Highlights
-
•
UAE is an efficient method to obtain betalains and polyphenols with high AA.
-
•
BT, BC, BX, TP, AA, b*, KLa, and A were affected by temperature and UPD.
-
•
Optimal UAE conditions were 41.80 °C and 188.84 mW/mL.
-
•
Optimal conditions showed that BC, BX, BT, TP, AA, L*, a*, b*, KLa, A, and IA agreed with predicted values.
Keywords: Ultrasound-assisted extraction, Amaranthus, Betalains, Polyphenols
Abbreviations: UAE, ultrasound assisted extraction; BT, total betalains (mg/100 g d.m); BC, betacyanins (mg/100 g d.m); BX, betaxanthins (mg/100 g d.m); TP, total polyphenols (mg GAE/100 g d.m); AA, antioxidant activity (mmol TE/100 g d.m); , mass transfer coefficient (m3/s); A, amaranthine (mg/100 g d.m); IA, isoamaranthine (mg/100 g d.m); C, concentration of total betalains at time t (mg/100 g d.m); Cs, concentration of total betalains at saturation (mg/100 g d.m); UPD, ultrasonic power density (mW/mL); m, mass (g); Cp, specific heat (J/g °C); T, temperature (°C); dT/dt, change in temperature over time (°C/s); V, volume (mL); t, time (min); d.m, dry matter; w.m, wet matter; GAE, gallic acid equivalent; TE, trolox equivalent
Abstract
The present study optimised the ultrasound-assisted extraction (UAE) of bioactive compounds from Amaranthus hypochondriacus var. Nutrisol. Influence of temperature (25.86–54.14 °C) and ultrasonic power densities (UPD) (76.01–273.99 mW/mL) on total betalains (BT), betacyanins (BC), betaxanthins (BX), total polyphenols (TP), antioxidant activity (AA), colour parameters (L*, a*, and b*), amaranthine (A), and isoamaranthine (IA) were evaluated using response surface methodology. Moreover, betalain extraction kinetics and mass transfer coefficients (KLa) were determined for each experimental condition. BT, BC, BX, TP, AA, b*, KLa, and A were significantly affected (p < 0.05) by temperature extraction and UPD, whereas L*, a*, and IA were only affected (p < 0.05) by temperature. All response models were significantly validated with regression coefficients (R2) ranging from 87.46 to 99.29%. BT, A, IA, and KLa in UAE were 1.38, 1.65, 1.50, and 29.93 times higher than determined using conventional extraction, respectively. Optimal UAE conditions were obtained at 41.80 °C and 188.84 mW/mL using the desired function methodology. Under these conditions, the experimental values for BC, BX, BT, TP, AA, L*, a*, b*, KLa, A, and IA were closely related to the predicted values, indicating the suitability of the developed quadratic models. This study proposes a simple and efficient UAE method to obtain betalains and polyphenols with high antioxidant activity, which can be used in several applications within the food industry.
1. Introduction
Current trends in the industrialised foods’ consumer market made with natural ingredients are due, in part, to consumers’ preference for functional foods, free of preservatives, and chemical additives with beneficial effects on human health. This has led to changes and innovations in the food industry, so they meet physical characteristics, such as texture, colour, acidity, and sensory attributes that are acceptable to the consumer.
Colour is the first attribute of the product perceived by the consumer and a decisive factor in the acceptance or rejection of food [1]. A normal practice in food processing is the pigmentation with synthetic dyes rather than natural pigments because of such advantages as low cost and greater stability during processing and storage. Nevertheless, they have been questioned and must be approved for use with certain restrictions because they have been associated with allergic reactions and harmful effects on the health of consumers [2]. Therefore, the synthetic dye market has decreased in favour of natural dyes [1]. However, the production of natural pigments is limited because of a series of factors, ranging from the shortage of large amounts of highly pigmented plants or fruits to the lack of efficient extraction and purification methods for these compounds [3].
Amaranth belongs to the Amaranthaceae family and to the Amaranthus genus, is predominantly tropical and includes approximately 60 species native to the tropics and temperate regions [4], [5], [6], [7]. Furthermore, it has high resistance to different soil conditions, such as drought, salinity, alkalinity and acidity [5]. The plant is characterized as a source of betalains and other bioactive compounds, such as polyphenols with high antioxidant power activity [8], [9], [10]. Betalains possess anticancer, antiviral, and antimicrobial properties that enhance their potential in the food industry [3].
Several studies have been carried out for betalain extraction in some amaranth species and fruits using conventional methods, such as maceration and Soxhlet extraction [11], [12]. Li et al. [9] determined the betalain content in three amaranth species and in different parts of the plant, obtaining the maximum content of 20.93 mg/100 g d.m in Amaranthus caudatus leaves in an extraction time of 15 h. Das et al. [13] reported a maximum content of 159.09 mg/100 g d.m in 60 min from foliage of Amaranthus cruentus. However, Maran et al. [14] reported a maximum content of total betalains of 41.54 mg/100 g d.m in an extraction time of 115 min in Opuntia ficus-indica fruit, whereas Zin et al. [15] obtained maximum concentrations of betacyanins and betaxanthins in 1 h in beetroot peels. Although these studies have been of great importance regarding the extraction and characterisation of betalains in different sources, it is noteworthy that high pigment concentration is achieved in long extraction times, which leads to higher energy consumption. Therefore, conventional methods are sometimes considered not viable because long extraction times are directly related to higher energy consumption during operation. Thus, non-conventional extraction methods have countered the disadvantages of conventional methods [16].
Using emerging technologies such as ultrasound-assisted extraction (UAE) is an alternative that allows the reduction of the extraction time and the volume of solvents, favouring the reduction of energy consumption during the extraction process. The physical phenomenon of acoustic cavitation is the key to the positive effects of ultrasonic extraction of bioactive compounds. The passage of the waves in the liquid medium creates a series of compressions and rarefactions in the medium, which induces the formation, growth, and collapse of the bubbles formed in the liquid medium [17]. This violent collapse allows temperatures and pressures of 5000 K and 50 MPa to be reached at the molecular level, increasing the permeability of plant cell walls and the diffusivity of the solute in the solvent, which positively influences the release and extraction of compounds [18]. Factors such as temperature, power, and ultrasonic frequency, besides properties of the solvent, such as viscosity and surface tension, directly influence the phenomenon of cavitation and bioactive compound extraction. The interrelation of these variables needs to be evaluated in order to establish the best UAE conditions. Although increases in ultrasound power favour the extraction of compounds contained at the cellular level [18], this positive effect could be counteracted by the application of high levels of temperature in the medium. This is because of the impact of temperature on the minimisation of cavitation [17], [18], [19], as also reported in other studies [20], [21], [22]. However, researches about the influence of temperature and UPD on the UAE of bioactive compounds from amaranth are limited. Ahmed et al. [23] studied the influence of temperature on the UAE of bioactive compounds in Amaranthus cruentus leaves. Moreover, Roriz et al. [24] optimised the UAE of betacyanins from Amaranthus caudatus flowers, evaluating the influence of time and ultrasonic power. Although the importance of both studies, they do not show the impact of the interrelation of temperature and UPD on yields and changes of total and individual betalains. Both variables, temperature and UPD, have been related enhancing the mass transfer during the UAE process of different bioactive compounds present in plants and fruits [25], [26], [27]. However, studies that correlate mass transfer coefficients with individual bioactive compounds in amaranth are still scarce. Temperature extraction and UPD are important factors that are related to yields and changes in pigments and other bioactive compounds in plant tissues. Hence, the study of UAE could represent a technological alternative to maximise the extraction of total pigments and other bioactive compounds at minimum extraction times. Moreover, to evaluate the impact of UAE on individual betalains, such as amaranthine and isoamaranthine, which are bioactive compounds of interest and specific to this plant. Therefore, the objective of this study was to evaluate and optimise the UAE at different temperatures and UPD on betalains, polyphenols, antioxidant activity, and colour changes from Amaranthus hypochondriacus var. Nutrisol plant.
2. Materials and methods
2.1. Materials
2.1.1. Chemicals
Folin-Ciocalteu phenol reagent, gallic acid grade reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), betanin standard, and ß-glucuronidase from Helix pomatia were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) solvent methanol was obtained from J.T. Baker (Mexico City, Mexico). Deionized water was obtained using a deionizer (Ultrapure Type 1, Millipore, Bedford, MA, USA). All other analytical grade reagents and solvents were acquired from J.T. Baker (Mexico City, Mexico).
2.1.2. Plant material
The raw material used in this study was Amaranthus hypochondriacus var. Nutrisol. The Nutrisol plant is an improved, high-yielding variety. The seeds of this variety were supplied by the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), Estado de México. The seeds were sown in trays in the Facultad de Ciencias Agrícolas y Tecnológicas (FACIATEC) in September 2019 and later transplanted into bags 25 days after germination. For the transplant, a 33.3% compost, garden soil, and sand mixture were used as substrates. The harvest was made 63 days after sowing, at which time the highest total betalain content and colour parameters were found in the plant. The collected plant material was washed with distilled water to remove any impurities.
2.1.3. Sample preparation and conditioning
The aerial parts of the plant were then cut manually into small pieces using a stainless steel knife and subjected to conventional oven drying at 40 °C for 25 h [13]. The dehydrated material was ground using a blender and sieved to obtain a uniform particle size between 20 (0.85 mm) and 30 (0.60 mm) mesh sieves. The amaranth powder was stored in hermetically sealed plastic bags at −20 °C until further analysis.
2.2. Proximal analysis of plant material
Plant material was characterised using proximal analysis to determine the contents of moisture, ash, fat, protein, and crude fibre according to 950.02, 923.03, 920.39, 960.52, and 962.09 AOAC methods [28]. Carbohydrate content was determined by the difference between the total mass and the sum of the other components.
2.3. Experimental procedure in ultrasound-assisted extraction
UAE experiments were carried out with a 750 W Branson sonifier with variable power (GEX-750, Sonic, Newtown, CT, USA) at a frequency of 20 kHz. Amaranth powder was mixed with distilled water as a solvent at pH 5 adjusted with HCl 0.1 N and a solute–solvent ratio of 1/30 (g/mL). Subsequently, the mixture was subjected to UAE at different temperatures (25.86, 30, 40, 50, and 54.14 °C) and ultrasonic power densities (UPD) (76.01, 105, 175, 245, and 273.99 mW/mL) for 10 min according to the experimental design shown in Table 1. Temperature and UPD levels were established according to preliminary studies based on single factor experiments. The temperature was controlled using a thermocouple (Fisherbrand Traceable, Fisher Scientific, Pittsburgh, PA, USA) and a water bath. The solute–solvent ratio and extraction time were adjusted according to the preliminary studies. The extracts obtained for each experimental condition were centrifuged (5702-R, Eppendorf, Hamburg, Germany) at 2415 × g, followed by filtration using Whatman No. 1 paper. The filtered extract was used to determine BC, BX, BT, PT, AA, A, IA, and colour parameters L*, a*, and b*. Additionally, extraction kinetics of betalains were performed for each experimental condition (Table 1), where samples were taken at different extraction times to quantify BT. The collected data were used to determine the KLa for each treatment. Conventional extraction at 40 °C and 50 rpm for 1 h was performed as an experimental control in a water bath (Jeio Tech, BS-21, Korea). The initial BT and TP of the plant matrix were determined under the same conditions of temperature and agitation speed of the experimental control in 48 h. BT and TP yields were calculated based on the ratio of sample concentration in the treatment and its initial concentration.
Table 1.
Process variables and levels employed in the experimented design.
| Levels | |||||
|---|---|---|---|---|---|
| Process variables | −1.41421 | −1 | 0 | +1 | +1.41421 |
| Temperature (°C) | 25.86 | 30 | 40 | 50 | 54.14 |
| Ultrasonic power density (mW/mL) | 76.01 | 105 | 175 | 245 | 273.99 |
2.4. Ultrasonic power density (UPD) calculation
The ultrasonic power supplied to the liquid medium was calculated using the calorimetric method described by Rawson et al. [29]. The input power levels were digitally set as amplitude to 22, 25, 50, 75, and 80%. The temperature of the liquid medium during sonication was logged every 0.5 min by triplicate using a thermocouple (Fisherbrand Traceable, Fisher Scientific) under adiabatic conditions. In these calorimetric tests, the solvent temperature was not controlled and no samples were included [30]. The initial temperature increase (dT/dt) was determined by fitting to a second-order polynomial curve (Supplemental data).
The ultrasound power (UP) was calculated using equation (1). The UP was obtained for every amplitude tested.
| (1) |
where m is the mass of the solvent (g), Cp is the specific heat of water (4.1813 J/g °C), and (dT/dt) is the change in temperature over time (°C/s). The UPD was determined from the relationship between the ultrasonic power and the volume of the solvent, expressed in mW/mL. The corresponding UPD were 76.01, 105, 175, 245, and 273.99 mW/mL.
2.5. Betalains (BT)
Quantification of BT content was carried out as described by Cai et al. [31] in a spectrophotometer (Perkin-Elmer model Lambda 25 UV/VIS, Waltham, MA, USA). BT content was expressed as the sum of betacyanins and betaxanthins, calculated according to equations (2), (3).
| (2) |
| (3) |
where BC and BX are the content of betacyanins and betaxanthins, respectively, A538 and A480 are the absorbances at 538 and 480 nm, M are molar masses of amaranthine and indicaxanthin, respectively (726.6 and 308.29 g/mol), V is total volume of extract used (mL), FD is the dilution factor, is the molar extinction coefficient of amaranthine and indicaxanthin, (56,600 and 48,000 Lmol/cm, respectively), and L is the cell length (1 cm). Measurements were performed in triplicate and expressed in mg/100 g d.m for each extract.
2.6. Total polyphenols (TP)
TP content was determined by the Folin-Ciocalteu spectrophotometric method described by Singleton et al. [32] with modifications. For each experimental condition, 30 µL of extract, 200 µL of Folin-Ciocalteu reagent, and 3 mL of distilled water were mixed in a test tube and allowed to react for 8 min at 25 °C. Subsequently, 600 µL of a 20% sodium carbonate solution was added, and the mixture was shaken in a vortex and incubated at 45 °C for 15 min in a water bath (model 210, Fisher Scientific, Pittsburgh, PA, USA). After, the reaction was stopped in an ice bath, and the absorbance at 765 nm was measured using a spectrophotometer (Perkin-Elmer model Lambda 25 UV/VIS). Measurements were made in triplicate for each extract, and the results were calculated based on a gallic acid calibration curve and expressed as gallic acid equivalents (mg GAE/100 g d.m).
2.7. Antioxidant activity (AA)
AA was determined according to the DPPH radical spectrophotometric method developed by Brand-Williams et al. [33]. A 3.9 mL aliquot of 100 µM DPPH solution dissolved in 80% methanol was added to 0.1 mL, stirred, and stored at 25 °C in the dark for 3 h. The decrease in radical concentration was measured at 517 nm using a spectrophotometer (Perkin-Elmer model Lambda 25 UV/VIS). The results were expressed as mmol of Trolox equivalents (mmol TE/100 g d.m) based on a Trolox calibration curve. Measurements were performed in triplicates for each extract.
2.8. Colour parameters
The colour of the extracts was determined using a Konica Minolta CR-400/410 colorimeter (Minolta Co., Osaka, Japan) calibrated using a white ceramic plate. The colour parameters L* (luminosity), a* (green–red trend), and b* (blue-yellow trend) were determined from 10 measurements for each treatment.
2.9. Mass transfer coefficient
The mass transfer coefficient was determined by obtaining extraction kinetics for each condition of the experimental design in the UAE and experimental control. BT was measured at intervals of 2 min of sonication time and 12 min for the experimental control, considering the change in total betalain content as a function of time is described by equation (4).
| (4) |
where KLa represents the global mass transfer coefficient (m3/s), C (mg/100 g d.m), and Cs (mg/100 g d.m) the concentration of total betalains in the solvent at a time t (s) and in the saturation condition, respectively [34]. Considering the extraction process is carried out in batch mode, where the volume of solvent (V) remains constant, equation (5) is obtained.
| (5) |
| (6) |
linearising equation (5) using natural algorithms gives equation (6), allowing the volumetric mass transfer coefficients to be determined, as the slope of the line corresponding to each experimental condition.
2.10. HPLC of individual betalains
HPLC analysis of individual betalains was carried out as described by Cai et al. [31] with modifications. A volume of 20 µL of extract, previously filtered with a 0.22 µm membrane filter (Millipore, MA, USA) was injected into a Thermo Scientific Dionex Ultimate 3000 UHPLC equipped with a Thermo Scienitific reverse phase nucleosil column (C18 150 × 4.6 mm; 0.5 μm particle size) and a UV detector. Mobile phase A: CH3OH/KH2PO4 0.05 M (18:82 v/v) pH = 2.75 with H3PO4, and mobile phase B: CH3OH, were adjusted to a gradient from 100% A to 80% A and 20% B in 20 min at a flow of 1 mL/min and a temperature of 25 °C in the column. Detection was made at 538 and 480 nm for BC and BX, respectively. Quantification of A and IA was performed in duplicate correcting the spectrophotometric determinations by the respective chromatogram areas at 538 nm according to Stintzing et al. [35].
2.10.1. Enzymatic hydrolysis
Individual betalains were identified by enzimatic hydrolysis. Enzymatic hydrolysis of the Amaranthus betalains was conducted using ß-glucuronidase according to the method of Cai et al. [31] with modifications. Prior to enzymatic hydrolysis, purification was carried out by gel filtration on Sephadex G-25 (St. Louis MO, USA) column (20 × 1.0 cm), pre-equilibrated and eluted with deionized water. To 1.5 mL of the purified Amaranthus betacyanins, 50 µL of ß-glucuronidase was added. Solution was hydrolyzed at pH 5.0 and 37 °C for 30 min in a water bath (model 210, Fisher Scientific). The samples with and without ß-glucuronidase treatment were assayed by HPLC to compare the change of peaks. Betanin was employed as control and reference compound to identify amaranth betacyanins.
2.11. Experimental design and statistical analysis
A second-order rotatable central composite design was used to analyze the results using surface response analysis. The extraction temperature and UPD were established as the independent variables. The fitted second-order model is given by equation (7):
| (7) |
where Yi is the response variable of the experiment, X1 and X2 are the extraction temperature and the UPD corresponding to experiment i, respectively; b0, b1, b2, b11, b22, and b12 are the estimated regression coefficients of the intercept, linear effects, quadratic effects, and interaction, respectively, and ε is the error. The levels and combinations of variables are shown in Table 1, Table 2, respectively. Design Expert software v. 11 (State Ease Inc., Minneapolis, USA) was used to fit the response surface models to the experimental data, while Pearson correlation analysis was performed using Minitab software v. 17 (Minitab Inc., State College, PA, USA). Differences were considered significant at p < 0.05. The established confidence level was set at 95%.
Table 2.
BC, BX, BT, TP, AA, L*, a*, b*, KLa, A, and IA from A. hypochondriacus var. Nutrisol at different temperatures and UPD.
| Treatment | T (°C) | UPD (mW/mL) | BC (mg/100 g d.m) | BX (mg/100 g d.m) | BT (mg/100 g d.m) | TP (mg GAE/100 g d.m) | AA (mg/100 gTE/100 g d.m) | L* | a* | b* | Aa (mg/100 g d.m) | IAa (mg/100 g d.m) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25.86 | 175 | 53.260 ± 0.607 | 25.482 ± 0.358 | 78.742 ± 0.621 | 840.305 ± 2.117 | 2.42 ± 0.116 | 26.021 ± 0.059 | 8.093 ± 0.025 | 0.258 ± 0.023 | 3.365 ± 1.485*10-8 | 57.490 ± 1.269 | 3.177 ± 0.251 |
| 2 | 30 | 245 | 84.975 ± 0.696 | 27.453 ± 0.485 | 112.429 ± 1.181 | 1005.200 ± 1.904 | 2.66 ± 0.027 | 25.980 ± 0.067 | 8.186 ± 0.028 | 0.663 ± 0.021 | 3,370 ± 2.263*10-8 | 87.149 ± 3.283 | 4.593 ± 0.040 |
| 3 | 40 | 175 | 102.261 ± 0.649 | 42.052 ± 0.399 | 144.313 ± 1.046 | 1226.860 ± 3.703 | 3.35 ± 0.019 | 25.328 ± 0.067 | 9.617 ± 0.023 | 0.906 ± 0.026 | 4,205 ± 1.485*10-8 | 101.856 ± 8.674 | 11.357 ± 0.469 |
| 4 | 40 | 76.01 | 65.857 ± 0.804 | 15.739 ± 0.440 | 81.596 ± 1.237 | 944.400 ± 3.711 | 2.59 ± 0.022 | 26.245 ± 0.008 | 7.710 ± 0.016 | 0.058 ± 0.008 | 3.370 ± 2.970*10-8 | 55.702 ± 2.002 | 6.609 ± 0.312 |
| 5 | 50 | 105 | 88.541 ± 1.015 | 27.411 ± 0.412 | 115.952 ± 1.415 | 995.318 ± 2.202 | 2.72 ± 0.023 | 27.046 ± 0.031 | 7.292 ± 0.021 | 0.713 ± 0.028 | 3,620 ± 2.687*10-8 | 85.051 ± 0.197 | 4.250 ± 0.500 |
| 6 | 30 | 105 | 47.764 ± 0.462 | 12.781 ± 0.381 | 60.545 ± 0.081 | 872.610 ± 0.917 | 2.47 ± 0.025 | 25.744 ± 0.026 | 8.227 ± 0.027 | −0.174 ± 0.024 | 3.040 ± 3.111*10-8 | 42.890 ± 3.913 | 2.352 ± 0.116 |
| 7 | 40 | 175 | 103.458 ± 0.529 | 39.820 ± 0.377 | 143.279 ± 0.905 | 1238.700 ± 3.175 | 3.38 ± 0.019 | 25.445 ± 0.027 | 9.426 ± 0.031 | 0.973 ± 0.023 | 4,020 ± 8.485*10-8 | 109.143 ± 2.583 | 11.562 ± 0.648 |
| 8 | 54.14 | 175 | 79.453 ± 1.242 | 34.565 ± 0.253 | 114.017 ± 1.495 | 1198.730 ± 5.753 | 3.33 ± 0.025 | 26.894 ± 0.037 | 7.430 ± 0.026 | 0.877 ± 0.026 | 3,910 ± 5.233*10-8 | 84.891 ± 0.169 | 9.110 ± 0.661 |
| 9 | 40 | 175 | 104.749 ± 0.720 | 40.484 ± 0.829 | 145.234 ± 1.525 | 1266.590 ± 5.571 | 3.48 ± 0.030 | 25.303 ± 0.029 | 9.574 ± 0.027 | 1.087 ± 0.017 | 4,285 ± 7.000*10-8 | 110.363 ± 3.019 | 11.655 ± 0.051 |
| 10 | 40 | 273.99 | 101.857 ± 1.267 | 26.789 ± 0.237 | 128.647 ± 1.377 | 1123.240 ± 2.417 | 2.92 ± 0.019 | 26.192 ± 0.010 | 7.755 ± 0.022 | 0.745 ± 0.020 | 3.900 ± 1.480*10-8 | 99.090 ± 0.810 | 3.892 ± 0.264 |
| 11 | 50 | 245 | 90.140 ± 0.835 | 32.888 ± 0.547 | 123.028 ± 1.312 | 1251.180 ± 1.853 | 3.43 ± 0.025 | 26.577 ± 0.028 | 7.546 ± 0.025 | 0.882 ± 0.032 | 4.085 ± 4.031*10-8 | 84.004 ± 3.288 | 6.890 ± 0.887 |
| 12 | 40 | 175 | 101.965 ± 0.621 | 41.797 ± 0.512 | 143.762 ± 1.118 | 1255.080 ± 6.765 | 3.35 ± 0.018 | 25.313 ± 0.030 | 9.587 ± 0.023 | 0.889 ± 0.018 | 3.760 ± 4.384*10-8 | 107.347 ± 1.464 | 11.616 ± 0.488 |
| 13 | 40 | 175 | 105.594 ± 0.378 | 43.446 ± 0.464 | 149.040 ± 0.842 | 1254.580 ± 1.615 | 3.34 ± 0.101 | 25.308 ± 0.028 | 9.574 ± 0.030 | 1.186 ± 0.054 | 4,040 ± 7.071*10-8 | 103.374 ± 0.316 | 11.580 ± 0.042 |
| Control | 40 | – | 79.280 ± 1.325 | 28.251 ± 0.784 | 107.532 ± 2.108 | 2533.192 ± 16.185 | 3.51 ± 0.365 | 26.128 ± 0.039 | 8.370 ± 0.030 | 2.900 ± 0.023 | 0.138 ± 0.495*10-9 | 66.207 ± 2.116 | 7.779 ± 0.312 |
*Values are the average of triplicate measurements ± standard deviation. aValues are the average of duplicate measurements ± standard deviation. T, extraction temperature; UPD, ultrasonic power density; BC, betacyanins; BX, betaxanthins; BT, total betalains; TP, total polyphenols; AA, antioxidant activity; L*, a*, b*, color parameters; KLa, mass transfer coefficient; A, amaranthine; IA, isoamaranthine.
2.12. Experimental validation
Once the conditions of optimal extraction of temperature and UPD for the different response variables were determined with regression analysis and the desirability function, the results were verified performing experiments in triplicate. The contents of the response variables were determined in the extract and compared with the values predicted by the mathematical model obtained to determine its validity. The criterion employed to demonstrate the validity of the optimised UAE condition was the relative error (equation (8)):
| (8) |
where n is the number of experimental data, xe is the experimental value and xp is the predicted value.
3. Results and discussion
3.1. Proximal analysis
The chemical composition of the dehydrated A. hypochondriacus var. Nutrisol is shown in Table 3. It is observed that proximal composition is in agreement with different studies in several varieties of amaranth [36], [37], [38].
Table 3.
Proximal composition of A. hypochondriacus var. Nutrisol.
| Component | Proximate composition (g/100 g d.m) |
|---|---|
| Moisture | 5.83 ± 0.22 |
| Ash | 24.55 ± 0.83 |
| Fat | 2.83 ± 0.10 |
| Proteina | 31.57 ± 2.29 |
| Crude Fiber | 13.92 ± 0.83 |
| Carbohydratesb | 21.30 ± 1.89 |
| BT | 0.172 ± 0.003 |
| TP | 6.633 ± 1.056 |
| AA | 1.210 ± 0.008 |
*Mean ± standard error. a N × 6.25. b Calculated by difference.
In relation to the bioactive compounds, BT (0.172 ± 0.003 g/100 g d.m) was higher than those reported by Li et al. [9], Nana et al. [39] and Sarker et al. [40] in the aerial parts of A. hypochondriacus, A. caudatus, A. cruentus, A. hybridus, and A. tricolor leaves. TP (6.633 ± 1.056 g GAE/100 g d.m) value agreed with those reported by Nana et al. [39], which were higher than measured by Sarker et al. [40].
3.2. Model fitting
The results obtained in the UAE under different temperature and UPD conditions for the quantification of BC, BX, BT, TP, AA, colour parameters, KLa, A, and IA are shown in Table 2. The influence of the factors under study and the regression coefficients obtained for each response variable are shown in Table 4, Table 5. The analysis of variance indicated an excellent adjustment for all the dependent variables under study, with the determination coefficients (R2) greater than 90%, whereas for KLa it reached an R2 of 87.46 (Table 4), which shows that these adjusted models adequately explain the variability of all the response variables.
Table 4.
Analysis of variance of BC, BX, BT, TP, AA, L*, a*, b*, KLa, A, and IA from A. hypochondriacus var. Nutrisol.
| Mean squares | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | BC | BX | BT | TP | AA | L* | a* | b* | A | IA | |
| Model | 5 | 950.84* | 238.78* | 1996.65* | 59896.88* | 0.3909* | 0.8970* | 2.10* | 0.3810* | 3.087 × 10-15* | 1160.47* | 29.72* |
| T | 1 | 860.80* | 135.39* | 1678.88* | 95830.17* | 0.6652* | 1.23* | 0.7892* | 0.4907* | 5.334 × 10-15* | 755.96* | 19.80* |
| UPD | 1 | 1006.25* | 159.99* | 1968.79* | 51419.20* | 0.2335* | 0.0119 | 0.0096 | 0.4888* | 2.982 × 10-15* | 1366.91* | 0.1348 |
| T × UPD | 1 | 317.05* | 21.14* | 501.94* | 3798.75 | 0.0676* | 0.1243* | 0.0218 | 0.1116* | 4.556 × 10-17 | 513.16* | 0.0398 |
| T2 | 1 | 2238.26* | 240.17* | 3944.94* | 89330.51* | 0.4391* | 2.17* | 5.39* | 0.3104* | 3.978 × 10-15* | 2128.73* | 62.57* |
| UPD2 | 1 | 587.15* | 731.66* | 2629.65* | 78413.71* | 0.6739* | 1.34* | 5.57* | 0.6022* | 4.019 × 10-15* | 1140.55* | 82.59* |
| Residual | 7 | 7.58 | 2.52 | 10.13 | 813.51 | 0.0065 | 0.0104 | 0.0121 | 0.0105 | 3.162 × 10-16* | 13.65 | 2.08 |
| Lack of fit | 3 | 14.44 | 3.20 | 16.56 | 1574.31 | 0.0108 | 0.0194 | 0.0207 | 0.0032 | 1.924 × 10-16 | 13.88 | 4.83* |
| Pure error | 4 | 2.45 | 2.01 | 5.32 | 242.92 | 0.0034 | 0.0036 | 0.0056 | 0.0159 | 4.091 × 10-16 | 13.48 | 0.0134 |
| R2 | 98.90 | 98.55 | 99.29 | 98.13 | 97.71 | 98.41 | 99.20 | 96.30 | 87.46 | 98.38 | 91.09 | |
| Adjusted R2 | 98.11 | 97.51 | 98.79 | 96.80 | 96.07 | 97.27 | 98.64 | 93.66 | 78.50 | 97.22 | 84.73 | |
*Significance at p < 0.05. BC, betacyanins; BX, betaxanthins; BT, total betalains; TP, total polyphenols; AA, antioxidant activity; L*, a*, b*, color parameters; KLa, mass transfer coefficient; A, amaranthine; IA, isoamaranthine.
Table 5.
Regression coefficients of second order model for the response variables of extracts from A. hypochondriacus var. Nutrisol.
| Coefficients | BC | BX | BT | TP | AA | L* | a* | b* | KLa*10-7 | A | IA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| b0 | 103.61* | 41.52* | 145.13* | 1248.36* | 3.38* | 25.34* | 9.56* | 1.01* | 4.062* | 106.42* | 11.55* |
| b1 | 10.37* | 4.11* | 14.49* | 109.45* | 0.2884* | 0.3917* | −0.3141* | 0.2477* | 0.258* | 9.72* | 1.57* |
| b2 | 11.22* | 4.47* | 15.69* | 80.17* | 0.1708* | −0.0385 | 0.0346 | 0.2472* | 0.193* | 13.07* | 0.1298 |
| b12 | −8.90* | −2.30* | −11.20* | 30.82 | 0.1300* | −0.1762* | 0.0738 | −0.1670* | 0.034 | −11.33* | 0.0998 |
| b11 | −17.94* | −5.88* | −23.81* | −113.32* | −0.2512* | 0.5587* | −0.8806* | −0.2112* | −0.239* | −17.49* | −3.00* |
| b22 | −9.19* | −10.26* | −19.44* | −106.17* | −0.3112* | 0.4392* | −0.8951* | −0.2942* | −0.240* | −14.39* | −3.45* |
*Significance at p < 0.05. BC, betacyanins; BX, betaxanthins; BT, total betalains; TP, total polyphenols; AA, antioxidant activity; L*, a*, b*, color parameters; KLa, mass transfer coefficient; A, amaranthine; IA, isoamaranthine.
3.3. Total betalains, betacyanins and betaxanthins (BT, BC, and BX) extraction
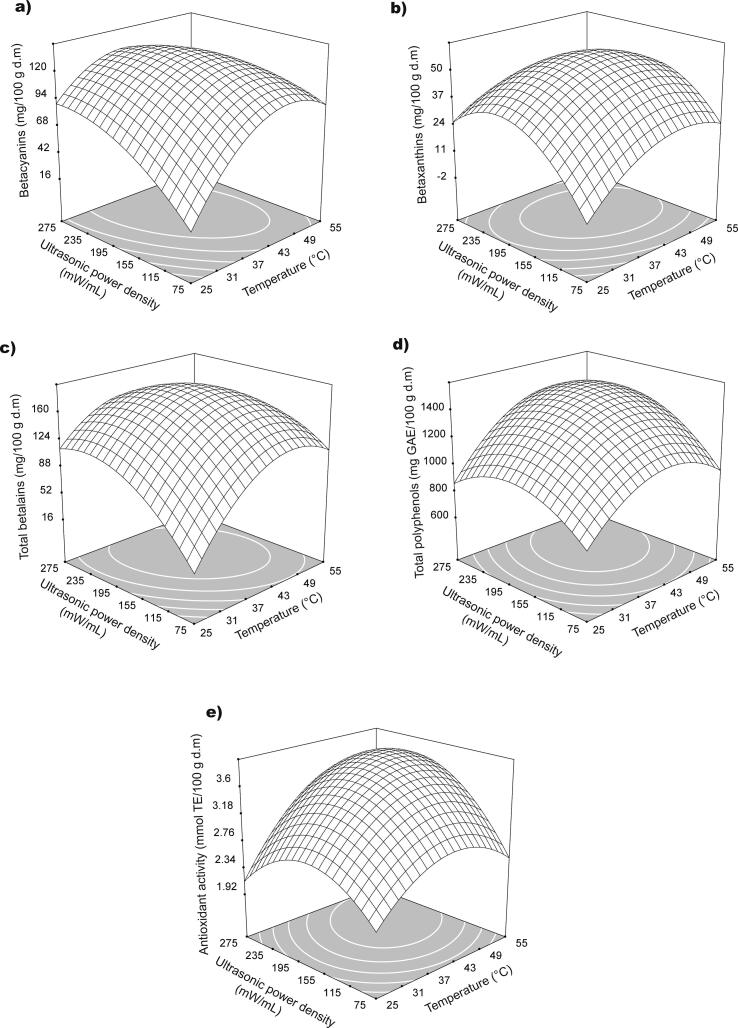
The BC, BX, and BT contents obtained in the UAE were significantly affected by the two factors under study: temperature and UPD, and the linear, quadratic, and interaction terms (p < 0.05). The second-order models obtained for these response variables allow an adequate prediction of the experimental data for BC, BX, and BT, resulting in R2 of 98.90, 98.55, and 99.29%, respectively. The effects of temperature and UPD on the contents of BC, BX, and BT are shown in Fig. 1a-c, respectively.
Fig. 1.
Response surface for the effects of temperature extraction and UPD on BC (a), BX (b), BT (c), TP (d), and AA (e) of A. hypochondriacus var. Nutrisol extracts.
The mean values for BC, BX, and BT content in the extracts for each treatment varied between 47.764–105.594, 12.781–43.446, and 60.545–149.040 mg/100 g d.m, respectively (Table 2). Fig. 1a-c show that for BC, BX, and BT content as temperature and UPD increased up to (41.57 °C, 212.42 mW/mL), (43.14 °C, 187.79 mW/mL), and (42.25 °C, 198.72 mW/mL), resulting in maximum BC, BX, and BT contents of 107.42, 42.58, and 149.41 mg/100 g d.m, respectively. A subsequent decrease in BC, BX, and BT content was observed at higher levels of temperature and UPD. It is known that an increase in the ultrasonic power supplied to the liquid medium increases the extraction performance by favouring sonochemical effects and cavitation; however high power values decrease cavitation in the liquid medium, prevailing agitation instead of cavitation, which results in a lower yield [17], [19]. Likewise, increases in temperature favour betalain diffusion into the solvent, favouring the extraction process, but too high values (in this case, temperature values greater than 40 °C) resulted in a decrease in BC, BX, and BT content. This can be explained because betalains are thermolabile compounds that degrade at high temperatures through isomerisation, deglycosilation, and carboxylation reactions, which induces a decrease in colour and therefore in pigment content [1], [41], [42], [43], [44]. Nevertheless, high temperature values affect sonication, reducing the cavitation phenomenon because an increase in the vapour pressure of the solvent favours the vapour to be inserted into the cavities of the bubbles formed, which causes a less violent collapse [17], [18], [45], [46], decreasing compounds extraction. Similar behaviours and trends of decreased pigment content at high temperatures and potencies were reported by Karchiyappan et al. [47], Maran et al. [48], Maran and Priya [49], and Zhong et al. [22] in ultrasonic extraction of betalains from fruits of Malabar spinach (S. oleracea), beet stems (Beta vulgaris L.), Bougainvillaea glabra flowers, and anthocyanins from Dioscorea cirrhosa tubers, respectively. Still, BT determined by conventional extraction control was 1.39 times lower than UAE treatment. Remarkably, UAE achieved high yields of betalains, reaching values between 35.20 and 86.65%. This demonstrates the suitability of UAE, achieving a high BT in a shorter extraction time. This can be attributed to the sonochemical effects produced by ultrasound extraction as cell wall disruption, which favours the diffusion of betalains, increasing mass transfer [16].
BC contents were higher than those reported by Khanam and Oba [50] for foliage of four varieties of A. hypochondriacus (0.018–0.024 mg/100 g w.m) and A. tricolor (0.04–0.08 mg/100 g w.m). However, results determined in this study were like values reported for 21 genotypes of seven amaranth species in different parts of the plant (46.10–199 mg/100 g w.m) by Cai et al. [31], A. cruentus foliage (15.79–159.09 mg/100 g d.m) by Das et al. [13], and for A. blitum and A. gangeticus (62.07; 152.50 mg/100 g d.m, respectively) according to Chong et al. [51].
BX contents were higher than those reported by Khanam and Oba [50] (0.04–0.07 mg/100 g w.m); Sarker et al. [10], [52] for 20 and 23 amaranth foliage genotypes 0.02–0.06 mg/100 g w.m and 0.01–0.04 mg/100 g w.m, respectively; and Sarker and Oba [53] for 25 amaranth foliage genotype morphology red (0.01–0.06 mg/100 g w.m). The differences found in these studies are mainly due to factors such as the variety and species under study, climate, and growing conditions [31], [54], [55].
3.4. Total polyphenol extraction (TP)
TP extraction was significantly affected by temperature and UPD (p < 0.05) (Table 4). The second-order model had a good fit, explaining the variability of TP content in 98.13%.
TP content for the different treatments varied between 840.305–1266.590 mg GAE/100 g d.m (Table 2). Fig. 1d shows the TP content trend at different temperatures and UPD levels, where an increase in TP was observed as both the temperature and the UPD increased. The highest TP content (1296.49 mg GAE/100 g d.m) was obtained at 45.45 °C and 206.97 mW/mL, decreasing subsequently at higher values of both factors. This trend may be attributed to the degradation of the phenolic compounds present in the extracts, as well as the decrease in the cavitation phenomenon at high power densities and temperatures during the extraction process. Similar trends have been described in the ultrasonic extraction of polyphenols from grape pomace (Vitis vinifera), pomace from cranberry (Vaccinium ashei), pomegranate peels (Punica granatum), and mandarin (Citrus reticulata) [27], [56], [57], [58]. Despite this result, TP obtained in the UAE was 1.95 times lower than the conventional treatment. This result may be attributed to the localisation of phenolics and betalains in the cell wall. Betalains are located in vacuoles in the cell wall [59], whereas phenolics are situated mostly in primary cell walls [60]. It is possible that conventional extraction increased the release of bound phenolics over long extraction times. This agrees with several studies [61], [62], [63] on the UAE of phenolics from Pistacia lentiscus leaves, red dragon fruit (Hylocereus polyrhisus), lemon balm, and peppermint leaves. Therefore, low TP yields were achieved (12.67–19.20%) in UAE. However, it is noteworthy that these polyphenol yields were obtained in a short extraction time.
The average contents of TP were higher than those reported by López-Mejía et al. [64] in leaves (619 mg GAE/100 g d.m) and seeds (25 mg GAE/100 g d.m) of A. hypochondriacus; Sarker and Oba [53], [65] in 25 and 3 genotypes of A. tricolor and A. lividus (10.240–26.084 mg GAE/100 g d.m, 7.162–24.004 mg GAE/100 g d.m, respectively), and Ozsoy et al. [66] in A. lividus (46–155 mg GAE/100 g d.m). In addition, the results agree with those determined by Li et al. [9] and Pulipati et al. [67] for different amaranth species, who reported values that ranging from to 104–1494 mg GAE/100 g d.m and 1380–1940 mg GAE/100 g d.m, respectively.
3.5. Antioxidant activity (AA)
Phenolics and betalains in the extracts were characterised by high antioxidant power. Table 4 shows that the AA of the extracts was significantly affected by temperature and UPD. The second-order model had a good level of adjustment (R2 = 97.71).
AA in the different treatments varied between 2.42–3.48 mmol TE/100 g d.m (Table 2). Increases in temperature and UPD resulted in an increase in AA as seen in Fig. 1e. Notably, behaviour of AA was like that of TP, reaching a maximum AA value (3.51 mmol TE/100 g d.m) at 46.82 °C and 204.17 mW/mL. TP is present in a greater proportion of compounds that contain a high AA, and there must be a high correlation between the two parameters. The AA value determined by conventional extraction corresponded with those obtained at intermediate temperatures and UPD and high values of both factors. AA is highly correlated with BT and TP (Table 7), which demonstrates that both BT and TP contribute to AA. This indicates the suitability of UAE for the simultaneous extraction of betalains and phenolics, which contribute directly to the AA of the extracts. AA determined correspond with values reported for A. hypochondriacus foliage by López-Mejía et al. [64] (4.28 mmol TE/100 g d.m); and Jiménez-Aguilar and Grusak [68] in leaves of 15 amaranth species (3.80–9.00 mmol TE/100 g w.m). However, the results obtained were superior to those corresponding to the leaves of 20 amaranth genotypes (0.006–0.013 mmol TE/100 g d.m), leaves and stems of 17 genotypes for A. lividus (0.004–0.011 mmol TE/100 g d.m), three genotypes for A. tricolor and A. lividus (0.005–0.018 mmol TE/100 g d.m), and 25 amaranth genotypes of red morphology (0.004–0.013 mmol TE/100 g d.m) [10], [40], [53], [65].
Table 7.
Correlation coefficients for BC, BX, BC, TP, AA, L*, a*, b*, KLa, A, and IA in extracts of A. hypochondriacus var. Nutrisol.
| BC | BX | BT | TP | AA | L* | a* | b* | KLa | A | IA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BX | 0.836* 0.000* |
← Pearson’s coefficient ←Probability |
|||||||||
| BT | 0.982* 0.000 |
0.925* 0.000 |
|||||||||
| TP | 0.874* 0.000 |
0.879* 0.000 |
0.909* 0.000 |
||||||||
| AA | 0.802* 0.001 |
0.877* 0.017 |
0.859* 0.000 |
0.986* 0.000 |
|||||||
| L* | −0.346 0.247 |
−0.458 0.116 |
−0.398 0.178 |
−0.336 0.261 |
−0.322 0.284 |
||||||
| a* | 0.538** 0.058 |
0.691* 0.009 |
0.612* 0.026 |
0.539** 0.057 |
0.535** 0.059 |
−0.944* 0.000 |
|||||
| b* | 0.914* 0.000 |
0.929* 0.000 |
0.955* 0.000 |
0.895* 0.000 |
0.864* 0.000 |
−0.228 0.454 |
0.469 0.106 |
||||
| KLa | 0.846* 0.000 |
0.850* 0.000 |
0.880* 0.000 |
0.910* 0.000 |
0.915* 0.000 |
−0.250 0.410 |
0.459 0.114 |
0.889* 0.000 |
|||
| A | 0.974* 0.000 |
0.895* 0.000 |
0.984* 0.000 |
0.871* 0.000 |
0.815* 0.001 |
−0.392 0.185 |
0.596* 0.032 |
0.937* 0.000 |
0.838* 0.000 |
||
| IA | 0.719* 0.006 |
0.867* 0.000 |
0.798* 0.001 |
0.855* 0.000 |
0.871* 0.000 |
−0.560* 0.046 |
0.762* 0.002 |
0.742* 0.004 |
0.765* 0.002 |
0.753* 0.003 |
*Significance at p < 0.05, **Significance at p < 0.1 according to Pearson’s correlation. BC, betacyanins; BX, betaxanthins; BT, total betalains; TP, total polyphenols; AA, antioxidant activity; L*, a*, b*, color parameters; KLa, mass transfer coefficient; A, amaranthine; IA, isoamaranthine.
3.6. Colour parameters L*, a*, and b*
The average values for the colour parameters L*, a*, and b* in the extracts for each treatment varied between 25.303–27.046, 7.292–9.617, and −0.174–1.186, respectively (Table 2). The two factors under study, its linear, quadratic, and interaction terms significantly influenced the parameter b* [blue (-) - yellow (+)]. There was a significant influence of linear and quadratic terms of temperature extraction on L* and a* parameters, and the influence of UPD was not significant. However, the quadratic term of the UPD factor significantly influenced (p < 0.05) the L* and a* parameters. The models obtained for the three-colour parameters exhibited a high coefficient of determination. The corresponding surface response graphs for the colour parameters L*, a*, and b* are shown in Fig. 2a-c.
Fig. 2.
Response surface for the effects of temperature extraction and UPD on L* (a), a* (b), b* (c), and KLa (d) of A. hypochondriacus var. Nutrisol extracts.
As the temperature and the UPD increase, the parameter L* decreases until a minimum value (25.27) is reached at 36.45 °C and 173.05 mW/mL (Fig. 2a). Darker extracts (lower values of L*) corresponded to those with higher contents of BT, which were also found at intermediate values of temperature and UPD (Fig. 1c).
The same trend is observed for the parameters a* and b* (Fig. 2b, c) where an increase in temperature and UPD up to 38.22 °C, 175.83 mW/mL and 44.73 °C, 195.00 mW/mL, respectively, guarantee the maximum values of 9.58 and 1.10 for a* and b*, respectively. This behaviour is due to and correlates with the trends observed in Fig. 1a, b for BC and BX, respectively, where their content decreases because of degradation reactions at high temperatures. In general, the extracts showed dark, red, and blue colour predominance. Similar values have been reported in A. blitum, A. gangeticus, and A. cruentus extracts [13], [51]. Colour parameters corresponded with the experimental control, except for b*, which was higher in the control. This result can be attributed to the higher TP obtained in conventional extraction, which contributes directly to the yellowness of the extracts. Furthermore, Table 7 shows a significant positive correlation between TP and b*.
3.7. Mass transfer coefficient during betalains extraction (KLa)
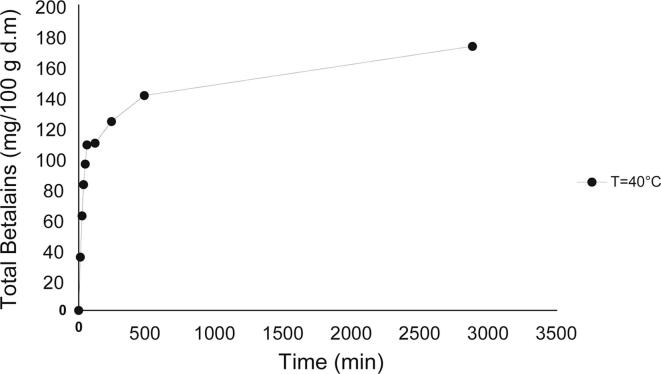
The kinetics of extraction for BT at different temperatures and UPD are shown in Fig. 3, where the BT content increases with increasing temperature and UPD. At a low extraction temperature (30 °C) and by varying the power density, there is a significant difference in performance, not so for an extraction temperature of 50 °C, where the extraction time increased, and the curve corresponding to the lowest UPD was closer to the one with the highest power density. The same trend was observed by varying the extraction temperature and maintaining the power density at the two factor levels. This is because, as explained in the previous sections, extractions at extremely high temperatures and UPD negatively affect the ultrasonic extraction process by reducing the cavitation effect [16], [17], [18] while thermally degrading betalains.
Fig. 3.
Kinetics of betalains extraction of A. hypochondriacus var. Nutrisol at different extraction temperatures and UPD.
The extraction rate evaluated as KLa was significantly affected by temperature and UPD (p < 0.05). The model adequately predicted variable response KLa (R2 = 87.46). Fig. 2d shows as the temperature and the power density increases, KLa increases, reaching its maximum value (4.18 × 10-7 m3/s) at 45.71 °C and 205.93 mW/mL, but decreasing for the experimental conditions outside that interval. This same trend corresponded to the maximum BT content (Fig. 1c). As explained in the previous sections, a greater UPD and temperature produce a greater effect on the rupture of the cell wall, thus favouring the extraction of different bioactive compounds. The kinetic of the BT extraction in the control treatment at 40 °C is shown in Fig. 4. Compared with the conventional extraction control, the KLa calculated in the UAE was 30.29 times higher. This demonstrates the known advantages of UAE, enhancing high BT release over a short period.
Fig. 4.
Kinetic of betalains extraction of A. hypochondriacus var. Nutrisol for control treatment at 40 °C.
3.8. Identification of individual betalains (A, IA)
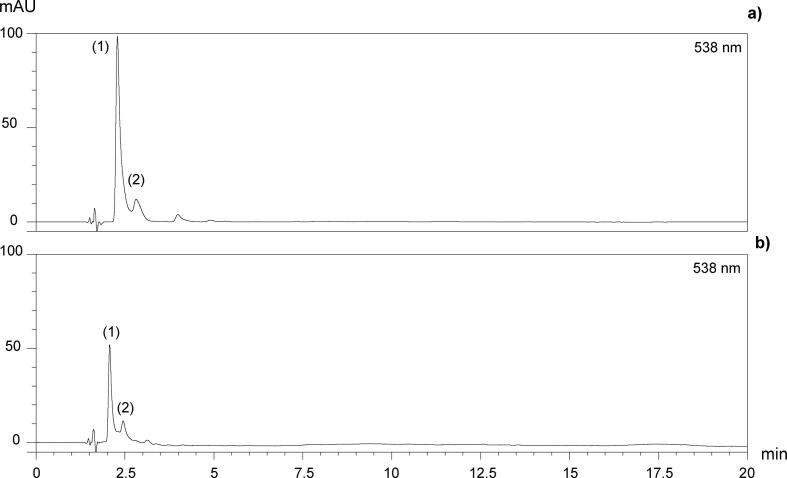
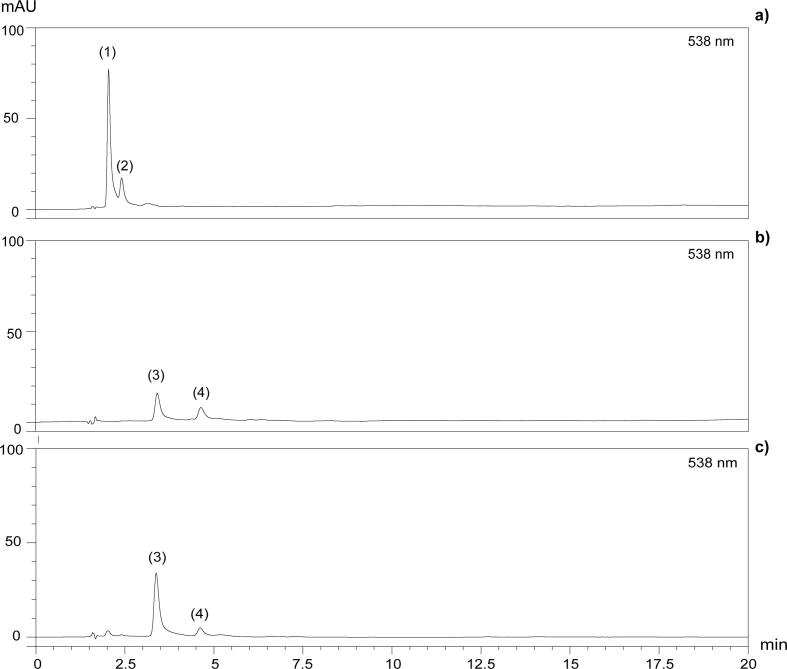
The presence of two peaks detected at 538 nm is observed in the chromatograms corresponding to individual betalains at 40 °C, 175 mW/mL and control treatment (Fig. 5). According to several studies [9], [31], [69], there are two characteristic peaks in amaranth, corresponding to betacyanins A (peak 1) and its isomer IA (peak 2). However, this statement must be confirmed despite the tentative identification based on the different specialised studies. It is shown in Fig. 6a, b the chromatograms of betacyanins fraction before enzymatic treatment and betanin standard, respectively. It is observed that only two peaks are present in both samples. For betanin standard, these peaks are betanin (peak 3) and its isomer isobetanin (peak 4). Enzymatic hydrolysis of amaranth betacyanins confirmed our identification. After ß-glucuronidase hydrolysis, peaks 1, 2 were hydrolyzed in peaks 3, 4, respectively (Fig. 6c). These two peaks coincided exactly with the corresponding to betanin (peak 3) and isobetanin (peak 4) of the betanin standard (Fig. 6b). It demonstrates the correct specificity of ß-glucuronidase with A and IA, which allow the release of ß-glucuronic acid yielding betanin and isobetanin, respectively. Therefore, it is proved that betalains present in Amaranthus hypochondriacus var Nutrisol are A and IA, corresponding to peaks 1 and 2, respectively.
Fig. 5.
HPLC chromatograms of A. hypochondriacus var. Nutrisol extracts determined at 40 °C and 175 mW/mL (a), in comparison with control treatment at 40 °C (b).
Fig. 6.
HPLC chromatograms of A. hypochondriacus var. Nutrisol betacyanins before ß-glucuronidase hydrolysis (a), betanin standard (b), and after ß-glucuronidase hydrolysis (c).
3.9. Amaranthine and isoamaranthine (A, IA) extraction
The A content determined in the UAE was significantly affected by the two factors under study: temperature and UPD, and the linear, quadratic, and interaction terms (p < 0.05), whereas IA was not significantly affected by UPD and the interaction term. There was a good model fit for A and IA, resulting in R2 of 98.38 and 91.09%, respectively. However, there was a significant lack of fit (p < 0.05) for IA. The effects of temperature and UPD on the contents of A, IA are shown in Fig. 7a, b.
Fig. 7.
Response surface for the effects of temperature extraction and UPD on A (a) and IA (b) of A. hypochondriacus var. Nutrisol extracts.
The mean values for A and IA content ranged between 42.890–110.363 mg/100 g d.m and 2.352–11.655 mg/100 g d.m, respectively. Fig. 7a, b show that for A and IA content as temperature and UPD increased up to (41.57 °C, 201.99 mW/mL) and (42.71 °C, 176.93 mW/mL), resulting in maximum A and IA contents of 109.727 and 11.762 mg/100 g d.m. A subsequent decrease in A and IA content was observed at higher levels of both factors. Behaviours of A and IA were like that of BC, because these are the betacyanins present in amaranth extracts.
Changes of A and IA during heat processing are due to isomerisation, decarboxylation or cleavage reactions [1]. The isomerisation of A to IA is performed in C15 and it is induced by acidic or alkaline conditions at high temperatures, resulting in A diminution. Betacyanins decarboxylation is possible at C2, C15, and C17, being most pronounced at C2 in aqueous solutions [70]. A and IA diminution may be attributed to the cleavage of a carboxyl group from A and IA at high temperatures values, resulting in molecules with smaller absorption maxima compared to the amaranth betalains [42]. Otherwise, increases in UPD favour sonochemical effects and cavitation, resulting in higher A and IA contents. However, high UPD resulted in a diminution of A and IA. This may be attributed to the cavitation phenomenon diminution, which affect the cell wall disruption, and therefore, the release of A and IA. Decreased amaranthine and isoamaranthine contents from A. caudatus flowers at high temperatures and potencies were reported by Roriz et al. [24], and gomphrenin, isogomphrenin from floral parts of Gomphrena globosa L. by Roriz et al. [71]. This reaffirms, that in order to guarantee maximum extraction yields of A and IA, UAE should be carried out at intermediate temperatures and UPD. Still, A and IA determined by conventional extraction control were 1.66 and 1.51 times lower than UAE treatment. This could be corroborated in Fig. 5, where peaks area of UAE treatment were greater than those of conventional extraction control. This demonstrates the effectiveness of the UAE in obtaining higher A and IA than conventional method in a shorter extraction time.
A and IA contents were higher than those reported by Li et al. [9] (7.75–9.67, 5.13–6.38 mg/100 g d.m, respectively) and Stintzing et al. [35] (15.13, 5.87 mg/100 g d.m, respectively). However, results determined in this study were lower than reported for A. caudatus flowers (4887–5570, 1652–1956 mg/100 g d.m, respectively) by Roriz et al. [24].
3.10. Numerical optimisation
A numerical optimisation technique was performed to give the optimum response variables in the UAE. Optimum levels of temperature and UPD were obtained to maximise all variables, except L* which was minimised by applying the methodology of the desired function [72]. This function searches for a combination of factor levels that simultaneously satisfy the requirements for each response in the design. Response variables were classified according to their order of importance. BC, BX, BT, A, IA, and a* were selected as the most important variables, followed by KLa, TP, AA, L*, and b* which were the response variables with minor importance. The optimal extraction conditions were an extraction temperature of 41.80 °C and UPD of 188.84 mW/mL. This condition was determined to be optimum by surface response methodology optimisation with an overall desirability value of 0.963. The corresponding predicted contents and values of BC, BX, BT, PT, AA, L*, a*, b*, KLa, A, and IA were 106.434, 42.472, 148.906 mg/100 g d.m, 1277.206 mg GAE/100 g d.m, 3.450 mmol TE/100 g d.m, 25.431, 9.445, 1.077, 4.131 × 10-7 m3/s, 109.219, and 11.635 mg/100 g d.m, respectively. Under these optimal conditions, BT, A, IA and KLa were 1.38, 1.65, 1.50, and 29.93 times higher than determined by conventional extraction, respectively.
3.11. Verification of optimal extraction conditions
The validity of the optimised condition was confirmed by conducting experiments to compare the experimental results with the values predicted by the quadratic models. Table 6 lists the experimental values determined, and the values predicted by the quadratic models for the optimisation process. The experimental and predicted values for most of the response variables agree. This demonstrates the suitability of the quadratic models by testing the optimum conditions for predicting the optimum response. However, the colour parameter b* presented a relative error higher than 10%. Thus, the results prove the usefulness of the surface response methodology with simultaneous optimisation using the desirability function.
Table 6.
Experimental and predicted values of response variables.
| Response variables | Experimental value | Predicted value | Relative error (%) |
|---|---|---|---|
| BC | 97.447 ± 1.299 | 106.434 | 9.22 |
| BX | 45.978 ± 0.644 | 42.472 | 7.63 |
| BT | 143.425 ± 1.943 | 148.906 | 3.82 |
| TP | 1374.826 ± 23.991 | 1277.206 | 7.10 |
| AA | 3.29 ± 0.095 | 3.450 | 4.86 |
| L* | 25.144 ± 0.067 | 25.431 | 1.14 |
| a* | 9.971 ± 0.016 | 9.445 | 5.28 |
| b* | 0.947 ± 0.034 | 1.077 | 13.73 |
| KLa | 3.885 × 10-7 ± 0.495 × 10-8 | 4.131 × 10-7 | 6.33 |
| A | 100.225 ± 1.507 | 109.219 | 8.97 |
| IA | 12.478 ± 0.423 | 11.635 | 6.76 |
*Values presented are the average of triplicate measurements.
3.12. Correlation study
A correlation analysis was performed to investigate the relationship between the different response variables (Table 7). In the correlation study, there was a positive and significant correlation between BC, BX, and BT; the correlation was stronger with BC. BT is the sum of BC and BX; in this study, BC was higher than BX. Moreover, there was a strong and significant (p < 0.05) correlation of BC, BX, and BT with all colour parameters except L*. Likewise, there was a strong significant (p < 0.05) correlation of the variables previously mentioned with TP, AA, and KLa, which shows that ultrasonic extraction allows not only the extraction of natural pigments as betalains, but also compounds with antioxidant activity, such as polyphenols. As expected, there was a strong significant (p < 0.05) correlation of BC with A and IA; the correlation was stronger with A, the mayor betacyanin in amaranth. Moreover, A and IA were strongly correlated with AA, which shows that these compounds are the betalains responsible of the antioxidant activity in amaranth.
TP was not correlated with parameter L*, although it was significant (p < 0.05) and positive for a* and b*, where the correlation with parameter b* was stronger. This is due to many phenolic compounds having a yellow colour. However, there was a significant (p < 0.05), strong, and positive correlation between TP and AA. This result corroborates that polyphenols are compounds characterised by high antioxidant power. Likewise, it was significantly and positively related to KLa. This last response variable was significantly correlated with all the response variables analysed, except for L* and b*. This demonstrates the effectiveness of the ultrasonic extraction process in obtaining not only extracts with high pigment content and adequate colour parameters but also polyphenols with high antioxidant power.
4. Conclusions
The second-order model proposed using the surface response methodology efficiently predicted the ultrasonic extraction of betalains and polyphenols with high antioxidant power from A. hypochondriacus var. Nutrisol at different temperatures and UPD. Furthermore, these factors influenced most of the response variables in this study. To achieve the maximum performance of ultrasound extraction for optimising different response variables, temperature and UPD of 41.80 °C and 188.84 mW/mL were selected as the optimal conditions, achieving BT and TP yields of 83.39 and 20.73%, respectively. Under these optimal conditions, the experimental values for BC, BX, BT, TP, AA, L*, a*, b*, KLa, A, and IA agreed with the predicted values, indicating the suitability of the quadratic models. The results obtained suggest that UAE is a favourable alternative guaranteeing high concentrations of betalains and polyphenols in a short period than conventional methods applied in plants with high nutritional and functional value such as amaranth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the National Council of Science and Technology (CONACYT, Mexico) for financial support via a research project grant (No. 250804, PROINNOVA, 2018).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105680.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Azeredo H.M.C. Betalains: Properties, sources, applications, and stability - A review. Int. J. Food Sci. Technol. 2009;44:2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- 2.Swaroop V.R., Roy D.D., Vijayakumar T. Genotoxicity of Synthetic Food Colorants. J. Food Sci. Eng. 2011;1:128–134. [Google Scholar]
- 3.Delgado-Vargas F., Jiménez A.R., Paredes-López O. Natural pigments: Carotenoids, anthocyanins, and betalains - Characteristics, biosynthesis, processing. and stability. 2000;40(3):173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- 4.Coelho L.M., Silva P.M., Martins J.T., Pinheiro A.C., Vicente A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018;9(11):5499–5512. doi: 10.1039/C8FO01422A. [DOI] [PubMed] [Google Scholar]
- 5.Corke H., Cai Y.Z., Wu H.X. Amaranth: Overview. Encycl. Food Grains Second Ed. 2015;1–4:287–296. doi: 10.1016/B978-0-12-394437-5.00032-2. [DOI] [Google Scholar]
- 6.E. Espitia-Rangel, C. Mapes-Sánchez, D. Escobedo-López, M. De la O-Olán, P. Rivas-Valencia, G. Martínez-Trejo, L. Cortés-Espinoza, J.M. Hernández-Casillas, Conservación y uso de los recursos genéticos de amaranto en México., 2010.
- 7.Venskutonis P.R., Kraujalis P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013;12(4):381–412. doi: 10.1111/crf3.2013.12.issue-410.1111/1541-4337.12021. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y.-Z., Sun M., Corke H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005;16(9):370–376. doi: 10.1016/j.tifs.2005.03.020. [DOI] [Google Scholar]
- 9.Li H., Deng Z., Liu R., Zhu H., Draves J., Marcone M., Sun Y., Tsao R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015;37:75–81. doi: 10.1016/j.jfca.2014.09.003. [DOI] [Google Scholar]
- 10.Sarker U., Islam M.T., Rabbani M.G., Oba S. Variability in total antioxidant capacity, antioxidant leaf pigments and foliage yield of vegetable amaranth. J. Integr. Agric. 2018;17(5):1145–1153. doi: 10.1016/S2095-3119(17)61778-7. [DOI] [Google Scholar]
- 11.Castro L.M.G., Alexandre E.M.C., Pintado M., Saraiva J.A. Bioactive compounds, pigments, antioxidant activity and antimicrobial activity of yellow prickly pear peels. Int. J. Food Sci. Technol. 2019;54(4):1225–1231. doi: 10.1111/ijfs.14075. [DOI] [Google Scholar]
- 12.Kushwaha R., Kumar V., Vyas G., Kaur J. Optimization of Different Variable for Eco-friendly Extraction of Betalains and Phytochemicals from Beetroot Pomace. Waste and Biomass Valorization. 2018;9(9):1485–1494. doi: 10.1007/s12649-017-9953-6. [DOI] [Google Scholar]
- 13.Das M., Saeid A., Hossain M.F., Jiang G.-H., Eun J.B., Ahmed M. Influence of extraction parameters and stability of betacyanins extracted from red amaranth during storage. J. Food Sci. Technol. 2019;56(2):643–653. doi: 10.1007/s13197-018-3519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash Maran J., Manikandan S., Mekala V. Modeling and optimization of betalain extraction from Opuntia ficus-indica using Box-Behnken design with desirability function. Ind. Crops Prod. 2013;49:304–311. doi: 10.1016/j.indcrop.2013.05.012. [DOI] [Google Scholar]
- 15.Zin M.M., Márki E., Bánvölgyi S.z. Conventional Extraction of Betalain Compounds From Beetroot Peels With Aqueous Ethanol Solvent. Acta Aliment. 2020;49(2):163–169. doi: 10.1556/066.2020.49.2.5. [DOI] [Google Scholar]
- 16.Wen L.e., Zhang Z., Sun D.-W., Sivagnanam S.P., Tiwari B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020;60(11):1826–1841. doi: 10.1080/10408398.2019.1602823. [DOI] [PubMed] [Google Scholar]
- 17.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari B.K. Ultrasound: A clean, green extraction technology. TrAC - Trends Anal. Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- 19.Wen C., Zhang J., Zhang H., Dzah C.S., Zandile M., Duan Y., Ma H., Luo X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops – A review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Righi Pessoa da Silva H., da Silva C., Bolanho B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.) J. Food Process Eng. 2018;41(6):e12833. doi: 10.1111/jfpe.12833. [DOI] [Google Scholar]
- 21.Tao Y., Zhang Z., Sun D. Ultrasonics Sonochemistry Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc : Influence of acoustic energy density and temperature. Ultrason. - Sonochemistry. 2014;21:1461–1469. doi: 10.1016/j.ultsonch.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Zhong M., Huang S., Wang H., Huang Y., Xu J., Zhang L. Optimization of ultrasonic-assisted extraction of pigment from Dioscorea cirrhosa by response surface methodology and evaluation of its stability. RSC Adv. 2019;9(3):1576–1585. doi: 10.1039/C8RA07455K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed M., Ramachandraiah K., Jiang G., Eun J.B. Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract. Foods. 2020;9:1–18. doi: 10.3390/foods9081116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C. Lobo Roriz, V. Xavier, S.A. Heleno, J. Pinela, M.I. Dias, R.C. Calhelha, P. Morales, I.C.F.R. Ferreira, L. Barros, Chemical and Bioactive Features of Amaranthus caudatus L. Flowers and Optimized Ultrasound-Assisted Extraction of Betalains, Foods. 10 (2021) 1–13. https://doi.org/https:// doi.org/10.3390/foods10040779 Academic. [DOI] [PMC free article] [PubMed]
- 25.Dias A.L.B., Arroio Sergio C.S., Santos P., Barbero G.F., Rezende C.A., Martínez J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017;198:36–44. doi: 10.1016/j.jfoodeng.2016.11.020. [DOI] [Google Scholar]
- 26.Prakash Maran J., Manikandan S., Vigna Nivetha C., Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017;10:S1145–S1157. doi: 10.1016/j.arabjc.2013.02.007. [DOI] [Google Scholar]
- 27.Nipornram S., Tochampa W., Rattanatraiwong P., Singanusong R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018;241:338–345. doi: 10.1016/j.foodchem.2017.08.114. [DOI] [PubMed] [Google Scholar]
- 28.AOAC, 1998. https://doi.org/10.32741/fihb.3.honey.
- 29.Rawson A., Tiwari B.K., Tuohy M.G., O’Donnell C.P., Brunton N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011;18(5):1172–1179. doi: 10.1016/j.ultsonch.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 30.González-Centeno M.R., Knoerzer K., Sabarez H., Simal S., Rosselló C., Femenia A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.) - A response surface approach. Ultrason. Sonochem. 2014;21(6):2176–2184. doi: 10.1016/j.ultsonch.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Cai Y., Sun M., Wu H., Huang R., Corke H. Characterization and Quantification of Betacyanin Pigments from Diverse Amaranthus Species. J. Agric. Food Chem. 1998;46(6):2063–2070. doi: 10.1021/jf9709966. [DOI] [Google Scholar]
- 32.Singleton V.L., Orthofer R., Lamuela-Ramontós R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1007/BF02530903. [DOI] [Google Scholar]
- 33.Brand-Williams W., Cuvelier M.E., Berset C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT- Food Sci. Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 34.D.R. Heldman, R.P. Singh, Food Process Engineering, AVI Publishing Company, INC. Westport, Connecticut, 1981. https://doi.org/10.1007/978-94-010-9337-8.
- 35.F.C. Stintzing, D. Kammerer, A. Schieber, H. Adama, O.G. Nacoulma, R. Carle, Betacyanins and Phenolic Compounds from Amaranthus spinosus L . and Boerhavia erecta L ., Zeitschrift Fur Naturforsch. - Sect. C J. Biosci. C 59 (2004) 1–8. https://doi.org/https://doi.org/10.1515/znc-2004-1-201. [DOI] [PubMed]
- 36.Aletor O., Oshodi A.A., Ipinmoroti K. Chemical composition of common leafy vegetables and functional properties of their leaf protein concentrates. Food Chem. 2002;78(1):63–68. doi: 10.1016/S0308-8146(01)00376-4. [DOI] [Google Scholar]
- 37.W. Biel, E. Jendrzejczak, A. Jaroszewska, R. Witkowicz, E. Piatkowska, A. Telesiński, Nutritional content and antioxidant properties of selected species of Amaranthus L., Ital. J. Food Sci. 29 (2017) 728–740. https://doi.org/10.14674/IJFS-712.
- 38.Ngugi C.C., Oyoo-Okoth E., Manyala J.O., Fitzsimmons K., Kimotho A. Characterization of the nutritional quality of amaranth leaf protein concentrates and suitability of fish meal replacement in Nile tilapia feeds. Aquac. Reports. 2017;5:62–69. doi: 10.1016/j.aqrep.2017.01.003. [DOI] [Google Scholar]
- 39.Nana F.W., Hilou A., Millogo J.F., Nacoulma O.G. Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals. 2012;5:613–628. doi: 10.3390/ph5060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarker U., Oba S., Daramy M.A. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-60252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celli G.B., Brooks M.S.L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins — A current review. Food Res. Int. 2017;100:501–509. doi: 10.1016/j.foodres.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Herbach K.M., Stintzing F.C., Carle R. Betalain stability and degradation - Structural and chromatic aspects. J. Food Sci. 2006;71(4):R41–R50. doi: 10.1111/j.1750-3841.2006.00022.x. [DOI] [Google Scholar]
- 43.Khan M.I. Stabilization of betalains: A review. Food Chem. 2016;197:1280–1285. doi: 10.1016/j.foodchem.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Amaya D.B. Update on natural food pigments - A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019;124:200–205. doi: 10.1016/j.foodres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 45.Pardo-Rueda A.J., Quintero-Ramos A., Genovese D.B., Camacho-Dávila A., Zepeda-Rodríguez A., Contreras-Esquivel J.C., Bizarro A.P. Efficient extraction of fructans from sotol plant (Dasylirion leiophyllum) enhanced by a combination of enzymatic and sonothermal treatments. Food Bioprod. Process. 2015;94:398–404. doi: 10.1016/j.fbp.2014.05.005. [DOI] [Google Scholar]
- 46.H.M. Santos, C. Lodeiro, J.L. Capelo-Martínez, The Power of Ultrasound, in: J.-L. Capelo-Martínez (Ed.), Ultrasound Chem. Anal. Appl., WILEY-VCH, 2009: pp. 1–16. https://doi.org/10.1002/9783527623501.
- 47.T. Karchiyappan, Betalain Pigment Extraction from Malabar Spinach Fruit Using Ultrasound: Modeling and Validation, Env. Sci Ind J. 13 (2017) 1–10. http://www.tsijournals.com/articles/betalain-pigment-extraction-from-malabar-spinach-fruit-using-ultrasound-modeling-and-validation.pdf.
- 48.Maran J.P., Priya B., Nivetha C.V. Optimization of ultrasound-assisted extraction of natural pigments from Bougainvillea glabra flowers. Ind. Crops Prod. 2015;63:182–189. doi: 10.1016/j.indcrop.2014.09.059. [DOI] [Google Scholar]
- 49.Maran J.P., Priya B. Multivariate statistical analysis and optimization of ultrasound-assisted extraction of natural pigments from waste red beet stalks. J. Food Sci. Technol. 2016;53(1):792–799. doi: 10.1007/s13197-015-1988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.K.S. Khanam, S. Oba, Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus, Can. J. Plant Sci. 93 (2013) 47–58. https://doi.org/10.4141/CJPS2012-117.
- 51.Chong P.H., Yusof Y.A., Aziz M.G., Mohd Nazli N., Chin N.L., Syed Muhammad S.K. Evaluation of solvent extraction of Amaranth betacyanins using multivariate analysis. Int. Food Res. J. 2014;21:1569–1573. [Google Scholar]
- 52.Sarker U., Islam M.T., Rabbani M.G., Oba S. Antioxidant Leaf Pigments and Variability. 2018;50:209–220. doi: 10.2298/GENSR1801209S. [DOI] [Google Scholar]
- 53.Sarker U., Oba S., Elfalleh W. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS One. 2019;14(12):e0222517. doi: 10.1371/journal.pone.0222517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khanam U.K.S., Oba S. Phenotypic plasticity of vegetable amaranth, amaranthus tricolor L. under a natural climate. Plant Prod. Sci. 2014;17(2):166–172. doi: 10.1626/pps.17.166. [DOI] [Google Scholar]
- 55.Khandaker L., Ali M.B., Oba S. Influence of cultivar and growth stage on pigments and processing factors on betacyanins in Red Amaranth (Amaranthus tricolor L.) Food Sci. Technol. Int. 2009;15(3):259–265. doi: 10.1177/1082013209341119. [DOI] [Google Scholar]
- 56.Drevelegka I., Goula A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. - Process Intensif. 2020;149:107845. doi: 10.1016/j.cep.2020.107845. [DOI] [Google Scholar]
- 57.He B., Zhang L.L., Yue X.Y., Liang J., Jiang J., Gao X.L., Yue P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016;204:70–76. doi: 10.1016/j.foodchem.2016.02.094. [DOI] [PubMed] [Google Scholar]
- 58.Kaderides K., Goula A.M., Adamopoulos K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015;31:204–215. doi: 10.1016/j.ifset.2015.08.006. [DOI] [Google Scholar]
- 59.Grotewold E. the Genetics and Biochemistry of Floral Pigments. Annu. Rev. Plant Biol. 2006;57(1):761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z., Li S., Ge S., Lin S. Review of Distribution, Extraction Methods, and Health Bene fi ts of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020;68(11):3330–3343. doi: 10.1021/acs.jafc.9b06574. [DOI] [PubMed] [Google Scholar]
- 61.Dahmoune F., Spigno G., Moussi K., Remini H., Cherbal A., Madani K. Pistacia lentiscus leaves as a source of phenolic compounds : Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014;61:31–40. doi: 10.1016/j.indcrop.2014.06.035. [DOI] [Google Scholar]
- 62.Ramli N.S., Ismail P., Rahmat A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus) Sci. World J. 2014;2014:1–7. doi: 10.1155/2014/964731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Žlabur J.Š., Voća S., Dobričević N., Pliestić S., Galić A., Boričević A., Borić N. Ultrasound-assisted extraction of bioactive compounds from lemon balm and peppermint leaves. Int. Agrophysics. 2016;30:95–104. doi: 10.1515/intag-2015-0077. [DOI] [Google Scholar]
- 64.López-Mejía O.A., López-Malo A., Palou E. Antioxidant capacity of extracts from amaranth (Amaranthus hypochondriacus L.) seeds or leaves. Ind. Crops Prod. 2014;53:55–59. doi: 10.1016/j.indcrop.2013.12.017. [DOI] [Google Scholar]
- 65.Sarker U., Oba S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-52033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozsoy N., Yilmaz T., Kurt O., Can A., Yanardag R. In vitro antioxidant activity of Amaranthus lividus L. Food Chem. 2009;116(4):867–872. doi: 10.1016/j.foodchem.2009.03.036. [DOI] [Google Scholar]
- 67.S. Pulipati, P.S. Babu, U. Naveena, S.K.R. Parveen, S.K.S. Nausheen, M.T.N. Sai, Determination of Total Phenolic, Tannin, Flavonoid Contents and Evaluation of Antioxidant Property of Amaranthus tricolor (L), Int. J. Pharmacogn. Phytochem. Res. 9 (2017) 814–819. https://doi.org/10.25258/phyto.v9i6.8184.
- 68.Jiménez-Aguilar D.M., Grusak M.A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017;58:33–39. doi: 10.1016/j.jfca.2017.01.005. [DOI] [Google Scholar]
- 69.Biswas M., Dey S., Sen R. Betalains from Amaranthus tricolor L. J. Pharmacogn. Phytochem. 2013;1:87–95. [Google Scholar]
- 70.Wybraniec S., Mizrahi Y. Generation of decarboxylated and dehydrogenated betacyanins in thermally treated purified fruit extract from purple pitaya (Hylocereus polyrhizus) monitored by LC-MS/MS. J. Agric. Food Chem. 2005;53(17):6704–6712. doi: 10.1021/jf050700t. [DOI] [PubMed] [Google Scholar]
- 71.Roriz C.L., Barros L., Prieto M.A., Morales P., Ferreira I.C.F.R. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017;229:223–234. doi: 10.1016/j.foodchem.2017.02.073. [DOI] [PubMed] [Google Scholar]
- 72.Derringer G., Suich R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980;12(4):214–219. doi: 10.1080/00224065.1980.11980968. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.