Abstract
Anticoagulant rodenticides (ARs) deployed to control rodent pest populations can increase the risk of pathogen infection for some wildlife. However, it is unknown whether ARs also increase infection risk for target rodents, which are common hosts for zoonotic (animal-to-human transmitted) pathogens. In this study, we tested whether rats exposed to ARs were more likely to be infected with zoonotic pathogens, specifically Leptospira spp. or Escherichia coli, after controlling for known predictors of infection (i.e. sex, age, body condition). We collected biological samples from 99 rats trapped in Chicago alleys and tested these for Leptospira infection, E. coli shedding and AR exposure. We found that rats that had been exposed to ARs and survived until the time of trapping, as well as older rats, were significantly more likely to be infected with Leptospira spp. than other rats. We found no significant association between E. coli shedding and any predictors. Our results show that human actions to manage rats can affect rat disease ecology and public health risks in unintended ways, and more broadly, contribute to a growing awareness of bidirectional relationships between humans and natural systems in cities.
Keywords: rodenticide, zoonotic infection, Leptospira, urban ecology, Rattus
1. Introduction
Anticoagulant rodenticides (ARs) are one of the most common types of substance used to control rodent pest populations; however, little is known about potential unintended, sublethal AR effects on rodents. In other species, AR exposure has been associated with numerous sublethal effects (in addition to acute toxicity). For example, sublethal AR exposure can increase infection risk in urban predators (e.g. bobcats, Lynx rufus; mountain lions, Puma concolor; coyotes, Canis latrans; [1–3]) and has been linked to higher parasite and pathogen burdens in birds (e.g. great bustards, Otis tarda; [4]). Wildlife exposed to ARs may be more susceptible to infection because ARs have been shown to disrupt immune function [5]. Like the species above, rodents might also experience greater infection owing to AR exposure; in turn, this is relevant to human health as rodents are common hosts for zoonotic pathogens [6–8], especially in human-dominated areas [9]. ARs do not kill immediately; first-generation ARs require multiple feedings to provide a lethal dose, and second-generation ARs—more potent compounds that can kill after a single dose—typically lead to death in 5–10 days [10]. If infection risk is heightened during the period between AR exposure and death, widespread AR use might increase population transmission of pathogens among rodents. Additionally, this could pose a risk of zoonotic pathogen transmission.
Understanding any unintended effects of rodent control on rodent disease dynamics is important because commensal rats carry dozens of zoonotic pathogens [11,12], come in close proximity to people [13], and have a near-global distribution [14]. Brown rats (Rattus norvegicus) and black rats (R. rattus) can carry several environmentally transmitted pathogens that cause human disease (e.g. Leptospira interrogans, pathogenic Escherichia coli; [15]). Leptospirosis in particular poses a large public health burden, causing an estimated 434 000–1 750 000 cases and 23 800–95 900 deaths in humans annually [16]. Among major cities in the USA, Leptospira seroprevalence in rats ranges from 44.1 to 65.3% [17]. Environmental features and management practices can modulate Leptospira prevalence. For example, in Chicago, IL, rats trapped in high-income areas with more standing water complaints were more likely to be infected with Leptospira spp. [18], while in Vancouver, Canada, rodent control via rat trapping was associated with higher Leptospira prevalence [19]. Importantly, low-income urban residents can be disproportionately exposed to rat-associated zoonoses [20] and lower-income countries are often reliant on ARs for rodent control [21]. It is thus crucial to understand how other widespread management practices such as use of ARs could also influence infection dynamics in rats.
In this study, we tested if rats exposed to ARs were more likely to be infected with zoonotic pathogens, specifically Leptospira spp. or E. coli, after controlling for known physiological predictors of infection. We focused on these pathogens because they are zoonotic, transmitted through the environment, and present in our study population [18]. Based on previous work in urban carnivores, we predicted the probability of Leptospira spp. infection and E. coli shedding would be higher for rats with detectable concentrations of common ARs in liver tissue relative to other rats. We also predicted the probability of Leptospira spp. infection and E. coli shedding would be higher for rats that were female, older, and in poorer body condition because these biological factors are known predictors of infection [18,22–24]. Our results will help design best practices for rodent management to protect public health and advance our understanding of how pest management affects urban wildlife ecology.
2. Methods
As part of a previous study [18], 254 rats were trapped in 13 community areas in Chicago, a city with numerous rat complaints (figure 1). Trapped rats were measured, examined for injuries, weighed, and sexed. Rats were considered to be brown rats based on ear and tail morphology, but this assumption was not verified with genetic analyses. A subset of 202 rats were necropsied and screened for environmentally-transmitted bacterial pathogens [18]. Rat kidney tissue was tested for Leptospira spp. using polymerase chain reaction (PCR) and rat colon contents (i.e. faeces) were tested for E. coli using aerobic culture [18] at Wyoming State Veterinary Laboratory. From these rats, we selected 99 (table 1) to be screened for seven commonly used ARs (first-generation: chlorophacinone, coumachlor, diphacinone, warfarin; second-generation: brodifacoum, bromadiolone, difethialone). Rats were chosen for screening such that sample sizes would be roughly balanced by capture location, sex, age and infection status. Liver screening was performed by the Animal Disease Diagnostic Laboratory at Purdue University (West Lafayette, IN) using high performance liquid chromatography. Method detection limits (lowest concentration that can be confidently identified) for each AR in liver tissue were as follows: chlorophacinone and diphacinone: 0.25 ppm; coumachlor and warfarin: 0.5 ppm; brodifacoum, bromadiolone and difethialone: 1.00 ppm. Animal use was deemed exempt from Lincoln Park Zoological Society IACUC approval because rat samples were procured through pest management professionals (protocol number 2019–005).
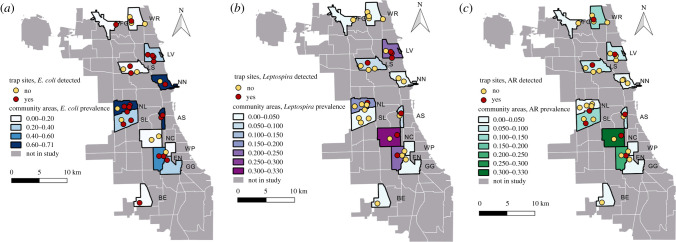
Figure 1.
Maps of study community areas (polygons) and trap sites (circles) in Chicago. Colours show the prevalence (shading) or the presence (darker circles) of rats with (a) E. coli shedding, (b) Leptospira spp. infection, and (c) anticoagulant rodenticide (AR) exposure. Abbreviations correspond to table 1.
Table 1.
Sex, age class and anticoagulant rodenticide poisoning status of rats, separated by trapping location (community area).
| community area | sex |
age class |
poisoning status |
|||
|---|---|---|---|---|---|---|
| F | M | younger (30–65 days) | older (>65 days) | AR detected | AR not detected | |
| Armour Square (AS) | 5 | 10 | 14 | 1 | 1 | 14 |
| Beverly (BE) | 1 | 0 | 1 | 0 | 0 | 1 |
| Edge Water (ED) | 1 | 1 | 1 | 1 | 1 | 1 |
| Englewood (EN) | 0 | 4 | 3 | 1 | 1 | 3 |
| Forest Glen (FG) | 1 | 0 | 1 | 0 | 0 | 1 |
| Greater Grand Crossing (GG) | 2 | 2 | 2 | 2 | 0 | 4 |
| Lake View (LV) | 14 | 6 | 16 | 4 | 2 | 18 |
| Logan Square (LS) | 11 | 5 | 14 | 2 | 1 | 15 |
| Near North Side (NN) | 5 | 2 | 7 | 0 | 0 | 7 |
| New City (NC) | 2 | 1 | 1 | 2 | 1 | 2 |
| North Lawndale (NL) | 4 | 1 | 4 | 1 | 0 | 5 |
| South Lawndale (SL) | 11 | 2 | 8 | 5 | 2 | 11 |
| Washington Park (WP) | 0 | 1 | 1 | 0 | 0 | 1 |
| West Ridge (WR) | 7 | 0 | 6 | 1 | 1 | 6 |
We used generalized linear mixed models (GLMMs; binomial distribution, logit link) to test whether infection status varied by rodenticide exposure status (binary; we considered a rat exposed to poison if at least one AR was detected in the liver) as well as other biological predictors previously found to influence rat infection status. We constructed two GLMMs, one with a response variable of Leptospira infection status (positive or negative) and the other with a response variable of E. coli shedding status (positive or negative). Explanatory variables for each model included AR exposure status, sex, age class and body condition. We estimated rat age in days based on their mass using growth curve equations, following the methods of [25], and binned rats as younger (30–65 days) or older (greater than 65 days; electronic supplementary material, dataset). We quantified body condition using the scaled mass index [26] using tip-to-tip length (i.e. tip of nose to tip of tail) because it was most highly correlated with mass (see the electronic supplementary material for more detail). While injuries have also been found to be associated with infection [15], we did not include this as a variable because we observed only a few, mild wounds in the study population. Given the low sample size, only main effects of the explanatory variables were considered. We also included capture location (i.e. community area) as a random effect to account for non-independence among samples from the same neighbourhood. Analyses were performed using the glmmTMB package [27] in the R statistical environment v. 4.0.3 [28].
3. Results and discussion
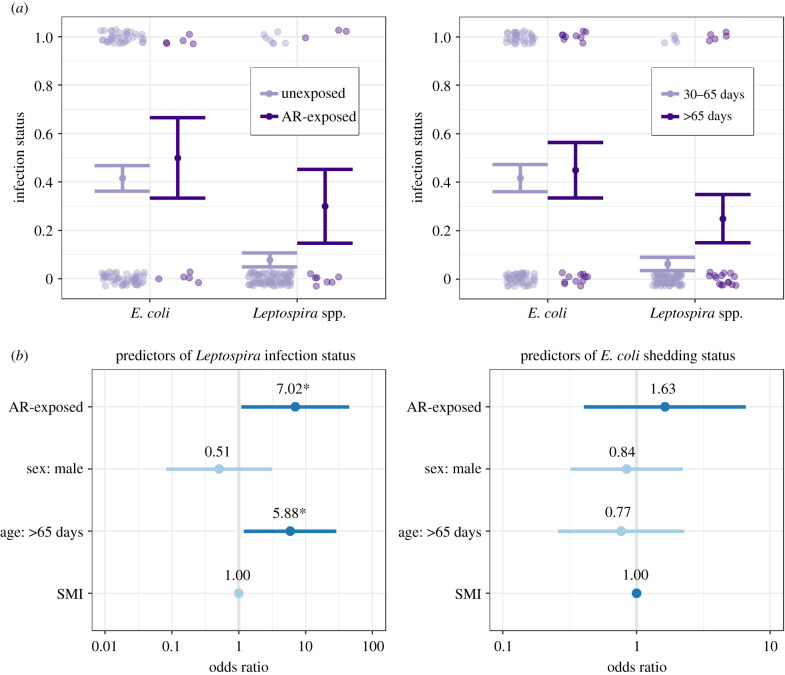
We analysed infection status as a function of AR exposure, sex and age class for 99 rats that were trapped in 14 community areas (table 1). Ten liver samples were positive for AR residues (6 females, 4 males; 2 older, 8 younger). Specifically, seven were positive for second-generation ARs (brodifacoum: n = 3, bromadiolone: n = 3, difethialone: n = 1) and three were positive for first-generation ARs (diphacinone: n = 3). Leptospira prevalence was higher for AR-exposed rats (30%, 3/10) than for unexposed rats (7.9%, 7/89), and E. coli prevalence was higher for AR-exposed rats (50%, 5/10) than for unexposed rats (42%, 37/89; figure 2).
Figure 2.
(a) Pale-shaded points display binary infection status and solid points and lines represent means and standard errors of infection prevalence. (b) Points and lines represent odds ratios and 95% confidence intervals for predictors of infection status from GLMMs. Darker blue lines indicate odds ratios greater than 1, while lighter blue lines indicate odds ratios less than 1. 95% confidence intervals that cross the vertical line at 1 indicate that a predictor is not significant. Asterisks indicate p < 0.05.
GLMMs indicated that AR exposure status was a significant predictor of Leptospira infection status (odds ratio = 7.02, 95% CI = 1.10–45.0, p = 0.04), as was age class (figure 2 and electronic supplementary material, table S1). Older rats (greater than 65 days) were significantly more likely to be infected with Leptospira spp. than younger rats (30–65 days; odds ratio = 5.88, 95% CI = 1.20–28.9, p = 0.03). Neither sex nor SMI was a significant predictor in the model. The marginal R2 (i.e. proportion of variance explained by fixed effects) for the Leptospira infection model was 0.21, while the conditional R2 (i.e. proportion of variance explained by both fixed and random effects) was 0.33. No explanatory variables were significant predictors of E. coli shedding status. The marginal R2 for this model was 0.01, while the conditional R2 was 0.12.
We found that rats exposed to ARs that survived until the time of trapping were significantly more likely to be infected with Leptospira spp. than other rats. Though it is known that ARs can promote infection risk in non-target wildlife, our results demonstrate increased zoonotic infection risk in target rodents. This result is significant for public health and urban ecology because commensal rodents are abundant reservoirs of zoonotic pathogens in cities. More generally, this relationship between rodenticide exposure and infection risk demonstrates an unintended effect of wildlife management on a target species that can feed back to human health.
AR-exposed rats may be more susceptible to infection in the period between exposure and death because of immunomodulatory effects of ARs. Rats exposed to warfarin for 30 days exhibit increased lymphocytes, basophils and monocytes [29,30], suggesting immune dysfunction. In carnivores, AR exposure has been associated with immune dysfunction consistent with cytokine-mediated inflammatory processes, including the suppression of neutrophils [31]. These phenotypic changes might interfere with rodents' ability to mount an effective defence when exposed to infectious leptospires in the environment. Although we quantified rat exposure to rat poison as a binary status, the detection limit in our study exceeded concentrations deemed indicative of acute AR poisoning in other species (200 ng g−1 or 0.2 ppm; [4]), suggesting they were high enough to interfere with physiological processes. If rats are more likely to become infected with Leptospira spp. after consuming ARs, infection would have to occur before the poison kills the rat (approx. 1 week). Experimental work has demonstrated successful Leptospira infection 7 days post-infection [32,33], yet further work is needed to examine Leptospira spp. infection dynamics at a shorter timescale and determine how long rats can survive following AR exposure.
Alternatively, infected rats might be more likely to consume poisoned bait. For instance, infected rats could be more attracted to bait stations if they have less energy to actively forage for other food. However, rats are considered asymptomatic, chronic carriers of Leptospira ([17]; though see [34]), suggesting it is unlikely that infected rats are more likely to consume AR bait. Future work could also investigate behavioural and physiological changes in poisoned and infected rats to clarify causal mechanisms.
Interestingly, the only other study, to our knowledge, to examine AR poisoning and infection risk in target rodents found that common voles (Microtus arvalis) infected with Francisella tularensis had lower concentrations of the AR chlorophacinone relative to uninfected voles [35]. These results likely differ from ours because all poisoned voles were found dead rather than trapped and F. tularensis infection is fatal in voles. However, these differences highlight the need to understand interactions among ARs, pathogens, and hosts with different ecologies. Future epidemiological surveys and experimental work could help identify which types of pathogenic infections are affected by AR exposure.
We also found that older rats were significantly more likely to be infected with Leptospira spp. than younger rats. This aligns with previous research and is likely attributable to a greater chance of exposure and infection over time [22]. We might not have found significant associations with other biological factors because of small sample size, which could also explain the relatively large confidence intervals around the odds ratios (figure 2). Contrary to our predictions, we found no association between AR exposure and E. coli infection. We may not have detected an increased risk of E. coli infection in poisoned rats because our methods could only detect active shedding of E. coli in faeces, rather than true infection. Although this is informative for public health, rats could have been infected with E. coli but not actively shedding, which might have confounded our results. In addition, while we accounted for non-independence among rats within the same community area using a random effect (under the assumption that community areas are statistically independent from one another, supported by the small home ranges of rats (less than 200 m) [36]), our results may have been confounded by spatial autocorrelation.
Our results add to a growing literature showing environmental hazards of managing rats using ARs, and highlight potential unintended and unpredicted effects of AR exposure on the ecology of rat-associated pathogens of public health importance. Apart from disease ecology, urban rats have exhibited genetic resistance to ARs for decades. Resistant rats carry genetic mutations in the Vkorc1 gene that interfere with anticoagulant effects on blood clotting [37], rendering the rats less susceptible to anticoagulants. Rats have exhibited genetic resistance even as new generations of ARs are developed [38,39], demonstrating how lethal management can have evolutionary consequences for zoonotic hosts [40]. AR resistance may have important consequences for leptospiral shedding if ARs act as modulators of immune and inflammatory responses and resistant rats are less likely to die following AR exposure. Instead of relying on ARs, integrated pest management might offer a more sustainable approach by improving urban sanitation and rodent exclusion [41]. Such an approach would align with One Health principles and prevent mortality of urban carnivores, which provide ecosystem services such as rodent population control. More broadly, our results contribute to a growing awareness of bidirectional relationships between humans and natural systems in cities: in our case, that human actions to manage rats can affect rat disease ecology and public health risks in unintended ways.
Acknowledgements
We thank Landmark Pest Management for providing rat carcasses and Matthew Mulligan, Jazmín Rios and Gabriella Barnas for their assistance with rat necropsies. We also thank Dr Kaylee Byers for insightful comments.
Ethics
Animal use was deemed exempt from Lincoln Park Zoological Society IACUC approval because rat samples were procured through pest management professionals (protocol no. 2019-005).
Data accessibility
The dataset used in our analysis is available on Zenodo at https://zenodo.org/badge/latestdoi/387547164 [42].
Authors' contributions
M.H.M. led the conceptualization of the study and the collection of biological samples. C.A.S. contributed to project design and led the statistical analysis. M.H.M. and C.A.S. wrote and edited the manuscript, approved the final version of the manuscript agree to be held accountable for the content therein.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by the Grant Healthcare Foundation and based upon work supported by the National Science Foundation under grant no. 1923882. Partial funding for C.A.S. was provided by the National Science Foundation through an INTERN grant (grant no. DEB-1717282). The funding bodies played no role in the design of the study or in the collection, analysis, and interpretation of data or in writing the manuscript.
References
- 1.Riley SPD, Bromley C, Poppenga RH, Uzal FA, Whited L, Sauvajot RM. 2007. Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban Southern California. J. Wildl. Manage. 71, 1874-1884. ( 10.2193/2005-615) [DOI] [Google Scholar]
- 2.Poessel SA, Breck SW, Fox KA, Gese EM. 2015. Anticoagulant rodenticide exposure and toxicosis in coyotes in the Denver Metropolitan Area. J. Wildl. Dis. 51, 265-268. ( 10.7589/2014-04-116) [DOI] [PubMed] [Google Scholar]
- 3.Serieys LEK, et al. 2018. Widespread anticoagulant poison exposure is linked with immune dysregulation and severe notoedric mange in urban bobcats. Vertebr. Pest Conf. 28, 258-263. ( 10.5070/v42811047) [DOI] [Google Scholar]
- 4.Lemus JA, Bravo C, García-Montijano M, Palacín C, Ponce C, Magaña M, Alonso JC. 2011. Side effects of rodent control on non-target species: rodenticides increase parasite and pathogen burden in great bustards. Sci. Total Environ. 409, 4729-4734. ( 10.1016/j.scitotenv.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 5.Fraser D, Mouton A, Serieys LEK, Cole S, Carver S, Vandewoude S, Lappin M, Riley SPD, Wayne R. 2018. Genome-wide expression reveals multiple systemic effects associated with detection of anticoagulant poisons in bobcats (Lynx rufus). Mol. Ecol. 27, 1170-1187. ( 10.1111/mec.14531) [DOI] [PubMed] [Google Scholar]
- 6.Meerburg BG, Singleton GR, Kijlstra A. 2009. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 35, 221-270. ( 10.1080/10408410902989837) [DOI] [PubMed] [Google Scholar]
- 7.Morand S, Jittapalapong S, Kosoy M. 2015. Rodents as hosts of infectious diseases: biological and ecological characteristics. Vector Borne Zoonotic Dis. 15, 1-2. ( 10.1089/vbz.2015.15.1.intro) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luis AD, et al. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280, 20122753. ( 10.1098/rspb.2012.2753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, Jones KE. 2020. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398-402. ( 10.1038/s41586-020-2562-8) [DOI] [PubMed] [Google Scholar]
- 10.Erickson W, Urban D. 2004. Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach. Washington, DC: United States Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances.
- 11.Firth C, et al. 2014. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York city. MBio 5, e01933-14. ( 10.1128/mBio.01933-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strand TM, Lundkvist Å. 2019. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995–2016. Infect. Ecol. Epidemiol. 9, 1553461. ( 10.1080/20008686.2018.1553461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puckett EE, Orton D, Munshi-South J. 2020. Commensal rats and humans: integrating rodent phylogeography and zooarchaeology to highlight connections between human societies. Bioessays 42, 1900160. ( 10.1002/bies.201900160) [DOI] [PubMed] [Google Scholar]
- 14.Puckett EE, et al. 2016. Global population divergence and admixture of the brown rat (Rattus norvegicus). Proc. R. Soc. B 283, 20161762. ( 10.1098/rspb.2016.1762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Himsworth CG, Parsons KL, Jardine C, Patrick DM. 2013. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 13, 349-359. ( 10.1089/vbz.2012.1195) [DOI] [PubMed] [Google Scholar]
- 16.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. 2015. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl. Trop. Dis. 9, e0003898. ( 10.1371/journal.pntd.0003898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boey K, Shiokawa K, Rajeev S. 2019. Leptospira infection in rats: a literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 13, e0007499. ( 10.1371/journal.pntd.0007499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray MH, et al. 2020. City sanitation and socioeconomics predict rat zoonotic infection across diverse neighbourhoods. Zoonoses Public Health 67, 673-683. ( 10.1111/zph.12748) [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Byers KA, Donovan CM, Bidulka JJ, Stephen C, Patrick DM, Himsworth CG. 2018. Effects of culling on Leptospira interrogans carriage by rats. Emerg. Infect. Dis. 24, 356-360. ( 10.3201/eid2402.171371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibler JH, Zakhour CM, Gadhoke P, Gaeta JM. 2016. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990–2014. Vector Borne Zoonotic Dis. 16, 435-444. ( 10.1089/vbz.2015.1863) [DOI] [PubMed] [Google Scholar]
- 21.Jacob J, Buckle A. 2018. Use of anticoagulant rodenticides in different applications around the world. In Anticoagulant rodenticides and wildlife (eds van den Brink NW, Elliott JE, Shore RF, Rattner BA), pp. 11-43. Cham, Switzerland: Springer. [Google Scholar]
- 22.Himsworth CG, et al. 2013. Ecology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, Canada. PLoS Negl. Trop. Dis. 7, e2270. ( 10.1371/journal.pntd.0002270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minter A, Himsworth CG, Byers KA, Childs JE, Ko AI, Costa F. 2019. Tails of two cities: age and wounding are associated with carriage of Leptospira interrogans by Norway rats (Rattus norvegicus) in ecologically distinct urban environments. Front. Ecol. Evol. 7, 14. ( 10.3389/fevo.2019.00014) [DOI] [Google Scholar]
- 24.Desvars-Larrive A, Smith S, Munimanda G, Bourhy P, Waigner T, Odom M, Gliga DS, Walzer C. 2020. Prevalence and risk factors of Leptospira infection in urban brown rats (Rattus norvegicus), Vienna, Austria. Urban Ecosyst. 23, 775-784. ( 10.1007/s11252-020-00957-9) [DOI] [Google Scholar]
- 25.Minter A, Diggle PJ, Costa F, Childs J, Ko AI, Begon M. 2017. Evidence of multiple intraspecific transmission routes for Leptospira acquisition in Norway rats (Rattus norvegicus). Epidemiol. Infect. 145, 3438-3448. ( 10.1017/S0950268817002539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883-1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 27.Brooks M, Kristensen K, van Benthem KJ, Magnusson A, Berg C, Nielsen A, Skaug H, Maechler M, Bolker B. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 28.R Core Team. 2019. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 29.Kataranovski M, Mirkov I, Zolotarevski L, Popov A, Belij S, Stosic J, Kataranovski D. 2009. Basic indices of spleen immune activity in natural populations of Norway rats (Rattus norvegicus Berkenhout, 1769) in Serbia. Arch. Biol. Sci. 61, 723-732. ( 10.2298/ABS0904723K) [DOI] [Google Scholar]
- 30.Mikhail MW, Abdel-Hamid YM. 2007. Effect of warfarin anticoagulant rodenticide on the blood cell counts of Rattus norvegicus and Rattus rattus. J. Egypt. Soc. Parasitol. 37, 853-861. [PubMed] [Google Scholar]
- 31.Serieys LEK, et al. 2018. Urbanization and anticoagulant poisons promote immune dysfunction in bobcats. Proc. R. Soc. B 285, 20172533. ( 10.1098/rspb.2017.2533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucunduva de Faria M, Athanazio DA, Gonçalves Ramos EA, Silva EF, Reis MG, Ko AI. 2007. Morphological alterations in the kidney of rats with natural and experimental Leptospira infection. J. Comp. Pathol. 137, 231-238. ( 10.1016/j.jcpa.2007.08.001) [DOI] [PubMed] [Google Scholar]
- 33.Cordonin C, Turpin M, Bringart M, Bascands JL, Flores O, Dellagi K, Mavingui P, Roche M, Tortosa P. 2020. Pathogenic Leptospira and their animal reservoirs: testing host specificity through experimental infection. Scient. Rep. 10, 7239. ( 10.1038/s41598-020-64172-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agudelo-Flórez P, Murillo VE, Londoño AF, Rodas JD. 2013. Histopathological kidney alterations in rats naturally infected with Leptospira. Biomedica 33, 82-88. ( 10.7705/biomedica.v33i0.686) [DOI] [PubMed] [Google Scholar]
- 35.Vidal D, Alzaga V, Luque-Larena JJ, Mateo R, Arroyo L, Viñuela J. 2009. Possible interaction between a rodenticide treatment and a pathogen in common vole (Microtus arvalis) during a population peak. Sci. Total Environ. 408, 267-271. ( 10.1016/j.scitotenv.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 36.Byers K, Lee MJ, Patrick DM, Himsworth CG. 2019. Rats about town: a systematic review of rat movement in urban ecosystems. Front. Ecol. Evol. 7, 13. ( 10.3389/fevo.2019.00013) [DOI] [Google Scholar]
- 37.Meerburg BG, van Gent-Pelzer MPE, Schoelitsz B, van der Lee TAJ. 2014. Distribution of anticoagulant rodenticide resistance in Rattus norvegicus in the Netherlands according to Vkorc1 mutations. Pest Manag. Sci. 70, 1761-1766. ( 10.1002/ps.3809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Runge M, Von Keyserlingk M, Braune S, Becker D, Plenge-Bönig A, Freise JF, Pelz HJ, Esther A. 2013. Distribution of rodenticide resistance and zoonotic pathogens in Norway rats in lower Saxony and Hamburg, Germany. Pest Manag. Sci. 69, 403-408. ( 10.1002/ps.3369) [DOI] [PubMed] [Google Scholar]
- 39.Haniza MZH, Adams S, Jones EP, MacNicoll A, Mallon EB, Smith RH, Lambert MS. 2015. Large-scale structure of brown rat (Rattus norvegicus) populations in England: effects on rodenticide resistance. PeerJ 2015, e1458. ( 10.7717/peerj.1458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schell CJ, Stanton LA, Young JK, Angeloni LM, Lambert JE, Breck SW, Murray MH. 2020. The evolutionary consequences of human–wildlife conflict in cities. Evol. Appl. 14, 178-197. ( 10.1111/eva.13131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn N, Kenmuir S, Krueger L. 2019. A California without rodenticides: challenges for commensal rodent management in the future. Hum. Wildl. Interact. 13, 212-225. ( 10.5070/v42811007) [DOI] [Google Scholar]
- 42.Murray MH, Sánchez CA. 2021. Data from: Urban rat exposure to anticoagulant rodenticides and zoonotic infection risk. Zenodo. ( 10.5281/zenodo.5115097) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Murray MH, Sánchez CA. 2021. Data from: Urban rat exposure to anticoagulant rodenticides and zoonotic infection risk. Zenodo. ( 10.5281/zenodo.5115097) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The dataset used in our analysis is available on Zenodo at https://zenodo.org/badge/latestdoi/387547164 [42].