Abstract
Anthropogenic environmental change can underpin major shifts in natural selective regimes, and can thus alter the evolutionary trajectories of wild populations. However, little is known about the evolutionary impacts of deforestation—one of the most pervasive human-driven changes to terrestrial ecosystems globally. Absence of forest cover (i.e. exposure) has been suggested to play a role in selecting for insect flightlessness in montane ecosystems. Here, we capitalize on human-driven variation in alpine treeline elevation in New Zealand to test whether anthropogenic deforestation has caused shifts in the distributions of flight-capable and flightless phenotypes in a wing-polymorphic lineage of stoneflies from the Zelandoperla fenestrata species complex. Transect sampling revealed sharp transitions from flight-capable to flightless populations with increasing elevation. However, these phenotypic transitions were consistently delineated by the elevation of local treelines, rather than by absolute elevation, providing a novel example of human-driven evolution in response to recent deforestation. The inferred rapid shifts to flightlessness in newly deforested regions have implications for the evolution and conservation of invertebrate biodiversity.
Keywords: rapid evolution, flight loss, Plecoptera, Zelandoperla
1. Introduction
Human-driven environmental change is increasingly recognized as an important evolutionary force [1,2]. Indeed, examples of evolution over contemporary timescales are accumulating at an accelerating rate [3,4], with many of the most dramatic cases resulting from anthropogenic disturbances [5,6]. Perhaps the best known of these rapid shifts is ‘industrial melanism’ in the peppered moth [7], wherein melanic morphs rapidly increased in frequency with the onset of industrial pollution. While the biological effects of some anthropogenic pressures (e.g. urbanization, pollution, overfishing) have been well studied, relatively little is known about the potential evolutionary impacts of human-driven deforestation.
Loss of forest habitats represents one of the most significant human-driven alterations of terrestrial ecosystems globally [8]. This ecological transformation can result in local extinctions [9] but also has the potential to alter the evolutionary trajectories of surviving biota through isolation of populations and alteration of selection regimes [10,11]. New Zealand, as the last major landmass to be colonized by humans, remained almost entirely covered with forest in areas below the alpine treeline prior to Polynesian (Māori) settlement around 750 years ago [12]. Widespread burning of native forest commenced shortly after Māori arrival and transformed more than 40% of forests in the South Island to grassland and fern-shrubland [12–15]. The dominant forest species of the South Island (Nothofagus spp.) are poorly adapted to fire and in many areas forests have not recovered [16], providing unique opportunities to study evolutionary responses to human-driven environmental change.
Loss of flight is a common characteristic of montane insect assemblages globally [17], and the absence of forest cover in many montane ecosystems has been suggested to play a role in the evolution of flightlessness [18]. In New Zealand, for example, wingless stonefly species are found almost exclusively above the alpine treeline, with increased exposure (e.g. strong winds) thought to impose a strong selection pressure against flight [18]. Genomic and transcriptomic evidence support a genetic basis for wing reduction in stoneflies from the polymorphic Zelandoperla fenestrata species complex (Plecoptera: Gripopterygidae) [19,20], where fully winged morphs predominantly occur in lowland forest and wing-reduced morphs are associated with high elevations [21–24]. Intriguingly, recent transect sampling has revealed localized clines in morph frequencies that appear to correspond with the elevation of the alpine treeline [24], supporting a possible role for exposure in directly selecting for flight reduction. More broadly, the role of wind exposure as a key driver of insect flight loss is strongly supported by quantitative analyses of Southern Ocean insect assemblages [25].
In this study, we take advantage of variation in treeline elevation at deforested and undisturbed sites in southern New Zealand (figure 1) to assess the association between treeline position and flight loss in wing-polymorphic stoneflies. Specifically, by undertaking elevational transects in parallel across sites that vary in the contemporary positions of their treelines (ranging from approx. 600 to 1000 m.a.s.l.), we tested for rapid evolutionary and biogeographic effects of environmental change following human arrival in New Zealand. If phenotype frequencies were not affected by recent deforestation, transitions to flight loss were expected to occur at a consistent elevation. Anthropogenic shifts in the distributions of flight-capable and flightless phenotypes were predicted based on strong biogeographic associations previously found between treeline elevation and flightless stonefly species [18].
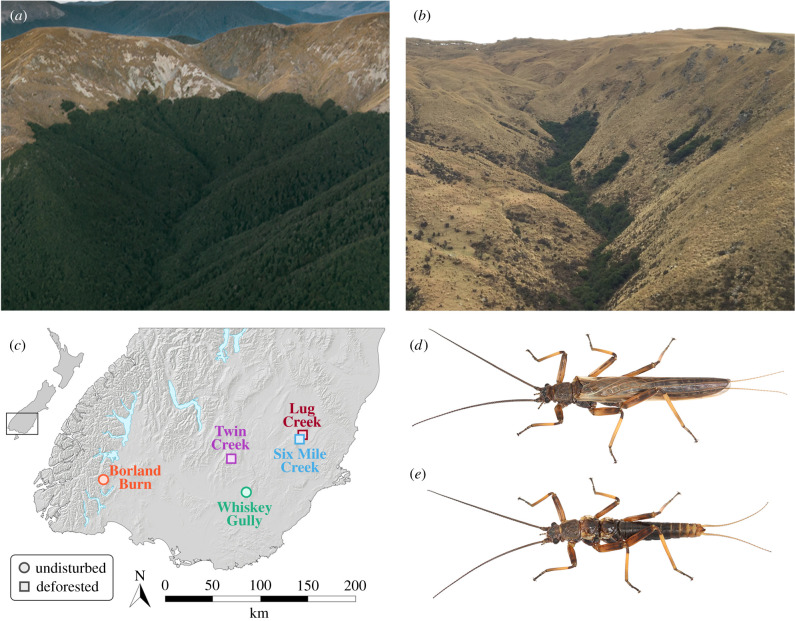
Figure 1.
Testing for the effect of treeline elevation on the distribution of insect ecotypes. (a) A forested stream (treeline 1090 m.a.s.l.) in southwestern New Zealand; (b) a largely deforested stream (treeline 710 m.a.s.l.) in southeastern New Zealand. (c) Locations of elevational transects in undisturbed (circles) and deforested (squares) montane streams, sampling the relative frequencies of flight phenotypes in the Z. fenestrata species complex; (d) example of a flight-capable adult and (e) a flightless adult.
2. Material and methods
(a) . Study system
Wing-polymorphic populations of the Z. fenestrata complex are only known to occur in southern New Zealand [22]. Wing length variation within polymorphic populations is typically bimodal, although the extent of wing reduction varies among populations [21,26]. We conducted flight assays using an assemblage of individuals covering the spectrum of wing reduction in the Z. fenestrata complex (n = 383), so that we could identify flight-capable and flightless forms in other populations based on morphological data. Evaluation of selective forces interacting with wing reduction in this complex has previously relied on the availability of individuals in the terrestrial adult stage [24]. However, the degree of wing development can also be observed during the final instars of the more commonly encountered aquatic nymph stage. We, therefore, carried out an additional study on wing pad growth patterns [27] in nymphs from a fully winged lowland population, allowing the classification of flight-capable and flightless phenotypes to be extended to late-instar nymphs sampled in elevational transects.
(b) . Transect sampling
Transects were undertaken across multiple stream systems that differed primarily in the elevational position of the local treeline. Prospective transect sites were excluded if populations lacked sufficient numbers of nymphs passing a minimum size threshold, or lacked evidence for the existence of a polymorphism (see supplementary methods in the electronic supplementary material for details). Five transects of wing-polymorphic populations were completed, ranging in treeline elevation from 607 to 965 m.a.s.l. (electronic supplementary material, table S1), representing three deforested sites and two undisturbed sites (figure 1). All five transects were carried out in comparably sized montane streams, at similar latitudes (figure 1c), and over similar elevational ranges (figure 2) in order to equitably compare sites with undisturbed and deforested treelines. In each stream, treeline elevation was measured using an altimeter and corroborated against the digital elevation model of Google Earth. Recently emerged adults and large nymphs were collected opportunistically from stones, wood and moss in riffles at discrete elevational intervals above and below the treeline of each stream. Collecting effort at each elevation was spread over several metres and across multiple areas of riffle habitat to avoid sampling of closely related individuals. Adults were immediately preserved in absolute ethanol and nymphs were returned to the laboratory and reared to later instars or adults.
Figure 2.
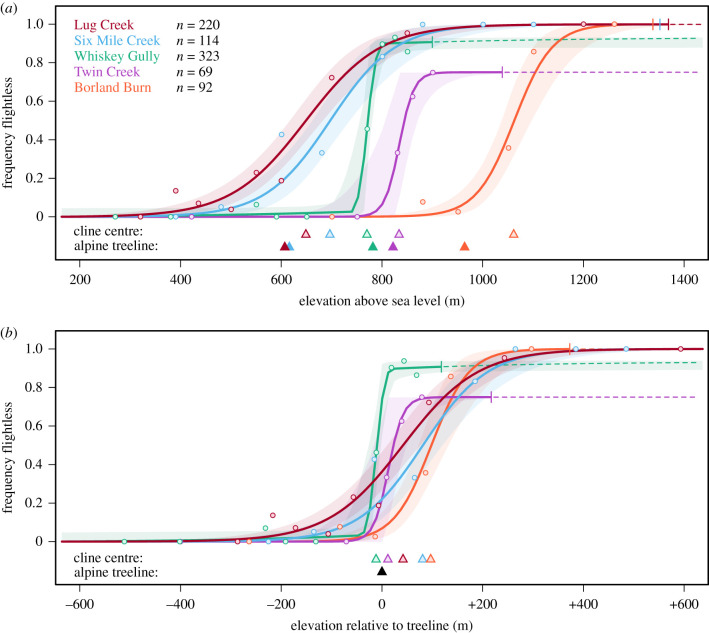
Evidence for the role of the treeline in constraining the local distributions of flight-capable versus flightless insect ecotypes. Maximum-likelihood clines (with 95% credible intervals) and observed data depict the frequency of flightless individuals along five independent elevational transects of wing-polymorphic stoneflies (Z. fenestrata complex) in southern New Zealand. At each location, separate clines were fitted to frequency data over (a) elevation above sea level and (b) elevation relative to the position of the local treeline. Vertical strokes represent the upper limits of streams. In all transects cline centres were found to correspond to the elevation of the local treeline rather than to absolute elevation. Individual clines are detailed in electronic supplementary material, figure S4, and tables S3 and S4.
(c) . Phenotype classification
Flight assays were used to identify the degree of wing reduction conferring a loss of flight ability in wing-polymorphic populations (see supplementary methods for details). From these assays, a ‘flightless threshold’ was identified (electronic supplementary material, figure S1), which we subsequently used to classify the flight capability of adults originating from elevational transects of treeline populations (n = 454; electronic supplementary material, figure S2). Next, we fitted the flightless threshold to nymph data by scaling adult wing length variation to nymph wing pad size variation based on a series of linear regression models (see supplementary methods for details). In this way, by projecting the future flight capability of nymphs (electronic supplementary material, figure S3), we were able to integrate data from both nymphs (n = 364) and adults sampled along elevational transects. The sums of flight-capable and flightless adults and nymphs from each elevational sample were then used to calculate phenotype frequencies.
(d) . Data analysis
We examined how frequencies of flight-capable and flightless phenotypes change in relation to absolute elevation and the position of the local treeline across each transect. Previous elevational sampling of wing-polymorphic populations in the Z. fenestrata complex indicate that the transition to vestigial-winged populations at high elevations fits a clinal model [24]. Accordingly, we fitted geographical clines to phenotype frequency data using the Metropolis–Hastings Markov chain Monte Carlo (MCMC) algorithm implemented in the R package HZAR [28]. Five separate models that varied in the number of estimated cline shape parameters were fitted to the frequency data of each transect (with pmin and pmax set to observed values) over the two elevation variables: (i) elevation above sea level and (ii) elevation relative to the position of the treeline. Each run consisted of 500 000 iterations after an initial burn-in phase of 50 000 iterations. Convergence was assessed by visualizing sampling trajectories with the R package coda [29]. Each model estimated cline centre (c, the location along a transect where the frequency of a variable changes most rapidly) and width (w, the distance over which that rapid change occurs), but could also fit different combinations of the exponential decay curve parameters δ and τ [28]. The best-fitting model for each transect was selected based on Akaike's information criterion scores corrected for small sample sizes.
Maximum-likelihood estimates of cline centre and width were obtained from best-fitting models, and confidence intervals of two log-likelihood scores were used to assess coincidence of cline centres, where non-overlapping confidence intervals were considered statistical support for differences in cline location [30]. Additionally, we assessed coincidence between clines by constructing likelihood profiles based on 20 000 random changes in cline centre and visualized them as normalized distributions using loess regression of the maximum log-likelihood values in the R package ggplot2 [31].
3. Results
All transects displayed clinal transitions from exclusively flight-capable populations at low elevations to populations increasingly dominated by flightless individuals at higher elevations (figure 2). Cline locations, however, varied substantially among transects (cline centres 649–1062 m.a.s.l.) but were tightly linked to the position of the local treeline in each case (linear regression: R2 = 0.90, F1,3 = 35.16, p = 0.01) (electronic supplementary material, tables S2 and S3). Notably, local elevational offsets between cline centre and the treeline were always less than 100 m. Cline widths were highly variable (35–297 m) with two localities (Twin Creek and Whiskey Gully) exhibiting more sudden transitions in phenotypic frequency compared to other sampling sites (figure 2). Overall, clinal variation supported substantially higher coincidence in cline centre when clines were measured relative to treeline position compared to those measured using absolute elevation (electronic supplementary material, figure S4).
4. Discussion
Our results reveal that the position of the treeline, rather than elevation per se, influences the relative frequencies of flight-capable versus flightless phenotypes in polymorphic populations of the Z. fenestrata complex. Specifically, the consistent associations detected between treeline position and phenotypic transitions implicate the treeline as a key driver of ecotype differentiation. While numerous environmental parameters covary across the treeline (e.g. wind, light, productivity, riparian vegetation), wind exposure is a particularly prominent feature of New Zealand's montane ecosystems [32] and has been previously suggested to explain the prevalence of flightless insects on oceanic islands [25,33]. In the current study, flight assays confirm that even individuals with moderate forms of wing reduction (e.g. Whiskey Gully) lack flight capability. Crucially, the finding that ecotype clines are consistently delineated by the treeline, even in recently deforested regions (see below), appears to provide a novel example of rapid, human-driven evolutionary change [2,3].
Prior to human arrival in New Zealand approximately 750 years ago, the South Island was almost completely forested below the alpine treeline [15,34]. While forests have remained largely intact at the sites of two transects in this study (Borland Burn and Whiskey Gully) [35], the low treeline elevations of the other three transects reflect anthropogenic deforestation. Radiocarbon dating of charcoal sampled in close proximity to the Twin Creek transect [15], for instance, indicates that clearance of forest in this area resulted from fire events within the first two centuries of Māori arrival. Similarly, subfossil logs found at high elevations on the Rock and Pillar Range [36,37] indicate that the treelines of Lug Creek and Six Mile Creek have been lowered by some 400 m since human settlement. Thus, the strong associations found between phenotypic transitions and the contemporary positions of treelines (figure 2b) suggest that substantial shifts in ecotype frequencies have occurred since the onset of forest burning approximately 600 years ago [15]. Such shifts may have occurred in fewer than 300 generations considering the relatively long generation times (2–3 years) of montane populations of related stoneflies [38].
Forested versus grassland habitats present contrasting selective regimes for stonefly assemblages [18], and such differentiation may be an important driver of ecological speciation and biogeographic disjunction in New Zealand (and elsewhere). Notably, at three of the five elevational transects analysed here (Borland, Lug, Six Mile), fully winged and wing-reduced morphs also exhibit substantial genomic divergence [24,26], with strong genetic evidence for independent ecotype divergence events among distinct stream populations. In contrast, ecotypes within the Twin and Whiskey clines lack genome-wide divergence, with wing polymorphism likely reflecting simple genetic polymorphisms [19,26]. In the former cases, repeated divergence highlights the repeatability of incipient speciation processes driven by parallel ecological gradients [39]. Given the relatively recent arrival of humans in New Zealand [40], our findings also indicate that ecotype distributions can respond rapidly to sudden environmental disturbances. Such shifts could potentially involve the redistribution of lineages (i.e. a biogeographic response involving already-diverged ecotypes) [24,26], and/or rapid, in situ shifts in allele frequencies within lineages driven by selection (i.e. a microevolutionary response involving simple genetic polymorphisms) [1,7,19]. Future detailed genetic analyses promise to shed further light on these intriguing possibilities. Regardless, these recent biological shifts represent a novel example of human-driven evolution [7], highlighting the ability of natural populations to respond rapidly to anthropogenic change [2,41].
While numerous studies have reported macroecological contrasts between upland versus lowland insect assemblages [39,42,43], ours may be the first analysis to provide strong evidence that such clines can shift rapidly in response to anthropogenic ecological change. In addition to the local shifts inferred here, it is likely that widespread deforestation has increased the proportion of flightless lineages across large areas of southern New Zealand [18]. Furthermore, as reduced dispersal ability has potential to increase rates of lineage diversification and also their extinction risk [39,44], the inferred human-driven expansion of flightless versus flight-capable lineages potentially carries conservation implications. These findings may thus lend support to recent suggestions that anthropogenic pressures can potentially affect both speciation and extinction processes [1,2,45]. Future ecological genomic analyses likely hold the key for better understanding the evolutionary dynamics and trajectories of flightless insect populations in recently deforested regions.
Acknowledgements
We thank Nicholas Foster and Andrew Oliphant for assistance in the field, and Danilo Hegg for permission to use the image in figure 1a. We also thank three anonymous reviewers for their constructive comments. Specimens were collected under DOC permit 59871-RES.
Data accessibility
Data and code associated with this manuscript are available on Figshare: https://dx.doi.org/10.6084/m9.figshare.14452788 [46].
Authors' contributions
B.J.F. and J.M.W. designed the study. B.J.F., G.A.M. and J.M.W. conducted fieldwork. B.J.F. and M.F.S.V. collected the data. B.J.F. analysed the data. G.A.M., T.I. and J.M.W. contributed to data interpretation. B.J.F. wrote the manuscript with input from all authors. All authors approved the final version of the manuscript and agree to be held accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Marsden contracts UOO1412 and UOO2016 (Royal Society of New Zealand) awarded to J.M.W. and G.A.M., and a University of Otago Doctoral Scholarship awarded to B.J.F.
References
- 1.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372, 20160028. ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catullo RA, Llewelyn J, Phillips BL, Moritz CC. 2019. The potential for rapid evolution under anthropogenic climate change. Curr. Biol. 29, R996-R1007. ( 10.1016/j.cub.2019.08.028) [DOI] [PubMed] [Google Scholar]
- 3.Allendorf FW, Hard JJ. 2009. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl Acad. Sci. USA 106, 9987-9994. (doi:10.1073pnas.0901069106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill BJ, Dornelas M, Gotelli NJ, Magurran AE. 2015. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30, 104-113. ( 10.1016/j.tree.2014.11.006) [DOI] [PubMed] [Google Scholar]
- 5.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367-387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti MA. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114-126. ( 10.1016/j.tree.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 7.Cook LM, Saccheri IJ. 2013. The peppered moth and industrial melanism: evolution of a natural selection case study. Heredity (Edinb). 110, 207-212. ( 10.1038/hdy.2012.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of Earth's ecosystems. Science 277, 494-499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 9.Hanski I, Koivulehto H, Cameron A, Rahagalala P. 2007. Deforestation and apparent extinctions of endemic forest beetles in Madagascar. Biol. Lett. 3, 344-347. ( 10.1098/rsbl.2007.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson B, Van Dyck H. 2005. Does habitat fragmentation affect temperature-related life-history traits? A laboratory test with a woodland butterfly. Proc. R. Soc. B 272, 1257-1263. ( 10.1098/rspb.2005.3074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willi Y, Hoffmann AA. 2012. Microgeographic adaptation linked to forest fragmentation and habitat quality in the tropical fruit fly Drosophila birchii. Oikos 121, 1627-1637. ( 10.1111/j.1600-0706.2011.20156.x) [DOI] [Google Scholar]
- 12.McGlone MS. 1983. Polynesian deforestation of New Zealand: a preliminary synthesis. Archaeol. Ocean. 18, 11-25. ( 10.1002/arco.1983.18.1.11) [DOI] [Google Scholar]
- 13.McGlone MS. 1989. The Polynesian settlement of New Zealand in relation to environmental and biotic changes. N. Z. J. Ecol. 12, 115-129. ( 10.2307/24053254) [DOI] [Google Scholar]
- 14.McWethy DB, Whitlock C, Wilmshurst JM, McGlone MS, Li X. 2009. Rapid deforestation of South Island, New Zealand, by early Polynesian fires. Holocene 19, 883-897. ( 10.1177/0959683609336563) [DOI] [Google Scholar]
- 15.McWethy DB, et al. 2010. Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement. Proc. Natl Acad. Sci. USA 107, 21 343-21 348. ( 10.1073/pnas.1011801107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGlone MS, Wilmshurst JM. 1999. Dating initial Maori environmental impact in New Zealand. Quat. Int. 59, 5-16. ( 10.1016/S1040-6182(98)00067-6) [DOI] [Google Scholar]
- 17.Roff DA. 1994. Habitat persistence and the evolution of wing dimorphism in insects. Am. Nat. 144, 772-798. ( 10.1086/285706.) [DOI] [Google Scholar]
- 18.McCulloch GA, Foster BJ, Ingram T, Waters JM. 2019. Insect wing loss is tightly linked to the treeline: evidence from a diverse stonefly assemblage. Ecography (Cop.). 42, 811-813. ( 10.1111/ecog.04140) [DOI] [Google Scholar]
- 19.Veale AJ, Foster BJ, Dearden PK, Waters JM. 2018. Genotyping-by-sequencing supports a genetic basis for wing reduction in an alpine New Zealand stonefly. Sci. Rep. 8, 1-12. ( 10.1038/s41598-018-34123-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCulloch GA, Oliphant A, Dearden PK, Veale AJ, Ellen CW, Waters JM. 2019. Comparative transcriptomic analysis of a wing-dimorphic stonefly reveals candidate wing loss genes. Evodevo 10, 1-9. ( 10.1186/s13227-019-0135-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLellan ID. 1999. A revision of Zelandoperla Tillyard (Plecoptera: Gripopterygidae: Zelandoperlinae). New Zeal. J. Zool. 26, 199-219. ( 10.1080/03014223.1999.9518190) [DOI] [Google Scholar]
- 22.McCulloch GA, Wallis GP, Waters JM. 2009. Do insects lose flight before they lose their wings? Population genetic structure in subalpine stoneflies. Mol. Ecol. 18, 4073-4087. ( 10.1111/j.1365-294X.2009.04337.x) [DOI] [PubMed] [Google Scholar]
- 23.Dussex N, Chuah A, Waters JM. 2016. Genome-wide SNPs reveal fine-scale differentiation among wingless alpine stonefly populations and introgression between winged and wingless forms. Evolution 70, 38-47. ( 10.1111/evo.12826) [DOI] [PubMed] [Google Scholar]
- 24.McCulloch GA, Foster BJ, Dutoit L, Ingram T, Hay E, Veale AJ, Dearden PK, Waters JM. 2019. Ecological gradients drive insect wing loss and speciation: the role of the alpine treeline. Mol. Ecol. 28, 3141-3150. ( 10.1111/mec.15114) [DOI] [PubMed] [Google Scholar]
- 25.Leihy RI, Chown SL. 2020. Wind plays a major but not exclusive role in the prevalence of insect flight loss on remote islands. Proc. R. Soc. B 287, 20202121. ( 10.1098/rspb.2020.2121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCulloch GA, Foster BJ, Dutoit L, Harrop TWR, Guhlin J, Dearden PK, Waters JM. 2020. Genomics reveals widespread ecological speciation in flightless insects. Syst. Biol. syaa094. ( 10.1093/sysbio/syaa094) [DOI] [PubMed] [Google Scholar]
- 27.Beer-Stiller A, Zwick P. 1995. Biometric studies of some stoneflies and a mayfly (Plecoptera and Ephemeroptera). Hydrobiologia 299, 169-178. ( 10.1007/BF00017568) [DOI] [Google Scholar]
- 28.Derryberry EP, Derryberry GE, Maley JM, Brumfield RT. 2014. HZAR: hybrid zone analysis using an R software package. Mol. Ecol. Resour. 14, 652-663. ( 10.1111/1755-0998.12209) [DOI] [PubMed] [Google Scholar]
- 29.Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6, 7-11. [Google Scholar]
- 30.While GM, et al. 2015. Sexual selection drives asymmetric introgression in wall lizards. Ecol. Lett. 18, 1366-1375. ( 10.1111/ele.12531) [DOI] [PubMed] [Google Scholar]
- 31.Wickham H. 2016. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 32.Chinn WGH, Chinn TJH. 2020. Tracking the snow line: responses to climate change by New Zealand alpine invertebrates. Arctic, Antarct. Alp. Res. 52, 361-389. ( 10.1080/15230430.2020.1773033) [DOI] [Google Scholar]
- 33.Darwin C. 1859. On the origin of the species. London, UK: John Murray. [Google Scholar]
- 34.Hall GMJ, Mcglone MS. 2001. Forest reconstruction and past climatic estimates for a deforested region of south-eastern New Zealand. Landsc. Ecol. 16, 501-521. ( 10.1023/A:1013199209388) [DOI] [Google Scholar]
- 35.McGlone MS. 2001. The origin of the indigenous grasslands of southeastern South Island in relation to pre-human woody ecosystems. N. Z. J. Ecol. 25, 1-15. [Google Scholar]
- 36.Fulton RV. 1922. Medical practice in the early days. Dunedin, New Zealand: Otago Daily Times and Witness Newspapers. [Google Scholar]
- 37.Thomson H. 1949. East of the Rock and Pillar: a history of the Strath Taieri and Macraes districts. Dunedin, New Zealand: Otago Centennial Publications. [Google Scholar]
- 38.Hynes HBN, Hynes ME. 1975. The life histories of many of the stoneflies (Plecoptera) of south-eastern mainland Australia. Mar. Freshw. Res. 26, 113-153. ( 10.1071/MF9750113) [DOI] [Google Scholar]
- 39.Waters JM, Emerson BC, Arribas P, McCulloch GA. 2020. Dispersal reduction: causes, genomic mechanisms, and evolutionary consequences. Trends Ecol. Evol. 35, 512-522. ( 10.1016/j.tree.2020.01.012) [DOI] [PubMed] [Google Scholar]
- 40.Hogg AG, Higham TFG, Lowe DJ, Palmer JG, Reimer PJ, Newnham RM. 2003. A wiggle-match date for Polynesian settlement of New Zealand. Antiquity 77, 116-125. ( 10.1017/S0003598X00061408) [DOI] [Google Scholar]
- 41.Hendry AP, Farrugia TJ, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20-29. ( 10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 42.Hendrickx F, Backeljau T, Dekoninck W, Van Belleghem SM, Vandomme V, Vangestel C.. 2015. Persistent inter- and intraspecific gene exchange within a parallel radiation of caterpillar hunter beetles (Calosoma sp.) from the Galápagos. Mol. Ecol. 24, 3107-3121. ( 10.1111/mec.13233) [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Suzuki N, Tojo K. 2019. Parallel evolution of an alpine type ecomorph in a scorpionfly: independent adaptation to high-altitude environments in multiple mountain locations. Mol. Ecol. 28, 3225-3240. ( 10.1111/mec.15119) [DOI] [PubMed] [Google Scholar]
- 44.Burridge CP, Waters JM. 2020. Does migration promote or inhibit diversification? A case study involving the dominant radiation of temperate Southern Hemisphere freshwater fishes. Evolution 74, 1954-1965. ( 10.1111/evo.14066) [DOI] [PubMed] [Google Scholar]
- 45.Thompson KA, Rieseberg LH, Schluter D. 2018. Speciation and the city. Trends Ecol. Evol. 33, 815-826. ( 10.1016/j.tree.2018.08.007) [DOI] [PubMed] [Google Scholar]
- 46.Foster BJ, McCulloch GA, Vogel MFS, Ingram T, Waters J. 2021. Anthropogenic evolution in an insect wing polymorphism following widespread deforestation. Figshare. 10.6084/m9.figshare.14452788. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Foster BJ, McCulloch GA, Vogel MFS, Ingram T, Waters J. 2021. Anthropogenic evolution in an insect wing polymorphism following widespread deforestation. Figshare. 10.6084/m9.figshare.14452788. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data and code associated with this manuscript are available on Figshare: https://dx.doi.org/10.6084/m9.figshare.14452788 [46].