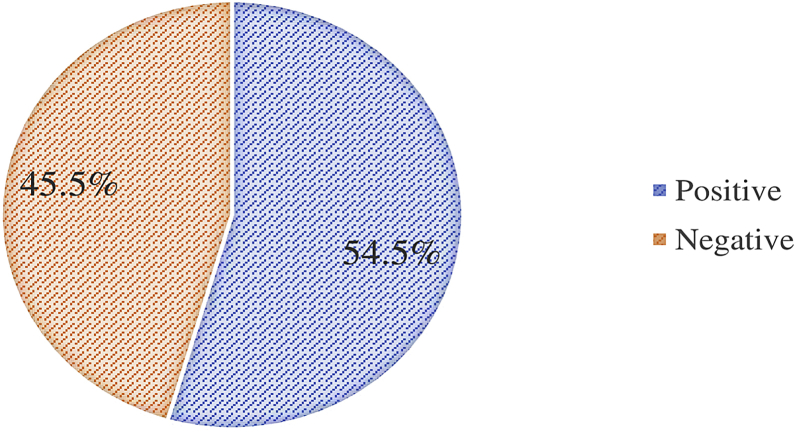Abstract
Background
Meningiomas that are progesterone receptor positive have a low recurrence rate and good prognosis compared to those that are progesterone receptor negative. This study aimed to determine the prevalence of expression of progesterone in meningiomas and its association with clinicopathological characteristics.
Materials and methods
This was a cross-sectional laboratory-based study that was conducted at Muhimbili National Hospital. The study included 112 formalin-fixed paraffin-embedded tissue blocks of patients who were confirmed to have meningiomas on histological basis from January 2010 to December 2014. Immunohistochemical expression of progesterone receptor was tested using a primary monoclonal progesterone receptor antibody ready to use (IR 068 Dako). The χ2 test was used to determine the association between clinicopathological characteristics and progesterone receptor expression. A 2-tailed P < 0.05 was considered significant.
Results
The mean age of the patients was 45.5 ± 3.601 years, and majority (66.1%, n = 74) were in the age group between 31 and 60 years. Also, majority of the patients (60%, n = 67) in this study were females. Over one-third of the cases (34.8%, n = 39) comprised of meningotheliomatous subtype, and majority of the cases (89.3%, n = 100) were of grade I. The prevalence of progesterone expression was 54.5% (n = 61), and only age was associated with progesterone receptor expression (P = 0.043).
Conclusion
The finding of high expression of the progesterone receptor for grade I cases in this study indicates that progesterone receptor expression in meningiomas is of prognostic value and may be considered when evaluating patients for management. Lack of expression of progesterone receptor in all the malignant cases is intriguing and needs further studies that can investigate its prognostic role.
Key words: Clinicopathological characteristics, Meningioma, Progesterone receptor
Abbreviations and Acronyms: CNS, Central nervous system; FFPE, Formalin-fixed paraffin-embedded; IHC, Immunohistochemistry; PR, Progesterone receptor; TBS, Tris buffer solution; WHO, World Health Organization
Introduction
Meningiomas are the most common primary benign tumors of the central nervous system (CNS) as well as intradural part of the spinal cord.1 These are slow growing tumors; however, they may recur and cause significant morbidity and mortality.2 According to the World Health Organization (WHO) report of 2007, meningiomas account for approximately 20% and 25% of all primary CNS and spinal cord tumors, respectively. Furthermore, the report revealed that approximately 30% of the diagnoses are usually established during autopsy.3 The Central Brain Tumor report, which was produced in the United States among patients with meningiomas between 2004 and 2008, revealed that the age-adjusted incidence rate of meningioma was 3.76 per 100,000 person-years for men and 8.44 per 100,000 person-years for women.4 In the United Kingdom, the epidemiology of meningiomas has remained constant for over 12 years from 1996 to 2008 where women have been reported to have a 2-fold increased risk of developing meningioma compared with men.5 The incidence of meningioma in Africa has been reported to stand at 30%.6 A prevalence of 26% of meningiomas among intracranial tumors was reported in a study that was previously conducted in Tanzania.7
The prevalence of expression of progesterone receptor (PR) among patients with meningioma has been reported to be determined by different clinicopathologic factors particularly tumor grade as it was established by the WHO.8 For example, Shayanfar et al9 reported that the expression of PR for grades I, II, and III was 96.8%, 20%, and 0%, respectively. Another study that was performed in German reported that only WHO grade I meningiomas were PR positive and all cases with WHO grades II and III were negative.10 Other studies have shown that meningiomas that are positive for PRs usually have better prognosis and they have very limited chances of recurrence.11,12
Because tumor biology has been reported to be influenced by genetical composition, which in turn is usually determined by the race of the individuals and also their geographical location,13,14 we thought of conducting this study among Tanzanians with meningiomas so as to determine the level of PR expression using formalin-fixed paraffin-embedded (FFPE) tissue blocks of patients who were diagnosed with meningiomas between 2010 and 2014. This was done for the purpose of addressing the knowledge gap that exists regarding the level of expression of PR and the way it may show association with the clinicopathological factors.
Materials and Methods
Study Design and Setting
This was a cross-sectional descriptive laboratory-based study that included 112 FFPE tissue blocks of meningioma cases diagnosed at Muhimbili National Hospital from January 2010 to December 2014. This hospital is the national referral hospital that receives patients from different parts of the country and neighboring countries including Kenya, Uganda, Burundi, and Rwanda. In addition, the hospital is used as the teaching hospital for the Muhimbili University of Health and Allied Sciences.
Study Population
The current study included 112 FFPE tissue blocks of patients who were diagnosed on histological basis for a period of 5 years (2010–2014). All cases diagnosed histologically as meningioma during the study period with available intact FFPE tissue blocks as well as clinical information were included in the present study. On the other hand, all cases with missing or spoilt FFPE tissue blocks and/or missing clinical information were excluded from the study.
Sample Size and Selection of the Cases
The sample size was obtained through retrospective reviewing of all the recorded FFPE tissue blocks for both spinal cord and CNS tumors at the Central Pathology Laboratory of Muhimbili National Hospital.
Immunohistochemistry Staining for Progesterone Receptor
The obtained FFPE tissue blocks were sectioned at the thickness of 4.0 μm. Dewaxing was done by placing the slides on a hot plate at 60°C for 30 minutes; then they were placed in 3 changes of xylene solution. This was followed by rehydration by dipping them in descending concentration of ethanol (absolute, 95%, 80%, and 70%). Thereafter, the tissue slides were rinsed in distilled water. A ring was made around the section using Dako pen to limit spreading of the primary antibody. Two drops of peroxidase blocking solution (SM 8OI; Dako, Glostrup, Denmark) were added to each section and kept for 15 minutes so as to block endogenous peroxidase activity.
Then the slides were rinsed in distilled water for 3 minutes. The antigen retrieval solution consisting of 10.0 mM/dL of citrate buffer at pH 7.6 (Batch DM 828; Dako) was heated in a pressure cooker until it started to boil; then the slides were incubated in the boiling antigen retrieval solution for 2 minutes of full pressure and thereafter placed in distilled water at room temperature. Tris buffer solution (TBS) (DM 831; Dako) was added to each section for 3 minutes. The TBS was drained from the section, and the primary monoclonal PR antibody ready to use (IR 068; Dako) was added to the tissue sections and incubated for 1 hour. The slides were washed in TBS for 5 minutes followed by the addition of 2 drops of horseradish peroxidase (SM 802; Dako) in each section for 30 minutes.
Subsequently, the glass slides were rinsed in TBS for 5 minutes; then a detection system consisting of 2,3-diaminobenzidin (Batch DM 927; Dako) was added to the tissue sections for 5 minutes. The tissue sections were rinsed in TBS and counterstained with Harris hematoxylin solution for 3 minutes and differentiated in 1% acid-alcohol for 2 dips. Then the tissue sections were blued in tap running water for 2 minutes. This was followed by dehydrating them through the ascending concentration of ethanol (70%, 80%, 95%, and absolute); they were then cleared in 3 changes of xylene and the slides were ready for interpretation.
The positivity was interpreted according to the nuclear staining of the tumor cells using a light microscope at a high power field as it was done in a previous study.15 A total number of 100 tumor cells were counted, of which the percentage of positive cells was derived in nonconsecutive 10 fields at high magnification. Staining of the tumor cells was reported as follows: 0 = 0% tumor cells, 1 = 1%–29% tumor cells, 2 = 30%–59% tumor cells, and 3 = 60%–100% tumor cells. The intensity was graded as 0 = absent, 1 = weak, 2 = moderate, and 3 = strong. Cases with intensity of 0 and 1 were considered negative, whereas those with intensity of 2 and 3 were regarded to be positive. Positive control was obtained from a known case of breast cancer, whereas negative control was obtained by omitting the primary antibody. Reporting of the immunohistochemistry (IHC) stained tissue slides was done by 2 independent experienced pathologists who were first blinded to the clinical history of the patients.
Data Collection Methods
Data regarding age, sex, and location of the tumor were extracted from the patients' clinical files. Moreover, the histological types, WHO grades, and PR status were recorded after reporting of the hematoxylin and eosin and IHC stained tissue slides, respectively.
Statistical Analysis
We analyzed the data collected using SPSS statistics version 20.0 software (IBM, USA). Categorical and continuous variables were summarized as percentages and mean ± standard deviation, respectively. The χ2 and Fisher's exact tests were used to determine the association of the clinicopathological characteristics with PR expression. The association was considered significant when the P value was found to be less than 5%.
Results
Selection Process of the Cases Included in the Study
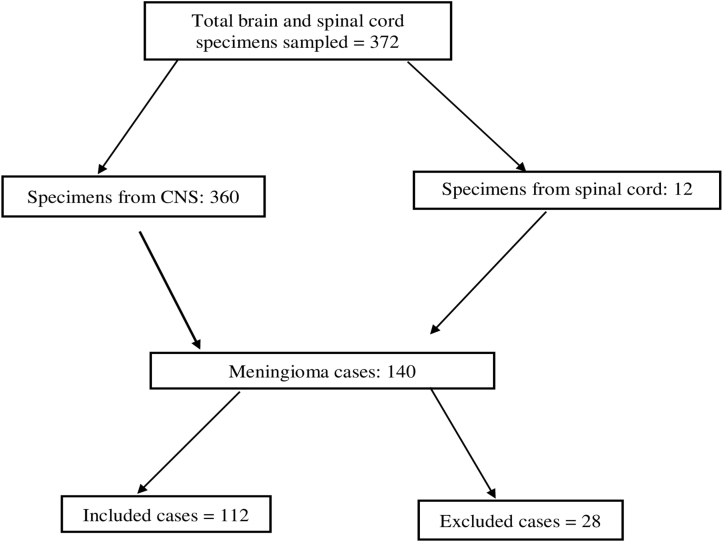
Figure 1 shows the steps through which the cases included in the present study were selected and excluded. A total of 372 specimens from patients with both CNS and spinal cord tumors were reported at the histopathology unit from January 2010 to December 2014. Of these, 37.6% (n = 140) were histopathologically confirmed to be meningioma. On the basis of the inclusion criteria, we found that 20% (n = 28) of the cases whom were confirmed meningiomas could not meet the inclusion criteria and therefore were excluded from the study.
Figure 1.
Flow chart indicating the process of selection of the cases included in the study. CNS, central nervous system.
Demographic Characteristics of the Patients
The mean age of the patients in this study was 45.5 ± 3.601 years (range: 4–72 years). Of all the meningioma cases, the majority (60%, n = 67) were found among females and 40% (n = 45) were in males. The male-to-female ratio of meningiomas in our study was 1:1.5. However, the peak age of occurrence of meningiomas was between 31 and 60 years, which consisted of 66.1% (n = 74) of all the cases.
Frequency Distribution of the Histological Types of Meningiomas in the Study
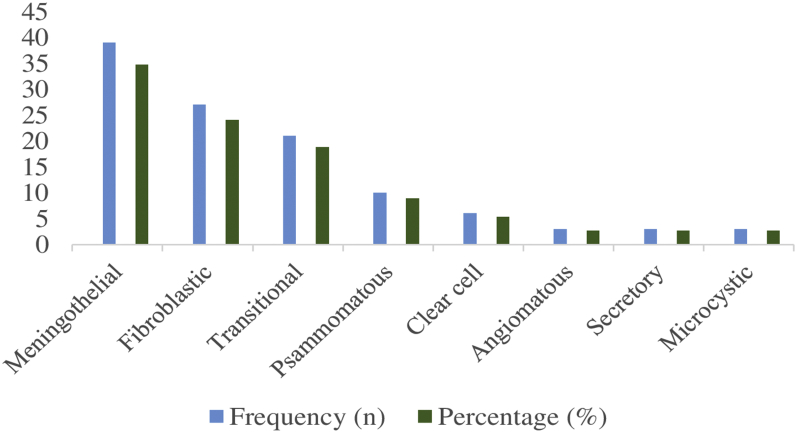
By far meningotheliomatous variant of meningioma was the most common histological type (34.8%, n = 39) followed by fibroblast type that comprised 24.1% (n = 27), and microcystic, secretory, and angiomatous types were the least types that comprised 2.7% (n = 3). Other histological variants are detailed in Figure 2.
Figure 2.
Frequency distribution of the histological types of meningiomas in the study.
Tumor Grading Among the Cases Included in the Study
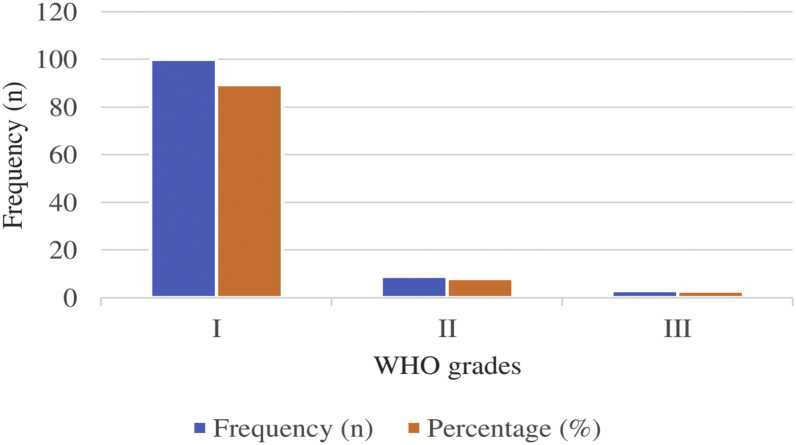
Regarding the WHO grading of meningiomas in this study, we observed that majority of them (89.3%, n = 100) were of grade I (typical meningiomas) followed by grade II cases (atypical meningiomas) that comprised 8% (n = 9) cases and grade III or malignant meningiomas that comprised 2.7% (n = 3) cases (Figure 3).
Figure 3.
World Health Organization (WHO) grading of the meningiomas included in the study.
Frequency Distribution of Meningiomas in the Study by Anatomical Sites
A relatively high proportion (18.8%, n = 21) of the meningiomas reported in the present study were located in the temporoparietal region followed by 14.3% (n = 16) cases that were located in the olfactory area. The spinal area was the region with a least number of meningiomas that comprised only 3.6% (n = 4) (Table 1).
Table 1.
Frequency Distribution of Meningiomas in the Study by Anatomical Sites (N = 112)
| Anatomical Site | Frequency (n) | Percentage (%) |
|---|---|---|
| Temporoparietal | 21 | 18.8 |
| Olfactory | 16 | 14.3 |
| Frontal | 14 | 12.5 |
| Sphenoid wing | 12 | 10.7 |
| Falx | 11 | 9.8 |
| Parasagittal | 10 | 8.9 |
| Posterior fossa | 7 | 6.3 |
| Cerebellum | 5 | 4.5 |
| Spine | 4 | 3.6 |
| Others | 12 | 10.7 |
Prevalence of Expression of Progesterone Receptor in the Study
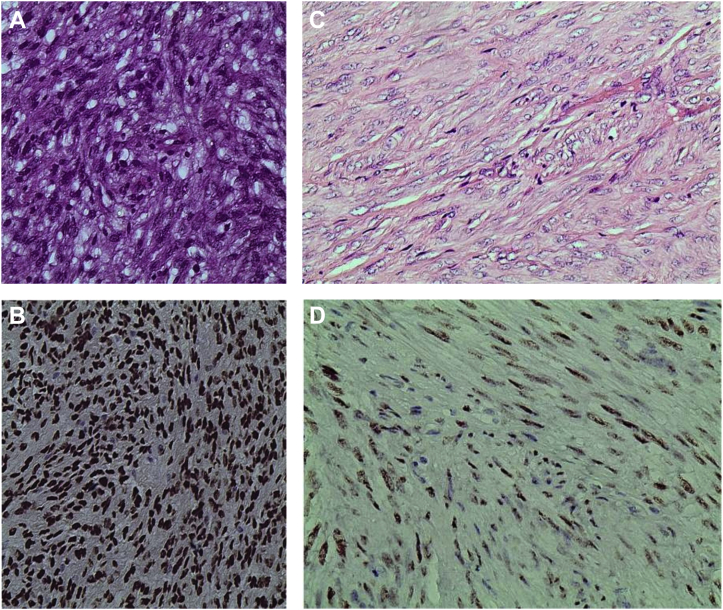
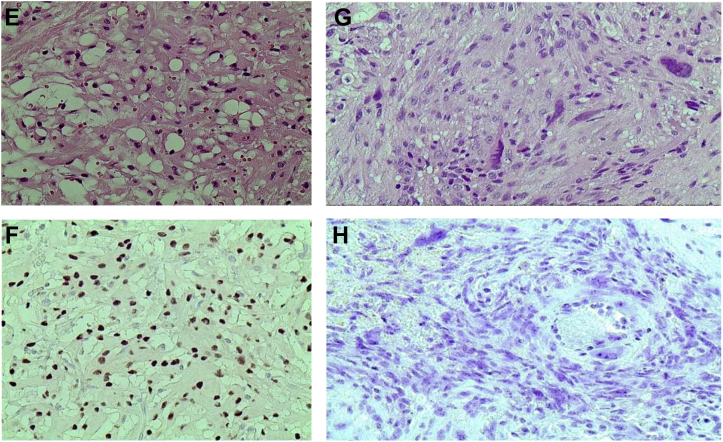
Figure 4 presents the prevalence of PR expression in the study. Of 112 cases of meningiomas, 54.5% (n = 61) were PR positive and 45.5% (n = 51) were PR negative. Among the PR positive cases, 95% (n = 58) were typical meningiomas (WHO grade I) and the remaining (5%, n = 3) were atypical (WHO grade II), and surprisingly, all the malignant meningiomas were PR negative (0%, n = 3). Figure 5 shows the IHC staining of the various histological subtypes of meningioma in the study.
Figure 4.
The frequency of progesterone receptor expression among meningioma biopsies at Muhimbili National Hospital.
Figure 5.
(A) A case of meningotheliomatous meningioma (hematoxylin and eosin stains, ×100). (B) The same case as in (A) showing strong intranuclear diffuse staining (3+) of progesterone receptor antibody (immunohistochemical stain, ×100). (C) A case of fibroblastic meningioma (hematoxylin and eosin stains, ×400). (D) The same case as in (C) showing moderate intranuclear diffuse staining (2+) of progesterone receptor antibody (immunohistochemical stain, ×400). (E) A case of microcystic meningioma (hematoxylin and eosin stains, ×400). (F) The same case as in (E) showing moderate intranuclear diffuse staining (2+) of progesterone receptor antibody (immunohistochemical stain, ×400). (G) A case of malignant meningioma rhabdoid type (hematoxylin and eosin stains, ×400). (H) The same case as in (G) showing negative staining (0) of progesterone receptor antibody (immunohistochemical stain, ×400).
Association of Progesterone Receptor Expression with Clinicopathological Prognostic Factors Among Patients with Meningiomas
We found that there was high expression of the PRs among typical meningiomas compared to atypical and even malignant meningiomas (58% vs. 33.3% and 0%, respectively); however, the difference in expressing the PRs regarding the WHO grades of the meningioma cases in this study was not significant (P = 0.122). By considering the expression of the PRs in relation to the different histological types of meningioma, we observed that the meningothelial types expressed more PRs (50.8%) than, for example, fibroblast type (14.8%) and even all other histological types (34.4%); however, the difference in expression of the PRs for all the histological types was insignificant (P = 0.091). Nevertheless, we found more females expressing the PRs than males (57.4% vs. 42.6%), but the difference in expression of the PRs was not significant (P = 0.177). Moreover, the number of patients aged >40 years expressing the PRs was outweighing that of patients aged ≤40 years (70.5% vs. 29.5%), and the difference was significant (P = 0.043). In addition, tumor location in our study was not associated with PR expression (P = 0.554) although tumors that were located in the cerebral convexity (olfactory groove, posterior fossa, and temporoparietal and frontal lobe) had higher expression of the PRs than those that were located elsewhere (63.9% vs. 36.1%) (Table 2).
Table 2.
Association of Progesterone Receptor Expression With Clinicopathological Characteristics
| Variable | PR Status |
P Value | |
|---|---|---|---|
| Positive, n (%) | Negative, n (%) | ||
| Age (years) | 0.043 | ||
| ≤40 | 18 (29.5) | 33 (64.7) | |
| >40 | 43 (70.5) | 18 (35.3) | |
| Sex | 0.177 | ||
| Male | 26 (42.6) | 19 (37.3) | |
| Female | 35 (57.4) | 32 (62.7) | |
| WHO grade | 0.122 | ||
| Typical (grade I) | 58 (95.1) | 42 (82.4) | |
| Atypical (grade II) | 3 (4.9) | 6 (11.8) | |
| Malignant (grade III) | 0 (0.0) | 3 (5.9) | |
| Histological types | 0.091 | ||
| Meningothelial | 31 (50.8) | 8 (15.7) | |
| Fibroblast | 9 (14.8) | 18 (35.3) | |
| Others∗ | 21 (34.4) | 25 (49.0) | |
| Tumor location | 0.554 | ||
| Cerebral convexity | 39 (63.9) | 19 (37.3) | |
| Others† | 22 (36.1) | 32 (62.7) | |
PR, progesterone receptor; WHO, World Health Organization.
Others include transitional, psammomatous, clear cell, angiomatous, secretory, and microcystic.
Others, sphenoid wing, falx, parasagittal, cerebellum, and spine.
Discussion
Reporting on the prognostic role of the PR among patients with meningiomas is of utmost importance due to the fact that it may help invention of PR inhibitors that can be of therapeutic benefit to the patients. Furthermore, the discovery of such drugs may be used for personalized medicine that in turn helps to prevent unnecessary exposure to chemotoxicity and also it reduces the possibility of incurring unnecessary expenses.
Prevalence of Expression of Progesterone Receptor for the FFPE Tissue Blocks of the Cases Included in the Study
PRs have been found to be highly expressed in meningiomas. This was also reflected in the present study in which over 50% of the cases were PR positive. However, previous studies performed in Caucasians and Africans have reported higher prevalence of PR expression than the prevalence reported in our study. For example, the studies that were performed in Iran, the United States, Nigeria, and India reported the prevalence of PR expression in FFPE tissue blocks of patients with meningiomas of 96%, 82.9%, 87.5%, and 65%, respectively.12,15, 16, 17 Lower prevalence of PR in meningiomas than the one observed in the present study has also been reported elsewhere. The studies that were performed in the United Kingdom, North Korea, and Brazil reported the prevalence of PR expression of 48%, 31.9%, and 53.4%, respectively.12,18,19 The difference in expression of PRs across the studies may have various reasons including the difference in the methodology used, tumor biology, and genetical constitution of the individual included in the different studies. Studies have shown that delayed fixation and long-term storage of the FFPE tissue blocks may render the FFPE tissue blocks negative for IHC staining.20,21 Therefore, timely fixation of the specimens and optimal storage time of the FFPE tissue blocks help to increase the level of IHC antibodies including PRs.
Association Between Expression of Progesterone Receptor and Clinicopathological Characteristics
The expression of PRs in meningiomas has been found to be associated with different prognostic factors such as age of the patients, sex, tumor grade, and tumor location among many others.22,23 Regarding the association of sex with PR expression in our study, we found that over half of the cases showing PR expression were females. However, there was no association between PR expression and sex. Lack of association between the expression of PR and sex among patients with meningiomas in spite of female preponderance has also been reported in other studies.10,23,24
The association of age with expression of the PRs in meningiomas seems to be contradicting. Some studies have reported a positive association between age and expression of the PR, whereas other studies did not find any association between the 2 variables. Some studies have reported that there is a high trend of expression of PR among older patients compared with younger patients with meningiomas.25,26 We found a positive association between age and expression of PR (P = 0.043), and the expression of the biomarker was increasing with the increase in the age of the patients. This is similar to the finding of the study performed by Roser et al,10 who reported that patients who were <37 years had less rate of expression of PRs compared with those who were ≥37 years.
When the expression of PRs in our study was compared among the cases based on their anatomical location, we found that there was no difference. This is contrary to the findings of a previous study27 in which when the expression of PR was compared among the cases based on their anatomical location, it was found that there was a marked difference in terms of expression of the PRs.27 In their study, there was 81%, 71.4%, and 66.7% level of PR expression for the meningiomas that were anatomically located in the olfactory groove, posterior fossa, and temporoparietal region.27 Also Fewing et al12 and Roser et al10 reported no correlation of PR expression with the anatomical location of the meningiomas in their studies. This is because of modern microsurgical techniques in which only tumor parts with no precisely named anatomical location are usually submitted for histopathological evaluation. Therefore, focal accumulation of biological activity within the meningioma may be missed or misjudged.
Expression of PRs among patients with meningiomas has been found to predict good prognosis with less possibility of recurrence and/or malignant transformation.16 But meningiomas that are PR negative have been reported to have a high chance of recurrence and/or ability of becoming malignant.15 Both atypical (WHO grade II) and anaplastic (WHO grade III) meningiomas have a high recurrence rate and poor prognosis, and they have a low rate of PR or not at all compared with typical ones (WHO grade I).6 Other studies also have reported the fact that benign meningiomas tend to express the PRs and have good prognosis unlike the ones that are negative for the PRs and usually have poor prognosis.4,10, 11, 12 In this study, we found that all the 61 cases that were PR positive were all benign. Similar findings have also been reported in the studies by Fewing et al12 and Fakhrjou et al.28 Hsu et al16 and Roser et al10 also reported a positive association between WHO grade and expression of PR. However, Kim et al18 reported that there was no association between WHO grade of meningioma and PR expression.
The histopathological subtypes have been reported to be associated with the expression of the PRs although with some discrepancy between studies. In this study, we observed that there was no association between histopathological subtypes and PR expression despite many cases of meningothelial and fibroblast histopathological subtypes that were PR positive. This is similar to the findings in the studies by Roser et al10 and Dora et al,16 which also found increased expression of PR in meningothelial and fibroblast histopathological subtypes compared with other variants but without a significant difference. Other studies also have shown similar findings that suggested that meningotheliomatous meningiomas tend to express more PR than other histopathological subtypes.15,23 A study conducted in Egypt by Shayanfar et al9 showed that 57% of meningiomas were mixed with psammoma bodies and a few of them could express the PRs similar to the finding in the current study, in which 25% of the meningiomas had psammoma bodies and showed either negative or weak intranuclear staining for the PRs. This has been explained by the fact that psammomatous meningiomas are more likely not to express the PRs because of being calcified by virtual of the presence of psammoma bodies that require decalcification so as to unmask the epitopes for the PR antibody to stain easily.29
Conclusion
The vast majority of the patients in this study were benign, and the prevalence of PR expression of 54.5% was 100% found in benign cases. There was a converse positive association between age and PR expression in our study in which there was a higher level of PR expression among older patients than younger ones. In addition, there was a higher proportion of PR expression in cases with WHO grade I compared with other grades. Therefore, the expression of PR observed in this study may help in determining the prognosis of patients with meningiomas.
Limitations of the Study
Financial constraints contributed to limitation of use of Ki67 for determining the prognosis of the patients with meningiomas in comparison with the expression of PRs and also the clinicopathological characteristics. Failure to include survival analysis with regard to the expression of PRs, which was due to lack of follow-up data, was another limitation of our study.
Availability of Data and Materials
The datasets used during in this study are available from the corresponding author and they may be provided when requested.
Declaration of competing interest
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CRediT authorship contribution statement
Leah Mnango: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft. Angela Mwakimonga: Writing – review & editing. Advera I. Ngaiza: Writing – review & editing. James J. Yahaya: Writing – original draft, Writing – review & editing. Edda Vuhahula: Methodology, Supervision, Writing – original draft. Amos R. Mwakigonja: Writing – review & editing.
Acknowledgments
We appreciate every support given to us by all staff at the Central Pathology Laboratory of Muhimbili National Hospital.
Footnotes
Received 12 March 2021; accepted 3 July 2021
References
- 1.Abul-Kasim K., Thurnher M.M., McKeever P., Sundgren P.C. Intradural spinal tumors: current classification and MRI features. Neuroradiology. 2008;50:301–314. doi: 10.1007/s00234-007-0345-7. [DOI] [PubMed] [Google Scholar]
- 2.Arnautovic K., Arnautovic A. Extramedullary intradural spinal tumors: a review of modern diagnostic and treatment options and a report of a series. Bosn J Basic Med Sci. 2009;9(suppl 1):40–45. doi: 10.17305/bjbms.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis D.N., Ohgaki H., Wiestler O.D. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrom Q.T., Gittleman H., Farah P. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cea-Soriano L., Wallander M.A., Garca Rodrguez L.A. Epidemiology of meningioma in the United Kingdom. Neuroepidemiology. 2012;39:27–34. doi: 10.1159/000338081. [DOI] [PubMed] [Google Scholar]
- 6.Ibebuike K., Ouma J. Demographic profile of patients diagnosed with intracranial meningiomas in two academic hospitals in Johannesburg, South Africa: a 12-month prospective study. Afr Health Sci. 2014;14:939–945. doi: 10.4314/ahs.v14i4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A.P., Fisher J.L., Nichols E. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:376–393. doi: 10.1016/S1474-4422(18)30468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhopadhyay M., Das C., Kumari M., Sen A., Mukhopadhyay B., Mukhopadhyay B. Spectrum of meningioma with special reference to prognostic utility of ER, PR and Ki67 expression. J Lab Physicians. 2017;9:308–313. doi: 10.4103/JLP.JLP_158_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shayanfar N., Mashayekh M., Mohammadpour M. Expression of progestrone receptor and proliferative marker ki 67 in various grades of meningioma. Acta Med Iran. 2010;48:142–147. [PubMed] [Google Scholar]
- 10.Roser F., Nakamura M., Bellinzona M., Rosahl S.K., Ostertag H., Samii M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004;57:1033–1037. doi: 10.1136/jcp.2004.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy B.J., Davis F.G., Freels S. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88:831–839. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- 12.Fewings P.E., Battersby R.D.E., Timperley W.R. Long-term follow up of progesterone receptor status in benign meningioma: a prognostic indicator of recurrence? J Neurosurg. 2000;92:401–405. doi: 10.3171/jns.2000.92.3.0401. [DOI] [PubMed] [Google Scholar]
- 13.Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q., Baudis M. Enabling population assignment from cancer genomes with SNP2pop. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-61854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee S., Ghosh S.N., Chatterjee U., Chatterjee S. Detection of progesterone receptor and the correlation with Ki-67 labeling index in meningiomas. Neurol India. 2011;59:817–822. doi: 10.4103/0028-3886.91357. [DOI] [PubMed] [Google Scholar]
- 16.Hsu D.W., Efird J.T., Hedley-Whyte E.T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86:113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 17.Fakhrjou A, Meshkini A, Shadrvan S. Status of Ki67, estrogen and progesterone receptors in various subtypes of intracranial meningioma. Pakistan J Biol Sci. 15:530-535. [DOI] [PubMed]
- 18.Kim J.H., Suh J.H., Kwun B.D. Immunohistochemical analysis of progesterone receptor in intracranial meningiomas. J Korean Neurosurg Soc. 1998;27:1525–1532. [Google Scholar]
- 19.Hilbig A., Barbosa-Coutinho L.M. Meningiomas and hormonal receptors. Immunohistochemical study in typical and non-typical tumors. Arq Neuropsiquiatr. 1998;56:193–199. doi: 10.1590/s0004-282x1998000200005. [DOI] [PubMed] [Google Scholar]
- 20.Ramos-Vara J.A., Webster J.D., DuSold D., Miller M.A. Immunohistochemical evaluation of the effects of paraffin section storage on biomarker stability. Vet Pathol. 2014;51:102–109. doi: 10.1177/0300985813476067. [DOI] [PubMed] [Google Scholar]
- 21.Bass B.P., Engel K.B., Greytak S.R., Moore H.M. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med. 2014;138:1520–1530. doi: 10.5858/arpa.2013-0691-RA. [DOI] [PubMed] [Google Scholar]
- 22.Wiemels J., Wrensch M., Claus E.B. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelzaher E., El Deeb N.M.F., Gowil A.G., Yehya A. Biological and demographic profile of meningiomas in a cohort of Egyptian patients: impact on tumor recurrence. Sci World J. 2013;2013:375139. doi: 10.1155/2013/375139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatch E.E., Linet M.S., Zhang J. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer. 2005;114:797–805. doi: 10.1002/ijc.20776. [DOI] [PubMed] [Google Scholar]
- 25.Brokinkel B., Hess K., Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro Oncol. 2017;19:1298–1307. doi: 10.1093/neuonc/nox071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Carvalho G.T.C., da Silva-Martins W.C., de Magalhães K.C.S.F. Recurrence/regrowth in grade I meningioma: how to predict? Front Oncol. 2020;10:1–15. doi: 10.3389/fonc.2020.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiuri F., Mariniello G., Guadagno E., Barbato M., Corvino S., Del Basso De Caro M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir (Wien) 2019;161:2553–2561. doi: 10.1007/s00701-019-04084-z. [DOI] [PubMed] [Google Scholar]
- 28.Fakhrjou A., Meshkini A., Shadrvan S. Status of Ki67, estrogen and progesterone receptors in various subtypes of intracranial meningiomas. Pak J Biol Sci. 2012;15:530–535. doi: 10.3923/pjbs.2012.530.535. [DOI] [PubMed] [Google Scholar]
- 29.Kunimatsu A., Kunimatsu N., Kamiya K., Katsura M., Mori H., Ohtomo K. Variants of meningiomas: a review of imaging findings and clinical features. Jpn J Radiol. 2016;34:459–469. doi: 10.1007/s11604-016-0550-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during in this study are available from the corresponding author and they may be provided when requested.