Abstract
Primary adrenal lymphoma (PAL) and primary renal lymphoma (PRL) are rare extranodal lymphomas, predominantly of diffuse large B-cell lymphoma subtype. Primary adrenal and renal lymphomas (PARL) exhibit a high predilection for the central nervous system (CNS). Therefore, current guidelines support the use of CNS prophylaxis in PARL, particularly in cases of high-risk Central Nervous System International Prognostic Index (CNS-IPI). However, the route of administration (i.e. systemic vs. intrathecal chemotherapy) has not been clearly elucidated. With this in mind, we initiated an international collaboration and literature review to analyze 50 patient cases, 20 of which received CNS prophylaxis. Based on our analysis, we conclude that PARL may indicate a need for CNS chemo-prophylaxis in the form of systemic high-dose methotrexate (HD-MTX) over intrathecal methotrexate (IT-MTX), although IT-MTX may still have utility in certain cases.
Keywords: PAL, PRL, Methotrexate, CNS, Prophylaxis, CNS relapse
1. Introduction
Primary adrenal lymphoma (PAL) and primary renal lymphoma (PRL) are both extremely rare types of non-Hodgkin lymphoma, mostly composed of the diffuse large B-cell lymphoma (DLBCL) subtype [1, 2]. PAL and PRL (referred to as PARL hereafter) are defined as lymphomas histologically proven in the adrenal gland or kidney, respectively, with no evidence of primary involvement elsewhere [1, 3]. Because of their scarcity and poor prognosis, conclusions about epidemiology, overall prognosis, pathogenesis, and best treatment options remain unsettled.
Central nervous system (CNS) involvement in DLBCL occurs in less than 5% of patients, resulting in an overall survival (OS) of less than six months [4]. The Central Nervous System International Prognostic Index (CNS-IPI) is the most commonly used tool in determining the risk of CNS relapse in DLBCL. It was developed and validated to predict the development of CNS relapse and progression in DLBCL patients in order to identify high-risk patients where CNS prophylaxis is indicated [5]. Risk factors used to calculate the CNS-IPI are age greater than 60 years old, poor performance status, high lactate dehydrogenase (LDH), more than one extranodal site, stage III/IV, and adrenal/renal involvement. High risk CNS-IPI is defined by having 4–6 of the risk factors. Traditional guidelines state that patients with high-risk CNS-IPI scores have a >10% risk of CNS relapse and should be given CNS prophylaxis, while patients with low and intermediate-risk scores have <5% risk of CNS relapse and prophylaxis can be deferred [5]. More recent studies have refined this approach, and state that prophylaxis should also be given for activated B-cell-like (ABC) cell-of-origin [6], MYC/BCL2 double expressers [7], high-grade B-cell lymphoma with translocation of MYC and BCL2 and/or BCL6 (also known as double/triple hit lymphoma) [8], and certain high-risk locations (e.g. primary testicular)[9]. However, guidelines regarding the optimal route of administration have not been clearly elucidated. Here, we provide data analysis of 50 cases of PARL on disease biology, risk of CNS relapse, CNS prophylaxis, and outcomes.
2. Materials and methods
Data collection includes patients from Tulane University (1) and Italy (16). Additionally, we conducted a comprehensive literature review on PARL cases reported from January 1st 1998 to January 1st 2021 in PubMed. Keywords used (single or in combination) included: primary adrenal lymphoma, PAL, primary renal lymphoma, PRL, diffuse large B-cell lymphoma, DLBCL, and CNS prophylaxis. Excluded cases include PARL with CNS involvement at onset, unknown or unidentified staging, unclear if CNS prophylaxis used, unclear individual OS outcomes, and histology other than DLBCL (e.g. NK/T-cell, follicular, etc.). Patients under the age of 18 were also excluded. Finally, only cases that used rituximab-based chemotherapy were included in this analysis to limit a possible confounder to OS (Fig. 1A). Data was tabulated for the following 12 variables: age, gender, Ann Arbor stage, CNS-IPI score, laterality, cell of origin (germinal center B-cell-like (GCB) vs. ABC), chemotherapy, CNS prophylaxis (and type), disease relapse, CNS relapse, prognosis (alive or dead), and OS in months. Patients were then stratified by either stage (early-stage [Ann Arbor I and II] vs. advanced-stage [Ann Arbor III and IV] or CNS-IPI risk [low-intermediate vs. high risk]). Fisher's Exact Test was used to compare all categorical variables, with a two-tailed p-value equal or less to 0.05 set as statistically significant. Student's t-test was used for statistical comparison of continuous variables. OS was calculated using the Kaplan–Meier method, and comparison between OS curves made using the log-rank test. Statistical significance was set as p-value < 0.05 for all tests.
Fig. 1.
Flowchart of patient selection process.
3. Case report
A 39-year-old man presented with gross hematuria and right flank pain for two weeks. Vital signs were stable, and the physical exam was unremarkable. All labs drawn within the first few weeks of presentation were within normal limits, including complete blood count, complete metabolic profile, serum renin, aldosterone, cortisol, plasma normetanephrine and total metanephrine, and urine metanephrine and normetanephrine. A CT scan revealed a 5.1 × 4.2 × 3.8 cm mass in the left adrenal gland. Due to the concern for adrenocortical carcinoma, an en-bloc resection with left adrenalectomy and distal pancreatectomy, splenectomy, and diaphragm resection with aortic lymph node dissection was performed. Surprisingly, pathology revealed an ABC-DLBCL (CD10 negative, BCL6 negative, MUM1 positive) expressing BCL2 but not MYC (30%) with a high Ki-67 proliferation marker expression of 70–80%. Fluorescence in situ hybridization (FISH) was abnormal for BCL6 rearrangement. The spleen and resected aortic lymph nodes were negative for involvement by lymphoma. Bone marrow biopsy showed normal cytogenetics and was negative for malignancy. The patient was diagnosed with a stage IE PAL. The patient received six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). He also received one dose of intrathecal methotrexate and three doses of systemic high-dose methotrexate for CNS prophylaxis. PET scan six months after surgery showed complete remission, and he remained disease free and alive at last follow-up two years after the diagnosis.
4. Results
Literature review yielded 704 cases available for review. Of these, 30 cases had CNS involvement at onset, 241 case had unknown/ unidentified staging, 114 cases had unclear CNS prophylaxis usage, 168 cases had unclear survival outcomes per case, 72 cases had non-DLBCL histology, and 46 cases did not use rituximab-based chemo (Fig. 1). 33 cases of PARL from 21 distinct sources remained [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], along with 17 institutional cases (50 cases total; Supp. Table 1).
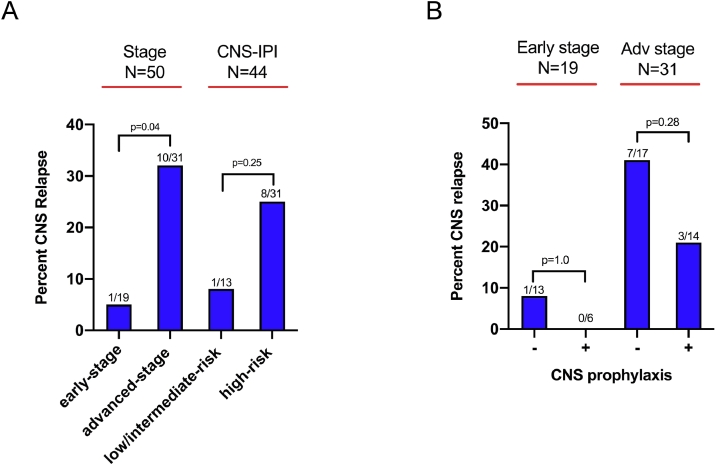
Twenty of 50 (40%) cases received CNS prophylaxis (Table 1). The CNS prophylaxis group had a higher male predominance compared to the Non-CNS prophylaxis group (p = 0.03), along with a slightly younger age that was not significant (58.4 versus 65.3 years; p = 0.06). There was no difference between the prophylaxis and non-prophylaxis groups with regards to GCB cell of origin (p = 0.69), advanced stage (p = 0.39), mean CNS IPI (p = 0.65), or rate of CNS relapse (p = 0.49). Rates of CNS relapse were lower in the early-stage (1/19; 5.3%) and low-intermediate risk groups (1/13; 7.7%), although the difference was only significant in the early-stage group (p = 0.04) (Fig 2A). Notably, CNS prophylaxis did not have an effect on CNS relapse in either the early stage (p = 1.0) or advanced-stage groups (p = 0.28) (Fig 2B).
Table 1.
Baseline characteristics of CNS Prophylaxis vs. Non-CNS Prophylaxis groups.
| CNS prophylaxis (n = 20) | Non-CNS prophylaxis (n = 30) | p-value | |
| General | |||
| Age - mean ± SD | 58.4 ± 12.4 | 65.3 ± 13.6 | 0.06 |
| Male - no. (%) | 17 (85) | 16 (53.3) | 0.03 |
| Lymphoma characteristics - no. (%) | |||
| DLBCL | 20 (100) | 30 (100) | 1 |
| GCB cell of origin | 6 (30) | 2/17 (11.8) | 0.69 |
| Adrenal | 13 (65) | 22 (73.3) | 0.55 |
| Renal | 7 (35) | 8 (26.7) | |
| Rituximab chemo | 20 (100) | 30 (100) | 1 |
| Advanced stage | 14 (70) | 17 (56.7) | 0.39 |
| CNS-IPI, mean ± SD | 3.8 ± 0.8 | 4.0 ± 1.2 | 0.65 |
| CNS relapse | 3 (15) | 8 (26.7) | 0.49 |
| Prophylaxis - no. (%) | |||
| Intrathecal only | 10 (50) | 0 (0) | |
| HD-MTX ± Intrathecal | 10 (50) | 0 (0) |
Fig. 2.
CNS relapse Percentage. (A), Percent relapse according to category (Early vs. Advanced stage, Low vs. Intermediate/High risk). (B), Percent relapse according to CNS prophylaxis and stage.
In terms of OS, there was no difference between the adrenal and renal groups (median OS of 24 months in adrenal vs. unreached in renal; p = 0.08, Fig. 3A). We also did not observe a significant difference between the early vs. advanced-stage groups (median OS of 45 months for early and 28 months for advanced-stage; p = 0.91, Fig. 3B) or the low/intermediate vs. high-risk CNS-IPI groups (median OS of 45 months for early and 28 months for advanced-stage; p = 0.91, Fig. 4A).
Fig. 3.
Probabilities of OS. (A), Probabilities of OS of all Adrenal and Renal DLBCL patients using the Kaplan-Meier method and compared using the log-rank test. (B), Probabilities of OS were compared in subgroups dichotomized by stage (Early vs. Advanced stage) using the log-rank test. (C), Probabilities of OS were compared in the Early stage subgroup dichotomized by use of CNS prophylaxis using the log-rank test. (D), Probabilities of OS were compared in the Advanced stage subgroup dichotomized by use of CNS prophylaxis using the log-rank test.
Fig. 4.
Probabilities of OS. (A), Probabilities of OS were compared in subgroups dichotomized by CNS-IPI (Low vs. Intermediate/High risk) using the log-rank test. (B), Probabilities of OS were compared in the low/intermediate-risk CNS-IPI group dichotomized by use CNS prophylaxis using the log-rank test. (C), Probabilities of OS were compared in the high-risk CNS-IPI group dichotomized by use CNS prophylaxis using the log-rank test. (D), Probabilities of OS were compared in subgroups dichotomized by route of CNS prophylaxis (IT-chemo vs. HD-MTX) using the log-rank test.
CNS prophylaxis showed a survival benefit that extended to both advanced-stage (p = 0.04, Fig. 3D) and high-risk CNS-IPI (p = 0.01, Fig. 4C) diseases. However, there was no difference noted in either the early-stage (p = 0.13, Fig. 3C) or low/intermediate risk CNS-IPI groups (p = 0.19, Fig. 4B).
Finally, we analyzed whether the type of CNS prophylaxis used - intrathecal methotrexate or cytarabine (IT-chemo) versus high dose systemic methotrexate with or without IT-chemo (HD-MTX) affected OS. Notwithstanding the small number of cases (10 HD-MTX and 10 IT-chemo), we observed a significant trend in improved survival in the HD-MTX group compared to IT-chemo only group (median OS: 28 months in IT-chemo and unreached in HD-MTX; p = 0.02; Fig. 4D).
5. Discussion
Here, we provide data analysis of 50 cases of PARL, on disease biology, CNS prophylaxis, and outcomes. Lower rates of relapse were seen in early stage and low/intermediate-risk CNS-IPI, which is consistent with previous studies on DLBCL in general [5, 6]. Notably, the median OS was not reached with CNS prophylaxis across all stage and CNS-IPI categories. CNS prophylaxis showed a survival benefit in both advanced-stage and high-risk CNS-IPI groups. CNS prophylaxis did not reach significance in the early-stage and low/intermediate groups, but analysis was likely limited by small sample size. Finally, there was a significant trend in improved survival in the HD-MTX group compared to IT-chemo. This may indicate a need for CNS chemo-prophylaxis in the form of systemic HD-MTX over IT-MTX, but larger scale studies should be used to confirm these findings.
Our analysis showed that CNS prophylaxis did not significantly affect the rate of CNS relapse in any of the CNS-IPI or stage groups. The effect of CNS prophylaxis on early stage and low/intermediate-risk CNS-IPI groups was likely hindered by small sample sizes. With the use of CNS prophylaxis, no cases of CNS relapse were observed within the early stage and low/intermediate-risk CNS-IPI groups, which suggests a potential benefit to CNS prophylaxis within this patient population. Despite the fact that CNS prophylaxis failed to improve rates of CNS relapse in patients with advanced stage disease, we still detected a better OS in patients who received prophylaxis in this group. One plausible explanation could be that prophylaxis delays the onset of CNS relapse, resulting in longer OS, but similar relapse rates. Progression-free survival (PFS) analysis would be useful to confirm this finding, however this information was not available within our data set. In addition, the type of CNS prophylaxis might have played a role in this discrepancy, as all patients who suffered a CNS relapse within the IT-MTX group had advanced stage disease. Given these potential variables, we argue that while CNS prophylaxis did not significantly affect CNS relapse rates in our analysis, its benefit on OS is a better indicator of its utility in this population.
Due to the limited number of PARL cases in the community, the preferred route of administration for CNS prophylaxis (IT-chemo vs. HD-MTX) remains uncertain and mirrored after general DLBCL in most cases. Methotrexate is a poor penetrator of the blood brain barrier (BBB), unless given in high doses to reach a therapeutic level in the brain parenchyma [31, 32]. IT-chemo attempts to bypass the BBB, but the actual concentrations of drug penetration into parenchyma via this method are quite low [33]. HD-MTX has been demonstrated to be safely administered in high risk DLBCL patients, with a low (3%) CNS recurrence rate when combined with R-CHOP [34]. Although there is no standard protocol for administration, the most common regimen for HD-MTX prophylaxis involves 2–4 courses of 3–3.5 g/m2 given between days 11 to 15 of alternating cycles [35], [36], [37], [38]. On the other hand, numerous studies have concluded that intrathecal methotrexate is insufficient to prevent CNS relapse [9, 39, 40]. Our retrospective analysis corroborates these findings in a limited sample size, as HD-MTX therapy resulted in OS that was significant over intrathecal therapy alone (p = 0.02; Fig 4D). Additionally, none of the 10 patients receiving HD-MTX developed CNS relapse, while 30% (3/10) of the IT-chemo group suffered CNS relapse at some point (not shown). Larger data sets are required to further confirm the benefit of HD-MTX over IT-chemo in preventing CNS relapse and improving OS of patients with PARL.
Overall, HD-MTX is usually well-tolerated by most patients. However, renal toxicity, hepatotoxicity, and to some degree neutropenia could be encountered especially in the setting of older and frail patients. Nevertheless, CNS prophylaxis with HD-MTX has been successfully used in older patients up to 80 years old [[34], [35], [36], 41]. Most practices limit the use of HD-MTX in patients older than 80 years, in the setting of MTHFR 677TT genotype polymorphism, active HBV/HCV infections, and chronic renal insufficiency especially when creatinine is greater than 2.0 mg/dL [37].
IT-chemo, in addition to its unclear benefit in reducing CNS relapse in DLBCL, is not without side effects. It can result in neurotoxicity including paraplegia, cauda equina syndrome, spinal cord lesions, seizures, encephalopathy [42]. In 690 R-CHOP treated patients aged ≥70 years, when IT-chemo prophylaxis was given in addition to R-CHOP, the admission rate from all-cause infection increased (no CNS infections noted), while no benefit was observed [40]. A single-institution study of complications of IT-chemo used as either prophylaxis or treatment had a few patients experience significant neurological events including paresthesias and paralysis, while almost one-third of patients had common symptoms of nausea, vomiting, headache, and fever suggestive of chemical arachnoiditis secondary to the infused medication [43]. It has been noted that the incidence of chemical arachnoiditis increases with the number of cycles and dosage of IT-chemo, but is lower in those who receive it for prophylaxis rather than treatment [44]. Despite these potential side effects, in patients with a relative contraindication to systemic HD-MTX, intrathecal prophylaxis remains an acceptable option.
As a retrospective study based on systematic review, we concede that our conclusions can be limited by confounders, reporting bias and differing protocols for treatment. However, given the rarity of PARL and the scarcity of available data, prospective trials are currently lacking. Therefore, we offer an attempt to analyze outcomes based on the literature available in hopes of aiding clinical decision-making in future cases.
6. Conclusion
PARL are rare presentations of DLBCL, more likely to be associated with high-risk features such as ABC cell-of-origin and advanced-stage disease. The current literature and our findings support the use of HD-MTX over IT-MTX, particularly in advanced-stage disease and high-risk CNS IPI. Larger sample sizes are needed to confirm this in the early-stage and low/intermediate-risk CNS IPI groups. In the event that a patient meets exclusion criteria or cannot tolerate HD-MTX, intrathecal prophylaxis remains an acceptable option.
7. Financial support
There was no funding for this project
Declaration of Competing Interest
The authors declare no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
J.X., S.C., M.U. and S.N. searched for cases in the literature. J.X. analyzed the data and performed statistics. J.X. and A.J. wrote the manuscript. T.C.B. confirmed the cytogenetics. N.S.S., M.U., S.N., T.C., and A.. provided clinical data. N.S.S., F.S., J.S., T.C.B. and H.S. critically reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2021.100263.
Appendix. Supplementary materials
References
- 1.Rashidi A., Fisher S.I. Primary adrenal lymphoma: a systematic review. Ann. Hematol. 2013;92(12):1583–1593. doi: 10.1007/s00277-013-1812-3. [DOI] [PubMed] [Google Scholar]
- 2.Chen J. Primary renal lymphoma: a population-based study in the United States, 1980-2013. Sci. Rep. 2019;9(1):15125. doi: 10.1038/s41598-019-51635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasunaga Y. Malignant lymphoma of the kidney. J. Surg. Oncol. 1997;64(3):207–211. doi: 10.1002/(sici)1096-9098(199703)64:3<207::aid-jso6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C.D., Kahl B.S. Central nervous system involvement in diffuse large B-cell lymphoma: an analysis of risks and prevention strategies in the post-rituximab era. Leuk. Lymphoma. 2014;55(10):2228–2240. doi: 10.3109/10428194.2013.869326. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz N. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J. Clin. Oncol. 2016;34(26):3150–3156. doi: 10.1200/JCO.2015.65.6520. [DOI] [PubMed] [Google Scholar]
- 6.Klanova M. Integration of cell of origin into the clinical CNS international prognostic index improves CNS relapse prediction in DLBCL. Blood. 2019;133(9):919–926. doi: 10.1182/blood-2018-07-862862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage K.J. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–2188. doi: 10.1182/blood-2015-10-676700. [DOI] [PubMed] [Google Scholar]
- 8.Dunleavy K. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609–e617. doi: 10.1016/S2352-3026(18)30177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qualls D., Abramson J.S. Advances in risk assessment and prophylaxis for central nervous system relapse in diffuse large B-cell lymphoma. Haematologica. 2019;104(1):25–34. doi: 10.3324/haematol.2018.195834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichikawa S. Clinicopathological analysis of primary adrenal diffuse large B-cell lymphoma: effectiveness of rituximab-containing chemotherapy including central nervous system prophylaxis. Exp. Hematol. Oncol. 2013;2(1):19. doi: 10.1186/2162-3619-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada K. The Clinical and hormonal characteristics of primary adrenal lymphomas: the necessity of early detection of adrenal insufficiency. Intern. Med. 2017;56(17):2261–2269. doi: 10.2169/internalmedicine.8216-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masood A. The diverse clinical presentations of adrenal lymphoma. AACE Clin. Case Rep. 2017;3(4):e307–e312. [Google Scholar]
- 13.Mozos A. Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod. Pathol. 2009;22(9):1210–1217. doi: 10.1038/modpathol.2009.87. [DOI] [PubMed] [Google Scholar]
- 14.De Miguel Sanchez C. Acute adrenal insufficiency secondary to bilateral adrenal B-cell lymphoma: a case report and review of the literature. Ecancermedicalscience. 2016;10:634. doi: 10.3332/ecancer.2016.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons Michael J., J.K. A, Huang Stephen N., Kahnoski Richard J. Complete remission of rapidly-progressing, unilateral, primary adrenal diffuse large B-cell lymphoma with surgery and rituximab-chop chemotherapy. BJU Int. 2013 [Google Scholar]
- 16.Kacem K. Primary adrenal lymphoma. Turk. J. Haematol. 2014;31(2):188–191. doi: 10.4274/tjh.2012.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyyur Aravamudan V. A rare case of primary bilateral adrenal lymphoma. Case Rep. Med. 2017;2017 doi: 10.1155/2017/1251950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzo C. Primary bilateral non-Hodgkin's lymphoma of the adrenal gland presenting as incidental adrenal masses. Case Rep. Med. 2015;2015 doi: 10.1155/2015/620381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horiguchi K. Primary bilateral adrenal diffuse large B-cell lymphoma demonstrating adrenal failure. Intern. Med. 2010;49(20):2241–2246. doi: 10.2169/internalmedicine.49.3941. [DOI] [PubMed] [Google Scholar]
- 20.Alireza Ahmadi S.N., Daneshyar Sajjad. Bilateral primary adrenal lymphoma in a 59- year-old female. Rev.Clin. Med. 2010 [Google Scholar]
- 21.Radhakrishnan R.K. Unilateral Primary adrenal lymphoma: uncommon presentation of a rare disease evaluated using (18)F-fluorodeoxyglucose positron emission tomography/computed tomography. World J. Nucl. Med. 2018;17(1):46–48. doi: 10.4103/1450-1147.222288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E.Y. Primary bilateral adrenal non-hodgkin's lymphoma presented with adrenal insufficiency: a case report. Endocrinol. Metab. 2011;26(1):101–105. [Google Scholar]
- 23.Grigg A.P., Connors J.M. Primary adrenal lymphoma. Clin. Lymphoma. 2003;4(3):154–160. doi: 10.3816/clm.2003.n.024. [DOI] [PubMed] [Google Scholar]
- 24.Zomas A. Primary renal lymphoma presenting with chronic low-grade fever. Int. J. Hematol. 2004;79(4):361–363. doi: 10.1532/ijh97.e0320. [DOI] [PubMed] [Google Scholar]
- 25.Belbaraka R. Primary renal non-Hodgkin lymphoma: an unusual diagnosis for a renal mass. Indian J. Cancer. 2011;48(2):255–256. doi: 10.4103/0019-509X.82880. [DOI] [PubMed] [Google Scholar]
- 26.Hu R. Central nervous system involvement of primary renal lymphoma with diffuse large B-cell type lymphoma. Am. J. Case Rep. 2013;14:292–294. doi: 10.12659/AJCR.889308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okubo T. Diffuse large B-cell lymphoma presenting as bilateral renal infiltration leading to acute kidney injury. CEN Case Rep. 2017;6(2):140–147. doi: 10.1007/s13730-017-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rissman C.M. Primary renal lymphoma: an unusual finding following radical nephrectomy. Clin. Nephrol. Case Stud. 2017;5:1–4. doi: 10.5414/CNCS108955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vazquez-Alonso F. Primary renal lymphoma: long-term results of two patients treated with a chemotherapy + rituximab protocol. Case Rep. Oncol. Med. 2012;2012 doi: 10.1155/2012/726424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakar D. Rapidly progressive renal failure in a patient with extranodal non-Hodgkin's lymphoma. Indian J. Nephrol. 2015;25(1):43–45. doi: 10.4103/0971-4065.140723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glantz M.J. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J. Clin. Oncol. 1998;16(4):1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- 32.Lassman A.B. Systemic high-dose intravenous methotrexate for central nervous system metastases. J. Neurooncol. 2006;78(3):255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 33.Blasberg R.G., Patlak C., Fenstermacher J.D. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther. 1975;195(1):73–83. [PubMed] [Google Scholar]
- 34.Abramson J.S. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283–4290. doi: 10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]
- 35.Lee K. Systemic HD-MTX for CNS prophylaxis in high-risk DLBCL patients: a prospectively collected, single-center cohort analysis. Int. J. Hematol. 2019;110(1):86–94. doi: 10.1007/s12185-019-02653-7. [DOI] [PubMed] [Google Scholar]
- 36.Goldschmidt N. Addition of high-dose methotrexate to standard treatment for patients with high-risk diffuse large B-cell lymphoma contributes to improved freedom from progression and survival but does not prevent central nervous system relapse. Leuk. Lymphoma. 2019;60(8):1890–1898. doi: 10.1080/10428194.2018.1564823. [DOI] [PubMed] [Google Scholar]
- 37.Ferreri A.J. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br. J. Haematol. 2015;168(5):654–662. doi: 10.1111/bjh.13194. [DOI] [PubMed] [Google Scholar]
- 38.Savage K.J. Secondary CNS relapse in diffuse large B-cell lymphoma: defining high-risk patients and optimization of prophylaxis strategies. Hematol. Am. Soc. Hematol. Educ. Program. 2017;2017(1):578–586. doi: 10.1182/asheducation-2017.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomita N. Intrathecal methotrexate prophylaxis and central nervous system relapse in patients with diffuse large B-cell lymphoma following rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk. Lymphoma. 2015;56(3):725–729. doi: 10.3109/10428194.2014.931953. [DOI] [PubMed] [Google Scholar]
- 40.Eyre T.A. Stand-alone intrathecal central nervous system (CNS) prophylaxis provide unclear benefit in reducing CNS relapse risk in elderly DLBCL patients treated with R-CHOP and is associated increased infection-related toxicity. Br. J. Haematol. 2019;187(2):185–194. doi: 10.1111/bjh.16070. [DOI] [PubMed] [Google Scholar]
- 41.Cheah C.Y. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br. J. Cancer. 2014;111(6):1072–1079. doi: 10.1038/bjc.2014.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong Y.L., Yeung D.Y., Chan J.C. Intrathecal chemotherapy for hematologic malignancies: drugs and toxicities. Ann. Hematol. 2009;88(3):193–201. doi: 10.1007/s00277-008-0645-y. [DOI] [PubMed] [Google Scholar]
- 43.Byrnes D.M. Complications of intrathecal chemotherapy in adults: single-institution experience in 109 consecutive patients. J. Oncol. 2019;2019 doi: 10.1155/2019/4047617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob L.A. Methotrexate-induced chemical meningitis in patients with acute lymphoblastic leukemia/lymphoma. Ann. Indian Acad. Neurol. 2015;18(2):206–209. doi: 10.4103/0972-2327.150586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.