Highlights
-
•
The incidence of ES of the bone is very low, reaching a peak in adolescence.
-
•
We assessed the patterns of DM and the prognostic factors in this patient subset.
-
•
Lung and bone are the most frequently distant metastatic sites.
-
•
Bone metastasis is an independent risk factor for inferior survival.
Keywords: Ewing sarcoma, Distant metastasis, Bone metastasis, Prognosis, SEER
Abstract
Background
Ewing sarcoma (ES) of bone is accounting for the second most common type of primary bone cancer in children and adolescents. However, the patterns of distant metastasis (DM) and the effect of the sites of DM on survival outcomes were not investigated.
Aims
This study aimed to investigate the patterns of DM and the prognostic factors related to outcomes in primary metastatic ES of the bone.
Methods
Patients who were diagnosed with primary metastatic ES between 2010 and 2018 were identified from the Surveillance, Epidemiology, and End Results database. Kaplan–Meier analysis, log-rank tests, and Cox proportional-hazards regression models were used for statistical analyses.
Results
We identified 277 patients in this study and 95.3% of them (n = 264) receiving chemotherapy. A total of 371 sites of DM were observed. Lung was the most common distant metastatic site (n = 182, 49.1%), followed by bone (n = 139, 37.5%), distant lymph node (n = 26, 7.0%), liver (n = 14, 3.8%), and brain (n = 10, 2.7%). Three-year cause-specific survival (CSS) was 56.1% in the entire cohort. Older age (hazard ratio [HR] 2.210, P < 0.001) and bone metastasis (HR 1.903, P = 0.002) were the independent prognostic factors associated with inferior CSS. Similar results were found in those with bone-only metastasis (n = 80) or lung-only metastasis (n = 117), which showed that patients with bone-only metastasis had an inferior CSS compared to those with metastases only to the lung (HR 1.926, P = 0.005).
Conclusions
Lung and bone are the most frequently distant metastatic sites in patients with primary metastatic ES of bone. Bone metastasis is an independent risk factor for inferior survival.
1. Introduction
Ewing sarcoma (ES) of bone is accounting for the second most common type of primary bone cancer in children and adolescents [1], [2]. It was observed more frequently in Asians and Whites than in Blacks [2]. Despite the application of multidisciplinary treatment improves survival outcomes substantially [3], 30%-40% of patients will eventually develop local or distant failure after treatment [4]. In addition, approximately 20%–30% of the ES patients presenting with de novo stage IV metastatic disease [5], which would modify the survival outcomes with a poor prognosis [6].
Previous studies from the Several Surveillance, Epidemiology, and End Results (SEER) studies had found several predictive indicators related to the increased risk of detectable metastatic disease in ES, including older age, larger tumor diameter, and axial tumor location [7], [8]. Moreover, the prognostic factors affecting the prognosis of metastatic ES have been identified in several studies, including older age, larger tumor volume, axial tumor location, and the rise of serum lactate dehydrogenase [9], [10], [11]. However, the patterns of distant metastasis (DM) and the effect of the sites of DM on survival outcomes were not investigated. The current staging of ES was based on the American Joint Committee on Cancer staging system. However, the risk stratification for ES patients with primary metastatic disease is limited. There were heterogeneous survival outcomes in patients with DM, with a survival rate of 22%-62% [12], [13], [14], [15]. Therefore, the reasonable classification of the patients with metastatic disease must be investigated to better risk-stratify patients for subsequent surveillance and guide appropriate treatment.
In recent years, the patterns of DM as prognostic indicators in de novo stage IV metastatic cancers have been investigated increasingly, which provided additional information for clinical practice [16], [17], [18]. However, the progression of understanding the epidemiology of primary metastatic ES is limited. In addition, whether the prognostic factors in ES patients who occur distant failure after multidisciplinary treatment can be applied to patients who present with primary metastatic disease remains unclear. In light of this, our study aimed to conduct a retrospective analysis to determine the patterns of DM and the prognostic factors in patients with primary metastatic ES of the bone.
2. Materials and methods
2.1. Study population
Patients data were identified from the SEER program [19]. The SEER program includes 18 cancer registries, which represent approximately 35% of the United States population. Patients diagnosed with primary metastatic (de novo stage IV) ES of the bone between 2010 and 2018 were identified. Patients with available patterns of DM were identified in this study, including bone, lung, liver, brain, and distant lymph node metastasis. Because of the de-identified information of patient data in the SEER program, informed consent or ethical approval was no requirement of this study.
2.2. Variables
We investigated the effect of the following variables, including age, race/ethnicity, gender, tumor size, tumor location, regional nodal status, specific sites of DM, and clinical management, upon the survival of primary metastatic ES patients. Age was classified into two categories: <25 years and ≥25 years. Tumor location was divided into axial and extremity. The sites of DM comprised lung, brain, bone, liver, and distant lymph nodes. The primary endpoint of this study was cause-specific survival (CSS), which was determined by the time of diagnosis of ES to death from malignancy of bones and joints.
2.3. Statistical analyses
CSS were estimated using Kaplan-Meier methods, and log-rank tests were carried to compare survival differences among subgroups. Cox proportional hazards models were performed to determine the independent prognostic factors related to CSS. Multivariate analyses were performed including the variables positively associated with prognosis in univariate analysis (P<0.05 as a cutoff). SPSS 21.0 (IBM Corporation, Armonk, NY) and MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium) were used for statistical analyses, and P<0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
We identified 277 patients in this study with a median age of 17 years (range, 0-87 years). Table 1 lists the details of patient’s baseline characteristics. In patients with available tumor size (n=211) or regional nodal status (n=226), 91.5% (n=193) were tumor size >5cm and 81.4% (n=184) were regional node-negative. Regarding the tumor location, 58.1% (n=161) and 51.9% (n=116) of patients were tumor located in axial and extremity, respectively.
Table 1.
Patient’s baseline characteristics.
| Variables | Number (%) |
|---|---|
| Age (years) | |
| <25 | 195 (70.4) |
| ≥25 | 82 (29.6) |
| Gender | |
| Male | 172 (62.1) |
| Female | 105 (37.9) |
| Race/ethnicity | |
| White | 232 (83.8) |
| Black | 14 (5.1) |
| Other | 31 (11.2) |
| Tumor location | |
| Axial | 161 (58.1) |
| Extremity | 116 (41.9) |
| Tumor size | |
| ≤5 cm | 18 (6.5) |
| >5–10 cm | 95 (34.3) |
| >10 cm | 98 (35.4) |
| Unknown | 66 (23.8) |
| Regional nodal status | |
| Negative | 184 (66.4) |
| Positive | 42 (15.2) |
| Unknown | 51 (18.4) |
| Distant lymph node metastasis | |
| No | 251 (90.6) |
| Yes | 26 (9.4) |
| Bone metastasis | |
| No | 13 8(49.8) |
| Yes | 139 (50.2) |
| Lung metastasis | |
| No | 95 (34.3) |
| Yes | 182 (65.7) |
| Liver metastasis | |
| No | 263 (94.9) |
| Yes | 14 (5.1) |
| Brain metastasis | |
| No | 267 (96.4) |
| Yes | 10 (3.6) |
| Primary surgery | |
| No | 198 (71.5) |
| Yes | 79 (28.5) |
| Radiation therapy | |
| No | 94 (33.9) |
| Yes | 183 (66.1) |
| Chemotherapy | |
| No | 13 (4.7) |
| Yes | 264 (95.3) |
| Number of metastatic sites | |
| 1 | 204 (73.6) |
| 2 | 54 (19.5) |
| 3 | 17 (6.1) |
| 4 | 2 (0.7) |
| 5 | 0 (0) |
Regarding the treatment at the diagnosis of de novo stage IV disease, most patients (n=264, 95.3%) received chemotherapy. In addition, 28.5% (n=79) and 66.1% (n=183) of them were treated with primary surgery and primary radiotherapy, respectively.
3.2. The patterns of DM
The distributions of DM sites are shown in Table 2. Among the 277 patients, a total of 371 sites of DM were observed. Lung was the most common site of DM (n=182, 49.1%), followed by bone (n=139, 37.5%), distant lymph node (n=26, 7.0%), liver (n=14, 3.8%), and brain (n=10, 2.7%). In addition, 204 (73.6%), 54 (19.5%), 17 (6.1%), and 2 (0.7%) patients had one, two, three, and four sites of DM, respectively. No patients had five sites of DM.
Table 2.
The distribution of distant metastases sites.
| The specific site of distant metastasis | Number (%) |
|---|---|
| Lung alone | 117 (42.2) |
| Bone alone | 80 (28.9) |
| Distant lymph node alone | 5 (1.8) |
| Liver alone | 1 (0.4) |
| Brain alone | 1 (0.4) |
| Bone + Lung | 35 (12.6) |
| Lung + Distant lymph node | 9 (3.2) |
| Liver + Lung | 3 (1.1) |
| Bone + Brain | 3 (1.1) |
| Bone + Distant lymph node | 2 (0.7) |
| Brain + Distant lymph node | 1 (0.4) |
| Brain + Lung | 1 (0.4) |
| Bone + Lung + Distant lymph node | 6 (2.2) |
| Bone + Lung + Liver | 6 (2.2) |
| Bone + Brain + Lung | 3 (1.1) |
| Bone + Liver + Distant lymph node | 1 (0.4) |
| Bone + Brain + Liver | 1 (0.4) |
| Bone + Liver + Lung + Distant lymph node | 2 (0.7) |
3.3. Survival
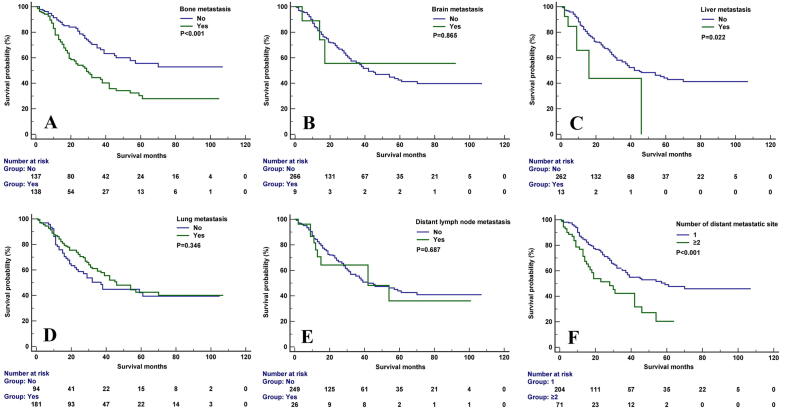
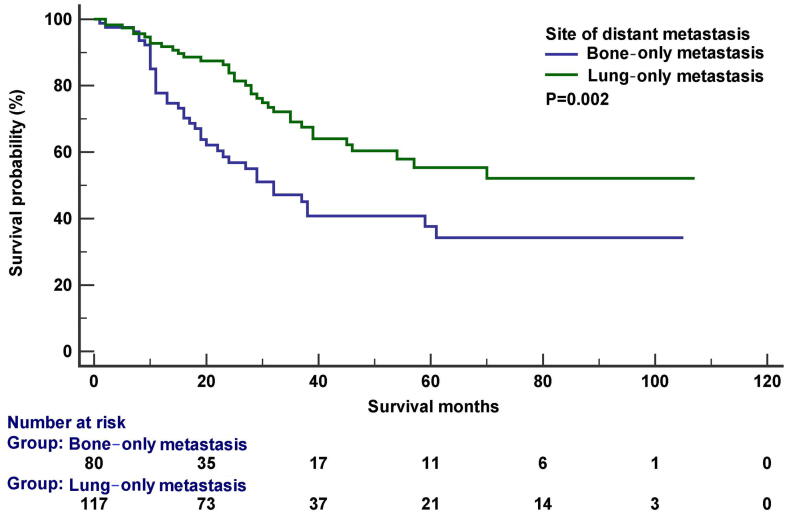
The median follow-up was 20 months (range, 0-107 months). In the entire cohort, 137 death were observed, including 111 patients were cancer-specific death. The 5-year CSS was 56.1% in the entire cohort. In the Kaplan–Meier survival analysis, bone metastasis was associated with inferior CSS (P<0.001), the 3-year CSS was 44.4% and 67.7% in patients with and without bone metastasis, respectively. Liver metastasis was also related to lower CSS (P=0.022), the 3-year CSS was 43.9% and 56.7% in patients with and without liver metastasis, respectively. Moreover, the number of metastatic sites had significantly impact CSS (P<0.001), the 3-year CSS was 60.3% and 42.3% in patients with single and two or more sites of DM, respectively. However, lung (P=0.346), brain (P=0.865), and distant lymph node metastases (P=0.687) had no significant effect on CSS. Figure 1 displays the survival curves for CSS after stratification by the specific site of DM. In patients with bone-only metastasis (n=80) or lung-only metastasis (n=117), the 3-year CSS was 47.1% and 69.1%, respectively (P=0.002) (Figure 2).
Fig. 1.
The cancer-specific survival curves stratified by the sites of distant metastasis and the number of metastatic sites (A, bone metastasis; B, brain metastasis; C, liver metastasis; D, lung metastasis; E, distant lymph node metastasis; F, the number of metastatic sites).
Fig. 2.
The cancer-specific survival curves between those with bone-only metastasis and lung-only metastasis.
3.4. Prognostic analyses
The results of prognostic analyses in the entire cohort are shown in Table 3. The univariate analysis showed that age, race/ethnicity, tumor size, bone metastasis, liver metastasis, and the number of DM sites were the prognostic factors related to CSS (all P<0.05). Next, we conducted multivariate analysis after adjustment for the aforementioned prognostic factors, older age (hazard ratio [HR] 2.210, 95% confidence interval [CI] 1.490-3.279, P<0.001) and bone metastasis (HR 1.903, 95%CI 1.254-2.887, P=0.002) remained the independent prognostic factors for inferior CSS. However, race/ethnicity, liver metastasis, and the number of DM sites were not associated with CSS in the multivariate analysis.
Table 3.
Univariate and multivariable Cox regression analyses for independent prognostic factors affecting cause-specific survival in the entire cohort (n = 277).
| Variables | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (years) | ||||||
| <25 | 1 | 1 | ||||
| ≥25 | 2.305 | 1.558–3.411 | <0.001 | 2.210 | 1.490–3.279 | <0.001 |
| Gender | ||||||
| Male | 1 | — | ||||
| Female | 1.013 | 0.687–1.493 | 0.948 | — | — | — |
| Race/ethnicity | ||||||
| White | 1 | 1 | ||||
| Black | 2.184 | 1.058–4.506 | 0.035 | 1.595 | 0.734–3.467 | 0.238 |
| Other | 1.199 | 0.656–2.192 | 0.554 | 0.862 | 0.458–1.622 | 0.645 |
| Tumor location | ||||||
| Axial | 1 | — | ||||
| Extremity | 0.989 | 0.679–1.440 | 0.952 | — | — | — |
| Tumor size | ||||||
| ≤5 cm | 1 | 1 | ||||
| >5–10 cm | 0.386 | 0.177–0.844 | 0.017 | 0.420 | 0.186–0.944 | 0.036 |
| >10 cm | 0.555 | 0.259–1.192 | 0.131 | 0.586 | 0.263–1.306 | 0.191 |
| Unknown | 0.743 | 0.342–1.612 | 0.452 | 0.568 | 0.250–1.290 | 0.176 |
| Regional nodal status | ||||||
| Negative | 1 | — | ||||
| Positive | 1.105 | 0.678–1.802 | 0.688 | — | — | — |
| Unknown | 0.920 | 0.441–1.919 | 0.824 | — | — | — |
| The sites of distant metastases | ||||||
| Bone yes vs. no | 2.183 | 1.482–3.216 | <0.001 | 1.903 | 1.254–2.887 | 0.002 |
| Lung yes vs. no | 0.833 | 0.567–1.223 | 0.351 | |||
| Liver yes vs. no | 2.531 | 1.102–5.814 | 0.029 | 1.436 | 0.582–3.541 | 0.433 |
| Brain yes vs. no | 0.906 | 0.288–2.855 | 0.866 | |||
| Distant lymph node yes vs. no | 1.142 | 0.596–2.189 | 0.69 | |||
| The number of distant metastases | ||||||
| 1 | 1 | 1 | ||||
| ≥2 | 2.063 | 1.376–3.095 | <0.001 | 1.458 | 0.943–2.255 | 0.090 |
CI, confidence interval; HR, hazard ratio.
Moreover, we conducted univariate and multivariate prognostic analyses among those with bone-only metastasis or lung-only metastasis (Table 4). The results of the univariate analysis showed that age, tumor size, and the sites of DM were the prognostic factors related to CSS (all P<0.05). Multivariate analysis was conducted with adjustment for the aforementioned prognostic factors. The results showed that older age (HR 2.372, 95%CI 1.453-3.871, P=0.001) and bone-only metastasis (HR 1.926, 95%CI 1.216-3.048, P=0.005) were the independent prognostic factors associated with inferior CSS.
Table 4.
Univariate and multivariable Cox regression analyses for independent prognostic factors affecting cause-specific survival in those with bone-only metastasis or lung-only metastasis (n = 197).
| Variables | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (years) | ||||||
| <25 | 1 | 1 | ||||
| ≥25 | 2.472 | 1.519–4.023 | <0.001 | 2.372 | 1.453–3.871 | 0.001 |
| Gender | ||||||
| Male | 1 | — | ||||
| Female | 0.969 | 0.607–1.547 | 0.896 | — | — | — |
| Race/ethnicity | ||||||
| White | 1 | — | ||||
| Black | 1.890 | 0.685–5.214 | 0.219 | — | — | — |
| Other | 1.340 | 0.665–2.701 | 0.414 | — | — | — |
| Tumor location | ||||||
| Axial | 1 | — | ||||
| Extremity | 1.020 | 0.644–1.613 | 0.934 | — | — | — |
| Tumor size | ||||||
| ≤5 cm | 1 | 1 | ||||
| >5–10 cm | 0.385 | 0.165–0.899 | 0.027 | 0.510 | 0.216–1.201 | 0.123 |
| >10 cm | 0.481 | 0.206–1.121 | 0.090 | 0.623 | 0.266–1.460 | 0.276 |
| Unknown | 0.638 | 0.267–1.524 | 0.312 | 0.716 | 0.298–1.718 | 0.455 |
| Regional nodal status | ||||||
| Negative | 1 | — | ||||
| Positive | 1.035 | 0.529–2.024 | 0.920 | — | — | — |
| Unknown | 0.929 | 0.369–2.338 | 0.876 | — | — | — |
| The sites of distant metastases | ||||||
| Lung-only | 1 | 1 | ||||
| Bone-only | 2.009 | 1.272–3.175 | 0.003 | 1.926 | 1.216–3.048 | 0.005 |
CI, confidence interval; HR, hazard ratio.
4. Discussion
In the present study, we sought to determine the patterns of DM and the prognostic factors associated with survival in primary metastatic ES using a population-based cohort in recent years. Our results showed that lung and bone were the most common sites of DM in patients with primary metastatic ES. However, only bone metastasis was associated with inferior survival for this population. Our study could help clinicians to improve patient counseling, “tailor” subsequent surveillance, better risk stratification in staging study, and guide appropriate treatment for this patient subset.
A previous study from the SEER program showed that tumor size >5cm and tumor located in the pelvic were the independent prognostic factors for DM [7]. In this study, we also found that 91.5% of patients were tumor size >5cm. Moreover, in patients with available regional lymph node status, only 18.6% of them had node-positive disease. Several previous studies also showed a lower risk of regional lymph node metastasis in non-metastatic ES patients [20], [21]. These results indicated that the presence of regional lymph node metastasis may not be associated with a higher risk of hematogenous dissemination, which may prove useful in risk stratification for this patient subset.
The incidence of ES is very low, reaching a peak in adolescence [22]. It is challenging to include a large cohort to investigate the patterns of DM for this patient subset. Several studies from the SEER program have investigated the survival outcome of primary metastatic ES [7], [8], [23]. However, the patterns of DM were not analyzed in these studies [7], [8], [23]. A study from Paulino et al. included 30 patients with primary metastatic ES, a total of 52 sites of DM were observed. Of these patients, 19 (36.5%), 18 (34.6%), 2 (3.8%), and 2 (3.8%) patients were presented with lung, bone, liver, and brain metastases, respectively [15]. Another study from Children’s Oncology Group included 110 patients with primary metastatic ES, 38.5%, 38.5%, and 23.1% of patients had lung metastasis, bones/bone marrow metastases, and combinations or others, respectively [24]. Moreover, Paulussen et al. included 171 primary metastatic ES patients from the European Intergroup Cooperative Ewing Sarcoma Studies (EICESS), 35.7%, 37.4%, and 21.1% of patients had lung, bone, and lung+bone metastases. However, only 5 (2.9%), 2 (1.2%), and 0 patients had distant lymph node, brain, and liver metastases, respectively [25]. In our study, 49.1%, 37.5%, 7.0%, 3.8%, and 2.7% of patients presented with lung, bone, distant lymph node, liver, and brain metastases, which were similar to the previous studies [15], [24], [25]. In patients with distant metastatic disease after definitive treatment, lung (48.7%) and bone (33.3%) were the most frequent metastatic sites [26]. These results suggest that ES is a heterogeneous subtype and more prone to have lung and bone metastases, and less potential for liver and brain metastases compared to the epithelial tumor.
Due to the different number of patients in various studies [15], [24], [27], the role of metastatic sites for survival in ES remains controversial. Similar to our results, several studies have found that bone metastasis portended a particularly inferior survival outcome than those with lung metastasis [25], [28], [29], [30]. The results from EICESS studies also showed that patients with bone metastasis had inferior outcomes than those with lung metastasis (P=0.0087) [25]. In addition, the findings from Euro-EWING 99 trial showed that bone metastasis confer a poorer 5-year relapse-free survival than those with lung/pleural metastases (<21% vs. 55%) [31]. In our study, lung metastasis was also not related to inferior survival, while patients with bone-only metastasis had significantly lower CSS compared to those with lung-only metastasis.
As part of the curative treatment in stage IV ES, whole lung radiotherapy has been routinely used to treat lung metastasis, and it has significantly improved the long-term outcomes [12], [32], [33], [34]. The better prognosis for patients with lung metastasis may reflect the unique biological characteristics or the distinctive microenvironment of the cancer cells. Tumor cells that located in the bone may have a higher malignant potential compared to tumor cells that merely settled in the first capillary bed after detachment from the primary tumor [25]. The study from the EICESS also showed an inferior relapse-free survival in those with bone metastasis compared to those with lung metastasis, with a 5-year relapse-free survival was 19% and 29%, respectively [14]. In ES patients with relapsed disease after local treatment and systemic chemotherapy, the 5-year event-free survival was also significantly related to the site of first DM, the 5-year event-free survival was 1.5% for those with bone metastasis and was 11.5% for those with lung metastasis [35]. This phenomenon has important implications for the treatment of specific organ metastasis and the prediction of the prognosis with primary metastatic ES.
In patients with non-metastatic ES after definitive treatment, older age was an independent prognostic factor for survival outcomes [21], [36], [37], [38], [39]. In patients with primary metastatic ES, our study also found that older age was associated with a higher risk of death. The underlying reason for this phenomenon is the efficacy of native tumor suppression pathways in the pediatric immune system, which can inhibit tumor growth and delay DM [40], [41]. Moreover, older ES has a special biological behavior than younger patients, thereby leading to a high tendency to DM by different host immune system evasion mechanisms [36].
Several limitations should be acknowledged in our study. First, inherently biased in any retrospective studies. Second, although most patients (95.3%) received chemotherapy in this study, the specific chemotherapeutic agents and intensity of chemotherapy used in the treatment for this patient subset were not recorded in the SEER program. Moreover, radiotherapy dose, Radiotherapy target volume definition, and detailed surgical information were also missing in the SEER database. Finally, the response to treatment and the treatment after disease progression are also not available in the SEER program. Despite these shortcomings, the SEER program can be an unparalleled resource when studying rare cancers such as ES. Moreover, we included patients diagnosed after 2010, which was more representative of contemporary multidisciplinary treatment of ES.
5. Conclusion
In conclusion, lung and bone are the most frequently distant metastatic sites in patients with primary metastatic ES of bone. Bone metastasis is an independent risk factor for inferior survival. The findings from our study would provide additional information for follow-up strategies, patient counseling, risk stratification, and treatment decision-making for this patient subset.
6. Funding statement
This work was partly supported by the Scientific Research Project of Hainan General Hospital (No. QN 202003).
7. Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: the Surveillance, Epidemiology and End Results database (www.seer.cancer.gov).
CRediT authorship contribution statement
Lei Zhang: Writing - review & editing. Lu Xiong: Writing - review & editing. Li-Mei Wu: Writing - review & editing. Wen-Hui Shen: Writing - review & editing. Ping Zhou: Software. Chen-Lu Lian: Software. Wen-Tong Zhang: Writing - review & editing. San-Gang Wu: Data curation, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was partly supported by the Scientific Research Project of Hainan General Hospital (No. QN 202003).
Contributor Information
Wen-Tong Zhang, Email: zhang.wentong@zsxmhospital.com.
San-Gang Wu, Email: wusg@xmu.edu.cn.
References
- 1.Marina N. Malignant bone tumors. In: Lanzkowsky P., editor. Manual of Pediatric Hematology and Oncology. 5th ed. Academic Press; London, UK: 2010. pp. 739–757. [Google Scholar]
- 2.National Cancer Institute. Ewing sarcoma treatment (PDQ)—health professional version. April 4, 2018. https://www.cancer.gov/types/bone/hp/ewing-treatment-pdq. Accessed Nov 7, 2020.
- 3.Balamuth N.J., Womer R.B. Ewing's sarcoma. Lancet Oncol. 2010;11(2):184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 4.Potratz J., Dirksen U., Jürgens H., Craft A. Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol. 2012;29(1):1–11. doi: 10.3109/08880018.2011.622034. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Galindo C., Navid F., Liu T., Billups C.A., Rao B.N., Krasin M.J. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19(4):814–820. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari S., Luksch R., Hall K.S., Fagioli F., Prete A., Tamburini A., Tienghi A., DiGirolamo S., Paioli A., Abate M.E., Podda M., Cammelli S., Eriksson M., Brach del Prever A. Post-relapse survival in patients with Ewing sarcoma. Pediatr Blood Cancer. 2015;62(6):994–999. doi: 10.1002/pbc.v62.610.1002/pbc.25388. [DOI] [PubMed] [Google Scholar]
- 7.Shi J., Yang J., Ma X., Wang X.u. Risk factors for metastasis and poor prognosis of Ewing sarcoma: a population based study. J Orthop Surg Res. 2020;15(1) doi: 10.1186/s13018-020-01607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramkumar D.B., Ramkumar N., Miller B.J., Henderson E.R. Risk factors for detectable metastatic disease at presentation in Ewing sarcoma - An analysis of the SEER registry. Cancer Epidemiol. 2018;57:134–139. doi: 10.1016/j.canep.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G, Ferrari S, Bertoni F et al (2000) Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol 18:4-11. https://doi.org/10.1200/JCO.2000.18.1.4. [DOI] [PubMed]
- 10.Leavey P.J., Mascarenhas L., Marina N., Chen Z., Krailo M., Miser J., Brown K., Tarbell N., Bernstein M.L., Granowetter L., Gebhardt M., Grier H.E. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51(3):334–338. doi: 10.1002/pbc.v51:310.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacci G., Forni C., Longhi A., Ferrari S., Donati D., De Paolis M., Barbieri E., Pignotti E., Rosito P., Versari M. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer. 2004;40(1):73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Elghazawy H., Nasr A., Zaky I., Zamzam M., Elgammal A., Farid N., Zaghloul M.S. Whole lung irradiation for completely responding pulmonary metastases in pediatric Ewing sarcoma. Future Oncol. 2020;16(15):1043–1051. doi: 10.2217/fon-2020-0066. [DOI] [PubMed] [Google Scholar]
- 13.Robinson SI, Ahmed SK, Okuno SH et al (2014) Clinical outcomes of adult patients with relapsed Ewing sarcoma: a 30-year single-institution experience. Am J Clin Oncol 37:585-591. https://doi.org/10.1097/COC.0b013e318281d6ab. [DOI] [PubMed]
- 14.Cotterill S.J., Ahrens S., Paulussen M., Jürgens H.F., Voûte P.A., Gadner H., Craft A.W. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 15.Paulino A.C., Mai W.Y., Teh B.S. Radiotherapy in metastatic ewing sarcoma. Am J Clin Oncol. 2013;36:283–286. doi: 10.1097/COC.0b013e3182467ede. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Huang T., Mao M. Metastatic Patterns and Prognosis of de novo Metastatic Nasopharyngeal Carcinoma in the United States. Laryngoscope. 2021;131:E1130–E1138. doi: 10.1002/lary.28983. [DOI] [PubMed] [Google Scholar]
- 17.Wu S.G., Zhang W.W., He Z.Y. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res. 2017;9:781–788. doi: 10.2147/CMAR.S150350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X.u., Zhang C., Ma W., Tian F., Xu G., Han X., Sun P., Baklaushev V.P., Bryukhovetskiy A.S., Wang G., Ma Y., Wang X. Patterns of bone metastases in newly diagnosed colorectal cancer: a real-world analysis in the SEER database. Int J Colorectal Dis. 2019;34(3):533–543. doi: 10.1007/s00384-018-3213-5. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
- 20.Applebaum M.A., Goldsby R., Neuhaus J., DuBois S.G. Clinical features and outcomes in patients with Ewing sarcoma and regional lymph node involvement. Pediatr Blood Cancer. 2012;59(4):617–620. doi: 10.1002/pbc.v59.410.1002/pbc.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma V., Denniston K.A., Lin C.J. A Comparison of Pediatric vs. Adult Patients with the Ewing Sarcoma Family of Tumors. Front. Oncol. 2017;7(82) doi: 10.3389/fonc.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mascarenhas L, Siegel S, Spector L et al (2026) Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Bethesda, MD: National Cancer Institute; 2006. p. 97–109.
- 23.Duchman K.R., Gao Y., Miller B.J. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39(2):189–195. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein M.L., Devidas M., Lafreniere D., Souid A.-K., Meyers P.A., Gebhardt M., Stine K., Nicholas R., Perlman E.J., Dubowy R., Wainer I.W., Dickman P.S., Link M.P., Goorin A., Grier H.E. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children's Cancer Group Phase II Study 9457–a report from the Children's Oncology Group. J Clin Oncol. 2006;24(1):152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- 25.Paulussen M., Ahrens S., Burdach S., Craft A., Dockhorn-Dworniczak B., Dunst J., Fröhlich B., Winkelmann W., Zoubek A., Jürgens H. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 1998;9(3):275–281. doi: 10.1023/A:1008208511815. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y., Lim D.H., Lee S.H., Lyu C.J., Im J.H., Lee Y.-H., Suh C.-O. Role of Radiotherapy in the Multimodal Treatment of Ewing Sarcoma Family Tumors. Cancer Res Treat. 2015;47(4):904–912. doi: 10.4143/crt.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosma S.E., Rueten‐Budde A.J., Lancia C., Ranft A., Dirksen U., Krol A.D., Gelderblom H., Sande M.A.J., Dijkstra P.D.S., Fiocco M. Individual risk evaluation for local recurrence and distant metastasis in Ewing sarcoma: A multistate model: A multistate model for Ewing sarcoma. Pediatr Blood Cancer. 2019;66(11) doi: 10.1002/pbc.27943. [DOI] [PubMed] [Google Scholar]
- 28.Carli M., Colombatti R., Oberlin O., Bisogno G., Treuner J., Koscielniak E., Tridello G., Garaventa A., Pinkerton R., Stevens M. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol. 2004;22(23):4787–4794. doi: 10.1200/JCO.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 29.Oberlin O., Rey A., Lyden E., Bisogno G., Stevens M.C.G., Meyer W.H., Carli M., Anderson J.R. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26(14):2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey D.L., Wexler L.H., Meyers P.A., Magnan H., Chou A.J., Wolden S.L. Radiation for bone metastases in Ewing sarcoma and rhabdomyosarcoma. Pediatr Blood Cancer. 2015;62(3):445–449. doi: 10.1002/pbc.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladenstein R., Pötschger U., Le Deley M.C., Whelan J., Paulussen M., Oberlin O., van den Berg H., Dirksen U., Hjorth L., Michon J., Lewis I., Craft A., Jürgens H. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28(20):3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 32.Jaffe N., Paed D., Traggis D. Improved outlook for Ewing's sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy. Cancer. 1976;38:1925–1930. doi: 10.1002/1097-0142(197611)38:5<1925::aid-cncr2820380510>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Bölling T., Schuck A., Paulussen M., Dirksen U., Ranft A., Könemann S., Dunst J., Willich N., Jürgens H. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Toxicity analysis and treatment results of the EICESS-92 trialGanzlungenbestrahlung bei Patienten mit ausschließlicher Lungenmetastasierung von Ewing-Tumoren. Toxizitätsanalyse und Behandlungsergebnisse der EICESS-92-Studie. Strahlenther Onkol. 2008;184(4):193–197. doi: 10.1007/s00066-008-1810-x. [DOI] [PubMed] [Google Scholar]
- 34.Rodeberg D., Arndt C., Breneman J., Lyden E., Donaldson S., Paidas C., Andrassy R., Meyer W., Wiener E. Characteristics and outcomes of rhabdomyosarcoma patients with isolated lung metastases from IRS-IV. J Pediatr Surg. 2005;40(1):256–262. doi: 10.1016/j.jpedsurg.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 35.Bacci G, Longhi A, Ferrari S (1999) Pattern of relapse in 290 patients with nonmetastatic Ewing's sarcoma family tumors treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. Eur J Surg Oncol 32:974-979. https://doi.org/doi: 10.1016/j.ejso.2006.01.023. [DOI] [PubMed]
- 36.Baldini E.H., Demetri G.D., Fletcher C.D.M., Foran J., Marcus K.C., Singer S. Adults with Ewing's sarcoma/primitive neuroectodermal tumor: adverse effect of older age and primary extraosseous disease on outcome. Ann Surg. 1999;230(1):79. doi: 10.1097/00000658-199907000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craft A., Cotterill S., Malcolm A., Spooner D., Grimer R., Souhami R., Imeson J., Lewis I. Ifosfamide-containing chemotherapy in Ewing's sarcoma: The Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. J Clin Oncol. 1998;16(11):3628–3633. doi: 10.1200/JCO.1998.16.11.3628. [DOI] [PubMed] [Google Scholar]
- 38.Grier H.E., Krailo M.D., Tarbell N.J., Link M.P., Fryer C.J.H., Pritchard D.J., Gebhardt M.C., Dickman P.S., Perlman E.J., Meyers P.A., Donaldson S.S., Moore S., Rausen A.R., Vietti T.J., Miser J.S. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 39.Marina N., Granowetter L., Grier H.E., Womer R.B., Randall R.L., Marcus K.J., McIlvaine E., Krailo M. Age, Tumor Characteristics, and Treatment Regimen as Event Predictors in Ewing: A Children's Oncology Group Report. Sarcoma. 2015;2015:1–8. doi: 10.1155/2015/927123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beheshti A., Wage J., McDonald J.T., Lamont C., Peluso M., Hahnfeldt P., Hlatky L. Tumor-host signaling interaction reveals a systemic, age-dependent splenic immune influence on tumor development. Oncotarget. 2015;6(34):35419–35432. doi: 10.18632/oncotarget.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beheshti A., Benzekry S., McDonald J.T., Ma L., Peluso M., Hahnfeldt P., Hlatky L. Host age is a systemic regulator of gene expression impacting cancer progression. Cancer Res. 2015;75(6):1134–1143. doi: 10.1158/0008-5472.CAN-14-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: the Surveillance, Epidemiology and End Results database (www.seer.cancer.gov).