Highlights
-
•
Novel ceramide drug for breast cancer.
-
•
Toxicological profile was studied.
-
•
Single dose of 80 mg/kg dose was safe.
-
•
Single dose of 120 mg/kg caused minor liver and cardiac tissue damage.
Keywords: Ceramide, Toxicology, Drug, Breast cancer, Biochemical parameters
Abstract
We have previously reported that treating triple-negative tumor bearing nude mice with intraperitoneal (ip) 10 mg/kg body weight of (S,E)-3-hydroxy-2-(2-hydroxybenzylidene)amino-N-tetradecylpropanamide, a ceramide analog, 5 days per week for 3 weeks, was shown not only to suppress tumor growth but also to reduce metastasis. Studies reported here focus on determining the toxicity of this drug in the nude mice. During the first study, treated animals (single intraperitoneal (ip) injection, 0, 40, 80 and 120 mg/kg body weight) were closely monitored for 14 days for any signs of illness or death. No mice were lost in any animal groups; however, hepatic serum enzymes were elevated, and hepatic and heart tissue damages were found in the highest dosage group. The subsequent study was performed using a lower dosage range (single ip injection, 0, 25, 50 and 75 mg/kg body weight), which resulted in no significant toxicity. All tested parameters were within normal ranges, with no observed irregularities. Our findings show that a single ip dose of this ceramide analog induced liver and heart toxicity at 120 mg/kg but not at doses of 80 mg/kg body weight or lower.
1. Introduction
Breast, lung, and colorectal cancers are the most common cancers in females [1]. Globally, there were 2.2 million newly diagnosed cancer cases in 2020, with 30 % contributed to breast cancer. In 2020 alone, there were approximately 685,000 deaths reported from breast cancer worldwide. The 5-year prevalence of breast cancer in all ages was reported to be 7.7 million [1]. It was estimated that about 3.8 million women were living with breast cancer in the US as of January 1, 2019 [2].
Cancer Today provides a comprehensive assessment of the cancer burden worldwide in 2020, based on the Globocan estimates of incidence, mortality, and prevalence for 36 cancer types, each reported by sex and age group [1]. Metastatic breast cancer (MBC) has been identified in about 20–30 % of breast cancer patients [3]. Even though MBC can be treated, it cannot be cured [4]. About 22 % of Stage 4 breast cancer patients survive for five years, with the average survival period being only three years [5]. Although there has been a significant amount of progress in cancer prevention and treatment, there are still major challenges to overcome. Since cancer cells often become resistant to commonly used chemotherapeutic agents [6], it is crucial to continue to develop more potent tumor-selective cytotoxic agents that can avoid drug-resistance and metastasis in cancer cells. To this end, using ceramide drugs has emerged as a potential solution.
Ceramides were first recognized as apoptosis [7] and cellular senescence [8] regulators in the 1990s. Radiation therapy and treatment with many chemotherapeutic agents result in the intracellular buildup of ceramides [9], while higher ceramide metabolism, and low ceramide levels are detected in drug-resistant cells [10]. Thus, an attractive cancer treatment strategy is to increase ceramide levels through delivery of exogenous ceramides or by de novo synthesis stimulation [9]. The involvement of ceramides [11] and sphingosine-1-phosphate (S1P) [12] in different types of cancer including breast cancer [13] have been confirmed. In 2018, the clinical significance of ceramide levels in breast cancer patients was reported by Moro et.al [14]. Use of ceramides is currently a novel trend in cancer treatment [15].
Researchers from our laboratory have synthesized, evaluated, and performed structure-activity relationship studies on over 50 ceramide analogs as potential breast cancer therapeutics [16]. Of these synthesized compounds, ceramide analog 315, (S,E)-3-hydroxy-2-(2-hydroxybenzylidene)amino-N-tetradecylpropanamide, was found to be the most effective and efficacious compound in all three cell lines tested (MCF7 (ERα-positive cell line), MCF7-TNR (chemo-resistant cell line), and MDA-MB-231 triple-negative cell line (ERα/PR/HER2-negative, and resistant to hormone and endocrine therapies)) with IC50 values of 14–22 μM [17,18]. This particular ceramide drug contains an amide backbone, a functional imine group, and a phenolic hydroxyl group. Due to these structural features, analog 315 is expected to have an increased permeability through the lipid bilayer as well as increased water-solubility compared to other ceramides. We have previously reported that treatment of mice with intraperitoneal (ip) 10 mg/kg body weight of ceramide analog 315, 5 days per week for 3 weeks, inhibited tumor growth and reduced metastasis in triple-negative breast cancer tumors [19]. The objective of the studies reported here was to determine the toxicological and physiological effects that result from administering this compound to the nude mice.
2. Materials and methods
2.1. Synthesis
(S,E)-3-hydroxy-2-(2-hydroxybenzylidene)amino-N-tetradecylpropanamide was synthesized as previously reported [20].
2.2. Study 1
Twenty female nude mice (Nu/Nu) ages 8–10 weeks were purchased from Charles River Laboratories, USA. They were maintained in germ-free and pathogen-free clean rooms with 12-hours light/dark cycle. Autoclaved food and water were available ad libitum. Animal Research Reporting of in vivo Experiments (ARRIVE) guidelines were followed as described by Percie du Sert et al. [21]. The ethical protocol used was approved by the Xavier University of Louisiana’s Institutional Animal Care and Use Committee. (Protocol Number #:0080415-05CH). The mice were monitored closely, not only for changes in their behavior, but also for any signs of pain, suffering, illness, or weakness.
All twenty animals purchased were included in the study. After acclimatization, the mice were randomly distributed into four groups (Sample size was 5 mice per cage). Simple randomization method was used. Group 1 was the control group, and received 50 μL of DMSO (dimethyl sulfoxide, which was the solvent used for dissolving the drug). Groups 2, 3, and 4 received a low dose of 40 mg/kg body weight, intermediate dose of 80 mg/kg body weight, or high dose of 120 mg/kg body weight of the drug, respectively. The drug doses were given once as an intraperitoneal (ip) injection. During the 14-day acute toxicity study period, mice were observed daily for mortality, morbidity, and changes in behavior. Animal weights were recorded weekly. At the end of 14 days, mice were weighed and then deeply anesthetized with xylazine hydrochloride and ketamine hydrochloride (10 /100 mg/kg body weight injection). Blood was collected from the heart in small Eppendorf tubes without ethylenediaminetetraacetic acid (EDTA), and the serum was collected and stored at −80 °C for biochemical assays.
2.3. Necropsy procedures
Mice were closely inspected for any external injury. Vital organs after isolation were cleared, blotted dry, and checked for any external abnormalities. After recording the organ weights, they were stored in 10 % neutral-buffered formalin and submitted to the Department of Pathology at Tulane University School of Medicine for preparation of histological slides. Slides were stained with Hematoxylin and Eosin.
2.4. Serum biochemical assays
Serum biochemical evaluations were performed according to the manufacturers’ protocols using the corresponding kits purchased from Bioassay Systems and Thermo Fisher Scientific companies. The kidney function was analyzed by testing serum protein and creatinine (Crea); the liver function was analyzed by aspartate transaminase (AST), and alanine transaminase (ALT); and the electrolyte panel (calcium (Ca++) and phosphate (PO4−−) levels) were determined. Serum calcium and phosphate, kidney function, and liver function were measured using the QuantiChromTM assay kits (BioAssay Systems, Hayward, CA): Calcium Assay Kit (cat. no. DICA-500), Phosphate Assay Kit (DIPI, 500 Assays), EnzyChromTM Alanine Transaminase Assay Kit (EALT-100, 100assays), Aspartate Transaminase Assay Kit (EASTR-100, EnzyChromTM, 100 assays), and Creatinine Assay Kit (DICT-500, 500 assays). Micro BCA Protein Assay Kit was purchased from Thermo Fisher Scientific (23235). Liver function was found to be altered, and liver tissue damage was observed in the high-dosage group of Study 1. Therefore, a second acute toxicity study with a lower dosage range was planned.
2.5. Study 2
The subsequent study was performed using the same protocols and under similar conditions with lower dosages, in which the mice were treated with intraperitoneal injection of a single drug dose of 0, 25, 50, or 75 mg/kg body weight.
2.6. Statistical analysis
Values reported are given as mean ± SE. P values <0.05 were considered significant. The mean and SE were calculated using Microsoft Excel or Prism software (GraphPad, La Jolla, CA, USA). Statistical significance (P < 0.05) was done using Student t-tests Graph Pad Prism Two-way ANOVA followed by Tukey’s multiple comparison test was used for multiple comparisons.
3. Results
3.1. Body and organ weights
Tukey multiple comparison test comparisons between all the groups were made for different days and none of the groups exhibited statistical significance in the whole animal body weights either in Study 1 or 2. In Study 1, the control group’s body weight average was 31.20 g vs. 34.69, 34.58, and 32.86 g, respectively, in the low-, mid-, and high-dose groups and the difference was not significant in any group. In Study 2, the control group’s average body weight was 31.69 g vs. 32.86, 31.66, and 32.22 g, respectively, in the low-, mid-, and high-dose groups; all not significantly different. Organ weights of the treated versus control groups were not significantly different in either toxicity study (Fig. 1A and B).
Fig. 1.
(A) Animal Weights (B) Tissue Weights.
A: Whole animal weights of female nude mice (Nu/Nu) aged between 8 and 10 weeks (n = 5) after treatment with analog 315. A single intraperitoneal dose of 0 mg/kg (control), 40 mg/kg (low), 80 mg/kg (mid), or 120 mg/kg (high) was administered in Study 1. In Study 2, a single intraperitoneal dose of 0 mg/kg (control), 25 mg/kg (low), 50 mg/kg (mid), or 75 mg/kg (high) was administered. (B) Animals were sacrificed on day 14 and tissues were isolated and weighed. Values shown are mean ± SE. Two-way ANOVA followed by Tukey’s multiple comparison was used for statistical analysis.
3.2. Results of serum biochemical assays
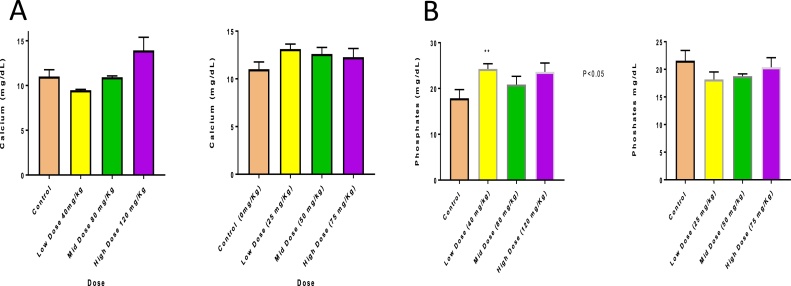
Biochemical testing showed no abnormal toxicity signs. Calcium levels were within normal ranges for the first and second studies at all doses (ranging from 11.01 mg/dL to 13.93 mg/dL), and no hypocalcemia or hypercalcemia was observed (Fig. 2A). Serum phosphate levels were within the normal ranges at all doses tested in both studies (Fig. 2B). Biochemical analysis of liver function showed AST levels of 38.47 U/L (control) vs. 16.85 U/L (Study 1, high-dose group) (P < 0.001) indicating a significant change in liver function. However, no significant changes in the ALT levels were observed in either study (Figs. 3A & B and 4 A & B). Total protein concentration was significantly higher in mid- and high-dose groups of Study 1 (control group levels of 34.61 μg/mL vs. 41.53, 45.61, and 47.37 μg/mL in the low-, mid- and high-dose groups, respectively (P < 0.01)), but showed no significant changes in Study 2 (Fig. 4A). No Changes in the levels of creatinine were observed (Fig. 4B).
Fig. 2.
(A) Calcium Levels (B) Phosphate levels.
A: Calcium levels and (B) phosphate levels, (n = 5), after a single intraperitoneal injection of different doses of ceramide analog 315. Animals were sacrificed on day 14, serum was collected, and calcium and phosphate assay kits purchased from BioAssay Systems, Hayward, CA, were used for the measurements. Assays were conducted according to the manufacturer’s protocols. Values shown are mean ± SE (p < 0.05).
Fig. 3.
Aspartate and Alanine Transaminase A: Study 1 B: Study 2.
A: Aspartate and Alanine Transaminase levels, (n = 5), after a single intraperitoneal injection of different doses of ceramide analog 315. Animals were sacrificed on day 14, serum was collected, and aspartate and alanine transaminase assay kits purchased from BioAssay Systems, Hayward, CA were used for the measurements. Assays were conducted according to the manufacturer’s protocols. Values shown are mean ± SE (p < 0.05).
Fig. 4.
(A)Total Protein Levels (B) Creatinine levels.
A: Total protein levels, (n = 5), after a single intraperitoneal injection of different doses of ceramide analog 315. Animals were sacrificed on day 14, and serum was collected. Micro BCA protein assay kits were purchased from Thermo Fisher Scientific Company, and assays were performed according to manufacturer’s protocol. (B) Creatinine assay kits were purchased from BioAssay Systems, Hayward, CA, and assays were conducted according to the manufacturer protocol. Values shown are mean ± SE (p < 0.05).
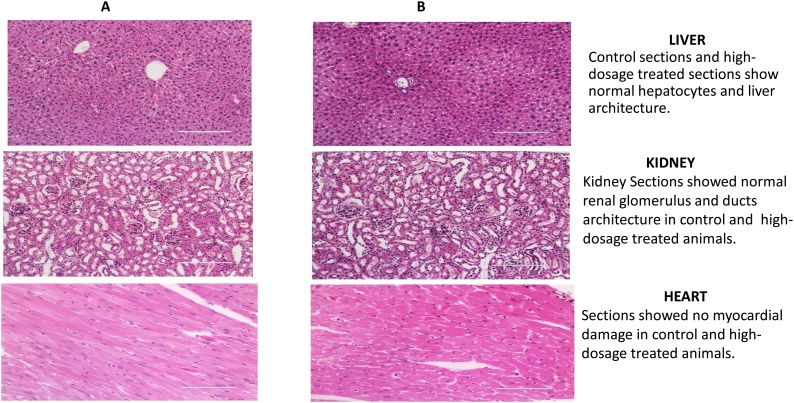
3.3. Histopathological results
There were no histological changes in vital tissues such as spleen, lungs, and kidneys with any treatment in either study (data not shown); however, the histological analysis of liver and heart tissues showed minor changes in the high-dose (120 mg/kg) group in Study 1 (Fig. 5B). No signs of hepatitis, cirrhosis, bile duct obstruction, cholestasis, or any sort of drug-induced liver disease or damage to any other organ were observed with any of the other dosages tested (Fig. 6B).
Fig. 5.
A: Control. B: High dose (120 mg/kg).
Female nude mice (Nu/Nu) were treated with a single intraperitoneal dose of 0 mg/kg, 40 mg/kg (low), 80 mg/kg (mid), or 120 mg/kg (high) of the ceramide analog in Study 1 and sacrificed after 14 days. Tissues were fixed in 10 % formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. (Low- and mid-dosage figures are not shown).
Fig. 6.
A: Control. B: High dose (75 mg/kg).
Female nude mice (Nu/Nu) were treated with a single intraperitoneal dose of 0 mg/kg, 25 mg/kg (low), 50 mg/kg (mid), or 75 mg/kg (high) of the ceramide analog and sacrificed after 14 days. Tissues were fixed in 10 % formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. (Low- and mid-dose figures are not shown).
4. Discussion
Rodents are commonly used in laboratory experiments because of their ready availability, inexpensiveness to breed and house, and capability to produce reliable results. Mice are frequently used for efficacy testing due to the abundance of standard, athymic, transgenic, and knock-out strains. For toxicological studies usually male and female sexes are used. However, changes in toxic response between the two sexes are minimal, except for hormonal substances. Therefore, only female mice were selected for our studies. A 1999 rodent toxicological study with 25 cancer drugs during preclinical and clinical experimental stages established that rodent studies provide a means to calculate a safe dosage for potential clinical trials [22].
Acute toxicity studies are conducted to provide data as to whether brief exposure to a drug will cause any adverse effects. These studies are necessary for assessing the safety of novel drugs. In the drug discovery process, acute toxicity studies follow the initial study to assess for the efficaciousness, and general toxicity. Acute toxicity evaluates different markers for overall health. This is typically done by biochemical analyses of molecules associated with the optimal function of different body systems. The information gained from acute toxicity studies is used to guide the selection of dosages suitable for potential clinical studies. In these acute toxicity studies, four groups of animal subjects were used: control, low-, intermediate-, and high-dosage treated groups. The reason for using a dosage range is to determine little to no observable adverse effect level (NOAEL) or no observable effect level (NOEL). Following an acute toxicity study, a chronic toxicity study is performed, where subjects are exposed to the drug for a longer period to determine the safety of the drug after an extended exposure.
Doses for the preclinical studies are based on the prior experiments within a research group; therefore, our dosages were based on our previous experiments. In our first toxicity study, nude mice were treated with a single dose of 0, 40, 80, or 120 mg/kg body weight of the ceramide analog. In the subsequent toxicity study, mice were treated with a single dose of 0, 25, 50, or 75 mg/kg body weight of the ceramide analog (lower dosages than used in Study 1). AST levels were altered in mice treated with 120 mg/kg body weight dosage. These results are in agreement with the histological analysis of the liver of the animals, in which alterations were observed only in the high-dose treated group. On the contrary, the ALT levels remained in the reported normal levels for mice [23,24]. These relatively easy, inexpensive, and reliable blood tests indicated no serious liver damage, confirming no drug-induced liver toxicity in concentrations up to 75 mg/kg body weight. Due to their function of removing toxins from circulation, kidneys are highly exposed to chemicals in the blood and are especially vulnerable to xenobiotic toxicity. Thus, monitoring biomarkers that track kidney failure and kidney histology are primary end points in preclinical toxicology. In our studies, analysis of the biomedical assay results showed no alterations in the creatinine levels. Further the kidneys did not show any histological alterations, indicating no toxicity to this organ upon treatment. The cardiac tissue also did not show any adverse effects.
Attrition of drug candidates has been mainly attributed to the toxicity of the drugs, and is a major contributor to the high cost of drug development [25]. Safety concerns can arise at any stage of drug development. Thus, our goal was to improve ways to predict the safe tolerable dosage of the ceramide analog at initial stages of drug development. Our results demonstrated that ceramide analog 315 is safe and can be further studied as a new drug candidate for the treatment of breast cancer. In this study, the maximum tolerable dose of the analog (MTD) was found to be 120 mg/kg body weight, and the no observed adverse effect level (NOAEL was determined to be at a concentration of 75 mg/kg body weight. At this dosage, no significant changes were observed, thus establishing a safety profile for treatment with this novel drug candidate. Complete comprehensive toxicity and pharmacokinetic studies are planned with rats, which will enable us to test more clinical parameters with the availability of the higher serum quantity. This will also allow us to determine any cross-species differences. Further studies are also required to determine the detailed intracellular pathway(s) involved, and to determine whether the mild injury caused by the single high-dose treatment is reversible. A chronic toxicity study is also planned.
Funding
Portions of this work were supported by the NIH AREA Grant 1R15CA159059, the DoD Breast Cancer Research AwardW81XWH-11-1-0105, BC102922, the Louisiana Cancer Research Center, the NIH-RCMI Award5G12MD007595, and the NIH BUILD AwardsTL4GM118968 and 5RL5GM118966.
Declaration of Competing Interest
The authors declare no conflict of interest.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Globocan . 2020. Cancer Today.https://wwwuiccorg/news/globocan-2020-new-global-cancer-data [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. Epub 2019/06/12. PubMed PMID: 31184787. [DOI] [PubMed] [Google Scholar]
- 3.Rugo H.S. The importance of distant metastases in hormone-sensitive breast cancer. Breast. 2008;17(Suppl. 1):S3–S8. doi: 10.1016/s0960-9776(08)70002-x. Epub 2008/02/19. PubMed PMID: 18279764. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Y.C., Ueno N.T. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer (Tokyo, Japan) 2012;19(3):191–199. doi: 10.1007/s12282-011-0276-3. Epub 2011/05/14. PubMed PMID: 21567170; PubMed Central PMCID: PMCPMC3860359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Angelis R., Tavilla A., Verdecchia A., Scoppa S., Hachey M., Feuer E.J. Breast cancer survivors in the United States: geographic variability and time trends, 2005–2015. Cancer. 2009;115(9):1954–1966. doi: 10.1002/cncr.24217. Epub 2009/02/28. PubMed PMID: 19248047. [DOI] [PubMed] [Google Scholar]
- 6.Alfarouk K.O., Stock C.M., Taylor S., Walsh M., Muddathir A.K., Verduzco D. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. Epub 2015/07/17. PubMed PMID: 26180516; PubMed Central PMCID: PMCPMC4502609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obeid L.M., Linardic C.M., Karolak L.A., Hannun Y.A. Programmed cell death induced by ceramide. Science (New York, NY) 1993;259(5102):1769–1771. doi: 10.1126/science.8456305. Epub 1993/03/19.PubMed PMID: 8456305. [DOI] [PubMed] [Google Scholar]
- 8.Venable M.E., Lee J.Y., Smyth M.J., Bielawska A., Obeid L.M. Role of ceramide in cellular senescence. J. Biol. Chem. 1995;270(51):30701–30708. doi: 10.1074/jbc.270.51.30701. Epub 1995/12/22. PubMed PMID: 8530509. [DOI] [PubMed] [Google Scholar]
- 9.Barth B.M., Cabot M.C., Kester M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med. Chem. 2011;11(9):911–919. doi: 10.2174/187152011797655177. Epub 2011/06/29. PubMed PMID: 21707481. [DOI] [PubMed] [Google Scholar]
- 10.Senchenkov A., Litvak D.A., Cabot M.C. Targeting ceramide metabolism--a strategy for overcoming drug resistance. J. Natl. Cancer Inst. 2001;93(5):347–357. doi: 10.1093/jnci/93.5.347. Epub 2001/03/10.PubMed PMID: 11238696. [DOI] [PubMed] [Google Scholar]
- 11.Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Tumor suppressive functions of ceramide: evidence and mechanisms. Apoptosis. 2015;20(5):689–711. doi: 10.1007/s10495-015-1109-1. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel S., Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat. Rev. Mol. Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. Epub 2003/05/03. PubMed PMID: 12728273. [DOI] [PubMed] [Google Scholar]
- 13.Nagahashi M., Tsuchida J., Moro K., Hasegawa M., Tatsuda K., Woelfel I.A. High levels of sphingolipids in human breast cancer. J. Surg. Res. 2016;204(2):435–444. doi: 10.1016/j.jss.2016.05.022. Epub 2016/08/28. PubMed PMID: 27565080; PubMed Central PMCID: PMCPMC5002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moro K., Kawaguchi T., Tsuchida J., Gabriel E., Qi Q., Yan L. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget. 2018;9(28):19874–19890. doi: 10.18632/oncotarget.24903. Epub 2018/05/08. PubMed PMID: 29731990; PubMed Central PMCID: PMCPMC5929433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moro K., Nagahashi M., Gabriel E., Takabe K., Wakai T. Clinical application of ceramide in cancer treatment. Breast Cancer (Tokyo, Japan) 2019;26(4):407–415. doi: 10.1007/s12282-019-00953-8. Epub 2019/04/10. PubMed PMID: 30963461; PubMed Central PMCID: PMCPMC7315770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Beckman B.S., Foroozesh M. A review of ceramide analogs as potential anticancer agents. Future Med. Chem. 2013;5(12):1405–1421. doi: 10.4155/fmc.13.107. Epub 2013/08/08 06:00. PubMed PMID: 23919551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponnapakam A.P., Liu J., Bhinge K.N., Drew B.A., Wang T.L., Antoon J.W. 3-Ketone-4,6-diene ceramide analogs exclusively induce apoptosis in chemo-resistant cancer cells. Bioorg. Med. Chem. 2014;22(4):1412–1420. doi: 10.1016/j.bmc.2013.12.065. Epub 2014/01/25 06:00. PubMed PMID: 24457089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Antoon J.W., Ponnapakkam A., Beckman B.S., Foroozesh M. Novel anti-viability ceramide analogs: design, synthesis, and structure-activity relationship studies of substituted (S)-2-(benzylideneamino)-3-hydroxy-N-tetradecylpropanamides. Bioorg. Med. Chem. 2010;18(14):5316–5322. doi: 10.1016/j.bmc.2010.05.044. Epub 2010/07/20. PubMed PMID: 20639111. [DOI] [PubMed] [Google Scholar]
- 19.Ponnapakkam T., Saulsberry T., Hill T., Hill-Odom M., Goyal N., Anbalagan M. Inhibition of breast tumor growth in mice after treatment with ceramide analog 315. Anticancer Drugs. 2018;29(9):898–903. doi: 10.1097/cad.0000000000000675. Epub 2018/07/26. PubMed PMID: 30044300; PubMed Central PMCID: PMCPMC6136967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foroozesh M., Goyal N., Jackson T., Do C., Booker S., Hill T. Optimization of scale-up synthesis of anti-cancer ceramide analog 315. J. Undergrad. Chem. Res. 2017;16(3):89–90. Epub 2017/07/01. PubMed PMID: 30220887; PubMed Central PMCID: PMCPMC6138050. [PMC free article] [PubMed] [Google Scholar]
- 21.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMC Vet. Res. 2020;16(1):242. doi: 10.1186/s12917-020-02451-y. Epub 2020/07/15. PubMed PMID: 32660541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell D.R., Burtles S.S., Fox B.W., Jodrell D.I., Connors T.A. Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br. J. Cancer. 1999;81(5):760–768. doi: 10.1038/sj.bjc.6690761. Epub 1999/11/11. PubMed PMID: 10555743; PubMed Central PMCID: PMCPMC2374299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm O., Zur B., Koch A., Tran N., Freyenhagen R., Hartmann M. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol. Chem. 2007;388(5):547–554. doi: 10.1515/bc.2007.061. Epub 2007/05/23. PubMed PMID: 17516851. [DOI] [PubMed] [Google Scholar]
- 24.Charles R. 2011. CD-1 Nude Mice Biochemistry. Charles River-Technical Sheet. [Google Scholar]
- 25.Guengerich F.P. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011;26(1):3–14. doi: 10.2133/dmpk.dmpk-10-rv-062. Epub 2010/10/28. PubMed PMID: 20978361; PubMed Central PMCID: PMCPMC4707670. [DOI] [PMC free article] [PubMed] [Google Scholar]