Graphical abstract
Keywords: Toombak, Smokeless tobacco, Sudan, pH, Microbiome, Metabolome, Heavy metal, Scanning electron microscopy, Composition
Highlights
-
•
Toombak, a form of moist smokeless tobacco from Sudan is placed as a dip in the oral cavity most commonly used by males.
-
•
The microbiome of Toombak predominantly consists of the phyla, Firmicutes and Actinobacteria while abundant species include Corynebacterium casei, Atopostipes suicloacalis and Oceanobacillus chironomi.
-
•
High concentrations of iron, volatile aldehydes and tobacco specific nitrosamines were found in Toombak and can lead to toxicity.
-
•
Toombak has a non-homogenous abrasive surface with a high sodium level in the ready to buy form that can damage the oral mucosa.
-
•
New measures must be taken in Sudan to limit harmful compounds in Toombak.
Abstract
Toombak is a smokeless tobacco produced from the Nicotiana rustica tobacco plant from Sudan. Pre-prepared and ready to buy Toombak samples were analysed using mass spectrometry (heavy metals), gas and liquid chromatography (metabolomics), 16S rRNA metagenomic sequencing (microbiome) and Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) and pH analysis.
Chromium, cobalt, and copper were high in the pre-prepared form of Toombak while iron, tobacco specific nitrosamines (TSNAs), formaldehyde and acetaldehyde were high in both types. Firmicutes and Actinobacteria dominated Toombak. Samples of ready to buy Toombak showed inter-variational differences depending on place of purchase. We found Virgibacillus were increased in the pre-prepared form while Corynebacterium casei, Atopococus tabaci, Atopostipes suicloacalis, Oceanobacillus chironomi and Staphylococcus gallinarum were the most abundant species in the ready to buy forms. PICRUSt analysis highlighted increased activity of metal transport systems in the ready to buy samples as well as an antibiotic transport system. SEM-EDX highlighted large non-homogenous, irregular particles with increased sodium, while pH of samples was in the alkaline range.
The final composition of Toombak is affected by its method of preparation and the end product has the potential to impart many negative consequences on the health of its users. TSNA levels observed in Toombak were some of the highest in the world while the micro-environment of Toombak supports a distinct microbiota profile.
1. Introduction
Smokeless tobacco refers to the use of unburnt or pyrolysis free tobacco, differing around the world in tobacco plant source, nicotine content and additives. Products are often applied orally (chewed, sucked, or placed passively to the gingivae) and include forms such as chewing tobacco, moist forms, dry snuff, and snus, while fine powdered types are administered nasally. It is thought that more than 300 million people use a form of smokeless tobacco worldwide, in over 70 countries that include Southeast Asia, many regions of Africa, the Middle East, Europe (Scandinavian countries), the Americas and Russia [1]. In Sudan, there are an estimated 4–10 million users of Toombak; a moist smokeless tobacco used primarily by males. Idris et al. (1998) concluded that 60 % of Sudanese households contain at least one family member with Toombak use, while it has been estimated that Toombak prevalence of use amongst adolescents, young adults and those above 60 years of age is; 34 %, 32 % and 47 % respectively [2].
Toombak is produced from the Solanaceae species, Nicotiana rustica, a plant with characteristic yellow flowers, that contains up to nine times more nicotine compared to Nicotiana tabacum, that is more widely utilised in the production of tobacco products worldwide [3]. Nicotiana rustica is also used to make smokeless tobacco products in Turkey (Maras powder) [4], South America (Rapé) [5], Vietnam (Thuốc lào) and Russia (Makhorka). Plantation begins around November in western Sudan, leaves are cultivated from February to March, and air curing occurs during April and May. During this latter stage, temperatures can reach 45 °C–60 °C. Leaves change from yellow to brown in a process of natural or ‘compost’ like fermentation and the Toombak is then further prepared by milling to non-homogenous particles.
Toombak production is finalised by the addition of alkaline carbonates, flavourings, and other additives. Sodium bicarbonate is commonly incorporated to the final mix in order to improve taste and promote effective nicotine absorption into the bloodstream. There are limited regulatory laws for Toombak production and sale and so it is highly likely that it is contaminated by human, animal, soil, and other environmental factors, which have not been fully reported.
Toombak is sold in polythene bags (Fig. 1A), is a dark brown colour with a moist coarse consistency and has a distinct pungent aroma (Fig. 1B). A regular Toombak user would place approximately 6−10 g of a dip of Toombak in various areas of the oral cavity but mainly the upper or lower mucolabial folds and gingivobuccal sulci (Figs. 1C and 1D) as well as the floor of the mouth. The dip is often replaced around 10–15 times per day, usually when the nicotine effect becomes bland, and it may also be retained during sleep [6].
Fig. 1.
A: the smokeless tobacco, Toombak locally known as ‘sultan alkeef’ in Sudan is sold in common polythene bags from local vendors around the country. B: Toombak is of a brown coarse structure ranging from finely ground to macro-sized particles. C: a user placing the Toombak dip in the upper and D: the lower mucolabial folds.
Smokeless tobacco use including Toombak has been attributed to cardiovascular disease, male infertility, psychological disturbances, periodontal disease, premalignant lesions and oral, oro-pharyngeal, oesophageal and numerous other cancers [[7], [8], [9]]. 81% of oral cancers in Sudan are thought to occur amongst Toombak users and present at the site of Toombak placement [10]. In fact, in a recent systematic review it was reported that Sudan has the second highest oral cancer incidence in the Middle East, primarily attributed to Toombak use [11].
Current knowledge is lacking as to why the properties of Toombak adversely affect human health. This study was undertaken to better understand the physical, biological and chemical properties of Toombak, including its microbial-ecological niche and how these findings may reflect on its final composition and users health.
2. Methods
2.1. Sample collection
Two types of Toombak were collected; pre-prepared and ready to buy samples. S01 was sourced from Darfur, western Sudan, just after completion of the fermentation process in order to evaluate the smokeless tobacco prior to the preparatory stages. Twenty samples of ready to buy Toombak (S2-S21), were then collected from different vendors of Toombak sale; S2-S11 from Khartoum North and S12-S21 from Omdurman located in the capital Khartoum, Sudan. These were chosen to represent common points of Toombak purchase in these two cities.
Ready to buy samples were initially stored in a similar fashion to how Toombak users would store their product, at room temperature in airtight polythene bags. Samples were then shipped to Cork, Ireland, less than one week after sample collection and refrigerated, to prevent any changes to the product. The pre-prepared form was also refrigerated.
2.2. Heavy metals
Analysis of nine heavy metals/major cations (mg/kg) was performed on two of the 21 samples; the pre-prepared and a ready to buy sample of Toombak by ALS Life Sciences Ltd, (ALS Czech Republic). Samples were homogenized and mineralized by acids and hydrogen peroxide prior to analysis. Arsenic, cadmium, chromium, cobalt, copper, lead and nickel were analysed using mass spectrometry with inductively coupled plasma. Iron analysis followed the same protocol although atomic emission spectrometry was used while mercury was determined by atomic absorption spectrometry.
2.3. Metabolomics
Metabolomic analysis was performed on all samples of Toombak by MS-OMICS (Vedbaek, Denmark). Samples were extracted by shaking (2 rpm/sec) for one hour in 100 mM ammonium acetate buffer. For quality control, a mixed pooled sample was prepared by taking a small aliquot from each sample. This sample was analysed at regular intervals throughout the sequence. Matrix effects were tested for quantified compounds by spiking the quality control sample at a minimum of two levels. The volatile organic compound method is a gas chromatography – mass spectrometry method targeted to volatile compounds using a low-mid polarity column. Urethane and N-nitrosodimethylamine were analysed without derivatization by adding methyl tert-butyl ether (MTBE) to the samples and injecting the organic phase to the instrument.
Formaldehyde and acetaldehyde required derivatization which was performed using a protocol similar to the one described by Bao et al. (DOI: 10.2478/cttr-2014−0017) [12]. The raw gas chromatography – mass spectrometry data were processed by software developed by MS-OMICS and collaborators which use a powerful PARAFAC2 model.
For liquid chromatography metabolites, samples were diluted ten times in eluent A prior to analysis. The data were analysed using both a targeted and an untargeted approach. The targeted approach was used to extract the response of compounds included in a standard list covering 85 compounds. For the untargeted approach, feature extraction was conducted using mzMine, an open-source software for mass spectrometry data processing.
Levels of the heavy metals arsenic, cadmium, chromium, lead, mercury and nickel as well as formaldehyde, acetaldehyde, and the tobacco specific nitrosamines (TSNAs) in Toombak were compared with the GothiaTek standard for Swedish snus and chewing tobacco production adopted by the members of the trade organisation, European Smokeless Tobacco Council (ESTOC) and is the regulatory manufacturing standard for the production of Swedish snus and chewing tobacco [13]. The GothiaTek standard ensures the levels of constituents in smokeless tobacco are present in amounts that do not pose harm to the user [14]. Where the components in Toombak could not be compared with this standard, they were compared with other findings in the literature.
2.4. Microbiome
Thirteen samples were analysed by metagenomic sequencing for microbiome composition. These were S01, the pre-prepared form, and the ready to buy samples; S2−11, from Khartoum North and S12 -S13 from Omdurman.
2.4.1. DNA extraction
Toombak samples and a positive and negative control, were subjected to the DNA extraction protocol provided by the manufacturer of the DNeasy Powersoil Kit (Qiagen, Hilden, Germany). Eluted DNA was transferred to Eppendorf tubes and frozen at −80 °C for further analysis.
2.4.2. Amplicon sequencing
Amplicon PCR was carried out on the Toombak DNA extracted samples using 2x KAPA HiFi HotStart Ready Mix and universal forward and reverse primers, to target the V3 – V4 hypervariable regions of 16S rRNA selected for next generation sequencing based diversity studies.
The following thermal cycling conditions were used: 3 min of initial denaturation at 95 °C followed by 30 cycles each at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and then at 72 °C for 5 min. Electrophoresis using 1.5 % agarose gel with Invitrogen DNA loading dye (Thermo Fisher Scientific), verified base pair size which was expected at 550 base pairs. All samples were positive on the agarose gel while the control samples were negative. This was followed by a clean up stage using AMPure XP beads (Beckman Coulter) to purify the amplicon from free primers and primer dimer species.
Index PCR was then continued by attaching dual indices and Illumina sequencing adapters using Nextera XT index kits and the following PCR thermal cycling conditions were used: 3 min of initial denaturation at 95 °C followed by 8 cycles each at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and then at 72 °C for 5 min. AMPure XP beads (Beckman Coulter) were utilised for final clean-up, and the library quantified using the Qubit® 2.0 Fluorometer and Qubit dsDNA high sensitivity assay kit (Thermo Fisher Scientific). Samples were sequenced using the V3-V4 regions of the 16S rRNA gene on an Illumina MiSeq device 2 × 300 platform (Illumina, Inc. San Diego).
2.4.3. Bioinformatic analysis
The 300 base paired end FastQ products generated from 16S rRNA sequencing were merged using FLASH (fast length adjustment of short reads) using default parameters [15]. QIIME’s split_libraries_fastq.py script was used for demultiplexing and filtering of the FastQ sequence data. Further quality filtering was performed using the USEARCH analysis tool. Briefly, single unique reads were removed following denoising, chimera removal and grouping into operational taxonomic units (OTUs) at 97 % similarity and was performed using USEARCH v7 (64 bit) [16]. Alignment of OTUs was performed using Pynast (PyNAST: python nearest alignment space termination), a flexible tool for aligning sequences to a template alignment [17]. Further, assigning of taxonomic ranks was performed by using the Basic Local Alignment Search Tool (BLAST®), against the SILVA SSURef database release 132 [18]. The U.S National Library of Medicine, National Centre for Biotechnology Information was then accessed in order to assign species identity to those FASTA sequences with > 98 % percentage homogeneity with BLASTn [19].
2.4.4. Metagenomic statistical analysis
Statistical analysis with graphical outputs was undertaken using Microbiome AnalystR package. Data normalisation followed by compositional and functional profiling, comparative analysis, and Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) for the Toombak samples [20, 21] were performed.
Quantitative visualisation using stacked bar charts representing abundant phyla, families, and genera within all Toombak samples was achieved. Alpha (α) diversity was used to identify community profile and any significant variation in the microbiome of Toombak. Beta (β) diversity evaluated the relative abundance of OTUs and the clustering and outliers amongst samples. This was formulated using Bray – Curtis matrices and visualised by principal coordinate analysis (PCoA) in the ready to buy forms only. Correlations were assessed through pattern search between the two ready to buy groups while classic univariate analysis (ANOVA) evaluated the variation of microbiome between Khartoum North and Omdurman samples. DEseq2 was also employed to assess differential abundance between the market groups using shrinkage estimation for dispersion and fold change to improve estimates of differential expression [22]. False detection rates (FDR) or q values were reported to limit false positive results [23].
2.5. Scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX)
Structural analysis was achieved by scanning electron microscopy at 35, 800 and 4000 x magnification of two of the 21 samples; S01 and a ready to buy sample of Toombak. A form of snuff bought in the Republic of Ireland (McChrystals snuff), was also used as a comparison in this assessment. Samples were mounted onto aluminium stubs using double sided carbon tape. Samples were sputter-coated with a 5 nm layer of gold palladium (80:20) using a Quorum Q150 RES Sputter Coating System (Quorum Technologies, UK), before being examined using a JEOL JSM 5510 Scanning Electron Microscope (JEOL Ltd., Japan). Digital electron micrographs were obtained of areas of interest. Energy dispersive X-ray spectroscopy (EDX) analysis was performed using an INCA x-sight EDX Detector (Oxford Instruments, Buckinghamshire, UK) on samples that were not sputter coated to determine the characteristic elements within the sample.
2.6. pH
pH was determined on all 21 samples by weighing equal parts of Toombak (3 g) at room temperature and adding 3 mL dH2O to each sample. The mixtures were vortexed for 5 min, decanted into separate Eppendorf tubes and centrifuged at 21,380 Χ g for 10 min. The supernatants were then transferred to fresh bijou tubes and analysed using a laboratory grade pH meter that was fully calibrated.
3. Results
3.1. Heavy metals
Arsenic, cadmium, lead, and mercury were at acceptable levels in the Toombak samples. Nickel levels were at the upper range of acceptable limits while chromium, cobalt and copper were high in the pre-prepared form. Iron was found to be the most abundant heavy metal in Toombak. The International Agency for Research on Cancer (IARC) categorisation for heavy metal carcinogen classification is listed alongside the GothiaTek standard or references from the literature (Table 1).
Table 1.
Heavy metal content in one pre-prepared and one ready to buy sample of Toombak.
| Metals/ major cations | Limit of reporting | Pre-prepared sample (mg/kg) | Ready to buy sample (mg/kg) | GothiaTek standard limits (mg/kg) | Other reference | IARC carcinogen classification |
|---|---|---|---|---|---|---|
| Arsenic | 0.10 | 0.12 | < 0.10 | 0.25 | – | Group 1 |
| Cadmium | 0.04 | 0.34 | 0.09 | 0.5 | – | Group 1 |
| Chromium | 0.20 | 1.86 | 1.50 | 1.5 | – | Group 1 |
| Cobalt | 0.05 | 0.99 | 0.64 | – | 0.98 mg/kg [25] | Group 2B |
| Iron | 1 | 1660 | 1270 | – | 8–18 mg/d* [26] | Group 2B |
| Copper | 0.10 | 4.80 | 2.73 | – | 2-3 mg/d* [27] | Group 3 |
| Lead | 0.05 | 0.39 | 0.35 | 1.0 | – | Group 2B |
| Mercury | 0.003 | 0.014 | 0.004 | 0.02 | – | Group 2B |
| Nickel | 0.20 | 2.03 | 1.36 | 2.25 | – | Group 1 |
*mg/d = mg/ day.
3.2. Metabolomics of Toombak
Using the volatile organic compound method; Benzaldehyde, benzyl alcohol, phenylethyl alcohol, formaldehyde, acetaldehyde, propionaldehyde and 3 – methyl – valeric acid were detected in the Toombak samples while urethane and N-nitrosodimethylamine were not detected.
Both Benzaldehyde and benzyl alcohol were markedly increased in the pre-prepared sample S01, while in the ready to buy samples, lower concentrations were found of the former and variable concentrations of the latter. Phenylethyl alcohol was elevated in the pre-prepared sample as well as in samples from both Khartoum North S08, S09 and Omdurman S13, S14, S15 and S20, while 3 – methyl – valeric acid was low in all samples except in the ready to buy sample S21 from Omdurman where it was markedly increased.
Volatile aldehydes were increased in Toombak compared to European standards (Table 2). Formaldehyde ranged from 8−17 mg/kg in samples from Omdurman and 7−12 mg/kg from those found in Khartoum North. Compared to the allowed levels of this aldehyde in Swedish snus; only two of the 21 samples of Toombak, contained formaldehyde levels below 7.5 mg/kg. Acetaldehyde and propionaldehyde were also elevated in all samples. Acetaldehyde was found to be in the range of 500–1300 mg/kg in Toombak.
Table 2.
Levels of formaldehyde and acetaldehyde in Toombak samples compared to GothiaTek standards.
| Compound (aldehyde) | Pre- prepared Toombak | Omdurman ready to buy Toombak range | Khartoum North ready to buy Toombak range | GothiaTek standard limits of aldehydes in Swedish snus |
|---|---|---|---|---|
| Formaldehyde (mg/kg) | 25 mg/kg | 8−17 mg/kg | 7−12 mg/kg | 7.5 mg/kg |
| Acetaldehyde (mg/kg) | 900 mg/kg | 500−1300 mg/kg | 600−1200 mg/kg | 25 mg/kg |
The pre-prepared Toombak had a distinct metabolomic composition from all the remaining ready to buy samples as highlighted on the principal component analysis model (PCA) (Fig. 2). Quality control samples were tightly grouped together indicating analytical variance greatly exceeded biological variance. Quality control samples were also located close to the centre of the plot indicating that matrix effects were not an issue with these samples and that data were of high quality [24]. Metabolomic findings were then compared for the 16 compounds included in the reduced dataset using separate bar charts for each of the compounds. These compounds were amino acids (valine, carnitine, citrulline, proline and alanine), nicotine, tobacco specific nitrosamines [(N-nitrosonornicotine (NNN), nicotine-derived nitrosamine ketone (NNK), N-nitrosoanabasine (NAB), N′-nitrosoanatabine (NAT)], hexose, adenine, hypoxanthine, inosine, pantothenic acid and succinic acid. Additionally, 1058 features were extracted using mzMine.
Fig. 2.
Multivariate score plot: PCA model describing two dimensional LC/MS data of Toombak. Data have been auto scaled. S01 = pre-prepared Toombak, S02 – S11 = Khartoum North samples and S12 –S21= Omdurman samples. Distinct variation between S01 and the ready to buy samples in the plot suggests handmade preparation of Toombak greatly modifies the final product. No major variation was found between the ready to buy groups.
Highest levels of adenine were found in the pre-prepared sample while ready to buy Toombak samples all had low levels. Hypoxanthine was lowest in S01 and highest in Khartoum North samples S05, S07, S08, and S09. In particular, inosine was negligible in the pre-prepared sample as well as in S08 from Khartoum North and Omdurman samples S16, S17, S18, S19 and S21, while it was markedly elevated in Khartoum North samples S03, S05 and S10 and Omdurman samples S13 and S20.
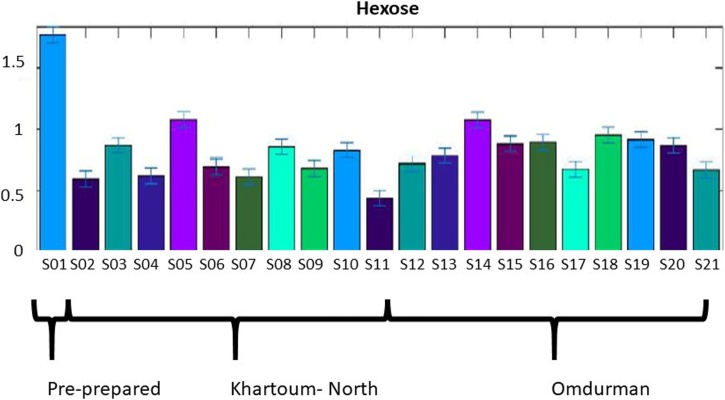
All samples contained the amino acids carnitine, citrulline, valine, alanine, and proline in varying amounts. Carnitine was found to be highly variable while citrulline was highest in the pre-prepared sample and S12 (Omdurman). Valine was high in all samples while alanine was increased only in the pre-prepared form and one sample from Omdurman; S14. Proline was found to be elevated in the pre-prepared form and reduced in the remaining ready to buy samples. Hexose (Fig. 3) was markedly elevated in the pre-prepared form (S01) but lower in all ready to buy samples while pantothenic acid showed a similar pattern. Succinic acid was highest in the pre-prepared form but was also elevated in the ready to buy samples.
Fig. 3.
Bar graph: hexose monosaccharide in Toombak samples was found to be significantly elevated in the pre-prepared sample S01, compared to the remaining ready to buy samples.
Nicotine in the pre-prepared form was 31 mg/g but ranged lower; between 16−25 mg/g in the ready to buy samples. Mean levels of the individual TSNAs in the ready to buy samples of Toombak were found to be as follows: NAB, 10-40 mg/kg, NAT, 5-14mg/kg, NNK, 1200-4700 mg/kg and NNN, 60-130 mg/kg. The highest concentrations of nicotine (31 mg/g), NNN (160 mg/kg), NAT (28 mg/kg) and NAB (46 mg/kg) were found in the pre-prepared sample while concentrations of NNK were consistent throughout both pre-prepared and ready to buy Toombak. Cumulative NNN and NNK in the ready to buy samples of Toombak were further compared to the GothiaTek standard (Table 3) and were found to range between 1270−4830 mg/kg amongst samples.
Table 3.
Levels of nicotine and the tobacco specific nitrosamines (TSNAs), NNK, NAB, NAT and NNN in the pre-prepared and ready to buy Toombak samples. NNN and NNK were cumulatively compared in mg/kg with GothiaTek standard maximum limits allowed.
| Type | Sample | Compound mg/g |
GothiaTek standard NNN + NNK mg/kg = (0.95) | ||||
|---|---|---|---|---|---|---|---|
| Nicotine | NNK | NAB | NAT | NNN | NNN + NNK converted from mg/g to mg/kg in Toombak samples | ||
| Pre-prepared Toombak | S01 | 31 | 3.5 | 0.046 | 0.028 | 0.16 | 3660 |
| Khartoum North Toombak | S02 | 22 | 1.8 | 0.012 | 0.006 | 0.07 | 1870 |
| S03 | 21 | 2.6 | 0.017 | 0.007 | 0.09 | 2690 | |
| S04 | 22 | 1.9 | 0.014 | 0.006 | 0.08 | 1980 | |
| S05 | 19 | 2.4 | 0.017 | 0.008 | 0.08 | 2480 | |
| S06 | 25 | 1.5 | 0.014 | 0.006 | 0.08 | 1580 | |
| S07 | 24 | 1.2 | 0.015 | 0.006 | 0.08 | 1280 | |
| S08 | 19 | 1.2 | 0.011 | 0.005 | 0.07 | 1270 | |
| S09 | 20 | 2.0 | 0.022 | 0.008 | 0.09 | 2090 | |
| S10 | 22 | 2.3 | 0.021 | 0.011 | 0.10 | 2400 | |
| S11 | 22 | 1.9 | 0.021 | 0.011 | 0.10 | 2000 | |
| Omdurman Toombak | S12 | 20 | 4.7 | 0.037 | 0.011 | 0.13 | 4830 |
| S13 | 20 | 2.9 | 0.024 | 0.011 | 0.10 | 3000 | |
| S14 | 22 | 2.7 | 0.029 | 0.014 | 0.12 | 2820 | |
| S15 | 19 | 3.0 | 0.022 | 0.008 | 0.10 | 3100 | |
| S16 | 20 | 3.6 | 0.025 | 0.007 | 0.11 | 3710 | |
| S17 | 20 | 2.4 | 0.021 | 0.007 | 0.11 | 2510 | |
| S18 | 23 | 3.4 | 0.040 | 0.013 | 0.12 | 3520 | |
| S19 | 22 | 1.9 | 0.015 | 0.007 | 0.08 | 1980 | |
| S20 | 23 | 2.0 | 0.026 | 0.010 | 0.11 | 2110 | |
| S21 | 16 | 1.4 | 0.010 | 0.005 | 0.06 | 1460 | |
| Total range | 16−25 mg/g | 1.2−4.7 mg/g | 0.010 – 0.040 mg/g | 0.005 – 0.014 mg/g | 0.06 – 0.13 mg/g | 1270 – 4830 mg/kg | |
| 0.01 | 0.02 | 0.003 | 0.002 | 0.002 | |||
3.3. Microbiome of Toombak
Overall, 258 assigned OTUs or features from a total read count of 1,203,891 were obtained. The maximum count was 171,130 while the minimum count per sample was 61,860 with an average of 92,607 reads. A total of 66 low abundance features were removed, based on their prevalence being under 10 %, while a further eight low variance features were removed based on a standard deviation under 5%. The remaining OTUs or features after data filtering were 141. In total, 13 phyla, 12 classes, 47 orders, 85 families and 134 genera were characterised in Toombak. Firmicutes and Actinobacteria where the most abundant phyla amongst all Toombak samples. Cyanobacteria were high in the sample obtained from western Sudan (pre-prepared form), while Proteobacteria and Halenaerobiaeota were the least abundant phyla in all samples (Fig. 4A). The families Carnobacteriaceae and Corynebacteriaceae dominated in ready to buy forms while Bacillaceae dominated in the pre-prepared Toombak (Fig. 4B). Fig. 4C highlights the genera distribution in the ready to buy samples from Khartoum North and Omdurman as well as the pre-prepared form. Relative abundance amongst the ready to buy Toombak included Corynebacterium_1 (24 %), Atopostipes (16 %), Atopococcus (12 %), Oceanobacillus (8%), Yaniella (7%), Virgibacillus (7%), Staphylococcus (6%) and Aerococcus (5%).
Fig. 4.
A: Microbiome Analyst R programming of abundant phyla within Toombak samples. Firmicutes are the most abundant phylum, followed by Actinobacteria. Pre-prepared samples harboured increased Cyanobacteria; due to the less degraded plant composition of the pre-prepared form. B: Stacked bar chart representing 16S rRNA compositional data of families within Toombak. Corynebacteriaceae and Carnobacteriaceae are predominant in the ready to buy forms while in the pre-prepared Toombak; Bacillaceae are markedly increased. C: Stacked bar chart of genera composition amongst Toombak samples. Corynebacterium_1, Atopostipes and Atopococcus are the most abundant genera in Khartoum North samples. Atopostipes and Alloiococcus were found to be abundant amongst Omdurman samples while Virgibacillus was the most abundant genus in the pre-prepared form. Oceanobacillus, Staphylococcus, Yaniella and Aerococcus are other genera identified in the ready to buy Toombak samples.
When comparing both ready to buy groups, Corynebacterium_1 was the most abundant genus in Toombak from Khartoum North (26 %), followed by Atopostipes (13 %), Atopococcus (13 %) and Oceanobacillus (9%), while Atopostipes was the most abundant genus in Toombak from Omdurman (34 %) followed by Alloiococcus (24 %) and Corynebacterium_1 (13 %). Other variations included Staphylococcus that accounted for 6% of the composition of Toombak from Khartoum North but only 3% of Omdurman samples and Atopococcus which accounted for 13 % of Khartoum North samples but only 7% of Omdurman samples. On the other hand, Alloiococcus was highly distributed amongst Omdurman samples (24 %), while it was minimally detected in Toombak samples from Khartoum North. Virgibacillus was the most abundant genus in the pre-prepared form.
Alpha diversity was similar amongst ready to buy Toombak samples, attributed to OTU feature domination as indicated by a non-significant Chao1 (P = 0.46), observed richness (p = 0.16), and Shannon diversity index (p = 0.25) (Fig. 5). Beta diversity, however was statistically significant (p < 0.025) with an R2 of 0.61. Samples from Khartoum North exhibited intra – group commonality compared to those from Omdurman (Fig. 6).
Fig. 5.
α Diversity as measured by Chao1 (p = 0.46), observed richness (p = 0.16), and Shannon diversity index (p = 0.25) comparing ready to buy samples of Toombak (pink = Khartoum North, blue = Omdurman). Non-significant α diversity highlights that the microbial ecological niche is the same amongst all ready to buy Toombak and is attributed to OTU feature domination. Shannon scores > 2 indicate an increased number of rare species.
Fig. 6.
PCoA model highlighting β diversity of ready to buy Toombak in relation to place of purchase at feature level (OTUs). Predominant inter-group microbiome variation of Toombak samples (dependant variable) due to location of purchase (independent variable). Samples from Khartoum North exhibit intra – group commonality compared to those from Omdurman. This variation is statistically significant (p <0.025). R2= 0.61, thus 61 % of the microbiome in Toombak ready to buy samples can be related to place of purchase most likely affected by multi-local handlers and the social and geographical considerations that are unique between Khartoum North and Omdurman.
Utilising BLASTn, OTUs were interrogated at species level in the ready to buy Toombak samples, for those sequences > 98 % identity to the NCBI database of rRNA bacterial and archaeic species identification. This revealed that Corynebacterium casei, (98.89 % identity), Atopococcus tabaci (99.34 % identity), Atopostipes suicloacalis (98.49 % identity), Oceanobacillus chironomi (98.49 % identity), Staphylococcus gallinarum (99.35 % identity), Enteractinococcus lamae (98.45 % identity) and Facklamia tabacinasalis (99.35 % identity) were some of the most represented species amongst the ready to buy Toombak samples.
Furthermore, the species Corynebacterium casei and the genus Lentibacillus were highly correlated with Toombak samples from Khartoum North, with correlation scores of 0.94 and 0.83, respectively. The species, Atopostipes suicloacalis and genus Alloiococcus were positively correlated with Omdurman samples with correlation scores of 0.93 and 0.71, respectively.
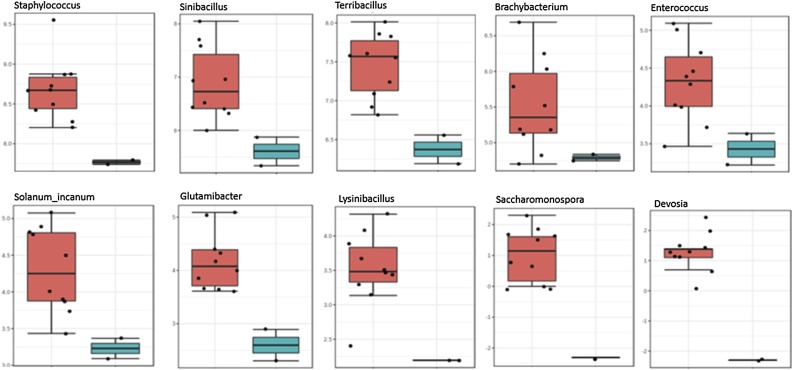
Classic univariate analysis (Anova) highlighted significant variations in genera abundance between the two Toombak ready to buy groups. Fig. 7 highlights the most enriched genera amongst Khartoum North samples which included Staphylococcus (q = 0.002), Sinibacillus (q = 0.028), Terribacillus (q = 0.028), Brachybacterium (q = 0.046), Enterococcus (q = 0.032), Glutamibacter (q = 0.025), Lysinibacillus (q = 0.021), Saccharomonospora (q = 0.025) and Devosia (q = 0.004). Shrub addition (Solanum_incanum) in the final ready to buy Toombak from Khartoum North was also apparent, most likely incorporated during the early production stages (q = 0.006).
Fig. 7.
Classic univariate analysis (Anova), log scaled, highlights significant variation in microbiome between the two Toombak ready to buy groups; FDR adjusted p values or q values. Staphylococcus (q = 0.002), Sinibacillus (q = 0.028), Terribacillus (q = 0.028), Brachybacterium (q = 0.046), Enterococcus (q = 0.032), Glutamibacter (q = 0.025), Lysinibacillus (q = 0.021), Saccharomonospora (q = 0.025) and Devosia (q = 0.004) were the most abundant genera in Toombak obtained from Khartoum North. Shrub contamination (Solanum_incanum) in the final ready to buy Toombak from Khartoum North was also found (q = 0.006) and reflects the varied production techniques of Toombak. Pink = Khartoum North, blue = Omdurman.
In ready to buy Toombak, it was found that Staphylococcus gallinarum, Devosia albogilva (99.77 % identity), Enteractinococcus lamae, Lysinibacillus telephonicus, (99.35 % identity), Oceanobacillus chironomi, Saccharomonsospora viridis (99.33 % identity), Glutamibacter_mysorens (99.10 % identity), Terribacillus saccharophilus (99.78 % identity) and Terribacillus halophilus (99.14 % identity), were significantly enriched in Khartoum North samples. Deseq2 was further employed to assess differentially abundant OTUs particularly amongst Omdurman samples. The genus Alloiococcus and the three species Atopostipes suicloacalis, Alkalibacterium iburiense (99.57 % identity) and Serratia quinivorans (99.14 % identity) were increased in those samples from Omdurman (Fig. 8).
Fig. 8.
Deseq2 was used to assess differentially abundant OTUs amongst Omdurman samples. Deseq2 determines the mean expression of various shrinkage estimates and fold changes to improve final interpretability, while also correcting dispersion estimates that may be too low through modelling or under sampling. OTU_3 was the most significantly aligned species utilising BLASTn and was found to be 98.49 % identical to Atopostipes suicloacalis. Omdurman samples also contained increased abundances of Alkalibacterium iburiense, Serratia quinivorans and the genus Alloiococcus. Pink = Khartoum North, blue = Omdurman.
PICRUSt assimilations were determined using the Kyoto Encyclopaedia for Genes and Genomes (KEGG) pathways to assess activity for metagenome functions amongst ready to buy samples and pre-prepared Toombak and profiled using a heatmap (Fig. 9). Ready to buy Toombak samples had an abundance of functions in the ATP binding proteins, metal transport mechanisms that included cobalt/nickel transport (K02006), iron complex transports (K02013 and K02015), and peptide/nickel transport system substrate binding protein (K02035). The ATP binding protein antibiotic transport system (K09687) was highly expressed in all samples while the F420H(2) dependent quinone reductase (K00540) pathway was present amongst the ready to buy samples. Signalling and cellular process pathways in Toombak included the polar amino acid transport system substrate binding protein (K02030), putative ABC transport system permease proteins (K02003 and K02004), found to be low in the pre-prepared sample and the ABC 2 type transport system (K01990), found to be high in all samples. Finally, sucrose 6 phosphate (K07024), associated with starch and sucrose metabolism was reduced in the pre-prepared form.
Fig. 9.
PICRUSt data assimilation of the top 20 KEGG pathways in pre-prepared and ready to buy Toombak with visualisation of abundance activity using heatmap profiling. Increased peptide/nickel (K02035), cobalt/ nickel (K02006), and iron transport systems (K02013 and K02015) amongst ready to buy Toombak as well as active antibiotic transport systems (K09687) amongst all samples were identified.
3.4. Microscopic analysis
At low magnification (35x), the large non-homogenous and irregular particles were highlighted in the Toombak samples (Fig. 10A and 10B) compared to a pasteurised regulated European tobacco powder with a uniform structure (Fig. 10C). A ready to buy sample of Toombak at 4000x magnification yielded evidence of bacterial cocci and rod contamination (Fig. 10D).
Fig. 10.
A: pre-prepared Toombak before handmade preparation with large, wooden and leaf like components at 35x magnification. B: ready to buy Toombak highlighting a non-homogenous large particle size product that is more closely packed together and may be quite abrasive to the oral mucosa (35x). C: evenly dispersed European snuff particles at 35x and D: ready to buy Toombak with abundance of bacterial rods and cocci at 4000x magnification.
3.5. Energy dispersive X-ray spectroscopy
Elemental composition was analysed amongst 26 spectra within the Toombak samples. Carbon, oxygen, magnesium, aluminium, silicon, sulphur, chlorine, calcium, molybdenum, beryllium, sodium, and potassium were all found in the samples and were calculated in weight %. Oxygen and carbon were the most predominant elements in Toombak and European standard, however, calcium, potassium, sodium, and silicon were also present.
While the pre-prepared Toombak sample had only a maximum of 0.55 % sodium, ready to buy Toombak contained a maximum of 4.93 %. Potassium was increased in the pre-prepared form (max 3.17 %), while a high percentage of calcium was present in the ready to buy sample (max 5.67 %). Table 4 summarises the main elements in Toombak samples compared to the control. While the weight % of elements in Toombak were more variable and of increased total weight % (max 0.44 %–5.67 %), the control sample exhibited consistent levels and a lower total weight % of the most common six elements (max 0.21 %–2.52 %).
Table 4.
The main elements found in the pre–prepared Toombak and one ready to buy sample compared to a control (European smokeless tobacco) using energy dispersive X ray spectroscopy.
| Pre-prepared Toombak |
Ready to buy Toombak |
Control |
||||
|---|---|---|---|---|---|---|
| Weight % | Min % | Max % | Min % | Max % | Min % | Max % |
| Elements | ||||||
| Calcium | 0.56 % | 1.08 % | 0.36 % | 5.67 % | 0.98 % | 2.52 % |
| Potassium | 0.98 % | 3.17 % | 0.40 % | 2.23 % | 1.06 % | 2.11 % |
| Aluminium | 0.50 % | 2.03 % | 0.55 % | 0.71 % | 0.14 % | 0.21 % |
| Sodium | 0.21 % | 0.55 % | 2.08 % | 4.93 % | 1.13 % | 2.27 % |
| Magnesium | 0.20 % | 0.90 % | 0.24 % | 0.44 % | 0.28 % | 0.72 % |
| Silicon | 0.57 % | 1.99 % | 0.80 % | 2.09 % | 0.20 % | 0.74 % |
3.6. pH
pH of the original sample was 8.73, while pH of the ready to buy samples ranged from 9.08–9.92.
4. Discussion
Compared to a European regulated product, Toombak was a course, non-homogenous smokeless tobacco containing visible bacterial cocci and rods under microscopy. SEM-EDX showed high elemental sodium in the ready to buy Toombak samples, but other elements could also be found. All ready to buy samples were at pH 9 or above attributed to the addition of sodium bicarbonate, primarily to enhance nicotine bioavailability.
The pre-prepared and ready to buy Toombak samples had similar heavy metal concentrations, of which there were four cations classified as group 1 carcinogens, four cations classified as group 2 carcinogens, and one cation classified as a group 3 carcinogen in the samples. Six of the nine cation concentrations were available for comparison with the GothiaTek standard and were found to be of acceptable values. The remaining elements, cobalt, copper and iron were compared with other references [[25], [26], [27]] (Table 1). Arsenic, cadmium, lead, and mercury were found to be of acceptable values in the Toombak. Nicotiana rustica has been previously shown to accumulate sulphur which may support arsenic and cadmium detoxification [28], however nickel levels were closer to the upper range of acceptable limits.
Chromium, cobalt, and copper were high in the pre-prepared form while iron was by far the most abundant heavy metal in Toombak, at 1270 mg/kg in the ready to buy sample. Therefore, an average consumer using 90 g of Toombak (6 g of Toombak X 15 uses per day) would be exposed to an average of 114.3 mg of iron a day, while the reported acceptable dietary intake of iron is 8−18 mg per day [26].
Such levels of iron in Toombak are higher than the iron content in home-brewed beer (48−62 mg/l) prepared in south and central Africa in iron pots, the consumption of which has been associated with the condition Africa iron overload/Bantu siderosis. Manifestations of this disease include liver damage, diabetes, osteoporosis and cardiac abnormalities [29]. Interestingly, Toombak is stored and prepared for long periods in stainless steel pots containing at least 50 % iron and whether this undertaking has a direct effect on the iron concentration found in Toombak requires further investigation. Other sources of heavy metals include the use of pesticides such as fungicides and insecticides, fertilisers, sludge irrigation with polluted water and settled aerosols [30].
True bioavailability of heavy metals in Toombak or in any other smokeless tobacco, can be difficult to accurately determine. Bioavailability may be as low as 6% and as high as 100 % in some forms of smokeless tobacco [31]. Metals such as cadmium, cobalt and nickel have been proven to be quite efficient at being extracted by artificial saliva and thus could potentially pose greater harm to Toombak users [25]. The oral, oesophageal and gut linings can allow for absorption of cations but biological damage depends on the total concentration of the metal in the smokeless tobacco, the proximal transfer by epithelial contact, saliva and digestive juices as well as the individual health status [32]. Heavy metals also exhibit the property of bioaccumulation in the body over time, and thus a chronic Toombak user may be at risk of long-term chronic disease or side effects associated with heavy metal exposure.
Cadmium exposure in humans has been associated with respiratory, skeletal (osteomalacia and osteoporosis), renal and cardiovascular disease. Precancerous lesions, oral ulceration and progressive fibrosis of the oral tissues as well as liver damage have been associated with copper exposure [33] while the development of leukaemia, cancers of the bladder, kidney, and skin, neurological disturbances and renal failure are associated with arsenic toxicity. Neurological and haemoglobin synthesis disturbances can occur from mercury exposure. Users of Toombak are at particular risk of neurotoxic effects due to starting the product at a young age [34].
A diverse range of metabolomic compounds were isolated from Toombak samples with variations between the pre-prepared and ready to buy Toombak (Fig. 2). This highlights that the different stages of Toombak processing and storage can create a significant change to the final product. Formaldehyde, acetaldehyde and propionaldehyde were all detected in the Toombak samples. Only two of the 21 Toombak samples were found to be below the recommended level set out by the GothiaTek standards, acceptable for formaldehyde while acetaldehyde levels were 20–52 times higher in all the Toombak samples (Table 2). Aldehydes are mutagenic and carcinogenic and can be found naturally in smokeless tobacco or may be added. They are formed by the incomplete degradation of tobacco leaves as well as being a metabolic end-product of many microorganisms that are numerous in Toombak. Formaldehyde is a group 1 IARC carcinogen while acetaldehyde is now classed as a group 2B carcinogen. Formaldehyde has been associated with cancers of the nasopharynx, nasal sinuses as well as the oral cavity, brain, pancreas and non-solid cancers which include leukaemia, and multiple myeloma [35]. Acetaldehyde can affect both the oesophageal and gut mucosa [36] while animal models have shown that cardiovascular tissues are highly sensitive to aldehydes [37]. Aldehydes have also been shown to cause direct DNA damage in the buccal cells of cigarette smokers [38] and can activate the Jun/AP-1 pathway in oral keratinocytes leading to the transcription of oncogenes [39].
It is unclear if aldehydes are added to Toombak as a preservative or a flavouring as is done with some other forms of smokeless tobacco [40]. Most worryingly, acetaldehyde in itself is potently addictive, and can act in synergy with nicotine to increase the addictive potential of the latter [41].
Propionaldehyde was found in all Toombak samples. It is more likely introduced from drinking water during its preparation but may also be added. It is used in the manufacture of plastics and utilised as a disinfectant and preservative. In small amounts (140 μg/day), both the FDA and WHO have approved propionaldehyde as a synthetic flavouring, known to possess a fruity odour. Propionaldehyde has been shown however to increase arterial blood pressure and heart-rate by sympathomimetic activities [42].
Nicotine levels are high in the Nicotiana rustica plant as opposed to other Nicotiana species due to an additionally expressed putrescine methyltransferase gene which allows for its efficient production, while nicotine transport from the root to the shoot is more efficient [28]. Nicotine levels in the ready to buy Toombak samples in this study ranged between 16–25 mg/g (average 20.5 mg/g) but were highest in the pre-prepared form (31 mg/g) [43] (Table 3). Thus, in a Toombak user, utilising 90 g of the ready to buy product a day (6 g × 15 times/day), nicotine exposure would be 1440 – 2250 mg/g per day (average 1845 mg/g). Nicotine levels in Toombak in the literature are highly variable and range from 5.16 to 102.4 mg/g [44]. It is absorbed by passive diffusion into the blood, where the more alkaline the pH, the more unionised nicotine that becomes available and can be easily absorbed by epithelial linings [45]. Addition of water to Toombak also improves nicotine absorption and permeability through the oral mucosa as well as better transfer to saliva. Short term effects of nicotine exposure from smokeless tobacco include increased heart-rate (within the first 15 min), nausea and vomiting, while long term effects include the development of cardiovascular disease such as atherosclerosis [46] through increased low density lipoproteins and decreased high density lipoproteins [47]. Nicotine has also been associated with delayed wound healing, prevention of apoptosis of cancer cells and the promotion of reactive oxygen species and oxidative damage to cells [48].
Mean levels of the individual TSNAs amongst ready to buy Toombak samples were all found to be high (NAB; 10-40 mg/kg, NAT; 5-14 mg/kg, NNK; 1200-4700 mg/kg and NNN; 60-130 mg/kg). NNN and NNK levels in Toombak were compared to the maximum limits of these compounds in Swedish snus (NNN + NNK =0.95 mg/kg). Concentrations of NNN and NNK in ready to buy Toombak ranged from 1270−4830 mg/kg, values thousands fold higher than the maximum limits of these compounds in Swedish snus (Table 3), a trend also reported by Idris et al. [49]. Mean levels of NNN and NNK in Indian forms of smokeless tobacco are reported to be 22.9 mg/kg and 2.6 mg/kg, respectively [50], while it has been proposed by the FDA that NNN levels in smokeless tobacco sold in the USA should not exceed 1 mg/kg [51]. Thus, TSNA concentrations in Toombak may be the highest found amongst all smokeless tobacco products in the world. These are carcinogenic compounds produced when nitrate is converted to nitrite by both aerobic and anaerobic metabolic routes that involve nitrate or nitrite reductase as the two key enzymes. This is followed by nitrosation which involves a chemical reaction between nitrite and nicotine [52,53].
TSNAs are absent in the green leafy tobacco plant and their accumulation occurs during the post cultivation period of Toombak production, harvesting, fermentation, storage and even after purchase. TSNA formation is also closely related to the triad of nicotine, moisture content and elevated temperatures. Moist forms of smokeless tobacco with added water such as Toombak, promote bacterial contamination and their growth [54]. The high environmental temperatures then allow for the chemical reduction of nitrate to nitrite, where the latter is originally elevated in Toombak due to the high nicotinic alkaloid content in the Nicotiana rustica plant.
The metabolism and activation of NNN and NNK in particular, lead to the pyridyloxobutylation of DNA, where in the setting of proto-oncogenes and tumour suppressor genes, can cause activation and inactivation of these critical genes, respectively, and thus tumour growth [55,56]. Furthermore, NNN and NNK are thought to act in a synergistic manner, binding to nicotinic acetylcholine receptors, further facilitating tumour development [57]. NNN is a powerful oral and oesophageal carcinogen in laboratory animals as well as in humans [58, 59], and together, NNN and NNK have been strongly linked with the development of oral cancer when administered to rats [60]. Another consideration is that the enzymatic activity of saliva facilitates the release and extraction of TSNAs in the oral cavity [61] and the TSNA content in saliva of Toombak users has been shown to be elevated [62]. The NNK specific metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), excreted in urine is highly elevated amongst Toombak users [63] and is supported by the findings of the largely increased TSNA content reported in this paper.
Adenine is a purine nucleobase that was found in high amounts in the pre–prepared sample and at lower levels in the ready to buy Toombak, most likely reflecting lack of its degradation at this stage. Hypoxanthine and inosine were found at higher concentrations in the ready to buy samples compared to the pre-prepared form, suggesting the deamination of adenine and purine metabolism that are a natural metabolic process of plant metabolism. Amino acids in Toombak accumulate in parallel with the curing stage, where breakdown of tobacco proteins and hydrolysis of pigments occur by enzymes and microorganisms releasing such amino acids [64]. Citrulline was elevated in the pre-prepared sample and lower in the ready to buy Toombak and is derived from the catabolism of arginine by lactic acid bacteria as well as being a nitrogenous precursor of urethane, a group 2A IARC carcinogen. No urethane was detected in any of the Toombak samples. Urethane content has been poorly linked to the process of fermentation where its formation is associated with increased temperatures during pasteurisation and presence of added ethanol in smokeless tobacco, however these features are not part of Toombak preparation [65]. Carnitine presence in Toombak may provide bacteria with thermal and osmotic protection [66] promoting their survival but its presence was found to be highly variable. Proline, found in all samples in Toombak, may induce yeast to hyphal form in the oral cavity and promote their pathogenic capability [67] while alanine and valine are not known to cause harm.
Benzylaldehyde and benzyl alcohol, aroma compounds in Nicotiana rustica, were highest in the pre-prepared sample, but variable in the ready to buy Toombak and contribute to the tobacco flavour [68, 69]. Phenylethyl alcohol, which has a rose odour, is found naturally amongst various human nutritional consumables [70] but is also known to be added to Gutka, a type of smokeless tobacco used in India [71]. All samples of Toombak contained phenylethyl alcohol, however, levels were variable where six of the 21 samples had high concentrations including the pre–prepared Toombak, while the remaining samples had reduced or negligible amounts. This may suggest that this compound occurs both naturally and is added during the manufacturing process. Phenylethyl alcohol may have some selective inhibitory action on gram negative bacteria [72] thus may be a contributing factor to Toombak microorganism composition. The flavour of Toombak may also arise from the presence of proline and hexose which contribute to the Maillard reaction in tobacco, a non-enzymatic reaction that leads to the development of flavour compounds [73].
Bacteria can transform glucose and other sugars into succinic acid during fermentation [74] while Lactobacillus and Bacillus strains can produce succinic acid from citric acid in particular [75,76]. Citric acid is a prominent component of Nicotiana rustica after curing while Toombak contained abundant Bacillus [68]. Interestingly, Corynebacterium and Enterococcus species are some of the main studied succinic acid producers, both found in Toombak [77]. Succinic acid contributes to the taste of Toombak, by adding a bitter salt-like flavour and is also high in some fermented beverages (e.g sake from Japan) but is not known to harm human health. It also has many other uses in industry and is either produced chemically or through fermentation by fungi and bacteria. 3 – Methyl – valeric acid, found in Toombak, has a strong, pungent, fruity and cheesy flavour [78] and is formed from the alkaline hydrolysis of glycolipids found in Nicotiana species including Nicotiana rustica [79]. Pantothenic acid (vitamin B5) most likely originates from the plant structure with no harmful addition to users. Hexose (glucose and fructose) was elevated only in the pre-prepared sample and was considerably reduced in all the ready to buy Toombak samples (Fig. 3), a finding supported by the air curing process of Toombak, which contributes to the low sugar levels. Hexose may also contribute to the taste of Toombak. Low sugar content may however, allow bacterial growth and prevent acid metabolites from occurring, thus keeping Toombak at alkaline pH [80,81]. At least, in part, the low sugar content of Toombak should not contribute to the risk of caries development from extrinsic sugars, amongst Toombak users.
In total, 13 phyla, 12 classes, 47 orders, 85 families and 134 genera were characterised in Toombak of which Firmicutes and Actinobacteria were the predominant phyla amongst ready to buy and pre-prepared forms, although in the latter, Actinobacteria were markedly reduced while Cyanobacteria were increased. Proteobacteria were the least abundant in all forms of Toombak. Proteobacteria are more adapted to, and thus are generally found in cold environments, explaining their low abundance in Toombak obtained from Sudan, a country of a hot and temperate climate [82]. Bacillaceae family were enriched in the pre-prepared form while Virgibacillus was the most abundant genus (Fig. 4A, 4B and 4C).
The microbiome of Toombak has been previously reported by three authors (ElHebshi, Smyth and Tyx et al) with the studies reporting on one to five Toombak samples. ElHebshi et al. (2017) analysed one Toombak sample where Facklamia, Desemzia, Atopostipes, Lysinibacillus and Corynebacterium were the most abundant genera found, while Facklamia tabacinasalis, Atopostipes suicloacalis, Lysinibacillus chungkukjangi and Desemzia incerta predominated in the Toombak sample [83]. Tyx et al. (2016) analysed two Toombak samples and found a high relative abundance of Corynebacteriaceae and Staphylococcaceae and a low α diversity [84], while Smyth et al. (2017) analysed five Toombak samples and one alkalinising agent and found Enteractinococcus and Corynebacterium to be significantly increased [85]. Our results are aligned with some of these previous data, in particular that Corynebacterium is one of the most abundant genus in Toombak. This study is the first to report Toombak microbiome composition with respect to inter-locational variations, Khartoum North and Omdurman. Although Corynebacterium are enriched amongst Toombak including the species Corynebacterium casei, they are not the dominant genus or species, respectively. Atopostipes suicloacalis, Atopococcus tabaci, Oceanobacillus chironomi, Staphylococcus gallinarum, Enteractinococcus lamae, Facklamia tabacinasalis as well as Alloiococcus genera also predominantly defined the final composition of Toombak. Furthermore, Staphylococcus cohnii was found to be highly abundant amongst Toombak samples analysed by Smyth et al. [85], while Staphylococcus gallinarum was the most abundant species in our samples; known to be highly salt tolerant [86].
We found a high Shannon diversity index score (>1) indicating that Toombak has an increased number of microorganisms, particularly rare species. This trend is similar amongst samples since non-significant findings were obtained when comparing both ready to buy groups. Chao1 was found to be similar between the two ready to buy groups due to certain OTUs dominating in the samples (Fig. 5). This reflects the natural plant composition and alkalinic niche of Toombak, as confirmed by our pH findings [87]. Therefore, abundant bacteria in Toombak can survive alkalinity or even prefer it, such as the Bacilli class. These likely become predominant in the setting of elevated temperatures during Toombak production (fermentation and storage). Their ability to produce endospores allows them to be highly resistant to harsh conditions while Bacilli can also drive alkalinity and improve nicotine absorption, contributing to the addictive nature of Toombak. Bacilli isolated from American forms of smokeless tobacco have been shown to injure the oral mucosa of hamster cheek via the kallikrein/kinin metabolic pathway and thus may directly impact on chronic inflammation in the mouth [88].
The alkalinic nature of Toombak is further appreciated with the presence of alkaphiles such as Oceanobacillus chironomi, a facultative alkaphile and Alkalibacterium known to possess superior cellulose hydrolytic and carbohydrate fermentation properties [89], while Alkalibacterium iburiense identified in increased abundance amongst Omdurman samples is an obligate alkaphile. This allows for a self-sustainable fermentation system within Toombak over long periods and past the original air-curing period [90]. Furthermore, the contribution of bacteria to the potently elevated TSNA levels in Toombak are an important addition to the role of the microbiome. Corynebacterium and Staphylococceae species are known to be potent nitrate reducing bacteria as well as efficient nitrogen metabolisers [91], and may contribute to the high TSNA content of Toombak.
This study is the first to assess β diversity amongst numerous Toombak samples, particularly the socio – geographical dynamics which was found to significantly affect (p < 0.025) the final composition of the ready to buy groups (Fig. 6). The microbiome of the ready to buy Toombak is affected by the preparatory techniques, continuous unsterile human handling of the product and poor storage qualities, that allow for microbiome changes that may yet remain underestimated in the implications for human health. Furthermore, Toombak contained several animal and insect microbes including Enteractinoccocus lamae isolated from animal faeces [92], Staphylococcus gallinarum first isolated from chickens [93] and Oceanobacillus chironomi, isolated from chironomid egg mass [94]. Lysinibacillus telephonicus has been isolated from the screen of a mobile phone [95]. Alloiococcus is regarded as a commensal of the external auditory meatus, nasopharynx and nasal cavity but is also associated with development of otitis media [[96], [97], [98]] and was found to be abundant in samples from Omdurman. Health concerns arise from long term Toombak use due to the potential gateway for entry of novel infection causing bacteria as well as the presence of antibiotic resistant genes amongst them. Metagenomic pathways of ready to buy samples were active for metal transport systems particularly peptide/nickel, cobalt/nickel and iron transport compared to the pre-prepared form. These active transport systems may reflect the increased metal bioavailability in Toombak and active metal transport systems have been associated with carcinogenic smokeless tobacco products [83]. The K09687 pathway, expressed in all samples is an antibiotic transport system while F420H(2), a dependant quinone reductase pathway found to be active in the ready to buy samples may protect against bactericidal agents [99] (Fig. 9). Further analysis is required through whole genome sequencing and may be an important area in the future to better understand which species may relate to specific metagenomic pathways within Toombak that may have an impact on important diseases such as cancer and infection.
The physical structure of Toombak is a suitable niche for the survival of bacteria, fungi and the harbouring of other contaminants. The rough surface of Toombak can encourage chronic mucosal abrasion and epithelial cell damage and can promote inflammation and entry of opportunistic pathogens into the body. Compared to a European sample of smokeless tobacco, Toombak had a non-homogenous consistency and high levels of a number of elements. The SEM-EDX also highlights the large, non-homogenous structure of Toombak (Fig. 10A and B), compared to a regulated European sample (Fig. 10C) and provides visual evidence of bacterial rods and cocci at high magnification (Fig. 10D). Oxygen and carbon were the most predominant organic elements in Toombak, however, calcium, potassium, sodium, silicon, and aluminium were also abundant. Magnesium, beryllium, sulphur, and chlorine were less abundant within the samples. Sodium was present at 0.55 weight % in the pre-prepared form while it was 4.93 % in the ready to buy sample, suggesting that the addition of sodium bicarbonate may be the causative factor for this increase. Such elements can have hazardous local and systemic effects on users, particularly chronic abrasion as well as allergic and inflammatory predisposition to disease [100]. Sodium compounds may also lead to inflammation and cancer development [7].
All Toombak samples were in the alkaline pH range. The pre-prepared Toombak was of lower pH value, 8.73, compared to the remaining ready to buy samples that were above pH 9. The alkalinity of Toombak is a result of the cultural addition of sodium bicarbonate in order to convert the total nicotine to a free or unprotonated form which is more readily absorbed in the body. This allows Toombak to be highly addictive as nicotine becomes more potently available and is rapidly transported to the central nervous system. The addition of alkaline products such as ash, ammonium or calcium bicarbonate, slaked lime, and sodium citrate, can also be observed in other forms of smokeless tobacco.
5. Conclusion
In this study, we have shown that the sudanese smokeless tobacco, Toombak, contains numerous potential biohazardous components. Chromium, cobalt and copper were found to be high in the pre-prepared sample while iron concentration was alarmingly high in both pre-prepared and ready to buy Toombak. This may be a hidden cause of disease that includes liver damage, diabetes, osteoporosis, and cardiac disease.
High levels of volatile aldehydes were also found in all the ready to buy Toombak samples. Aldehydes are both carcinogenic and addictive and may be produced naturally, added in Toombak or present as contaminants from water. High levels of heavy metals and aldehydes in Toombak are an alarming cause of concern. Furthermore, samples of Toombak were found to contain some of the highest TSNA content in smokeless tobacco in the world, most likely a contribution of many factors. These include the high content of nicotinic alkaloid in the Nicotiana rustica plant, the elevated temperatures used during production, prolonged storage, and the high abundance of nitrate-reducing bacteria. TSNAs are among the major cancer-causing agents in Toombak, leading to mutational DNA damage of cells. Ready to buy Toombak was found not to contain a high extrinsic sugar content while flavour-related compounds are likely naturally developed in Toombak.
Toombak microbiome consisted of a core set of microbiota, consisting of the two main phyla Actinobacteria and Firmicutes. The most abundant genera within the ready to buy Toombak were Corynebacterium_1, Atopostipes, Atopococcus, Oceanobacillus and Staphylococcus, while Virgibacillus was the most abundant genus in the pre-prepared form. BLASTn revealed Corynebacterium casei as the most abundant species in Toombak from Khartoum North samples, while Atopostipes suicloacalis was the most abundant species in Toombak from Omdurman. Other species included Staphylococcus gallinarum, Atopococcus tabaci, Enteractinococcus lamae, Oceanobacillus chironomi and Facklamia tabacinasalis. α Diversity was similiar amongst all Toombak samples (i.e. the same genera were present), however their distribution and relative abundance were variable, dependant on place of purchase as shown by a significant β diversity and Deseq2 analysis. PICRUSt data showed metagenomic activity related to enriched peptide/nickel, cobalt/nickel and iron transport pathways in the ready to buy samples, an active antibiotic transport system and numerous active KEGG pathways related to survival in diverse environments.
This paper is the first to represent a wide scope analysis of numerous Toombak samples, a form of smokeless tobacco used extensively in Sudan incorporating metabolomic, metagenomic, microscopic, heavy metal and pH analysis. While smokeless tobacco sold in Europe and the USA is considerably regulated, Toombak is produced via individual manufacturing processes in Sudan. Use of the product is often initiated at a young age due to social difficulties and its low cost. Toombak harbours extensive biohazardous properties and it is prudent that urgent new and extensive measures be taken by the relevant government ministries in the Sudan to reassess its production laws as well as to take steps to reduce its use amongst the population.
CRediT authorship contribution statement
Amel Sami: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Visualization, Writing - review & editing. Imad Elimairi: Conceptualization, Project administration, Supervision, Validation, Writing - review & editing. Dhrati Patangia: Formal analysis. Claire Watkins: Formal analysis, Investigation. C. Anthony Ryan: Conceptualization, Methodology, Validation, Writing - review & editing. R. Paul Ross: Conceptualization, Funding acquisition, Project administration, Validation, Writing - review & editing. Catherine Stanton: Conceptualization, Methodology, Funding acquisition, Project administration, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding from APC Microbiome Ireland.
The authors would like to acknowledge the Department of Anatomy & Neuroscience Imaging Centre, BioSciences Institute, University College Cork, for assistance in preparing and imaging specimens for this research.
Handling Editor: Aristidis Tsatsakis
References
- 1.Siddiqi K., Vidyasagaran A.L., Readshaw A., Croucher R. A policy perspective on the global use of smokeless tobacco. Curr. Addict. Rep. 2017;4:503–510. doi: 10.1007/s40429-017-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idris A.M., Ibrahim Y.E., Warnakulasuriya K.A., Cooper D.J., Johnson N.W., Nilsen R. Toombak use and cigarette smoking in the Sudan: estimates of prevalence in the Nile state. Prev. Med. 1998;27:597–603. doi: 10.1006/pmed.1998.0331. [DOI] [PubMed] [Google Scholar]
- 3.Roberts K. 2008. Nicotiana Sp.https://web.archive.org/web/20080906115744/http://artsci.wustl.edu/ ∼gjfritz/Nicotiana_sp.html. Accessed 07/06/2020. [Google Scholar]
- 4.Güven A., Köksal N., Büyükbeşe M.A., Cetinkaya A., Sökmen G., Aksu E., Cağlayan C.E. Effects of using a different kind of smokeless tobacco on cardiac parameters: “Maraş Powder”. Anadolu Kardiyol. Derg. 2003;3:230–235. [PubMed] [Google Scholar]
- 5.Stanfill S.B., Oliveira da Silva A.L., Lisko J.G., Lawler T.S., Kuklenyik P., Tyx R.E., Peuchen E.H., Richter P., Watson C.H. Comprehensive chemical characterization of Rapé tobacco products: nicotine, un-ionized nicotine, tobacco-specific N’-nitrosamines, polycyclic aromatic hydrocarbons, and flavor constituents. Food Chem. Toxicol. 2015;82:50–58. doi: 10.1016/j.fct.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idris A.M., Ibrahim S.O., Vasstrand E.N., Johannessen A.C., Lillehaug J.R., Magnusson B., Wallström M., Hirsch J.M., Nilsen R. The Swedish snus and the Sudanese toombak: are they different? Oral Oncol. 1998;34:558–566. doi: 10.1016/s1368-8375(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 7.Boffetta P., Hecht S., Gray N., Gupta P., Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–675. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 8.Critchley J.A., Unal B. Health effects associated with smokeless tobacco: a systematic review. Thorax. 2003;58:435–443. doi: 10.1136/thorax.58.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunanda P., Panda B., Dash C., Ray P.K., Padhy R.N., Routray P.K. Prevalence of abnormal spermatozoa in tobacco chewing sub-fertile males. J. Hum. Reprod. Sci. 2014;7:136–142. doi: 10.4103/0974-1208.138873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbeshir E.I., Abeen H.A., Idris A.M., Abbas K. Snuff dipping and oral cancer in Sudan: a retrospective study. Br. J. Oral Maxillofac. Surg. 1989;27:243–248. doi: 10.1016/0266-4356(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 11.Patil S., Arakeri G., Alamir A.W.H., Patil S., Awan K.H., Baeshen H., Raj T., Fonseca F.P., Brennan P.A. Is toombak a risk factor for oral leukoplakia and oral squamous cell carcinoma? A systematic review. J. Oral Pathol. Med. 2020;49:103–109. doi: 10.1111/jop.12954. [DOI] [PubMed] [Google Scholar]
- 12.Bao M., Joza P.J., Masters A., Rickert W.S. 2014. Analysis of Selected Carbonyl Compounds in Tobacco Samples by Using Pentafluorobenzylhydroxylamine Derivatization and Gas Chromatography-Mass Spectrometry; p. 86. 26. [Google Scholar]
- 13.Rutqvist L.E., Curvall M., Hassler T., Ringberger T., Wahlberg I. Swedish snus and the GothiaTek® standard. Harm Reduct. J. 2011;8:11. doi: 10.1186/1477-7517-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SwedishMatch . 2020. January 15th 2020, January 15th Gothiatek Standard.https://www.swedishmatch.com/Snus-and-health/GOTHIATEK/GOTHIATEK-standard/ Accessed. [Google Scholar]
- 15.Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 17.Caporaso J.G., Bittinger K., Bushman F.D., DeSantis T.Z., Andersen G.L., Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGinnis S., Madden T.L. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20–5. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong M., Li L., Chen M., Kusalik A., Xu W. Predictive analysis methods for human microbiome data with application to Parkinson’s disease. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broadhurst D., Goodacre R., Reinke S.N., Kuligowski J., Wilson I.D., Lewis M.R., Dunn W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics. 2018;14:72. doi: 10.1007/s11306-018-1367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappas R.S., Stanfill S.B., Watson C.H., Ashley D.L. Analysis of toxic metals in commercial moist snuff and Alaskan iqmik. J. Anal. Toxicol. 2008;32:281–291. doi: 10.1093/jat/32.4.281. [DOI] [PubMed] [Google Scholar]
- 26.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 27.Turnlund J.R. Copper nutriture, bioavailability, and the influence of dietary factors. J. Am. Diet. Assoc. 1988;88:303–308. [PubMed] [Google Scholar]
- 28.Sierro N., Battey J.N.D., Bovet L., Liedschulte V., Ouadi S., Thomas J., Broye H., Laparra H., Vuarnoz A., Lang G., Goepfert S., Peitsch M.C., Ivanov N.V. The impact of genome evolution on the allotetraploid Nicotiana rustica – an intriguing story of enhanced alkaloid production. BMC Genomics. 2018;19:855. doi: 10.1186/s12864-018-5241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker A.R., Arvidsson U.B. Iron overload in the south african bantu. Trans. R. Soc. Trop. Med. Hyg. 1953;47:536–548. doi: 10.1016/s0035-9203(53)80006-4. [DOI] [PubMed] [Google Scholar]
- 30.Dhaware D., Deshpande A., Khandekar R.N., Chowgule R. Determination of toxic metals in Indian smokeless tobacco products. ScientificWorldJournal. 2009;9:1140–1147. doi: 10.1100/tsw.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakar V., Jayakrishnan G., Nair S.V., Ranganathan B. Determination of trace metals, moisture, pH and assessment of potential toxicity of selected smokeless tobacco products. Indian J. Pharm. Sci. 2013;75:262–269. doi: 10.4103/0250-474X.117398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO study group on tobacco product regulation : report on the scientific basis of tobacco product regulation : fourth report of a WHO study group. (WHO Technical report series; 967). [PubMed]
- 33.Trivedy C., Meghji S., Warnakulasuriya K.A., Johnson N.W., Harris M. Copper stimulates human oral fibroblasts in vitro: a role in the pathogenesis of oral submucous fibrosis. J. Oral Pathol. Med. 2001;30:465–470. doi: 10.1034/j.1600-0714.2001.030008465.x. [DOI] [PubMed] [Google Scholar]
- 34.Jarup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 35.Formaldehyde, https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-29.pdf.
- 36.Seitz H.K., Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010;5:121–128. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keith R.J., Fetterman J.L., Riggs D.W., O’Toole T., Nystoriak J.L., Holbrook M., Lorkiewicz P., Bhatnagar A., DeFilippis A.P., Hamburg N.M. Protocol to assess the impact of tobacco-induced volatile organic compounds on cardiovascular risk in a cross- sectional cohort: cardiovascular Injury due to Tobacco Use study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M-w Weng, Lee H.-W., Park S.-H., Hu Y., Wang H.-T., Chen L.-C., Rom W.N., Huang W.C., Lepor H., Wu X.-R., Yang C.S., Tang M.-s. Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. 2018;115:E6152–E6161. doi: 10.1073/pnas.1804869115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timmons S.R., Nwankwo J.O., Domann F.E. Acetaldehyde activates Jun/AP-1 expression and DNA binding activity in human oral keratinocytes. Oral Oncol. 2002;38:281–290. doi: 10.1016/s1368-8375(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 40.Kostygina G., Ling P.M. Tobacco industry use of flavourings to promote smokeless tobacco products. Tob. Control. 2016;25:ii40–ii49. doi: 10.1136/tobaccocontrol-2016-053212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belluzzi J.D., Wang R., Leslie F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 42.Stanek J.G.S. 2008. Toxicological Review of Propionaldehydewww.epa.gov/iris . Washington, DC. [Google Scholar]
- 43.Sami A., Stanton C., Ross P., Ryan T., Elimairi I. Ultra- structure of Toombak; smokeless tobacco of Sudan and its effects on oral and systemic health. Access Microbiology. 2020:2. [Google Scholar]
- 44.Idris A.M., Prokopczyk B., Hoffmann D. Toombak: a major risk factor for cancer of the oral cavity in Sudan. Prev. Med. 1994;23:832–839. doi: 10.1006/pmed.1994.1141. [DOI] [PubMed] [Google Scholar]
- 45.Singh I., Singh A., Kour R., Singh A., Singh R., Bali A. Is sodium carbonate in snuff a causative factor for oral mucosal lesions: a cross-sectional analysis. J. Int. Soc. Prev. Community Dent. 2018;8:339–342. doi: 10.4103/jispcd.JISPCD_134_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fant R.V., Henningfield J.E., Nelson R.A., Pickworth W.B. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob. Control. 1999;8:387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cluette-Brown J., Mulligan J., Doyle K., Hagan S., Osmolski T., Hojnacki J. Oral nicotine induces an atherogenic lipoprotein profile. Proc. Soc. Exp. Biol. Med. 1986;182:409–413. doi: 10.3181/00379727-182-3-rc1. [DOI] [PubMed] [Google Scholar]
- 48.Campain J.A. Nicotine: potentially a multifunctional Carcinogen? Toxicol. Sci. 2004;79:1–3. doi: 10.1093/toxsci/kfh106. [DOI] [PubMed] [Google Scholar]
- 49.Idris A.M., Nair J., Ohshima H., Friesen M., Brouet I., Faustman E.M., Bartsch H. Unusually high levels of carcinogenic tobacco-specific nitrosamines in Sudan snuff (toombak) Carcinogenesis. 1991;12:1115–1118. doi: 10.1093/carcin/12.6.1115. [DOI] [PubMed] [Google Scholar]
- 50.Warnakulasuriya S., Straif K. Carcinogenicity of smokeless tobacco: evidence from studies in humans & experimental animals. Indian J. Med. Res. 2018;148:681–686. doi: 10.4103/ijmr.IJMR_149_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berman M.L., Hatsukami D.K. Reducing tobacco-related harm: FDA’s proposed product standard for smokeless tobacco. Tob. Control. 2018;27:352–354. doi: 10.1136/tobaccocontrol-2016-053612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehra R., Mohanty V., Balappanavar A.Y., Kapoor S. Bacterial contamination of packaged smokeless tobacco sold in India. Tob. Prev. Cessat. 2020:6. doi: 10.18332/tpc/115064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priyanja J.K.M. Diversity study of nitrate reducing Bacteria from soil Samples-A metagenomics approach. J. Comput. Sci. Syst. Biol. 2015:8. [Google Scholar]
- 54.Han J., Sanad Y.M., Deck J., Sutherland J.B., Li Z., Walters M.J., Duran N., Holman M.R., Foley S.L. Bacterial populations associated with smokeless tobacco products. Appl. Environ. Microbiol. 2016;82:6273–6283. doi: 10.1128/AEM.01612-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakdash A. Shammah (Smokeless tobacco) and public health. Asian Pac. J. Cancer Prev. 2017;18:1183–1190. doi: 10.22034/APJCP.2017.18.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang M., Cheng G., Sturla S.J., Shi Y., McIntee E.J., Villalta P.W., Upadhyaya P., Hecht S.S. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens. Chem. Res. Toxicol. 2003;16:616–626. doi: 10.1021/tx034003b. [DOI] [PubMed] [Google Scholar]
- 57.Xue J., Yang S., Seng S. Mechanisms of Cancer induction by tobacco-specific NNK and NNN. Cancers (Basel) 2014;6:1138–1156. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan J.M., Knezevich A.D., Wang R., Gao Y.T., Hecht S.S., Stepanov I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stepanov I., Sebero E., Wang R., Gao Y.T., Hecht S.S., Yuan J.M. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: remarkable coherence with rat tumor sites. Int. J. Cancer. 2014;134:2278–2283. doi: 10.1002/ijc.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hecht S.S., Rivenson A., Braley J., DiBello J., Adams J.D., Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46:4162–4166. [PubMed] [Google Scholar]
- 61.Prokopczyk B., Wu M., Cox J.E., Hoffmann D. Bioavailability of tobacco-specific N-nitrosamines to the snuff dipper. Carcinogenesis. 1992;13:863–866. doi: 10.1093/carcin/13.5.863. [DOI] [PubMed] [Google Scholar]
- 62.Idris A.M., Nair J., Friesen M., Ohshima H., Brouet I., Faustman E.M., Bartsch H. Carcinogenic tobacco-specific nitrosamines are present at unusually high levels in the saliva of oral snuff users in Sudan. Carcinogenesis. 1992;13:1001–1005. doi: 10.1093/carcin/13.6.1001. [DOI] [PubMed] [Google Scholar]
- 63.Murphy S.E., Carmella S.G., Idris A.M., Hoffmann D. Uptake and metabolism of carcinogenic levels of tobacco-specific nitrosamines by Sudanese snuff dippers. Cancer Epidemiol. Biomarkers Prev. 1994;3:423–428. [PubMed] [Google Scholar]
- 64.Yang S.S., Smetena I. Determination of free amino acids in tobacco by HPLC with fluorescence detection and precolumn derivatization. Chromatographia. 1993;37:593–598. [Google Scholar]
- 65.McAdam K., Vas C., Kimpton H., Faizi A., Liu C., Porter A., Synnerdahl T., Karlsson P., Rodu B. Ethyl carbamate in Swedish and American smokeless tobacco products and some factors affecting its concentration. Chem. Cent. J. 2018;12:86. doi: 10.1186/s13065-018-0454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meadows J.A., Wargo M.J. Carnitine in bacterial physiology and metabolism. Microbiology. 2015;161:1161–1174. doi: 10.1099/mic.0.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali A.K.S. Tobacco exract induces yeast to Hyphal form transition in the human pathogen, Candida albicans. Am J Clin Microbiol Antimicrob. 2018;1:1013. [Google Scholar]
- 68.Popova V., Ivanova T., Stoyanova A., Nikolova V., Docheva M., Hristeva T., Damyanova S., Nikolov N. Chemical constituents in leaves and aroma products of Nicotiana rustica L. Tobacco. Int. J. Food Stud. 2020:9. [Google Scholar]
- 69.LaVoie E.J., Tucciarone P., Kagan M., Adams J.D., Hoffmann D. Analyses of steam distillates and aqueous extracts of smokeless tobacco. J. Agric. Food Chem. 1989;37:154–157. [Google Scholar]
- 70.Burdock G. CRC Press; Boca Raton: 2010. Fenaroli’s Handbook of Flavor Ingredients. [Google Scholar]
- 71.Kellian C. Drugs in american society: an encyclopedia of history, politics, culture, and the law. Ref. Rev. Inc. Aslib Book Guide. 2015;29:455. [Google Scholar]
- 72.Lilley B.D., Brewer J.H. The selective antibacterial action of phenylethyl alcohol. J. Am. Pharm. Assoc. 1953;42:6–8. doi: 10.1002/jps.3030420103. [DOI] [PubMed] [Google Scholar]
- 73.Ali H., Pätzold R., Brueckner H. Determination of L- and D-amino acids in smokeless tobacco products and tobacco. Food Chem. 2006;99:803–812. [Google Scholar]
- 74.Harrison R., Todd P., Rudge S., Petrides D. 2015. Bioseparations Science and Engineering. [Google Scholar]
- 75.Kaneuchi C., Seki M., Komagata K. Production of succinic Acid from citric Acid and related acids by lactobacillus strains. Appl. Environ. Microbiol. 1988;54:3053–3056. doi: 10.1128/aem.54.12.3053-3056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruce W.F. The decomposition of citric acid by bacillus aertrycke. J. Biol. Chem. 1934;107:119–129. [Google Scholar]
- 77.Song H., Lee S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006;39:352–361. [Google Scholar]
- 78.Bedoukian research . 2018. Technical Data Sheet - 3-METHYL PENTANOIC ACID.http://search.bedoukian.com/flavorfragrance/ff_product.asp?method=POP&id=477 [Google Scholar]
- 79.Matsuzaki T., Shinozaki Y., Suhara S., Ninomiya M., Shigematsu H., Koiwai A. Isolation of glycolipids from the surface lipids of Nicotiana bigelovii and their distribution in Nicotiana species. Agric. Biol. Chem. 1989;53:3079–3082. [Google Scholar]
- 80.Andersen R.A., Fleming P.D., Hamilton-Kemp T.R., Hildebrand D.F. pH Changes in smokeless tobaccos undergoing nitrosation during prolonged storage: effects of moisture, temperature, and duration. J. Agric. Food Chem. 1993;41:968–972. [Google Scholar]
- 81.Banožić M., Jokic S., Ackar D., Blažić M., Šubarić D. Carbohydrates-key players in tobacco aroma formation and quality determination. Molecules. 2020;25:1734. doi: 10.3390/molecules25071734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yadav A.N., Verma D.P., Kumar M., Pal K., Dey R., Gupta A., Padaria J., Gujar G., Kumar S., Suman A., Prasanna R., Saxena A. Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann. Microbiol. 2015;65:611–629. doi: 10.1007/s13213. [DOI] [Google Scholar]
- 83.Al-Hebshi N.N., Alharbi F.A., Mahri M., Chen T. Differences in the bacteriome of smokeless tobacco products with different oral carcinogenicity: compositional and predicted functional analysis. Genes (Basel) 2017:8. doi: 10.3390/genes8040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyx R.E., Stanfill S.B., Keong L.M., Rivera A.J., Satten G.A., Watson C.H. Characterization of bacterial communities in selected smokeless tobacco products using 16S rDNA analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smyth E.M., Kulkarni P., Claye E., Stanfill S., Tyx R., Maddox C., Mongodin E.F., Sapkota A.R. Smokeless tobacco products harbor diverse bacterial microbiota that differ across products and brands. Appl. Microbiol. Biotechnol. 2017;101:5391–5403. doi: 10.1007/s00253-017-8282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia Y., Niu C.-T., Lu Z.-M., Zhang X.-J., Chai L.-J., Shi J.-S., Xu Z.-H., Li Q., Elkins C.A. A bottom-up approach to develop a synthetic microbial community model: application for efficient reduced-salt broad bean paste fermentation. Appl. Environ. Microbiol. 2020;86:e00306–20. doi: 10.1128/AEM.00306-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qian X.-B., Chen T., Xu Y.-P., Chen L., Sun F.-X., Lu M.-P., Liu Y.-X. A guide to human microbiome research: study design, sample collection, and bioinformatics analysis. Chin. Med. J. 2020:133. doi: 10.1097/CM9.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubinstein I., Pedersen G.W. Bacillus species are present in chewing tobacco sold in the United States and evoke plasma exudation from the oral mucosa. Clin. Diagn. Lab. Immunol. 2002;9:1057–1060. doi: 10.1128/CDLI.9.5.1057-1060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yumoto I., Hirota K., Nodasaka Y., Tokiwa Y., Nakajima K. Alkalibacterium indicireducens sp. nov., an obligate alkaliphile that reduces indigo dye. Int. J. Syst. Evol. Microbiol. 2008;58:901–905. doi: 10.1099/ijs.0.64995-0. [DOI] [PubMed] [Google Scholar]
- 90.Okamoto T., Aino K., Narihiro T., Matsuyama H., Yumoto I. Analysis of microbiota involved in the aged natural fermentation of indigo. World J. Microbiol. Biotechnol. 2017;33:70. doi: 10.1007/s11274-017-2238-1. [DOI] [PubMed] [Google Scholar]
- 91.Walter B., Hänssler E., Kalinowski J., Burkovski A. Nitrogen metabolism and nitrogen control in corynebacteria: variations of a common theme. J. Mol. Microbiol. Biotechnol. 2007;12:131–138. doi: 10.1159/000096468. [DOI] [PubMed] [Google Scholar]
- 92.Chen X., Li G.-D., Li Q.-Y., Hu C.-J., Qiu S.-M., Jiang Y., Jiang C.-L., Han L., Huang X.-S. Enteractinococcus lamae sp. Nov. And Enteractinococcus viverrae sp. nov., isolated from animal faeces. Antonie Van Leeuwenhoek. 2015;108:1477–1483. doi: 10.1007/s10482-015-0603-3. [DOI] [PubMed] [Google Scholar]
- 93.Devriese L., Poutrel B., Kilpper-Bälz R., Schleifer K. Staphylococcus gallinarum and Staphylococcus caprae, two new species from animals. Int. J. Syst. Evol. Microbiol. 1983;33:480–486. [Google Scholar]
- 94.Raats D., Halpern M. Oceanobacillus chironomi sp. nov., a halotolerant and facultatively alkaliphilic species isolated from a chironomid egg mass. Int. J. Syst. Evol. Microbiol. 2007;57:255–259. doi: 10.1099/ijs.0.64502-0. [DOI] [PubMed] [Google Scholar]
- 95.Rahi P., Kurli R., Khairnar M., Jagtap S., Pansare A.N., Dastager S.G., Shouche Y.S. Description of Lysinibacillus telephonicus sp. nov., isolated from the screen of a cellular phone. Int. J. Syst. Evol. Microbiol. 2017;67:2289–2295. doi: 10.1099/ijsem.0.001943. [DOI] [PubMed] [Google Scholar]
- 96.Lappan R., Jamieson S.E., Peacock C.S. Reviewing the pathogenic potential of the otitis-associated Bacteria Alloiococcus otitidis and Turicella otitidis. Front. Cell. Infect. Microbiol. 2020:10. doi: 10.3389/fcimb.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harimaya A., Takada R., Somekawa Y., Fujii N., Himi T. High frequency of Alloiococcus otitidis in the nasopharynx and in the middle ear cavity of otitis-prone children. Int. J. Pediatr. Otorhinolaryngol. 2006;70:1009–1014. doi: 10.1016/j.ijporl.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Proctor D.M., Relman D.A. The landscape ecology and microbiota of the human nose, Mouth, and throat. Cell Host Microbe. 2017;21:421–432. doi: 10.1016/j.chom.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gurumurthy M., Rao M., Mukherjee T., Rao S.P.S., Boshoff H.I., Dick T., Barry C.E., 3rd, Manjunatha U.H. A novel F420-dependent anti-oxidant mechanism protects Mycobacterium tuberculosis against oxidative stress and bactericidal agents. Mol. Microbiol. 2013;87:744–755. doi: 10.1111/mmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Halstead M.M., Watson C.H., Pappas R.S. Electron microscopic analysis of surface inorganic substances on oral and combustible tobacco products. J. Anal. Toxicol. 2015;39:698–701. doi: 10.1093/jat/bkv097. [DOI] [PMC free article] [PubMed] [Google Scholar]