Abstract
BLZ-100 (tozuleristide) is an intraoperative fluorescent imaging agent that selectively detects malignant tissue and can be used in real time to guide tumor resection. The purpose of this study was to assess the safety, tolerability, and pharmacokinetics of BLZ-100 and to explore the pharmacodynamics of fluorescence imaging of skin tumors. In this first-in-human study, BLZ-100 was administered intravenously to 21 adult patients 2 days before excising known or suspected skin cancers. Doses were 1, 3, 6, 12, and 18 mg, with 3–6 patients/cohort. Fluorescence imaging was conducted before and up to 48 h after dosing. BLZ-100 was well tolerated. There were no serious adverse events, deaths, or discontinuations due to adverse events, and no maximum tolerated dose (MTD) was identified. Headache (n = 2) and nausea (n = 2) were the only BLZ-100 treatment-related adverse events reported for >1 patient. Median time to maximal serum concentration was <0.5 h. Exposure based on maximal serum concentrations increased in a greater than dose-proportional manner. For intermediate dose-levels (3–12 mg), 4 of 5 basal cell carcinomas and 4 of 4 melanomas were considered positive for BLZ-100 fluorescence. BLZ-100 was well tolerated at all dose levels tested and these results support further clinical testing of this imaging agent in surgical oncology settings. Clinicaltrials.gov: NCT02097875.
Keywords: Skin neoplasms, Fluorescent dyes, Cystine-knot miniproteins
Abbreviations: CTX, chlorotoxin; NMSC, non-melanoma skin cancer; NCI CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events; PK, pharmacokinetic(s)
1. Introduction
There has been rapid development of fluorescent molecular imaging of solid tumors for cancer diagnosis and image-guided surgery. Conventional anatomical and molecular imaging technologies such as magnetic resonance imaging, computed tomography and positron emission tomography have improved diagnostic accuracy and preoperative surgical planning. However, there are limitations. The spatial and temporal resolution are not sensitive enough to detect occult nodal tumors, and they require hazardous ionizing radiation. In addition, they are expensive to operate and require a complex infrastructure. Therefore, they cannot be applied easily in the surgical view field. In contrast, fluorescent molecular imaging can provide high spatial resolution and real-time capabilities and is a more adaptable imaging technology for tumor detection and image-guided surgery in a theater [1]. Many biomarkers that are unique to cancer cells including metabolism, hypoxia, secreted proteases, and cell proliferation have been characterized and used to distinguish between normal and cancer tissue.
As an imaging agent, indocyanine green (ICG) has historically been the most widely used fluorophore in oncogenic image-guided surgery [1]. When an ICG fluorophore is excited by light, it fluoresces in the near-infrared range, which is minimally absorbed by water or hemoglobin, making it well-suited as a non-specific agent for intraoperative imaging when used with an appropriate detection device. As such, an indocyanine green fluorophore has traditionally been also frequently employed in vascular and ophthalmic surgical procedures and for sentinel lymph node mapping [2].
Like ICG, Methylene blue or 5-Aminolevulinic acid (5-ALA) have been used for non-targeted optical imaging for many years. Methylene blue is often used to map sentinel nodes. In 2014, Tummers et al. showed methylene blue can be used intraoperatively to detect breast cancer via NIR [3]. Schucht et al. conducted a feasibility study where they used 5-ALA to image highly metabolic brain cancers to aim to achieve complete resection of the tumor [4]. Folate-FITC is the earliest example of ligand-based tumor imaging to improve intraoperative staging on folate receptor α-positive ovarian cancer. When the analog of folate was conjugated with NIR fluorescent dye, the sensitivity and definitive contrast was improved to 1 cm beneath the tissue [3].
Blaze Bioscience, Inc. has developed an agent, BLZ-100 (a.k.a. tozuleristide or Tumor Paint®), which is composed of a modified chlorotoxin peptide and a covalently attached ICG moiety as a candidate to provide real-time guidance during surgical tumor resection, for example in brain or breast cancer surgeries. The natural chlorotoxin peptide has been shown to bind tumors via a molecular interaction with protein components of cholesterol-rich lipid rafts, including Annexin A2 and matrix metalloproteinase 2 (MMP2) (reviewed in Ref. [5]). In preclinical imaging studies, BLZ-100 bound selectively to human and animal tumors with fluorescence signal and contrast being observed [[6], [7], [8]]. BLZ-100 was well-tolerated in definitive toxicology and safety pharmacology studies in rats and non-human primates [9]. The BLZ-100 Phase 1 clinical development program has included studies in adult glioma (NCT02234297), pediatric CNS tumors (NCT02462629), and breast cancer [10] and other solid tumors (NCT02496065). The results from Phase I adult glioma have been published in 2020 [11].
This report describes the first-in-human initial clinical experience of BLZ-100 in adult skin cancer patients. This patient population was chosen because skin cancer is more readily accessible and non-invasive imaging was possible to aid in pharmacodynamic assessments. The study was designed to assess the safety, tolerability, and pharmacokinetics (PK) of BLZ-100.
2. Materials & methods
2.1. Study design
This was a non-randomized, single-dose, open-label, dose-escalation/expansion study (Dec 2013–Mar 2015). After screening, eligible patients were sequentially assigned to a BLZ-100 dosing cohort (1, 3, 6, 12, or 18 mg). BLZ-100 was manufactured synthetically and supplied as sterile, frozen liquid drug product. BLZ-100 was administered by intravenous infusion in a Phase 1 residential unit (Q-Pharm Pty Ltd, Herston, QLD, Australia) on Day 1. The first patient in any cohort received BLZ-100 ≥ 2 h before the other patients. On Day 3, patients underwent study-specific procedures in the residential unit and then had their skin lesion(s) removed at a dermatology clinic (Veracity Clinical Research Pty Ltd, Specialist Connect, Woolloongabba, QLD, Australia). Follow-up safety visits occurred on Days 5 and 8. Clinical laboratory assessments (hematology, coagulation, and biochemistry), HIV and hepatitis serology, and pregnancy and follicle stimulating hormone (FSH) tests were performed by a central laboratory (Sullivan Nicolaides Pathology, Taringa, Brisbane, QLD, Australia).
Dose escalation used a 3 + 3 design with a Protocol Steering Committee reviewing the results of each dosing cohort. If 1 of the first 3 patients in a cohort had experienced a dose-limiting toxicity (DLT), 3 additional patients were to be treated. If ≥ 33% of the patients experienced a DLT, the dose below that was to be considered the maximum tolerated dose (MTD). Dose escalation was to continue until the MTD was determined or the highest dose level was tested. Adverse events were classified by Medical Dictionary for Regulatory Activities (MedDRA) preferred terms, version 17.0. DLTs were defined as any BLZ-100-related adverse event of ≥Grade 3 severity (according to National Cancer Institute's Common Terminology Criteria for Adverse Events [NCI CTCAE] version 4.0). Two expansion cohorts of 3 patients each were enrolled after the dose-escalation phase.
The study was conducted in accordance with the protocol, the Declaration of Helsinki and its amendments, and the requirements of national drug and data protection laws and was conducted in accordance with the principles of Good Clinical Practice as outlined in regulation 12AB(2)(a) of the Australian Therapeutic Goods Regulations and the National Statement on Ethical Conduct in Research Involving Humans and any other local guidelines or regulations. The study was approved by a Human Research Ethics Committee before patients were recruited and written informed consent was obtained from each participant or from his/her legally acceptable representative before any study-related procedures were performed.
2.2. Eligibility
Adult patients with known or suspected non-metastatic basal cell or squamous cell carcinoma (≥10 mm longest diameter) or non-metastatic melanoma (≥6 mm longest diameter) scheduled for excision, without advanced disease and who agreed to use an effective contraceptive for 30 days after treatment were eligible. Exclusion criteria included: life expectancy <6 months; Karnofsky Performance Status ≤70; history of hypersensitivity or allergic reactions requiring corticosteroids, epinephrine, and/or hospitalization; asthma (uncontrolled or requiring oral corticosteroids); clinically significant chronic inflammatory skin conditions; unstable angina, myocardial infarction, transient ischemic events, or stroke during 24 weeks before screening; uncontrolled hypertension; QTc prolongation >450 msec; receipt of photosensitizing drugs during 30 days before screening; HIV, HBV, or HCV positive serology; protocol-specified laboratory abnormalities; pregnancy; lactating/breastfeeding; known or suspected sensitivity to study products or excipients; or other conditions which the Investigator thought would adversely impact the patient or interpretation of study data. Patients were not to take medication which might have generated near-infrared fluorescence or take medication that, according to its product label, might have generated a photochemical reaction.
2.3. Disposition
Twenty-one eligible patients were assigned to dosing cohorts, treated with BLZ-100 (via 15-min IV infusion), and completed the study. Three patients were enrolled into each of the 5 pre-specified BLZ-100 dosing cohorts during the dose-escalation portion of the study (1, 3, 6, 12, and 18 mg). As dose limiting toxicities (DLTs) were not observed, dosing continued to the highest planned dose (18 mg BLZ-100). Based on the study emergent pharmacodynamic imaging and safety data, two dose levels (6 and 12 mg) were chosen for expansion into cohorts of 3 patients (to 6 patients total in each of these dose levels).
2.4. Objectives
The primary objective was to evaluate the safety and tolerability of BLZ-100 following a single IV administration. Secondary objectives were to determine the PK of BLZ-100 and a dose level for Phase 2 studies. Exploratory pharmacodynamic objectives included evaluating the fluorescent signal from skin tumor lesions and in urine and determining the expression of other potential biomarkers of response.
2.5. Pharmacokinetics (PK)
During the dose escalation portion of the study, blood samples were collected before and 1, 5, 15, 30, and 60 min, and 2, 4, 8, 12, 24, 48, 96, and 168 h after BLZ-100 infusions. During the expansion portion of the study, samples were collected before and 1, 5, 15, 30, 60, and 90 min, and 2, 3, 4, 6, 8, 12, and 24 h after BLZ-100 infusions. Urine samples were collected at baseline and 0–4, 4–8, 8–12, 12–24 and 24–48 h after the end of BLZ-100 infusions.
Serum samples were analyzed for BLZ-100 concentration with a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method at TetraQ (The University of Queensland, Herston, QLD, Australia). The resulting concentration vs time profiles for each subject were evaluated by noncompartmental analysis using WinNonlin Phoenix version 6.3 software (Pharsight Corporation, Cary, NC). The start of BLZ-100 administration was defined as T = 0. Any sample that was not collected or assayed was treated as missing. Any predose sample below the level of quantitation was assigned a value of zero; such samples that occurred after the time of Tmax, or between 2 quantifiable points, were treated as missing. No imputations were performed.
2.6. Pharmacodynamic imaging
Using the Fluobeam® 800 instrument (Fluoptics) with excitation at 750 nm and a long-pass filter (>800 nm), fluorescence image data were collected in situ from the target lesions during the 48 h after the BLZ-100 infusions. Fluorescence was measured for a 0.5 cm diameter circular region that included the clinically identified lesion and for five 0.5 cm diameter circular regions surrounding the lesion. For each time point (baseline [pre-dose] and 2, 4, 24, and 48 h after administration of BLZ-100), the amount of fluorescence was recorded for exposure times of 1, 10, 83, 167, 333, 500, and 1000 msec. The mean gray value for each of the 6 selected areas was measured using Image J software (http://imagej.nih.gov/ij/, accessed January 03, 2016). The average mean gray value for the 5 peri-lesional regions was used for comparisons and the ratio of lesional/peri-lesional signals were summarized qualitatively (“Was fluorescence signal higher in lesion than in peri-lesional region at various times after BLZ-100 dosing?” “Yes” or “No”).
2.7. Pathology correlations
Excised skin lesions were formalin fixed and examined using standard histopathology techniques. Clinical identification of the lesion was used to guide both pathology and image analysis.
2.8. Data management
Data management and statistical analyses were performed by Asia Pacific INC Research (Oakleigh, VIC, Australia). Results were summarized using descriptive statistics. Up to 30 patients were planned for enrollment, depending on the number of patients per cohort.
3. Results
3.1. Safety
The study population (Table 1) was older adults (median age of 58.0 and a range from 44 to 80 years) with clinically suspicious skin lesions/tumors. Baseline demographics (i.e., height, weight) were generally similar across the five cohorts. Approximately half the subjects were female (52.4%), and all were Caucasians. Twenty-eight treatment emergent adverse events were reported in 13 of the 21 subjects during the 7-day safety observation period. Adverse events occurring in more than 1 subject include procedural pain (n = 5/21, 24%), nausea (n = 3/21, 14%), dizziness (n = 2), headache, (n = 2) and muscle stiffness (n = 2). One case of procedural pain reached Grade 3 level, was unrelated to BLZ-100 and occurred during the dose expansion in a subject with a relatively large lesion (25 × 20 mm) on their forearm.
Table 1.
Patient and lesion characteristics.
| Cohort 1 1 mg BLZ-100 (N = 3) | Cohort 2 3 mg BLZ-100 (N = 3) | Cohort 3 6 mg BLZ-100 (N = 6) | Cohort 4 12 mg BLZ-100 (N = 6) | Cohort 5 18 mg BLZ-100 (N = 3) | All Subjects (N = 21) | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Median (range): | 53.0 (48, 67) | 67.0 (56, 72) | 56.5 (45, 78) | 53.5 (44, 68) | 60.0 (53, 80) | 58.0 (44, 80) |
| Gender, n (%) | ||||||
| Male: | 2 (66.7) | 2 (66.7) | 3 (50.0) | 2 (33.3) | 1 (33.3) | 10 (47.6) |
| Female: | 1 (33.3) | 1 (33.3) | 3 (50.0) | 4 (66.7) | 2 (66.7) | 11 (52.4) |
| Weight (kg) | ||||||
| Median (range): | 68.4 (62.9, 150.0) | 99.5 (88.6, 102.9) | 80.0 (55.9, 133.4) | 84.4 (64.5, 162.2) | 64.5 (51.1, 74.9) | 83.5 (51.1, 162.2) |
| Cohort 1 (N = 6 lesions) | Cohort 2 (N = 3 lesions) | Cohort 3 (N = 9 lesions) | Cohort 4 (N = 6 lesions) | Cohort 5 (N = 5 lesions) | All Lesions (N = 29) | |
| Final skin cancer diagnosis1, n (%) | ||||||
| BCC: | 2 (33.3) | 2 (66.6) | 4 (44.4) | 0 | 5 (100) | 13 (44.8) |
| SCC: | 2 (33.3) | 0 | 0 | 0 | 0 | 2 (6.9) |
| Melanoma: | 0 | 0 | 3 (33.3) | 1 (16.7) | 0 | 4 (13.8) |
| Other2: | 2 (33.3) | 1 (33.3) | 2 (22.2) | 5 (83.3) | 0 | 10 (34.5) |
max = maximum; min = minimum; BCC = basal cell carcinoma; SCC = squamous cell carcinoma.
1Final diagnoses were determined on Day 3 post-excision by histopathology. A total of 29 lesions from 21 patients were analyzed.
2Unidentified or non-cancerous.
Adverse events considered possibly related to BLZ-100 administration were reported in 5 subjects (24%) and all were grade 1 (Table 2). The two most common BLZ-10 related adverse events were headache (n = 2) and nausea (n = 2), reported by 1 subject at 6 mg and another at 18 mg dose levels. Other adverse events possibly related to BLZ-100 were abdominal pain, dysgeusia, frequent bowel movements, pruritus, and upper abdominal pain, all occurring in subjects at the 1 mg dose level. No treatment-emergent or clinically significant abnormal hematology or lab measures were observed. Subjects were frequently monitored after dosing for changes in vital signs, including heart rate, blood pressure and ECGs. BLZ-100 treatment had no effect on these parameters during the study period.
Table 2.
Adverse events considered possibly related to BLZ-100.
| Cohort 1 1 mg BLZ-100 (N = 3) n (%) |
Cohort 2 3 mg BLZ-100 (N = 3) n (%) |
Cohort 3 6 mg BLZ-100 (N = 6) n (%) |
Cohort 4 12 mg BLZ-100 (N = 6) n (%) |
Cohort 5 18 mg BLZ-100 (N = 3) n (%) |
All Subjects (N = 21) n (%) | |
|---|---|---|---|---|---|---|
| Subjects with at least one AE considered possibly related to BLZ-100 | 3 (100) | 0 (0.0) | 1 (16.7) | 0 (0) | 1 (33.3) | 5 (23.8) |
| Headache | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (33.3) | 2 (9.5) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (33.3) | 2 (9.5) |
| Abdominal pain | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Abdominal pain upper | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Dysgeusia | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Frequent bowel movements | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
| Pruritus | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) |
This table presents the number of subjects with an adverse event considered possibly related to BLZ-100 and the percentage of total subjects. Percentages are based on the total number of subjects. Events are presented by decreasing incidence in the “All Subjects” column.
Adverse events are coded according to Medical Dictionary for Medical Activities (MedDRA) Version 17.1 system organ class and preferred terms.
3.2. PK
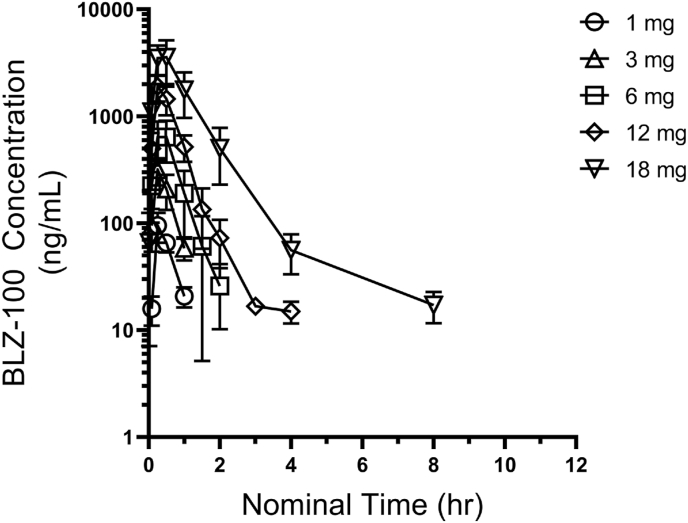
After a single 15-min IV infusion of BLZ-100, mean BLZ-100 serum concentrations were measurable until 1 h after dosing at the 1 and 3 mg dose levels, and until 2, 4, and 8 h after dosing at the 6, 12, and 18 mg dose levels, respectively (Fig. 1).
Fig. 1.
Mean (SD) Serum BLZ-100 Concentration vs Time Profiles after a Single IV Infusion of BLZ-100.
The 1 mg dose level had too few post dose data points to estimate pharmacokinetic parameters. Mean half-life (t1/2) was consistent in the 3 and 6 mg dose cohorts (0.33 and 0.36 h, respectively), but had longer observed values in the 12 and 18 mg cohorts (0.50 and 1.75 h, respectively). This corresponded to a decrease in clearance (CL) at the 12 and 18 mg dose levels relative to the 3 and 6 mg dose levels. The steady-state volume of distribution (Vss) did not appear to change substantially with dose. Exposure based on maximum observed concentration (Cmax) and area under the plasma concentration vs time curve from 0 to the last measurable time point (AUC0-t) increased in a greater than dose-proportional manner. PK parameters are summarized in Table 3.
Table 3.
Pharmacokinetic parameters after a single IV infusion of BLZ-100.
| Cohort 1 |
Cohort 2 |
Cohort 3 |
Cohort 4 |
Cohort 5 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg BLZ-100 (N = 3) |
3 mg BLZ-100 (N = 3) |
6 mg BLZ-100 (N = 6) |
12 mg BLZ-100 (N = 6) |
18 mg BLZ-100 (N = 3) |
|||||||||||
| Parameter, units | n | Mean | SD | na | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD |
| t1/2, hours | 0 | NE | – | 1 | 0.33 | – | 6 | 0.36 | 0.07 | 6 | 0.50 | 0.12 | 2 | 1.75 | – |
| CL, mL/hour | 0 | NE | – | 1 | 10800 | – | 6 | 12100 | 4460 | 6 | 8830 | 3060 | 2 | 5260 | – |
| Vss, mL | 0 | NE | – | 1 | 4770 | – | 6 | 5710 | 1530 | 6 | 4740 | 1100 | 2 | 6110 | – |
| Cmax, ng/mL | 3 | 95.5 | 29.7 | 3 | 315 | 89.5 | 6 | 783 | 208 | 6 | 1900 | 529 | 3 | 3830 | 1230 |
| Tlast, hours | 3 | 1.00 | 0.00 | 3 | 1.34 | 0.57 | 6 | 2.17 | 0.41 | 6 | 3.34 | 0.82 | 3 | 8.02 | 3.98 |
| AUC0-t, hr*ng/mL | 0 | NE | – | 3 | 184 | 77.5 | 6 | 548 | 197 | 6 | 1470 | 450 | 3 | 4430 | 1790 |
a. n = number of subjects for whom data were sufficient for estimation of each parameter. AUC0-t = area under the curve from 0 to the last measurable time point; CL = apparent clearance; Cmax = maximum observed concentration; NE = not estimable; SD = standard deviation; t1/2 = half-life based on the terminal portion of the concentration vs time curve; Tlast = time of the last observed measurable concentration; Vss = apparent steady-state volume of distribution.
3.3. Tumor imaging
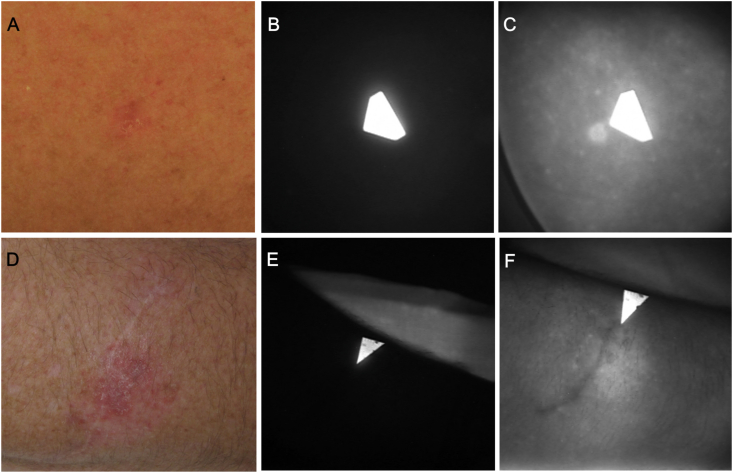
Fluorescence image data were collected in situ at several time points during the 48 h after the BLZ-100 infusions using a Fluobeam® 800 imaging device. Examples of imaging results from two patients, one with basal cell carcinoma treated at 3 mg and another with amelanotic melanoma in situ in the 6 mg BLZ-100 cohort, are provided in Fig. 2. Fluorescence was clearly visible in the lesions at 2 h after the BLZ-100 infusion.
Fig. 2.
NIR imaging of a basal cell carcinoma from the 3 mg cohort (top row) and an amelanotic melanoma in situ from the 6 mg cohort (bottom row). (A, D) Clinical photographs. The image in panel A was adjusted for color balance and brightness. (B, E) NIR images taken before BLZ-100 administration. The arrows are paper pointers with fluorescing pigment indicating the clinically identified lesion. (C, F) NIR images (500 msec exposures) acquired 2 h after dosing with BLZ-100. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fluorescence imaging data were available for 29 known or suspected skin cancer lesions from 21 patients (Table 1). Disease status of the lesions was confirmed after excision on Day 3 by histopathology. The lesional and peri-lesional fluorescence signals increased with the increased dose. Likewise, the duration of fluorescence signal appears to last longer with the higher BLZ-100 doses. The image analysis was able to illustrate this trend and to also identify lesions that preferentially accumulated BLZ-100. Signals in the 1 mg cohort were generally low and resulted in no obvious contrast between lesional and peri-lesional skin. In the 18 mg cohort, signal from the surrounding skin had increased such that discriminating the target lesion from peri-lesional skin was difficult, although contrast was noted in 2 of 5 lesions (both BCC) in this cohort. For intermediate doses (3–12 mg), 5 of 6 basal cell carcinomas were highlighted by fluorescence at 1 or more time points after the infusion of BLZ-100, as were 4 of 4 melanomas, and 4 of 7 other lesions (including 1 with actinic keratosis and 1 nevus with melanocytic atypia) (Table 4).
Table 4.
Pharmacodynamics summary for 3–12 mg BLZ-100 dose cohorts.
| Subject | Lesion No. | Dose (mg) | Pathology of formalin-fixed samples | BLZ-100 uptake in lesion | Kinetics – Contrast observed: |
|
|---|---|---|---|---|---|---|
| Day of dosing | Day after dosing | |||||
| R201 | 1 | 3 | BCC | Yes | Yes | Yes |
| R202 | 1 | 3 | Scar; no residual viable lesion identified | No | – | – |
| R203 | 1 | 3 | BCC | Yes | Yes | Yes |
| R301 | 1 | 6 | BCC | Yes | Yes | No |
| 2 | Minor scar + upper dermal lymphocytic infiltration | Yes | Yes | No | ||
| R302 | 1 | 6 | BCC | No | – | – |
| R303d | 1 | 6 | BCC | Yes | Yes | Yes |
| 2 | BCC | Yes | Yes | No | ||
| R304 | 1 | 6 | Melanoma in situ | Yes | Yes | Yes |
| R305 | 1 | 6 | Melanoma in situ | Yes | No | Yes |
| R306 | 1 | 6 | Melanoma in situ | Yes | Yes | No |
| R401 | 1 | 12 | Multifocal lichenoid solar keratosis | No | – | – |
| R402 | 1 | 12 | No dysplasia, tumor, or other specific lesion identified | Yes | Yes | Yes |
| R403 | 1 | 12 | Dermal scar; mild junctional melanocytic atypia | Yes | Yes | Yes |
| R404 | 1 | 12 | No residual melanoma identified | No | – | – |
| R405 | 1 | 12 | No residual melanoma; incidental solar keratosis; large cell acanthoma present | Yes | Yes | Yes |
| R406 | 1 | 12 | Biopsy site and residual melanoma in situ (level 1) | Yes | Yes | Yes |
4. Discussion
Fluorescence-guided surgery is a tremendously active field of investigation, and a variety of imaging agents have reached clinical investigation stages in the past few years. Strategies include targeting with antibodies to known tumor antigens such as EGFR or CEA, targeting with peptides such as cRGD or small molecules such as folate that interact with tumor antigens or tumor vasculature, creating cleavable constructs that are activated by proteases within the tumor milieu, creating pH-sensitive constructs that are activated in the low pH environment of the tumor, metabolic labeling with 5-ALA, as well as continued study of non-targeted agents that accumulate in tumor tissue due to the enhanced permeability and retention (EPR) effect (reviewed in Refs. [[12], [13], [14]]). Indications with high unmet need, such as head and neck cancer and brain tumors, have received particularly intense attention (reviewed in Refs. [[15], [16], [17]]). Factors that will influence clinical utility include safety, dose schedule, and specificity for a given tumor type.
This study was the initial clinical experience with BLZ-100, an investigational tumor targeting imaging agent. BLZ-100 was well tolerated in this study; no maximum tolerated dose (MTD) was identified and dosing continued to 18 mg, the highest planned dose. Headache (n = 2) and nausea (n = 2) were the only BLZ-100 treatment-related adverse events reported for more than one patient. Other adverse events, such as abdominal pain and pruritis, were considered possibly related to BLZ-100 but were reported only once in this study and at the lowest dose tested, suggesting these adverse events were probably not related to BLZ-100 dosing. It is worth noting that administration of BLZ-100 was not associated with immediate infusion like reactions, such as those seen with some fluorescence imaging agents under development for oncologic surgical applications [18].
Exposure to BLZ-100 based on Cmax and AUC0-t appeared to increase in a greater than dose-proportional manner, potentially due to decreased clearance at the higher dose levels. However, the observed differences in PK parameters may be due, in part, to a more complete characterization of the PK profiles at the higher dose levels. As these results were based on ≤6 patients for each cohort, dose-related differences should be interpreted with caution.
Imaging of the benign and malignant skin neoplasms appeared to be dose related. In the 1 mg BLZ-100 cohort, the signal was generally low, at or near the level of detection of the imaging device, making visual assessments of contrast difficult and suggesting that the 1 mg dose may be too low for effective imaging. Further, both histopathologically confirmed squamous cell carcinomas were in the 1 mg cohort, such that the potential for BLZ-100 to highlight this tumor type is unclear and should be studied further at higher doses. In the 18 mg BLZ-100 cohort, the background signal from the surrounding skin had increased such that discriminating tumor and peritumoral tissue was difficult. The pharmacokinetic exposure at the 18 mg was also 3 times the next lowest dose of 12 mg, which likely contributed to the higher background seen at 18 mg. There were also cases of the target lesion displaying no fluorescent contrast because no tumor at all was found by histopathology. The small sample size and use of several dose levels in this study precludes drawing meaningful conclusions as to the accuracy of BLZ-100 to identify skin tumors; however, the data suggest there is sufficient potential for this approach to warrant further study. In particular for skin cancer applications, it would be useful to further study doses between 3 and 12 mg, simplify the drug administration to an IV bolus (currently being tested in other clinical trials with BLZ-100), consider imaging sooner than 2 h post dose, and explore imaging devices more specific to the optical properties of BLZ-100.
The results of this study support the safety of BLZ-100 at doses up to 18 mg after a 15-min IV infusion. The half-life appears to be 20–30 min and fluorescence imaging is feasible within a few hours of dosing at doses between 3 and 12 mg. The ability to serially image the cutaneous lesions provided data regarding signal and contrast durability. These data were important in the design of subsequent clinical trials with BLZ-100, which demonstrated efficacy with dosing the day before or day of surgery in CNS [11] or non-CNS [10] tumors. A Phase II/III study in pediatric CNS tumors is ongoing.
Declaration of competing interest
DMM, JPN, and LI are employees or former employees of and hold equity in Blaze Bioscience, Inc. ML, KBB, and GB are consultants for Blaze Bioscience, Inc. and Blaze Bioscience Australia Pty Ltd. JMO is a co-founder of and holds equity in Blaze Bioscience, Inc. LS was a paid clinical investigator on the trial. HPS is a shareholder of MoleMap NZ Limited and e-derm consult GmbH and undertakes regular teledermatological reporting for both companies. HPS is a Medical Consultant for Canfield Scientific Inc., MetaOptima and Revenio Research Oy and also a Medical Advisor for First Derm.
Acknowledgments
We thank the patients, their families, and the clinical site personnel. We thank Sally Kinrade, Kurt Davidson, Teagan Holland, Lauren Kunde, Suzanne Elliott, and Carolyn Gombotz for clinical support. We thank Kate Loughney for medical writing assistance.
References
- 1.Harmsen S., Teraphongphom N., Tweedle M.F., Basilion J.P., Rosenthal E.L. Optical surgical navigation for precision in tumor resections. Mol. Imag. Biol. 2017;19:357–362. doi: 10.1007/s11307-017-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alander J.T., Kaartinen I., Laakso A., Pätilä T., Spillmann T., Tuchin V.V., Venermo M., Välisuo P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imag. 2012:940585. doi: 10.1155/2012/940585. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tummers Q.R., Verbeek F.P., Schaafsma B.E., Boonstra M.C., van der Vorst J.R., Liefers G.J., van de Velde C.J., Frangioni J.V., Vahrmeijer A.L. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur. J. Surg. Oncol. 2014;40:850–858. doi: 10.1016/j.ejso.2014.02.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schucht P., Murek M., Jilch A., Seidel K., Hewer E., Wiest R., Raabe A., Beck J. Early re-do surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PloS One. 2013;8 doi: 10.1371/journal.pone.0079846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanyile S., Masamba P., Oyinloye B.E., Mbatha L.S., Kappo A.P. Current biochemical applications and future prospects of chlorotoxin in cancer diagnostics and therapeutics. Adv. Pharmaceut. Bull. 2019;9:510–520. doi: 10.15171/apb.2019.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik F.M., Hansen S., Knoblaugh S.E., Sahetya D., Mitchell R.M., Xu C., Olson J.M., Parrish-Novak J., Mendez E. Fluorescence identification of head and neck squamous cell carcinoma and high-risk oral dysplasia with BLZ-100, a chlorotoxin-indocyanine green conjugate. JAMA Otolaryngol. Head Neck Surg. 2016;142:330–338. doi: 10.1001/jamaoto.2015.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel J., Kennedy K.C., Dernell W.S., Hansen S., Wiss V., Stroud M.R., Molho J.I., Knoblaugh S.E., Meganck J., Olson J.M., Rice B., Parrish-Novak J. Preclinical validation of the utility of BLZ-100 in providing fluorescence contrast for imaging spontaneous solid tumors. Canc. Res. 2015;75:4283–4291. doi: 10.1158/0008-5472.CAN-15-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butte P.V., Mamelak A., Parrish-Novak J., Drazin D., Shweikeh F., Gangalum P.R., Chesnokova A., Ljubimova J.Y., Black K. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus. 2014;36:E1. doi: 10.3171/2013.11.FOCUS13497. [DOI] [PubMed] [Google Scholar]
- 9.Parrish-Novak J., Byrnes-Blake K., Lalayeva N., Burleson S., Fidel J., Gilmore R., Gayheart-Walsten P., Bricker G.A., Crumb W.J., Jr., Tarlo K.S., Hansen S., Wiss V., Malta E., Dernell W.S., Olson J.M., Miller D.M. Nonclinical profile of BLZ-100, a tumor-targeting fluorescent imaging agent. Int. J. Toxicol. 2017;36:104–112. doi: 10.1177/1091581817697685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dintzis S.M., Hansen S., Harrington K.M., Tan L.C., Miller D.M., Ishak L., Parrish-Novak J., Kittle D., Perry J., Gombotz C., Fortney T., Porenta S., Hales L., Calhoun K.E., Anderson B.O., Javid S.H., Byrd D.R. Real-time visualization of breast carcinoma in pathology specimens from patients receiving fluorescent tumor-marking agent tozuleristide. Arch. Pathol. Lab Med. 2019;143:1076–1083. doi: 10.5858/arpa.2018-0197-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patil C.G., Walker D.G., Miller D.M., Butte P., Morrison B., Kittle D.S., Hansen S.J., Nufer K.L., Byrnes-Blake K.A., Yamada M., Lin L.L., Pham K., Perry J., Parrish-Novak J., Ishak L., Prow T., Black K., Mamelak A.N. Phase 1 safety, pharmacokinetics, and fluorescence imaging study of tozuleristide (BLZ-100) in adults with newly diagnosed or recurrent gliomas. Neurosurgery. 2019;85:E641–E649. doi: 10.1093/neuros/nyz125. [DOI] [PubMed] [Google Scholar]
- 12.Azari F., Kennedy G., Bernstein E., Hadjipanayis C., Vahrmeijer A., Smith B., Rosenthal E., Sumer B., Tian J., Henderson E., Lee A., Nguyen Q., Gibbs S., Pogue B., Orringer D., Charalampaki C., Martin L., Tanyi J., Lee M., Lee J.Y., Singhal S. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. J. Biomed. Opt. 2021;26 doi: 10.1117/1.JBO.26.5.050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debie P., Hernot S. Emerging fluorescent molecular tracers to guide intra-operative surgical decision-making. Front. Pharmacol. 2019;10:510. doi: 10.3389/fphar.2019.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R., Xu Y., Xu K., Dai Z. Current trends and key considerations in the clinical translation of targeted fluorescent probes for intraoperative navigation. Aggregate. 2021:1–23. 2021. [Google Scholar]
- 15.Lee Y.J., Krishnan G., Nishio N., van den Berg N.S., Lu G., Martin B.A., van Keulen S., Colevas A.D., Kapoor S., Liu J.T.C., Rosenthal E.L. Intraoperative fluorescence-guided surgery in head and neck squamous cell carcinoma. Laryngoscope. 2021;131:529–534. doi: 10.1002/lary.28822. [DOI] [PubMed] [Google Scholar]
- 16.Belykh E., Martirosyan N.L., Yagmurlu K., Miller E.J., Eschbacher J.M., Izadyyazdanabadi M., Bardonova L.A., Byvaltsev V.A., Nakaji P., Preul M.C. Intraoperative fluorescence imaging for personalized brain tumor resection: current state and future directions. Front Surg. 2016;3:55. doi: 10.3389/fsurg.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schupper A.J., Yong R.L., Hadjipanayis C.G. The neurosurgeon's armamentarium for gliomas: an update on intraoperative technologies to improve extent of resection. J. Clin. Med. 2021;10:236. doi: 10.3390/jcm10020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoogstins C.E., Tummers Q.R., Gaarenstroom K.N., de Kroon C.D., Trimbos J.B., Bosse T., Smit V.T., Vuyk J., van de Velde C.J., Cohen A.F., Low P.S., Burggraaf J., Vahrmeijer A.L. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin. Canc. Res. 2016;22:2929–2938. doi: 10.1158/1078-0432.CCR-15-2640. [DOI] [PubMed] [Google Scholar]