Abstract
The incidence of urinary tract infections (UTIs) caused by Klebsiella pneumoniae has exhibited an increasing trend and has become a high burden for many public health systems, especially in hospital settings. Multidrug resistance associated with the production of extended-spectrum β-lactamases (ESBL) among K. pneumoniae isolates is endemic in Southeastern Europe. We retrospectively analyzed 75 cases admitted to ‘St. Parascheva’ Clinical Hospital of Infectious Diseases in Iasi, Romania, during the first 6 months of 2019 (January 1 to June 30), who had a confirmed diagnosis of K. pneumoniae UTI at discharge. From a total of 75 patients, 34 (45.3%) presented ESBL+ K. pneumoniae. The mean age was 66 years (70.1 for the ESBL+ patients vs. 62.6 for the ESBL- patients, P=0.0365). There was a symmetrical sex distribution (37 men vs. 38 women). Of these, 22 men had ESBL+ K. pneumoniae UTIs, compared to only 15 with an ESBL- strain, P=0.0087. Another risk factor for ESBL+ K. pneumoniae UTIs was the presence of hospitalization in the past 6 months; 20 (58.82%) patients with ESBL+ infections were previously hospitalized, compared to only 5 (12.19%) patients with ESBL- strains, P<0.0001. The urinary catheter carriers presented an increased prevalence of ESBL+ infections (15/34 vs. 5/41, P=0.0012). Regarding mortality, ESBL+ infections caused 6 fatalities, compared to only 1 death in the ESBL- group, P=0.0166. ESBL+ K. pneumoniae strains represent an important cause of healthcare-related UTIs, with a significantly higher mortality rate compared to ESBL- strains. Early identification and adequate management of the risk factors incriminated in ESBL+ UTIs should be a priority for physicians in order to limit the dissemination of the ESBL-producing strains and thus to improve the outcome of these patients.
Keywords: ESBL-producing, K. pneumoniae, susceptibility, risk factors, antibiotics
Introduction
Klebsiella pneumoniae (KP) is a relatively common component of the normal microbiota, having the capacity to colonize various tissues and anatomical structures (e.g., distal urethra, upper respiratory tract, or the gastrointestinal tract). It is considered an opportunistic pathogen, being incriminated in the etiology of severe infections in hospitalized patients, especially in those immunocompromised or who present severe comorbidities (1,2).
The multidrug-resistance of KP, in particular to carbapenems due to the production of carbapenemases, has increased dramatically over the past 10 years and has become an important worldwide burden for the public health systems (3,4). First recognized almost four decades ago, extended-spectrum β-lactamases (ESBL) are enzymes produced by multiple gram-negative bacteria, being significantly involved in the resistance of these bacteria to almost all β-lactam antibiotics, except for cephamycins and carbapenems (5,6).
The ESBL mechanism of action is based upon their capacity to hydrolyze the β-lactam ring of the third-generation cephalosporins and aztreonam, but are inhibited by clavulanic acid (7).
The ESBL producers can also develop co-resistance to other classes of antibiotics, such as fluoroquinolones, cotrimoxazole or aminoglycosides, which are frequently used in the treatment of urinary tract infections (UTIs), thus inducing a severe limitation to their therapeutic approach (8,9). This aspect is due to the coexistence, on large plasmids, of the genes that encode ESBL with similar genes for resistance to other antimicrobial agents, subsequently creating a multidrug-resistant phenotype that is more and more associated with ESBL-producing Enterobacteriaceae (10). As a result, the judicious choice of empirical antibiotic therapy for UTI treatment is of paramount importance as it must be approached in accordance to the risk factors for infections due to ESBL-producing K. pneumoniae (ESBL+ KP), such as recurrent UTIs, diabetes mellitus (DM), previous antibiotic usage, female sex or urinary catheterization (11).
The epidemiological magnitude determined by ESBL-producing KP (ESBL+ KP) isolates is based on their significantly increased morbidity and mortality rates, prolonged hospitalization, and important financial burden (12). Additionally, there is a major concern regarding the increasing prevalence of ESBL+ KP's; a multicenter study conducted over a period of 10 years revealed a worldwide prevalence of 16%, with higher rates among patients hospitalized in intensive care units (ICU) (13). A similar increasing trend over time was noted in the case of colonization with ESBL+ KP, with rates in ICU patients ranging from 2.6% in the US to almost 50% in India (14). However, is it likely that the real prevalence is higher than the reported numbers, due to the paucity of reported data and difficulties in laboratory assessment as many testing kits fail to detect all ESBL producers.
In this challenging context, healthcare providers aim to find the ideal, thin balance between an effective antibiotic treatment against ESBL+ KP and to avoid the selection of new multidrug-resistant strains, the current paradigm being focused on preventing their spread both in the community and in healthcare facilities.
Patients and methods
Study design and population
We conducted this retrospective study aiming to assess the incidence, risk factors and antibiotic susceptibility of K. pneumoniae urinary strains isolated from patients admitted to the ‘St. Parascheva’ Clinical Hospital of Infectious diseases in Iasi, Romania, a University Clinic with 300 beds, between January 1, 2019 and June 30, 2019.
We performed a comparative assessment between patients with ESBL+ KP infections and the ESBL-negative (ESBL-) ones, by dividing them into two distinct cohorts: group 1, patients with ESBL+ KP UTIs and group 2, patients with non-ESBL producing KP (ESBL- KP) UTIs. The main objectives were to assess the individual epidemiological characteristics, risk factors for antibiotic resistance and overall patient mortality.
In the present study, we included all hospitalized patients presenting with UTIs, with the following additional inclusion criteria: i) suggestive clinical syndrome (dysuria, pollakiuria); ii) pyuria [≥10 white blood cell count (WBC)/mm3]; iii) isolation of K. pneumoniae in urine culture [≥105 colony forming units (CFU)/ml)]. We excluded patients with K. pneumoniae colonization and those with a urinary CFU count <105/ml.
Study and laboratory measurements
The following antimicrobial agents were tested by disk diffusion method: Ampicillin + sulbactam, cefixime, cefuroxime, cefotaxime, cefepime, ceftazidime, cefuroxime, cefoxitin, gentamicin, amikacin, ciprofloxacin, imipenem, meropenem, ertapenem, and colistin. EUCAST clinical breakpoint table v8.0 (15) was used for the interpretation of the minimal inhibitory concentrations and zone diameters.
We analyzed several parameters from the patient medical hospital records. Demographic characteristics (including age, sex), a full medical history, previous conditions (hospitalizations in the past 6 months, history of antibiotic use within the previous 30 days), clinical aspects (underlying diseases, invasive procedures) were fully recorded. Important comorbidities (e.g., chronic renal failure, cardiovascular diseases, diabetes mellitus, malignancy, chronic liver diseases, neurological pathologies) were previously documented or diagnosed during the current hospitalization. Charlson comorbidity index was used for assessing the overall mortality risk, based on patients coexisting comorbidities. Recurrent UTI in adults is defined as 2 or more UTIs in the last 6 months or 3 or more UTIs in the last 12 months. Diabetes mellitus was defined as a fasting blood glucose level ≥126 mg/dl, a HbA1c ≥6.5% or current consumption of antidiabetic medication. Obesity was established in all patients presenting a body mass index ≥30 kg/m2.
Routine investigations as part of the admission approach (complete blood count, inflammatory markers), assessment of the antibiotic susceptibility, hospitalization length, therapeutic management (including inappropriate empirical antibiotics and targeted single therapy/association of antibiotics) and clinical outcome were registered as well.
Statistical analysis
Categorical variables are presented as numbers and percentages, with continuous variables being presented as means and standard deviations. We used the 95% confidence interval in parameter estimation. Independent t-tests were used to compare continuous variables, while Chi-squared tests were used to compare categorical variables. Mann-Whitney U test was used for abnormally distributed categorical variables. A P-value of <0.05 was considered statistically significant for all of the analyses. Statistical analysis was performed with SPSS v20 (IBM Corp.) and EpiInfo v7.2 softwares (developer: Centers for Disease Control and Prevention).
Results
A total of 75 patients met the inclusion criteria for the study. Group 1 was comprised of 34 patients (45.3%) who had an ESBL+ KP UTI, while group 2 was made up of 41 patients (54.7%) with an ESBL- KP UTI. Out of the 34 ESBL+ KP cases, 6 (17.6%) were OXA48-producers.
Mean age of the total participants was 66±17.72 years (70.1±18.68 for group 1 vs. 62.6±7.21 for group 2; P=0.0365). The mean age of patients infected with OXA48+ strains was 69.3 years. We found a symmetrical sex distribution in the entire cohort (37 males and 38 females), but with a significant difference concerning the sex distribution in the two groups: (64.7% males in group 1 vs. 36.5% in group 2, P=0.087) (Table I). Out of the 6 OXA48-producing cases, 4 were males.
Table I.
Age distribution of the patients with K. pneumoniae UTI.
| Age interval | ESBL+ KP (n=34) | ESBL- KP (n=41) | P-value | OR (95% CI) |
|---|---|---|---|---|
| 0-20 years | 1 (2.9%) | 5 (12.1%) | 0.0855 | 0.218 (0.024-1.966) |
| 21-50 years | 3 (8.8%) | 4 (9.7%) | 0.4528 | 0.895 (1.186-4.307) |
| 51-70 years | 10 (29.4%) | 17 (41.4%) | 0.1464 | 0.588 (0.224-1.543) |
| ≥71 years | 20 (58.8%) | 15 (36.5%) | 0.0303 | 2.476 (0.974-6.294) |
| Mean age | 70.1 | 62.6 | 0.0365 |
All data are expressed as n (%). UTI, urinary tract infections; KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamase; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP. OR, odds ratio; CI, confidence interval. Significant P-values are presented in bold print.
The baseline patient characteristics are summarized in Table II. In addition to the demographic aspects, we also found statistically significant differences between the two groups concerning the underlying conditions, such as the recurrent UTIs, chronic kidney disease, cardiovascular pathologies and malignancies, which were more commonly associated with ESBL+ KP. This aspect was furtherly confirmed by an increased Charlson comorbidity index among patients with ESBL+ KP UTI (5.7 vs. 3.5, P=0.0005). Moreover, both the presence of an indwelling urinary catheter (15 vs. 5, P=0.0011) and previous hospitalizations in the past six months (20 vs. 5, P<0.0001) or a history of antibiotic use within the last 30 days (9 vs. 4, P=0.034) were significant predictors for the presence of ESBL+ KP (Table II).
Table II.
Baseline characteristics of the patients with K. pneumoniae UTI.
| Characteristics | ESBL+ KP (n=34) | ESBL- KP (n=41) | P-value | OR (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 70.1 | 62.6 | 0.0365 | |
| Male sex | 22 (64.7%) | 15 (36.5%) | 0.0080 | 3.177 (1.231-8.200) |
| Previous conditions | ||||
| Hospitalizations in the past 6 months | 20 (58.8%) | 5 (12.1%) | <0.0001 | 10.285 (3.23-32.752) |
| Antibiotic treatment in the last 30 days | 9 (26.4%) | 4 (9.7%) | 0.0340 | 3.330 (0.923-12006) |
| Underlying conditions | ||||
| Chronic kidney disease | 8 (23.5%) | 3 (7.31%) | 0.0296 | 3.897 (0.944-16.085) |
| Recurrent UTIs | 12 (35.2%) | 7 (17.0%) | 0.0404 | 2.694 (0.903-7.765) |
| Cardiovascular diseases | 24 (70.5%) | 20 (48.7%) | 0.0309 | 2.520 (0.966-6.573) |
| Diabetes mellitus | 10 (29.4%) | 13 (31.7%) | 0.4180 | 0.897 (0.334-2.411) |
| Chronic liver disease | 6 (17.6%) | 13 (31.7%) | 0.0880 | 0.461 (0.153-1.386) |
| Malignancy | 11 (32.3%) | 5 (12.1%) | 0.0203 | 3.443 (1.058-11.201) |
| Cerebrovascular disease | 11 (32.3%) | 9 (21.9%) | 0.1630 | 1.700 (0.606-4.768) |
| Obesity | 7 (20.5%) | 9 (21.9%) | 0.4470 | 0.921 (0.303-2.804) |
| Charlson comorbidity index, median (IQR) | 5.7 | 3.5 | 0.0005 | |
| Urinary catheterization | 15 (44.1%) | 5 (12.1%) | 0.0011 | 5.684 (1.791-18.036) |
| Laboratory findings | ||||
| Hemoglobin <11.7 g/dl | 16 (47.0%) | 11 (26.8%) | 0.0385 | 2.424 (0.923-6.361) |
| White blood cells >10,000/mm3 | 19 (55.8%) | 19 (46.3%) | 0.2113 | 1.466 (0.588-3.657) |
| Erythrocyte sedimentation rate >12 mm/h | 24 (32.0%) | 36 (48.0%) | 0.0372 | 0.333 (0.101-1.097) |
| Treatment | ||||
| Inappropriate empirical therapy | 18 (52.9%) | 19 (46.3%) | 0.3334 | 1.226 (0.496-3.025) |
| Targeted therapy | 17 (47.0%) | 22 (53.6%) |
All data are expressed as n (%). UTI, urinary tract infections; KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamase; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP. IQR, interquartile range; OR, odds ratio; CI, confidence interval. Significant P-values are presented in bold print.
Another important aspect of the study was to highlight the relationship between the incidence of ESBL+ KP and the use of specific antibiotics. Noteworthy, we observed that patients without ESBL-producing strains were more commonly treated with one single antibiotic (P=0.046), the use of regimens consisting of multiple antibiotic molecules (≥3 antibiotics) being observed in the lot infected with ESBL+ KP (32.3 vs. 17%, P=0.0683). Keeping in mind the resistance profile of ESBL+ KP, we identified a positive correlation between the administration of ciprofloxacin, amikacin, colistin or 3rd generation cephalosporins and the absence of ESBL producers. Contrary, the presence of ESBL+ KP was significantly associated with the large-scale use of carbapenems, indirectly reflecting both the susceptibility profile and the severity of infection (Table III).
Table III.
Antibiotic treatment of the enrolled patients with K. pneumoniae UTI.
| Variables | ESBL+ KP (n=34) (%) | ESBL- KP (n=41) (%) | P-value | OR (95% CI) |
|---|---|---|---|---|
| Single antibiotic therapy | 7 (20.5) | 16 (39.0) | 0.0462 | 0.405 (0.143-1.147) |
| 2 antibiotic therapy | 16 (47.0) | 18 (43.9) | 0.3949 | 1.135 (0.455-2.830) |
| ≥3 antibiotic therapy | 11 (32.3) | 7 (17.0) | 0.0683 | 2.323 (0.784-6.877) |
| Most commonly used antibiotics | ||||
| Ciprofloxacin | 6 (17.6) | 21 (51.2) | 0.0014 | 0.204 (0.069-0.597) |
| Ampicillin + sulbactam | 6 (17.6) | 13 (31.7) | 0.0882 | 0.461 (0.153-1.386) |
| 3rd generation cephalosporins | 6 (17.6) | 21 (51.2) | 0.0014 | 0.204 (0.069-0.597) |
| Carbapenems | 18 (52.9) | 6 (14.6) | 0.0002 | 6.562 (2.190-19.657) |
| Amikacin | 19 (55.8) | 7 (17.0) | 0.0002 | 6.152 (2.135-17.728) |
| Trimethoprim/sulfamethoxazole | 5 (14.7) | 8 (19.5) | 0.302 | 0.711 (0.209-2.417) |
| Colistin | 6 (17.6) | 1 (2.4) | 0.0166 | 8.571 (0.977-75.178) |
UTI, urinary tract infections; KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamases; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP. OR, odds ratio; CI, confidence interval. Significant P-values are presented in bold print.
In terms of evolution, the patients with ESBL+ KP presented a severe prognosis, characterized both by prolonged hospitalization (13.1 vs. 9.4 days, P=0.0012) and by a much higher mortality rate compared to those without ESBL-producing strains (17.6 vs. 2.4%, P=0.0166; Table IV).
Table IV.
Evolution of the patients with K. pneumoniae UTI.
| Variables | ESBL+ KP (n=34) | ESBL- KP (n=41) | P-value | OR (CI) |
|---|---|---|---|---|
| Length of hospital stay (IQR) | 13.1 days | 9.4 days | 0.0012 | |
| Mortality | 6 (17.6%) | 1 (2.4%) | 0.0166 | 8.571 (0.977-75.178) |
UTI, urinary tract infections; KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamases; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP; IQR, interquartile range; OR, odds ratio; CI, confidence interval. Significant P-values are presented in bold print.
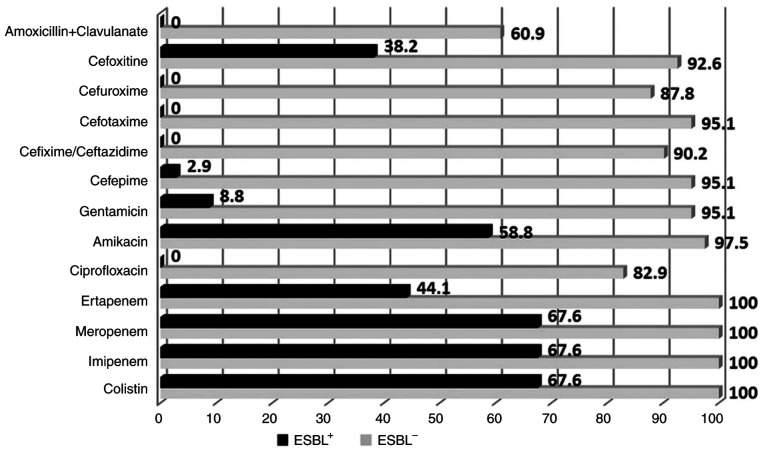
The differences between ESBL and non-ESBL susceptibility profiles are illustrated in Fig. 1, revealing the quasi-total resistance of ESBL+ KP to cephalosporins, but also to certain other classes of antibiotics, such as fluoroquinolones (ciprofloxacin) or aminoglycosides (gentamicin).
Figure 1.
ESBL+ and ESBL- KP susceptibility profiles (%) to various classes of antibiotics. KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamase; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP.
Furthermore, our study aimed to identify the risk factors for a poor outcome among the included patients. In this regard, we performed a comparative analysis between the surviving patients and the ones who were deceased. When assessing certain data from the personal history, we observed that elderly patients >70 years present a high risk for a fatal outcome (P=0.019), a similar negative evolution being also associated with previous antibiotic therapy (P=0.008) or other hospitalizations in the past six months (P=0.021). Very importantly, even if the share of severe comorbidities (except for diabetes mellitus) was higher among the deceased patients, the differences did not reach the threshold of statistical significance.
Evaluating the classic risk factors for UTIs, we found a significant association between the mortality rate and the presence of an indwelling urinary catheter (P=0.044) or a prolonged hospitalization (P=0.033). Moreover, a severe course of the infection, translated as the initiation of an aggressive antibiotic treatment (e.g., carbapenems) was significantly correlated with an increased fatality rate (P=0.018). Finally, when analyzing the ESBL production among deceased patients, we noted that 6 out of 7 fatalities presented an ESBL+ KP infection (P=0.016), suggesting the important negative prognostic value of these strains in the outcome of patients with UTI (Table V).
Table V.
Risk factors for in-hospital mortality of the patients with K. pneumoniae UTI.
| Factors | Deceased N=7 | Surviving N=68 | P-value | OR (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Age >70 years | 6 (85.7%) | 29 (42.6%) | 0.019 | 8.069 (0.95-75.734) |
| Age, median (IQR) | 75.8 | 65 | 0.142 | |
| Male sex | 3 (42.8%) | 34 (50.0%) | 0.370 | 0.750 (0.155-3.607) |
| Previous conditions | ||||
| Hospitalizations in the past 6 months | 5 (71.4%) | 20 (29.4%) | 0.021 | 6.000 (1.073-33.534) |
| Previous antibiotic therapy | 4 (57.1%) | 9 (13.2%) | 0.008 | 8.740 (1.673-45.656) |
| Comorbidities | ||||
| Chronic kidney disease | 2 (28.5%) | 9 (13.2%) | 0.165 | 2.622 (0.44-15.604) |
| Cardiovascular pathology | 5 (71.4%) | 39 (57.3%) | 0.256 | 1.859 (0.336-10.266) |
| Diabetes mellitus | 2 (28.5%) | 22 (32.3%) | 0.838 | 0.836 (0.15-4.655) |
| Chronic liver disease | 2 (28.5%) | 17 (25.0%) | 0.836 | 1.200 (0.212-6.763) |
| Malignancy | 2 (28.5%) | 15 (22.0%) | 0.695 | 1.413 (0.248-8.029 |
| Cerebrovascular disease | 2 (28.5%) | 18 (26.4%) | 0.904 | 1.111 (0.197-6.242) |
| Obesity | 2 (28.5%) | 14 (20.5%) | 0.623 | 1.542 (0.27-8.808) |
| Charlson comorbidity index, median (IQR) | 6 | 4.3 | 0.153 | |
| Urinary catheterization | 4 (57.1%) | 16 (23.5%) | 0.044 | 4.333 (0.876-21.429) |
| Length of hospital stay (IQR) | 15.2 | 10.6 | 0.033 | |
| Carbapenem treatment after laboratory diagnosis | 5 (71.4%) | 19 (27.9%) | 0.018 | 6.447 (1.15-36.124) |
| ESBL production | ||||
| ESBL+ | 6 (17.6%) | 28 (82.3%) | 0.0166 | 8.571 (0.977-75.178) |
| ESBL- | 1 (2.4%) | 40 (97.5%) |
UTI, urinary tract infections; KB, Klebsiella pneumoniae; ESBL, extended-spectrum β-lactamases; ESBL+ KP, ESBL-producing K. pneumoniae; ESBL- KP, non-ESBL producing KP; IQR, interquartile range; OR, odds ratio; CI, confidence interval. Significant P-values are presented in bold print.
Discussion
The current situation of antimicrobial resistance has reached a crucial point since 2005. In UTIs, multidrug-resistant Enterobacteriaceae, including ESBL-producing microorganisms, can be readily encountered, emerging as a growing challenge in many infectious disease clinical setups (16). In addition to the fact that patients with ESBL-producing bacteria are generally prone to a more severe course of infection compared to those without ESBL isolates, another serious issue is represented by the paucity of antibiotics that can be used for the treatment of multidrug-resistant K. pneumoniae (KP) UTIs. The persistent and often inappropriate exposure of KP strains to various β-lactam antibiotics has created the background for dynamic mutations and enhanced synthesis of β-lactamases in these bacteria, thus inducing multidrug resistance even against the extremely useful, broad-spectrum, 3rd generation cephalosporins (17,18).
Previous studies have reported a variable, geographic-related incidence of ESBL+ KP. This regional variability is explained by several factors, ranging from the specific, local pattern of resistance to some socio-economic aspects, such as healthcare access or household income, with higher rates being prevalent in developing, low-income countries from Asia or Eastern Europe (19-21). Given the scarce data in the literature concerning ESBL-producing strains in the North East region of Romania (22), our study aimed to unravel the local resistance profile in an area with certain socio-epidemiological particularities (23,24).
We found a very high prevalence of ESBL-producing isolates (45.3%) among all K. pneumoniae strains detected in urinary specimens from a university hospital serving a region with ~4 million inhabitants.
Urinary catheterization is a well-known risk factor for UTIs, this aspect being confirmed by multiple previous studies, starting from Platt et al study in 1986, when about 20% of UTIs were associated with Foley catheterization, due to contamination at the insertion of the catheter (25). Additionally, Lee et al (26) found that ESBL-producing bacteria were approximately 2.4 times more frequent in patients with Foley catheterization. In line with these findings, our study revealed that 20 patients (26.6%) had an indwelling urinary catheter, which was significantly associated with the presence of ESBL+ KP (15 ESBL+ KP vs. 5 ESBL- KP, P=0.0011).
A past history of exposure to antibiotics and hospitalizations in the last 6 months may represent another risk factor for the emergence of ESBL-producing strains. In the literature, particularly the use of cefaclor and cefminox was incriminated for the emergence of resistant strains (26). In our study, we identified a previous antibiotic exposure in 26.4% of the patients with ESBL+ KP, compared to only 9.7% with an ESBL- KP (P=0.0340). Regarding recent contact with healthcare facilities, we also found a noteworthy difference among the two groups, 20 (58.8%) for the ESBL producers vs. 5 (12.1%) for the non-ESBL producers (P<0.0001) presenting a documented hospitalization in the last six months. Similarly, Yilmaz et al (27) found that 90% of the patients with an ESBL producer had a history of previous hospitalization in the past three months vs. only 16.7% for the ESBL-negative ones.
Basically, when reviewing the literature (28-30), we noted a plethora of risk factors for infection with ESBL-producing bacteria that were also identified in our research, such as recent antibiotic use, residence in long-term care facilities, recent hospitalization, old age and male sex. In our study, elderly patients (>70 years), male sex, hospitalization in the past 6 months, previous antibiotic treatment, chronic renal failure, recurrent UTIs, urinary catheterization, longer hospital stay, underlying cardiovascular pathologies, malignancies, and anemia were considerably more prevalent among the patients with ESBL+ KP UTIs. The latter aspects may be explained by the mutual and dynamic relationship between serious comorbidities and the occurrence and severity of KP infections. Frail patients with impaired immunity due to a severe associated pathology are more prone to develop a KP infection, and vice versa, the KP infection may negatively influence the outcome of such a patient. For example, a recent study highlighted the significant incidence and poor prognosis of carbapenemase-producing KP infections among patients with hematological malignancies or aplastic anemia (31).
Antimicrobial susceptibility was investigated for all isolates. Our results demonstrate that, among the ESBL+ KP patients, susceptibility rates to ciprofloxacin, gentamicin, amikacin, imipenem, meropenem and colistin were extremely low: 0, 8.9, 58.9, 67.7, 67.7 and 67.7%, respectively. On the other hand, the ESBL- KP strains showed lower susceptibility to amoxicillin + clavulanic acid (61%), ciprofloxacin (83%) and cefuroxime (87.8%), while high susceptibility was identified for carbapenems (100%), amikacin (97.6%), gentamicin (95.1%), cefepime (95.1%), ceftazidime (90.3%), cefotaxime (95.1%), cefixime (90.3%) and cefoxitin (92.7%), these findings being in accordance with other results that have identified a similar resistance pattern, thus raising awareness both on the adequate therapeutic approach and nosocomial or community spread of ESBL+ KP (32). It is noteworthy to point out that the antibiotic combination between a β-lactam and an aminoglycoside is synergistic and is widely used as a first-line therapy prior to antibiogram results, especially in patients with established risk factors for multidrug-resistant infections. Given the local susceptibility profile, in addition to a β-lactam, in our setting we prefer the use of amikacin instead of gentamicin given the higher resistance rate associated with the latter.
However, despite the similarities, there are also some susceptibility-related differences, mainly due to particular local factors. Even if some of the results from a Pakistani study (33) were similar to ours concerning the susceptibility profile of ciprofloxacin, amikacin, imipenem and meropenem they also found a significantly lower susceptibility to gentamicin for the ESBL- KP (63.1 vs. 95.1% in our study). A more recent multicentric study from the same country furtherly confirmed this regional pattern of resistance, thus bringing attention to the geographical polymorphism of ESBL+ KP (34). Coinciding results were found in another study performed by Müller-Schulte et al (35) regarding the differences between the susceptibility profiles depending on the ESBL production in a sub-Saharan country; for the ESBL+ KP the susceptibility to ciprofloxacin, gentamicin, meropenem, cefuroxime, ceftazidime and cefotaxime was 38, 21, 100, 0, 0 and 0%, respectively, while for the ESBL- KP the susceptibility was 88, 94, 100, 100, 100 and 100%, respectively.
Numerous studies have examined whether ESBL production has an adverse effect on clinical outcomes. Although most of the reported data revealed an association between unfavorable outcome and infections with ESBL+ KP (36-40), other studies have failed to demonstrate such a link (41). In our patients we identified a significant association between the presence of ESBL+ KP and the morality rate (P=0.016). Noteworthy, of the six patients with OXA48-producing K. pneumoniae UTI, we recorded two deaths.
Furthermore, we found that the risk factors for all-cause mortality were similar to those for ESBL+ KP infection and included: Age >70 years (P=0.019), hospitalizations in the past 6 months (P=0.024), previous antibiotic treatment (P=0.003), urinary catheterization (P=0.044), length of hospital stay (P=0.033), carbapenem treatment based on antibiogram result (P=0.018) and ESBL production (P=0.0166).
In terms of therapeutic approach, we could not quantify accurately the superiority of any particular antibiotic regimen in the prognosis of K. pneumoniae UTI mainly due to the heterogenicity of the regimens (often consisting of at least 3 different antibiotics), but also due to some patient-related specific aspects which led to the use of only certain antibiotics. As a general algorithm, before initiating antibiotic treatment in ESBL+ patients, of paramount importance, is to assess the in vitro susceptibilities, the degree of the infection's source control and, finally, the clinical condition of each patient (7).
Carbapenems exhibit the broadest spectrum of β-lactam antibiotics, presenting the highest potency against Gram-negative bacteria, but being also characterized by stability to hydrolysis by the majority of β-lactamases. The majority of available data have demonstrated that carbapenem treatment is associated with improved outcomes in patients with severe ESBL+ KP infections and remains the ‘gold standard’ especially in critically ill patients (7,42,43). The research spectrum of ESBL+ KP has focused mainly on meropenem or imipenem, although a recently published multicentric study also assessed the efficacy of ertapenem (44), finding similar cure rates (90.6% with ertapenem and 75.5% with other carbapenems in empiric and 89.8 and 82.6% in targeted treatment), without significant differences concerning mortality rates. Importantly, in our study we observed a rather low ESBL+ KP susceptibility rate to ertapenem (44.1%), compared to imipenem or meropenem (67.6% each), thus outlining bleak future prospects when compared to a 100% susceptibility rate to both ertapenem and imipenem reported less than two decades ago (45,46).
Our study has limitations related to the relatively small size of the research group and the retrospective nature. Given the continuously increasing trend of ESBL+ KP prevalence, this means that our results could underestimate the actual burden of these strains, therefore requiring careful interpretation of the data. Despite these limitations, our study provides several insights on the association between the ESBL+ KP and the outcome of the UTI patients with this etiology, as well as the implications regarding susceptibility profiles and local risk factors from the Northeast region of Romania. In conclusion, our data may have a significant impact for further antibiotic prescription in patients with community- and/or hospital-acquired UTIs, mainly focusing on the early identification or even prevention of certain risk factors associated with ESBL-producing KP.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Data used in this original study were obtained from the patient personal medical records admitted to the ‘St. Parascheva’ Clinical Hospital of Infectious Diseases in Iasi, Romania. Any further information regarding the present study is available from the corresponding authors upon reasonable request.
Authors' contributions
All authors presented an equal contribution to this paper. ILM, OSD and EGM designed the study and collected data from the included patients. OSD, CL and LSI performed the susceptibility tests and other laboratory determinations. DTAP, EVN, IIC and CSS analyzed data and performed the statistics. ILM, OSD and RSM analyzed and wrote the Results and Discussion sections including the literature review. RSM and EVN prepared the manuscript, translated it and managed all the correspondence for publishing. All authors were actively involved in conceiving the paper and they read and approved the final version of the manuscript for publication.
Ethics approval and patient consent
This study was approved by the local Ethics Commission of ‘St. Parascheva’ Clinical Hospital of Infectious Diseases in Iasi, Romania (approval no. 8/2019) and it was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki Principles. Patient consent was not required due to the retrospective character of the study.
Patient consent for publication
Not applicable.
Competing interests
There are no competing interests regarding the authors of this research.
References
- 1.Shimasaki T, Seekatz A, Bassis C, Rhee Y, Yelin RD, Fogg L, Dangana T, Cisneros EC, Weinstein RA, Okamoto K, et al. Increased relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae within the gut microbiota is associated with risk of bloodstream infection in long-term acute care hospital patients. Clin Infect Dis. 2019;68:2053–2059. doi: 10.1093/cid/ciy796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navon-Venezia S, Kondratyeva K, Carattoli A . Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 3.Lee BY, Bartsch SM, Wong KF, McKinnell JA, Slayton RB, Miller LG, Cao C, Kim DS, Kallen AJ, Jernigan JA, Huang SS. The potential trajectory of carbapenem-resistant Enterobacteriaceae, an emerging threat to health-care facilities, and the impact of the centers for disease control and prevention toolkit. Am J Epidemiol. 2016;183:471–479. doi: 10.1093/aje/kwv299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun SD, Dorneanu OS, Vremeră T, Reißig A, Monecke S, Ehricht R. Carbapenemase-producing Enterobacteriaceae: A 2-year surveillance in a hospital in Iaşi, Romania. Future Microbiol. 2016;11:391–401. doi: 10.2217/fmb.15.148. [DOI] [PubMed] [Google Scholar]
- 5.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Committee on Antimicrobial Susceptibility Testing (EUCAST): EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2.01. EUCAST, Sweden, 2017. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf. Accessed Febrary 20, 2021. [Google Scholar]
- 7.Pana ZD, Zaoutis T. Treatment of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Res. 2018;7(F1000 Faculty Rev-1347) doi: 10.12688/f1000research.14822.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miftode E, Dorneanu O, Leca D, Teodor A, Mihalache D, Filip O, Luca V. Antimicrobial resistance profile of E. coli and Klebsiella spp. From urine in the infectious diseases hospital Iaşi. Rev Med Chir Soc Med Nat Iasi. 2008;112:478–482. (In Romanian) [PubMed] [Google Scholar]
- 10.Brolund A, Edquist PJ, Mäkitalo B, Olsson-Liljequist B, Söderblom T, Wisell KT, Giske CG. Epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in Sweden 2007-2011. Clin Microbiol Infect. 2014;20:O344–O352. doi: 10.1111/1469-0691.12413. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 12.Maslikowska JA, Walker SA, Elligsen M, Mittmann N, Palmay L, Daneman N, Simor A. Impact of infection with extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. J Hosp Infect. 2016;92:33–41. doi: 10.1016/j.jhin.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 2013;6:1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients-review of the literature from a clinical perspective. Crit Rev Microbiol. 2016;42:1–16. doi: 10.3109/1040841X.2013.875515. [DOI] [PubMed] [Google Scholar]
- 15. EUCAST Clinical breakpoints-breakpoints and guidance, 2018. https://www.eucast.org/clinical_breakpoints/. Accessed December 10, 2019. [Google Scholar]
- 16.Kader AA, Kumar A. Prevalence and antimicrobial susceptibility of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a general hospital. Ann Saudi Med. 2005;25:239–242. doi: 10.5144/0256-4947.2005.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarojamma V, Ramakrishna V. Prevalence of ESBL-producing Klebsiella pneumoniae isolates in tertiary care hospital. ISRN Microbiol. 2011;2011(318348) doi: 10.5402/2011/318348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 19.Goossens H. MYSTIC program: Summary of European data from 1997 to 2000. Diagn Microbiol Infect Dis. 2001;41:183–189. doi: 10.1016/s0732-8893(01)00320-0. MYSTIC Study Group (Europe) [DOI] [PubMed] [Google Scholar]
- 20.Hoşoğlu S, Gündes S, Kolayli F, Karadenizli A, Demirdağ K, Günaydin M, Altindis M, Caylan R, Ucmak H. Extended-spectrum beta-lactamases in ceftazidime-resistant Escherichia coli and Klebsiella pneumoniae isolates in Turkish hospitals. Indian J Med Microbiol. 2007;25:346–350. doi: 10.4103/0255-0857.37336. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Z, Zhu D, Zhang Y, Wang F. Extended-spectrum beta-lactamase in Klebsiella pneumoniae and Escherichia coli isolates. Zhonghua Yi Xue Za Zhi. 2002;82:1476–1479. (In Chinese) [PubMed] [Google Scholar]
- 22.Miftode E, Dorneanu O, Badescu A, Ghibu L, Leca D, Vremera T, Mereuţă A. Emergence of a new group CTX-M enzyme in Romania and risk factors for extended spectrum beta-lactamase producing E. coli infections. Rev Med Chir Soc Med Nat Iasi. 2012;116:477–480. [PubMed] [Google Scholar]
- 23.Anton-Paduraru DT, Miftode EG, Iliescu ML, Pricop C, Carauleanu A, Boiculese LV. Knowledge of adolescent girls regarding sexually transmitted diseases a study in a rural area from North-Eastern Romania. Rev Cercet Interv Soc. 2020;69:143–155. [Google Scholar]
- 24.Costache II, Miftode E, Mitu O, Aursulesei V. Sex differences in cardiovascular risk factors in a rural community from North Romania Region. Rev Cercet Interv Soc. 2016;55:204–214. [Google Scholar]
- 25.Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol. 1986;124:977–985. doi: 10.1093/oxfordjournals.aje.a114487. [DOI] [PubMed] [Google Scholar]
- 26.Lee DS, Lee CB, Lee SJ. Prevalence and risk factors for extended spectrum beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol. 2010;51:492–497. doi: 10.4111/kju.2010.51.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz E, Akalin H, Ozbey S, Kordan Y, Sinirtaş M, Gürcüoglu E, Ozakin C, Heper Y, Mistik R, Helvaci S. Risk factors in community-acquired/onset urinary tract infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Chemother. 2008;20:581–585. doi: 10.1179/joc.2008.20.5.581. [DOI] [PubMed] [Google Scholar]
- 28.Vading M, Nauclér P, Kalin M, Giske CG. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS One. 2018;13(e0195258) doi: 10.1371/journal.pone.0195258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen ML, Toye B, Kanji S, Zvonar R. Risk factors for and outcomes of bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can J Hosp Pharm. 2015;68:136–143. doi: 10.4212/cjhp.v68i2.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koksal E, Tulek N, Sonmezer MC, Temocin F, Bulut C, Hatipoglu C, Erdinc FS, Ertem G. Investigation of risk factors for community-acquired urinary tract infections caused by extended-spectrum beta-lactamase Escherichia coli and Klebsiel species. Investig Clin Urol. 2019;60:46–53. doi: 10.4111/icu.2019.60.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tofas P, Skiada A, Angelopoulou M, Sipsas N, Pavlopoulou I, Tsaousi S, Pagoni M, Kotsopoulou M, Perlorentzou S, Antoniadou A, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: Analysis of 50 cases. Int J Antimicrob Agents. 2016;47:335–339. doi: 10.1016/j.ijantimicag.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Parveen RM, Khan MA, Menezes GA, Harish BN, Parija SC, Hays JP. Extended-spectrum β-lactamase producing Klebsiella pneumoniae from blood cultures in Puducherry, India. Indian J Med Res. 2011;134:392–395. [PMC free article] [PubMed] [Google Scholar]
- 33.Ullah F, Malik SA, Ahmed J. Antimicrobial susceptibility pattern and ESBL prevalence in Klebsiella pneumoniae from urinary tract infections in the North-West of Pakistan. Afr J Microbiol Res. 2009;3:676–680. [Google Scholar]
- 34.Abdullah FE, Mushtaq A, Irshad M, Rauf H, Afzal N, Rasheed A. Current efficacy of antibiotics against Klebsiella isolates from urine samples-a multi-centric experience in Karachi. Pak J Pharm Sci. 2013;26:11–15. [PubMed] [Google Scholar]
- 35.Müller-Schulte E, Tuo MN, Akoua-Koffi C, Schaumburg F, Becker SL. High prevalence of ESBL-producing Klebsiella pneumoniae in clinical samples from central Côte d'Ivoire. Int J Infect Dis. 2020;91:207–209. doi: 10.1016/j.ijid.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on on outcomes. Clin Infect Dis. 2001;32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 37.Shanthi M, Sekar U. Extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomes. J Assoc Physicians India. 2010;58 (Suppl):S41–S44. [PubMed] [Google Scholar]
- 38.MacVane SH, Tuttle LO, Nicolau DP. Impact of extended-spectrum β-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med. 2014;9:232–238. doi: 10.1002/jhm.2157. [DOI] [PubMed] [Google Scholar]
- 39.Shamsrizi P, Gladstone BP, Carrara E, Luise D, Cona A, Bovo C, Tacconelli E. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum beta-lactamases: A systematic review and meta-analysis. BMJ Open. 2020;10(e030266) doi: 10.1136/bmjopen-2019-030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vata A, Hunea IM, Dorneanu O, Harja-Alexa IA, Plesca C, Leonte-Enache G, Ciocan A, Ghiciuc CM, Esanu I, Manolache M, Luca CM. Biochemical changes and risk factors in the prognosis of antibiotics susceptibility in urinary tract infections. Rev Chim. 2019;70:1822–1825. [Google Scholar]
- 41.Kola A, Maciejewski O, Sohr D, Ziesing S, Gastmeier P. Clinical impact of infections caused by ESBL-producing E. coli and K. pneumoniae. Scand J Infect Dis. 2007;39:975–982. doi: 10.1080/00365540701466140. [DOI] [PubMed] [Google Scholar]
- 42.Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29:583–594. doi: 10.1097/QCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 43.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015;60:1319–1325. doi: 10.1093/cid/civ003. Antibacterial Resistance Leadership Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutiérrez-Gutiérrez B, Bonomo RA, Carmeli Y, Paterson DL, Almirante B, Martínez-Martínez L, Oliver A, Calbo E, Peña C, Akova M, et al. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: A multinational pre-registered cohort study. J Antimicrob Chemother. 2016;71:1672–1680. doi: 10.1093/jac/dkv502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betriu C, Salso S, Sánchez A, Culebras E, Gómez M, Rodriguez-Avial I, Picazo JJ. Comparative in vitro activity and the inoculum effect of ertapenem against Enterobacteriaceae resistant to extended-spectrum cephalosporins. Int J Antimicrob Agents. 2006;28:1–5. doi: 10.1016/j.ijantimicag.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Mody RM, Erwin DP, Summers AM, Carrero HA, Selby EB, Ewell AJ, Moran KA. Ertapenem susceptibility of extended spectrum beta-lactamase-producing organisms. Ann Clin Microbiol Antimicrob. 2007;6(6) doi: 10.1186/1476-0711-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this original study were obtained from the patient personal medical records admitted to the ‘St. Parascheva’ Clinical Hospital of Infectious Diseases in Iasi, Romania. Any further information regarding the present study is available from the corresponding authors upon reasonable request.