Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: prospective results from a multi-center study. Thromb Res. 2021;198:135–138. doi: 10.1016/j.thromres.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chaudhri I, Koraishy FM, Bolotova O, Yoo J, Marcos LA, Taub E, et al. Outcomes associated with the use of renin-angiotensin-aldosterone system blockade in hospitalized patients with SARS-CoV-2 infection. Kidney 360. 2020;1(8):801–809. doi: 10.34067/KID.0003792020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huh K, Ji W, Kang M, Hong J, Bae GH, Lee R, et al. Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea. IJID. 2021;104:7–14. doi: 10.1016/j.ijid.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Orioli L, Servais T, Belkhir L, Laterre PF, Thissen JP, Vandeleene B, et al. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium. Diab Metab Syndr. 2021;15(1):149–157. doi: 10.1016/j.dsx.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gude-Sampedro F, Fernández-Merino C, Ferreiro L, Lado-Baleato Ó, Espasandín-Domínguez J, Hervada X, et al. Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study. Int J Epidemiol. 2021;50(1):64–74. doi: 10.1093/ije/dyaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Monteiro AC, Suri R, Emeruwa IO, Stretch RJ, Cortes-Lopez RY, Sherman A, et al. Obesity and smoking as risk factors for invasive mechanical ventilation in COVID-19: A retrospective, observational cohort study. PloS one. 2020;15(12):e0238552. doi: 10.1371/journal.pone.0238552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lano G, Braconnier A, Bataille S, Cavaille G, Moussi-Frances J, Gondouin B, et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J. 2020;13(5):878–888. doi: 10.1093/ckj/sfaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lanini S, Montaldo C, Nicastri E, Vairo F, Agrati C, Petrosillo N, Scognamiglio P, Antinori A, Puro V, Di Caro A, De Carli G, Navarra A, Agresta A, Cimaglia C, Palmieri F, D’Offizi G, Marchioni L, Kobinger GP, Maeurer M, Girardi E, Capobianchi MR, Zumla A, Locatelli F, Ippolito G, Ricci S. COVID-19 disease—Temporal analyses of complete blood count parameters over course of illness, and relationship to patient demographics and management outcomes in survivors and non-survivors: a longitudinal descriptive cohort study. PLOS ONE. 2020;15(12):e0244129. doi: 10.1371/journal.pone.0244129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schwartz KL, Achonu C, Buchan SA, Brown KA, Lee B, Whelan M, et al. Epidemiology, clinical characteristics, household transmission, and lethality of severe acute respiratory syndrome coronavirus-2 infection among healthcare workers in Ontario, Canada. PloS one. 2020;15(12):e0244477. doi: 10.1371/journal.pone.0244477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sun Y, Guan X, Jia L, Xing N, Cheng L, Liu B, et al. Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID-19: A retrospective cohort study of Huoshen Mountain Hospital and Guanggu Fangcang Shelter Hospital. J Clin Hypertens. 2021;23(2):218–231. doi: 10.1111/jch.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diab Endocrinol. 2021;9(2):82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cetinkal G, Kocas BB, Ser OS, Kilci H, Yildiz SS, Ozcan SN, et al. The association between chronic use of renin-angiotensin-aldosterone system blockers and in-hospital adverse events among COVID-19 patients with hypertension. Sisli Etfal Hastanesi tip bulteni. 2020;54(4):399–404. doi: 10.14744/SEMB.2020.15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu M, Yang W, Huang T, Zhou J. Diabetic patients with COVID-19 need more attention and better glycemic control. World J Diab. 2020;11(12):644–653. doi: 10.4239/wjd.v11.i12.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lv Z, Lv S. Clinical characteristics and analysis of risk factors for disease progression of COVID-19: a retrospective cohort study. Int J Biol Sci. 2021;17(1):1–7. doi: 10.7150/ijbs.50654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Guerra Veloz MF, Cordero Ruiz P, Ríos-Villegas MJ, Del Pino BP, Bravo-Ferrer J, Galvés Cordero R, et al. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig. 2021;113(2):103–109. doi: 10.17235/reed.2020.7627/2020. [DOI] [PubMed] [Google Scholar]
- 217.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. IJID. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, Checchi F, Perel P, Joseph S, Gibbs HP, Banerjee A, Eggo RM, Nightingale ES, O'Reilly K, Jombart T, Edmunds WJ, Rosello A, Sun FY, Atkins KE, Bosse NI, Clifford S, Russell TW, Deol AK, Liu Y, Procter SR, Leclerc QJ, Medley G, Knight G, Munday JD, Kucharski AJ, Pearson CAB, Klepac P, Prem K, Houben RMGJ, Endo A, Flasche S, Davies NG, Diamond C, van Zandvoort K, Funk S, Auzenbergs M, Rees EM, Tully DC, Emery JC, Quilty BJ, Abbott S, Villabona-Arenas CJ, Hué S, Hellewell J, Gimma A, Jarvis CI. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Guan W-j, Liang W-h, Zhao Y, Liang H-r, Chen Z-s, Li Y-m, Liu X-q, Chen R-c, Tang C-l, Wang T, Chun-quan O, Li L, Chen P-y, Sang L, Wang W, Li J-f, Li C-c, Li-min O, Cheng B, Xiong S, Ni Z-y, Jie Xiang YH, Liu L, Shan H, Lei C-l, Peng Y-x, Wei L, Liu Y, Hu Y-h, Peng P, Wang J-m, Liu J-y, Chen Z, Li G, Zheng Z-j, Qiu S-q, Luo J, Ye C-j, Zhu S-y, Cheng L-l, Ye F, Li S-y, Zheng J-p, Zhang N-f, Zhong N-s, He J-x. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table A1. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Fig. A1. Subgroup analysis stratified by sample size. Fig. A2. Subgroup analysis stratified by type of disease. Fig. A3. Subgroup analysis stratified by age. Fig. A4. Subgroup analysis stratified by the proportion of male. Fig. A5. Subgroup analysis stratified by study design. Fig. A6. Subgroup analysis stratified by region. Fig. A7. Subgroup analysis stratified by outcome of disease.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.