Abstract
The metabolic heat production of modern pigs has increased by an average of 16%, compared with sows of 30 years ago. Therefore, it is likely that temperature recommendations require updating to meet the needs of modern pigs. The objective of this study was to evaluate whether different reproductive stages of sows altered thermal preference and if current recommendations required updating. Twenty multiparous sows (3.4 ± 1.2 parity) in different reproductive stages (nonpregnant: n = 7; mid-gestation: 58.5 ± 5.68 d, n = 6; and late-gestation: 104.7 ± 2.8 d, n = 7) were tested. Thermal preference was individually tested, and sows could freely choose a temperature, using a thermal gradient between 10.4 and 30.5 °C. Sows were given 24 h to acclimate to the thermal apparatus. Before testing began, sows were given daily feed allotment and returned to the apparatus. Video from the 24-h test period was used to record sow behavior (time spent inactive), posture (upright and sternal and lateral lying), and location using instantaneous scan samples every 15 min. Data were analyzed using PROC MIXED procedure in SAS 9.4. A cubic regression model was used to calculate the sow’s most preferred temperature based on the location, or temperature, in which they spent the most time. The preference range was calculated using peak temperature preference ±SE for each sow. The reproductive stage altered where sows spent their time within the thermal gradient (P < 0.01). Late-gestation sows preferred cooler temperatures (14.0 °C) than mid-gestation (14.8 °C; P < 0.01) and nonpregnant sows (14.8 °C; P < 0.01). In summary, sow thermal preferences were within the lower half of the current recommended range (10 to 25 °C). This indicates that temperatures at the higher end of the recommended range could be uncomfortable to sows and that the thermal comfort zone of sows may be narrower than recommendations indicate.
Keywords: reproductive stage, sows, thermal comfort zone, thermal preference, thermal recommendations
Introduction
Heat stress (HS) causes infertility in sows, characterized by anestrus, increased wean-to-estrus interval, reduced farrowing rate, and reduced litter size (e.g., number of piglets born per litter) and litter weight (Peltoniemi and Virolainen, 2006; Bertoldo et al., 2012; Muns et al., 2016). Due to reduced heat tolerance from the high metabolic heat load during lactation, studies have focused on understanding HS during this period (Williams et al., 2013). However, the impact of HS is not limited to lactation; it also affects the sow during gestation. For example, HS during the early stages of gestation can result in increased embryo mortality (Tompkins et al., 1967; Edwards et al., 1968; Wildt et al., 1975) and reduced farrowing rates and litter size (Nardone et al., 2006). Additionally, exposure to HS in late gestation can result in an increased number of stillborn piglets and reduced piglet birth weight (as reviewed by Lucy and Safranski, 2017). Furthermore, HS during in utero development can permanently alter postnatal phenotypes and negatively affect future animal performance (Johnson and Baumgard, 2019; Johnson et al., 2020). Thus, HS has a profound impact on the swine industry both economically (St-Pierre et al., 2003) and in terms of animal health and welfare (as reviewed by Johnson, 2018).
Although HS can alter various reproductive measures, current temperature recommendations do not reflect thermal preference differences based the on reproductive stage. The Guide for the Care and Use of Agricultural Animals in Research and Teaching (hereon referred to as the Ag Guide; Federation for Animal Science Societies, 2020) indicates that sows or boars above 100 kg prefer temperatures between 10 and 25 °C. However, this may not be accurately reflecting preferences during different stages of reproduction since data indicate that physiological changes occur during maternal adaptation to pregnancy in mammals (Noblet and Etienne, 1987; Fewell, 1995) that may increase HS sensitivity. Furthermore, sows near parturition have an increased body (rectal) temperature compared with sows early in the reproductive stage or gilts indicating increased heat production (King et al., 1972; Hendrix et al., 1978). Therefore, a greater metabolic rate in late gestation could result in a cooler temperature preference to increase heat loss.
In addition to not accounting for reproductive stage differences in recommended temperatures, previous studies took a theoretical approach to determining the thermoneutral zone (TNZ) of sows, using mathematical formulas to calculate the preferred temperature of sows based on previous work in pigs (Heitman et al., 1958; Bond et al., 1959; Bianca, 1968; Holmes and Close, 1977; Hahn, 1985). For example, Curtis (1983) estimated a sow’s lower limit was between 18 and 20 °C, based on the calculations on still air conditions in barns and insulation of the pig. Since the publication of these studies, genetic selection of modern pigs has focused on larger litter sizes and a higher lean-growth pig, which creates an animal with higher metabolic heat production compared with swine of 30 years ago (Brown-Brandl et al., 2014). Consequently, modern sows have likely become more sensitive to HS (Brown-Brandl et al., 2014), and, as such, their TNZ has likely shifted.
The TNZ reflects the range of ambient temperature (TA), where no regulatory changes in metabolic heat production or evaporative heat loss occur. Indications exist that the TA range, wherein an animal feels comfortable, referred to as the thermal comfort zone (TCZ) is larger compared with the TNZ (Kingma et al., 2014). This is due, in part, since an animal is likely to take behavioral action before the physiological mechanisms for thermoregulation. As such, researchers can determine an animal’s TCZ by offering an animal a choice of temperatures that are comfortable to them by placing them inside a thermal gradient (Ogilvie and Stinson, 1966; Robbins et al., 2020). This type of experiment utilizes an animal’s innate motivation to seek an TA where it does not have to utilize any physiological mechanisms for thermoregulation, thus heat loss equals heat production (i.e., thermopreferrendum; Gordon, 1993). This technique provides information about the animals’ preferred temperatures, or TCZ, from the animal’s perspective (Gordon, 1993). From this type of research, we can extrapolate the fundamental temperatures of an animal’s TNZ.
The study objective was to determine the preferred TA of sows at three stages of reproduction (nonpregnant, mid-gestation, and late gestation). Based on previous research, we hypothesized that the reproductive stage would alter thermal preference in sows. Due to an increase in metabolic heat production during gestation and an increase in mass from growing piglets (Noblet et al., 1997), it is expected that the preferred ambient temperatures will be cooler for sows later in gestation compared with nonpregnant sows. Specifically, both late- and mid-gestation sows will prefer cooler temperatures compared with nonpregnant sows, and late-gestation sows will prefer cooler temperatures compared with mid-gestation sows.
Materials and Methods
All procedures involving animal care and use were approved by the Institutional Animal Care and Use Committee at Purdue University (protocol # 1712001652), and animal care and use standards were based upon the Ag Guide (Federation of Animal Science Societies, 2020).
Animals and housing
Twenty multiparous (3.4 ± 1.2 parity) sows (Yorkshire × Landrace) were selected for temperature preference testing based on reproductive stage (nonpregnant sows; mid-gestation: 58.5 ± 5.7 d pregnant; and late-gestation: 104.7 ± 2.8 d pregnant; Table 1 and Supplementary Table 1). Before being brought to the thermal preference testing location, all sows were housed in a 3-sided enclosure on concrete, outdoors, at the Animal Sciences Research and Education Center at Purdue (ASREC; West Lafayette, IN). All sows, whether they were in this study or not, were housed in groups of similar reproductive stages between 8 and 10 animals per pen and limit fed based on the recommendations for gestating sows (NRC, 2012). Prior to being moved to the preference testing location, sows were fed their 24 h ration (approximately 1.82 kg) and had access to water ad libitum.
Table 1.
Number of sows based on reproductive stage with average BW prior to placement inside a thermal apparatus
| Parameter | Number of sows used | Average weight, kg, mean ± SD |
|---|---|---|
| Nonpregnant | 7 | 202.69 ± 19.19 |
| Mid | 5 | 233.83 ± 28.08 |
| Late | 6 | 237.94 ± 33.65 |
Experimental design—thermal preference
Mead’s resource equation was used a priori to determine the number of sows required for this study (Mead, 1990). Twenty sows were randomly assigned (via random integer generator; random.org) to be tested individually in one of two thermal apparatuses, such that the testing of each reproductive stage would be relatively balanced between the two apparatuses. Testing began on February 8, 2018, and ran until April 4, 2018. Body weight (BW) was documented prior to acclimation to the thermal gradient.
For temperature preference testing, two sows at a time were transported from ASREC to the United States Department of Agriculture-Agricultural Research Service Farm Animal Behavior Laboratory (FABL: West Lafayette, IN; 3.38 km from ASREC) where the thermal apparatuses were located. Sows were fed and weighed prior to transport and individually placed inside their assigned thermal apparatus upon arrival. Sows were allowed 24 h to acclimate to the new enclosure and thermal gradient. During acclimation and temperature preference testing, sows were able to explore the entirety of the thermal apparatus. Each apparatus was cleaned in between acclimation, testing, and prior to new sows being delivered. Between acclimation and testing, sows were removed from the thermal apparatus and individually housed in an adjacent pen (5.5 m2) for approximately 3 h to clean and reestablish the thermal gradient. Furthermore, sows were given their daily food ration upon entering the pen, which took them approximately 30 min to consume. Waste was removed from within the thermal apparatus with a pressure washer and disinfected (LYSOL disinfectant all-purpose cleaner, Reckitt Benckiser LLC, NJ, USA). After cleaning, the sows were returned to their assigned thermal apparatus. During experimental set-up and testing, researchers were not blinded to the sows’ reproductive stage. However, during video coding, observers were blinded to this information.
Prior to placement inside the thermal apparatus, a calibrated Thermochron temperature recorder (iButton model 1921H, calibrated accuracy ± 0.10 °C; resolution = 0.125 °C; Dallas Semi-conductor, Maxim, Irving, TX) attached to a blank controlled internal drug-releasing device (Eazi-Breed; Zoetis, New York, NY) was inserted intravaginally into each sow. This allowed for continuous recording of a sow’s vaginal temperature (method previously described by Johnson and Shade, 2017). Unfortunately, data were lost from 14 sows due to the Thermochrons being dislodged or not inserted due to a miscommunication. Thus, data from the Thermochrons are not reported here but was used where available as an exclusion criterion if any sows had a vaginal temperature that appeared abnormal (≥39.0 °C; Tummaruk and Sang-Gassanee, 2013).
Thermal apparatus
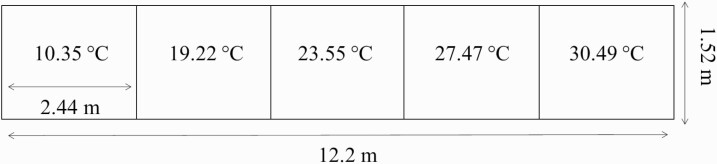
The materials and methods were adapted in part from Robbins et al. (2018, 2020). Two identical thermal gradient apparatuses were built (12.2 × 1.52 × 1.86 m; L × W × H) to provide the required space per sow and create a desired temperature gradient (Figure 1). To create the thermal gradient (10.35 ± 0.42 to 30.49 ± 0.45 °C), ceramic heating lamps (herein referred to as heating elements (Floureon 100 to 250W Multi Basking IR Heat Bulb) were placed at strategic locations 1.80 m above the floor (Supplementary Figure 1). Some heating elements were on constantly, whereas others would turn on/off depending on internal thermostat readings, which were ultimately influenced by the external TA. During validation of the thermal gradients, prior to the research starting, data indicated that the gradient could not be held constant when outdoor temperatures were below −10 °C or above 12.78 °C. Therefore, if outdoor temperatures were predicted to be outside of this range, testing was halted until temperatures returned to our operational range (this happened only once during the experiment). While animals were being tested, the ambient outdoor temperature averaged −1.83 °C with ± 10.33 °C.
Figure 1.
Top view of a single thermal apparatus showing average temperature per thermal zone.
Light, humidity, and temperature were monitored using data loggers (HOBO Data Logger; U12-012, Onset Computer Corporation, MA, USA; temperature range of −20 to 70 °C with an accuracy of ± 0.35 °C and relative humidity range of 5% to 95% with an accuracy of ±2.5% to max 3.5%) to assure that the two apparatuses were as identical as possible. The data loggers were placed on the wall of each apparatus approximately 0.94 m above the floor and 0.61 m apart to calculate the average temperature within each thermal zone. An additional set of data loggers was placed inside a black globe, at a similar location on the opposite wall (Supplementary Figure 1).
The thermal apparatus was fully enclosed with overhead lighting. Led lighting strips (10,500 lumen, 12.19 m length, CB concept, CA, USA) were used to illuminate the apparatuses with a light:dark cycle similar to their housing conditions at ASREC (10:14 [L:D] h cycle). To create the cool end of both thermal apparatuses, a conditioning box (1.52 × 1.52 × 0.76 m, L × W × H; Supplementary Figure 2) was designed with an air conditioning unit (10,000 BTU, LG Electronics, Seoul, South Korea) attached to a Coolbot (Store It Cold LLC, FL USA; this device allows for a standard air conditioner to drop below normal operating temperatures and can transform a well-insulated room into a walk-in cooler) to circulate 5 °C air into the apparatuses. Both apparatuses had ad libitum water supplied using waterers (AquaChief, Hog Slat, Inc. NC, US) located within each thermal zone.
Two weeks prior to sows being placed into either thermal apparatus, temperatures were taken every 15 min using data loggers and recorded on-site using an LCD screen temperature probe within the black globes to ensure the gradient remained constant. While the study ran, data loggers continued to monitor the temperature inside the thermal apparatuses every 15 min (Supplementary Table 2).
Behavior and posture observations
The sows were video recorded continuously over the 24-h testing period for behavior, location, and posture using infrared bullet CCTV cameras (Versiton Video Technology; SuperVue BKRSP-L355) and video surveillance software (GeoVision, Taiwan). The location, behavior, and posture were recorded for each sow using instantaneous scan samples every 15 min. The scan interval used for recording data from the video was determined by comparing the proportion of observations at each location with different subsampling intervals (5, 10, 15, 20, 25 and 30 min). Data from each subsample were compared pairwise and considered to accurately estimate the behavior or posture if the intervals were not significantly different from each other compared with the 5-min interval (Ledgerwood et al., 2010). Based on these data, a 15-min sampling interval was selected for this study. The ethogram contained three simple behavior categories: active, inactive, and other (Table 2). If sows were observed in more than one thermal zone (location), the proportion of the sow in each zone was documented in 25% increments (head, front quarter, mid-section, and rump; Supplementary Figure 3). Postures can provide an indication of thermal comfort (Mount, 1960); therefore, posture was also documented at each scan sample (Table 3).
Table 2.
Ethogram used for behavioral observations (Robbins et al., 2020)
| Category | Behavior | Description |
|---|---|---|
| Active | Active | Sow is walking about and can be seen actively engaged with the environment. Sow can be observed interacting with water drinkers located in each thermal zone, such as biting, scratching, or chewing on. |
| Drinking | Sow’s head is in the water drinker, located on the same wall to lighting strips (10 total), and can only see back of head and ears while within in the water drinker. | |
| Inactive | Inactive | Sow is motionless and assumed to be sleeping. The animal may be inactive if sitting, standing, or lying still and alert. Animal is stationary; slow and small head movements may be seen but their body is motionless. |
| Other | Other | Sow’s behavior cannot be determined; camera angles or glare does not allow for accurate assessment. |
| Defecation | Sow is stationary or in a dog-sit position and can see fecal matter being excreted. |
Table 3.
Ethogram used for posture observations (Robbins et al., 2020)
| Posture | Description |
|---|---|
| Upright | Sow’s body is erect and the top line (back) is to the camera. This includes sow standing on all four hoofs on the ground and dog-sitting where sow has rump on floor. |
| Sternal Laying | Sow lies upright with stomach and chest touching the ground, and the top line is facing the camera. This includes when a sow is sternal on her anterior body and lateral on her posterior body. Sternal includes the medial plane of the head and body being perpendicular to a 45-degree angle to the ceiling. |
| Lateral Laying | Sow lies on the side with shoulder and rump touching the ground, and the top line is facing a wall. The medial plane of the head and body is greater than 45 degrees and approximately 90 degrees to the ceiling. |
| Other | Any posture that could not be determined due to viewing angle (e.g., interference with camera data, glare or sow was hidden behind a waterer). When sow was in a transition posture (e.g., she was moving from standing to laying or sitting to upright) or in a half stance where either her hind or front legs were down with the opposite end of her body in an upright posture. |
The proportion of behaviors was calculated for each sow by counting the total number of times each behavior was observed in each location during testing. This calculation was repeated separately for posture data. Since sows were observed inactive in ~85% of observations, only inactivity was included in the behavioral analysis. For the postural data, any observations of sows documented in the “other” category were similarly excluded from the analysis. Thus, the time budgets do not total 100%, and the independent variables are not colinear and a change in one category will not directly influence the level of another category.
Analyses
All analyses were performed using the PROC MIXED (general linear mixed model: GLM) procedure in SAS 9.4 (SAS Institute INC., Cary, NC). The assumptions of the GLM (normality of error, homogeneity of variance, and linearity) were confirmed post hoc, and data were transformed when necessary to meet these assumptions (Grafen and Hails, 2002). This model was then subjected to backward elimination to remove nonsignificant variables (i.e., previous temperature exposure based on sows being housed outdoors) leaving a more parsimonious model. The threshold for significance P < 0.05 was used and Bonferroni corrected for multiple tests.
Behavior and posture by location
Originally, sow was nested within reproductive stage, weight category (classified as above or below the mean split of all sows’ weight), and parity. When the blocking factors of parity and weight category was included in the original model, the Akaike Information Criterion (AIC; is an estimator of prediction error and gives a relative quality of statistical models for a given set of data, the lower the AIC score the better the model) was increased, and the R-squared was lower, compared to when these factors were removed. Thus, a better and simplified statistical model model was to remove these factors. Further, these were found to be nonsignificant. A cubic regression model was used for both behavior (Supplementary Figure 4) and posture data (Supplementary Figure 5), and both were log10 + 0.001 transformed to meet the assumption of a GLM. Main effects plus second-order interactions of the reproductive stage, time spent inactive and posture (where applicable), and location (i.e., temperature) were tested. Location was used as a cubic variable. Since the data were not orthogonal, nonsignificant higher-order interactions were dropped from the final analysis.
Data from three sows (not reported here) were also excluded from the analyses. Complications within the thermal gradient required us to drop data from two sows. An additional sow was removed from the study due to her vaginal temperature being 39.25 °C, an indication of a fever (≥39 °C; Tummaruk and Sang-Gassanee, 2013). Since fever may alter thermal preference, this sow was excluded from the study.
The cubic curve from the final model above was generated in 0.2 °C increments starting with the coldest thermal zone temperature (10.4 °C) and increased to the warmest temperature (30.4 °C). Peak temperatures for inactive behavior and posture were calculated by identifying the temperature with the greatest proportion of time spent in each location. The thermal preference range was then calculated from the peak temperature ± SE. Tukey tests for differences in least square means between reproductive stages were run in each thermal zone. Since Tukey tests were run five times (for each thermal zone), the alpha was Bonferroni corrected for the multiple tests (α = 0.05/5 = 0.01).
BW, number of piglets, and peak thermal preference
First, we wanted to determine if the number of piglets gestating (that is number of piglets the sow had in total, including mummified and stillborn, referred to hereon as piglets) and stage of reproduction altered the overall sow BW. Only the main effect of reproductive stage and piglets were tested in this GLM analysis.
BW is such an influential variable on thermal comfort; it was necessary to see if it affected peak thermal preference. To test this, the peak thermal preference for each sow was identified from the raw inactive behavior data, by determining the location (temperature) where sows spent most of their time. For this GLM, only the main effect of the reproductive stage and BW were included in the model. The number of piglets was tested initially as a covariate, but it was not significant, thus it was removed from the model.
Results
Behavior and thermal preference
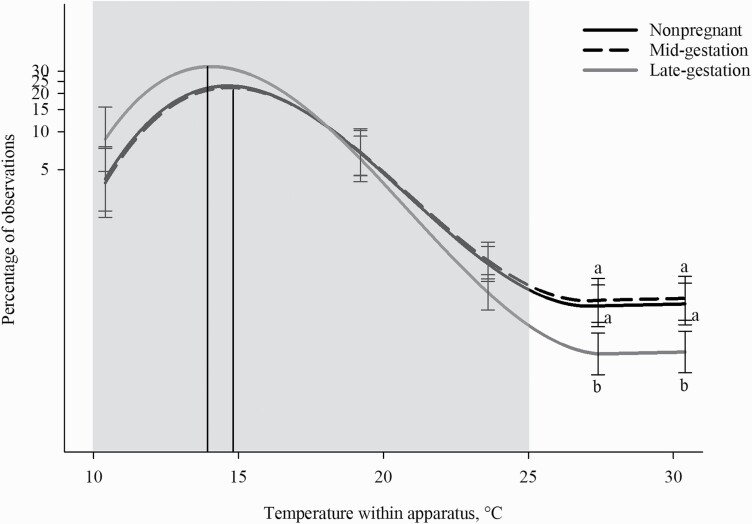
The reproductive stage altered the amount of time sows spent in the different thermal zones (P = 0.015; Table 4). Late-gestation sows had a peak thermal preference of 14.0 °C (spending 32.59% of their time at this temperature) with a range between 12.6 and 15.6 °C. This peak temperature was cooler than mid-gestation sows with a peak preference of 14.8 °C (22.31%, α/3: F1,75 = 6.83; P = 0.011; Figure 2) and ranged between 13.2 and 16.4 °C. Peak thermal preference was also different between late and nonpregnant sows (α/3: F1,75 = 6.37; P = 0.014; Figure 2), with nonpregnant sows having the same peak thermal preference (14.8 °C, 22.96%) and range as mid-gestation sows (α/3: F1,75 = 0.04; P = 0.852).
Table 4.
Statistical terms included in the inactive behavior model which tested for differences in the percentage of observations
| Effect | F | P-value |
|---|---|---|
| Reproductive stage | F 2,17 = 1.33 | 0.290 |
| Location | F 1,75 = 74.36 | <0.001 |
| Location * Reproductive stage | F 2,75 = 4.46 | 0.015 |
| Location * Location | F 1,75 = 4.25 | 0.043 |
| Location * Location * Location | F 1,75 = 30.67 | <0.001 |
Figure 2.
Percentage of observations in different temperatures within the thermal gradient based on the reproduction stage and during inactive behaviors. The effects of the reproductive stage (nonpregnant; mid-gestation: 58.5 ± 5.68 d; and late gestation: 104.7 ± 2.8 d) on thermal preference. Temperature within the thermal apparatus is plotted on the0 x-axis, and the percentage of time observed during inactive behaviors is plotted on the y-axis as a log10 + 0.001 scale. Cubic peaks are indicated by vertical lines corresponding to the reproductive stage. Standard error bars are located at the temperatures of the five thermal zones (10.4, 19.2, 23.6, 27.5, and 30.5 °C); different letters denote significant Tukey pairwise comparisons (P < 0.01), and no letters given where no significance was found between the three reproductive stages. The gray box indicates the recommended temperatures (10 to 25°C) for sows or boars > 100 kg (Federation for Animal Science Societies, 2020).
BW and peak thermal preference
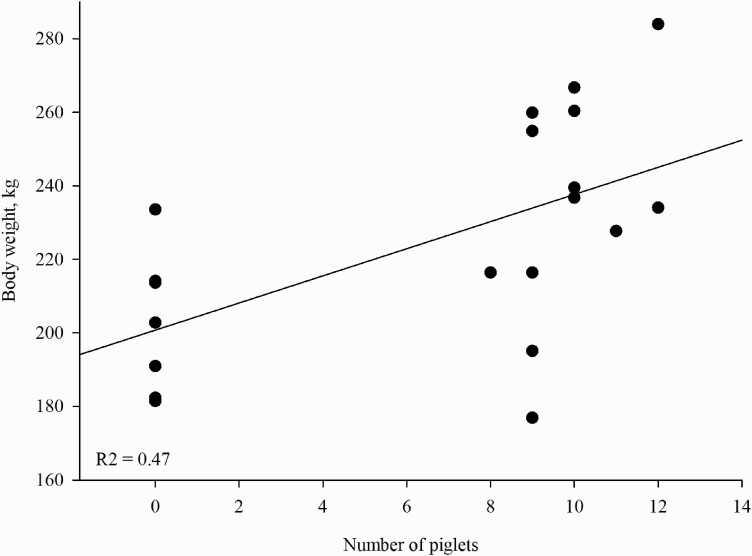
No significant differences in BW were detected based on the main effect of reproductive stage (P = 0.199: Table 5). Piglet numbers did affect BW (Table 5), where sows with increasing number of piglets had increased BW (Figure 3).
Table 5.
Statistical terms included in the model which tested for differences in the percentage of observations in sows at peak temperature with BW as a covariate
| Effect | F ratio | P-value |
|---|---|---|
| Reproductive stage | F 2,16 = 1.79 | 0.199 |
| BW, kg (Least square means± SE) | Number of piglets (mean ± SD) | |
| Nonpregnant | 202.69 ± 10.43 | N/A |
| Mid | 233.83 ± 11.27 | 9.42 ± 1.27 |
| Late | 237.94 ± 10.43 | 10.00 ± 1.58 |
| Effect | F ratio | P-value |
| Number of piglets | F 1,16 = 5.47 | 0.033 |
Figure 3.
BW in kilograms per sow based on the number of piglets (piglets). Symbols represent individual sows.
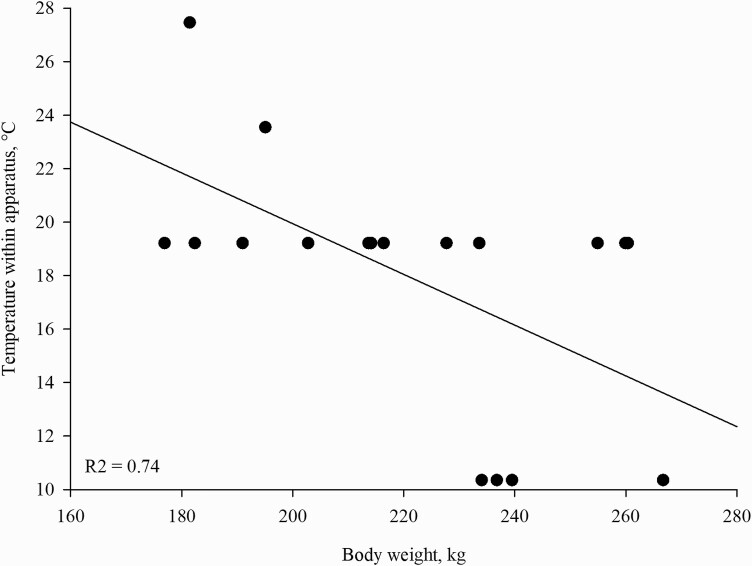
BW (P = 0.001) and stage of reproduction (P = 0.005) affected peak thermal preference (Table 6). Late-gestation sows had a cooler peak thermal preference compared with mid-gestation and nonpregnant sows (Table 6). Sows, regardless of reproductive stage, with a higher BW preferred cooler temperatures (Figure 4).
Table 6.
Reproductive stage and BW influence peak thermal preference (Least square means ± SE)
| Effect | Peak temperature, °C | SE | F-value | P-value |
|---|---|---|---|---|
| Reproductive stage | F 2,15 = 10.92 | .001* | ||
| Nonpregnant | 14.80* | 1.12 | ||
| Mid | 14.80* | 1.11 | ||
| Late | 14.00+ | 1.05 | ||
| Weight, kg | F 1,15 = 10.42 | 0.005* |
Different symbols and * denote a significant difference in peak thermal preference between parameters Tukey tests (P < 0.01).
Figure 4.
Weight in kilograms per sow and preferred peak thermal preference based on the amount of time spent within the five thermal zones (10.4, 19.2, 23.6, 27.5, and 30.5 °C) during inactivity. Symbols represent individual sow weights.
Posture and thermal preference
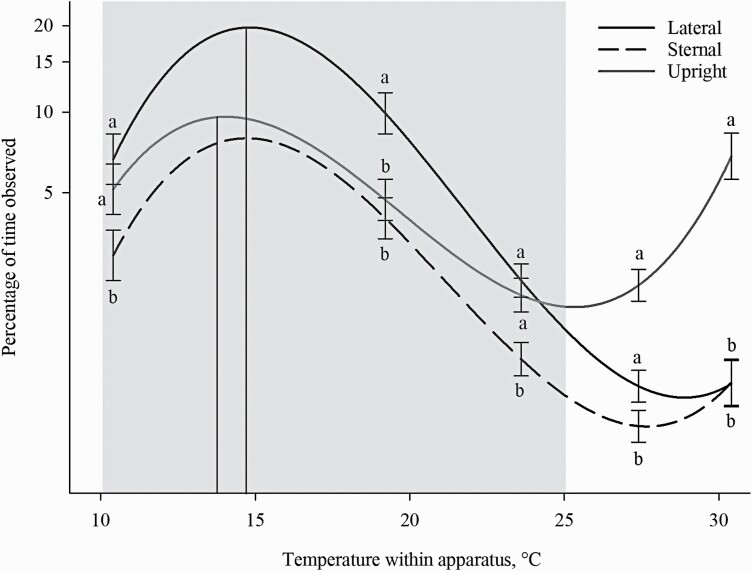
The percentage of time spent in various postures differed across the thermal gradient (P < 0.001; Table 7). Lateral and sternal lying were observed most often at 14.8 °C (spending 19.74% and 8.04% of their time, respectively) and had a preference range between 10.8 to 19.8 °C and 10.6 to 20.0 °C, respectively. Upright posture was observed most often at 14.0 °C (9.61%) with a range between 10.4 and 20.2 °C; this peak differed from sternal (α/3: F1,267 = 11.19; P < 0.001) and lateral lying (α/3: F1,267 = 24.56; P < 0.001: Figure 5).
Table 7.
Statistical terms included in the model which tested for differences in the percentage of observations in sows at various locations (temperatures) while in different postures
| Effect | F-value | P-value |
|---|---|---|
| Reproductive stage | F 2,17 = 1.90 | 0.179 |
| Posture | F 2,267 = 8.06 | <0.001 |
| Location | F 1,267 = 115.22 | <0.001 |
| Location * Reproductive stage | F 2,267= 11.73 | < 0.001 |
| Location * Posture | F 2,267 = 12.79 | <0.001 |
| Location * Location | F 1,267 = 24.25 | <0.001 |
| Location * Location * Posture | F 2,267 = 0.438 | 0.646 |
| Location * Location * Location | F 1,267 = 65.27 | <0.001 |
| Location * Location * Location * Posture | F 2,267= 3.21 | 0.042 |
Figure 5.
Percentage of observations in different temperatures within the thermal gradient based on posture. Data are plotted by postures: lateral and sternal lying, and upright. Temperature within the thermal apparatus is plotted on the x-axis, and the percentage of time observed is plotted on the y-axis as a log10 + 0.001 scale. Cubic peaks are indicated by solid vertical lines. Standard error bars are located at the temperatures of the five thermal zones (10.4, 19.2, 23.6, 27.5, and 30.5 °C). Different letters denote significant Tukey pairwise comparisons (P < 0.01). The gray box indicates the recommended temperatures (10 to 25 °C) for sows or boars > 100 kg (Federation for Animal Science Societies, 2020).
Discussion
This study examined the preferred TA of sows, by reproductive stage, to the best of our knowledge, for the first time by utilizing the animal’s innate motivation to seek out their preferred TA. Reproduction is an energetically demanding process, with the greatest energetic costs occurring during late pregnancy and lactation (Kaczmarski, 1966; Millar, 1978; Close et al., 1985; Noblet et al., 1997). Therefore, this study hypothesized that reproductive stage would alter the thermal preference of sows.
The reproductive stage affected the temperature preference of sows when inactive. Late-gestation sows preferred a temperature that was 0.8 °C cooler than both nonpregnant sows and mid-gestation sows. Additionally, late-gestation sows spent less time at the hot end of the thermocline (27.5 and 30.5°C) than both mid-gestation and nonpregnant sows, indicating an aversion to these temperatures. Interestingly, mid-gestation and nonpregnant sows preferred similar temperatures while inactive indicating that their TCZ is like each other despite the potential increased metabolic heat production in mid-gestation compared with nonpregnant sows. Although the metabolic rate was not directly measured in this study, it is known that late-gestation sows have increased total heat production (THP) due to rapidly developing fetuses and increased energetic demands (Kaczmarski, 1966; Millar, 1978; Close et al., 1985; Noblet et al., 1997; Feyera et al., 2018). Therefore, this THP increase likely explains the late-gestation sows’ preference for cooler temperatures relative to mid-gestation and nonpregnant sows. However, the THP differences between mid-gestation and nonpregnant sows were likely minimal resulting in similar temperature preferences. Although differences were found, we want to draw attention to the fact that the sows used in this study were smaller than typical commercial sows and produced fewer piglets. Despite this, our data illustrate that the metabolic demands vary based on the reproductive stage and highlight the importance of incorporating these data into temperature recommendations, which may be exacerbated in larger sows.
During times of HS, pigs alter their posture and increase lateral lying to increase skin contact with the floor (Mount, 1979; Huynh et al., 2005) and increase heat loss through the skin. These postural adjustments are energetically cheap methods for heat loss; however, they cannot eliminate heat load as temperatures increase beyond the upper critical temperature. Regardless, these postures can give us information about a sow’s thermal comfort. Typically, there is a linear relationship between lateral lying and environmental temperature in pigs (Huynh et al., 2005). Alternatively, a decrease in TA increases sternal lying because this posture reduces exposed skin surface area to the floor and helps reduce heat loss (Mount, 1960). In the present study, sows spent most of their time in the lateral posture (20%) compared with the sternal posture (9%). Both postures were observed at temperatures within the Ag Guide recommendations (Federation of Animal Science Societies, 2020). However, both postures were observed in a narrower temperature range (10.6 to 19.8 °C for sternal and 10.6 to 20.0 °C for lateral laying) compared with 10 to 25 °C as outlined in the Ag Guide (Federation of Animal Science Societies, 2020). The fact that the sows chose temperatures at the lower end of the recommended range (10 to 25 °C) and were lying in a posture that indicates comfort, supports that the temperatures selected fall within their TCZ. Unfortunately, 10.35 °C was the lowest temperature zone that we could consistently maintain, which may have limited their ability to choose their true preferred temperature. Thus, currently, it is unknown if sows prefer temperatures cooler than 10.35 °C.
Sows were individually tested in this study, which does not accurately reflect normal living conditions on commercial farms. As such, solitary conditions are less likely to represent a typical sow’s thermoregulatory environment. In group housing, sows tend to spend a considerable amount of time near each other, often huddling together (Kittawornrat and Zimmerman, 2011). Although no data related to thermal preference in group-housed sows exists, groups of mice typically prefer a cooler temperature than those housed individually (Gordon et al., 1998). Thus, the temperature preference ranges observed here might be warmer than if sows were tested in groups. Although it is currently unknown to what extent temperature preference may be reduced for group-housed sows, future research should investigate the effects of group size and temperature preference to provide TCZ guidelines for various housing scenarios. In addition, sows in this study were housed outdoors between January and April in Indiana, which are the cooler months and may have resulted in sows acclimating to cooler temperatures, thus impacting their thermal preference. It would be prudent to replicate this study with sows housed in warmer months; however, the thermal apparatus was at the mercy of external TA and is unable to run in warmer months. This is due to the R value (e.g., insulation value) that was used; though effective in cooler months, it allowed for heat to enter and thus disrupt the thermal gradient in warmer months.
Finally, this study did not acknowledge the radiant temperature load from the heat lamps and how that may have contributed to the reported TA. Had we added radiant heat into the "real-feel" temperature for the sow the temperatures reflected here may have been warmer. To account for this, the study used black globes in determining the internal TA, a common method in demonstrating real feel temperatures (Graves, 1974; as reviewed by Guo et al., 2020). However, it should be noted that TA can be calculated in a variety of ways to determine real feel including using the surface area of the sow, dew point, relative humidity, airflow, and wet black globes.
Conclusions
This study is the first to look at temperature preference differences based on the reproductive stage of sows. The temperature preferences demonstrate that further research is required to produce accurate guidelines on preferred temperature ranges. The results of this study indicate that late-gestation sows prefer a temperature range of 12.6 to 15.6 °C and may have a cooler upper critical limit than what is currently recommended (approximately, 25 °C; Federation of Animal Science Societies, 2020). This indicates that sows might experience HS at cooler temperatures than expected based on the stage of reproduction and invoke behavioral and physiological adjustments sooner to mitigate the negative effects of HS. Based on the research conducted in this study, individual sows should be housed in temperatures between 12.6 and 16.4 °C to provide their TCZ and could optimize production and improve the overall well-being.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute of Food and Agriculture (NIFA; grant 2018-67015-28130) Washington, DC. We would also like to thank the swine farm staff at Purdue University for animal care, especially Brian Ford, Katlyn Ade, and Aaron Nally. Additionally, thank you to the employees at the United States Department of Agriculture-Agricultural Research Service Livestock Behavior Research Unit, Morgan Garvy, and undergraduate staff, Mackenzie Rossman, for assistance in daily animal care and data collection. All opinions expressed in this paper are the authors and do not necessarily reflect the policies and views of United States Department of Agriculture-Agricultural Research Service, or National Institute of Food and Agriculture. Mention of trade names or commercial products in this article is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture or Purdue University.
Glossary
Abbreviations
- AIC
Akaike Information Criterion
- BW
body weight
- HS
heat stress
- TA
ambient temperature
- TCZ
thermal comfort zone
- THP
total heat production
- TNZ
thermoneutral zone
Conflict of interest statement
No conflicts of interest, financial, or otherwise are declared by the authors.
Literature Cited
- Bertoldo, M. J., Holyoake P. K., Evans G., and Grupen C. G.. . 2012. Seasonal variation in the ovarian function of sows. Reprod. Fertil. Dev. 24:822–834. doi: 10.1071/RD11249 [DOI] [PubMed] [Google Scholar]
- Bianca, W. 1968. Thermoregulation. In: Hafez E. S. E., editor. Adaptation of domestic animals. Pullman, Washington/Philadelphia: Washington State University/Lea & Febiger; p. 97–18. [Google Scholar]
- Bond, T. E., C. F. Kelly, and H. Heitman Jr. 1959. Hog house air conditioning and ventilation data Trans. ASAE. 2(1):1–4. doi: 10.13031/2013.41147 [DOI] [Google Scholar]
- Brown-Brandl, T. M., Hayes M. D., Xin H., Nienaber J. A., and Li H.. . 2014. Heat and moisture production of modern swine. ASHRAE Trans. 120: 469–489. doi: 10.13031/trans.57.1071 [DOI] [Google Scholar]
- Close, W. H., Noblet J., and Heavens R. P.. . 1985. Studies on the energy metabolism of the pregnant sow. 2. The partition and utilization of metabolizable energy intake in pregnant and non-pregnant animals. Br. J. Nutr. 53:267–279. doi: 10.1079/bjn19850034 [DOI] [PubMed] [Google Scholar]
- Curtis, S. E. 1983. Environmental management in animal agriculture. Ames (IA):Iowa State University Press; p. 6–96. [Google Scholar]
- Edwards, R. L., Omtvedt I. T., Tuesman E. J., Stephens D. F., and Mahoney G. W. A.. . 1968. Reproductive performance of gilts following heat stress prior to breeding and in early gestation. J. Anim. Sci. 27:1634–1637. doi: 10.2527/jas1968.2761634x [DOI] [Google Scholar]
- Federation of Animal Science Societies . 2020. Guide for the care and use of agricultural animals in research and teaching. 4th ed. Chap. 9. Champaign (IL):Federation of Animal Science Societies; p. 291. [Google Scholar]
- Feyera, T., Pedersen T. F., Krogh U., Foldager L., and Theil P. K.. . 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. doi: 10.1093/jas/sky141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell, J. E. 1995. Body temperature regulation in rats near term of pregnancy. Can. J. Physiol. Pharmacol. 73:364–368. doi: 10.1139/y95-046 [DOI] [PubMed] [Google Scholar]
- Gordon, C. J. 1993. Temperature regulation in laboratory rodents. Cambridge (NY): Cambridge University Press;xii: 276 p. [Google Scholar]
- Gordon, C. J., Becker P., and Ali J. S.. . 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol. Behav. 65:255–262. doi: 10.1016/s0031-9384(98)00148-6 [DOI] [PubMed] [Google Scholar]
- Grafen, A., and Hails R.. . 2002. Modern statistics for the life sciences. Oxford (NY):Oxford University Press; p. 155–163. [Google Scholar]
- Graves, K. W. 1974. Globe thermometer evaluation. Am. Ind. Hyg. Assoc. J. 35:30–40. doi: 10.1080/0002889748507003 [DOI] [PubMed] [Google Scholar]
- Guo, H., Aviv D., Loyola M., Teitelbaum E., Houchois N., and Meggers, F. 2020. On the understanding of the mean radiant temperature within both the indoor and outdoor environment: a critical review. Renew. Sustain. Energy Rev. 117:109207. doi: 10.1016/j.rser.2019.06.014 [DOI] [Google Scholar]
- Hahn, G. 1985. Managing and housing of farm animals in hot environments in stress physiology in livestock. In: Yousef, M. K., editor. Vol II: Ungulates. Boca Raton (FL):CRC Press; p. 151–174. [Google Scholar]
- Heitman Jr, H., C. F. Kelly, and T. E. Bond. 1958. Ambient air temperature and weight gain in swine. J. Anim. Sci. 17(1):62–67. doi: 10.2527/jas1958.17162x [DOI] [Google Scholar]
- Hendrix, W. F., Kelley K. W., Gaskins C. T., and Bendel R. B.. . 1978. Changes in respiratory rate and rectal temperature of swine near parturition. J. Anim. Sci. 47:188–191. doi: 10.2527/jas1978.471188x [DOI] [PubMed] [Google Scholar]
- Holmes, C. W., and Close W. H.. . 1977. The influence of climatic variables on energy metabolism and associated aspects of productivity in the pigs. In: Haresign, W., Swan H., and Lewis D., editors. Nutrition and the climatic environment of pigs. London: Butterworths; p. 51–73. [Google Scholar]
- Huynh, T. T. T., Aarnink A. J. A., Gerrits W. J. J., Heetkamp M. J. H., Canh T. T., Spoolder H. A. M., Kemp B., and Verstegen M. W. A.. . 2005. Thermal behaviour of growing pigs in response to high temperature and humidity. Appl. Anim. Behav. Sci. 91:1–16. doi: 10.1016/j.applanim.2004.10.020 [DOI] [Google Scholar]
- Johnson, J. S. 2018. Heat stress: impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 58:1404–1413. doi: 10.1071/AN17725 [DOI] [Google Scholar]
- Johnson, J. S., and Baumgard L. H.. . 2019. PHYSIOLOGY SYMPOSIUM: Postnatal consequences of in utero heat stress in pigs. J. Anim. Sci. 97:962–971. doi: 10.1093/jas/sky472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. S., and Shade K. A.. . 2017. Characterizing body temperature and activity changes at the onset of estrus in replacement gilts. Livest. Sci. 199:22–24. doi: 10.1016/j.livsci.2017.03.004 [DOI] [Google Scholar]
- Johnson, J. S., Stewart K. R., Safranski T. J., Ross J. W., and Baumgard L. H.. . 2020. In utero heat stress alters postnatal phenotypes in swine. Theriogenology 154:110–119. doi: 10.1016/j.theriogenology.2020.05.013 [DOI] [PubMed] [Google Scholar]
- Kaczmarski, F. 1966. Bioenergetics of pregnancy and lactation in the bank vole. Acta Theriol. 11:409–417. doi: 10.4098/AT.arch.66-19 [DOI] [Google Scholar]
- King, G. J., Willoughby R. A., and Hacker R. R.. . 1972. Fluctuations in rectal temperature of swine at parturition. Can. Vet. J. 13:72–74. PMID: 5016929. [PMC free article] [PubMed] [Google Scholar]
- Kingma, B. R., Frijns A. J., Schellen L., and van Marken Lichtenbelt W. D.. . 2014. Beyond the classic thermoneutral zone: including thermal comfort. Temperature (Austin). 1:142–149. doi: 10.4161/temp.29702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittawornrat, A., and Zimmerman J. J.. . 2011. Toward a better understanding of pig behavior and pig welfare. Anim. Health Res. Rev. 12:25–32. doi: 10.1017/S1466252310000174 [DOI] [PubMed] [Google Scholar]
- Ledgerwood, D. N., Winckler C., and Tucker C. B.. . 2010. Evaluation of data loggers, sampling intervals, and editing techniques for measuring the lying behavior of dairy cattle. J. Dairy Sci. 93:5129–5139. doi: 10.3168/jds.2009-2945 [DOI] [PubMed] [Google Scholar]
- Lucy, M. C., and Safranski T. J.. . 2017. Heat stress in pregnant sows: thermal responses and subsequent performance of sows and their offspring. Mol. Reprod. Dev. 84:946–956. doi: 10.1002/mrd.22844 [DOI] [PubMed] [Google Scholar]
- Mead, R. 1990. The design of experiments: statistical principles for practical applications. Cambridge (NY): Cambridge University Press; p. 620. [Google Scholar]
- Millar, J. S. 1978. Energetics of reproduction in Peromyscus leucopus: the cost of lactation. Ecology 59:1055–1061. doi: 10.2307/1938558 [DOI] [Google Scholar]
- Mount, L. E. 1960. The influence of huddling and body size on the metabolic rate of the young pig. J. Agric. Sci. 55:101–105. doi: 10.1017/S0021859600021651 [DOI] [Google Scholar]
- Mount, L. E. 1979. Adaptation to thermal environment. Man and his productive animals. East Kilbride, Scotland: Edward Arnold (Publishers) Ltd. Chp. 14. [Google Scholar]
- Muns, R., Malmkvist J., Larsen M. L., Sørensen D., and Pedersen L. J.. . 2016. High environmental temperature around farrowing induced heat stress in crated sows. J. Anim. Sci. 94:377–384. doi: 10.2527/jas.2015-9623 [DOI] [PubMed] [Google Scholar]
- Nardone, A., Ronchi B., Lacetera N., and Bernabucci U.. . 2006. Climatic effects on productive traits in livestock. Vet. Res. Commun. 30:75–81. doi: 10.1007/s11259-006-0016-x [DOI] [Google Scholar]
- Noblet, J., Dourmad J. Y., Etienne M., and Le Dividich J.. . 1997. Energy metabolism in pregnant sows and newborn pigs. J. Anim. Sci. 75:2708–2714. doi: 10.2527/1997.75102708x [DOI] [PubMed] [Google Scholar]
- Noblet, J., and Etienne M.. . 1987. Metabolic utilization of energy and maintenance requirements in pregnant sows. Livest. Prod. Sci. 16:243–257. doi. 10.1016/0301-6226(87)90042-X [DOI] [PubMed] [Google Scholar]
- NRC . 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Ogilvie, D. M., and Stinson R. H.. . 1966. The effect of age on temperature selection by laboratory mice (Mus musculus). Can. J. Zool. 44:511–517. doi: 10.1139/z66-055 [DOI] [PubMed] [Google Scholar]
- Peltoniemi, O. A. T., and Virolainen J. V.. . 2006. Seasonality of reproduction in gilts and sows. J. Reprod. Fertil. Suppl. 6: 205–218. PMID: 16866319. [PubMed] [Google Scholar]
- Robbins, L., Green-Miller A. R., Johnson J. S., and Gaskill B. N.. . 2018. Thermocline design for thermal preference testing in piglets. In: Proceedings of the 10th International Livestock Environment Symposium (ILES X) September 25–27, 2018; American Society of Agricultural and Biological Engineers; p. 1. doi: 10.13031/iles.18-154 [DOI] [Google Scholar]
- Robbins, L., Green-Miller A. R., Johnson J. S., Gonzales C., and Gaskill B. N.. . 2020. Early life thermal stress: impacts on future temperature preference in weaned pigs (3 to 15 kg). J. Anim. Sci. 98(12). doi: 10.1093/jas/skaa327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre, N., Cobanov B., and Schnitkey G.. . 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Tompkins, E., Heidenreich C., and Stob M.. . 1967. Effect of post-breeding thermal stress on embyronic mortality in swine. J. Anim. Sci. 26: 377–380. doi: 10.2527/jas1967.262377x [DOI] [Google Scholar]
- Tummaruk, P., and Sang-Gassanee K.. . 2013. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: a clinical study. Trop. Anim. Health Prod. 45:1071–1077. doi: 10.1007/s11250-012-0315-x [DOI] [PubMed] [Google Scholar]
- Wildt, D. E., Riegle G. D., and Dukelow W. R.. . 1975. Physiological temperature response and embryonic mortality in stressed swine. Am. J. Physiol. 229:1471–1475. doi: 10.1152/ajplegacy.1975.229.6.1471 [DOI] [PubMed] [Google Scholar]
- Williams, A. M., Safranski T. J., Spiers D. E., Eichen P. A., Coate E. A., and Lucy M. C.. . 2013. Effects of a controlled heat stress during late gestation, lactation, and after weaning on thermoregulation, metabolism, and reproduction of primiparous sows. J. Anim. Sci. 91:2700–2714. doi: 10.2527/jas.2012-6055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.