Abstract
Throughout the history of reverse total shoulder arthroplasty, the extent of lateral offset has changed considerably from “too lateral” to “too medial” and has been lately swinging back towards a point somewhere in between. Nonlateralized designs minimize shear forces on the glenoid and decrease force required by the deltoid. Glenoid lateralization decreases impingement and scapular notching and improves range of motion. Humeral lateralization achieves a more anatomic position of the tuberosities while maintaining a nonlateralized center of rotation. Several factors play a role in choosing the extent of lateral offset and method of lateralization.
Keywords: Glenoid lateralization, humeral lateralization, lateral offset, lateralization, reverse shoulder arthroplasty
Introduction
The principles associated with reverse total shoulder arthroplasty (RTSA) are being continuously modified in order to optimize performance and minimize complications. The purpose of this review is to summarize the biomechanical concepts of lateralized and nonlateralized RTSA and outline the clinical outcomes of each.
Definitions
In the native shoulder, the lateral humeral offset is the distance between the base of the coracoid and the most lateral point of the greater tuberosity.1–3 Harman et al.4 defined the lateral offset in RTSA as the distance between the baseplate and the center of articular contact between the glenosphere and the polyethylene cup. The latter method measures the distance between different parts of the prosthesis, so it helps compare different RTSA designs. However, it does not consider humeral component influence on the lateral offset. Moreover, it does not measure the distance between bony landmarks which is important to assess changes in muscle tension. Valenti et al.5 defined the lateral offset in RTSA as the distance between the center of rotation (COR) and the greater tuberosity.
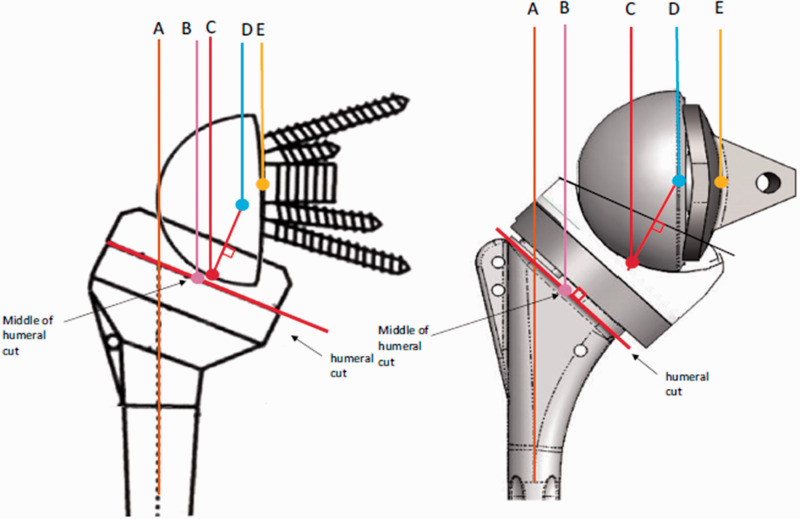
Werthel et al.6 provided a detailed description of different components of the total lateral offset, which is believed to be the most accurate in the literature. However, their definitions still do not account for differences in the amount of glenoid erosion and/or reaming. The measurements they described included humeral stem offset, humeral insert offset, perceived radius of the glenosphere, and COR offset. Humeral stem offset is the horizontal distance between the humeral stem diaphysis (point A) and the midpoint of the humeral implant bearing surface at the level of the humeral cut (point B). Humeral insert offset is the horizontal distance between point B and the deepest point of the articular surface of the humeral insert (point C). Perceived radius of the glenosphere is the distance between point C and the COR (point D). The COR offset, as defined by Werthel et al.,6 is the distance between the COR (point D) and the glenoid-baseplate interface (point E) (Figure 1). We suggest redefining the COR offset to be the distance between the COR and the native glenoid after glenoid reaming (point E) in order to incorporate the increase in lateral offset achieved by bony increased offset implants. Sum of humeral and glenoid lateralization is the global implant lateral offset. The greater tuberosity lateral offset is the horizontal distance between point E and the lateral-most point of the greater tuberosity with the shoulder at 0 degrees of abduction. There are large variations in current RTSA designs in terms of means and extent of lateralization. Total lateral offset can range from 13.1 mm (Delta III, Depuy International Ltd, Leeds, UK) to 50.7 mm (maximal lateral offset in Biomet Comprehensive RTSA (Biomet, Warsaw, IN, USA)).6
Figure 1.
Components of the lateral offset in RTSA. Republished from Werthel et al.6 with permission from Springer Nature.
Boutsiadis et al.7 described the lateralization shoulder angle (LSA) for postoperative radiographic evaluation of the lateral offset. On anteroposterior views, the LSA vertex is at the most lateral border of the acromion and the two rays of the angle pass through the superior glenoid tubercle medially and the most lateral border of the greater tuberosity laterally. In their study, higher LSA correlated with increased implant lateralization. Routman et al.8 considered a 5 mm COR offset the cutoff to classify glenoid components into medialized and lateralized glenoids and 15 mm humeral offset to categorize humeral components to medialized and lateralized designs.
In this review, as with most reports in the current literature, the term “lateralization” is used to describe any implant with a lateral offset greater than that of the Delta III prosthesis. Despite failure of the early lateralized RTSA implants, lateralization has regained much attention in the past few years owing to its potential advantages.
Methods of lateralization
Implants that follow Grammont’s principles are nonlateralized on both glenoid and humeral sides. Most implants in current use allow lateralization on the glenoid and/or the humerus.6 Factors affecting the lateral offset are summarized in Table 1.
Table 1.
Factors affecting the lateral offset in RTSA.
| Glenoid and glenoid component factors | Humerus and humeral component factors |
|---|---|
| Pre-existing glenoid erosion Extent of glenoid reaming Autograft bone spacers Thickness of baseplate Shape of glenosphere Glenosphere size (radius of curvature) | Thickness of polyethylene insert Level of humeral cut Inclination angle of humeral cut (neck shaft angle) Humeral bearing (inlay vs eccentric onlay) Humeral stem design (straight vs curved) |
Size of glenosphere
Lateral offset increases with increased glenosphere radius. Werthel et al.6 demonstrated that increasing the size of the glenosphere to the next available size increases glenoid lateralization by a mean of only 1.14 mm. This is a very limited increase in lateral offset relative to COR lateralization or humeral lateralization. A larger glenosphere has been shown to increase arc ranges of abduction, internal, and external rotation.9,10 A large glenosphere can introduce difficulties with surgical exposure and carries the potential risk of overstuffing the joint. Given the minimal lateralization that can be achieved and the other variables that are more sensitive to upsizing the glenosphere than the lateral offset, glenosphere size is rarely used as a method for lateralization. Glenosphere size is the only method of glenoid lateralization that does not lateralize the COR. Therefore, glenoid lateralization is often referred to as COR lateralization.
Center of rotation
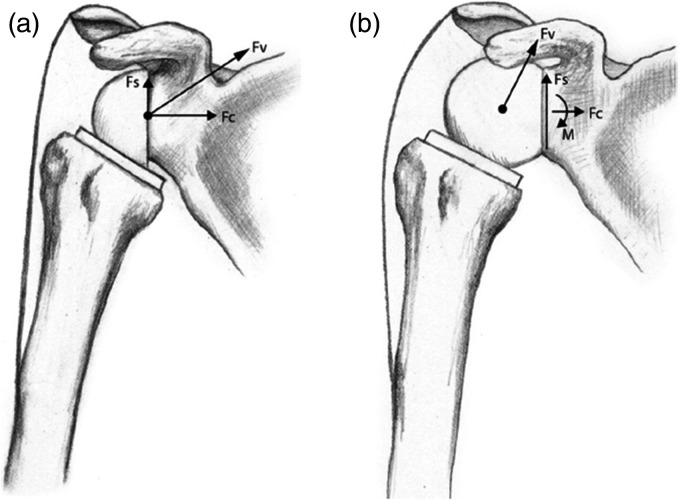
Unlike the native shoulder, the COR in RTSA is fixed due to increased constraint and matched radii of curvature. This creates a combination of compression and shear forces passing through the fixed COR to the glenoid-implant interface. Maximizing the compressive forces and minimizing the shear forces is encouraged for the durability of glenoid component fixation. Although a lateralized COR improves rotational movements,11 the lever arm through which destabilizing and shear forces act on the glenoid-implant interface increases, resulting in increased torque12 (Figure 2). The Grammont-style design medializes the COR and therefore converts shear forces into compressive forces.13
Figure 2.
(a) Compressive (Fc) and shear (Fs) components of the resultant force vector (Fv), which act through a fixed center of rotation (COR). (b) COR lateralization increases the lever arm length which decreases compressive forces, increases destabilizing shear forces, and creates a new moment (M) at the glenoid-implant interface. Republished from Berliner et al.14 with permission from Elsevier.
Gutiérrez et al.15 analyzed the in vitro effect of multiple implant factors on range of motion (ROM). COR offset had the greatest effect. COR can be lateralized either by the implant design or with insertion of bone graft.16 Lateralization of COR by implant design can be achieved by either glenosphere shape modification15 or baseplate lateralization.5,17 Bony lateralization of COR was developed with the hypothesis that increasing the length of the scapular neck would lateralize both, COR and glenoid-implant interface. This type of “biologic lateralization” would achieve the advantages of COR lateralization with minimizing the undesired forces generated at the glenoid-implant interface with prosthetic COR lateralization. Bony increased offset (BIO) RTSA was introduced by Boileau et al.16 in 2011 using Aequalis Reverse Shoulder Prosthesis (Tornier, Saint-Ismier Cedex, France) in which a cylinder of autologous bone graft was harvested from the resected humeral head and placed between the reamed glenoid surface and baseplate. This maintained the COR at the glenoid-implant interface minimizing the torque on the glenoid component.
Bony COR lateralization is not without drawbacks. Denard et al.18 compared stress and displacement in bony and prosthetic lateralization. Stress was lower with prosthetic lateralization through the glenosphere or baseplate. They concluded that the maximum mechanically acceptable limit is at least 10 mm for prosthetic glenoid lateralization and only 5 mm for bony lateralization. Other theoretical advantages of prosthetic over bony lateralization include less surgical time and more accurate control of lateralization in contrast to bone graft which adds surgical time for graft harvest and impaction and also can compress with impaction decreasing the desired extent of lateralization.
Despite the biomechanical advantages of glenoid lateralization, the extent of lateralization that can be achieved through the glenoid component is limited. In a review that included 28 currently used RTSA configurations, Werthel et al.6 demonstrated that none of the implants lateralizes more than 8.3 mm on the glenoid.
Humeral neck shaft angle
Valgus neck shaft angle (NSA) improves abduction but increases scapular notching and limits extension.19–21 Decreasing the NSA has been shown to increase lateral offset22 and improve impingement-free ROM,19 particularly inferior impingement, which helps avoid an adduction deficit20 and lowers the risk of scapular notching.23 Using computed tomography scan-based three-dimensional computer templating, Werner et al.19 analyzed the influence of NSA and bony COR lateralization on ROM. Impingement-free adduction, extension, and rotation were mostly influenced by NSA, whereas COR lateralization had the largest effect on impingement-free abduction and forward flexion. They concluded that a humeral NSA of 135° along with 5 mm glenoid lateralization provides the best combination for impingement-free abduction, adduction, and global function. Gutiérrez et al.15 studied the effect of glenosphere diameter, COR offset, glenosphere position on the glenoid, and humeral NSA on inferior scapular impingement. Reduction of NSA to 130° had the largest effect on inferior scapular impingement. Similarly, de Wilde et al.24 demonstrated that decreasing the NSA from 155° to 145° improved impingement-free adduction by 10°. In a systematic review of 2222 shoulders, scapular notching was significantly higher with 155° prostheses than with 135° prostheses23 (16.8% vs 2.8%). Lädermann et al.25 compared the effect of humeral stems with different NSAs on ROM. Stems with 155° NSA achieved better abduction but worse adduction, extension, and external rotation compared to 135° and 145° designs.
Polyethylene insert and level of humeral cut
Since the humeral surface upon which humeral bearing is inserted is oblique when the arm is in the anatomic position, changing the level of humeral cut or polyethylene cup thickness not only affects the lateral offset but also the distal offset. The distal offset has an influence on the deltoid length and tension. Virani et al.9 demonstrated that polyethylene thickness did not have a significant effect on ROM. Polyethylene thickness and level of humeral cut can be adjusted intraoperatively to optimize passive joint tension and stability. Care should be taken not to overstuff the joint with thicker polyethylene inserts as this may have negative effects on joint load and deltoid force required for abduction.26
Humeral bearing
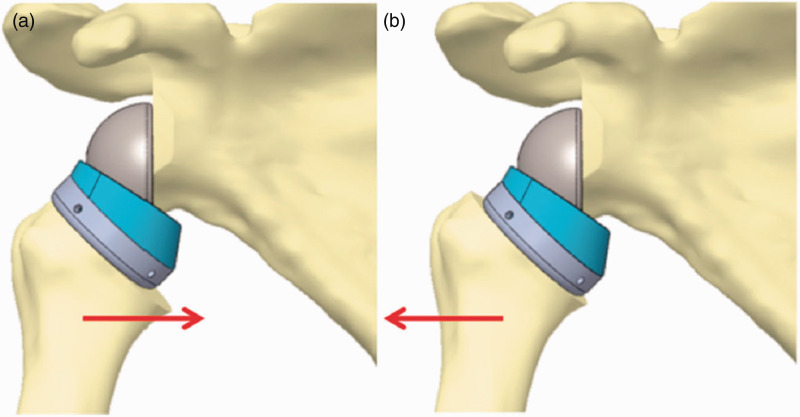
Grammont-style RTSA design features an inlay design of the humeral component. Onlay designs, in which the humeral tray rests on top of the humeral stem, were developed to facilitate conversion of an anatomic arthroplasty to RTSA without replacing the humeral stem. Another advantage of onlay designs is the ability to adjust the lateral offset by placing the tray eccentric over the stem (Figure 3). All designs with humeral lateralization are onlay designs.6 Potential disadvantages of onlay designs include the need to resect more bone from the humerus as well as the modularity which creates another potential junction for failure of the implant.22 Additionally, humeral lateralization using an onlay design decreases the acromiohumeral distance (AHD) which may lead to acromial impingement.22
Figure 3.
Illustration showing how eccentricity of an onlay humeral tray can lateralize the humerus. Republished from Lädermann et al.22 with permission from Springer Nature.
The position of the humeral tray does not change relative to the glenosphere.27 Therefore, a medially placed tray lateralizes the humerus whereas a laterally placed tray shifts the humerus medially. Similarly, the anterior and posterior offsets can be changed by eccentric placement of the tray in the sagittal plane. An anteriorly placed tray shifts the humerus posteriorly while posterior placement of the tray shifts the humerus anteriorly. Berhouet et al.,28 in a virtual model, studied the effect of onlay humeral tray position on impingement-free ROM and muscle moment arms. Placing the tray laterally (medializing the humerus) decreased superior impingement during abduction and elevation in the scapular plane. Placing the tray posteriorly shifted the humerus anteriorly increasing the subscapularis moment arm while anterior placement of the tray increased infraspinatus and teres minor moment arms. Therefore, eccentric placement of the tray in the sagittal plane can be adjusted according to preoperative deficits. A posteriorly placed tray maybe preferable in preoperative internal rotation deficit and an anteriorly placed tray may better correct preoperative external rotation deficit. Glenday et al.,27 in a similar study, demonstrated that a laterally placed tray increases abduction and forward flexion ranges of motion before acromial impingement. A 5 mm laterally placed tray increased abduction by 10° and increased forward flexion ROM by 6°. A posterolaterally placed tray maximized the overall impingement-free ROM, whereas a medially placed tray maximized muscle moment arms. They identified an antagonistic relationship between impingement-free ROM and muscle moment arms due to tray placement. Both studies showed that eccentric placement of the tray does not affect the position of the cup relative to the COR or to the inferior scapular neck. Therefore, there was no effect on inferior impingement. Lädermann et al.25 showed that a medially placed tray (lateralized humerus) decreased abduction due to decreased AHD.
Humeral stem design
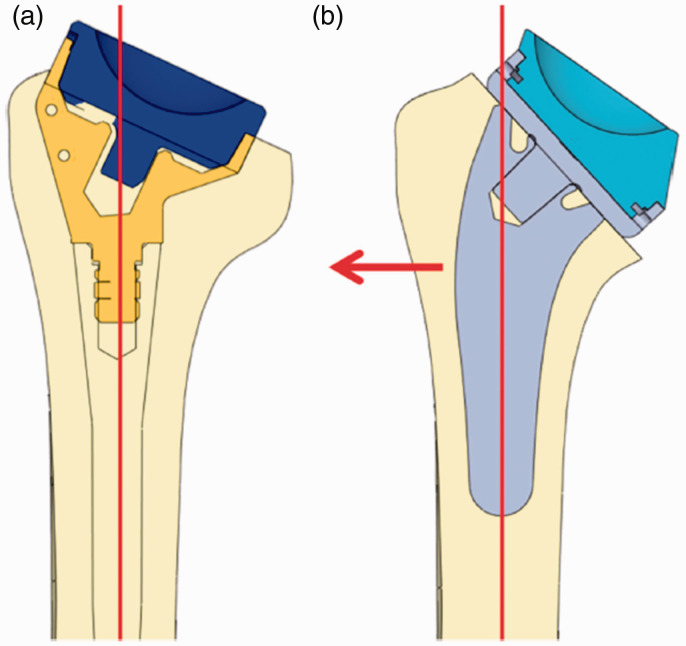
Lädermann et al.22 demonstrated that using a curved stem can increase the lateral offset to a greater extent than that could be achieved by decreasing NSA or by using an onlay design (Figure 4). However, unlike other factors that influence the lateral offset, lateralization by stem design (curved vs straight) is predetermined by the implant design and cannot be modified by the surgeon intraoperatively. Awareness of shoulder surgeons about these factors help choose the appropriate implant preoperatively and should be considered in future implant designs.
Figure 4.
Illustration showing how a curved stem can lateralize the humerus relative to the center of polyethylene. Republished from Lädermann et al.22 with permission from Springer Nature.
Effect of lateral offset on biomechanics of RTSA
Scapular notching
Inferior impingement leading to scapular notching is one of the main complications of RTSA. High rates of scapular notching have been reported with the use of nonlateralized prostheses, reaching up to 96% in some series.29 Notching occurs due to friction between the polyethylene humeral bearing and the scapular pillar.30 This can lead to foreign body reaction from polyethylene debris and higher risk of glenoid loosening.31,32 Clinically, scapular notching can result in increased pain, in addition to decreased strength, ROM and functional outcome scores.33–36
As mentioned earlier, Lateralization can minimize inferior impingement and scapular notching through decreasing NSA and/or COR lateralization.24 Eccentric placement of humeral tray does not affect inferior scapular impingement.27 de Wilde et al.24 demonstrated that prosthetic overhang is the most effective method to prevent scapular notching. Additionally, with only 1 mm overhang, the positive effect of COR lateralization in preventing scapular notching was eliminated. However, in another study, NSA had the largest effect on inferior scapular impingement.15
Range of motion
Gutierrez et al.37 demonstrated a positive linear correlation between COR offset and abduction ROM before impingement on the acromion. Werner et al.19 showed similar results in terms of increased impingement-free abduction with COR lateralization. Additionally, they demonstrated that superior impingement with abduction occurred at the superior edge of the glenoid with medialized COR and at the acromion with lateralized COR.
Several studies have shown the COR lateralization improves adduction ROM.15,20 However, Henninger et al.38 disputed this effect. Authors of the latter study explained the different outcomes were related to different types of implant (hemispherical glenosphere with a cylindrical spacer (Tornier Aequalis) versus a more spherical glenosphere (Reverse Shoulder Prosthesis; DJO Surgical, Austin, TX, USA)), experimental methods (accounting for native deltoid tension) and surgical technique (glenoid tilt).
Rotational movements improve with both glenoid16,39,40 and humeral lateralization.22 Both methods restore the tension of the anterior and posterior cuff. Additionally, glenoid (COR) lateralization recruits more deltoid fibers medial to the COR and increases rotational moment arm of the deltoid. It also decreases impingement on the coracoid with internal rotation and the scapular spine with external rotation. On the other hand, humeral lateralization, in addition to the improved cuff tension, increases the distance between the COR and cuff insertion which increases the moment arm of the anterior and posterior cuff and thereby improves rotation.
Giles et al.41 demonstrated that both internal and external rotation improved with either humeral lateralization or glenosphere lateralization. However, for internal rotation, combining both methods were not synergistic and resulted in internal rotation less than that achieved by each method alone. Overstuffing of the joint and overtensioning of the posterior soft tissues tether the posterior structures limiting internal rotation. They highlighted the effect of intraoperative assessment of internal rotation and the possibility of releasing the contracted posterior soft tissue. On the other hand, external rotation was not limited by overtensioning anterior soft tissues when both humeral and glenoid lateralization were applied.41 Similarly, Ferle et al.42 showed that increased soft tissue tension had no negative impact on passive external rotation ROM.
Using a virtual shoulder model, Virani et al.9 evaluated the effects of different methods of glenoid and humeral lateralization on ROM. They concluded that COR lateralization increases ROM in all planes. A higher NSA maximized abduction while a lower NSA maximized flexion/extension. In their study, inferior glenosphere placement had the most impact on rotational ROM.
Joint loads
Lateralization of COR increases the overall joint contact forces including compression, shear, and bending moments.26,43 Cuff repair in the setting of COR lateralization additionally increases joint load by 29%.41 The increased overall joint loads improve joint stability possibly by increased soft tissue tension.43 However, this can have a negative impact on prosthetic wear and implant survival.33 The increase in shear and bending moments creates a destabilizing torque at the glenoid-implant interface.43 Several studies demonstrated a linear correlation between increasing COR offset and baseplate micromotion.4,44,45 This potentially leads to a higher risk of baseplate loosening, glenosphere unscrewing, and migration.46 The importance of adequate screw fixation of the baseplate with COR lateralization has been emphasized in multiple studies.4,47,48
Studies have shown that joint load magnitude is either decreased or unchanged with humeral lateralization.41,49 Giles et al.41 demonstrated that humeral lateralization (eccentric tray) and rotator cuff loading significantly affect the joint load angle to become more compressive and therefore contribute to concavity compression and enhance joint stability. They found no effect of glenoid lateralization on joint load angle.41
Deltoid
Grammont named his second-generation prosthesis “Delta” in order to emphasize the importance of the deltoid in RTSA.50 All current RTSA designs have a medialized COR relative to the native shoulder. This increases the moment arm of the deltoid which helps decreasing the load required by the deltoid for abduction. Disadvantages of nonlateralized designs include impairment of normal shoulder contour16,40 and loss of physiological wrapping of the deltoid around the greater tuberosity.8 Lateralized COR designs usually minimize the positive influence of a medialized COR on the deltoid by decreasing the deltoid moment arm in elevation and abduction.51 This increases the abduction force required by the deltoid often leading to deltoid fatigue and acromial fracture.26,38,43,52–56 In contrast, humeral lateralization achieves the advantages of lateralizing the greater tuberosity and the deltoid insertion while keeping the COR in its medialized position. This increases the abductor lever arm of the deltoid decreasing the force required for abduction.57 Additionally, with humeral lateralization, more fibers of the anterior and posterior deltoid are lateral to COR, which can theoretically assist in abduction. Therefore, the influences of glenoid and humeral lateralization on the abduction force required by deltoid are opposite. This has been confirmed in several studies where the deltoid force required for abduction increases with COR lateralization26,38,58 and decreases with humeral lateralization.26,49 In two different in vitro studies by the same authors, Giles et al.26,41 reported that humeral lateralization using an eccentric humeral tray can partly reverse the negative effect of glenosphere lateralization on deltoid force. Additionally, rotator cuff load exacerbated the negative effect of glenosphere lateralization on the deltoid.41 They suggested that glenosphere lateralization causes the cuff to act as an adductor, antagonizing the effect of the deltoid. The negative effect of cuff repair in the setting of glenosphere lateralization on deltoid force and joint loads makes this combination undesirable.
Stability
Instability is the most common cause for RTSA revision with a prevalence up to 48%.59 Instability in nonlateralized designs is possibly due to insufficient soft tissue tension as well as inferior impingement on the scapular neck60 and loss of physiological wrapping angle of the deltoid.8 Inferior impingement can be minimized by glenoid lateralization or decreasing NSA. Any method of lateralization can provide more tension on the deltoid and rotator cuff muscles during flexion and abduction. Glenoid lateralization increases overall joint loads, including the compressive component of joint reaction force (JRF) which improves stability.43,49 Humeral lateralization via eccentric humeral tray placement has been shown to change the joint load angle to become more compressive.41 Ferle et al.42 compared anterior dislocation force with different magnitudes of glenosphere lateralization and with varying NSA. Higher dislocation forces were required with 6 mm and 9 mm lateralized compared to nonlateralized glenosphere. Configurations with NSA of 135° required higher dislocation forces than those with NSA of 145°.
The biomechanical aspects of different RTSA lateral offset designs are summarized in Table 2.
Table 2.
Summary of biomechanical aspects according to the magnitude of lateral offset and method of lateralization.
| Nonlateralized RTSA | Glenoid (COR) lateralization | Humeral lateralization (Nonlateralized COR) | |
|---|---|---|---|
| Biomechanical concept | Nonlateralized COR to minimize shear forces on glenoid and decrease force required by deltoid | Lateralized COR to decrease impingement and improve ROM | More anatomic position of tuberosities while maintaining a nonlateralized COR |
| Deltoid | Less force required by deltoid due to: • Higher abduction lever arm • More fibers of the anterior and posterior deltoid lateral to COR, thereby can assist in abduction | COR closer to deltoid line of pull leads to: • Decreased moment arm of deltoid in elevation and abduction • Increased abduction force required for the deltoid • Increased acromial stress fracture | Less force required by deltoid due to: • Higher abduction lever arm • More fibers of the anterior and posterior deltoid lateral to COR, thereby can assist in abduction |
| ROM | Less impingement-free ROM-Poor restoration of IR and ER due to: • Slack cough • Peripheral impingement • Less deltoid fibers medial to COR | Improves ROM: • Increased tension of the remaining cuff • Less peripheral impingement • More deltoid fibers medial to COR which can help with rotational movements | Improves ROM • Increased tension of the remaining cuff • Increased abductor lever arm • Increased rotational moment arm of anterior and posterior cuff-More acromial impingement due to less AHD (less abduction) |
| Scapular notching | Higher risk of scapular notching leading to • glenoid osteolysis, loosening • polyethylene wear | Lower risk of scapular notching | • Lower risk with decreasing NSA • No effect with eccentric humeral tray placement |
| Stability | Instability due to • slack cough • inferior impingement loss of physiological wrapping angle of the deltoid | Improved stability • Increased soft tissue tension which increases compressive force • Increased overall joint load magnitude • Partial restoration of physiologic wrapping of the deltoid • Less peripheral impingement | Improved stability • Increased soft tissue tension which increases compressive force • Affects joint load angle which increases compressive component of JRF • Partial restoration of physiologic wrapping of the deltoid |
| Joint loads | Increased compressive component of joint load | Increased shear forces which increases risk of glenoid loosening | Increased compressive component of joint load |
| Increased total JRF due to: • Increased soft tissue tension, including rotator cuff • Increased force required by deltoid • Deltoid wrapping around greater tuberosity Leads to: • Improved stability • Negative impact on prosthetic wear and implant survival | Increased total JRF due to: • Increased soft tissue tension, including rotator cuff • Deltoid wrapping around greater tuberosity Leads to: • Improved stability • Negative impact on prosthetic wear and implant survival | ||
| Shoulder contour | Loss of normal shoulder contour | More anatomic shoulder contour | |
| Soft tissue tension | Slack rotator cuff | • Retensions the remaining cuff. • A more anatomic vector of pull of the deltoid as it wraps around the humeral component | |
COR: center of rotation; RTSA: reverse total shoulder arthroplasty; ROM: range of motion; AHD: acromiohumeral distance; JRF: joint reaction force; IR: internal rotation; ER: external rotation.
The subscapularis
The influence of subscapularis repair on biomechanics of RTSA is controversial. Some advocate repair in order to decrease the risk of dislocation and improved internal rotation.21,61–64 Repair also improves joint protection with muscle closure around the joint. Other studies showed that subscapularis repair antagonizes the posterior cuff in external rotation and does not improve stability.65–67 Additionally, the distalization of the humerus creates an adductor moment arm of the subscapularis,63 which increases the abduction force required by the deltoid.67
The decision to repair the subscapularis may be influenced by the lateral offset. Werner et al. reported that subscapularis repair in the setting of glenosphere lateralization can negatively affect functional outcomes.68 In contrast, Roberson et al.,69 using a lateralized glenoid design, reported no differences in functional outcomes with or without repair. Humeral lateralization increases the distance between COR and humerus which increases the rotational moment arms. Friedman et al.,70 with a humeral lateralized design, reported better internal rotation and functional outcome scores but less active abduction and passive external rotation with subscapularis repair. Eno et al.71 demonstrated that repairing the subscapularis in a more superior position can help reduce its adductor moment arm and minimize the antagonistic effect on the deltoid.
Published outcomes in clinical studies
Nonlateralized RTSA
Sirveaux et al.,36 in a multicenter study, reported the outcomes of 80 RTSAs for treatment of glenohumeral arthritis with massive rotator cuff tears using the Delta III prosthesis at a mean follow-up of 44 months. The mean gain in active external rotation at the side was 7.7°. The mean gain in passive external rotation at the side was 9°. Both were not statistically significant. However, statistically significant improvements were achieved in active and passive external rotation at 90° of abduction. Rate of scapular notching was 63.6%. Several other studies reported high rates of scapular notching and limited improvements in external rotation with nonlateralized designs.29,72,73
Lateralized RTSA
Frankle et al.40 reported outcomes of RTSA using the first-generation Reverse Shoulder Prosthesis (Encore Medical, Austin, TX, USA) which has a lateralized COR. They achieved a 29.1° postoperative improvement in external rotation and no notching at a mean of 33 months follow-up. However, out of 60 shoulders, there were 7 cases of glenoid loosening and 2 cases of stress fracture of the metal baseplate. To minimize the incidence of baseplate failure that was observed in the latter study, a modification of the Encore Reverse Shoulder Prosthesis was made by designing a 5 mm peripheral locking screws for use with the baseplate instead of 3.5 mm nonlocking screws. Additionally, the surgical technique was modified to inferiorly tilt the glenosphere 10–15° which had been proved to produce the most uniform compressive forces and limit micromotion at the glenoid-implant interface.74
Following these important modifications, another study was performed in the same institution on 96 shoulders using the second-generation Reverse Shoulder Prosthesis. At a mean follow-up of 27.5 months, they reported no mechanical baseplate failure and no scapular notching. They achieved a 54.5° gain in forward flexion, 48.5° in abduction and 14.8° in external rotation.75 In their five-year follow-up report, there were no baseplate loosening or failure and 9% scapular notching. Additionally, forward flexion, abduction, and external rotation ranges of motion continued to improve significantly.76 However, they suggested that improved ROM may be due to different methods of assessment at the two- and five-year follow-up reports. In their 10-year follow-up report, 42 shoulders were available for follow-up. There was no mechanical baseplate failure or glenoid component loosening. There was a slight decrease in all planes of motion compared to the five-year report. Implant survivorship was 90.7% using revision as an endpoint.77 Following these reports, several studies reported improved external rotation and lower rates of scapular notching with COR lateralization compared to nonlateralized designs.5,16,17
Comparative studies
Streit et al.,78 in a prospective study compared the outcomes of 9 grammont-style prostheses (Aequalis) (nonlateralized COR and a 155° NSA) and 9 Encore Reversed Shoulder Prostheses (DJO) (6- or 10-mm lateralized COR and a 135° NSA). Mean follow-up was 8.1 months. Postoperative ER was 35° in the lateralized group and 28.3° in the nonlateralized group (p = 0.07). However, higher forward flexion values were achieved in the nonlateralized group (144° vs 116°) (p = 0.05). They explained the latter observation by the less distalization achieved with the Encore prosthesis. Despite the small sample size and the short follow-up period, the observed differences in ROM are worth considering.
Athwal et al.79 retrospectively compared outcomes of a standard Grammont-style RTSA with a BIO-RTSA (40 patients; 20 in each cohort). They utilized the Aequalis system in both groups. Scapular notching was 75% in the standard RTSA group and 40% in the BIO-RTSA group (p = 0.022). Rate of complete graft incorporation in the BIO-RTSA group was 80%. No differences were noted in active flexion, external rotation, internal rotation, or any of the functional outcomes tested.
Collin et al.80 compared outcomes of standard (69 shoulders) and BIO-RTSA (61 shoulders) by a single surgeon. They used the Aequalis system for both groups. There were no differences in internal/external rotation and in radiographic outcomes including scapular notching. Forward flexion was better in the standard group. However, the difference was not clinically important (145° vs 138°).
Franceschetti et al.81 recently performed a comparative study of 29 nonlateralized and 30 BIO-RTSAs. All surgeries were performed by a single surgeon using Aequalis Ascend Flex with a NSA 145°. Graft incorporation 90% in the BIO group. External rotation gain was 23° in BIO and 17° in standard (statistical analysis was not performed on external rotation gain)
Merolla et al.82 compared outcomes of 36 Aequalis Reverse II (155°, inlay, straight stem, follow up (FU) 35.1 months) versus 38 Aequalis Ascend flex (short curved stem, eccentric tray, FU 29.1 months). In the second group, 20 patients had BIO lateralization. External rotation gain was 15° in the first group and 33° in the second. (p = 0.003). Three scapular fractures occurred with the curved short stem and BIO implant. Scapular notching was 39% in the first group and 5% in the second.
Level I randomized controlled trials
Greiner et al.39 compared the outcomes of 10 mm BIO versus standard Aequalis Reverse Shoulder Prosthesis (34 patients; 17 in each group). Mean follow-up was 22 months. Two patients died and 1 was lost to follow-up leaving 15 patients in the standard group and 16 in the BIO group. External rotation gain in standard versus BIO at 0° was 18° versus 28° and at 90° was 33° versus 42°. Differences did not reach statistical significance, possibly due to the small sample size. However, it did reach statistical significance after excluding patients with degenerative changes of teres minor; 16° versus 42° at 0 and 27° versus 60° at 90. Graft healed in all BIO patients.
Gobezie et al.83 enrolled 100 primary reverse shoulder arthroplasty patients to compare outcomes according to NSA using otherwise identical implants (Univers Revers prosthesis (Arthrex, Naples, FL, USA) with a nonlateralized glenosphere). This prosthesis allows placing the humeral cup at either 135° or 155°. Thirty-seven patients in the 135° group and 31 patients in the 155° group completed a minimum follow-up of two years. No significant differences in ROM and functional outcomes. Postoperative external rotation was similarly not improved in both groups. Rate of scapular notching was significantly higher in the 155° group (58%) versus 21% in the 135° group. They attributed the lack of improvement in external rotation and the relatively high rate of scapular notching in the 135° group to the nonlateralized glenosphere which they now recommend against using.
Long-term outcomes
It is still not clear whether lateralization influences long-term outcomes and implant survivorship. Functional outcomes of nonlateralized RTSA were reported to deteriorate after six to eight years.36,53 Similarly, there was slight deterioration in all planes of motion at 10-year follow-up compared to the 5-year report in the study by Cuff et al.77 In two studies with minimum 10-year-follow-up, implant survivorship at 10 years was 90.7% with the lateralized Encore/DJO Reversed Shoulder Prosthesis77 and 93% with the nonlateralized Delta III prosthesis.84 Kennon et al.85 recently compared outcomes of nonlateralized and glenoid-lateralized RTSA at 10 years. There were no significant differences in complications or reoperations. Nonlateralized group had better active forward flexion and higher scapular notching rate.
Conclusion
The ideal extent and method of lateralization are yet to be determined and are probably variable from one patient to another. Surgeons can choose from several RTSA designs with various features. Patient factors usually influence this choice including age, bone quality, strength of glenoid fixation, cuff condition, patient activities, and patient expectations. Most current implants allow different degrees and methods of lateralization within the same implant to help individualize the lateral offset for different patients. Excessive lateralization can be problematic in smaller patients and those with soft tissue contractures. This can result in difficult joint reduction and subscapularis repair. Moreover, overstuffing can limit ROM and accelerate polyethylene wear.
In case glenoid-lateralized implants are used, glenoid component fixation should be optimal. Anterior or posterior screws are commonly omitted in small-sized glenoids or in patients with remarkable glenoid erosion. In this case, risk of glenoid complications might be higher and the surgeon’s threshold to not lateralize through the glenoid should be lowered. In case optimal baseplate fixation is achievable, maximizing the function and ROM may be reasonable to aim at. Humeral lateralization can be applied to improve soft tissue tension, improve stability, and decrease force required by deltoid. However, the risk of acromial impingement and overstuffing the joint should be considered.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: YHB*.
ORCID iD: Yehia H Bedeir https://orcid.org/0000-0002-3559-2005
Ethical approval and Patient Consent
Not needed.
References
- 1.Kadum B, Wahlström P, Khoschnau S, et al. Association of lateral humeral offset with functional outcome and geometric restoration in stemless total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: e285–e294. [DOI] [PubMed] [Google Scholar]
- 2.Kadum B, Sayed-Noor AS, Perisynakis N, et al. Radiologic assessment of glenohumeral relationship: reliability and reproducibility of lateral humeral offset. Surg Radiol Anat 2015; 37: 363–368. [DOI] [PubMed] [Google Scholar]
- 3.Iannotti JP, Gabriel JP, Schneck SL, et al. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am 1992; 74: 491–500. [PubMed] [Google Scholar]
- 4.Harman M, Frankle M, Vasey M, et al. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 2005; 14: 162S–167S. [DOI] [PubMed] [Google Scholar]
- 5.Valenti P, Sauzières P, Katz D, et al. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res 2011; 469: 2550–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werthel J-D, Walch G, Vegehan E, et al. Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop 2019; 43: 2349–2360. [DOI] [PubMed] [Google Scholar]
- 7.Boutsiadis A, Lenoir H, Denard PJ, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1226–1234. [DOI] [PubMed] [Google Scholar]
- 8.Routman HD, Flurin P-H, Wright TW, et al. Reverse shoulder arthroplasty prosthesis design classification system. Bull Hosp Jt Dis 2015; 73: S5–S14. [PubMed] [Google Scholar]
- 9.Virani NA, Cabezas A, Gutiérrez S, et al. Reverse shoulder arthroplasty components and surgical techniques that restore glenohumeral motion. J Shoulder Elbow Surg 2013; 22: 179–187. [DOI] [PubMed] [Google Scholar]
- 10.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 151–158. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Knutson Z, Choi D, et al. Effects of glenosphere positioning on impingement-free internal and external rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 807–813. [DOI] [PubMed] [Google Scholar]
- 12.Roche CP, Stroud NJ, Flurin P-H, et al. Reverse shoulder glenoid baseplate fixation: a comparison of flat-back versus curved-back designs and oval versus circular designs with 2 different offset glenospheres. J Shoulder Elbow Surg 2014; 23: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 13.Kontaxis A, Johnson GR. The biomechanics of reverse anatomy shoulder replacement – a modelling study. Clin Biomech 2009; 24: 254–260. [DOI] [PubMed] [Google Scholar]
- 14.Berliner JL, Regalado-Magdos A, Ma CB, et al. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 150–160. [DOI] [PubMed] [Google Scholar]
- 15.Gutiérrez S, Levy JC, Frankle MA, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg 2008; 17: 608–615. [DOI] [PubMed] [Google Scholar]
- 16.Boileau P, Moineau G, Roussanne Y, et al. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011; 469: 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz D, Valenti P, Kany J, et al. Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int Orthop 2016; 40: 99–108. [DOI] [PubMed] [Google Scholar]
- 18.Denard PJ, Lederman E, Parsons BO, et al. Finite element analysis of glenoid-sided lateralization in reverse shoulder arthroplasty. J Orthop Res 2017; 35: 1548–1555. [DOI] [PubMed] [Google Scholar]
- 19.Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1726–1731. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez S, Comiskey CA, Luo Z-P, et al. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. J Bone Joint Surg Am 2008; 90: 2606–2615. [DOI] [PubMed] [Google Scholar]
- 21.Oh JH, Shin S-J, McGarry MH, et al. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 22.Lädermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop 2015; 39: 2205–2213. [DOI] [PubMed] [Google Scholar]
- 23.Erickson BJ, Frank RM, Harris JD, et al. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2015; 24: 988–993. [DOI] [PubMed] [Google Scholar]
- 24.de Wilde LF, Poncet D, Middernacht B, et al. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop 2010; 81: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lädermann A, Denard PJ, Collin P, et al. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop. Epub ahead of print 3 January 2020. DOI: 10.1007/s00264-019-04463-2. [DOI] [PubMed] [Google Scholar]
- 26.Giles JW, Langohr GDG, Johnson JA, et al. Implant design variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res 2015; 473: 3615–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glenday J, Kontaxis A, Roche S, et al. Effect of humeral tray placement on impingement-free range of motion and muscle moment arms in reverse shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2019; 62: 136–143. [DOI] [PubMed] [Google Scholar]
- 28.Berhouet J, Kontaxis A, Gulotta LV, et al. Effects of the humeral tray component positioning for onlay reverse shoulder arthroplasty design: a biomechanical analysis. J Shoulder Elbow Surg 2015; 24: 569–577. [DOI] [PubMed] [Google Scholar]
- 29.Werner CML, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005; 87: 1476–1486. [DOI] [PubMed] [Google Scholar]
- 30.Day JS, MacDonald DW, Olsen M, et al. Polyethylene wear in retrieved reverse total shoulder components. J Shoulder Elbow Surg 2012; 21: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyffeler RW, Werner CML, Simmen BR, et al. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br 2004; 86: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 32.Mollon B, Mahure SA, Roche CP, et al. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017; 26: 1253–1261. [DOI] [PubMed] [Google Scholar]
- 33.Hoenecke HR, Flores-Hernandez C, D’Lima DD. Reverse total shoulder arthroplasty component center of rotation affects muscle function. J Shoulder Elbow Surg 2014; 23: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 34.Sadoghi P, Leithner A, Vavken P, et al. Infraglenoidal scapular notching in reverse total shoulder replacement: a prospective series of 60 cases and systematic review of the literature. BMC Musculoskelet Disord 2011; 12: 101–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simovitch RW, Helmy N, Zumstein MA, et al. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am 2007; 89: 934–939. [DOI] [PubMed] [Google Scholar]
- 36.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004; 86: 388–395. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez S, Levy JC, Lee WE, et al. Center of rotation affects abduction range of motion of reverse shoulder arthroplasty. Clin Orthop Relat Res 2007; PAP: 78–82. [DOI] [PubMed] [Google Scholar]
- 38.Henninger HB, Barg A, Anderson AE, et al. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 2012; 21: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 39.Greiner S, Schmidt C, Herrmann S, et al. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg 2015; 24: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 40.Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum of two year follow up study of sixty patients. J Bone Joint Surg Am 2005; 87: 1697–1697. [DOI] [PubMed] [Google Scholar]
- 41.Giles JW, Langohr GDG, Johnson JA, et al. The rotator cuff muscles are antagonists after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 1592–1600. [DOI] [PubMed] [Google Scholar]
- 42.Ferle M, Pastor M-F, Hagenah J, et al. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg 2019; 28: 966–973. [DOI] [PubMed] [Google Scholar]
- 43.Costantini O, Choi DS, Kontaxis A, et al. The effects of progressive lateralization of the joint center of rotation of reverse total shoulder implants. J Shoulder Elbow Surg 2015; 24: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 44.Virani NA, Harman M, Li K, et al. In vitro and finite element analysis of glenoid bone/baseplate interaction in the reverse shoulder design. J Shoulder Elbow Surg 2008; 17: 509–521. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins AR, Hansen UN, Bull AMJ, et al. Fixation of the reversed shoulder prosthesis. J Shoulder Elbow Surg 2008; 17: 974–980. [DOI] [PubMed] [Google Scholar]
- 46.Elwell J, Choi J, Willing R. Quantifying the competing relationship between adduction range of motion and baseplate micromotion with lateralization of reverse total shoulder arthroplasty. J Biomech 2017; 52: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahir SP, Walker PS, Squire-Taylor CJ, et al. Analysis of glenoid fixation for a reversed anatomy fixed-fulcrum shoulder replacement. J Biomech 2004; 37: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 48.Chebli C, Huber P, Watling J, et al. Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg 2008; 17: 323–327. [DOI] [PubMed] [Google Scholar]
- 49.Liou W, Yang Y, Petersen-Fitts GR, et al. Effect of lateralized design on muscle and joint reaction forces for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 564–572. [DOI] [PubMed] [Google Scholar]
- 50.Grammont PM, Trouilloud P, Laffay J, et al. Etude et réalisation d’une nouvelle prothèse d’épaule. Rheumatologie 1987; 39: 407–418. [Google Scholar]
- 51.Hamilton MA, Diep P, Roche C, et al. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res 2015; 33: 605–613. [DOI] [PubMed] [Google Scholar]
- 52.Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011; 469: 2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006; 88: 1742–1747. [DOI] [PubMed] [Google Scholar]
- 54.Walch G, Mottier F, Wall B, et al. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg 2009; 18: 495–502. [DOI] [PubMed] [Google Scholar]
- 55.Wong MT, Langohr GDG, Athwal GS, et al. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg 2016; 25: 1889–1895. [DOI] [PubMed] [Google Scholar]
- 56.Hess F, Zettl R, Smolen D, et al. Anatomical reconstruction to treat acromion fractures following reverse shoulder arthroplasty. Int Orthop 2018; 42: 875–881. [DOI] [PubMed] [Google Scholar]
- 57.Franceschetti E, de Sanctis EG, Ranieri R, et al. The role of the subscapularis tendon in a lateralized reverse total shoulder arthroplasty: repair versus nonrepair. Int Orthop 2019; 43: 2579–2586. [DOI] [PubMed] [Google Scholar]
- 58.Hettrich CM, Permeswaran VN, Goetz JE, et al. Mechanical tradeoffs associated with glenosphere lateralization in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 1774–1781. [DOI] [PubMed] [Google Scholar]
- 59.Boileau P, Melis B, Duperron D, et al. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 1359–1370. [DOI] [PubMed] [Google Scholar]
- 60.Boileau P, Watkinson DJ, Hatzidakis AM, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005; 14: 147S–161S. [DOI] [PubMed] [Google Scholar]
- 61.Edwards TB, Williams MD, Labriola JE, et al. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18: 892–896. [DOI] [PubMed] [Google Scholar]
- 62.Ackland DC, Roshan-Zamir S, Richardson M, et al. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am 2010; 92: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 63.Routman HD. The role of subscapularis repair in reverse total shoulder arthroplasty. Bull Hosp Jt Dis 2013; 71: 108–112. [PubMed] [Google Scholar]
- 64.Cheung EV, Sarkissian EJ, Sox-Harris A, et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1946–1952. [DOI] [PubMed] [Google Scholar]
- 65.Boulahia A, Edwards TB, Walch G, et al. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics 2002; 25: 129–133. [DOI] [PubMed] [Google Scholar]
- 66.Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg 2012; 21: 36–41. [DOI] [PubMed] [Google Scholar]
- 67.Hansen ML, Nayak A, Narayanan MS, et al. Role of subscapularis repair on muscle force requirements with reverse shoulder arthroplasty. Bull Hosp Jt Dis 2015; 73: S21–S27. [PubMed] [Google Scholar]
- 68.Werner BC, Wong AC, Mahony GT, et al. Clinical outcomes after reverse shoulder arthroplasty with and without subscapularis repair: the importance of considering glenosphere lateralization. J Am Acad Orthop Surg 2018; 26: e114–e119. [DOI] [PubMed] [Google Scholar]
- 69.Roberson TA, Shanley E, Griscom JT, et al. Subscapularis repair is unnecessary after lateralized reverse shoulder arthroplasty. JB JS Open Access 2018; 3: e0056–e0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedman RJ, Flurin P-H, Wright TW, et al. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg 2017; 26: 662–668. [DOI] [PubMed] [Google Scholar]
- 71.Eno JT, Kontaxis A, Novoa‐Boldo A, et al. The biomechanics of subscapularis repair in reverse shoulder arthroplasty: The effect of lateralization and insertion site. J Orthop Res 2020; 38: 888–894. [DOI] [PubMed] [Google Scholar]
- 72.Boileau P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15: 527–540. [DOI] [PubMed] [Google Scholar]
- 73.Lévigne C, Garret J, Boileau P, et al. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res 2011; 469: 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutiérrez S, Greiwe RM, Frankle MA, et al. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg 2007; 16: S9–S12. [DOI] [PubMed] [Google Scholar]
- 75.Cuff D, Pupello D, Virani N, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am 2008; 90: 1244–1251. [DOI] [PubMed] [Google Scholar]
- 76.Cuff D, Clark R, Pupello D, et al. Reverse Shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am 2012; 94: 1996–2000. [DOI] [PubMed] [Google Scholar]
- 77.Cuff DJ, Pupello DR, Santoni BG, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am 2017; 99: 1895–1899. [DOI] [PubMed] [Google Scholar]
- 78.Streit JJ, Shishani Y, Gobezie R. Medialized versus lateralized center of rotation in reverse shoulder arthroplasty. Orthopedics 2015; 38: e1098–e1103. [DOI] [PubMed] [Google Scholar]
- 79.Athwal GS, MacDermid JC, Reddy KM, et al. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg 2015; 24: 468–473. [DOI] [PubMed] [Google Scholar]
- 80.Collin P, Liu X, Denard PJ, et al. Standard versus bony increased-offset reverse shoulder arthroplasty: a retrospective comparative cohort study. J Shoulder Elbow Surg 2018; 27: 59–64. [DOI] [PubMed] [Google Scholar]
- 81.Franceschetti E, Ranieri R, Giovanetti de Sanctis E, et al. Clinical results of bony increased-offset reverse shoulder arthroplasty (BIO-RSA) associated with an onlay 145° curved stem in patients with cuff tear arthropathy: a comparative study. J Shoulder Elbow Surg 2020; 29: 58–67. [DOI] [PubMed] [Google Scholar]
- 82.Merolla G, Walch G, Ascione F, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg 2018; 27: 701–710. [DOI] [PubMed] [Google Scholar]
- 83.Gobezie R, Shishani Y, Lederman E, et al. Can a functional difference be detected in reverse arthroplasty with 135° versus 155° prosthesis for the treatment of rotator cuff arthropathy: a prospective randomized study. J Shoulder Elbow Surg 2019; 28: 813–818. [DOI] [PubMed] [Google Scholar]
- 84.Bacle G, Nové-Josserand L, Garaud P, et al. Long-term outcomes of reverse total shoulder arthroplasty. J Bone Joint Surg Am 2017; 99: 454–461. [DOI] [PubMed] [Google Scholar]
- 85.Kennon JC, Songy C, Bartels D, et al. Primary reverse shoulder arthroplasty: how did medialized and glenoid-based lateralized style prostheses compare at 10 years? J Shoulder Elbow Surg. Epub ahead of print 7 February 2020. DOI: 10.1016/J.JSE.2019.11.004. [DOI] [PubMed] [Google Scholar]