Abstract
Background:
Myeloid-derived suppressor cells (MDSC) are categorized as granulocytic (G-MDSCs) and monocytic (M-MDSCs) and their expansion play a role in cancer progression. Recruitment to the cancer site depends upon the presence of a chemoattractant. We aimed to investigate the presence of MDSC subtypes and of interleukin-8 (CXCL-8) in the peripheral blood in lung cancer subtypes including non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) patients.
Materials and Methods:
Peripheral blood samples of 26 NSCLC patients, 18 SCLC patients, and 8 healthy control donors (HDs) were harvested and the surface expression of CD14, CD15, CD11b, and HLA-DR on MDSCs was measured using flow cytometry. The level of serum CXCL8 was measured by the ELISA method.
Results:
The frequency of circulating M-MDSCs was significantly higher in patients with NSCLC than in SCLC and HDs. In contrast, there was no statistical difference concerning the frequency of circulating G-MDSCs between the three groups. The concentration of CXCL-8 was significantly higher in the NSCLC and SCLC patients than in HD control with no significant difference between NSCLC and SCLC groups. There was no correlation between serum CXCL8 and G-MDSC levels.
Conclusion:
Our data confirm a higher frequency of circulating M-MDSCs, but not G-MDSCs, in the blood of those suffering from NSCLC but not for SCLC cases. Measuring MDSC subtypes and serum chemotactic factors may have implications for the differential diagnosis of NSCLC.
Keywords: Myeloid-derived suppressor cells, Lung cancer, CXCL8, NSCLC, SCLC, MDSC
INTRODUCTION
Lung cancer not only is highly invasive but also rapidly metastasizes. As a highly prevalent disorder, it is the main cause of cancer-related death despite recent promising advances in diagnostics and therapeutics. Globally, lung cancer is the most prevalent cancer and the leading cause of cancer death in men and is the third most common cancer in women. According to the American Cancer Society, the annual new cases of lung cancer will reach 228,820 in the United States by 2020. In addition, it is reported that lung cancer will claim 135,720 lives (2). The 5-year survival of early-stage lung cancers is greater than 50%; however, most lung cancers are diagnosed at an advanced stage when survival is poor (3). Currently available treatments for lung cancer include surgery, radiation therapy, chemotherapy, and targeted therapy. Despite the improvements in diagnosis and treatment during the past 25 years, the prognosis of patients with lung cancer remains disappointing (4).
Traditionally, lung cancer is divided into two major histologic types that develop and spread differently: small-cell lung carcinomas (SCLC), which are subdivided into limited and extensive cancers, whilst non-SCLC (NSCLC) cancer is further divided into clinical stages of 1 to 4. NSCLC is further subdivided histopathologically into adenocarcinomas (ADC), squamous cell carcinomas (SqCC), and large cell carcinomas (4).
Therapeutic protocols for lung cancer depend on the individual subtype and stages of disease (5,6). For example, although advanced stage chemotherapy is the main treatment strategy for both NSCLC and SCLC, the response is much greater for patients with extensive-stage SCLC than patients with metastatic adenocarcinoma (6). Limited-stage SCLC and localized NSCLC are both potentially curable diseases, but their treatment strategies are substantially different (5,7–9).
Immune dysfunction is an important feature during cancer development (10). The immune system identifies and eradicates malignant cells, but dysregulation of these anti-tumor immune responses occurs as the malignancy develops (11). An important mechanism through which cancers escape immune destruction is the accumulation of the relatively immature, heterogeneous population of activated myeloid-derived suppressor cells (MDSC).
MDSCs have a potent immunosuppressive activity involving multiple mechanisms (12). MDSCs can be further divided into granulocytic or polymorphonuclear (G-MDSCs), which are morphologically similar to neutrophils and defined as CD11b+CD14-CD15+HLADR-, and monocytic (M-MDSCs), phenotypically similar to monocytes and defined as CD11b+CD14+CD15-HLADR- (13,14). MDSCs develop in the bone marrow (BM) from primary myeloid progenitor cells and are recruited to the site of malignancy via chemokines such as CCL2 (C-C chemokine ligand 2) (15), CCL5 (16), and CXCL-8 (17). The levels of these chemokines vary according to the type of cancer and their subtypes. CXCL-8 is important in most types of cancer and is linked to the recruitment and activation of myeloid cells such as MDSC and neutrophils (18). Indeed, serum levels of CXCL-8 may predict the responses to immunotherapies in melanoma and NSCLC (19,20). The anti-immune function of MDSCs involves several mechanisms including the production of nitric oxide (NO), reactive oxygen species (ROS), and the elimination of key nutrition factors like arginine that are required for T cell proliferation (21,22). While rare in healthy individuals, MDSC may accumulate in the setting of severe trauma, cancer, and sepsis (23,24).
The correlation between MDSC and increasing tumor burden suggests that they may be useful in monitoring disease progression and treatment response (25). We hypothesize that circulating levels of MDSC subtypes may be associated with different subtypes of lung cancer and that this association will also depend upon chemokine levels. We, therefore, aimed to, firstly, determine the circulating levels of M- and G-MDSCs and of CXCL-8 in patients with SCLC and NSCLC and, secondly, to compare the observed levels to that of healthy volunteers..
MATERIALS AND METHODS
Study population
A total of 26 NSCLC patients, 18 SCLC patients, and 8 healthy donor controls (HDs) were enrolled in this study. NSCLC and SCLC patients were admitted to the oncology wards at the Masih Daneshvari hospital (Tehran, Iran) from November 2018 to December 2019. Informed written consent was obtained from all participants. All experimental protocols, including blood sampling, were reviewed and approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1397.835). Blood samples were transferred into EDTA tubes (3mls) for flow cytometry analysis and 2mls blood was collected in the absence of anticoagulant for serum isolation.
The exclusion criteria included patients with current or previous use of immunomodulating drugs or oral steroids, a diagnosis of immune-related disease, suffering from infectious diseases, and a history of any cancer-specific treatment or a concomitant malignancy within the past 5 years. Patient demographics are provided in Table 1.
Cell staining and flow cytometry
Within an hour of sampling, blood samples were analyzed for MDSCs phenotype using flow cytometry. Peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Hypaque density gradient (lymphosep, biosera, UK), and then washed with cold PBS and incubated for 30 min at 4°C with anti-CD14 PercP-Clone: MQP9 (BD Biosciences, USA), anti-CD11b APC-Clone: ICRF44 (also known as 44) (BD Biosciences, USA), anti-HLA-DR PE-Clone: NL3 (Invitrogen, USA) and anti-CD15 FITC-Clone: MCS-1 (Cytognos, Spain). Immunoglobulin isotype controls were used to detect and remove background staining.
Afterward, 200,000 cells were analyzed by FACS (FACS Calibour, BD, USA) with cells being gated on CD11b+/HLA-DR-/dim and within this population CD14+/CD15- cells and CD14-/CD15+ cells were further analyzed as described previously (13). FlowJo-V10 software was used for data analysis.
Measurement of serum CXCL-8
Serums were harvested from blood tubes without anticoagulants and stored at −80°C. Serum CXCL-8 was assayed by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (BD Pharmangene, CA, USA) according to the Manufacturer’s instructions. The detection limit of this kit was 2pg/ml.
Statistical analysis
Statistical analysis was administered using GraphPad Prism version 8. Data from individual experiments are presented as the mean fluorescence intensity (MFI) in arbitrary units (AU) of at least 200,000 events. ANOVA was used to compare study groups (more than two). For non-normally-distributed parameters, the non-parametric Mann Whitney U test and Kruskal Wallis H test were used. Statistical significance was considered when p<0.05.
RESULTS
Patient characteristics
Twenty-six patients with NSCLC (17 male) and 18 (15 male) with SCLC were recruited (Table 1). The diagnosis was confirmed by chest computerized tomography (CT) scan and histopathological findings. There was no difference between the study groups concerning the mean age of the subjects. Also, for NSCLC groups, 23 NSCLC patients had Stage IIII disease, whilst only 1 patient in each group was classified in Stages I, II, and III. Nine patients were diagnosed with limited-stage SCLC and 9 had an extensive form of SCLC (Table 1). According to histopathological findings, 21/26 NSCLC patients had ADC and 5 were diagnosed with SqCC. The smoking history was similar between the two groups with 33 (0–90) pack-years for NSCLC subjects and 37(10–105) pack-years for the SCLC patients.
Flow cytometry of MDSC cells
Our gating strategy began with the gating M-MDSC (HLA-DR −/ dim , CD11b+, CD14−, CD15+) and then G-MDSC (HLA-DR −/ dim , CD11b+, CD14+, CD15−) cells. The percentages of M-MDSC and G-MDSC cells in healthy control subjects (Figure 1A), NSCLC (Figure 1B) and SCLC (Figure 1C) patients in representative samples is shown
Figure 1.
Representative gating strategy in order to the identification of monocytic myeloid-derived suppressor cells (M-MDSC) (HLA-DR-/dimCD11b+CD14+CD15- cells) and granulocytic (G-MDSCs) (HLA-DR-/dimCD11b+CD14-CD15+ cells) in blood samples from (A) a healthy donor, (B) a non-small cell lung cancer (NSCLC) patient and (C) a small cell lung cancer (SCLC) patient.
Abbreviations: HLA-DR = human leukocyte antigen-D–related.
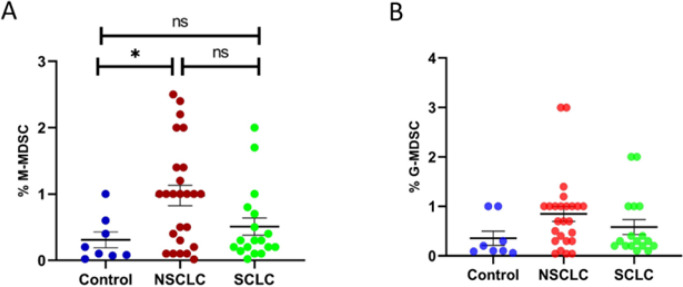
The percentage of the respective subset of live-gated leukocytes (% CD14+MDSC or % CD15+MDSC, respectively) was calculated and showed that the percentage of circulating M-MDSCs was significantly higher in patients with NSCLC than in the HDs (p≤0.0311) groups. We found no statistical difference in the level of M-MDSCs between NSCLC and SCLC (p≤0.1628) as well as SCLC and HDs (p≤0.8812) (Figure 2A). In contrast, there was no significant difference in the circulating percentage of G-MDSC between any of the groups (Figure 2B). Moreover, we found no statistical difference concerning the level of M-MDSCs, G-MDSCs, and CXCL-8 between ADC and SqCC patients as well as between different stages of SCLC.
Figure 2.
Frequencies of myeloid-derived suppressor cells (MDSC) in patients with lung cancer. Peripheral blood mononuclear cells (PBMCs) obtained from 26 patients with non–small cell lung cancer (NSCLC), 18 patients with small cell lung cancer (SCLC) and 8 healthy volunteers (control). The percentage of monocytic MDSCs (M-MDSCs)(A) and granulocytic MDSCs (G-MDSCs) (B) in each subject group are shown graphically. The results for each individual subject are shown along with the mean±SEM. *p≤0.05.
Serum levels of CXCL-8
The serum concentration of CXCL-8 was significantly higher in the NSCLC and SCLC patients than in healthy volunteers (p≤0.0001, Shown in Figure 3). There was no statistically significant difference concerning the level of CXCL-8 between NSCLC and SCLC patients (p=0.6180, Figure 3).
Figure 3.

Serum CXCL8 levels in 26 patients with non–small cell lung cancer (NSCLC), 18 patients with small cell lung cancer (SCLC) and 8 healthy volunteers (control). Results are presented as the mean±SEM. ****p<0.0001.
DISCUSSION
According to the findings of this small-scale pilot study, circulating M-MDSCs, but not G-MDSC, are elevated in NSCLC patients compared to healthy volunteers. There was a trend towards a greater expression of circulating M-MDSC in NSCLC compared to the SCLC, but it was not statistically significant. Furthermore, the percentage of neither of the circulating cell types was raised in SCLC. In addition, serum CXCL-8 level was higher in both NSCLC and SCLC patients compared to healthy controls.
According to the findings, the presence of circulating M-MDSCs in NSCLC patients is higher than in healthy controls, as demonstrated earlier (26, 27). However, establishing noninvasive methods for understanding the types of lung cancer remains challenging. MDSCs are tumor-related cells mediating immune escape that are detectable in the blood circulation of cancer patients. The properties of MDSCs in patients with different tumors have recently been studied (28–31).
In a study on 89 NSCLC patients, Huang et al. reported a significant increase in the numbers of circulating M-MDSCs, which was associated with tumor metastasis and degree of response to chemotherapy (27). In the same vein, in a study on 42 SCLC patients, Tian et al. reported significantly elevated numbers of peripheral blood M-MDSCs compared to the healthy controls (26). Furthermore, in patients with advanced stage of NSCLC, increased subpopulations of peripheral blood CD11b+/CD14−/CD15+/CD33+ cells with characteristics of MDSCs has been reported (31). In contrast, there was a higher percentage of circulating G-MDSCs in 90 naïve NSCLC patients who were receiving treatment than 25 healthy controls (32). In this latter study, the levels of M-MDSCs were not studied.
Barrera et al. (32) indicated that subjects with a low frequency of peripheral blood G-MDSCs had a better prognosis than subjects with a high frequency of blood G-MDSCs. They also were unable to show a correlation between CXCL-8 blood levels and G-MDSC numbers, despite a negative correlation between the expression of inflammatory cytokines such as IL-1β, IL-2, IL-27, and IL-29. Interestingly, a higher level of blood M-MDSCs is correlated with the response to PD-1 therapy (33). Further analysis of the levels of G-MDSCs is required across a greater range of patient’s severity and prognosis. This issue has been suggested by Yamauchi et al. (13), who showed monocytes and M-MDSC move actively toward the primary and metastatic tumor sites via the blood under the influence of chemotactic factors.
In the current study, we found similar elevated levels of circulating CXCL-8 in both NSCLC and SCLC patients. CXCL-8 is produced by lymphoid, myeloid, and tumor cells and is one of the main chemokines that regulate the recruitment of neutrophils and MDSCs to the site of the tumor (31,34). A previous study demonstrated that raised CXCL-8 levels are linked to tumor progression and stages in NSCLC patients (13). This reflects the fact that the long-term secretion and maintenance of inflammatory mediators during chronic inflammation or tumor progression may stimulate M-MDSC activation (13). However, although CXCL-8 is a neutrophil chemoattractant, we did not see a link between serum CXCL-8 levels and G-MDSC numbers. Indeed, raised serum CXCL-8 levels were associated with the greater presence of M-MDSCs. This suggests that another chemokine may be involved in the mobilization of M-MDSCs and that G-MDSCs from the current population of subjects studied do not express CXCL-8 receptors such as CXCR1/2.
In summary, we report in this study, frequency of the circulating M-MDSCs and G-MDSCs in both NSCLC and SCLC bearing patients. Based on the findings, it can be argued that those with NSCLC have increased levels of M-MDSCs, but it is not true for SCLC and G-MDSCs in either disease despite high expression of CXCL- 8 in NSCLC and SCLC. It is necessary to mention some limitations of our study including the sample size, range of disease severity in the NSCLC group, and the measurement of a single peripheral blood chemokine level. However, we suggest that a larger multi-centered study using a combination of blood MDSC levels and a wide range of chemokines may indicate a better combination of markers that may be useful in the diagnosis and prognosis of lung cancer and the potential response to the therapy.
REFERENCES
- 1.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69(3):211–33. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman RM, Sanchez R. Lung Cancer Screening. Med Clin North Am 2017;101(4):769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015December;1856(2):189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26(21):3543–51. [DOI] [PubMed] [Google Scholar]
- 7.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta 2015;1856(2):189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52(Pt 1):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnet I. Cancer bronchique à petites cellules. Rev Malad Respir Actual 2018;10(3):349–57. [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 11.Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27(45):5894–903. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol 2016;37(3):208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi Y, Safi S, Blattner C, Rathinasamy A, Umansky L, Juenger S, et al. Circulating and Tumor Myeloid-derived Suppressor Cells in Resectable Non-Small Cell Lung Cancer. Am J Respir Crit Care Med 2018;198(6):777–87. [DOI] [PubMed] [Google Scholar]
- 14.Yin Z, Li C, Wang J, Xue L. Myeloid-derived suppressor cells: Roles in the tumor microenvironment and tumor radiotherapy. Int J Cancer 2019;144(5):933–46. [DOI] [PubMed] [Google Scholar]
- 15.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 2012;72(4):876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu L, Chen J, Yao F, Zhou L, Zhang C, Wen W, et al. Percutaneous cryoablation for stage IV lung cancer: a retrospective analysis. Cryobiology 2013;67(2):151–5. [DOI] [PubMed] [Google Scholar]
- 17.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res 2011;71(24):7463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanmamed MF, Carranza-Rua O, Alfaro C, Oñate C, Martín-Algarra S, Perez G, et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res 2014;20(22):5697–707. [DOI] [PubMed] [Google Scholar]
- 19.Alfaro C, Teijeira A, Oñate C, Pérez G, Sanmamed MF, Andueza MP, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res 2016;22(15):3924–36. [DOI] [PubMed] [Google Scholar]
- 20.Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 2017;28(8):1988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 2013;123(10):4464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12(4):253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9(3):162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med 2011;17(3–4):281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angell TE, Lechner MG, Smith AM, Martin SE, Groshen SG, Maceri DR, et al. Circulating Myeloid-Derived Suppressor Cells Predict Differentiated Thyroid Cancer Diagnosis and Extent. Thyroid 2016;26(3):381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian T, Gu X, Zhang B, Liu Y, Yuan C, Shao L, et al. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark 2015;15(4):425–32. [DOI] [PubMed] [Google Scholar]
- 27.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother 2013;62(9):1439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AC, Groen HJM, et al. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013;81(3):468–74. [DOI] [PubMed] [Google Scholar]
- 29.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010;136(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, et al. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells’ Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11(8):1263–272. [DOI] [PubMed] [Google Scholar]
- 31.Feng PH, Lee KY, Chang YL, Chan YF, Kuo LW, Lin TY, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 2012;186(10):1025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrera L, Montes-Servín E, Hernandez-Martinez JM, Orozco-Morales M, Montes-Servín E, Michel-Tello D, et al. Levels of peripheral blood polymorphonuclear myeloid-derived suppressor cells and selected cytokines are potentially prognostic of disease progression for patients with non-small cell lung cancer. Cancer Immunol Immunother 2018;67(9):1393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng J, Chen S, Li S, Wu B, Lu J, Tan L, et al. The association between monocytic myeloid-derived suppressor cells levels and the anti-tumor efficacy of anti-PD-1 therapy in NSCLC patients. Transl Oncol 2020;13(12):100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev 2017;60:24–31. [DOI] [PubMed] [Google Scholar]