Abstract
9-cis-13,14-dihydroretinoic acid (9CDHRA), acts as an endogenous ligand of the retinoid X receptors (RXRs), and is an active form of a suggested new vitamin, vitamin A5/X. Nutritional-relevance of this pathway as well as its detailed role in vertebrate physiology, remain largely unknown. Since recent GWAS data and experimental studies associated RXR-mediated signaling with depression, we explored here the relevance of RXR and vitamin A5/X-mediated signaling in the control of stress adaptation and depressive-like behaviors in mice. We found that compromised availability of 9CDHRA in Rbp1−/− mice was associated with increased despair in the forced swim and anhedonia in the sucrose preference test. 9CDHRA similarly to synthetic RXR agonist, BMS649, normalized despair behaviors in Rbp1−/− but not Rxrγ−/− mice, supporting involvement of RXR signaling in anti-despair activity of these ligands. Importantly, similarly to BMS649, the 9CDHRA and its nutritional-precursor, 9-cis-13,14-dihydroretinol (vitamin A5/X alcohol), prevented development of depressive-like behaviors in mice exposed to chronic social defeat stress, revealing the beneficial role of RXRs and its endogenous ligand in stress adaptation process. These data point to the need for relevant nutritional, biochemical and pharmacological studies of this signaling pathway in human, both in physiological conditions and in pathologies of stress-related disorders.
Keywords: Vitamin A, Retinoid receptors, Depression, Chronic stress, Mouse models
1. Introduction
Depression is a highly prevalent multifactorial disorder, resulting from abnormal interactions of environmental and genetic factors. Whereas a number of environmental stimuli were associated with an increased risk of depression, including chronic stress, nutritional deficiencies or pharmacological agents, no causal genetic factor has been clearly identified so far owing probably to the diversity of such factors or weak, concomitant contribution of multiple genes with very small individual effect requiring therefore very large sample size and complex multifactorial analyses to be detected in clinical setting. Accordingly, recent genome wide association studies (GWAS) identified functionally connected sets of genes including targets of retinoid X receptors (RXRs) signaling pathway defined by RXR cistrome (i.e. direct transcriptional targets of RXR regulations) as significantly associated with major depression (Wray et al., 2018). The three RXR isotypes (RXRα, RXRβ and RXRγ) belong to a family of nuclear hormone receptors and act as ligand-dependent transcription factors (Mangelsdorf and Evans, 1995; Mangelsdorf et al., 1995). Furthermore, RXRs occupy a central place in nuclear hormone receptor-mediated signaling networks as compulsory permissive or non-permissive dimerization partners for a number of these receptors (Desvergne, 2007; Perez et al., 2012; Szanto et al., 2004). Therefore RXRs are particularly well suited to act as an interface between environment and genome by translating environmental signals, including food–derived stimuli into transcriptional adaptive programs (de Lera et al., 2016; Krężel et al., 2019; Rühl et al., 2015). Indeed, some of the RXR dimers, called permissive and including for example liver X receptors (LXRs)-RXR, peroxisome proliferator-activated receptors (PPARs)-RXR, nuclear receptor subfamily 4 group A member 2 (NR4A2/Nurr1)-RXR, and possibly RXR-RXR homodimers, can be directly activated by natural as well as synthetic RXR ligand (Krężel et al., 2019; Mangelsdorf et al., 1995; Perez et al., 2012). Of particular importance is 9-cis-13,14-dihydroretinoic acid (9CDHRA), identified recently as the first endogenously occurring retinoid acting as a physiologically relevant RXR ligand in mice (Rühl et al., 2015) and in humans (Krężel et al., 2021). Importantly, 9CDHRA cannot be efficiently generated from all-trans retinol, vitamin A1-alcohol, but was shown to be the active metabolite of 9-cis-13,14-dihydroretinol (9CDHROL), an alcohol form of vitamin A5/X, the novel sub-class of vitamin A recently identified in rodents and human and their respective food chains (Krężel et al., 2021). Deficit in 9CDHRA observed in retinol binding protein 1 null mutant (Rbp1−/−) mice was associated with working memory deficits (Rühl et al., 2015), and beneficial promnemonic effects of 9CDHRA and 9CDHROL supplementation were also demonstrated (Krężel et al., 2021; Rühl et al., 2015). The role of the vitamin A5/X pathway in the control of affective behaviors was not investigated, but can be hypothesized based on several lines of evidence which support an association of compromised RXR signaling with depression. Accordingly, null mutation of RXRγ led to depressive-like phenotype in mice (Krzyzosiak et al., 2010).
Clinical and experimental data indicate also that reduced nutritional import and circulating levels of n-3 polyunsaturated fatty acids (PUFAs), which in some conditions may act as RXR agonist in vivo (de Urquiza et al., 2000; Krężel et al., 2019; Wietrzych-Schindler et al., 2011), was associated with increased incidence of depression (Peet et al., 1998). In line with this observation, dietary supplementation with supra-physiological levels of n-3 PUFAs displayed beneficial anti-depressant activities in humans (Peet and Stokes, 2005). The anti-despair activity of docosahexaenoic acid (DHA), the major human relevant n-3 PUFA, or a synthetic RXR agonist BMS649 treatments were also observed in mice and were prevented by co-treatment with RXR antagonist or in null mutation of RXRγ in Rxrγ−/− mice (Wietrzych-Schindler et al., 2011). Such data indicate that memory as well as despair behavior are modulated by RXR-mediated signaling in a ligand-dependent manner. A direct link between RXR-mediated signaling and stress adaptation was also suggested by beneficial effects of bexarotene (Targretin®) a synthetic RXR agonist, on extinction of learned fear in mice (McCullough et al., 2018).
Here we used a pharmacogenetics approach to address the relevance of 9CDHRA for control of despair and hedonic behaviors and tested involvement of RXR-mediated signaling in such regulations. In absence of any validated nutritional models of vitamin A5/X or 9CDHRA deficiency, we used for such studies Rbp1−/− mice reported previously to display significant reduction of circulating and brain levels of 9CDHRA (Rühl et al., 2015). We hypothesized that reduced availability of 9CDHRA in these mice may lead to pro-depressive behaviors, whereas 9CDHRA supplementation should revert such behaviors supporting thereby causal link between 9CDHRA deficiency and depressive behaviors in mice. To furthermore address specificity of 9CDHRA, we also addressed the possibility that anti-depressive effects of 9CDHRA should be abolished in Rxrγ−/− mice if RXRγ is directly involved in mediating such activity. Next, we also investigated whether modulation of RXR-mediated signaling by using synthetic, BMS649, or endogenously relevant RXR ligand, 9CDHRA, may prevent induction of depressive-like behaviors in a social defeat stress model. Finally, considering that 9CDHROL is an efficient nutritional precursor of 9CDHRA, we also tested its potential in preventing adverse effects of social defeat stress.
2. Results
2.1. Affective deficits in Rbp1−/− mice are associated with deficient RXR signaling
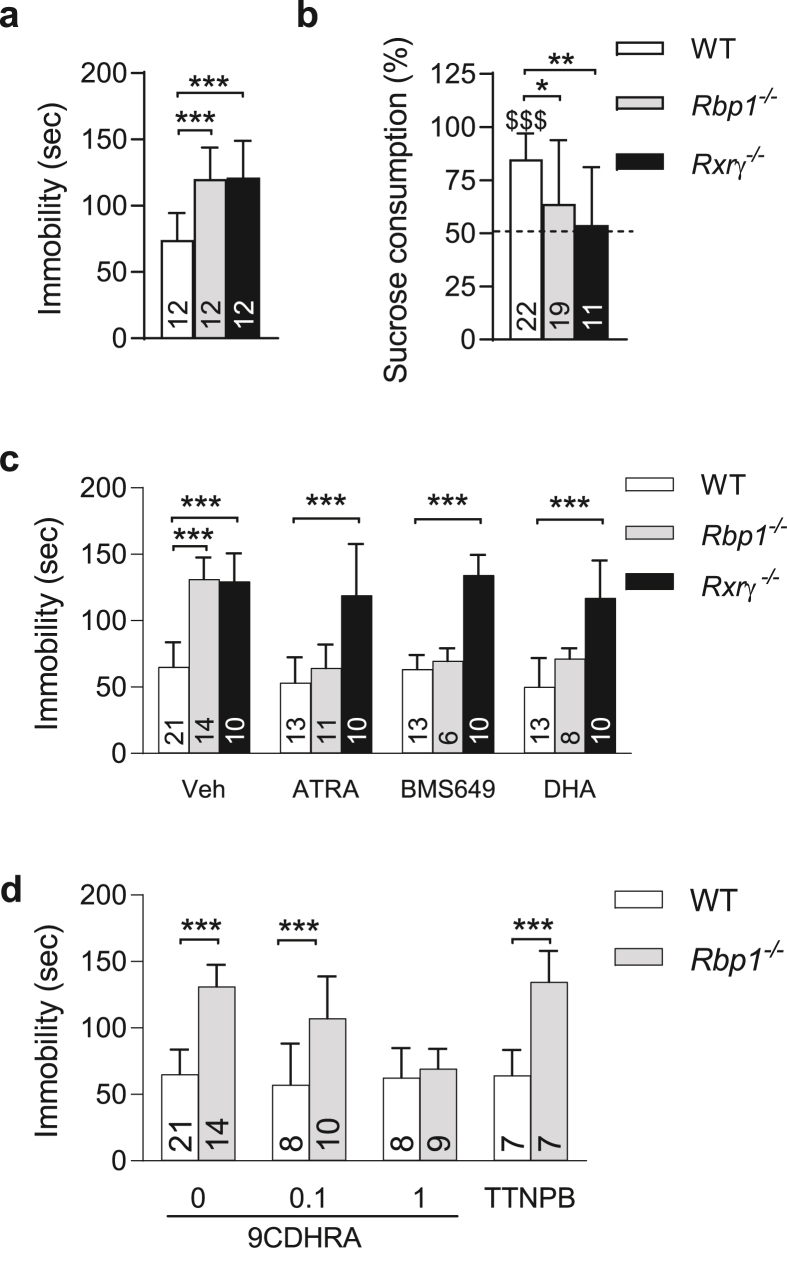
To investigate whether deficiency of endogenous RXR ligand, 9CDHRA, observed in Rbp1−/− mice is involved in the control of affective behaviors in mice, we first analyzed this mouse line in a series of behavioral tests sensitive to detect depressive-like behaviors. Rbp1−/− males displayed significant increase of the immobility states in the forced swim test suggesting enhanced despair behaviors (Fig. 1a). Consistent with a phenotype of increased depressive-like behavior, Rbp1−/− mice displayed also anhedonia in sucrose preference test as their average, 63.8%, consumption of sucrose solution was significantly lower than 84.8% preference in WT control mice (Fig. 1b) and showed only a tendency to differ from 50% of chance level (one-sample t-test t (18) = 2.0; p = 0.06). Such abnormalities resemble phenotype of mice carrying null mutation for RXRγ (Krzyzosiak et al., 2010). Indeed, increased immobility of Rxrγ−/− mice in the forced swim test (Fig. 1a) and reduced preference for sucrose drink (Fig. 1b) were comparable and not statistically different when compared to Rbp1−/− mice. This similarity together with reduced availability of 9CDHRA suggests that compromised RXR signaling may be at the origin of depressive-like behaviors in Rbp1−/− mice. To challenge this hypothesis functionally, we took advantage of the sensitivity of the forced swim test to reveal anti-depressant activities of acute treatments with RXR-agonists (Wietrzych-Schindler et al., 2011). All-trans-retinoic acid (ATRA), which after treatment in vivo can rapidly be transformed to the RXR agonist, 9-cis-retinoic acid, reduced significantly immobility time of Rbp1−/− mice in the forced swim test similarly to a synthetic RXR agonist BMS649 (Fig. 1c). A distinct, natural RXR agonist of nutritional origin, DHA, also normalized increased despair of Rbp1−/− mice (Fig. 1c). Rxrγ−/− mice were not responsive to any of these treatments further confirming previous observations that RXRγ is functionally predominant RXR in mediating anti-depressant activities of pan-RXR agonists (Wietrzych-Schindler et al., 2011). Effects of treatments were not evident in WT mice, which may be related to the low baseline immobility in this strain and in the consequence the floor effect.
Fig. 1.
9CDHRA deficiency in Rbp1−/− is associated with depressive-like behaviors. (a) Rbp1−/− and Rxrγ−/− mice display increased despair behaviors in the forced swim test as compared to WT mice. (b) Rbp1−/− and Rxrγ−/− mice consume sucrose drink at random, 50% level indicating sucrose preference deficit. (c) Treatment with RXR-agonists reverses despair in Rbp1−/−, but not in Rxrγ−/− mice. (d) 9CDHRA treatment reverses despair in Rbp1−/− in a dose-dependent manner. Numbers of analyzed animals were reported in corresponding bars graphs. Statistical differences were indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared to WT controls in respective groups; $$$, p < 0.001; in comparison with 50% of chance level. All the error bars represent standard error.
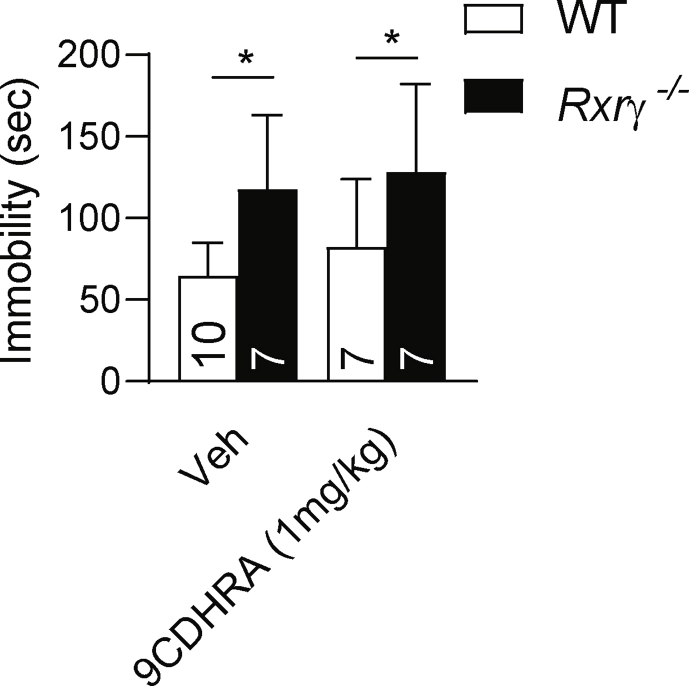
We previously reported that Rbp1−/− mice display significant decrease of circulating and brain levels of 9CDHRA, an endogenous RXR agonist, compromising thereby RXR signaling (Rühl et al., 2015). To investigate whether 9CDHRA deficits may underlie depressive-like abnormalities in Rbp1−/− mice we tested efficiency of 9CDHRA treatment in normalizing despair behaviors in these mice. Intraperitoneal injection of 9CDHRA 5–6 h before testing displayed dose-dependent decrease of immobility of Rbp1−/− mice in the forced swim test which was evident at 1 but not 0.1 mg/kg of 9CDHRA. This effect is supported by significant interaction of treatment x genotype (F (2,64) = 10, p < 0.01) and significant effect of treatment (F (2,64) = 12.2, p < 0.01) in two-way ANOVA analyses followed by Bonferroni post-hoc test (Fig. 1d). Effect of 9CDHRA, but also ATRA, BMS649 or DHA did not involve activation of RARs since TTNPB, a synthetic RAR agonist, did not display such activities (Fig. 1d). Finally, RXRγ appeared as functionally predominant RXR in mediating 9CDHRA effects in despair behaviors as increased immobility observed in Rxrγ−/− mice was not modified by 9CDHRA treatment (Fig. S1).
2.2. Synthetic RXR agonist prevents development of depressive-like behaviors in wild type mice subjected to chronic social defeat stress
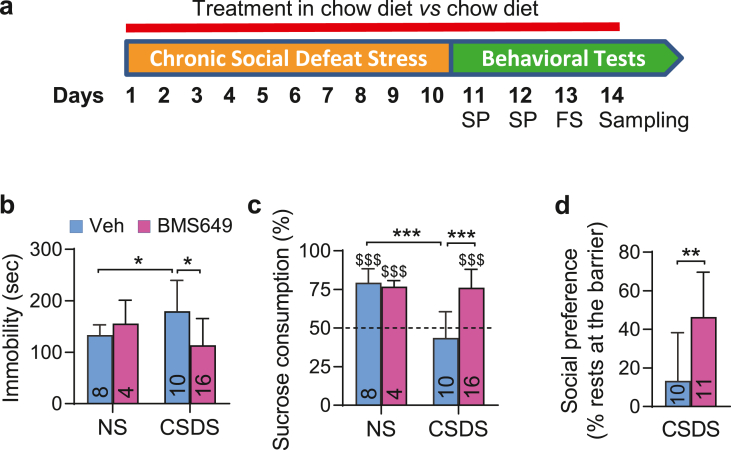
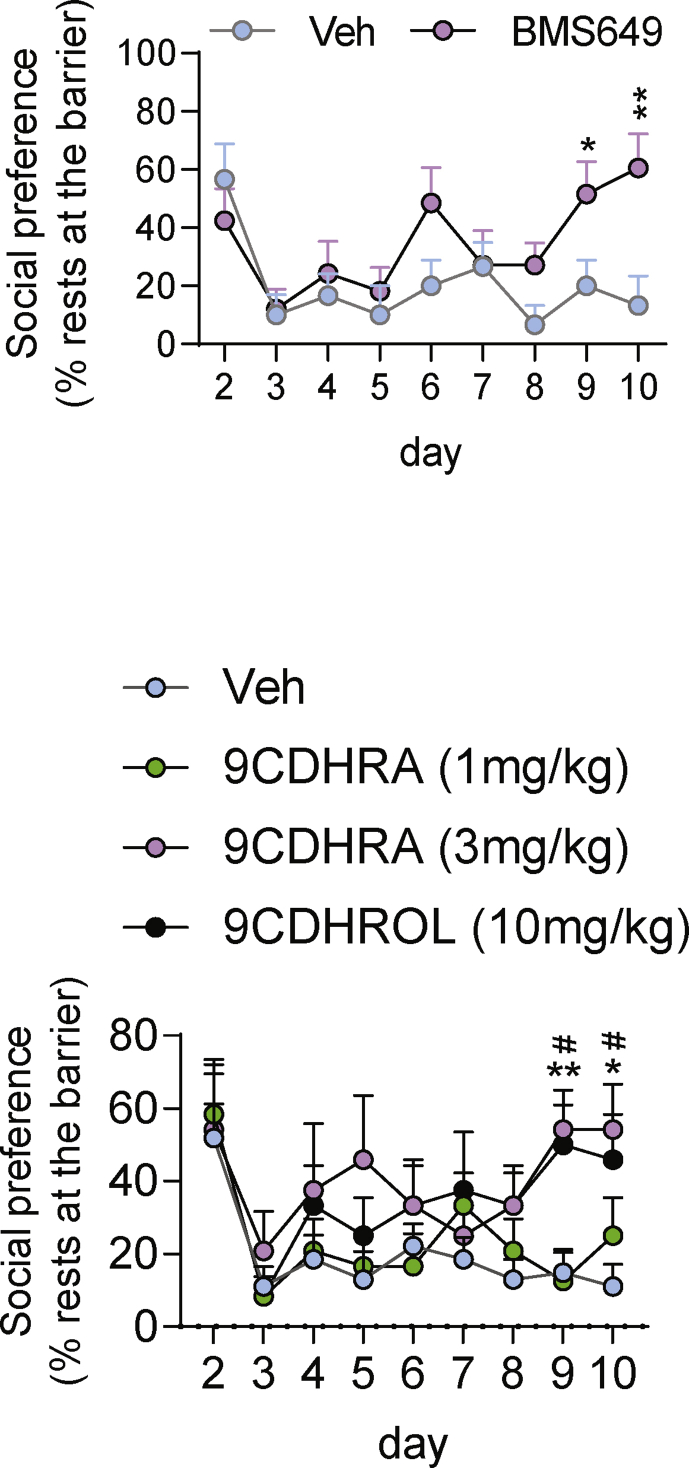
Since reduced availability of RXR ligand in Rbp1−/− mice or abolished RXRγ signaling in Rxrγ−/− mice display pro-depressive effects, we hypothesized that enhancing RXR signaling may have anti-depressant activity in mice. To address this possibility we pharmacologically enhanced RXR signaling in a mouse model of chronic social defeat stress (CSDS), an animal model widely used in research into depression (Golden et al., 2011). First, we tested the efficiency of BMS649 treatment in preventing depressive-like behaviors in CSDS model, according to the design depicted in Fig. 2a. In order to increase robustness of this protocol we used C57BL/6N mice known to be particularly susceptible to stress (Simon et al., 2013; Sturm et al., 2015). We found that mice subjected to stress displayed significantly increased immobility time in the forced swim test, but such effect was treatment dependent and was not observed in stressed mice treated chronically in chow diet with 1 mg/kg of BMS649 (Fig. 2b). This observation was supported by significant stress × treatment interaction in two-way ANOVA analysis (F (1, 34) = 6,088, p < 0.05) and post-hoc tests (Fig. 2b). In addition to anti-despair activity and in line with anti-depressant effect of BMS649 we also observed prevention of anhedonia in treated stressed mice. Accordingly, mice subjected to CSDS and maintained on standard chow diet (vehicle) displayed decreased preference to sweetened drink of 0.8% sucrose at the end of stress period, but mice receiving BMS649 throughout stress protocol preserved their normal preference to sucrose solution, which was comparable to that observed in control non-stressed (NS) mice as indicated by significant stress × treatment interaction in two-way ANOVA analyses (F (1, 34) = 14,5, p < 0.001) or treatment effect (F (1, 34) = 15,9, p < 0.001) and Bonferroni post-hoc test (Fig. 2c). Finally, BMS649 treatment reduced also social avoidance associated with chronic stress as after initial reduction of frequency of resting at the separation barrier observed in all stressed mice, only treated stressed mice were more frequently present next to the barrier at the later phases of the stress protocol which was supported by significant interaction of stress x BMS649 treatment for the evolution of this measure over consecutive days of experiment (F (8, 152) = 2,403, p < 0.05 in two-way ANOVA) and post-hoc analyses (Fig. S2). This was also illustrated by increased frequency of resting next to the barrier over the last 2 days of social defeat protocol (Fig. 2d).
Fig. 2.
Synthetic RXR agonist prevents development of depressive-like behaviors in social defeat stress mouse model. (a) Schematic representation of experimental design of chronic social defeat stress protocol, treatments and behavioral analyses in the sucrose preference (SP) and forced swim (FS) tests. (b) Immobility in the forced swim test was measured in non-stressed mice (NS) and mice subjected to chronic social defeat stress (CSDS) fed with standard chow diet (Veh) and diet with RXR agonist (BMS649). (c) Sucrose preference expressed as percent of consumed sucrose solution, was evaluated in the same groups of mice. (d) Social preference expressing a preference of stressed mice to rest next to the barrier separating it from resident, aggressor mouse was evaluated throughout the stress protocol. Numbers of analyzed animals were reported in corresponding bars graphs. All the error bars represent standard error. Statistical differences were indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001 and for comparison with 50% of chance level $$$, p < 0.001.
2.3. Vitamin A5/X and its bioactive form, 9CDHRA, protects from adverse, depressive-like effects of social defeat stress
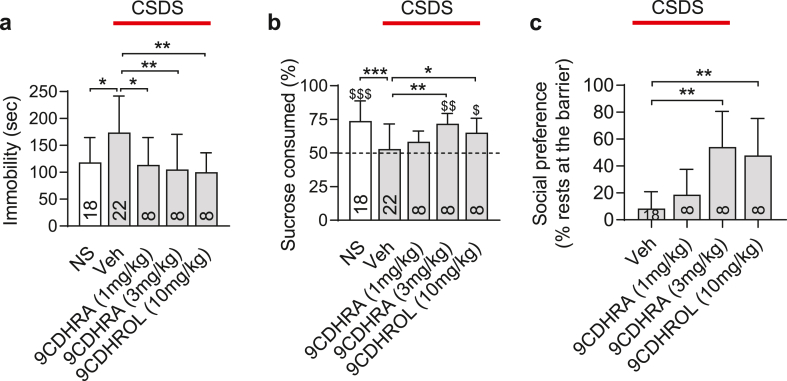
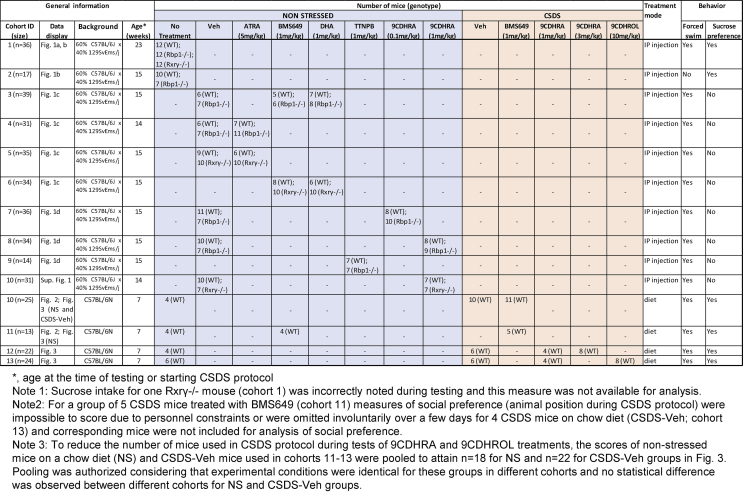
Considering that synthetic RXR ligand, BMS649, displays protective antidepressant-like activities in social defeat stress model, we speculated that also endogenous RXR ligand may display such activities. To this end we investigated behavioral effects of chronic 9CDHRA treatment in social defeat stress model using the same protocol as described (Fig. 2a). The treatment induced dose-dependent responses. Accordingly, a clear decrease of the immobility in the forced swim test (Fig. 3a) and an increase of sucrose preference (Fig. 3b) as well as increased frequency of resting at the barrier (Fig. 3c) were observed for 3 mg/kg, whereas lower dose of 1 mg/kg led only to significant decrease of the immobility in the forced swim test, without affecting sucrose preference or frequency of resting at the barrier, which remained comparable to scores of stressed mice maintained on standard chow diet, vehicle. Importantly, the higher dose of 3 mg/kg completely prevented stress-induced despair behaviors and anhedonia as treated mice performed at the level comparable with non-stressed mice (compare 3 mg/kg with NS in Fig. 3a and b).
Fig. 3.
Endogenous RXR agonist, 9CDHRA and its precursor, 9CDHROL, prevent development of depressive-like behaviors in chronic social defeat stress mouse model. (a) Immobility in the forced swim test was evaluated after chronic social defeat stress (CSDS) protocol in mice treated with 9CDHRA or 9CDHROL during stress protocol and compared to mice fed with standard chow diet (vehicle; Veh) and to non-stressed (NS) mice. (b) The effect of dihydroretinoid treatments on sucrose preference was compared to Veh and NS control mice. (c) Social preference monitored throughout the entire period of stress protocol was represented as mean and compared to non-treated mice. Error bars represent standard error. Statistical differences were indicated as: *, p < 0.05; **, p < 0.01; ***, p < 0.001 and for comparison with 50% of chance level $, p < 0.05; $$, p < 0.01; $$$, p < 0.001.
3. Discussion
While RXRs control multiple important physiological functions virtually no information is available on regulation of such functions by 9CDHRA, a recently identified physiologically and nutritionally-relevant endogenous RXR ligand (Krężel et al., 2021; Rühl et al., 2015). Recent genetic studies in humans revealed an association of RXR-mediated signaling pathways with depression. However, the type of deregulation and potential role of RXR ligands was not examined (Wray et al., 2018). Here we show that a compromised availability of 9CDHRA reported earlier in Rbp1−/− mice (Rühl et al., 2015) is associated with an increased immobility in the forced swim test and reduced sucrose preference consistent with depressive-like phenotype of increased despair and anhedonia respectively. Importantly, supplementation of Rbp1−/− mice with a synthetic RXR-ligand, BMS649, or an endogenous RXR-ligand, 9CDHRA, normalized despair behaviors. Furthermore, using a social defeat stress in wild type mice we benchmarked protective effects of RXR-stimulation against adverse effects of stress as mice treated with BMS649 during stress protocol did not display increased despair, anhedonia, but also loss of social preference measured here as resting at separation barrier in the proximity of resident mouse.
Importantly, 9CDHRA displayed comparable beneficial activity in a dose-dependent manner with best activity at 3 mg/kg of body weight. Similar effect was also observed with the 9CDHRA precursor, 9CDHROL, although at higher concentration of 10 mg/kg to allow its metabolism and potential storage (Krężel et al., 2021). Such data are important for several distinct reasons. They suggest more functional and mechanistic interpretation of the link between RXR-mediated transcriptional signaling and depression revealed by human genetic data. In particular, present findings converge with previous data to support that compromised RXR-mediated signaling has pro-depressive effects (Krzyzosiak et al., 2010), whereas enhancing RXR-mediated signaling displays anti-depressant activity (Krzyzosiak et al., 2010; Wietrzych-Schindler et al., 2011). Furthermore, we also show that RXR modulation of stress responses are ligand dependent, which has an immediate mechanistic relevance because it limits implicated RXRs to their homodimers or permissive heterodimers, i.e. dimers which can be activated by RXR ligand. RXRs form such permissive heterodimers with for example NR4A2/Nurr1, PPARs, LXRs or FXRs (Fadel et al., 2020; Krężel et al., 2021; Mangelsdorf et al., 1995). Although actual implication of RXR-mediated signaling in stress-related disorders remains to be investigated, we cannot exclude that chronic stress may directly decrease expression of specific RXRs, including RXRγ, or may negatively affect metabolism or transport of 9CDHROL or 9CDHRA.
Preventive and/or antidepressant activities of RXR agonists appear to be robust as they can be detected in different paradigms validated for research into depression or used previously for functional studies of RXRγ (Krzyzosiak et al., 2010). Furthermore, such effects were not biased by choice of genetic background as they were observed in mice on mixed C57BL/6J; 129SvEms/j and isogenic C57BL/6N backgrounds (this study) or in BALBcByJ mice as a spontaneous genetic model of increased despair behaviors (Wietrzych-Schindler et al., 2011). However, it is not clear whether such effects are sex-dependent as all studies were performed on males only in order to avoid confounding effects of hormonal changes in females, but also due to limitations of classical CSDS paradigm used in present study. Indeed, CSDS is an ethological model based on the aggressivity of resident male causing both physical and emotional stress in an intruder male and cannot be performed in females without major modifications possibly impacting robustness of this paradigm (Yohn et al., 2019).
From the epidemiological point of view, identification of endogenous RXR-ligand and its precursor of nutritional origin as involved in control of stress responses is also meaningful for understanding environmental factors like nutrition in stress related disorders (Marx et al., 2021). In particular, our recent data indicate that 9CDHROL is an endogenous and physiological precursor of 9CDHRA in mammals and can be considered as new type of vitamin A or even a new vitamin category, vitamin A5/X (Krężel et al., 2021). Accordingly, 9CDHROL treatments in mice were found to significantly and durably increase 9CDHRA in the brain (Krężel et al., 2021). Here we showed that administration of 9CDHROL throughout the stress period prevented development of depressive-like behaviors including despair behaviors in the forced swim, anhedonia in the sucrose preference test and social avoidance. Thus, a reduced intake of fruits and vegetables as a potential source of provitamin A5/X and thus a correlation of nutritional pattern frequent in Western society with an increased incidence of depression and other neuropsychiatric disorders may also depend on vitamin A5/X availability for enabling sufficient RXR-mediated signaling (Bishwajit et al., 2017; Joseph et al., 2009; Spencer et al., 2017). Furthermore, we cannot exclude that high fat, high sugar diets strongly associated with Western nutritional patterns may also impact vitamin A5/X availability similarly to that reported for compromised vitamin A1 signaling in the brain (Biyong et al., 2020; Buaud et al., 2010). As a solution, a more healthy dietary pattern with fruits/vegetables, rich in carotenoids and especially provitamin A5/X (Böhm et al., 2020) or alternatively direct food enrichment or a dietary supplementation might be a solution of this identified nutrition-dependent health problem.
We can thus speculate that nutritional availability of vitamin A5/X or Rbp1 haploinsufficiency as determinant of 9CDHRA availability and further compromised RXR-mediated signaling in the organism, or finally haploinsufficiency of RXRγ as a functionally predominant RXR in control of depressive-like behaviors are all susceptibility factors for depression. In addition, present study points to the role of RXR-mediated signaling in response to stress as a key element of such susceptibility. In line with this possibility, bexarotene was recently reported to facilitate extinction of fear in learned fear mouse model (McCullough et al., 2018).
Finally, although present study points to an importance of endogenous RXR ligand and its signaling through RXRs in the context of stress adaptation and stress-related disorders, such role may also concern other physiological functions and diseases in which RXR dysfunction has been implicated. Such a possibility is supported by beneficial effects of RXR-mediated signaling in animal models of Alzheimer disease (AD), Parkinson disease (PD), multiple sclerosis, glaucoma stroke or brain injury (Dheer et al., 2019; He et al., 2020; Huang et al., 2011; Mariani et al., 2017; Spathis et al., 2017; Ting et al., 2020). Beneficial activities of RXR-mediated signaling may involve different mutually non-exclusive mechanisms. For example, whereas activation of microglia and its phagocytic activity by RXR-dependent mechanism was suggested to underlie beneficial effects of RXR-mediated signaling in AD, multiple sclerosis or stroke and brain injury (He et al., 2020; Natrajan et al., 2015; Savage et al., 2015; Ting et al., 2020), neuroprotective effects were reported in models of PD, AD, glaucoma or stroke (Dheer et al., 2019; Mariani et al., 2017; Spathis et al., 2017; Ting et al., 2020). Although such mechanisms may also be relevant for vitamin A5/X and respective RXR-dependent control of depressive-like behaviors, their investigation should be the objective of future studies. Importantly, we also cannot exclude that some effects of RXR signaling on central nervous system (CNS) involve RXR-dependent control of metabolic processes outside brain. In particular, permissive RXR heterodimers with PPARs and LXRs are important regulators of lipid metabolism (Shulman and Mangelsdorf, 2005) whereas RXR-PPARγ heterodimers control glucose metabolism (Cesario et al., 2001). Accordingly, activation of RXR-dependent signaling appeared beneficial in models of atherosclerosis or metabolic syndrome (Parikh et al., 2014; Shulman and Mangelsdorf, 2005; Szanto et al., 2004) suggesting that vitamin A5/X signaling may also be directly relevant for such diseases. Furthermore, null mutation of Rbp1 was reported to affect not only retinoid metabolism, but also glucose homeostasis and energy metabolism as Rbp1−/− mice displayed hyperglycemia, high rate of gluconeogenesis and reduced glucose-stimulated insulin secretion (Kane et al., 2011). Although systemic effects of RXR-mediated signaling may potentially contribute to RXR-dependent control of depression-related behaviors, the direct control of such behaviors at the level of CNS was documented (Krzyzosiak et al., 2010). Such central control by RXRs is further supported by the possibility of rising 9CDHRA levels in the brain by systemic administration of different dihydroretinoids (Krężel et al., 2021). Specifically, administration of 9CDHROL, used also in present study, was shown as an efficient method for a long-term increase of 9CDHRA in the brain (Krężel et al., 2021).
4. Conclusions
In summary, our data indicate that enhancement of RXR-mediated signaling protects against effects of social defeat stress by displaying anti-depressant effects in mice. Essential for such adaptive mechanism is 9CDHRA, an endogenous RXR ligand or alternatively the availability of its nutritional precursor 9CDHROL, vitamin A5/X-alcohol. Thus nutritional availability of vitamin A5/X could be considered in future epidemiological studies on depression and other stress-related disorders in humans. Additionally the role of polymorphisms or rare genetic variants of RBP1 and RXRγ as potential genetic susceptibility factors should be examined in larger cohort studies.
5. Experimental procedures
5.1. Animals
Rbp1−/− and Rxrγ−/− mutants as well as their wild type (WT) control male mice were raised on a mixed genetic background (60% C57BL/6J and 40% 129SvEms/j) from heterozygous crosses as described (Ghyselinck et al., 1999; Krężel et al., 1996), and tested at the age of 3–6 months. All mice were housed in groups of 4–5 mice per cage in a 7a.m.-7p.m. light/dark cycle in individually ventilated cages (Techniplast, Italy). For chronic social defeat stress experiments MICE cages were used with CD1 male mice as residents and 7 weeks old C57BL/6N mice as intruders. Food and water were freely available. The experiments were approved by local ethics committee (authorisation No. 2016022411354542) and accredited by the French Ministry for Superior Education and Research in accordance with the Directive of the European Union Council (2010/63/EU), and were carried in compliance with the guidelines of CNRS and the French Agricultural and Forestry Ministry (decree 87848).
5.2. Behavioral procedures
All behavioral tests were carried out in the Institute Clinique de la Souris (http://www.ics-mci.fr/) according to standard operating procedures. Whereas some mice were tested only for despair behaviors in the forced swim paradigm (Fig. 1c and d) all the other mice were tested in sucrose preference and forced swim tests according to the timeline depicted in Fig. 2 (note that non-treated Rbp1−/− and Rxrγ−/− mice and their respective control groups [Fig. 1a and b] were not subjected to CSDS). Each mouse was tested only once in each type of task in order to avoid confounding effects of multiple exposures to the same test. All studied cohorts, their behavioral experience and representation of corresponding data within specific figures were listed and explained in table shown in supplementary data as Fig. S3.
Forced swim test: The forced swim paradigm (Dalvi and Lucki, 1999) was carried out between 1p.m. and 4p.m. in a 3-L glass beaker half-filled with water at 22–23 °C (the water depth was 17 cm). All mice were tested only once in this task. To this end, each mouse was lowered gently into the water and the time of immobility was scored during a 6-min testing period. The mouse was judged immobile when it floated in an upright position and made only small movements to keep its head above the water. After 6 min, the mouse was taken out of the water, left to dry under a red light lamp and returned to its home cage. The immobility scores of each animal were used as an index of despair behavior.
Sucrose preference test: This task, designed to measure hedonic behaviors in mice (Moreau, 1997; Nestler et al., 2002) is based on the palatable nature of sucrose observed in a number of mouse strains. Sucrose-naive mice were habituated to 0.8% sucrose water solution starting at 5p.m. over 3.5 h. At 8:30p.m. sucrose and water containing bottles were weighed and placed in cages until morning when they were weighted at 9a.m. The sucrose preference was expressed as the percent of sucrose solution consumed with respect to total liquid consumption. Mice were not water deprived at any moment, in order to measure spontaneous sucrose preference and exclude any potential emotional confounds induced by stress of water deprivation.
Chronic Social Defeat Stress (CSDS): Social defeat stress procedure was a modified version of the protocol previously described (Berton et al., 2006; Golden et al., 2011). Briefly C57BL/6N mice were housed in groups of 4 mice/cage in order to avoid any effect of differential group housing prior to CSDS on the effects of stress. At 7 weeks of age mice were weighted and distributed semi-randomly to different experimental groups to assure similar mean weight of each group ranging at 22g ± 1.5g. Mice were then defeated chronically for 10 consecutive days by resident CD1 males, which were previously preselected for aggressive behavior. Every day C57BL/6N mice were exposed to the physical contact with an unfamiliar CD1 aggressor male in its home cage for 5 min starting at 5–6p.m. After each session of physical stress, C57BL/6N and CD1 mice were separated by a perforated wall and maintained in sensory contact for 24h. After the last session of stress mice were transferred into new cages and housed separately throughout the behavioral testing period. C57BL/6N non-stressed mice were housed in pairs throughout stress period and equally manipulated as mice used for social defeat procedure. Social preference was calculated daily for each mouse as percent of time the mouse was found resting at the barrier separating it from the resident, aggressor mouse. Such measures were taken three times a day approximately at 9a.m., 1p.m. and before stress session between 5 and 6p.m.
5.3. Pharmacological treatments
(R)-9-cis-13,14-dihydroretinoic acid (called hereafter 9CDHRA), (R)-9-cis-13,14-dihydroretinol (9CDHROL) and BMS649 (known also as UVI2108) were synthetized according to previous procedures (Krężel et al., 2021; Lehmann et al., 1992; Rühl et al., 2015). For intraperitoneal (IP) injections all compounds including also ATRA (Sigma-Aldrich, France), DHA (Sigma-Aldrich, France), and TTNPB (Sigma-Aldrich, France), were dissolved in ethanol and then mixed with sunflower oil, so that the final solution contained 3% of ethanol. Vehicle treatments consisted of IP injection of 3% ethanol solution in sunflower oil. Treatments were administered by intraperitoneal injections at vol/wt ratio 3 ml/kg between 8 and 11a.m. and 5–6 h before the test as previously validated (Wietrzych-Schindler et al., 2011). For chronic treatment during social defeat stress BMS649 or dihydroretinoids were applied in chow diet food and doses were calculated considering that mice consume 4 g of diet over 24 h. Treatment-supplemented food pellets were prepared shortly before stress protocols, immediately lyophilized and stored at −20 °C until use. During treatment, standard chow diet were replaced by treatment-supplemented food pellets and were available ad libitum for treated mice throughout treatment period. The consumption of treatment-containing pellets did not differ from the consumption of non-supplemented food pellets in control mice. Treated mice were compared to mice fed with standard chow diet called vehicle throughout the text.
5.4. Statistical analysis
The comparisons of behavioral performance in Rbp1−/− and Rxrγ−/− mice as well as pharmacological data for the treatments in WT and Rbp1−/− or Rxrγ−/− mice were carried out using Dunnett's multiple comparison test (Fig. 1a–c). Effects of 9CDHRA and TTNPB treatments in Rbp1−/− mice (Fig. 1d) and data for BMS649 treatments in social defeat stress experiments (Fig. 2b and c) were analyzed using two-way ANOVA with treatment and stress as independent factors followed by Bonferroni post-hoc analyses. Global social performance in Fig. 2d was compared using Student t-test. Social preference (Fig. S2) was analyzed using two-way RMANOVA with time and treatment as independent factors whereas effects of dihydroretinoids in CSDS model (Fig. 3) were evaluated using one-way ANOVA followed by Fisher's least significant difference comparison of specific treatment, or dose, or absence of stress with CSDS vehicle-treated mice (Veh) as the only reference group without any multiple comparisons. Additionally, sucrose preference data were evaluated with respect to 50% chance level using one-sample t-test. Significant differences are indicated in the corresponding figures.
CRediT authorship contribution statement
Agnieszka Krzyżosiak: Experimentation, Data curation, Formal analysis, Methodology. Anna Podleśny-Drabiniok: Experimentation, Data curation, Formal analysis, Methodology, Writing – original draft. Belén Vaz: Experimentation. Rosana Alvarez: Resources. Ralph Rühl: Conceptualization. Angel R. de Lera: Conceptualization, Funding acquisition, Resources. Wojciech Krężel: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
A.K., A.P.-D., B.V., R.A. report no conflict of interests. W.K., A.R.d.L. and R.R. are inventors of the patent family “Precursor compounds for providing retinoids of the vitamin A5 pathway and uses thereof” PCT/HU 2017/091937, US16102137, 127356JP-18340, 127578AU-18340, 127579CN-18340, 128446CA-18340. W.K., A.R.d.L. and R.R. are shareholders of CISCAREX UG.
Acknowledgments
We thank Brigitte Schuhbaur for mouse genotyping and Marta Wietrzych-Schindler for help in behavioral analyses. We acknowledge financial support from the University of Strasbourg Institute for Advanced Study (W.K.), LabEx ANR-10-LABX-0030-INRT, a French State fund managed by the Agence Nationale de la Recherche as part of the program Investissements d’Avenir ANR-10-IDEX-0002-02 (A.P-D., A.K., W.K.), Spanish MINECO (SAF 2016-77620-R-FEDER; PID 2019-107855RB-I00) and Xunta de Galicia (Consolidación GRC ED431C 2017/61 from DXPCTSUG; ED-431G/02 INBIOMED-FEDER “Unha maneira de facer Europa”) (B.V., R.A., A.R.dL.). A.K. was supported by PhD fellowship of French Goverment from French Embassy in Poland.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100375.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- Berton O., McClung C.A., Dileone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolanos C.A., Rios M. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bishwajit G., O'Leary D.P., Ghosh S., Sanni Y., Shangfeng T., Zhanchun F. Association between depression and fruit and vegetable consumption among adults in South Asia. BMC Psychiatr. 2017;17:15. doi: 10.1186/s12888-017-1198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyong E.F., Alfos S., Dumetz F., Helbling J.C., Aubert A., Brossaud J., Foury A., Moisan M.P., Layé S., Richard E. Dietary vitamin A supplementation prevents early obesogenic diet-induced microbiota, neuronal and cognitive alterations. Int. J. Obes. 2020;45(3):588–598. doi: 10.1038/s41366-020-00723-z. [DOI] [PubMed] [Google Scholar]
- Böhm V., Lietz G., Olmedilla-Alonso B., Phelan D., Reboul E., Bánati D., Borel P., Corte-Real J., de Lera A.R., Desmarchelier C. From carotenoid intake to carotenoid blood and tissue concentrations - implications for dietary intake recommendations. Nutr. Rev. 2020;79(5):544–573. doi: 10.1093/nutrit/nuaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaud B., Esterle L., Vaysse C., Alfos S., Combe N., Higueret P., Pallet V. A high-fat diet induces lower expression of retinoid receptors and their target genes GAP-43/neuromodulin and RC3/neurogranin in the rat brain. Br. J. Nutr. 2010;103:1720–1729. doi: 10.1017/S0007114509993886. [DOI] [PubMed] [Google Scholar]
- Cesario R.M., Klausing K., Razzaghi H., Crombie D., Rungta D., Heyman R.A., Lala D.S. The rexinoid LG100754 is a novel RXR:PPARgamma agonist and decreases glucose levels in vivo. Mol. Endocrinol. 2001;15:1360–1369. doi: 10.1210/mend.15.8.0677. [DOI] [PubMed] [Google Scholar]
- Dalvi A., Lucki I. Murine models of depression. Psychopharmacology (Berlin) 1999;147:14–16. doi: 10.1007/s002130051131. [DOI] [PubMed] [Google Scholar]
- de Lera A.R., Krężel W., Rühl R. An endogenous mammalian retinoid X receptor ligand. At Last! ChemMedChem. 2016;11:1027–1037. doi: 10.1002/cmdc.201600105. [DOI] [PubMed] [Google Scholar]
- de Urquiza A.M., Liu S., Sjoberg M., Zetterstrom R.H., Griffiths W., Sjovall J., Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Desvergne B. RXR: from partnership to leadership in metabolic regulations. Vitam. Horm. 2007;75:1–32. doi: 10.1016/S0083-6729(06)75001-4. [DOI] [PubMed] [Google Scholar]
- Dheer Y., Chitranshi N., Gupta V., Sharma S., Pushpitha K., Abbasi M., Mirzaei M., You Y., Graham S.L., Gupta V. Retinoid x receptor modulation protects against ER stress response and rescues glaucoma phenotypes in adult mice. Exp. Neurol. 2019;314:111–125. doi: 10.1016/j.expneurol.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Fadel L., Rehó B., Volkó J., Bojcsuk D., Kolostyák Z., Nagy G., Müller G., Simandi Z., Hegedüs É., Szabó G. Agonist binding directs dynamic competition among nuclear receptors for heterodimerization with retinoid X receptor. J. Biol. Chem. 2020;295:10045–10061. doi: 10.1074/jbc.RA119.011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck N.B., Bavik C., Sapin V., Mark M., Bonnier D., Hindelang C., Dierich A., Nilsson C.B., Hakansson H., Sauvant P. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Huang Y., Liu H., Sun X., Wu J., Zhang Z., Liu L., Zhou C., Jiang S., Huang Z. Bexarotene promotes microglia/macrophages - specific brain - derived Neurotrophic factor expression and axon sprouting after traumatic brain injury. Exp. Neurol. 2020;334:113462. doi: 10.1016/j.expneurol.2020.113462. [DOI] [PubMed] [Google Scholar]
- Huang J.K., Jarjour A.A., Nait Oumesmar B., Kerninon C., Williams A., Krężel W., Kagechika H., Bauer J., Zhao C., Baron-Van Evercooren A. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Cole G., Head E., Ingram D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009;29:12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M.A., Folias A.E., Pingitore A., Perri M., Krois C.R., Ryu J.Y., Cione E., Napoli J.L. CrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrations. Mol. Cell Biol. 2011;31:3277–3285. doi: 10.1128/MCB.05516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krężel W., Dupé V., Mark M., Dierich A., Kastner P., Chambon P. RXR gamma null mice are apparently normal and compound RXR alpha +/-/RXR beta -/-/RXR gamma -/- mutant mice are viable. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krężel W., Rivas A., Szklenar M., Ciancia M., Alvarez R., de Lera A.R., Rühl R. Vitamin A5/X, a new food to lipid hormone concept for a nutritional ligand to control RXR-mediated signaling. Nutrients. 2021;13(3):925. doi: 10.3390/nu13030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krężel W., Ruhl R., de Lera A.R. Alternative retinoid X receptor (RXR) ligands. Mol. Cell. Endocrinol. 2019;491:110436. doi: 10.1016/j.mce.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak A., Szyszka-Niagolov M., Wietrzych M., Gobaille S., Muramatsu S., Krężel W. Retinoid x receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron. 2010;66:908–920. doi: 10.1016/j.neuron.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Lehmann J.M., Jong L., Fanjul A., Cameron J.F., Lu X.P., Haefner P., Dawson M.I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992;258:1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J., Evans R.M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani M.M., Malm T., Lamb R., Jay T.R., Neilson L., Casali B., Medarametla L., Landreth G.E. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer's disease. Sci. Rep. 2017;7:42270. doi: 10.1038/srep42270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx W., Lane M., Hockey M., Aslam H., Berk M., Walder K., Borsini A., Firth J., Pariante C.M., Berding K. Diet and depression: exploring the biological mechanisms of action. Mol. Psychiatr. 2021;26(1):134–150. doi: 10.1038/s41380-020-00925-x. [DOI] [PubMed] [Google Scholar]
- McCullough K.M., Daskalakis N.P., Gafford G., Morrison F.G., Ressler K.J. Cell-type-specific interrogation of CeA Drd2 neurons to identify targets for pharmacological modulation of fear extinction. Transl. Psychiatry. 2018;8:164. doi: 10.1038/s41398-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau J.L. Reliable monitoring of hedonic deficits in the chronic mild stress model of depression. Psychopharmacology (Berlin) 1997;134:357–358. doi: 10.1007/s002130050467. discussion 371-357. [DOI] [PubMed] [Google Scholar]
- Natrajan M.S., de la Fuente A.G., Crawford A.H., Linehan E., Nunez V., Johnson K.R., Wu T., Fitzgerald D.C., Ricote M., Bielekova B. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E.J., Gould E., Manji H., Buncan M., Duman R.S., Greshenfeld H.K., Hen R., Koester S., Lederhendler I., Meaney M. Preclinical models: status of basic research in depression. Biol. Psychiatr. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Parikh M., Patel K., Soni S., Gandhi T. Liver X receptor: a cardinal target for atherosclerosis and beyond. J. Atherosclerosis Thromb. 2014;21:519–531. [PubMed] [Google Scholar]
- Peet M., Murphy B., Shay J., Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol. Psychiatr. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Peet M., Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65:1051–1059. doi: 10.2165/00003495-200565080-00002. [DOI] [PubMed] [Google Scholar]
- Perez E., Bourguet W., Gronemeyer H., de Lera A.R. Modulation of RXR function through ligand design. Biochim. Biophys. Acta. 2012;1821:57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rühl R., Krzyzosiak A., Niewiadomska-Cimicka A., Rochel N., Szeles L., Vaz B., Wietrzych-Schindler M., Alvarez S., Szklenar M., Nagy L. 9-cis-13,14-Dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J.C., Jay T., Goduni E., Quigley C., Mariani M.M., Malm T., Ransohoff R.M., Lamb B.T., Landreth G.E. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer's disease. J. Neurosci. 2015;35:6532–6543. doi: 10.1523/JNEUROSCI.4586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman A.I., Mangelsdorf D.J. Retinoid x receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- Simon M.M., Greenaway S., White J.K., Fuchs H., Gailus-Durner V., Wells S., Sorg T., Wong K., Bedu E., Cartwright E.J. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spathis A.D., Asvos X., Ziavra D., Karampelas T., Topouzis S., Cournia Z., Qing X., Alexakos P., Smits L.M., Dalla C. Nurr1:RXRα heterodimer activation as monotherapy for Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2017;114:3999–4004. doi: 10.1073/pnas.1616874114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.J., Korosi A., Layé S., Shukitt-Hale B., Barrientos R.M. Food for thought: how nutrition impacts cognition and emotion. NPJ science of food. 2017;1:7. doi: 10.1038/s41538-017-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm M., Becker A., Schroeder A., Bilkei-Gorzo A., Zimmer A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Gene Brain Behav. 2015;14:292–300. doi: 10.1111/gbb.12208. [DOI] [PubMed] [Google Scholar]
- Szanto A., Narkar V., Shen Q., Uray I.P., Davies P.J., Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl. 2):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- Ting S.M., Zhao X., Sun G., Obertas L., Ricote M., Aronowski J. Brain cleanup as a potential target for poststroke recovery: the role of RXR (retinoic X receptor) in phagocytes. Stroke. 2020;51:958–966. doi: 10.1161/STROKEAHA.119.027315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietrzych-Schindler M., Szyszka-Niagolov M., Ohta K., Endo Y., Pérez E., de Lera A.R., Chambon P., Krężel W. Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. Biol. Psychiatr. 2011;69:788–794. doi: 10.1016/j.biopsych.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A., Adams M.J., Agerbo E., Air T.M., Andlauer T.M.F. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn C.N., Dieterich A., Bazer A.S., Maita I., Giedraitis M., Samuels B.A. Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology. 2019;44:2220–2229. doi: 10.1038/s41386-019-0520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]