Abstract
Tau is a protein that associates with microtubules (MTs) and promotes their assembly and stability. The protein loses its ability to bind MTs in tauopathies, and detached tau can misfold and induce the pathological changes that characterize Alzheimer’s disease (AD). A growing body of evidence indicates that tauopathies can spread between cells or connected regions. Pathological tau transmission in the brain of patients with AD and other tauopathies is due to the spread of various tau species along neuroanatomically connected regions in a “prion-like” manner. This complex process involves multiple steps of secretion, cellular uptake, transcellular transfer, and/or seeding, but the precise mechanisms of tau pathology propagation remain unclear. This review summarizes the current evidence on the nature of propagative tau species and the possible steps involved in the process of tau pathology spread, including detachment from MTs, degradations, and secretion, and discusses the different mechanisms underlying the spread of tau pathology.
Keywords: tau, tauopathy, spread, toxicity, mechanism, Alzheimer’s disease
Introduction
The microtubule (MT)-binding protein tau is mainly expressed in the cytoplasm of neurons (Pérez et al., 2016) and plays key roles in regulating MT dynamics, axonal transport, and neurite outgrowth (Johnson and Stoothoff, 2004). Tau protein changes affect its MT-binding ability and consequently alter its normal physiological functions. For example, the phosphorylation of tau protein in and around its microtubule-binding domain (MBD) may neutralize its positive charges (Jho et al., 2010), alter MBD conformation, and lead to its detachment from MTs (Fischer et al., 2009). Once detached, tau accumulates in neurites and neuronal cell bodies, where it forms insoluble intracellular aggregates or inclusion bodies such as neurofibrillary tangles (NFTs), which are one of the major pathological features of Alzheimer’s disease (AD) (Lee et al., 2001; von Bergen et al., 2005; Zhang et al., 2009). Following detachment from MTs, tau can undergo structural transition, misfolding, and degradation (Frost et al., 2009). Tau can also be secreted into the extracellular space (Riemenschneider et al., 2003; Barthélemy et al., 2016) either in its naked form (Chai et al., 2012) or packaged in exosomes or other membranes (Saman et al., 2012; Simón et al., 2012; Polanco et al., 2016) following neuronal activity in mature neurons (Pooler et al., 2013; Dujardin et al., 2014b), neuron death (Gómez-Ramos et al., 2006), and/or when accumulated tau reaches a certain level in non-neuronal cells. In agreement with these findings, exogenous misfolded tau protein can be internalized by cells (Guo and Lee, 2011; Wu et al., 2013), a process that is mediated by heparin sulphate proteoglycans (HSPGs) and cell membrane receptors such as muscarinic (M1, M3) and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, as well as via endocytosis (Gómez-Ramos et al., 2008; Holmes et al., 2013; Tian H. et al., 2013). Once internalized, pathogenic misfolded tau proteins act as “seeds” that recruits soluble endogenous tau into larger aberrant conformations (Jucker and Walker, 2013) that slowly propagate across interconnected brain regions, as shown in various animal models (Clavaguera et al., 2009, 2013; Lasagna-Reeves et al., 2012). Fibrillar tau species can also transfer between cells and then recruit endogenous tau proteins onto their ends (Kfoury et al., 2012), a mechanism that may be responsible for the intracerebral spread of tau pathology (de Calignon et al., 2012; Iba et al., 2013).
Tau pathology spreading between neuronal cells and adjacent brain regions is a complex process involving many physiological and pathological aspects of tau protein, including its degradation, secretion, transmission, and toxicity. However, the exact mechanism underlying the spread of tau pathology after its release from cells remains unclear, and understanding these processes is the focus of an increasing number of studies (Le et al., 2012; Mohamed et al., 2013). There is some evidence that progressive accumulation of tau pathology in affected brain regions during AD development is due to the spread of aggregated tau along anatomically connected pathways (Hanger et al., 2014; Clavaguera et al., 2015; Lewis and Dickson, 2016). Accumulation of aggregates leads to neuronal loss and trans-synaptic spread of tau aggregates to more distal regions of the brain (Liu et al., 2012; Croft et al., 2017). The spread of extracellular species is the main pathway propagating neurofibrillary lesions and tau toxicity throughout different brain regions in neurodegenerative diseases (Iba et al., 2013; Pérez et al., 2018). A better understanding of the precise molecular mechanisms underlying tau propagation will contribute to the development of new therapeutic approaches for halting this process and provide new perspectives for the early diagnosis and prevention of tau pathologies (Fuster-Matanzo et al., 2018; Pérez et al., 2018). This review covers the most recent advances in our understanding of tau-spreading mechanisms, as well as the underlying implications of tauopathy-associated toxicity in AD. We further outline the possible mechanisms involved in pathology propagation including tau protein detachment from MTs; tau cleavage; tau degradation; and the release, uptake, and movement of pathogenic tau among synaptically connected neurons (Usenovic et al., 2015).
Physiological Characteristics and Dissociation of Tau Protein From MTs
Tau protein can be divided into four functional domains: an N-terminal projection region, a proline-rich domain, an MBD, and a C-terminal region (Goedert and Spillantini, 2011). Tau can bind to the outside—and possibly also the inside—of MTs with the N- and C-terminal regions projecting out (Kar et al., 2003; Santarella et al., 2004). The N-terminal region can associate with the cell membrane and may be as a part of a membrane-associated complex; it also regulates the spacing between MTs (Maas et al., 2000; Al-Bassam et al., 2002). The proline-rich domain includes multiple phosphorylation sites (Augustinack et al., 2002) and can bind to Src homology 3 (SH3) domains of other proteins (Reynolds et al., 2008) such as the tyrosine kinase Fyn (Lee et al., 1998; Figure 1). Tau protein not only plays a crucial role in regulating MT dynamics but also promotes MT assembly and stabilization, processes that are required for morphogenesis and axonal transport in the nervous system (Johnson and Hartigan, 1999). However, the ability of tau to stabilize MTs is due in large part to its MBD (Gustke et al., 1994). Tau is thought to directly bind MTs through positively charged tandem repeat sequences within its MBD that are attracted to tubulin’s negatively charged residues (Kar et al., 2003; Jho et al., 2010).
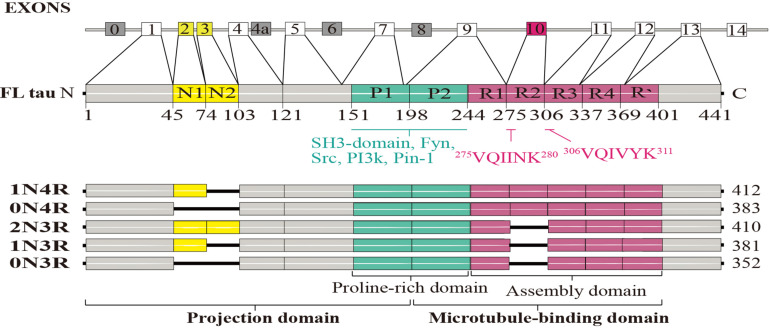
FIGURE 1.
Structural features of the tau protein. The human tau gene, microtubule-associated protein tau (MAPT), contains 16 exons; alternative splicing of exons 2, 3, and 10 generates six tau protein isoforms: oN3R, 1N4R, 2N3R, 0N4R, 1N4R, and 2N4R. Full-length human tau protein (2N4R) contains 441 amino acids and four functional domains: an N-terminal projection region, a proline-rich domain, a microtubule-binding domain (MBD), and a C-terminal region. The N-terminal inserts are N1 and N2. The proline-rich domains P1 and P2 contain many phosphorylation sites and can bind to SH3 domains of other proteins, such as the tyrosine kinase Fyn. R1-R4 make up the repeat domain, which, together with the R’-flanking region, constitute the MT-binding domain. Two sequences are necessary for tau aggregation: 275VQIINK280 and 306VQIVYK311.
The human tau gene, microtubule-associated protein tau (MAPT), is located on chromosome 17q21 and comprises 16 exons. Alternative splicing of exons 2, 3, and 10 generates six isoforms of the tau protein (Goedert et al., 1989). They are equally expressed in central nervous system neurons of a healthy adult brain (Goedert et al., 1989; Garcia and Cleveland, 2001), and can be grouped into tau-3R class members, which contain three MT-binding repeats (MTBRs), and tau-4R class members contain four MTBRs (Figure 1). Because of the extra repeat, 4R isoforms have a higher affinity for MTs and can therefore bind and stabilize MTs more efficiently (Goedert and Spillantini, 2011; Morris et al., 2011; Chen and Jiang, 2019).
Tau protein functions are regulated by complex post-translational modifications including phosphorylation, glycation, isomerization, sumoylation, nitration, acetylation, and truncation (Morris et al., 2011). Moreover, tau contains numerous serine and threonine residues, so almost 20% of the protein has the potential to be phosphorylated (Wang and Mandelkow, 2016). The phosphorylation state of tau and its MT-binding affinity are controlled by a balance between kinase and phosphatase activity (Brandt et al., 1995; Shackelford and Yeh, 1998). Tau phosphorylation is mediated by MT affinity-regulating kinases (also known as PAR1 kinases), cyclic AMP-dependent protein kinase A, calcium (Ca2+), or calmodulin-dependent protein kinase II (CaMKII), and tyrosine kinases like Src family members (Hanger et al., 2009). The activation of tau phosphorylation-associated kinases (e.g., CDK-5 and GSK-3β) can induce tau hyperphosphorylation, which drives dissociation of tau protein from MTs (Hanger et al., 2009). Dissociated tau can misfold and become toxic seeds that are secreted from the cell. In contrast, fully dephosphorylated tau binds to MTs with high affinity (Shackelford and Yeh, 1998). Tau dephosphorylation is mediated by protein phosphatases 1, 2A, 2B, 2C, and 5 (Hanger et al., 2009; Pérez et al., 2018). In addition, detached tau can accumulate in neurites and neuronal cell bodies, first forming insoluble filaments and eventually NFTs (Lee et al., 2001; von Bergen et al., 2005; Figure 2). Abnormal tau phosphorylation decreases MT binding and likely increases tau-tau interactions (Morris et al., 2011). Physiologically, tau is continuously phosphorylated and dephosphorylated to ensure its proper function; however, when the balance shifts toward phosphorylation, tau affinity for MTs decreases (Alonso et al., 1997), resulting in higher cytosolic tau levels, which facilitates tau aggregation (Wegmann et al., 2018). Additionally, aberrantly phosphorylated tau protein appears to sequester other microtubule-associated proteins (MAPs), further destabilizing MTs (Alonso et al., 1997). Similarly, aberrant tau phosphorylation and self-aggregation lead to the formation of oligomers and higher-order aggregates that can lead to tau detachment from MTs and disturb the binding of other MAPs to MTs (von Bergen et al., 2000).
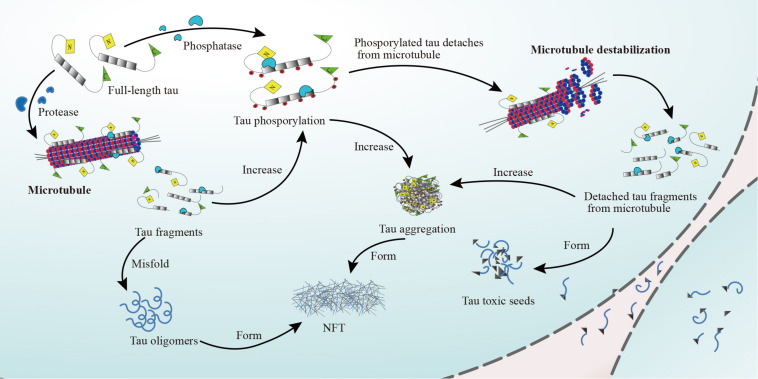
FIGURE 2.
Detachment of tau protein from MTs. The full-length tau protein binds to MTs, stabilizing them under the regulation of phosphatases (e.g., PP1, PP2A, PP2B, PP2C, and PP5). When tau protein phosphorylates, tau protein will detach from MTs. The microtubules will destabilize and dissociate. Detached tau fragments form tau aggregation, which ultimately leads to the formation of NFTs. At the same time, phosphorylated tau protein and detached tau fragments misfold and form tau oligomers, which are the precursors of NFTs, or toxic tau seeds.
Tau Protein Cleavage and Degradation
Tau cleavage and degradation are closely related to its pathological transmission and aggregation. The cleavage of tau generates seeds that promote tau aggregation (Wang et al., 2014), alter tau clearance, and can impair cognition and motor ability (Bondulich et al., 2016). Tau cleavage occurs at its N- and C-terminals (García-Sierra et al., 2008) and depends on associated proteases, mainly caspase-3, calpain, and cathepsin L (Wang et al., 2009; de Calignon et al., 2010; Table 1). Tau truncation can be initiated by caspase-3, which cleaves tau at residue D421, its predominant target (Chung et al., 2001). Caspase-3 can be activated by amyloid-beta (Aβ) and caspase-2 (Gamblin et al., 2003), following which caspase-3 can cleave tau at D25-Q26 (Corsetti et al., 2008) and D421-S422 (Gamblin et al., 2003). Cleavage at D421-S422 produces the N-terminal fragment (NTF) tau1–421 (Tau-C) (Nicholls et al., 2017). Caspase-2 is a protease that initiates activation of other caspases; it cleaves tau at D314-L315 to produce a soluble, toxic NTF (tau1–314; △tau314; Zhao et al., 2016). Tau1–314 levels were elevated in the brains of mice with mild cognitive impairment (MCI) and in the brains of AD patients compared with healthy controls (Zhao et al., 2016). Moreover, memory deficits were ameliorated following the application of anticaspase-2 morpholino oligonucleotides (Zhao et al., 2016). However, in vitro experiments using recombinant tau preparations suggested that caspase-2 preferentially cleaves tau at D421-S422 (Zhao et al., 2016). Another effector caspase, caspase-6, and puromycin-sensitive aminopeptidase (PSA) were reported to cleave recombinant human tau441 at D13-H14 (Sengupta et al., 2006), which was sufficient to cause axonal degeneration (Sokolowski et al., 2014). Caspase-6 cleavage at D402-T403 produces the NTF tau1–402, a cerebrospinal fluid (CSF) biomarker for AD (Ramcharitar et al., 2013). In addition, calpain-1 and -2 can both cleave tau (Chesser et al., 2013) and play opposing roles in regulating synaptic plasticity and promoting neurodegeneration (Baudry and Bi, 2016). The 17-kDa tau45–230 fragment is generated through cleavage by calpain-1 at K44-E45 (Yang and Ksiezak-Reding, 1995) or via calpain-1 (Park and Ferreira, 2005) or -2 (Garg et al., 2011) action at R230-T231. Tau is also cleaved by calpain-1 at R242-L243 to produce the 24-kDa C-terminal fragment (CTF) tau243–441 (Matsumoto et al., 2015). The levels of the tau243–441 fragment increase with aging in a tauopathy mouse model (Tg601 mice expressing wild-type human tau), and CTFs with sizes ranging from 20 to 28 kDa are present in brain samples from patients with AD and familial frontotemporal dementia (Matsumoto et al., 2015). Tau243–441 can proficiently propagate to other tau-expressing cells, leading to further seeding and tau441 phosphorylation (Matsumoto et al., 2015). Interestingly, tau441 build up can activate calpain-2, which leads to the degradation of nicotinic acetylcholine receptor subunit 4 (Yin et al., 2016), a crucial component of cholinergic signaling. Calpain-2 activation by tau441 creates a positive feedback loop that enhances neurotoxic tau fragment generation. Cathepsins B, D, and L can also proteolytically cleave tau. One study reported that cathepsin B was associated with intracellular NFTs, and its expression was elevated around Aβ plaques (Ii et al., 1993). Cathepsin D can cleave recombinant tau at F8-E9, M419-V420, and L436-A437; there is another potential cleavage site at either T427-L428 or L428-A429 and additional cleavage sites in D34-G161, P200-K257, and K267-D358 (Kenessey et al., 1997). In Neuro-2A murine cells, cathepsin L can cleave tau244–372 (lacking K280; tauRD△K), which is a mutated version of the aggregation-prone MBD tau fragment (García-Sierra et al., 2008). TauRD△K cleavage by cathepsin L depends on an initial cleavage at K257-S258 by an unknown cytosolic protease that generates tau258–372; this fragment is further cleaved by cathepsin L at V363-P364 to produce tau258–363 that is subsequently cleaved at I360-T361 to produce tau258–360 (García-Sierra et al., 2008). Tau258–360 and tau258–363 induce aggregation of intact tauRD△K and full-length tau, which is coincident with lysosomal leakage (García-Sierra et al., 2008). Asparagine endopeptidase-mediated tau cleavage occurs at both N255-V256 and N368-K369 and produces five tau fragments. Among them, tau1–368 and tau256–368 were the only critical drivers of enhanced apoptosis in rat primary neurons, while only tau1–368 has been found in the brains of patients with AD (Zhang et al., 2014; Quinn et al., 2018).
TABLE 1.
Tau protein cleavage at different sites by known proteases.
| Protease | Cleavage site | Cleavage domain | Tau fragment | Effect on AD | References |
| Caspase-2 | D314-L315 | MBD | Tau1-314 (△tau314) | Caspase-2 preferentially cleaves recombinant tau at D421-S422 compared to D314-L315. Detached from MTs, tau invades healthy dendritic spines. This impaired synaptic transmission and drove hippocampal neuronal loss, but spatial memory deficits and toxicity were only observed when tau1–314 promoted mislocalization of full-length tau to dendritic spines. |
Zhao et al., 2016 |
| Tau315–441 | Unclear | Zhao et al., 2016 | |||
| Caspase-3 | D421-S422 | C-terminus | Tau1–421 (Tau-C) | Linked to other tauopathies | Gamblin et al., 2003; Corsetti et al., 2008; Zhao et al., 2015 |
| D25-Q26 | N-terminus | Tau1–25 | No toxicity to neurons | Corsetti et al., 2008 | |
| Caspase-3 Calpain-1 | D25-Q26 K44-E45 | N-terminus | Tau26–44 | Caused NMDAR-mediated cell death in rat CGCs | Gamblin et al., 2003; Park and Ferreira, 2005; Corsetti et al., 2008; Garg et al., 2011; Zhao et al., 2015 |
| Caspase-3 Calpain-1 Calpain-2 | D25-Q26 | N-terminus | Tau26–230 (20–22kDa fragment) | Enriched in synaptic mitochondria; binds to Aβ peptides and exacerbates mitochondrial dysfunction. Induced NMDAR-mediated death of rat CGCs. | Gamblin et al., 2003; Park and Ferreira, 2005; Corsetti et al., 2008; Garg et al., 2011; Zhao et al., 2015 |
| R230-T231 | MBD | ||||
| Caspase-6 | D13-H14 | N-terminus | Tau1–13 | Caused axonal degeneration | Sengupta et al., 2006; Sokolowski et al., 2014 |
| Tau14–441 | Possible role in tangle maturation | Sengupta et al., 2006; Sokolowski et al., 2014 | |||
| D402-T403 | C-terminus | Tau1–402 (Tau△Casp6) | Serves as a CSF biomarker of neurodegeneration in AD | Ramcharitar et al., 2013 | |
| Tau403–441 | Unclear | Ramcharitar et al., 2013 | |||
| Caspase-1, -3, -6, -7, -8 | D421-S422 | C-terminus | Tau422–441 | Unclear | Quinn et al., 2018 |
| D421-S422 | C-terminus | Tau151–421 (△tau) | Led to tau aggregation and disrupted axonal transport, mitochondrial function, Golgi apparatus, and synaptic protein levels | ||
| K150-I151 | N-terminus | ||||
| PSA | D13-H14 | N-terminus | Tau1–13 | Caused axonal degeneration | Sengupta et al., 2006; Sokolowski et al., 2014 |
| Calpain-1 | K44-E45 | N-terminus | Tau1–44 | Caused NMDAR-mediated cell death in rat CGCs | Park and Ferreira, 2005; Garg et al., 2011 |
| Tau45–441 | Unclear | Yang and Ksiezak-Reding, 1995 | |||
| R242-L243 | MBD | Tau243–441 (24kDa CTF) | Accelerated the propagation to other tau-expressing cells, causing further seeding of aggregates and tau441 phosphorylation; reduced capacity for promoting MT assembly compared with tau441 | Matsumoto et al., 2015 | |
| Tau1–242 | Unclear | Matsumoto et al., 2015 | |||
| Calpain-1 and thrombin, Calpain-1 and -2 | K44-E45 | N-terminus | Tau45–230 (17kDa fragment) | Caused synapse loss and behavioral abnormalities; impaired organelle transport | Yang and Ksiezak-Reding, 1995; Park and Ferreira, 2005; Garg et al., 2011; Quinn et al., 2018 |
| R230-T231 | MBD | ||||
| Calpain-2 and thrombin, Calpain-1 and -2 | A2-E3 | N-terminus | Tau3–230 | Unclear | Quinn et al., 2018 |
| R230-T231 | MBD | ||||
| R230-T231 | MBD | Tau125–230 | Not toxic | ||
| Q124-A125 | Projection domain | ||||
| Calpain-2 | A2-E3 | N-terminus | Tau3–124 | Unclear | Quinn et al., 2018 |
| Q124-A125 | Projection domain | ||||
| Cathepsin L | K257-S258 | MBD | Tau258–372 | Unclear | García-Sierra et al., 2008 |
| V363-P364 | MBD | Tau258–363 | Induced the aggregation of full-length tau and intact tauRD△K coincident with lysosomal leakage | ||
| I360-T361 | MBD | Tau258–360 | |||
| AEP | N255-V256 | MBD | Tau1–255 | Unable to promote MT polymerization or aggregation into PHFs, but tau1–255 had strong AT8 immunoreactivity (at phosphorylation sites S202 and T205) | Zhang et al., 2014; Quinn et al., 2018 |
| N255-V256 | MBD | Tau256–441 | Significantly reduced MT polymerization and showed increased propensity to aggregate into PHFs compared with tau441 | ||
| N255-V256 | MBD | Tau1–368, Tau256–368 | Enhanced apoptosis; increased ability to aggregate into PHFs | ||
| N368-K369 | MBD | ||||
| N368-K369 | MBD | Tau369–441 | Unable to cause MT polymerization and aggregation into PHFs | ||
| Thrombin | R155-G156 | Proline-rich domain | Tau156–441 | Unclear | Quinn et al., 2018 |
| R155-G156 | Proline-rich domain | Tau156–209 | Unclear | ||
| R209-S210 | MBD | ||||
| R209-S210 | MBD | Tau210–441 | Unclear | ||
| R209-S210 | MBD | Tau210–230 | Unclear | ||
| R230-T231 | MBD | ||||
| R230-T231 | MBD | Tau231–441 | Unclear | ||
| Chymotrypsin | Y197-S198 | Projection domain | Tau1–197 | Unclear | Quinn et al., 2018 |
| Y197-S198 | MBD | Tau198–441 | Unclear | ||
| ADAM10 | A152-T153 | Proline-rich domain | Tau1–152 | Unclear | Quinn et al., 2018 |
| Tau153–441 (Tau-A) | Unclear |
Aβ, amyloid-beta; AD, Alzheimer’s disease; ADAM10, a disintegrin and metalloprotease 10; AEP, asparagine endopeptidase; AMPA, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CGC, cerebellar granule cell; CSF, cerebrospinal fluid; CTF, C-terminal fragment; MBD, microtubule-binding domain; MT, microtubule; NFT, neurofibrillary tangle; NMDAR, N-methyl-D-aspartate receptor; NTF, N-terminal fragment; PHF, paired-helical filament; PSA, puromycin-sensitive aminopeptidase; PSP, progressive supranuclear palsy.
Intracellular tau degradation mainly involves two major proteolytic systems: ubiquitin-proteasome and autophagy-lysosomal (Chesser et al., 2013; Lee et al., 2013; Guo et al., 2017). Full-length tau is cleared via the former system (Liu et al., 2009; Dolan and Johnson, 2010), whereas its mutated and truncated forms appear to be degraded through the latter pathway (García-Sierra et al., 2008; Fernández-Montoya and Pérez, 2015). Moreover, tau phosphorylation can exacerbate its proteolytic degradation (Kenessey et al., 1997), while hyperphosphorylation is associated with impaired tau degradation via the ubiquitin-proteasome system (Dickey et al., 2007) and tau secretion. Tau can undergo natural self-degradation at cysteine residues by acetyl-coenzyme A-induced autoacetylation (Cohen et al., 2016) following its dissociation from MTs (Cohen et al., 2013, 2016). Tau accumulation can also result from increased expression or decreased degradation of the protein (Barton et al., 1990; Zhang et al., 2014), and degradation is impaired by a modified form of tau (Zhang et al., 2014). Thus, tau acetylation may both inhibit and facilitate its degradation and also suppress its phosphorylation and aggregation (Min et al., 2010; Cook et al., 2014). Acetylated tau has been found in brains from patients with AD and other tauopathies. For example, Lys174 acetylation was recently described in AD brains and may be a critical determinant for tau-induced toxicity by delaying tau turnover (Min et al., 2015). This result indicates that targeting tau acetylation could be a novel therapeutic option for AD and other human tauopathies.
Secretion and Release of Tau Fragments
Although tau is intracellular, recent studies have indicated that it is also present in the extracellular space both in vitro and in vivo (Kim et al., 2010a,b; Chai et al., 2012). Tau has been detected in both the CSF and interstitial fluid of tau transgenic mouse brains (Yamada et al., 2011; Barten et al., 2012). In vitro studies have shown that human tau is secreted by both neuronal and non-neuronal cell lines when the protein is overexpressed (Chai et al., 2012; Saman et al., 2012; Simón et al., 2012; Pérez et al., 2016). Extracellular tau may elicit toxicity (Gómez-Ramos et al., 2006; Díaz-Hernández et al., 2010) by binding to cellular receptors such as muscarinic receptors (Gómez-Ramos et al., 2008), but the mechanisms by which tau exits into the extracellular space remain unclear (Nickel and Rabouille, 2009). Another study showed that tau can be released into the extracellular space following neuronal death (Simón et al., 2012) and can subsequently be identified in the CSF (Iqbal et al., 2005). Secreted extracellular tau can be toxic to surrounding cells through interactions with specific cell receptors (Gómez-Ramos et al., 2008; Díaz-Hernández et al., 2010; Figure 3). This toxic effect may result in cell death and the subsequent detection of tau in the CSF of patients with disorders such as AD (Iqbal et al., 2005; Yamada et al., 2011). Additionally, in affected regions such as the hippocampus, there is an inverse relationship between the numbers of surviving cells and extracellular tangles (Cras et al., 1995; Fukutani et al., 1995). This suggests that degenerating neurons containing fibrillar lesions might release NFT contents into the extracellular environment (Goedert, 1999). Meanwhile, several in vitro and in vivo studies reported that stimulation of neuronal activity can regulate the physiological secretion of endogenous tau by cortical neurons and enhance the release of pathological tau, a process that is Ca2+-dependent and modulated by phosphorylation (Sokolow et al., 2015; Fá et al., 2016; Wang et al., 2017). AMPA receptor stimulation promotes tau release through a Ca2+-dependent mechanism and the exocytosis of presynaptic vesicles. AMPA receptor stimulation generates action potentials that increase presynaptic Ca2+ concentrations, evoking vesicle release (Schmitz et al., 2009), and this plays a role in Ca2+-mediated regulation of neuronal tau release (Pooler et al., 2013). The relationship between neuronal activity and tau release appears to be bidirectional; both extracellular tau and Aβ perpetuate further neuronal tau release through feedback mechanisms (Bright et al., 2015). These results indicate that tau release partially occurs in a neuronal excitability-dependent manner in response to regional changes in the AD brain.
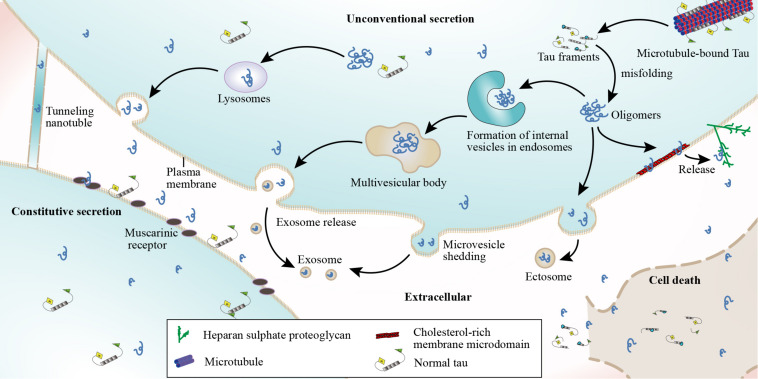
FIGURE 3.
Tau protein secretion and release. The possible mechanisms underlying the presence of tau in the extracellular space include cell death, neuroactive stimulation, constitutive secretion, and unconventional secretion. A small amount of tau can be released into the extracellular space in free form or by penetrating adjacent membranes, which can be achieved by stimulating neuroactivity or constitutive secretion. However, the methods involved in unconventional tau protein secretion include (1) non-vesicular direct translocation across the plasma membrane from the cytoplasm and extracellular trapping via HSPGs, (2) release via secretory lysosomes that fuse with the plasma membrane and release their contents into the extracellular space, (3) microvesicle shedding from the plasma membrane, and (4) vesicle-mediated exosome release. Tau can also be secreted by both neuronal and non-neuronal cells when tau protein is exogenously expressed, while extracellular tau can bind muscarinic (M1, M3) receptors. Tau may also be released through nanotubes that connect the cytoplasmic compartments of adjoining cells.
Tau can also be detected in the extracellular space before neurodegeneration, indicating that it can be released through mechanisms other than cell death (Yamada et al., 2011; Barten et al., 2012). Preliminary results demonstrated that tau can be released into the extracellular space via membrane vesicles in the absence of cell death (Simón et al., 2012). Tau secretion likely transpires via the unconventional vesicular- or non-vesicular-mediated secretory pathway since tau lacks an apparent endoplasmic reticulum-targeting sequence, which is necessary in the conventional secretory pathway (Yamada, 2017). Vesicle-mediated secretion might serve as a mechanism to regulate (proteostasis) cellular tau concentrations, maintaining them below a certain threshold level (Simón et al., 2012). Tau can also be transported through membrane vesicles after lysosomal degradation (García-Sierra et al., 2008). Four different mechanisms have been proposed for the unconventional secretion of soluble, cytoplasmic tau: (1) non-vesicular direct translocation from the cytoplasm across the plasma membrane, (2) release via secretory lysosomes that fuse with plasma membranes and release their contents into the extracellular space, (3) microvesicle shedding from the plasma membrane, and (4) vesicle-mediated exosome release (Nickel and Rabouille, 2009; Saman et al., 2012). In the last two scenarios, tau is surrounded by a membrane when it is released into the extracellular space (Nickel and Rabouille, 2009; Chai et al., 2012); Figure 3). It has also been proposed that tau may be released from cells through an exosome-independent pathway that requires heat shock cognate 70, its co-chaperone DnaJ (Hsp40), and synaptosomal-associated protein 23 (Fontaine et al., 2016). Additionally, tau secretion reportedly occurs through membrane vesicles when tau is overexpressed (Simón et al., 2012). Similarly, tau can be secreted in an exosome-dependent manner by Neuro2a cells overexpressing tau (Wang et al., 2017), as well as by microglia (Asai et al., 2015). Another vesicular-mediated mechanism involves large extracellular vesicles called exosomes that are directly shed from cells by plasma membrane budding (Théry et al., 2009; Figure 3). The third mechanism proposed to mediate tau release and spreading involves formation of thin membranous bridges called tunneling nanotubes (TNTs; Rustom et al., 2004). Moreover, cell depolarization was shown to induce the release of a 20-kDa tau fragment from AD synapses (Sokolow et al., 2015). These four mechanisms appear to be temperature dependent and are likely to be less efficient at low temperatures (Nickel and Rabouille, 2009).
Different forms of tau protein might be secreted through different mechanisms or vectors. Endogenous tau released from primary cortical rat neurons under basal conditions is predominantly full length (Pooler et al., 2013), but truncated species have also been identified (Dujardin et al., 2014a). Tau fragments lacking the proline-rich region are either not secreted or are secreted in a manner different from that of the full-length molecule (Pérez et al., 2016). Monomeric and aggregated tau have been detected in CSF, suggesting that they may be released following axonal degeneration and neuronal death (Hampel et al., 2004). However, other studies found that unstimulated human and rodent neurons only secrete C-terminally truncated forms of endogenous tau (Kanmert et al., 2015). Cell culture studies revealed that tau is released via the unconventional secretory pathway and that tau mutations influence the secretion rate, with 4R tau isoforms less abundant than 3R isoforms (Karch et al., 2012). Moreover, exogenously expressed hyperphosphorylated tau secreted by non-neuronal cells is cleaved at its C-terminus (Plouffe et al., 2012; Croft et al., 2017). For instance, tau cleavage at D421 can increase the secretion rate (Katsinelos et al., 2018). Aberrantly phosphorylated tau is secreted more efficiently than non-phosphorylated tau, at least in cultured cell lines (Plouffe et al., 2012; Katsinelos et al., 2018), possibly because abnormal phosphorylation impairs tau’s ability to interact with its partners, therefore altering the protein’s normal physiological properties (Yan et al., 2020). Meanwhile, endogenous tau is reportedly released either free and in a full-length, dephosphorylated form (Pooler et al., 2013) or as N-terminally truncated fragments (Bright et al., 2015; Kanmert et al., 2015). A small subset of tau released under these conditions is inside plasma membrane-derived vesicles called ectosomes (Dujardin et al., 2014a). Tau is also released from cells in association with exosomes, particularly when it is exogenously expressed or in a highly phosphorylated and misfolded state (Plouffe et al., 2012; Saman et al., 2012). One study suggested that tau hyperphosphorylation in AD may induce a vicious circle that amplifies its secretion (Plouffe et al., 2012). In this case, tau hyperphosphorylation would enhance its secretion, which would subsequently increase the level of dephosphorylated tau in the extracellular space. Dephosphorylated extracellular tau would then induce an increase in intracellular Ca2+ concentrations, which has been linked with elevated tau hyperphosphorylation (Díaz-Hernández et al., 2010). This vicious circle then promotes tau pathology propagation in the brain and CSF accumulation (Hampel et al., 2010; Plouffe et al., 2012). Golgi dynamics were also proposed to modulate tau secretion from both HeLa cells and primary cortical neurons (Mohamed et al., 2017). Tau cleavage and hyperphosphorylation increase its secretion from HeLa cells. Mitochondrial damage might reduce tau secretion (Shafiei et al., 2017), whereas impaired lysosomal function may increase it (Mohamed et al., 2014). Pathological tau in animal models appears to be more localized to synapses compared to non-pathological tau (Sahara et al., 2014), and synaptosomes isolated from human AD brains were shown to contain more phosphorylated and aggregated tau than those isolated from healthy controls (Tai et al., 2014). In summary, different tau species and isoforms including mutated (Hampel et al., 2004), hyperphosphorylated (Plouffe et al., 2012; Bright et al., 2015), and truncated forms of tau appear to be released via distinct mechanisms (Plouffe et al., 2012).
The Spread of Pathological Tau Protein
Although the specific routes and mechanisms underlying the spread of pathological tau remain unclear (Braak and Braak, 1991), the propagation of cytosolic tau to connected neurons consists of at least four phases. First, tau must be secreted or released from donor neurons; second, it must undergo aggregation before or after being released; third, tau must be taken up into recipient neurons; and fourth, tau aggregation must be induced in recipient cells (Kanmert et al., 2015). After the release and secretion of tau to the extracellular space following cell death (Simón et al., 2012), stimulation of neuronal activity (Pooler et al., 2013), or other mechanisms (e.g., associated with vesicles, secretory lysosomes, or microvesicle shedding from the plasma membrane) (Nickel and Rabouille, 2009; Saman et al., 2012), pathological tau oligomers, monomers, or aggregates must enter other cells via endocytosis. Subsequently, pathological tau seeds might be degraded, resecreted, or mediate the misfolding of wild-type tau molecules in recipient cells (Wu et al., 2013). Recipient cells appear to favor the uptake of short, low-molecular-weight, tau fibrils over monomers and larger fibrils (Wu et al., 2013). Consequently, the potencies of various tau aggregate species on cellular propagation may be different (Frost et al., 2009). For example, tau uptake is closely related to both the size and conformation of tau aggregates (Kanmert et al., 2015). Intracellular tau accumulation is dependent on the isoform composition of the extracellular tau oligomers (Swanson et al., 2017). However, tau aggregate uptake is not neuron specific; cell-to-cell transfer also occurs between glial cells and neurons. Tau aggregates can transfer between connected cells, induce templated misfolding, and be internalized from the extracellular space by a neighboring cell, which facilitates tauopathy propagation across different brain regions in a prion-like fashion (Jucker and Walker, 2013). Tau oligomers can be internalized by dynamin-dependent bulk endocytosis and are then transported through the endolysosomal pathway in recipient cells (Wu et al., 2013). One study demonstrated that neuronal uptake of α-synuclein and tau aggregate seeds can occur through macropinocytosis, a form of fluid-phase bulk endocytosis that represents the most likely mechanism for tau uptake. This process begins when aggregated proteins bind to HSPGs, a family of core proteins with cell-surface glycosaminoglycan polysaccharides. Interestingly, the internalization process is only initiated by aggregated species, not by monomeric tau (Holmes et al., 2013). Another study also suggested that HSPGs can mediate exosome internalization (Christianson et al., 2013; Fuster-Matanzo et al., 2018). Before internalization via micropinocytosis, tau binds to plasma membrane HSPGs, which promotes membrane rearrangement before endocytosis (Holmes et al., 2013). Tau binding to HSPGs seems to be essential for internalization, and the 6-O-sulfation pattern on heparan sulfate sidechains is an important determinant for tau binding (Rauch et al., 2018; Figure 4). However, heparan-like glycosaminoglycan (GAG) mimetics can hide tau’s HSPG binding site, which reduces cell-surface tau oligomer binding, uptake, and seeding (Holmes et al., 2013). HSPG-dependent macropinocytosis is instigated by small protein aggregates, and tau trimers were shown to be the smallest size able to initiate this mechanism (Mirbaha et al., 2015; Rauch et al., 2018). The results of a recent study also supported the hypothesis that different tau species could be internalized through different cellular mechanisms (Evans et al., 2018). For example, tau monomers and small oligomers are preferentially taken up by macropinocytosis, while dynamin-dependent endocytosis is the preferred route for larger aggregates. HSPGs and macropinocytosis may also play a role in the uptake of whole exosomes (Christianson et al., 2013), although exosomes are internalized because of this pathway’s non-specificity. In short, endocytosis and/or pinocytosis might be favored over direct fusion to the plasma membrane as an exosome internalization route (Tian T. et al., 2013; Polanco et al., 2018). During this process, vesicles can be endocytosed by neighboring cells, which might be involved in the propagation of misfolded or aggregated tau proteins in different neurodegenerative disorders (Goedert et al., 2010; Guo and Lee, 2011). Meanwhile, micropinocytosis might be critical for pathological tau uptake both in vitro and in vivo (Holmes et al., 2013; Brunello et al., 2016), as well as the uptake of other pathologically misfolded proteins including α-synuclein, TDP-43 (Zeineddine et al., 2015), and PrP (Hooper, 2011). Tau aggregates are reportedly internalized into primary neurons, where they are trafficked anterogradely and retrogradely along axons, and then spread to connected cells (Takeda et al., 2015; Wu et al., 2016; Wang et al., 2017), thereby propagating tau pathology (Calafate et al., 2015; Wu et al., 2016; Nobuhara et al., 2017). They can also seed the aggregation of native monomers, thereby initiating more aggregates that are then released and spread to neighboring cells (Kundel et al., 2018; Brunello et al., 2020; Figure 4). Recent reports found that inoculation of preformed tau fibrils into tau transgenic mice quickly induced an AD-like NFT pathology in connected brain regions (Clavaguera et al., 2013; Ahmed et al., 2014; Boluda et al., 2015). Moreover, misfolded tau proteins spread through anatomically connected neurons, presumably via trans-synaptic tau aggregate transmission (Harris et al., 2012; Liu et al., 2012). However, preformed tau aggregates can also spread by means other than synaptic connections, suggesting the existence of alternative (non-synaptic) propagation pathways (de Calignon et al., 2012; Peeraer et al., 2015).
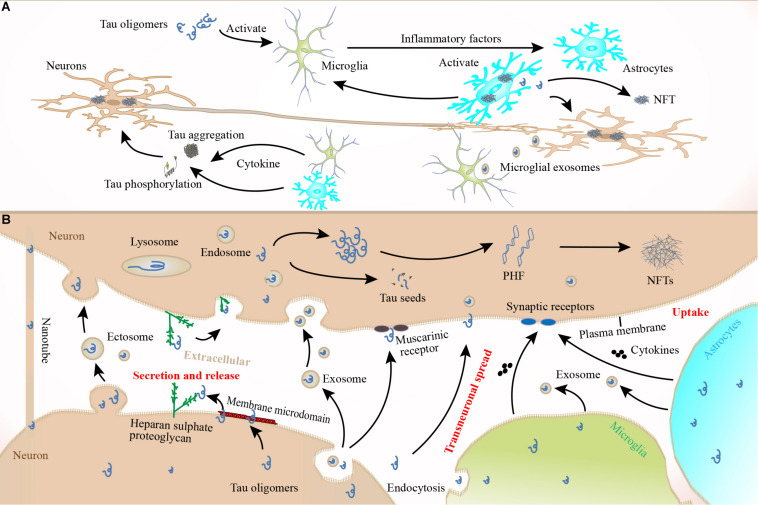
FIGURE 4.
Tau pathology spread. Once released into the extracellular space, tau can be transported to connected cells via exosomes or ectosomes. Tau can also be transported to other cells by binding to muscarinic (M1, M3) receptors or synaptic receptors or via special structures such as membrane microdomains and HSPGs. There may also be nanotubes between cells, which can also spread tau oligomers. The plasma membrane may play a role in tau transmission through endocytosis-mediated uptake of tau oligomers. Microglia can also phagocytose secreted tau oligomers that are then spread to healthy neurons in exosomes, and astrocytes may be involved in this process. Both microglia and astrocytes can secrete cytokines that act on connected neurons, which further accelerates tau pathology. However, the specific mechanisms underlying the roles of microglia and astrocytes in tau pathology spread remain unclear. When tau oligomers or monomers are released by lysosome or ingested by recipient cells, they will misfold and form toxic seeds, PHFs, or NFTs. Once the recipient cells spread the pathological tau fragments to other cells, this perpetuates a vicious cycle that exacerbates AD.
How neurons release tau that is then transmitted to recipient neurons to instigate tau propagation remains unclear. Although synaptic connections can facilitate tau’s transcellular spread, other cellular uptake routes cannot be excluded. Once internalized, tau can be located in both early and late endosomes, which links these tau species to lysosomal vesicles in a retrograde axonal pathway and provides further evidence for a transsynaptic route of transmission (Frost et al., 2009; Wu et al., 2013). Extracellular vesicles play a major role in cellular communication and the transport of pathogenic proteins related to AD (Vingtdeux et al., 2012). Notably, exosomal tau protein levels are elevated in prodromal AD (Fiandaca et al., 2015; Gibbons et al., 2019). Microglia also contribute to tauopathy progression via exosome secretion, such that microglia depletion and inhibition of exosome synthesis can dramatically suppress tau propagation in vitro and in vivo (Asai et al., 2015). Additionally, astrocytes can internalize both fibrillar and monomeric tau, implying that these cells are also be involved in tau pathology spread (Martini-Stoica et al., 2018; Perea et al., 2019). However, one study found that oligomers and short fibrils that bind to the membrane can be internalized by neuronal cells via a receptor-independent mechanism, while tau monomers, long fibrils, and long filaments cannot (Wu et al., 2013). Thus, these structures (exosomes, ectosomes, or TNTs) mediate neuron-to-neuron transfer of pathological tau protein assemblages, which is considered a fast manner of tau spread that is prion like (Abounit et al., 2016; Tardivel et al., 2016); Figure 4). Tau protein modifications can also affect the spread of tau pathology. Tau hyperphosphorylation can enhance spread, while partial dephosphorylation slows it (Alonso et al., 1996). This observation indicates that tau hyperphosphorylation may be a potential target to prevent tau pathology progression in AD and other tauopathies (Hu et al., 2016). Tau fibrils can propagate by incorporating unphosphorylated tau monomers that undergo conformational changes and are then hyperphosphorylated (Goedert et al., 2017). The extracellular domain of the amyloid precursor protein might be involved in tau fibril uptake into cells (Takahashi et al., 2015). It is well known that increasing Aβ42 oligomerization can activate protein kinases (including GSK-3β) that phosphorylate tau (Guo et al., 2013). Aβ aggregation can promote tau hyperphosphorylation, suggesting that Aβ might accelerate the spread of tau pathology (Pérez et al., 2018).
Toxicity Associated With the Spread of Tau Pathology
Tau is normally enriched on MTs within axons. In tauopathies, tau is hyperphosphorylated and accumulates in the somatodendritic compartment of brain cells, which is one of the pathological hallmarks of AD (Lee et al., 2001). Tau aggregates into insoluble filaments, forming NFTs (Morris et al., 2011) that are associated with cognitive deficits (Braak and Braak, 1991; Bierer et al., 1995; Plouffe et al., 2012). Tau phosphorylation, mislocalization, and conformational changes can alter Ca2+ homeostasis, induce dendritic spine loss, impair organelle trafficking (particularly mitochondria), and lead to cell death (Dixit et al., 2008; Zempel et al., 2010; Li et al., 2011; Spires-Jones et al., 2011). These phenotypes represent a pretangle stage, which is widely recognized as an early event in the pathological process of AD (Eckermann et al., 2007; Braak and Del Tredici, 2012). Three hypotheses have been proposed to underlie tau-mediated toxicity. First, insoluble NFTs may be toxic and lead to neuron death and cognitive dysfunction in AD; second, soluble species of misfolded, hyperphosphorylated tau may become toxic when they accumulate in inappropriate cellular compartments, whereas NFTs exert a protective effect by serving as a sink for these toxic species; and in the third view, soluble forms of pathological tau and insoluble NFTs are both toxic to cells in various ways and time scales (Kopeikina et al., 2012). We favor the third view. Neuronal transport disruption is considered an important form of tau toxicity, which is an early phenomenon and underlying cause of neurodegenerative conditions including AD (Lin and Beal, 2006; Morfini et al., 2009; Wang and Schwarz, 2009; Querfurth and LaFerla, 2010; Kopeikina et al., 2012). Recent studies have found that soluble tau species are related to synaptic or neuronal dysfunction (Berger et al., 2007; Polydoro et al., 2009; Hoover et al., 2010; Sydow et al., 2011), with results indicating that tau oligomers—but not monomers or fibrils—act as aggregation seeds in the brains of wild-type mice, leading to mitochondrial dysfunction, synaptic deficits, and memory impairment (Usenovic et al., 2015). Based on these results, the interneuronal spread of these soluble tau species might be involved in the spread of AD pathology through the brain (Braak and Del Tredici, 2012; de Calignon et al., 2012; Clavaguera et al., 2013). Furthermore, neurons can endocytose low-molecular-weight misfolded tau species (but not monomeric tau) that are transported anterogradely and retrogradely, resulting in endogenous tau pathology in vivo. However, they cannot endocytose fibrillar tau or brain-derived filamentous tau (Wu et al., 2013). This evidence strongly indicates that tau toxicity may be mediated by the cell-to-cell spread of trimeric and larger oligomeric forms in certain brain regions by endocytosis (Tian H. et al., 2013). Extracellular tau is neurotoxic (Gómez-Ramos et al., 2006) and contributes to the spread of AD pathology. When the extracellular level of free tau exceeds that inside the cell and reaches a critical concentration (Reynolds et al., 2005), tau protein will self-aggregate and induce extracellular toxicity. Paired-helical filament (PHF)-tau is less toxic than free tau. When tau interacts with muscarinic receptors, intracellular Ca2+ levels increase due to Ca2+ release from intracellular stores (Gómez-Ramos et al., 2006). Tau seeds can also cause toxicity and cell death via Ca2+ dysregulation (Querfurth and LaFerla, 2010; Tian H. et al., 2013; Hallinan et al., 2019); altered intracellular Ca2+ homeostasis results in tau phosphorylation, which is related to tau pathology progression in AD (Delacourte et al., 1999). Tau phosphorylation will increase tau detachment from MTs (Avila et al., 2004), increasing free tau levels. Free tau is then released during neurodegeneration or following cell death and binds to muscarinic receptors on surrounding cells, thereby inducing muscarinic toxicity and aggravating tau toxicity and transmission (Gómez-Ramos et al., 2008). In addition, different tau forms or isomers secreted into the extracellular space via different mechanisms may play varied roles in AD pathogenesis. Certain forms of tau released through cell death or neuroactive stimulation may be non-toxic, while misfolded tau fragments or seeds (induced by Aβ, kinases, and hydrolases) exert toxic effects on the extracellular space. Secreted vesicles containing tau protein inhibit tau binding to muscarinic receptors, thus reducing neurotoxicity.
The neurodegenerative consequences of tau hyperphos- phorylation include axonal transport impairment (Ittner et al., 2008), tau relocalization to the somatodendritic compartment, and synaptic loss (Di et al., 2016). Synaptic dysfunction can occur both presynaptically, where it can interfere with the transport of phosphorylated tau via synaptic vesicles (Zhou et al., 2017), and postsynaptically via the downregulation of AMPA receptors (Hoover et al., 2010). In the context of the prion hypothesis, tau assemblies that enter the cytoplasm can seed native monomer aggregation, and these species can be released and spread to neighboring cells (Clavaguera et al., 2013; Kundel et al., 2018). Under pathological conditions, aberrant posttranslational modifications such as hyperphosphorylation, truncation, deamidation, and others (Avila et al., 2004) can induce tau detachment from MTs and promote their accumulation in a free form. When neurons degenerate and die, this free tau enters the extracellular space, where it is free to diffuse in every direction (Guo and Lee, 2011; Hu et al., 2016). This is in line with the observation that neuron loss is progressive within brain areas affected by degeneration, and tau species can seed misfolding in human brains without tangles (DeVos et al., 2018). This implies that tau seeds are released from intact neurons prior to neuronal death (Pickett et al., 2017). Another possible explanation of tau pathology patterns in the brains of AD patients is that extracellular NFTs or other substances released by degenerating neurons accumulate in the extracellular space and damage nearby cells (Avila, 2006). These toxic compounds can act like extracellular Aβ peptides (Gómez-Ramos et al., 2006). Thus, both soluble tau species and insoluble NFTs may contribute to the spread of tau toxicity.
Conclusion and Prospects
The mechanism involved in the transcellular propagation of tau in neurodegeneration is still unclear. Studies on the molecular mechanisms underlying the release, propagation, and uptake of tau are needed; improving the ability to detect secreted tau species is also important. Future research should focus on reducing the secretion and generation of extracellular tau in a soluble or aggregated form and inhibiting cell uptake. However, tau aggregate species are diverse, and it is currently unclear if certain species prefer certain secretion pathways. It will be important to clarify whether the physiologic secretion of non-pathological tau from neurons occurs via the same or overlapping mechanisms as those for the pathologic forms. Moreover, both forms of tau secretion are connected to neuronal activity, so blocking synapse-mediated tau propagation and boosting the clearance of tau aggregates that are internalized at the synapse are equally important (Calafate et al., 2015). More attention should also be given to glial cells and the glymphatic system, which might play a role in clearing and propagating pathological protein aggregates. Inhibition of donor cell release and recipient cell uptake are also novel therapeutic directions worthy of consideration. For example, it might be possible to block exosome secretion pathways and apply tau antibodies that can act on pathological tau fragments in the extracellular space and inhibit tau aggregation on membranous structures. Recent studies have shown that anti-tau antibodies can reduce tau hyperphosphorylation and aggregation in the transgenic mouse brain (Yanamandra et al., 2013; Sankaranarayanan et al., 2015). In addition, preventing tau from binding to HSPGs precludes recombinant tau fibrils from inducing intracellular aggregation and blocks transcellular aggregate propagation. In vivo, the heparin mimetic F6 prevents neuronal uptake of tau fibrils injected stereotactically (Holmes et al., 2013). Moreover, microglia disseminate tau via exosome secretion, and hampering exosome synthesis significantly reduces tau propagation in vitro and in vivo, which implies that exosomes and microglia contribute to tauopathy progression. It also suggests that targeting the exosome secretion pathway could be therapeutically useful. Depleting microglia dramatically suppressed tau propagation and reduced excitability in the dentate gyrus in a mouse model of AD (Asai et al., 2015). In summary, tau release mechanics can be explored to develop new treatments for AD and other tauopathies.
Author Contributions
HZ wrote the manuscript. YC and LM assisted in the manuscript writing. YW and HL assisted in ideas and modification of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding. This review was funded by the National Natural Science Foundation of China (No. 81873350), the National Science and Technology Major Project for “Essential new drug research and development” (No. 2019ZX09301114), and the National Natural Science Foundation of China (No. 81904194).
References
- Abounit S., Wu J., Duff K., Victoria G., Zurzolo C. (2016). Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 10 344–351. 10.1080/19336896.2016.1223003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Cooper J., Murray T., Garn K., Mcnaughton E., Clarke H., et al. (2014). A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127 667–683. 10.1007/s00401-014-1254-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam J., Ozer R., Safer D., Halpain S., Milligan R. (2002). MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J. Cell Biol. 157 1187–1196. 10.1083/jcb.200201048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A., Grundke-Iqbal I., Iqbal K. (1996). Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat. Med. 2 783–787. 10.1038/nm0796-783 [DOI] [PubMed] [Google Scholar]
- Alonso A., Grundke-Iqbal I., Barra H., Iqbal K. (1997). Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. U. S. A. 94 298–303. 10.1073/pnas.94.1.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., et al. (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18 1584–1593. 10.1038/nn.4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack J., Schneider A., Mandelkow E., Hyman B. (2002). Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103 26–35. 10.1007/s004010100423 [DOI] [PubMed] [Google Scholar]
- Avila J. (2006). Tau phosphorylation and aggregation in Alzheimer’s disease pathology. FEBS Lett. 580 2922–2927. 10.1016/j.febslet.2006.02.067 [DOI] [PubMed] [Google Scholar]
- Avila J., Lucas J., Perez M., Hernandez F. (2004). Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 84 361–384. 10.1016/j.tcb.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Barten D., Fanara P., Andorfer C., Hoque N., Wong P., Husted K., et al. (2012). Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. J. Neurosci. 32 7137–7145. 10.1523/jneurosci.0188-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélemy N., Gabelle A., Hirtz C., Fenaille F., Sergeant N., Schraen-Maschke S., et al. (2016). Differential Mass Spectrometry Profiles of Tau Protein in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease, Progressive Supranuclear Palsy, and Dementia with Lewy Bodies. J. Alzheimers Dis. 51 1033–1043. 10.3233/jad-150962 [DOI] [PubMed] [Google Scholar]
- Barton A., Harrison P., Najlerahim A., Heffernan J., Mcdonald B., Robinson J., et al. (1990). Increased tau messenger RNA in Alzheimer’s disease hippocampus. Am. J. Pathol. 137 497–502. [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Bi X. (2016). Calpain-1 and Calpain-2: the Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 39 235–245. 10.1016/j.tins.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z., Roder H., Hanna A., Carlson A., Rangachari V., Yue M., et al. (2007). Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 27 3650–3662. 10.1523/jneurosci.0587-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer L., Hof P., Purohit D., Carlin L., Schmeidler J., Davis K., et al. (1995). Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 52 81–88. 10.1001/archneur.1995.00540250089017 [DOI] [PubMed] [Google Scholar]
- Boluda S., Iba M., Zhang B., Raible K., Lee V., Trojanowski J. (2015). Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 129 221–237. 10.1007/s00401-014-1373-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondulich M., Guo T., Meehan C., Manion J., Rodriguez Martin T., Mitchell J., et al. (2016). Tauopathy induced by low level expression of a human brain-derived tau fragment in mice is rescued by phenylbutyrate. Brain 139 2290–2306. 10.1093/brain/aww137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82 239–259. 10.1007/bf00308809 [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. (2012). Alzheimer’s disease: pathogenesis and prevention. Alzheimers Dement. 8 227–233. 10.1016/j.jalz.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Brandt R., Léger J., Lee G. (1995). Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J. Cell Biol. 131 1327–1340. 10.1083/jcb.131.5.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J., Hussain S., Dang V., Wright S., Cooper B., Byun T., et al. (2015). Human secreted tau increases amyloid-beta production. Neurobiol. Aging 36 693–709. 10.1016/j.neurobiolaging.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Brunello C., Merezhko M., Uronen R., Huttunen H. (2020). Mechanisms of secretion and spreading of pathological tau protein. Cell. Mol. Life Sci. 77 1721–1744. 10.1007/s00018-019-03349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello C., Yan X., Huttunen H. (2016). Internalized Tau sensitizes cells to stress by promoting formation and stability of stress granules. Sci. Rep. 6:30498. 10.1038/srep30498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafate S., Buist A., Miskiewicz K., Vijayan V., Daneels G., De Strooper B., et al. (2015). Synaptic Contacts Enhance Cell-to-Cell Tau Pathology Propagation. Cell Rep. 11 1176–1183. 10.1016/j.celrep.2015.04.043 [DOI] [PubMed] [Google Scholar]
- Chai X., Dage J., Citron M. (2012). Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis. 48 356–366. 10.1016/j.nbd.2012.05.021 [DOI] [PubMed] [Google Scholar]
- Chen X., Jiang H. (2019). Tau as a potential therapeutic target for ischemic stroke. Aging 11 12827–12843. 10.18632/aging.102547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser A., Pritchard S., Johnson G. (2013). Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front. Neurol. 4:122. 10.3389/fneur.2013.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson H., Svensson K., Van Kuppevelt T., Li J., Belting M. (2013). Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. U. S. A. 110 17380–17385. 10.1073/pnas.1304266110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C., Song Y., Kim I., Yoon W., Ryu B., Jo D., et al. (2001). Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis. 8 162–172. 10.1006/nbdi.2000.0335 [DOI] [PubMed] [Google Scholar]
- Clavaguera F., Akatsu H., Fraser G., Crowther R., Frank S., Hench J., et al. (2013). Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. U. S. A. 110 9535–9540. 10.1073/pnas.1301175110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Bolmont T., Crowther R., Abramowski D., Frank S., Probst A., et al. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11 909–913. 10.1038/ncb1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Hench J., Goedert M., Tolnay M. (2015). Invited review: prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 41 47–58. 10.1111/nan.12197 [DOI] [PubMed] [Google Scholar]
- Cohen T., Constance B., Hwang A., James M., Yuan C. (2016). Intrinsic Tau Acetylation Is Coupled to Auto-Proteolytic Tau Fragmentation. PLoS One 11:e0158470. 10.1371/journal.pone.0158470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T., Friedmann D., Hwang A., Marmorstein R., Lee V. (2013). The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat. Struct. Mol. Biol. 20 756–762. 10.1038/nsmb.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C., Carlomagno Y., Gendron T., Dunmore J., Scheffel K., Stetler C., et al. (2014). Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 23 104–116. 10.1093/hmg/ddt402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti V., Amadoro G., Gentile A., Capsoni S., Ciotti M., Cencioni M., et al. (2008). Identification of a caspase-derived N-terminal tau fragment in cellular and animal Alzheimer’s disease models. Mol. Cell. Neurosci. 38 381–392. 10.1016/j.mcn.2008.03.011 [DOI] [PubMed] [Google Scholar]
- Cras P., Smith M., Richey P., Siedlak S., Mulvihill P., Perry G. (1995). Extracellular neurofibrillary tangles reflect neuronal loss and provide further evidence of extensive protein cross-linking in Alzheimer disease. Acta Neuropathol. 89 291–295. 10.1007/bf00309621 [DOI] [PubMed] [Google Scholar]
- Croft C., Wade M., Kurbatskaya K., Mastrandreas P., Hughes M., Phillips E., et al. (2017). Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. Cell Death Dis. 8:e2671. 10.1038/cddis.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A., Fox L., Pitstick R., Carlson G., Bacskai B., Spires-Jones T., et al. (2010). Caspase activation precedes and leads to tangles. Nature 464 1201–1204. 10.1038/nature08890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D., Kopeikina K., et al. (2012). Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73 685–697. 10.1016/j.neuron.2011.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A., David J., Sergeant N., Buée L., Wattez A., Vermersch P., et al. (1999). The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52 1158–1165. 10.1212/wnl.52.6.1158 [DOI] [PubMed] [Google Scholar]
- DeVos S., Corjuc B., Oakley D., Nobuhara C., Bannon R., Chase A., et al. (2018). Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front. Neurosci. 12:267. 10.3389/fnins.2018.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di J., Cohen L., Corbo C., Phillips G., El Idrissi A., Alonso A. (2016). Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss. Sci. Rep. 6:20833. 10.1038/srep20833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Hernández M., Gómez-Ramos A., Rubio A., Gómez-Villafuertes R., Naranjo J., Miras-Portugal M., et al. (2010). Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J. Biol. Chem. 285 32539–32548. 10.1074/jbc.M110.145003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C., Kamal A., Lundgren K., Klosak N., Bailey R., Dunmore J., et al. (2007). The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 117 648–658. 10.1172/jci29715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Ross J., Goldman Y., Holzbaur E. (2008). Differential regulation of dynein and kinesin motor proteins by tau. Science 319 1086–1089. 10.1126/science.1152993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan P., Johnson G. (2010). A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J. Biol. Chem. 285 21978–21987. 10.1074/jbc.M110.110940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S., Bégard S., Caillierez R., Lachaud C., Delattre L., Carrier S., et al. (2014a). Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One 9:e100760. 10.1371/journal.pone.0100760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S., Lécolle K., Caillierez R., Bégard S., Zommer N., Lachaud C., et al. (2014b). Neuron-to-neuron wild-type Tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2:14. 10.1186/2051-5960-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckermann K., Mocanu M., Khlistunova I., Biernat J., Nissen A., Hofmann A., et al. (2007). The beta-propensity of Tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J. Biol. Chem. 282 31755–31765. 10.1074/jbc.M705282200 [DOI] [PubMed] [Google Scholar]
- Evans L., Wassmer T., Fraser G., Smith J., Perkinton M., Billinton A., et al. (2018). Extracellular Monomeric and Aggregated Tau Efficiently Enter Human Neurons through Overlapping but Distinct Pathways. Cell Rep. 22 3612–3624. 10.1016/j.celrep.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fá M., Puzzo D., Piacentini R., Staniszewski A., Zhang H., Baltrons M., et al. (2016). Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory. Sci. Rep. 6:19393. 10.1038/srep19393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Montoya J., Pérez M. (2015). Cathepsin D in a murine model of frontotemporal dementia with Parkinsonism-linked to chromosome 17. J. Alzheimers Dis. 45 1–14. 10.3233/jad-140456 [DOI] [PubMed] [Google Scholar]
- Fiandaca M., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J., et al. (2015). Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 11 600–607.e1. 10.1016/j.jalz.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D., Mukrasch M., Biernat J., Bibow S., Blackledge M., Griesinger C., et al. (2009). Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochemistry 48 10047–10055. 10.1021/bi901090m [DOI] [PubMed] [Google Scholar]
- Fontaine S., Zheng D., Sabbagh J., Martin M., Chaput D., Darling A., et al. (2016). DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 35 1537–1549. 10.15252/embj.201593489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B., Jacks R., Diamond M. (2009). Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284 12845–12852. 10.1074/jbc.M808759200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutani Y., Kobayashi K., Nakamura I., Watanabe K., Isaki K., Cairns N. (1995). Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci. Lett. 200 57–60. 10.1016/0304-3940(95)12083-g [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A., Hernández F., Ávila J. (2018). Tau Spreading Mechanisms; Implications for Dysfunctional Tauopathies. Int. J. Mol. Sci. 19:645. 10.3390/ijms19030645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin T., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A., et al. (2003). Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 100 10032–10037. 10.1073/pnas.1630428100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Cleveland D. (2001). Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 13 41–48. 10.1016/s0955-0674(00)00172-1 [DOI] [PubMed] [Google Scholar]
- García-Sierra F., Mondragón-Rodríguez S., Basurto-Islas G. (2008). Truncation of tau protein and its pathological significance in Alzheimer’s disease. J. Alzheimers Dis. 14 401–409. 10.3233/jad-2008-14407 [DOI] [PubMed] [Google Scholar]
- Garg S., Timm T., Mandelkow E., Mandelkow E., Wang Y. (2011). Cleavage of Tau by calpain in Alzheimer’s disease: the quest for the toxic 17 kD fragment. Neurobiol. Aging 32 1–14. 10.1016/j.neurobiolaging.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Gibbons G., Lee V., Trojanowski J. (2019). Mechanisms of Cell-to-Cell Transmission of Pathological Tau: a Review. JAMA Neurol. 76 101–108. 10.1001/jamaneurol.2018.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. (1999). Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354 1101–1118. 10.1098/rstb.1999.0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. (2011). Pathogenesis of the tauopathies. J. Mol. Neurosci. 45 425–431. 10.1007/s12031-011-9593-4 [DOI] [PubMed] [Google Scholar]
- Goedert M., Clavaguera F., Tolnay M. (2010). The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33 317–325. 10.1016/j.tins.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Goedert M., Eisenberg D., Crowther R. (2017). Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 40 189–210. 10.1146/annurev-neuro-072116-031153 [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M., Jakes R., Rutherford D., Crowther R. (1989). Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3 519–526. 10.1016/0896-6273(89)90210-9 [DOI] [PubMed] [Google Scholar]
- Gómez-Ramos A., Díaz-Hernández M., Cuadros R., Hernández F., Avila J. (2006). Extracellular tau is toxic to neuronal cells. FEBS Lett. 580 4842–4850. 10.1016/j.febslet.2006.07.078 [DOI] [PubMed] [Google Scholar]
- Gómez-Ramos A., Díaz-Hernández M., Rubio A., Miras-Portugal M., Avila J. (2008). Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol. Cell. Neurosci. 37 673–681. 10.1016/j.mcn.2007.12.010 [DOI] [PubMed] [Google Scholar]
- Guo J., Lee V. (2011). Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 286 15317–15331. 10.1074/jbc.M110.209296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Covell D., Daniels J., Iba M., Stieber A., Zhang B., et al. (2013). Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154 103–117. 10.1016/j.cell.2013.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Noble W., Hanger D. (2017). Roles of tau protein in health and disease. Acta Neuropathol. 133 665–704. 10.1007/s00401-017-1707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N., Trinczek B., Biernat J., Mandelkow E., Mandelkow E. (1994). Domains of tau protein and interactions with microtubules. Biochemistry 33 9511–9522. 10.1021/bi00198a017 [DOI] [PubMed] [Google Scholar]
- Hallinan G., Vargas-Caballero M., West J., Deinhardt K. (2019). Tau Misfolding Efficiently Propagates between Individual Intact Hippocampal Neurons. J. Neurosci. 39 9623–9632. 10.1523/jneurosci.1590-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Blennow K., Shaw L., Hoessler Y., Zetterberg H., Trojanowski J. (2010). Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp. Gerontol. 45 30–40. 10.1016/j.exger.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Teipel S., Fuchsberger T., Andreasen N., Wiltfang J., Otto M., et al. (2004). Value of CSF beta-amyloid1-42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol. Psychiatry 9 705–710. 10.1038/sj.mp.4001473 [DOI] [PubMed] [Google Scholar]
- Hanger D., Anderton B., Noble W. (2009). Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15 112–119. 10.1016/j.molmed.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Hanger D., Lau D., Phillips E., Bondulich M., Guo T., Woodward B., et al. (2014). Intracellular and extracellular roles for tau in neurodegenerative disease. J. Alzheimers Dis. 40 S37–S45. 10.3233/jad-132054 [DOI] [PubMed] [Google Scholar]
- Harris J., Koyama A., Maeda S., Ho K., Devidze N., Dubal D., et al. (2012). Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS One 7:e45881. 10.1371/journal.pone.0045881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., DeVos S., Kfoury N., Li M., Jacks R., Yanamandra K., et al. (2013). Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. U. S. A. 110 E3138–E3147. 10.1073/pnas.1301440110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. (2011). Glypican-1 facilitates prion conversion in lipid rafts. J. Neurochem. 116 721–725. 10.1111/j.1471-4159.2010.06936.x [DOI] [PubMed] [Google Scholar]
- Hoover B., Reed M., Su J., Penrod R., Kotilinek L., Grant M., et al. (2010). Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68 1067–1081. 10.1016/j.neuron.2010.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Zhang X., Tung Y., Xie S., Liu F., Iqbal K. (2016). Hyperphosphorylation determines both the spread and the morphology of tau pathology. Alzheimers Dement. 12 1066–1077. 10.1016/j.jalz.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Iba M., Guo J., Mcbride J., Zhang B., Trojanowski J., Lee V. (2013). Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 33 1024–1037. 10.1523/jneurosci.2642-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii K., Ito H., Kominami E., Hirano A. (1993). Abnormal distribution of cathepsin proteinases and endogenous inhibitors (cystatins) in the hippocampus of patients with Alzheimer’s disease, parkinsonism-dementia complex on Guam, and senile dementia and in the aged. Virchows Arch. A Pathol. Anat. Histopathol. 423 185–194. 10.1007/bf01614769 [DOI] [PubMed] [Google Scholar]
- Iqbal K., Flory M., Khatoon S., Soininen H., Pirttila T., Lehtovirta M., et al. (2005). Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann. Neurol. 58 748–757. 10.1002/ana.20639 [DOI] [PubMed] [Google Scholar]
- Ittner L., Fath T., Ke Y., Bi M., Van Eersel J., Li K., et al. (2008). Parkinsonism and impaired axonal transport in a mouse model of frontotemporal dementia. Proc. Natl. Acad. Sci. U. S. A. 105 15997–16002. 10.1073/pnas.0808084105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho Y., Zhulina E., Kim M., Pincus P. (2010). Monte carlo simulations of tau proteins: effect of phosphorylation. Biophys. J. 99 2387–2397. 10.1016/j.bpj.2010.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G., Hartigan J. (1999). Tau protein in normal and Alzheimer’s disease brain: an update. J. Alzheimers Dis. 1 329–351. 10.3233/jad-1999-14-512 [DOI] [PubMed] [Google Scholar]
- Johnson G., Stoothoff W. (2004). Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117 5721–5729. 10.1242/jcs.01558 [DOI] [PubMed] [Google Scholar]
- Jucker M., Walker L. (2013). Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501 45–51. 10.1038/nature12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmert D., Cantlon A., Muratore C., Jin M., O’malley T., Lee G., et al. (2015). C-Terminally Truncated Forms of Tau, But Not Full-Length Tau or Its C-Terminal Fragments, Are Released from Neurons Independently of Cell Death. J. Neurosci. 35 10851–10865. 10.1523/jneurosci.0387-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S., Fan J., Smith M., Goedert M., Amos L. (2003). Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 22 70–77. 10.1093/emboj/cdg001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch C., Jeng A., Goate A. (2012). Extracellular Tau levels are influenced by variability in Tau that is associated with tauopathies. J. Biol. Chem. 287 42751–42762. 10.1074/jbc.M112.380642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsinelos T., Zeitler M., Dimou E., Karakatsani A., Müller H., Nachman E., et al. (2018). Unconventional Secretion Mediates the Trans-cellular Spreading of Tau. Cell Rep. 23 2039–2055. 10.1016/j.celrep.2018.04.056 [DOI] [PubMed] [Google Scholar]
- Kenessey A., Nacharaju P., Ko L., Yen S. (1997). Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J. Neurochem. 69 2026–2038. 10.1046/j.1471-4159.1997.69052026.x [DOI] [PubMed] [Google Scholar]
- Kfoury N., Holmes B., Jiang H., Holtzman D., Diamond M. (2012). Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 287 19440–19451. 10.1074/jbc.M112.346072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Lee S., Hall G. (2010a). Secretion of human tau fragments resembling CSF-tau in Alzheimer’s disease is modulated by the presence of the exon 2 insert. FEBS Lett. 584 3085–3088. 10.1016/j.febslet.2010.05.042 [DOI] [PubMed] [Google Scholar]
- Kim W., Lee S., Jung C., Ahmed A., Lee G., Hall G. (2010b). Interneuronal transfer of human tau between Lamprey central neurons in situ. J. Alzheimers Dis. 19 647–664. 10.3233/jad-2010-1273 [DOI] [PubMed] [Google Scholar]
- Kopeikina K., Hyman B., Spires-Jones T. (2012). Soluble forms of tau are toxic in Alzheimer’s disease. Transl. Neurosci. 3 223–233. 10.2478/s13380-012-0032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundel F., Hong L., Falcon B., Mcewan W., Michaels T., Meisl G., et al. (2018). Measurement of Tau Filament Fragmentation Provides Insights into Prion-like Spreading. ACS Chem. Neurosci. 9 1276–1282. 10.1021/acschemneuro.8b00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves C., Castillo-Carranza D., Sengupta U., Guerrero-Munoz M., Kiritoshi T., Neugebauer V., et al. (2012). Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2:700. 10.1038/srep00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M., Kim W., Lee S., Mckee A., Hall G. (2012). Multiple mechanisms of extracellular tau spreading in a non-transgenic tauopathy model. Am. J. Neurodegener. Dis. 1 316–333. [PMC free article] [PubMed] [Google Scholar]
- Lee G., Newman S., Gard D., Band H., Panchamoorthy G. (1998). Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111 3167–3177. [DOI] [PubMed] [Google Scholar]
- Lee M., Lee J., Rubinsztein D. (2013). Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog. Neurobiol. 105 49–59. 10.1016/j.pneurobio.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Lee V., Goedert M., Trojanowski J. (2001). Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24 1121–1159. 10.1146/annurev.neuro.24.1.1121 [DOI] [PubMed] [Google Scholar]
- Lewis J., Dickson D. (2016). Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 131 27–48. 10.1007/s00401-015-1507-z [DOI] [PubMed] [Google Scholar]
- Li X., Kumar Y., Zempel H., Mandelkow E., Biernat J., Mandelkow E. (2011). Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO J. 30 4825–4837. 10.1038/emboj.2011.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Beal M. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443 787–795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Liu L., Drouet V., Wu J., Witter M., Small S., Clelland C., et al. (2012). Trans-synaptic spread of tau pathology in vivo. PLoS One 7:e31302. 10.1371/journal.pone.0031302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wei W., Yin J., Liu G., Wang Q., Cao F., et al. (2009). Proteasome inhibition increases tau accumulation independent of phosphorylation. Neurobiol. Aging 30 1949–1961. 10.1016/j.neurobiolaging.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Maas T., Eidenmüller J., Brandt R. (2000). Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J. Biol. Chem. 275 15733–15740. 10.1074/jbc.M000389200 [DOI] [PubMed] [Google Scholar]
- Martini-Stoica H., Cole A., Swartzlander D., Chen F., Wan Y., Bajaj L., et al. (2018). TFEB enhances astroglial uptake of extracellular tau species and reduces tau spreading. J. Expe. Med. 215 2355–2377. 10.1084/jem.20172158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Motoi Y., Ishiguro K., Tabira T., Kametani F., Hasegawa M., et al. (2015). The twenty-four KDa C-terminal tau fragment increases with aging in tauopathy mice: implications of prion-like properties. Hum. Mol. Genet. 24 6403–6416. 10.1093/hmg/ddv351 [DOI] [PubMed] [Google Scholar]
- Min S., Chen X., Tracy T., Li Y., Zhou Y., Wang C., et al. (2015). Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21 1154–1162. 10.1038/nm.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S., Cho S., Zhou Y., Schroeder S., Haroutunian V., Seeley W., et al. (2010). Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67 953–966. 10.1016/j.neuron.2010.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H., Holmes B., Sanders D., Bieschke J., Diamond M. (2015). Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J. Biol. Chem. 290 14893–14903. 10.1074/jbc.M115.652693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N., Desjardins A., Leclerc N. (2017). Tau secretion is correlated to an increase of Golgi dynamics. PLoS One 12:e0178288. 10.1371/journal.pone.0178288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N., Herrou T., Plouffe V., Piperno N., Leclerc N. (2013). Spreading of tau pathology in Alzheimer’s disease by cell-to-cell transmission. Eur. J. Neurosci. 37 1939–1948. 10.1111/ejn.12229 [DOI] [PubMed] [Google Scholar]
- Mohamed N., Plouffe V., Rémillard-Labrosse G., Planel E., Leclerc N. (2014). Starvation and inhibition of lysosomal function increased tau secretion by primary cortical neurons. Sci. Rep. 4:5715. 10.1038/srep05715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G., Burns M., Binder L., Kanaan N., Lapointe N., Bosco D., et al. (2009). Axonal transport defects in neurodegenerative diseases. J. Neurosci. 29 12776–12786. 10.1523/jneurosci.3463-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M., Maeda S., Vossel K., Mucke L. (2011). The many faces of tau. Neuron 70 410–426. 10.1016/j.neuron.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S., DeVos S., Commins C., Nobuhara C., Bennett R., Corjuc D., et al. (2017). Characterization of TauC3 antibody and demonstration of its potential to block tau propagation. PLoS One 12:e0177914. 10.1371/journal.pone.0177914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W., Rabouille C. (2009). Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10 148–155. 10.1038/nrm2617 [DOI] [PubMed] [Google Scholar]
- Nobuhara C., DeVos S., Commins C., Wegmann S., Moore B., Roe A., et al. (2017). Tau Antibody Targeting Pathological Species Blocks Neuronal Uptake and Interneuron Propagation of Tau in Vitro. Am. J. Pathol. 187 1399–1412. 10.1016/j.ajpath.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Ferreira A. (2005). The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J. Neurosci. 25 5365–5375. 10.1523/jneurosci.1125-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeraer E., Bottelbergs A., Van Kolen K., Stancu I., Vasconcelos B., Mahieu M., et al. (2015). Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol. Dis. 73 83–95. 10.1016/j.nbd.2014.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea J., López E., Díez-Ballesteros J., Ávila J., Hernández F., Bolós M. (2019). Extracellular Monomeric Tau Is Internalized by Astrocytes. Front. Neurosci. 13:442. 10.3389/fnins.2019.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez M., Cuadros R., Hernández F., Avila J. (2016). Secretion of full-length tau or tau fragments in a cell culture model. Neurosci. Lett. 634 63–69. 10.1016/j.neulet.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Pérez M., Medina M., Hernández F., Avila J. (2018). Secretion of full-length Tau or Tau fragments in cell culture models. Propagation of Tau in vivo and in vitro. Biomol. Concepts 9 1–11. 10.1515/bmc-2018-0001 [DOI] [PubMed] [Google Scholar]
- Pickett E., Henstridge C., Allison E., Pitstick R., Pooler A., Wegmann S., et al. (2017). Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer’s disease. Synapse 71:e21965. 10.1002/syn.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe V., Mohamed N., Rivest-Mcgraw J., Bertrand J., Lauzon M., Leclerc N. (2012). Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS One 7:e36873. 10.1371/journal.pone.0036873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco J., Li C., Durisic N., Sullivan R., Götz J. (2018). Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 6:10. 10.1186/s40478-018-0514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco J., Scicluna B., Hill A., Götz J. (2016). Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J. Biol. Chem. 291 12445–12466. 10.1074/jbc.M115.709485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydoro M., Acker C., Duff K., Castillo P., Davies P. (2009). Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J. Neurosci. 29 10741–10749. 10.1523/jneurosci.1065-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler A., Phillips E., Lau D., Noble W., Hanger D. (2013). Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14 389–394. 10.1038/embor.2013.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth H., LaFerla F. (2010). Alzheimer’s disease. N. Engl. J. Med. 362 329–344. 10.1056/NEJMra0909142 [DOI] [PubMed] [Google Scholar]
- Quinn J., Corbett N., Kellett K., Hooper N. (2018). Tau Proteolysis in the Pathogenesis of Tauopathies: neurotoxic Fragments and Novel Biomarkers. J. Alzheimers Dis. 63 13–33. 10.3233/jad-170959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharitar J., Albrecht S., Afonso V., Kaushal V., Bennett D., Leblanc A. (2013). Cerebrospinal fluid tau cleaved by caspase-6 reflects brain levels and cognition in aging and Alzheimer disease. J. Neuropathol. Exp. Neurol. 72 824–832. 10.1097/NEN.0b013e3182a0a39f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch J., Chen J., Sorum A., Miller G., Sharf T., See S., et al. (2018). Tau Internalization is Regulated by 6-O Sulfation on Heparan Sulfate Proteoglycans (HSPGs). Sci. Rep. 8:6382. 10.1038/s41598-018-24904-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C., Garwood C., Wray S., Price C., Kellie S., Perera T., et al. (2008). Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J. Biol. Chem. 283 18177–18186. 10.1074/jbc.M709715200 [DOI] [PubMed] [Google Scholar]
- Reynolds M., Berry R., Binder L. (2005). Site-specific nitration differentially influences tau assembly in vitro. Biochemistry 44 13997–14009. 10.1021/bi051028w [DOI] [PubMed] [Google Scholar]
- Riemenschneider M., Wagenpfeil S., Vanderstichele H., Otto M., Wiltfang J., Kretzschmar H., et al. (2003). Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol. Psychiatry 8 343–347. 10.1038/sj.mp.4001220 [DOI] [PubMed] [Google Scholar]
- Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H. (2004). Nanotubular highways for intercellular organelle transport. Science 303 1007–1010. 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- Sahara N., Murayama M., Higuchi M., Suhara T., Takashima A. (2014). Biochemical Distribution of Tau Protein in Synaptosomal Fraction of Transgenic Mice Expressing Human P301L Tau. Front. Neurol. 5:26. 10.3389/fneur.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., et al. (2012). Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287 3842–3849. 10.1074/jbc.M111.277061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S., Barten D., Vana L., Devidze N., Yang L., Cadelina G., et al. (2015). Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One 10:e0125614. 10.1371/journal.pone.0125614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarella R., Skiniotis G., Goldie K., Tittmann P., Gross H., Mandelkow E., et al. (2004). Surface-decoration of microtubules by human tau. J. Mol. Biol. 339 539–553. 10.1016/j.jmb.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Schmitz Y., Luccarelli J., Kim M., Wang M., Sulzer D. (2009). Glutamate controls growth rate and branching of dopaminergic axons. J. Neurosci. 29 11973–11981. 10.1523/jneurosci.2927-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S., Horowitz P., Karsten S., Jackson G., Geschwind D., Fu Y., et al. (2006). Degradation of tau protein by puromycin-sensitive aminopeptidase in vitro. Biochemistry 45 15111–15119. 10.1021/bi061830d [DOI] [PubMed] [Google Scholar]
- Shackelford D., Yeh R. (1998). Dephosphorylation of tau during transient forebrain ischemia in the rat. Mol. Chem. Neuropathol. 34 103–120. 10.1007/bf02815073 [DOI] [PubMed] [Google Scholar]
- Shafiei S., Guerrero-Muñoz M., Castillo-Carranza D. (2017). Tau Oligomers: cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 9:83. 10.3389/fnagi.2017.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón D., García-García E., Royo F., Falcón-Pérez J., Avila J. (2012). Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett. 586 47–54. 10.1016/j.febslet.2011.11.022 [DOI] [PubMed] [Google Scholar]
- Sokolow S., Henkins K., Bilousova T., Gonzalez B., Vinters H., Miller C., et al. (2015). Pre-synaptic C-terminal truncated tau is released from cortical synapses in Alzheimer’s disease. J. Neurochem. 133 368–379. 10.1111/jnc.12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski J., Gamage K., Heffron D., Leblanc A., Deppmann C., Mandell J. (2014). Caspase-mediated cleavage of actin and tubulin is a common feature and sensitive marker of axonal degeneration in neural development and injury. Acta Neuropathol. Commun. 2:16. 10.1186/2051-5960-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T., Kopeikina K., Koffie R., de Calignon A., Hyman B. (2011). Are tangles as toxic as they look? J. Mol. Neurosci. 45 438–444. 10.1007/s12031-011-9566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson E., Breckenridge L., Mcmahon L., Som S., Mcconnell I., Bloom G. (2017). Extracellular Tau Oligomers Induce Invasion of Endogenous Tau into the Somatodendritic Compartment and Axonal Transport Dysfunction. J. Alzheimers Dis. 58 803–820. 10.3233/jad-170168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow A., Van Der Jeugd A., Zheng F., Ahmed T., Balschun D., Petrova O., et al. (2011). Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J. Neurosci. 31 2511–2525. 10.1523/jneurosci.5245-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H., Wang B., Serrano-Pozo A., Frosch M., Spires-Jones T., Hyman B. (2014). Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer’s disease. Acta Neuropathol. Commun. 2:146. 10.1186/s40478-014-0146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Miyata H., Kametani F., Nonaka T., Akiyama H., Hisanaga S., et al. (2015). Extracellular association of APP and tau fibrils induces intracellular aggregate formation of tau. Acta Neuropathol. 129 895–907. 10.1007/s00401-015-1415-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Wegmann S., Cho H., DeVos S., Commins C., Roe A., et al. (2015). Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun. 6:8490. 10.1038/ncomms9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardivel M., Bégard S., Bousset L., Dujardin S., Coens A., Melki R., et al. (2016). Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 4:117. 10.1186/s40478-016-0386-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Ostrowski M., Segura E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9 581–593. 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- Tian H., Davidowitz E., Lopez P., Emadi S., Moe J., Sierks M. (2013). Trimeric tau is toxic to human neuronal cells at low nanomolar concentrations. Int. J. Cell Biol. 2013:260787. 10.1155/2013/260787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Zhu Y., Hu F., Wang Y., Huang N., Xiao Z. (2013). Dynamics of exosome internalization and trafficking. J. Cell. Physiol. 228 1487–1495. 10.1002/jcp.24304 [DOI] [PubMed] [Google Scholar]
- Usenovic M., Niroomand S., Drolet R., Yao L., Gaspar R., Hatcher N., et al. (2015). Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. 35 14234–14250. 10.1523/jneurosci.1523-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V., Sergeant N., Buée L. (2012). Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front. Physiol. 3:229. 10.3389/fphys.2012.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M., Barghorn S., Biernat J., Mandelkow E., Mandelkow E. (2005). Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim. Biophys. Acta 1739 158–166. 10.1016/j.bbadis.2004.09.010 [DOI] [PubMed] [Google Scholar]
- von Bergen M., Friedhoff P., Biernat J., Heberle J., Mandelkow E., Mandelkow E. (2000). Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. U. S. A. 97 5129–5134. 10.1073/pnas.97.10.5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gao X., Wang Z. (2014). The physiology and pathology of microtubule-associated protein tau. Essays Biochem. 56 111–123. 10.1042/bse0560111 [DOI] [PubMed] [Google Scholar]
- Wang X., Schwarz T. (2009). The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136 163–174. 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mandelkow E. (2016). Tau in physiology and pathology. Nat. Rev. Neurosci. 17 5–21. 10.1038/nrn.2015.1 [DOI] [PubMed] [Google Scholar]
- Wang Y., Balaji V., Kaniyappan S., Krüger L., Irsen S., Tepper K., et al. (2017). The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 12:5. 10.1186/s13024-016-0143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Martinez-Vicente M., Krüger U., Kaushik S., Wong E., Mandelkow E., et al. (2009). Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. 18 4153–4170. 10.1093/hmg/ddp367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S., Eftekharzadeh B., Tepper K., Zoltowska K., Bennett R., Dujardin S., et al. (2018). Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 37:e98049. 10.15252/embj.201798049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Herman M., Liu L., Simoes S., Acker C., Figueroa H., et al. (2013). Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 288 1856–1870. 10.1074/jbc.M112.394528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Hussaini S., Bastille I., Rodriguez G., Mrejeru A., Rilett K., et al. (2016). Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19 1085–1092. 10.1038/nn.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. (2017). Extracellular Tau and Its Potential Role in the Propagation of Tau Pathology. Front. Neurosci. 11:667. 10.3389/fnins.2017.00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Cirrito J., Stewart F., Jiang H., Finn M., Holmes B., et al. (2011). In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci. 31 13110–13117. 10.1523/jneurosci.2569-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Uronen R., Huttunen H. (2020). The interaction of α-synuclein and Tau: a molecular conspiracy in neurodegeneration? Semin. Cell Dev. Biol. 99 55–64. 10.1016/j.semcdb.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Yanamandra K., Kfoury N., Jiang H., Mahan T., Ma S., Maloney S., et al. (2013). Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80 402–414. 10.1016/j.neuron.2013.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ksiezak-Reding H. (1995). Calpain-induced proteolysis of normal human tau and tau associated with paired helical filaments. Eur. J. Biochem. 233 9–17. 10.1111/j.1432-1033.1995.009_1.x [DOI] [PubMed] [Google Scholar]
- Yin Y., Wang Y., Gao D., Ye J., Wang X., Fang L., et al. (2016). Accumulation of human full-length tau induces degradation of nicotinic acetylcholine receptor α4 via activating calpain-2. Sci. Rep. 6:27283. 10.1038/srep27283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine R., Pundavela J., Corcoran L., Stewart E., Do-Ha D., Bax M., et al. (2015). SOD1 protein aggregates stimulate macropinocytosis in neurons to facilitate their propagation. Mol. Neurodegener. 10:57. 10.1186/s13024-015-0053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H., Thies E., Mandelkow E., Mandelkow E. (2010). Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30 11938–11950. 10.1523/jneurosci.2357-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tian Q., Zhang Q., Zhou X., Liu S., Wang J. (2009). Hyperphosphorylation of microtubule-associated tau protein plays dual role in neurodegeneration and neuroprotection. Pathophysiology 16 311–316. 10.1016/j.pathophys.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Song M., Liu X., Kang S., Kwon I., Duong D., et al. (2014). Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med. 20 1254–1262. 10.1038/nm.3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Kotilinek L., Smith B., Hlynialuk C., Zahs K., Ramsden M., et al. (2016). Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med. 22 1268–1276. 10.1038/nm.4199 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tseng I., Heyser C., Rockenstein E., Mante M., Adame A., et al. (2015). Appoptosin-Mediated Caspase Cleavage of Tau Contributes to Progressive Supranuclear Palsy Pathogenesis. Neuron 87 963–975. 10.1016/j.neuron.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Mcinnes J., Wierda K., Holt M., Herrmann A., Jackson R., et al. (2017). Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8:15295. 10.1038/ncomms15295 [DOI] [PMC free article] [PubMed] [Google Scholar]